Abstract
Objective:
Inflammation is a well-known consequence of surgery. Although surgical debulking of tumor is beneficial to patients, the onset of inflammation in injured tissue may impede the success of adjuvant therapies. One marker for postoperative inflammation is IL-6, which is released as a consequence of surgical injuries. IL-6 is predictive of response to many cancer therapies, and it is linked to various molecular and cellular resistance mechanisms. The purpose of this study was to establish a murine model by which therapeutic responses to photodynamic therapy (PDT) can be studied in the context of surgical inflammation.
Materials and Methods:
Murine models with AB12 mesothelioma tumors were treated with either surgical resection or sham surgery with tumor incision but no resection. The timing and extent of IL-6 release in the tumor and/or serum was measured using enzyme-linked immunosorbent assay (ELISA) and compared to that measured in the serum of 27 consecutive, prospectively enrolled patients with malignant pleural mesothelioma (MPM) who underwent macroscopic complete resection (MCR).
Results:
MPM patients showed a significant increase in IL-6 at the time MCR was completed. Similarly, IL-6 increased in the tumor and serum of mice treated with surgical resections. However, investigations that combine resection with another therapy make it necessary to grow tumors for resection to a larger volume than those that receive secondary therapy alone. As the larger size may alter tumor biology independent of the effects of surgical injury, we assessed the tumor incision model. In this model, tumor levels of IL-6 significantly increased after tumor incision.
Conclusion:
The tumor incision model induces IL-6 release as is seen in the surgical setting, yet it avoids the limitations of surgical resection models. Potential mechanisms by which surgical induction of inflammation and IL-6 could alter the nature and efficacy of tumor response to PDT are reviewed. These include a wide spectrum of molecular and cellular mechanisms through which surgically-induced IL-6 could change the effectiveness of therapies that are combined with surgery. The tumor incision model can be employed for novel investigations of the effects of surgically-induced, acute inflammation on therapeutic response to PDT (or potentially other therapies). Lasers Surg. Med. 50:440–450, 2018. © 2018 Wiley Periodicals, Inc.
Keywords: surgery, inflammation, photodynamic therapy, resistance, malignant pleural mesothelioma, IL-6
INTRODUCTION
Malignant pleural mesothelioma (MPM) represents an aggressive cancer generated from asbestos exposure with a mortality rate of 38,400 to 43,000 deaths per year [1,2]. Despite recent advances in the field, treatment remains palliative in nature. Life expectancy is about 12 months for patients treated with pemetrexed and cisplatin chemo-therapies [3]. In an attempt to further improve outcomes, surgical debulking has been recommended as a component of multi-modality treatments by the International Mesothelioma Interest Group Congress for operable candidates [4]. However, surgical debulking does not provide for complete disease removal. Therefore, our lab has focused on addressing residual disease after surgery by applying photodynamic therapy (PDT) intraoperatively to macroscopic complete resections of MPM.
PDT involves the use of a photosensitizing drug alongside non-ionizing radiation, in the form of light energy, to eradicate diseased tissue. Once excited by light energy, the photosensitizer produces reactive oxygen species and leads to cell death. This direct killing of tumor cells is complemented by initiation of sterile inflammation and subsequent activation of anti-tumor immunity. In clinical trials of intraoperative PDT for MPM, surgical removal of bulk disease (extended pluerectomy/decorication, P/D) consisted of the removal of tumor while preserving the lung, phrenic nerve, and as much diaphragm as possible. This was followed by delivery of PDT to the entire chest cavity, including the following surfaces: apex of the heart, anterior chest wall, posterior chest wall, anterior sulcus, posterior sulcus, posterior mediastinum, pericardium, and diaphragm. Median overall survival was recently reported as 31.7 months for these patients, most of whom were treated for advanced (Stage III–IV) disease [5,6]. This is markedly longer than historical controls. We seek to further improve intraoperative PDT for MPM, as well as its application in treatment of disease at other locations where PDT could be combined with surgical resections. Toward this goal, we are considering the well-known immunosuppressive effects of surgery for their potential effect on PDT efficacy, which in many instances is dependent on an anti-tumor immune response [7,8].
Currently, little is known about the role of surgically-induced inflammation in the context of intraoperative PDT. Traditionally, the host immune response to surgery has been divided into two phases. The first represents a phase in which inflammatory cells are recruited to the site of injury via cytokine signaling [9]. This phase includes an influx of neutrophils and monocytes and is characterized by the release of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [9]. Prolongation or intensification of this phase due to postoperative complications or malnutrition can generate severe systemic inflammatory response syndrome (SIRS) [10], resulting in potentially life-threatening multiple organ distress syndrome (MODS) or acute respiratory distress syndrome (ARDS) [11]. Systemic inflammation is counteracted by the compensatory anti-inflammatory response syndrome (CARS), in which adaptive immunity is limited through decreases in antigen presentation and T cell proliferation, as well as induction of dendritic cell and T cell apoptosis [9]. During this second phase, signals are conducted by the cytokines IL-1 receptor agonist (IL1Ra), soluble TNF receptor 1 (sTNFR1), IL-4, IL-10, and transforming growth factor (TGF)-β. In the context of surgery, this second phase represents the conversion of macrophages to the “alternative” activation phenotype so as to promote wound healing and eradicate neutrophils [12,13]. These phases were initially thought to be in sequence; however, the observation of simultaneous pro- and anti-inflammatory cytokines at one day after surgery has complicated this interpretation [14]. Nevertheless, the above-defined divisions serve as a general basis for categorizing the body’s response to surgical trauma: a waxing activation phase coupled with a waning immunosuppression phase.
Among the above-mentioned effectors of post-surgical inflammation, IL-6 has received particular focus for several reasons. First, it may be predictive of outcome in surgical patients. In definitive surgery for non-small cell lung cancer (NSCLC), the level of IL-6 one day post-operatively was independently predictive of recurrence [15]. In a broader sense, IL-6 was also predictive of patient outcome in colorectal cancer (overall survival (OS), P < 0.001) [16], esophageal squamous cell carcinoma (OS, P < 0.001) [17], bladder cancer (OS, P = 0.004) [18], and neuroblastoma (event-free survival, P < 0.008) [19]. High circulating levels of IL-6 were predictive of poor response to chemotherapy in a cohort of NSCLC patients [20]. In oral squamous cell carcinoma, IL-6 was predictive of lymph node metastasis in response to preoperative chemoradiotherapy [21]. Second, IL-6 signaling can engage a broad spectrum of cellular resistance and immune function pathways. IL-6 release from cancer-associated fibroblasts (CAFs) initiated epithelial to mesenchymal transition (EMT) of lung cancer cells [22]. Also in NSCLC, high levels of IL-6 were associated with an increased expression of anti-apoptotic and DNA repair molecules and led to cisplatin resistance [23]. IL-6 signaling is commonly associated with activation of the transcription factor STAT-3, which can lead to transcription of anti-apoptotic factors Bcl-2 and survivin [24,25] and activation or overexpression of multi-drug resistant proteins [26,27]. In regard to cellular immunity, STAT-3 signaling can lead to numerous immunosuppressive effects such as increases in arginase expression by myeloid cells [28] and decreases in expression of major histocompatibility complex (MHC) Class II on dendritic cells (along with attenuation of antigen presentation) [29,30]. IL-6 also reduces IL-12 secretion by dendritic cells, accompanied by decreased proliferation of CD4+ and CD8+ T cells and decreased secretion of interferon (IFN)-γ [31]. This effect on dendritic cells was shown to decrease anti-tumoral immunity in tumor obtained from colorectal cancer patients [31]. IL-6 is also associated with the differentiation of naive CD4+ cells to T-helper 17 (Th17) cells that produce the cytokines IL-17 and IL-22 to attract neutrophils to sites of inflammation [32]. It can mediate acute phase reactions of an inflammatory response, including the synthesis of complement proteins [33].
A key question, therefore, is whether surgical inflammation (with special attention to IL-6) has an adverse effect on the efficacy of PDT. If so, understanding the mechanisms of this inhibitory effect will be important, as it might be possible to circumvent this negative effect pharmacologically to improve patient outcomes. The purpose of our study was to use clinical information to develop a novel preclinical model that enables isolation of the inflammatory consequences of surgery from its beneficial role in reducing tumor burden. In the present paper, we describe the development of such a preclinical model, which is to be employed in investigations of the effects of surgically-induced, acute inflammation on the therapeutic response to PDT (or potentially other therapies). We also review potential mechanisms by which surgical induction of inflammation and IL-6 could alter the nature and efficacy of tumor response to PDT.
MATERIALS AND METHODS
Clinical Trial
Serum IL-6 levels were studied in patients with epithelioid MPM who were enrolled on a randomized phase II prospective trial of P/D and post-operative chemotherapy with or without intraoperative PDT. All subjects were treated in accordance with protocols approved by the Institutional Review Board at the Hospital of the University of Pennsylvania. As defined by protocol, all subjects received lung-sparing P/D to achieve macroscopic complete resection (MCR) of MPM burden. A detailed description of this surgery has been published [34], but in brief, the technique involves preservation of the entire lung while MCR is achieved by mobilization of tumor and pleura from the lung and other thoracic surfaces. After the completion of MCR, some patients went on to receive light delivery for Photofrin-PDT as was dictated by the randomization scheme for this phase II randomized trial [5,6]. Notably, for the purpose of the present report, we evaluated blood samples that were obtained approximately 5–7 days prior to surgery (baseline) and at the completion of MCR. Therefore, both samples of interest were collected prior to light delivery for PDT. Blood was collected from a total of 26 of the first 27 consecutive subjects treated on this trial. Twenty-four of 27 subjects underwent surgery (and PDT if randomized to do so) at the Hospital of the University of Pennsylvania and three subjects at Roswell Park Cancer Institute. Twenty-two of 26 were male and 8 received neoadjuvant pemetrexed and platinum-based chemotherapy. Surgeries were performed between June 2014 and June 2017.
Collection and Analysis of Patient Serum
Blood samples were collected in EDTA-coated tubes from patients 5–7 days prior to surgery and at the achievement of MCR. Serum was isolated by centrifugation at 450g for 20 minutes and divided into 0.5 ml aliquots stored at −80°C. PGE2 and IL-6 levels in serum from study patients were defined by enzyme-linked immunosorbent assay (ELISA) per manufacturer’s specifications (R&D Systems Inc, Minneapolis, MN). Serum for testing PGE2 was diluted threefold according to manufacturer’s specifications. Each sample was aliquoted into two wells of the ELISA plate, representing a total of two replicates per sample. Lower limit of detection was 39 pg/ml for PGE2 and 3.13 pg/ml for IL-6.
Murine Surgical Resection and Tumor Incision Models
Animal studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and animal facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care. AB12 mouse mesothelioma cells were maintained in DMEM (ATCC) supplemented with 10% fetal bovine serum, 2 mm L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptavidin (Gibco, Carlsbad, CA). Tumors were propagated by injecting 1 × 106 cells intradermally over the right flank of BALB/c mice (Charles River Laboratories, Wilmington, MA). Tumor volume was calculated using the following formula: π/6* (tumor length) * (tumor width)2. Mice were anesthetized via inhalation of isofluorane in medical air (VetEquip anesthesia machine, Pleasanton, CA) and long-acting analgesia was provided via buprenorphine (Zoopharm, Windsor, CO) injection prior to initiation of surgery. At study endpoints, euthanasia was achieved by CO2 inhalation in a metered chamber (IMI Norgren, Littleton, CO).
For the surgical resection model, tumors were grown to a volume of ~300 mm3. For the purpose of resection, skin flaps were generated adjacent to the tumor, the tumor was exposed by inversion of these flaps, and resection was performed to a measured tumor volume of ~80 mm3 (residual tumor). Skin flaps were closed via sutures and mice were provided subcutaneous fluids.
For the sham tumor incision model, tumors were grown to a volume of ~80 mm3. The tumor was exposed as described above, but no resection was performed. Instead, tumors were incised one-half of their depth along their entire diameter. The skin was then closed with sutures as described above. All surgical procedures were performed using sterile technique.
Collection and Analysis of Murine Samples
Tumors were excised from euthanized animals at indicated times and frozen in a slurry of dry ice and 70% ethanol. Frozen tumors were homogenized on dry ice and then submitted to three freeze-thawcycles. Protein concentrations were measured by bicinchoninic acid (BCA) assay (Thermo Scientific, Hampton, NH). Additionally, prior to confirmatory euthanasia, blood was collected via cardiac puncture. Blood was collected using a syringe bearing a 22-gauge needle (BD, Franklin Lakes, NJ) and transferred to microcentrifuge tubes (Fisher Scientific, Hampton, NH). IL-6 ELISA of serum and tumor samples was performed per manufacturer’s instruction (R&D Systems, Minneapolis, MN). Serum was run undiluted. Tissue homogenate was run at a concentration of 1.0−1.2 mg/ml at dilutions of 1:1 and 1:2. Final values were adjusted for protein concentration and dilution factors. All samples were run in duplicate.
Statistics
Data are represented by box plots. The non-parametric Mann–Whitney U test was utilized to compare means using GraphPad Prism Version 7.03. Two-tailed P-values <0.05 were considered significant in this study.
RESULTS AND DISCUSSION
Resection of Mesothelioma Generates IL-6
The inflammatory response to surgery is characterized by an influx of immune cells and related changes in soluble factors that regulate innate immunity, among which the cytokine IL-6 is a well-known pro-inflammatory factor [33]. Among other prevalent inflammatory mediators, we also considered TNF-α and prostaglandin E2 (PGE2). However, in a previous study of patients treated with pleurectomy or extrapleural pneumonectomy, we found the level of TNF-α to be no different between pre-anesthesia, post-anesthesia, and post-surgical timepoints [35]. Similarly, in the present study, we found that the level of PGE2 did not change after MCR compared to baseline values (Supplemental Fig. S1). Therefore, based on its value as a prognostic marker in the surgical setting [20,21], we characterized the expression of IL-6 during P/D of MPM.
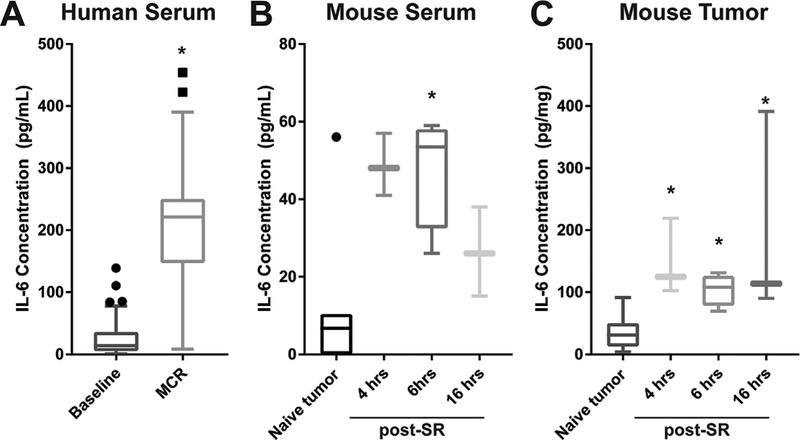
Figure 1A summarizes serum IL-6 levels in MPM patients before and after MCR. Serum level (mean SE) of IL-6 was 212 ± 22 pg/ml at the time that MCR was achieved, compared to a value of 30 ± 7 pg/ml at baseline. The paired baseline and MCR value for each patient is shown in Supplemental Figure S2. The IL-6 level after MCR for MPM is in agreement with the values reported by Amar et al. of 188 ± 123 pg/ml after thoracic surgery [36]. It is also similar to the values of 155.6 ± 13.6 pg/ml for non-recurring and 147.5 ± 10.8 pg/ml for recurring disease recorded by Kita et al. after lobectomy under either video-assisted thorascopic surgery (VATS) or thoracotomy (TH) [15]. In these studies, IL-6 was measured in the post-anesthesia care unit (PACU) and 1 hour after the completion of surgery, respectively. In the present report, IL-6 was measured immediately upon completion of MCR, that is, the time at which PDT would be delivered. The average length of P/D for MCR in these patients was 6 hours 27 minutes, with a minimum and maximum duration of 3 hours 54 minutes and 9 hours 36 minutes, respectively.
Fig. 1.
Surgical resection increases systemic and local IL-6. Surgical resection increased serum levels of IL-6 at the conclusion of macroscopic complete resection (MCR) as compared to baseline levels in a clinical trial that includes extended pleurectomy/decortication for treatment of malignant pleural mesothelioma (A). In flank tumors of AB12 murine mesothelioma, surgical resection (SR) increased serum (B) and tumor (C) levels of IL-6 (post-SR) as compared to untreated controls (naϊve tumor). Dots above box plots represent values deemed to be outliers by Tukey test. N = 27 for serum and n = 3−4 human for mouse tumor and serum, except in tumor controls where n = 7−10. *P < 0.05 for comparison to baseline or tumor control.
Having established that IL-6 levels were elevated at MCR in MPM patients, we next sought to replicate this finding in the preclinical setting. AB12 mesothelioma tumors were propagated on the flank of BALB/c mice, grown to a size of ~300 mm3 and then surgically resected to a tumor volume of ~80 mm3. A 4-hour delay was introduced after tumor resection in order to allow the inflammatory response to develop for a length of time similar to the minimum interval between first incision and light delivery for PDT in patients. At 4 hours after surgery, IL-6 levels in serum were 49 ± 4 pg/ml, compared to 11 ± 7 pg/ml in mice that were previously unresected (naϊve tumor), representing a 4–5 fold increase in circulating IL-6 after tumor resection (Fig. 1B). IL-6 levels remained similarly high at 6 hours after resection but declined at 16 hours. To access the local change in IL-6 at the site of tumor resection, which is importantly the site of PDT delivery, the residual tumor burden was collected at 4, 6, and 16 hours after surgery (Fig. 1C). Local IL-6 levels were elevated from 34 ± 8 pg/mg in unresected tumor to 149 ± 29 pg/mg in residual tumor burden at 4 hours after resection; levels in the residual tumor remained high through 16 hours after resection. Although the source of IL-6 is unknown in both clinical and preclinical studies, many cells express IL-6, including macrophages, monocytes, fibroblasts, keratinocytes, endothelial cells, T cells, B cells, granulocytes, mast cells, and tumor cells themselves [37].
Caveats of the Surgical Resection Model in a Combined Modality Setting
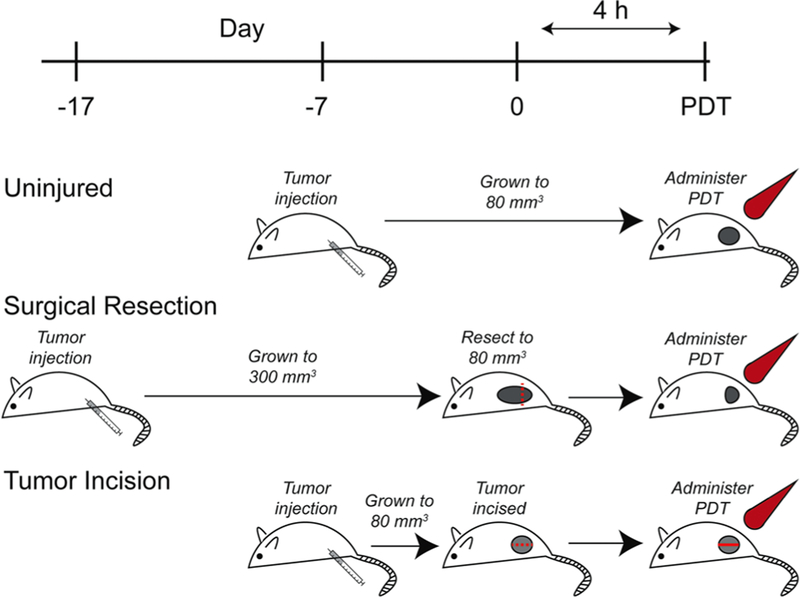
The above studies establish that murine tumor resection produces local increases in IL-6 at times that align with the delivery of PDT in our clinical trial. These results rationalize our proposed model to study the effects of surgically-induced inflammation on outcomes to intra-operative PDT. Nevertheless, such a model of surgical resection is accompanied by significant caveats when employed in combinational therapy with the intent to assess the efficacy of a subsequent therapy. For example, controlled investigation of the effects of surgical resection on PDT necessitates that surgically resected tumors and unresected tumors are administered light at a similar tumor volume. This requires that tumors destined for resection are grown to a larger overall volume than those to be treated with PDT alone. As depicted in Figure 2, animals that are to receive resection prior to PDT must have their tumors initiated about 7–10 days earlier than animals that receive stand-alone PDT. Tumors destined for surgery are then allowed to grow to a volume of ~300 mm3, which accommodates resection to a size of about ~80 mm3, that is, the size of tumors in the comparison group to receive PDT-alone. As tumors in the resection group are grown to a larger size, they may exhibit confounding variables outside of those induced by surgical trauma alone. For example, larger tumors could be associated with higher levels of vascular endothelial growth factor (VEGF) and other angiogenic or resistance factors that could independently alter PDT outcomes, as well as be characterized by increased deposition of extracellular matrix and greater numbers of fibroblasts [38–41]. In addition, the degree of immune infiltrate to the tumor may be different in larger versus smaller tumors due to prolonged exposure to the adaptive immune system in tumors grown over longer times [42]. To overcome these limitations, we employed a second model, tumor incision (TI), to replicate the inflammatory effects of surgical trauma in the absence of gross removal of tumor burden (Fig. 2). This model involves the exposure and incision of a tumor, as would be done for resection, but no tumor is removed and thus tumor size is unchanged. Consequently, tumors can be initiated at the same time and grown to the same size, yet the introduction of an incision provides for isolated investigation of the effects of surgical trauma to residual disease on its response to subsequent therapy.
Fig. 2.
Tumor incision model overcomes the limitations of surgical resection. Our research goal is to assess the effect of surgically-induced inflammation on PDT outcome, thus both resected tumor and uninjured tumor should undergo PDT at similar tumor volume (~80 mm3). Tumors to receive PDT alone are grown only to 80 mm3 (uninjured). However, the combination of PDT with resection (surgical resection) necessitates that tumors be allowed to grow to a larger size followed by resection to the desired 80 mm3. These differences in tumor sizes between the groups may introduce multiple variables that could alter PDT outcome, such as expression of resistance factors, immune tolerance, and levels of tumor hypoxia. The tumor incision model (TI) (tumor incision) allows for introduction of an inflammatory insult without tissue resection, allowing for controlled investigation of the consequences of surgically-induced inflammation by injuring the tumor without changing tumor volume by resection.
Tumor Incision (TI) Elicits an Inflammatory Response Without Need for Resection
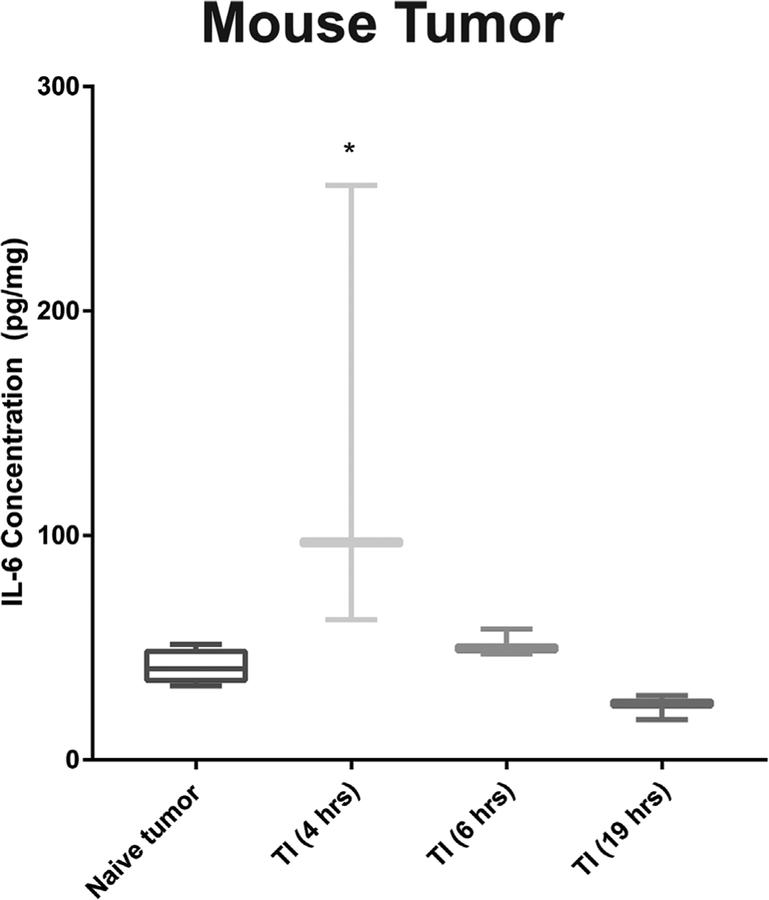
We next sought to confirm that TI generates a local inflammatory response in order to validate this model for investigations of the effects of surgically-induced inflammation on tumor response to PDT. The incision of tumor significantly increased local IL-6 (Fig. 3); IL-6 levels (mean ± SE) increased from 42 ± 3 pg/mg in naϊve tumors to 138 ± 38 pg/mg at 4 hours after TI, followed by a decline to baseline levels at times 6 hours and longer after TI. As described above, PDT would be introduced at 4 hours after TI in order to parallel the minimum delay between first incision and light delivery for PDT in clinical trials. Thus, PDT would be initiated under inflammatory conditions. It is also noteworthy that IL-6 levels decline from 6 to 19 hours after TI. This is advantageous in the combinational setting because IL-6 elevations that are detected in this timeframe may, therefore, be interpreted to result from the effects of PDT.
Fig. 3.
Tumor incision induces early increases in tumor IL-6. IL-6 spikes at 4 hours post-TI, and subsequently returns to levels detected in untreated tumors. Thus, the TI model can be used to investigate the effect of injury-induced IL-6 on subsequent therapy (such as PDT) that is administered 4 hours after TI. N = 3−6 mice.
Implications of an Inflammatory State on PDT Response
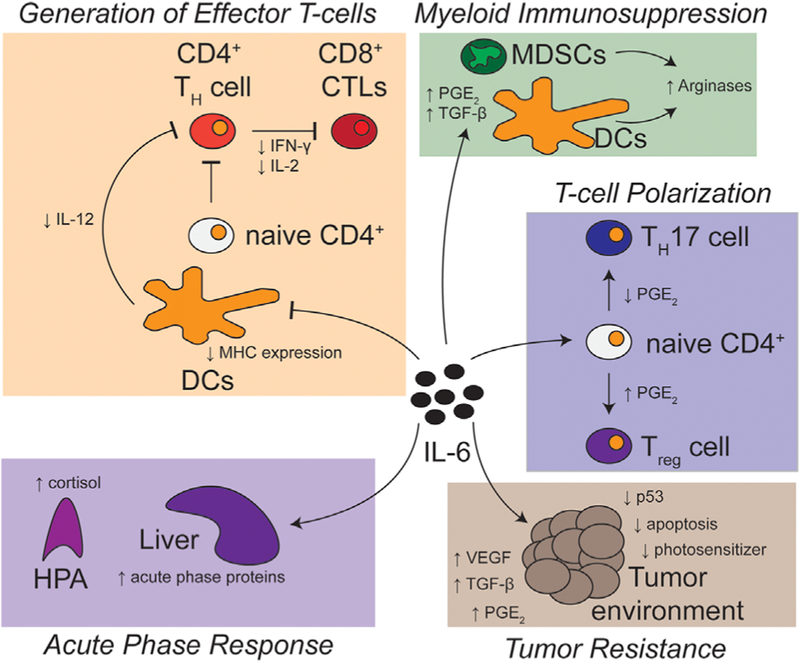
When PDT is performed intraoperatively, as for MPM, we expect that the inflammatory state accompanying tumor resection (modeled by TI) could alter numerous aspects of PDT response. Figure 4 reviews several facets of the multiple juxtaposed mechanisms through which high levels of IL-6 could alter response to PDT.
Fig. 4.
Potential effects of pre-PDT elevation of IL-6 on intraoperative PDT. Elevations in IL-6 at the time of light delivery for PDT may alter tumor response to PDT through multiple mechanisms. First, IL-6 may act at a molecular level on the tumor environment itself, generating increased resistance to PDT. IL-6 can also act to alter cell-based immunity in both the innate and adaptive systems of immune response. Increases in IL-6 can result in decreased antigen presentation by dendritic cells (DCs), as well as decreased IL-12 secretion, both of which may restrict the formation of anti-tumor immunity. IL-6-dependent release of TGF-b (prior to or after PDT delivery) and PGE2 (after PDT delivery) may result in the influx of myeloid-derived suppressor cells (MDSCs) and influence the differentiation of naϊve CD4+ T cells into regulatory T cells (Treg). Lastly, induction of cortisol can generate immunosuppressive phenotypes. This works in opposition to the acute phase proteins, such as complement and pentraxin, which induce neutrophilia and clear PDT-generated cell debris.
First, IL-6 can lead to increases in the expression of PDT resistance factors. For example, IL-6 has been demonstrated to increase tumor expression of transporters that promote efflux of drugs, including photosensitizers from cancerous cells [43]. Included among these transporters is the multidrug resistance transporter P-glycoprotein (P-gp, also known as MDR-1), as well as the breast cancer resistance protein (BCRP, also known as ABCG2) [44–46]. In osteosarcoma, it has been shown that IL-6 release by mesenchymal stem cells (MSCs) can lead to induction of P-gp in tumor cells [27]. Moreover, in prostate cancer cell lines, Yu et al. demonstrated that IL-6 leads to increased expression of BCRP [47], and others have reported IL-6 to induce 20- to 25-fold increases in BCRP expression in lung carcinoma cell lines [26]. Chu et al. demonstrated that P-gp reduced intracellular levels of protoporphyrin IX after administration of photosensitizer pro-drug, 5-aminolevulinic acid hexyl ester (Hexyl-ALA), and this efflux generated resistance to PDT [48]. Also, Usuda et al. demonstrated a negative correlation between response to Photofrin-PDT and BCRP levels in patients with centrally located, early lung cancer [49]. In contrast, BCRP-mediated drug efflux did not occur with several photosensitizers, such as hematoporphyrin IX and meso-tetra(3-hydroxyphenyl)porphyrin (m-THPP) [43]. Therefore, additional study is required to determine the effects of surgically-induced IL-6 on photosensitizer efflux as a mediator of PDT efficacy.
IL-6 can also elicit other mechanisms of tumor resistance to PDT, especially through its activation of transcription factor STAT-3. For example, STAT-3 regulates expression of apoptosis-controlling proteins such as those in the Bcl-2 family [24,50]. Bcl-2 expression is associated with resistance to PDT [51], and thus IL-6 could theoretically protect from PDT damage by altering the expression of Bcl-2 or its family members. Survivin is another anti-apoptotic protein that is upregulated by IL-6 [25] and has been found to confer PDT resistance [43]. Survivin enacts anti-apoptotic function by interacting with adaptors and cofactors for caspase activity. It can prevent signaling along the intrinsic and extrinsic apoptotic pathways [52], both of which play a role in PDT-mediated cytotoxicity [53].
Tumor suppressor protein p53 provides another potential node of interaction between IL-6 and PDT resistance. Dysregulation of p53 function desensitized human colon carcinoma cells to Photofrin-PDT [54]. Surgical trauma-induced inflammation may contribute to p53 dysregulation, since IL-6 was shown to downregulate p53 expression in various human cell lines by increasing the capacity for mouse double minute 2 homolog (MDM2) to degrade p53 proteins [55]. This degradation also led to epithelial-mesenchymal transition (EMT), which promotes escape from cellular stress. Consequently, EMT could contribute to inflammation-mediated resistance to PDT, but it is unlikely to be a sole effector since a recent investigation in pancreatic cell line monolayers demonstrated EMT induction alone did not generate PDT resistance [56].
Inflammation and IL-6 are also linked to activation of angiogenic pathways, with further potential contribution to therapy resistance. As tumors reach larger sizes, they require increased vascularization in order to maintain their supply of oxygen. Therefore, many tumors release proangiogenic signals in order to generate new blood vessels. The major constituent of this process is VEGF [57]. IL-6 has been shown to increase the expression of VEGF in various cancer models [58–60]. In the context of combination therapy, proangiogenic signals that are released as a result of surgical inflammation could serve to lessen the extent of tumor vascular damage that is attainable in tumors by PDT and consequently reduce the efficacy of treatment.
Separate from its potential effects on PDT at a molecular level, IL-6 that is characteristic of surgical inflammation may also modulate innate immune response to PDT. In the same tumor model that we have studied, namely AB12 murine mesothelioma, Fridlender et al. demonstrated that neutrophils were driven to a pro-tumoral state by the presence of transforming growth factor (TGF-β) [61]. TGF-β signaling is linked with IL-6 expression [62], thus providing a mechanism whereby elevations in IL-6 can alter myeloid cell function. Furthermore, STAT-3-dependent downregulation of the interferon regulatory factor 8 (IRF-8) can induce development of myeloid derived suppressor cells (MDSCs) that are responsible for functional suppression of T cells [63]. Yet, it is important to note that in this study STAT-3 signaling was attributed to the cytokine granulocytic-colony stimulating factor (G-CSF). In surgical investigations, thoracotomy of lung cancer patients increased the level of circulating MDSCs intra-operatively. Moreover, MDSC levels remained high at 7 days after treatment [64]. If MDSCs are present after MPM surgery, surgically-induced IL-6 may potentiate their immunosuppressive ability by increasing the expression of effector proteins such as arginase-1 and nitric oxide [28,65]. Further study is required to assess the function of myeloid cells in intraoperative PDT, as well as the role for surgically-induced inflammation in driving the commitment of myeloid cell precursors to an immunosuppressive phenotype prior to release into circulation.
IL-6 also has a wide array of effects on the transition to adaptive immunity. IL-6-dependent activation of STAT-3 suppresses the activation of murine-derived [29] and human-derived [31] dendritic cells, as witnessed by decreased expression of MHC Class II molecules and costimulatory signals CD80, CD86, and CD40 [29,30]. Dendritic cells act as antigen presenting cells, traveling from the local site of inflammation to the lymph nodes in order to activate T cell responses. MHC Class II interactions between dendritic cells and T cell receptors [66] are required in order to generate helper T cells from naϊve CD4+ T cells. Moreover, antigen presentation to naϊve CD8+ T cells in the absence of co-stimulatory signals generates tolerance rather than cytotoxic T cells [67]. Therefore, decreased presence of MHC Class II molecules/costimulatory signals would diminish the number of CD4+ (helper) and CD8+ (cytotoxic) T cells, as was observed in biopsies from colorectal cancer patients [31]. Treatment of human dendritic cells with IL-6 also decreased the secretion of IL-12 [31]. IL-12 diminishment is relevant in that it is necessary for the differentiation of Th1 responses [68]. The Th1 subtype of CD4+ T cells contributes to anti-tumor immunity by recruiting natural killer (NK) cells, type 1 macrophages, and antigen-presenting cells, as well as enhancing CD8+ T cell responses [69]. IL-12 is also linked to the formation of immune memory, in the form of CD8+ memory T cells, during vaccination with the vaccinia virus [70]. This link between IL-6-dependent decreases in IL-12 and the restriction of immune memory may explain why Brackett et al. showed IL-6 knockout mice (IL-6KO) had increased immune memory in colon and breast cancer models after PDT treatment with the photosensitizer 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) [71].
Another axis of potential interaction between surgery, inflammation, and immunosuppression is provided through cyclooxygenase-2 (COX-2). In cell culture studies, IL-6 has also been shown to induce the expression of COX-2 [72], an enzyme that is responsible for metabolizing arachidonic acid to prostaglandins as part of the inflammatory process [73]. Overall, COX-2 levels are prognostic in MPM [74–76]. It is known that both IL-6 and COX-2 increase after surgery in murine models [77], and the two were found to be co-expressed in patient samples [78]. Similarly, upregulation of COX-2 is also a well-described effect of PDT. Inhibition of COX-2 led to increased apoptosis, measured as an increase in poly (ADP-ribose) polymerase (PARP) cleavage and decrease in Bcl-2 expression [79]. Moreover, inhibition decreased the expression of inflammatory cytokines tumor necrosis factor (TNF)-α and IL-1β and increased the expression of the anti-inflammatory cytokine IL-10. PDT-induced upregulation of COX-2 also led to excesses in prostaglandin E2 (PGE2) after treatment [80–82]. This upregulation in PGE2 after PDT has been linked to increased expression of the hypoxia inducible factor (HIF)-1α, even in the absence of oxygen deprivation [83]. Therefore, the generation of PGE2 after PDT may be enhanced by the presence of IL-6 in the post-surgical setting, generating HIF-1 α response.
Among the effects of PGE2, it may circumvent the formation of anti-tumor adaptive immunity by negatively influencing the balance between T helper 17 (Th17) and regulatory T cells (Treg) [84,85]. Th17 cells are a subset of CD4þ helper cells that drive inflammation via the secretion of cytokines such as IL-17 and IL-22 [32]. In contrast, Treg cells derive from naϊıve CD4+ T cells in order to downregulate effector T cell function and are instrumental to the avoidance of autoimmunity [86]. Typically, IL-6 favors the generation of Th17 cells by inhibiting FOXP3, the transcription factor necessary for the differentiation of Treg cells [32,87]. However, Sharma et al. demonstrated that PGE2 generates a 12-fold increase in the expression of FOXP3. In addition to reducing effector T cell function, this favoring of Treg cells over Th17 cells may impact PDT by diminishing the recruitment of neutrophils to the tumor-draining lymph node after PDT administration, shown to be a necessary step in the establishment of anti-tumor immunity in PDT [88]. This group also demonstrated that inhibition with the selective COX-2 inhibitor SC58236 significantly improved the ability of naϊıve CD3+ T cells to proliferate in the presence of extracted Treg cells [84]. Therefore, PDT delivered in the setting of high IL-6 levels, induced due to surgery, could potentially increase levels of COX-2 and PGE2. This may result in therapy-dependent development of immunosuppression.
Finally, it is also possible that the known increases in cortisol caused by surgical stress could blunt the anti-tumor immune responses induced by PDT. IL-6 release stimulates the hypothalamic-pituitary axis (HPA), the eventual product of which is cortisol production. Cortisol enacts its immunosuppressive activity through a variety of steps. First, cortisol can inhibit the inflammatory transcriptional regulators nuclear factor-kappa-B (NF-κB) and activator protein-1 (AP-1) [89]. Furthermore, glucocorticoids induce the expression of the anti-inflammatory cytokine IL-10 [89]. Similar to the IL-6 mechanism described above, IL-10 suppresses the activation of macrophages and dendritic cells [90]. Therefore, it may limit the formation of effector Th cells. In a murine model of multiple sclerosis, glucocorticoids also acted on lymphocytes directly, reducing the adhesion of Th17 cells and inducing their apoptosis [91,92]. Surgically-induced cortisol release may, therefore, also negatively impact the formation of anti-tumor immunity by PDT.
Taken together, IL-6 may operate through a number of tumor cell- and immune-mediated mechanisms to generate resistance to PDT. However, IL-6 also has connections to PDT-enhancing effects via paracrine signaling to tumor cells. Administration of IL-6 or IL-6R-IL-6 complex (termed Hyper-IL-6) enhanced the response of Colon26 tumors to PDT with the photosensitizer HPPH. In vitro, there was a significant reduction in the cell cycle markers cyclin D1, cyclin E, Cdk2, Rb, and p27Kip1 after PDT in the presence of Hyper-IL-6 [93]. Addition of IL-6 during PDT of Lewis lung carcinoma (LLC) cells with the photosensitizer mono-L-aspartyl chlorin e6 (NPe6) increased apoptosis by inducing Bax expression [12,94]. IL-6 can also enhance PDT via endocrine signaling. More specifically, inflammatory cytokines released as a result of surgical inflammation, such as IL-6, act on the liver to induce the production of acute phase proteins [33]. These proteins include C-reactive protein (CRP), complement proteins (e.g., C3), and coagulation proteins (e.g., fibrinogen) [33,95]. Interestingly, two of these acute phase proteins (CRP and fibrinogen) were predictive of response to surgery of MPM. High levels of CRP were found to indicate worse overall survival in patients treated with radical surgery (82.5% extrapleural pneumonectomy, 11.8% pleurectomy) as part of a multimodal therapy. This was hypothesized to be due to its connection to inflammatory cytokines, such as IL-6, as a sign of tumor aggressiveness [96]. This same group later found that patients within the 75th percentile of pre-treatment circulating fibrinogen levels (i.e., >750 mg/dl) responded significantly worse to radical surgery as a component of multimodality treatment [97]. Acute phase proteins such as CRP, serum amyloid P, and C3 are increased in the tissue of PDT-treated tumors [98–100]. This increase was hypothesized to benefit PDT by opsonizing and clearing dead tumor cell debris [98]. In addition, acute phase proteins such as coagulation proteins and complement may induce neutrophilia [101,102]. Others have reported neutrophils to be linked to the formation of anti-tumor immunity after PDT administration [103], perhaps suggesting a positive impact of an acute phase response. Yet, the implication of timing is not currently understood but is likely very relevant. In other words, the potentially beneficial effects of a post-PDT inflammatory response may be negated when its mediators are present in the pre-PDT tumor environment. Additional studies are needed in this area to better discern the consequences of surgically-induced inflammation on acute phase response to PDT.
CONCLUSION
It has been previously shown that surgical inflammation can generate resistance to subsequent therapies, such as chemotherapy. IL-6 is likely an important mediator of this phenotype, as it may generate resistance to treatment on both the molecular (i.e., expression of resistance factors) and cellular (i.e., propagation of immunosuppressive cells) levels. In the current paper, we describe the design of a novel murine model that provides a means to study the consequence of surgically-induced inflammation on adjuvant therapy. This model avoids the need to grow tumors to a larger size in groups to receive resection and PDT compared to those to receive PDT alone. Moreover, this model separates the benefit of surgical resection from the expected negative consequences of surgically-induced inflammation. More specifically, the tumor incision model increases the expression of IL-6, just as observed in the clinical setting, without introducing growth delays secondary to surgical resection. Thus, the effects of inflammation on PDT outcome will not be masked by the benefit that is provided by surgical removal of tumor. In ongoing studies, we are utilizing this model to better understand the role of IL-6 and other mediators of inflammation in intraoperative applications of PDT.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH/NCI grants P01-CA087971 and R01-CA85831. We express thanks to Joann Miller and Min Yuan (Radiation Oncology, UPenn), as well as Matthew Fair and Surya Vadrevu (Wistar Institue) for technical assistance with the animal models and ELISA assays.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Contract grant sponsor: National Cancer Institute; Contract grant numbers: P01-CA087971, R01-CA85831.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
REFERENCES
- 1.Odgerel CO, Takahashi K, Sorahan T, et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup Environ Med 2017;74(12): 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, Pruss-Ustun A. The global burden of disease due to occupational carcinogens. Am J Ind Med 2005;48(6):419–431. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21(14):2636–2644. [DOI] [PubMed] [Google Scholar]
- 4.Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: Meeting summary of the International Mesothelioma Interest Group Congress, September 11–14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145(4):909–910. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93(5):1658–1665. Discussion 1665–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann Thorac Surg 2017;103(3): 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brackett CM, Gollnick SO. Photodynamic therapy enhancement of anti-tumor immunity. Photochem Photobiol Sci 2011; 10(5):649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gollnick SO, Brackett CM. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol Res 2010; 46(1–3):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabrowska AM, Slotwinski R. The immune response to surgery and infection. Cent Eur J Immunol 2014;39(4): 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). Jama 1992;268(24):3452–3455. [PubMed] [Google Scholar]
- 11.Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today 2010;40(9):793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184(7):3964–3977. [DOI] [PubMed] [Google Scholar]
- 13.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009;175(6):2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokart D, Capo C, Blache JL, Delpero JR, Houvenaeghel G, Martin C, Mege JL. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg 2002;89(11):1450–1456. [DOI] [PubMed] [Google Scholar]
- 15.Kita H, Shiraishi Y, Watanabe K, Suda K, Ohtsuka K, Koshiishi Y, Goya T. Does postoperative serum interleukin-6 influence early recurrence after curative pulmonary resection of lung cancer? Ann Thorac Cardiovasc Surg 2011;17(5): 454–460. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Ye Y, Zhang H, et al. Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine (Baltimore) 2016;95(2):e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MF, Chen PT, Lu MS, Lin PY, Chen WC, Lee KD. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer 2013;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MF, Lin PY, Wu CF, Chen WC, Wu CT. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS ONE 2013;8(4):e61901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egler RA, Burlingame SM, Nuchtern JG, Russell HV. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin Cancer Res 2008;14(21):7028–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CH, Hsiao CF, Yeh YM, et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer 2013;132(9):1977–1985. [DOI] [PubMed] [Google Scholar]
- 21.Jinno T, Kawano S, Maruse Y, et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep 2015;33(5):2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol 2016;11(9):1482–1492. [DOI] [PubMed] [Google Scholar]
- 23.Duan S, Tsai Y, Keng P, Chen Y, Lee SO. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget 2015;6(29): 27651–27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000;19(21):2548–2556. [DOI] [PubMed] [Google Scholar]
- 25.Gritsko T, Williams A, Turkson J, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res 2006;12(1):11–19. [DOI] [PubMed] [Google Scholar]
- 26.Yan HQ, Huang XB, Ke SZ, et al. Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer Sci 2014; 105(9):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu B, Zhu J, Liu S, et al. Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3. Oncotarget 2016;7(30):48296–48308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J Clin Invest 2015;125(9):3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SJ, Nakagawa T, Kitamura H, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 2004;173(6):3844–3854. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura H, Kamon H, Sawa S, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity 2005;23(5): 491–502. [DOI] [PubMed] [Google Scholar]
- 31.Ohno Y, Kitamura H, Takahashi N, et al. IL-6 downregulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4(þ) T cells. Cancer Immunol Immunother 2016;65(2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedberg JS. The state of the art in the technical performance of lung-sparing operations for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2013;25(2):125–143. [DOI] [PubMed] [Google Scholar]
- 35.Yom SS, Busch TM, Friedberg JS, Wileyto EP, Smith D, Glatstein E, Hahn SM. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem Photobiol 2003;78(1): 75–81. [DOI] [PubMed] [Google Scholar]
- 36.Amar D, Zhang H, Park B, Heerdt PM, Fleisher M, Thaler HT. Inflammation and outcome after general thoracic surgery. Eur J Cardiothorac Surg 2007;32(3):431–434. [DOI] [PubMed] [Google Scholar]
- 37.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol 1993;54:1–78. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher-Colombo SM, Maas AL, Yuan M, Busch TM. Photodynamic therapy-induced angiogenic signaling: Consequences and solutions to improve therapeutic response. Isr J Chem 2012;52(8–9):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407(6801):249–257. [DOI] [PubMed] [Google Scholar]
- 40.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol 2004;31(2 Suppl 7): 22–29. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto G, Muta M, Tsuruta K, Horiguchi S, Karasawa K, Okamoto A. Tumor size significantly correlates with postoperative liver metastases and COX-2 expression in patients with resectable pancreatic cancer. Pancreatology 2007;7(2–3):167–173. [DOI] [PubMed] [Google Scholar]
- 42.Lechner MG, Karimi SS, Barry-Holson K, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother 2013;36(9):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casas A, Di Venosa G, Hasan T, Al B. Mechanisms of resistance to photodynamic therapy. Curr Med Chem 2011;18(16):2486–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 1998;95(26):15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003;22(47):7340–7358. [DOI] [PubMed] [Google Scholar]
- 46.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol 1996;36:161–183. [DOI] [PubMed] [Google Scholar]
- 47.Yu D, Zhong Y, Li X, et al. ILs-3, 6 and 11 increase, but ILs-10 and 24 decrease stemness of human prostate cancer cells in vitro. Oncotarget 2015;6(40):42687–42703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu ES, Yow CM, Shi M, Ho RJ. Effects of photoactivated 5-aminolevulinic acid hexyl ester on MDR1 over-expressing human uterine sarcoma cells. Toxicol Lett 2008; 181(1):7–12. [DOI] [PubMed] [Google Scholar]
- 49.Usuda J, Tsunoda Y, Ichinose S, et al. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer 2010;67(2):198–204. [DOI] [PubMed] [Google Scholar]
- 50.Reed JC. Mechanisms of apoptosis. Am J Pathol 2000;157(5): 1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen XY, Zacal N, Singh G, Rainbow AJ. Alterations in mitochondrial and apoptosis-regulating gene expression in photodynamic therapy-resistant variants of HT29 colon carcinoma cells. Photochem Photobiol 2005;81(2):306–313. [DOI] [PubMed] [Google Scholar]
- 52.Jaiswal PK, Goel A, Mittal RD. Survivin: A molecular biomarker in cancer. Indian J Med Res 2015;141(4):389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta 2007;1776(1):86–107. [DOI] [PubMed] [Google Scholar]
- 54.Fisher AM, Rucker N, Wong S, Gomer CJ. Differential photosensitivity in wild-type and mutant p53 human colon carcinoma cell lines. J Photochem Photobiol B 1998;42(2): 104–107. [DOI] [PubMed] [Google Scholar]
- 55.Brighenti E, Calabrese C, Liguori G, Giannone FA, Trere D, Montanaro L, Derenzini M. Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: A new pathway connecting inflammation to cancer. Oncogene 2014;33(35):4396–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cramer GM, Jones DP, El-Hamidi H, Celli JP. ECM composition and rheology regulate growth, motility, and response to photodynamic therapy in 3D models of pancreatic ductal adenocarcinoma. Mol Cancer Res 2017;15(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrara N Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 2002;29(6 Suppl 16):10–14. [DOI] [PubMed] [Google Scholar]
- 58.Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol 2013;85(4):531–540. [DOI] [PubMed] [Google Scholar]
- 59.Dalwadi H, Krysan K, Heuze-Vourc’h N, et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res 2005;11(21):7674–7682. [DOI] [PubMed] [Google Scholar]
- 60.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003;22(10):1517–1527. [DOI] [PubMed] [Google Scholar]
- 61.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus ”N2” TAN. Cancer Cell 2009;16(3):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem 2005;280(13):12239–12245. [DOI] [PubMed] [Google Scholar]
- 63.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Su X, Yang L, et al. The influence of myeloid-derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int J Cancer 2016;138(11):2688–2699. [DOI] [PubMed] [Google Scholar]
- 65.Xu M, Zhao Z, Song J, et al. Interactions between interleukin-6 and myeloid-derived suppressor cells drive the chemo-resistant phenotype of hepatocellular cancer. Exp Cell Res 2017;351(2):142–149. [DOI] [PubMed] [Google Scholar]
- 66.Janeway CA, Jr. The priming of helper T cells. Semin Immunol 1989;1(1):13–20. [PubMed] [Google Scholar]
- 67.McDonnell AM, Robinson BW, Currie AJ. Tumor antigen cross-presentation and the dendritic cell: where it all begins? Clin Dev Immunol 2010;2010:539519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Athie-Morales V, Smits HH, Cantrell DA, Hilkens CM. Sustained IL-12 signaling is required for Th1 development. J Immunol 2004;172(1):61–69. [DOI] [PubMed] [Google Scholar]
- 69.Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol Res 2014;2(2):91–98. [DOI] [PubMed] [Google Scholar]
- 70.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol 2009;182(5):2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brackett CM, Owczarczak B, Ramsey K, Maier PG, Gollnick SO. IL-6 potentiates tumor resistance to photodynamic therapy (PDT). Lasers Surg Med 2011;43(7):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, Falcone DJ. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol 2014;192(1): 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 2013;35(2): 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Kane SL, Cawkwell L, Campbell A, Lind MJ. Cyclooxygenase-2 expression predicts survival in malignant pleural mesothelioma. Eur J Cancer 2005;41(11):1645–1648. [DOI] [PubMed] [Google Scholar]
- 75.Baldi A, Santini D, Vasaturo F, et al. Prognostic significance of cyclooxygenase-2 (COX-2) and expression of cell cycle inhibitors p21 and p27 in human pleural malignant mesothelioma. Thorax 2004;59(5):428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards JG, Faux SP, Plummer SM, Abrams KR, Walker RA, Waller DA, O’Byrne KJ. Cyclooxygenase-2 expression is a novel prognostic factor in malignant mesothelioma. Clin Cancer Res 2002;8(6):1857–1862. [PubMed] [Google Scholar]
- 77.Roh JL, Sung MW, Park SW, Heo DS, Lee DW, Kim KH. Celecoxib can prevent tumor growth and distant metastasis in postoperative setting. Cancer Res 2004;64(9):3230–3235. [DOI] [PubMed] [Google Scholar]
- 78.Maihofner C, Charalambous MP, Bhambra U, Lightfoot T, Geisslinger G, Gooderham NJ. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis 2003;24(4):665–671. [DOI] [PubMed] [Google Scholar]
- 79.Ferrario A, Fisher AM, Rucker N, Gomer CJ. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res 2005;65(20):9473–9478. [DOI] [PubMed] [Google Scholar]
- 80.Makowski M, Grzela T, Niderla J, et al. Inhibition of cyclooxygenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clin Cancer Res 2003;9(14): 5417–5422. [PubMed] [Google Scholar]
- 81.Ferrario A, Von Tiehl K, Wong S, Luna M, Gomer CJ. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy-mediated tumor response. Cancer Res 2002;62(14):3956–3961. [PubMed] [Google Scholar]
- 82.Hendrickx N, Volanti C, Moens U, et al. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J Biol Chem 2003;278(52):52231–52239. [DOI] [PubMed] [Google Scholar]
- 83.Mitra S, Cassar SE, Niles DJ, Puskas JA, Frelinger JG, Foster TH. Photodynamic therapy mediates the oxygen-independent activation of hypoxia-inducible factor 1alpha. Mol Cancer Ther 2006;5(12):3268–3274. [DOI] [PubMed] [Google Scholar]
- 84.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res 2005;65(12):5211–5220. [DOI] [PubMed] [Google Scholar]
- 85.Yuan XL, Chen L, Li MX, et al. Elevated expression of Foxp3 in tumor-infiltrating treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol 2010;134(3): 277–288. [DOI] [PubMed] [Google Scholar]
- 86.Kanamori M, Nakatsukasa H, Okada M, Lu Q. Yoshimura A. induced regulatory t cells: Their development, stability, and applications. Trends Immunol 2016;37(11):803–811. [DOI] [PubMed] [Google Scholar]
- 87.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441(7090):235–238. [DOI] [PubMed] [Google Scholar]
- 88.Brackett CM, Muhitch JB, Evans SS, Gollnick SO. IL-17 promotes neutrophil entry into tumor-draining lymph nodes following induction of sterile inflammation. J Immunol 2013; 191(8):4348–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol 2007;275(1–2):79–97. [DOI] [PubMed] [Google Scholar]
- 90.Couper KN, Blount DG, Riley EM. IL-10: The master regulator of immunity to infection. J Immunol 2008;180(9): 5771–5777. [DOI] [PubMed] [Google Scholar]
- 91.Wust S, van den Brandt J, Tischner D, et al. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol 2008;180(12): 8434–8443. [DOI] [PubMed] [Google Scholar]
- 92.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei LH, Baumann H, Tracy E, Wang Y, Hutson A, Rose-John S, Henderson BW. Interleukin-6 trans signalling enhances photodynamic therapy by modulating cell cycling. Br J Cancer 2007;97(11):1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Usuda J, Okunaka T, Furukawa K, et al. Increased cytotoxic effects of photodynamic therapy in IL-6 gene transfected cells via enhanced apoptosis. Int J Cancer 2001;93(4):475–480. [DOI] [PubMed] [Google Scholar]
- 95.Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85(1):109–117. [DOI] [PubMed] [Google Scholar]
- 96.Ghanim B, Hoda MA, Winter MP, et al. Pretreatment serum C-reactive protein levels predict benefit from multimodality treatment including radical surgery in malignant pleural mesothelioma: A retrospective multicenter analysis. Ann Surg 2012;256(2):357–362. [DOI] [PubMed] [Google Scholar]
- 97.Ghanim B, Hoda MA, Klikovits T, et al. Circulating fibrinogen is a prognostic and predictive biomarker in malignant pleural mesothelioma. Br J Cancer 2014;110(4):984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korbelik M, Cecic I, Merchant S, Sun J. Acute phase response induction by cancer treatment with photodynamic therapy. Int J Cancer 2008;122(6):1411–1417. [DOI] [PubMed] [Google Scholar]
- 99.Cecic I, Serrano K, Gyongyossy-Issa M, Korbelik M. Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett 2005;225(2):215–223. [DOI] [PubMed] [Google Scholar]
- 100.Merchant S, Huang N, Korbelik M. Expression of complement and pentraxin proteins in acute phase response elicited by tumor photodynamic therapy: The engagement of adrenal hormones. Int Immunopharmacol 2010;10(12):1595–1601. [DOI] [PubMed] [Google Scholar]
- 101.Cecic I, Stott B, Korbelik M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int Immunopharmacol 2006;6(8):1259–1266. [DOI] [PubMed] [Google Scholar]
- 102.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett 2002;183(1):43–51. [DOI] [PubMed] [Google Scholar]
- 103.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res 2007;67(21):10501–10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.