Abstract
The accurate measurement of blood pressure (BP) is essential for the diagnosis and management of hypertension. Restricted use of mercury devices, increased use of oscillometric devices, discrepancies between clinic and out-of-clinic BP, and concerns about measurement error with manual BP measurement techniques have resulted in uncertainty for clinicians and researchers. The National Heart, Lung, and Blood Institute of the US National Institutes of Health convened a Working Group of clinicians and researchers in October 2017 to review data on BP assessment among adults in clinical practice and clinic-based research. In this report, we review the topics discussed during a two-day meeting including the current state of knowledge on BP assessment in clinical practice and clinic-based research, knowledge gaps pertaining to current BP assessment methods, research and clinical needs to improve BP assessment, and the strengths and limitations of using BP obtained in clinical practice for research and quality improvement activities.
Keywords: blood pressure, hypertension, measurement
Condensed abstract
The National Heart, Lung, and Blood Institute convened a Working Group in October 2017 to review data on BP assessment in adults in clinical practice and clinic-based research. We report on outcomes from the working group meeting, including (i) evaluation of the current state of knowledge on BP assessment in clinical practice and clinic-based research for diagnosing hypertension and evaluating response to treatment, (ii) identifying knowledge gaps pertaining to current BP assessment methods, (iii) evaluating research and clinical needs to improve BP measurement, and (iv) using BP obtained in clinical practice for research and quality improvement activities.
Introduction
Hypertension affects about 103 million adults in the US and over a billion people worldwide (1-3). The accurate assessment of arterial blood pressure (BP) levels is needed for the diagnosis and treatment of hypertension. Researchers first measured BP in the 1700s, and by the late 1800s BP assessment was introduced into clinical practice (4). However, it was not until the 20th century that observational data showed that higher BP levels were associated with increased cardiovascular disease (CVD) risk. Subsequently, randomized trials demonstrated that lowering BP from levels which were previously considered “essential” (systolic/diastolic BP up to 210/100 mm Hg) reduced the risk of CVD and death.
The direct measurement of BP requires an intra-arterial assessment. In clinical practice and most clinic-based research studies, BP is estimated using non-invasive methods. In the current document, we use the term BP measurement for estimates obtained through non-invasive means. For much of the 20th century, BP was assessed through auscultation and the recognition of Korotkoff sounds, with mercury-based sphygmomanometer measurements serving as the reference standard. This non-invasive auscultatory approach remained the reference standard until the early 21st century.
More recently, restrictions on the use of mercury devices, increased availability of oscillometric devices, discrepancies between clinic BP and out-of-clinic BP, and an increasing recognition of the susceptibility of BP assessed using the auscultatory method to measurement error have resulted in uncertainty for clinicians and researchers. To date, hypertension treatment guidelines and quality control metrics have largely relied on BP measured in the clinic setting. Performance measures, which are often reported using data captured by electronic medical records (EMR), have expanded the role of clinic-based BP. However, different values are often obtained when BP is measured in the clinic versus outside of the clinic setting and it is recommended that out-of-clinic measurements be obtained to confirm the presence of hypertension based on clinic measurements and for the management of high BP(1,5).
The National Heart, Lung, and Blood Institute of the US National Institutes of Health convened a Working Group of clinicians and researchers in October 2017 to review data on BP assessment in adults in clinical practice and clinic-based research. BP assessment in children and adolescents is a complex topic that merits separate attention. The Working Group held a conference that was designed to complement ongoing American Heart Association (AHA) activities including the 2017 Task Force’s Guideline for Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults and an update of the 2005 AHA Scientific Statement on BP measurement in humans(1,6). This statement presents the discussion and recommendations from the Blood Pressure Measurement Working Group convened by the National Heart, Lung, and Blood Institute whose aims were (1) to evaluate the current state of knowledge on BP assessment in clinical practice and clinic-based research for diagnosing hypertension and evaluating response to antihypertensive treatment; (2) to identify knowledge gaps pertaining to current BP assessment methods; (3) to evaluate research and clinical needs to improve BP assessment for the aforementioned purposes; and (4) to evaluate the use of BP obtained in clinical practice for research and quality improvement activities. In addressing these objectives, the working group focused on 2 primary topics: (1) how different measurement methods can be integrated into clinic-based research and routine clinical practice to perform accurate BP assessment and (2) how the quality of BP measurements obtained in routine clinical practice can be improved to provide better patient care and to be suitable for clinic-based research (Central Illustration). It is outside the scope of this document to provide practice guidelines on blood pressure measurement.
BP assessment in ambulatory clinical practice and clinic-based research: current practice and challenges
Current approaches to BP measurement
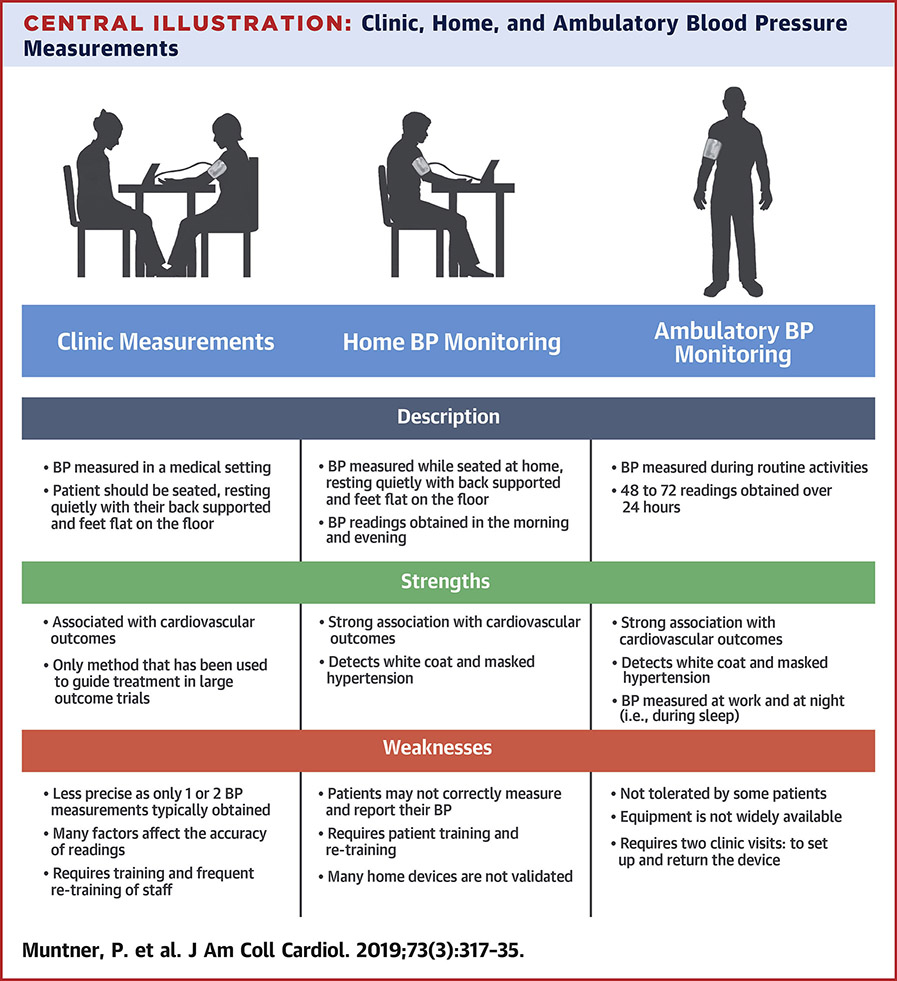
In the clinic setting, BP can be estimated by an observer listening to Korotkoff sounds with a stethoscope using the auscultatory approach and a manual manometer or with an electronic device using the oscillometric approach. Over the past 40 years, devices have been developed and validated to estimate BP outside of the clinic setting through ambulatory BP monitoring (ABPM) or self-monitoring. Self-monitored BP has been studied frequently using measurements taken in the home (referred to as home blood pressure monitoring [HBPM]), but also includes measurements taken in public settings (e.g., a pharmacy or grocery store) using a semi-automated or automated oscillometric device. Automated devices can take multiple BP readings at set intervals (e.g., one minute apart) with a single activation while semi-automated devices require manual initiation for each individual BP measurement. Most out-of-clinic BP measurements are obtained using the oscillometric approach.
BP measurement in the clinic setting
BP is typically assessed in routine clinical practice during outpatient visits. An initial BP measurement is most often performed by a medical assistant or nurse who frequently is also collecting information on other physical measurements (e.g., height, weight), relevant medical history, and current medications being taken before the patient is seen by a clinician. A follow-up BP measurement may be performed by a clinician to confirm the initial reading. The primary purposes of measuring BP in routine clinical practice are to screen for hypertension and hypotension and to monitor the response to antihypertensive treatment. There are often time constraints affecting the accuracy of completed BP measurements in clinical practice settings. Although contemporary data are sparse, training in BP measurement, equipment used, and measurement methods vary widely across clinics, and nearly always deviate from methods recommended by guidelines.
In clinical trials, the protocol used to measure BP is often standardized across sites to minimize systematic errors and variability. However, protocols often differ across trials and are not always reported in detail in publications. Differences in measurement techniques across clinical trials include the device employed, the use and duration of a rest period, the arm used, participant position during measurement, the presence of ambient noise, conversation with the participant, whether the measurement is observed or unobserved, time of day when BP is measured, trial activities that precede BP assessment, the number of measurements taken, and the number of measurement occasions(7). BP variability is increased when measurements do not follow a specific study protocol. As a result, BP readings measured in different research settings and between research and routine clinical practice can differ substantially from what they would be if a standardized protocol had been used(8).
The auscultatory approach requires good hearing, extensive training and retraining, and periodic certification to record the onset and disappearance of the Korotkoff sounds (9,10). Although rarely used anymore in clinical and research settings, the mercury sphygmomanometer is still considered the reference standard device. It remains useful for validating oscillometric devices (11). Calibrated aneroid manometers can be considered a substitute for mercury devices (12). However, aneroid manometers are easily damaged and require frequent re-calibration to ensure their measurement accuracy(13).
Oscillometric BP devices estimate systolic BP (SBP) and diastolic BP (DBP) from the mean arterial pressure using a device-specific algorithm and the oscillometric pulse waves detected in the BP cuff, typically during deflation although some devices assess BP during inflation. Each manufacturer of oscillometric devices incorporates its own undisclosed proprietary algorithm(s) for estimating SBP and DBP. Aging and other conditions that affect arterial compliance, such as pregnancy, diabetes, kidney disease and arrhythmias, may affect the accuracy of these algorithms (14). Any oscillometric BP measurement device used in clinical or research settings should have peer-reviewed published documentation of its rigorous validation with a mercury device or non-mercury device that meets the ISO 81060–1 requirements for accuracy of a non-invasive sphygmomanometer(15). The “black box” nature of proprietary algorithms used in oscillometric devices is a limitation(16). Some updated oscillometric devices may be using modified algorithms to estimate BP, and validation studies are not always performed to confirm their accuracy. Even after devices have been validated, they still need to be used in a standardized manner. This includes correctly positioning the patient, having the BP cuff at heart level on a bare-upper-arm, using an appropriate size and correctly fitting BP cuff, and having the patient rest before the first measurement and between repeat measurements (Table 1).
Table 1.
Key Steps and Instructions for the Proper Measurement of Clinic Blood Pressure
| Key Steps for Proper BP Measurements |
Specific Instructions |
|---|---|
| Step 1: Properly prepare the patient |
|
| Step 2: Use proper technique for BP measurements |
|
| Step 3: Take the proper measurements needed for diagnosis and treatment of elevated BP/hypertension |
|
| Step 4: Properly document accurate BP readings |
|
| Step 5: Average the readings | Use an average of ≥2 readings obtained on ≥2 occasions to estimate the individual’s level of BP. |
| Step 6: Provide BP readings to patient | Provide patients their SBP/DBP readings both verbally and in writing. Information to help patients interpret their BP values should also be provided. |
AOBP, Automated office blood pressure, BP, blood pressure; DBP, diastolic blood pressure; NHANES, National Health and Nutrition Examination Survey, SBP, systolic blood pressure.
Adapted with permission from Mancia et al. (Oxford University Press), Pickering et al. (American Heart Association, Inc.), Weir et al. (American College of Physicians, Inc.) and Whelton (American College of Cardiology/American Heart Association).
In the clinic setting, several oscillometric devices that take multiple BP measurements automatically, at set intervals, are available. These automated office blood pressure (AOBP) devices can measure BP with or without an observer present (17). Some studies have suggested that BP measured with staff present (attended BP assessment) result in higher readings than those obtained with staff absent during measurement (unattended AOBP). In a pooled analysis of 8,558 adults, the mean clinic BP was 10/7 mm Hg higher when recorded by a provider in clinical practice using the auscultatory method with a mercury sphygmomanometer versus with unattended AOBP(18-24). In 2017, the Hypertension Canada guideline recommended unattended AOBP as the preferred method of clinic BP measurement(25). Unattended and attended BP measurements should ideally be compared using the same device. In randomized studies comparing unobserved and observed AOBP using the same device, the difference in BP using these two approaches has been small(26,27). Also, a secondary analysis of the Systolic Blood Pressure Intervention Trial found no difference in BP levels measured in clinical sites that had staff present versus absent during BP measurement(28). These data suggest that no differences in BP may be present when assessed using attended versus unattended AOBP as long as a BP measurement protocol is rigorously followed and an oscillometric device is used. However, as these protocols are rarely followed in routine clinical practice, unattended AOBP is useful to minimize the occurrence of protocol violations (e.g., talking during the BP measurement, insufficient amount of rest prior to and between BP measurements).
BP measurement outside of the clinic setting
ABPM and HBPM are used to obtain BP measurements outside of medical settings, without a healthcare provider, researcher or observer present. Typically, more BP readings are obtained with ABPM and HBPM than with clinic measurements (29). Both ABPM and HBPM are typically used to assess average BP outside of the clinic, which enables the identification of mismatches between clinic hypertension and out-of-clinic hypertension including white coat hypertension and masked hypertension (defined below) (30). An important difference between ABPM and HBPM is that ABPM assesses daytime and nighttime BP typically over one 24-hour period, while a person goes about their routine daily activities. In contrast, HBPM typically assesses BP during the day, usually in the morning and early evening, over a period of days to weeks while the person is seated and resting but typically not during sleep (30).
ABPM and HBPM should not be considered interchangeable, as there is only moderate agreement between daytime values on ABPM and home BP on HBPM(31,32). Until recently, HBPM could not obtain BP readings during sleep preventing the determination of diurnal BP patterns. However, HBPM devices that can assess BP during sleep, typically three measurements at one hour intervals, have recently become available (33). There is currently insufficient evidence to decisively determine whether BP measured using ABPM or HBPM has a stronger association with risk for CVD events, although there are more data linking out-of-clinic BP on ABPM to CVD events (34). The selection of ABPM or HBPM may depend on the application. In clinical practice, ABPM may be most useful in identifying white coat, masked hypertension or nocturnal hypertension as multiple readings are taken throughout the day. HBPM may be more useful to monitor BP for patients taking antihypertensive medication as it can be used over prolonged periods of time. More data are needed on the use of HBPM devices to identify white coat and masked hypertension, phenotypes which are described below.
Oscillometric devices, often located in a booth or kiosk in pharmacies, grocery stores, fitness facilities, or other locations offer a convenient way to check BP. These devices may be configured to allow BP data to be directly transmitted to healthcare providers or to an EMR, which can then be used to help manage BP control. Although scarce evidence exists, there are some data suggesting that BP obtained in public settings may be similar to daytime ABPM (35). However, caution is needed. Many devices in public settings utilize a single size cuff that is considered too small (< 33 cm) for the many adults’ arm circumferences of many adults. Most of these devices have not undergone a validation study, or manufacturers have declined to share validation data when queried(36). The devices are frequently located in noisy, high traffic areas, which are not conducive to obtaining an accurate resting BP measurement. In addition, there are no data showing the association of BP measured in a booth or kiosk and CVD risk. Furthermore, thresholds for what should be considered a normal BP level have not been determined for this setting. When BP values from measurements in a kiosk are available, it is important that the device has undergone validation and is located in a quiet setting. An alternative approach is having pharmacists measure BP. There are some data suggesting pharmacist-measured BP aligns with awake BP on ABPM(37).
Despite recommendations from the United States Preventive Services Task Force and American College of Cardiology / American Heart Association (ACC/AHA) to assess out-ofclinic BP to identify white coat and masked hypertension, out-of-clinic BP is rarely obtained before a hypertension diagnosis is made in the US (1,5). In a qualitative research study conducted with primary care providers in Alabama and New York, cost, time requirements, lack of infrastructure including access to BP monitoring devices, and low reimbursement were reported as major barriers to performing ABPM (38). In contrast to ABPM, barriers preventing the more widespread use of HBPM relate to providers’ concerns that patients will not measure their BP correctly and that patients will become pre-occupied with their BP (39,40). Device affordability may be a barrier for conducting HBPM in some populations. Also, healthcare providers may lack confidence in their own skills and knowledge to interpret out-of-clinic BP assessments from ABPM and HBPM (38).
The successful incorporation of ABPM and HBPM into clinical practice may require additional equipment, staff training and time in conducting and understanding what constitutes satisfactory recordings. Additionally, knowledge about which devices are validated and how to interpret out-of-clinic BP readings and incorporate out-of-clinic BP into clinical decision making is needed(30). Currently, there are no core clinical competencies for conducting out-of-clinic BP assessment(30). Before undergoing ABPM, patients should be instructed on proper positioning and anticipating potential mild discomfort and disturbance of sleep associated with cuff inflation and deflation while BP is being measured, to prevent removal of the ABPM device and cuff(30,41). For HBPM, patients should be instructed on proper positioning, where and when to measure their BP and how to interpret the results.
Software for downloading BP readings from ABPM is included with each device and typically generates reports that include mean daytime, nighttime, and 24-hour values as well as daytime-to-nighttime BP ratio. In addition to PC-based software, some HBPM device manufacturers have developed mobile app and internet-based software that automatically stores BP readings, making it possible for patients to share HBPM data with their healthcare providers and to prevent misreporting of self-measurements. Ideally, individual ABPM and HBPM readings can be entered into structured fields of EMRs, using automated data transfer processes; such data could be useful for quality measures and to generate summary statistics, including average BP over time. Currently, few providers and patients have access to these resources.
White coat hypertension and masked hypertension
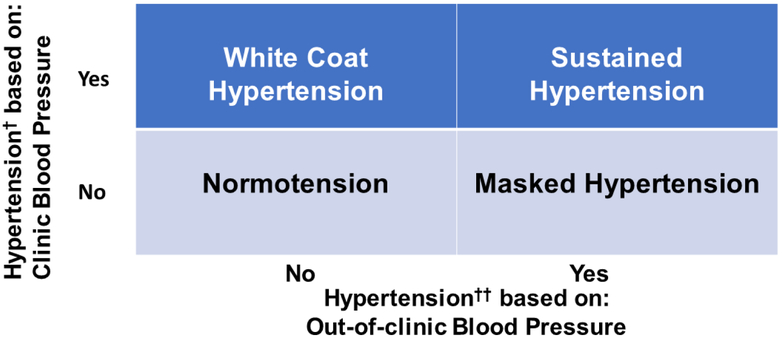
The difference between clinic and out-of-clinic BP measurements is often substantial (42-44). Thus, there are individuals who meet the criteria for hypertension based on their clinic BP but not based on their out-of-clinic BP, and vice versa. This results in four BP phenotypes defined by the possible combinations of hypertensive/non-hypertensive clinic BP and hypertensive /non-hypertensive out-of-clinic BP: normotension, white coat hypertension, masked hypertension, and sustained hypertension (Figure 1). European guidelines have suggested defining white coat and masked hypertension using mean out-of-clinic awake, sleep or 24-hour BP(45). Specifically, it is suggested to define white coat hypertension as BP in the hypertension range when measured in the clinic but mean awake, asleep and 24-hour BP not in the hypertension range. Analogously, masked hypertension is defined by BP not in the hypertension range when measured in the clinic but mean awake, asleep or 24-hour BP in the hypertension range.
Figure 1: Blood pressure phenotypes defined by combinations clinic and out-of-clinic blood pressure.
- White coat hypertension - white coat effect,
- Masked hypertension - masked uncontrolled hypertension,
- Sustained hypertension - uncontrolled hypertension
- Normotension - controlled hypertension
In a New York metropolitan area community sample (N=888) not taking antihypertensive medication, the prevalence of white coat hypertension among those with clinic SBP ≥140 mm Hg or DBP ≥ 90 mm Hg was 19.1%, while the prevalence of masked hypertension among those with clinic SBP <140 mm Hg and DBP < 90 mm Hg was 15.7%(42). In the Jackson Heart Study, a cohort comprised exclusively of African Americans, the prevalence of white coat hypertension and masked hypertension among those not taking antihypertensive medication was 30.2% and 25.4%, respectively(46).
When compared to normotension, masked hypertension has been associated with a two times higher risk for CVD(47,48). In some studies, the risk for CVD has been reported to be similar for individuals with masked hypertension and sustained hypertension (48,49). It is unclear if white coat hypertension is associated with a substantially increased risk for CVD compared with normotension (50,51). However, it should be recognized that a high proportion of participants with white coat hypertension in prior studies may have initiated antihypertensive medication during follow-up which would have resulted in a lower CVD risk compared to what might have been identified had they remained untreated. Also, the incidence of sustained hypertension is substantially higher for adults with white coat hypertension versus normotension (52,53).
The prevalence of white coat hypertension is higher in those who are older, female, and have lower BMI, and the prevalence of masked hypertension is higher in those who are older, male, have a higher BMI, have reduced kidney function, and are smokers (54-56). The strongest predictors of white coat hypertension and masked hypertension are clinic SBP and DBP, with the probability being highest when clinic BP is close to the threshold used for defining hypertension. Similar to BP measured in the clinic, the accuracy and reproducibility of out-of-clinic BP improves as the number of readings being averaged increases (57). However, the marginal benefit of each additional reading decreases as the total number of readings increases. The concern with diagnostic accuracy and reproducibility is compounded when using out-of-clinic BP in conjunction with clinic BP. Several studies have shown that diagnoses of white coat hypertension or masked hypertension are only moderately reproducible (58,59).
Emerging alternative approaches to BP assessment in ambulatory clinical settings
The explosion in iPhone and Android apps has made its way into the BP measurement arena. A number of apps measure BP directly while others allow for readings to be manually entered for storage (60,61). Many apps use a combination of finger plethysmography and pulse transit time calculations to estimate BP (62). Few rigorous studies assessing the validity of these apps have been conducted. One study showed poor performance for one of these apps (63). The US Food and Drug Administration (FDA) only oversees some forms of mobile health technology, and there are insufficient validation data and no outcome data supporting their use. The US FDA oversees mobile health technology that is used to diagnose and treat disease. Non-invasive BP monitors are considered moderate risk medical devices that must be cleared by the FDA. However, these devices, including mobile apps that measure BP, are only required to show “substantial equivalence” to another device that has been cleared by the FDA. There have been calls for laws requiring studies that demonstrate sufficient accuracy for new BP monitors.
Other “wearable” devices use a calibrated radial pulse waveform to estimate BP, and can do so over extended periods of time. Some devices appear to maintain calibration and be accurate for at least 24 hours and, thus, have been suggested as a surrogate for ABPM in some populations(64). An additional challenge is to ensure that measurements are obtained with the device at heart level; otherwise measured BP values will tend to under- or over-estimate actual BP depending on whether the wrist is above or below heart level, respectively.
Statistical considerations
The diagnosis of hypertension and evaluation of response to treatment require accurate assessment of BP to prevent under or over treatment. BP varies both within and between visits. While both sources of variability are important to recognize, variability is greater between versus within visits and the precision of observed mean BP depends on the number of visits and the number of measurements per visit (65). For example, in one study with one research-grade measurement obtained at a single visit, the standard errors of SBP and DBP were approximately 7.0 and 6.0 mm Hg, respectively, while three measurements at two visits resulted in standard errors of 3.7 and 3.2 mm Hg, respectively (Online Table 1). BP measured in routine clinic practice probably have larger standard errors. Hypertension screening algorithms can be determined based on a function of between-person and within-person variability, and two or more BP measurements on two or more occasions is required to accurately screen adults for high BP in the clinic (65,66). Similar approaches can be used to assess the response to treatment and require two or more pre- and post-treatment visits and a change of 5-7 mm Hg in DBP to be 80% confident that true change has occurred (67).
Special populations and clinical issues
Older adults
Because of its high prevalence in older adults, hypertension is a leading cause of preventable morbidity, mortality and premature disability and institutionalization in this population (68-71). Although mean SBP increases at older ages, the standard deviation of SBP and DBP is not substantially different when compared to younger persons with similar levels of BP (72). Additionally, in a recent meta-analysis, older age was not associated with the difference in BP when measured intra-arterially and non-invasively with a BP cuff (73). However, as in all ages, there is a subset of older adults in whom the accurate measurement of BP is challenging due to the presence of comorbidity, aging-related cardiovascular changes, arrhythmia and polypharmacy (74). For example, some older adults have non-compressible arteries which may make BP readings inaccurate (75). Additionally, an auscultatory gap might be present among older adults (76). The 2018 AAMI-ESH-ISO universal standard for the validation of BP monitors does not consider older adults a special population warranting a distinct validation of oscillometric BP monitors (15).
Orthostatic hypotension
Orthostatic hypotension is a risk factor for falls, syncope, CVD, stroke, and death (77-82). The prevalence of orthostatic hypotension increases with age, and is more common among patients with uncontrolled hypertension(79,83). While it is recommended that orthostatic hypotension be assessed in patients with a history of falls or postural dizziness, it has unclear utility in guiding BP management(84,85). Recent clinical trials have shown that lower versus higher BP goals may not increase the risk for having orthostatic hypotension, and that there is only modest overlap between measured orthostatic hypotension and symptoms of dizziness or lightheadedness on standing (86-88). However, methodologic limitations may be responsible for these null findings. First, multiple protocols exist for performing orthostatic hypotension assessments (e.g., seated versus supine versus tilt-table) (89). Second, there is substantial heterogeneity in guidelines as to when orthostatic hypotension should be assessed, ranging from within 1 minute to after 3 minutes of standing (25,45,90), and BP measurements performed sooner after rising appear to be stronger predictors of long-term risk for adverse outcomes including falls (80,91-94). Third, current cut-points used to define orthostatic hypotension based on change in SBP or DBP (i.e., a drop of 20 mm Hg in SBP or a drop of 10 mm Hg in DBP) do not reflect natural thresholds of risk, are insensitive for orthostatic symptoms and may perform poorly among adults with hypertension (81,85). Other definitions of orthostatic hypotension have been proposed for patients with hypertension (e.g., a change in SBP of >30 mm Hg or a standing SBP<90 mm Hg) (95). However, it is possible that orthostatic symptoms are more important for long-term outcomes than protocol-based changes in BP upon standing.
Obesity
The prevalence of obesity in US adults has increased substantially in recent years(96). The measurement of BP in obese adults, including those who are morbidly obese, is an increasingly common challenge (97). Obesity with its associated increase in arm circumference requires use of larger BP cuffs (98). Selecting an appropriately-sized cuff is a key component for obtaining valid BP measurements. An extra-large cuff or “thigh cuff” has been shown to provide accurate BP measurements in obese adults. However, there are few studies comparing BP measurement approaches using extra-large cuffs with direct intra-arterial measurements, and the 2018 AAMI/ESH/ISO device validation review recommends a separate validation be performed on individuals with an arm circumference > 42 cm (15). A challenge encountered with using larger cuffs is that large arm shapes are often conic. Some extra-large cuffs are available with a conic shape for these situations. When a sufficiently large cuff is not available to obtain BP measurements in the brachial artery, a properly used validated wrist device held at the level of the heart may be more accurate than measurements taken at the brachial artery (99).
Atrial fibrillation
Atrial fibrillation (AF) is a common arrhythmia which complicates the measurement of BP(100). There are no accepted non-invasive approaches for determining BP in AF, and the accuracy of the auscultatory method which, as mentioned above, is the reference for validating BP monitors, is unknown within this population. Inter- and intra-observer variation for measuring BP is higher in AF than in sinus rhythm (101). However, BP may be reasonably accurate in patients with AF if three or more readings are obtained (102). A meta-analysis of validation studies of automated (mostly oscillometric) BP monitors in AF showed no difference in SBP compared with manual auscultatory measurements but a small, yet consistent, overestimation of DBP (103). This overestimation of DBP may be less important since most people with AF are older, a population wherein SBP has more prognostic importance (100). ABPM is feasible in AF, with similar reliability as in sinus rhythm (104). Preliminary evidence suggests that for patients with AF both auscultatory and oscillometric BP measurements are clinically relevant, as they show similar associations with intra-arterial measurements and preclinical organ damage indices(102,105,106). Other arrhythmias, such as frequent premature atrial and ventricular contractions may also affect the accuracy of oscillometric BP measurements, but evidence is sparse.
Pregnancy
Due to hemodynamic changes and edema that often accompany pregnancy and complications including preeclampsia, oscillometric devices that are accurate in the general adult population may not be accurate in pregnant women, and it is recommended that they be separately tested for accuracy in this population(107). A systematic review of validation studies of clinic, ABPM and HBPM devices in pregnant women found 61% of devices specifically evaluated for use in pregnancy, including pre-eclampsia, met the validation criteria(108). However, only 34% of the studies wherein the device met the validation criteria were performed without any protocol violations (108). Current recommendations for the use of HBPM in pregnancy include at least weekly home measurements in women with gestational hypertension, and its use is also suggested for women with chronic hypertension and poorly controlled BP. The only recommendation for the use of ABPM in pregnancy is to rule out a diagnosis of suspected white coat hypertension prior to initiating antihypertensive medication (109). Although data are limited, approximately 30% of high-risk pregnant women have been reported to have masked hypertension on ABPM (110,111).
Pros and cons of using BP measurements obtained in routine clinical practice for research
The advantage of using BP measurements obtained in clinical practice for research is that over time, patients tend to have a large number of visits with BP readings, improving precision, and potentially reducing or even eliminating the need for research visits. Additionally, measurements obtained in clinical practice represent those used for management and decision making and are the basis for performance measures. Since BP is routinely measured at many encounters, especially in people with elevated BP or hypertension, the number and frequency of measurements may exceed those in research protocols. However, major concerns with using BP measurements obtained in clinical practice for research are the lack of standardization and the questionable accuracy of clinic BP measurements, with the potential for both systematic and random errors. Furthermore, unless BP measurements, including out-of-clinic readings, are recorded in an EMR, it may be impractical to extract them.
It is commonly believed that research-quality BP measurements are lower than the same individuals’ BP measurements in the routine practice setting. However, the pressure to score well on quality measures may increase the likelihood of bias in the opposite direction (112,113). In the Multi-Ethnic Study of Atherosclerosis, a large prospective observational study, research-grade SBP measurements were on average 6.3 mmHg lower than the most proximal clinic measurement, before or after the study visit, recorded in the EMR(114). This study highlights the high likelihood for misclassification of hypertension status of patients in settings without the use of standardized BP measurement protocols and validated devices. However, the association between routine clinic and research BPs may not be consistent across sites and may be modified by patient characteristics such as age, sex, race, and comorbidities. A standardized BP measurement protocol including the use of an oscillometric device, training of medical assistants, and monitoring compliance with BP protocols has shown promise in reducing systematic measurement errors(115). Also, when BP measurement in clinical practice is performed using established protocols and validated automated equipment, it may be acceptable for research purposes and yield similar conclusions as measurements obtained in research settings.
In clinical trials aimed at reducing BP, the statistical power to detect a between-group difference in BP change depends on two factors: the number of participants in each group and the standard deviation of the change in BP (SDΔBP). The SDΔBP can be reduced by averaging multiple readings taken over multiple visits for both the pre- and post-BP assessments. Using BP measurements from routine clinic visits, where BP from more visits are available, would result in a smaller SDΔBP and, therefore, a smaller sample size required for a clinical trial. However, increasing the number of measurements obtained will not overcome intra-person variability introduced by an inconsistent technique in how BP is measured and by concurrent clinical factors (e.g., acute medical conditions) that may be present when BP is measured in routine clinical practice. ABPM and HBPM are alternative approaches that provide many BP measurements.
Emerging approaches to obtaining BP measurements for clinical practice and clinic-based research
The availability of accurate and relatively inexpensive oscillometric devices that measure BP and transmit data wirelessly represents an emerging approach to BP assessment. Data may be transmitted from a device located in a clinic or out-of-clinic setting to a wireless hub or smartphone, and from there to a secure server. BP data may also be transmitted to an EMR, provided appropriate security procedures are in place, where it can then be used for clinical care and research purposes. The development of common data models has made it possible to pool BP and other clinical data from different research organizations. Databases that include BP measurements, diagnosis codes, and pharmacy data may be used to characterize the hypertension status of large populations and to create virtual registries. EMRs are likely to be used increasingly in observational studies and in trials for recruitment, delivery of interventions, and outcome ascertainment. These data will be greatly enhanced by standardization of the BP measurement method and the use of validated devices.
Optimization of measurements in clinical practice and in clinic-based research studies
Key principles
Key aspects of the measurement process include time of day, the staff member who prepared the participant and measured BP, location (emergency room, clinic, hospital, etc.), position (lying, seated, standing), site of cuff placement (right/left side, arm/leg), cuff size and the specific BP device utilized. All BPs should be recorded in the EMR in structured data fields as individual measurements rather than the average in order to facilitate monitoring of adherence to protocols that call for two or more BP measurements at each visit and for the purpose of using the data for ongoing clinical care and research. To avoid recording errors, BP values from the clinic, HBPM or ABPM should be directly transferred from the device to the EMR, whenever possible. If the average BP is recorded without notation, it is difficult to determine whether the appropriate number of BP measurements was obtained. Documentation of BPs in the EMR should include key components of the measurement process, listed above, along with the actual BP values (Table 2).
Table 2.
Key components of blood pressure measurement that should be documented in the electronic medical record.
| Component | Rationale/Notes/Necessary for: |
|---|---|
| Date/time | Allows for assessment of trends and diurnal variation |
| Location (emergency department, clinic, hospital ward, etc.) | Clinical interpretation |
| Staff member | Quality control including the monitoring of digit preference |
| Position (supine, seated, standing) | Clinical interpretation |
| Site of cuff placement (right/left side, arm/leg) | Clinical interpretation |
| Duration of quiet rest prior to the first measurement and between readings | Quality control/monitoring protocol adherence |
| Mid-arm circumference and cuff size used | Quality control |
| Device utilized | Quality control |
| Individual blood pressure levels | As opposed to just entering average of 2 or 3 blood pressure measurements. Allows for assessment of variability and quality control/monitoring protocol adherence (>1 blood pressure measured). |
| Blood pressure levels in both arms | Determine which arm is appropriate for future measurements (arm with the higher blood pressure should be used) |
| Pain level | Clinical interpretation |
Training and quality control
Clinical practice
Recommendations for standardization of BP assessment have not changed substantially from JNC7 in 2003 (116) and the 2005 AHA Scientific Statement on BP measurement (6) to the 2017 ACC/AHA BP guideline (1). However, a standardized approach is rarely followed in clinical practice (6,117). Increasingly, guidelines recommend use of validated upper arm oscillometric devices in place of auscultation(25,45), which can reduce human error and bias, but does not eliminate many of the factors that contribute to inaccurate measurements and the need for trained observers (Table 3) (118).
Table 3.
Sources of inaccuracy in the measurement of blood pressure in the clinic setting.
| Effect on SBP, mm Hg | Effect on DBP, mm Hg | |
|---|---|---|
| Before measurement | ||
| Acute meal ingestion | − 6 | −5 to −1.9 |
| Acute alcohol consumption | −23.6 to +24 | −14 to +16 |
| Acute caffeine consumption | +3 to +14 | +2.1 to +13 |
| Acute nicotine use or exposure | +2.8 to +25 | +2 to +18 |
| Bladder distension | +4.2 to +33 | +2.8 to +18.5 |
| Cold exposure | +5 to +32 | +4 to +23 |
| Insufficient rest period | +4.2 to +11.6 | +1.8 to +4.3 |
| Device | ||
| Use of a non-validated device | 0% to 70% with ≥ ±3† | 0% to 70% with ≥ ±3† |
| Device not calibrated | 0% to 70%† | 0% to 70%† |
| Patient positioning | ||
| Standing versus sitting | −2.9 to +5.0 | +7 |
| Supine versus sitting | −10.7 to +9.5 | −13.4 to +6.4 |
| Legs crossed at the knee | +2.5 to +14.9 | +1.4 to +10.8 |
| Unsupported back | Not significant effects | +6.5 |
| Unsupported arm | +4.9 | +2.7 to +4.8 |
| Arm lower than heart level | +3.7 to +23 | +2.8 to +12 |
| Attaching the device to the person | ||
| Paretic arm | +2 | +5 |
| Too small cuff size | +2.1 to +11.2 | +1.6 to +6.6 |
| Too large cuff size | −3.7 to −1.5 | −4.7 to −1.0 |
| Cuff placed over clothing | Not significant effects | Not significant effects |
| Stethoscope placed under cuff | +1.0 to +3.1 | −10.6 to −3.5 |
| Taking the measurement | ||
| White coat effect | −12.7 to +26.7 | −8.2 to +21 |
| Talking during the measurement | +4 to +19 | +5 to +14.3 |
| Use of stethoscope bell vs. diaphragm | −3.8 to −1.5 | −1.6 |
| Excessive pressure on stethoscope head | Not significant effects | −15 to −9 |
| Fast cuff deflation | −9 to −2.6 | +2.1 to +6.3 |
| Observer hearing deficit | −1.6 to −0.1 | +1.1 to +4.3 |
| Recording Korotkoff phase IV versus V for DBP | Not applicable | +12.5 |
| Short interval between measurements | Not significant effects | Not significant effects |
| Interpreting the measurement | ||
| Reliance on a single measurement | +3.3 to +10.4 | −2.4 to +0.6 |
| Inter-arm differences | 3.3 to 6.3†† | 2.7 to 5.1†† |
| Terminal digit preference | 1% to 79% over-representation of terminal of 0 | 3% to 79% over-representation of terminal of 0 |
DBP – diastolic blood pressure; SBP – systolic blood pressure
Depending on type of device used (mercury, aneroid or automated)
Values could be too low or too high depending on the arm used.
Adapted from N Kallioinen, A Hill, M Horswill et al. J Hypertens. 2017 Mar; 35 (3):421-441.
The technician or healthcare provider remains the most important component of accurate and reliable BP measurement, and standardization of training, re-training and certification at regular intervals are recommended for everyone who measures BP(6,117). While training may occur in ambulatory settings for clinical staff, physicians are not typically trained or tested in BP measurement accuracy after medical school. Although they may not be the primary person conducting BP measurements, physicians routinely confirm abnormal BP readings obtained in examination rooms, often using auscultation, the most technically demanding method of BP measurement. These manual backup BP readings are likely to be inaccurate in the absence of using a calibrated device, selection of an appropriately sized cuff, and regular retraining (117). After initial training, auscultatory skills decline rapidly without regular retraining and accuracy testing (117). Also, although guidelines recommend averaging BP within and across visits, informal polling of clinicians has found this is rarely done(1). Using AOBP devices may facilitate obtaining the average of multiple measurements during a visit. However, devices that provide individual readings, in addition to an average, should be used.
Research Studies
In research studies, rigorous standardized protocols for measurement are needed to ensure the comparability and accuracy of BP assessments because of measurement error and physiological BP variability (119,120). An international consortium for quality research (TRUE) was formed in 2015 to make recommendations to improve the quality of research BP assessment (121). Table 4 summarizes recommendations which constitute a minimum standard for clinical and epidemiological research.
Table 4.
Recommendations which constitute a minimum standard for clinical and epidemiological research.
| Observer Training and Testing |
|---|
|
| BP Measurement Devices |
|
| BP Assessment |
|
ABPM: ambulatory blood pressure monitoring, BP: blood pressure, HBPM: home blood pressure monitoring.
Regulatory approaches and partnerships
To date, efforts to improve the quality of BP measurements have focused on educating healthcare providers at an early stage of their career and on minimizing manual aspects of measurements (e.g., by using automated devices). Although contemporary evidence is sparse, prior studies have repeatedly documented poor quality of measurements as evidenced by digit preference and excess BP variability(122,123). Promulgation of recent guidelines is unlikely to be effective in improving BP measurement techniques, given prior lack of benefit when previous guidance has been published. In this context, a case can be made for regulatory and accreditation bodies, such as the Joint Commission: Accreditation, Health Care, Certification and National Committee for Quality Assurance, to develop and monitor basic standards for BP measurement, similar to those implemented in clinical research (124). Such standards could include requiring the use of validated devices, establishing criteria to assure continued device calibration, using appropriately sized cuffs, and training and re-training technicians and providers on key features of BP measurement. These requirements would reinforce the importance of accuracy in the measurement of BP.
An approach to ensure BP measurement procedures are followed will likely require collaboration between policymakers, insurers, health care systems, EMR vendors, device manufacturers, and professional organizations (American Academy of Family Physicians, ACC, American College of Physicians, AHA, American Medical Association [AMA] and others). Agencies such as the Centers for Medicare and Medicaid Services provide incentives to health care plans/organizations for meeting quality metrics including the percent of the population with hypertension who have controlled BP. Currently, however, BP control is determined using only a single BP measurement or the lowest SBP or DBP of two or more measurements taken on the same day, rather than averaging at least two BP measurements on two or more occasions, as is recommended in clinical practice guidelines. Also, the United States Preventive Services Task Force and ACC/AHA guideline recommend out-of-clinic BP measurement to confirm the diagnosis of hypertension (1,5). No quality assurance metrics guide the appropriate measurement of BP, and little reimbursement supports the clinical procedure of BP assessment, whether inclinic or out-of-clinic, despite the potential high cost of under- and over-treatment of a condition, hypertension, that affects about half of the US adult population (2). Additionally, health insurers should provide increased time and adequate reimbursement to correctly measure BP.
Use of resources to improve the quality of BP measurements and care of patients with high BP may produce better hypertension control rates, and, more importantly, lower cardiovascular morbidity and mortality. In the US, improved management of high BP, including encouragement of standardized BP measurement, is a central component of the Centers for Medicare and Medicaid Services Million Hearts (125) and the AHA/AMA TARGET BP(126) initiatives. Globally, the World Health Organization’s Global Hearts(127) and the Vital Strategies Resolve(128) projects have similar goals. Although as yet unproven, these initiatives hold promise for improving the health of adults in the US and other countries.
Summary and conclusions
Over the past two decades, there have been several developments in the approach to BP measurement that have provided opportunities, yet presented new challenges, for clinical practice and research.
BP measurements obtained in routine clinical practice are increasingly being used for research and quality improvement activities. Despite repeated guideline recommendations and educational efforts, it appears that the quality of BP measured routinely in clinical practice remains poor. Current limitations with clinic BP measurement include lack of standardization, infrequent technician/clinician training and re-training, use of devices that have not been validated and/or regularly calibrated, not using an appropriately-sized cuff, improper conditions and technique and inadequate documentation of the procedure. Also, despite guideline recommendations, the averaging of BP within and across visits is rarely done.
There is substantial evidence demonstrating that out-of-clinic BP measurements, using ABPM and HBPM, have stronger associations with risk for CVD events than clinic BP measurements(129). While guidelines recommend the use of ABPM and HBPM to guide the initiation and intensification of antihypertensive treatment, they are often not integrated into EMRs. Documenting out-of-clinic BP in the medical record could become more common with Healthcare Effectiveness Data and Information Set and National Committee for Quality Assurance recommendations to conduct ABPM or HBPM. It is important to recognize that despite strong observational data, ABPM and HBPM have not been used to determine eligibility for, or to guide antihypertensive treatment in large randomized controlled trials. Additionally, there has been rapid innovation with a burgeoning array of novel BP measurement devices being developed for out-of-clinic BP measurement, including some that measure BP without cuffs. However, few formal validation studies of the accuracy of these devices have been performed and, at present, these devices cannot be recommended.
EMRs provide an opportunity to document BP assessment and facilitate use of routine clinical BP measurements in research, but most of the concerns about obtaining high quality measurements persist. Efforts to standardize BP measurement procedures and improve their quality in routine clinical practice are needed. This may include documentation of BP training, selection of validated devices, and periodic device calibration by accreditation bodies. An example of this effort is the checklist used by Rakotz et al. to observe medical students measuring BP (130). Also, there is a need to develop and improve EMR functionality, including documentation of key features of BP measurement, seamless transmission of data from measurement devices, including out-of-clinic devices, to the EMR, tools to manipulate and average BP data at individual visits and over time, and improved data presentation to facilitate patient care, health system improvements, and research applications.
Supplementary Material
Central Illustration:
Clinic, home and ambulatory blood pressure measurements.
Research recommendations.
A robust research portfolio is needed to provide an evidence base for future clinical practice guidelines and clinical research, particularly research on the use of routinely collected clinic BP for research purposes. The working group has identified several objectives for future research with potentially high impact (Central Illustration).
Determine the variation in BP measurement approaches being used in routine clinical practice across the US and in clinical research protocols.
Identify the aspects of the BP measurement protocol (e.g., presence of an observer, duration of wait time) that have the most substantial impact on the accuracy and precision of clinic BP. This research can guide the development of simplified BP measurement protocols for implementation in routine clinical practice.
Evaluate the effect of interventions to improve BP measurements in routine clinical practice (e.g., use of automated devices that obtain and average multiple readings) on the accuracy and precision of clinic BP measurements and BP control rates.
Evaluate head-to-head comparative data on the association of standardized clinic vs. out-of-clinic BP with CVD outcomes and mortality.
Evaluate the impact of systematic and random errors on the diagnosis and management of hypertension and identify approaches to delineate real changes in BP from random error following treatment initiation.
Assess the associations between routine clinic and research BP measurements and determine in what circumstances measurements obtained in routine clinical practice are acceptable to be utilized in research.
Determine the optimal quality metric for BP control (e.g., using the average BP at an individual visit or across several visits, using only the last available BP reading) from the EMR.
- Evaluate the role of ABPM and HBPM in the diagnosis and treatment of hypertension; including:
- Whether BP from ABPM versus HBPM provides a more accurate estimate of CVD risk, including the contribution of sleep measurements.
- The utility of using unattended AOBP and HBPM as screening tools prior to conducting ABPM among adults not taking and taking antihypertensive medication.
- The CVD and all-cause mortality risk reduction benefit of initiating antihypertensive medication among adults with white coat hypertension and masked hypertension and intensifying antihypertensive medication among adults with white coat effect and masked uncontrolled hypertension.
- The utility of nighttime BP in the diagnosis of hypertension and the benefits of antihypertensive interventions among adults with asleep hypertension (e.g., chronotherapy).
- Effective ways to reduce barriers to conducting ABPM and HBPM in clinical practice.
- The optimal protocol for using HBPM readings to diagnose and assess control of hypertension (what time of day, how many BP measurements, minimum acceptable number of measurements, duration of measurement period)
- Quantifying the burden associated with conducting 24-hour ABPM, including sleep disturbances and restriction on daily activities.
Evaluate the role of BP measured in public locations for hypertension screening and conducting follow-up BP measurements that can be used to guide antihypertensive medication titration.
Determine the validity of novel approaches (e.g., cuff-less devices) for BP measurement and the association of BP measured with these devices and CVD risk.
Assess the value of using orthostatic hypotension as part of the protocol to guide antihypertensive therapy.
Evaluate the prognostic impact of different orthostatic hypotension definitions with an emphasis on position (supine vs seated), timing of BP measurements after standing, and thresholds of change in BP versus self-reported orthostatic symptoms.
Evaluate apps for simplifying and organizing the incoming data from out-of-clinic BP measurements.
Evaluate approaches to measuring BP in morbidly obese adults including where (e.g., forearm, finger or wrist) BP should be measured.
Acknowledgements:
This work was supported by the Division of Cardiovascular Sciences (DCVS) of the National Heart, Lung, and Blood Institute (NHLBI). The authors thank Dr. David Goff, Director, DCVS, and the Division leadership for their support. We also acknowledge the DCVS Planning Committee (Dr. Jeffrey Cutler, Dr. Lawrence Fine, Dr. Michael Mussolino, Ms. Joni Snyder, Mr. Michael Wolz, Dr. Jacqueline Wright) for their input into development of the Working Group objectives and the conference agenda. The authors thank Dr. Michael Lauer, Deputy Director for Extramural Research, NIH, for making a presentation on real-world clinical practice data in clinical research at the conference. Dr. Paul Muntner received grant support from the American Heart Association (15SFRN2390002). Dr. Paul K. Whelton received grant support from P20GM109036. Dr. Natalie Bello received grant support from the National Center for Advancing Translation Sciences (5KL2TR001874-02) and the National Heart, Lung, and Blood Institute (K23HL136853-01A1). Dr. Beverly B. Green received grant support from the Patient-Centered Outcomes Research Institute (CER-1511-32979 and IHS-1507-31146) and the National Heart, Lung, and Blood Institute (1R01HL136575). Dr. Daniel W. Jones received grant support from National Institute of General Medical Sciences (1U54GM115428). Dr. Karen L. Margolis received grant support from the Patient-Centered Outcomes Research Institute (IHS-1507-31146) and the National Heart, Lung, and Blood Institute (R01HL090965). Dr. Stephen P. Juraschek received grant support from the National Heart, Lung, and Blood Institute (7K23HL135273-02). Dr. Ann Marie Navar received grant support from the National Heart, Lung, and Blood Institute (K01 HL133416). Dr. Joseph E. Schwartz has received grant support from the National Heart, Lung, and Blood Institute (P01 HL47540). Dr. Daichi Shimbo has received grant support from the National Heart, Lung, and Blood Institute (K24-HL125704, R01HL117323, and P01 HL47540).
ABBREVIATIONS AND ACRONYMS
- ABPM
Ambulatory blood pressure monitoring
- AOBP
Automated office blood pressure
- BP
Blood pressure
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- FDA
Food and Drug Administration
- HBPM
Home blood pressure monitoring
- SBP
Systolic blood pressure
Footnotes
Disclosures: Dr. Muntner has received research support from Amgen. Dr. Cushman has received institutional grant support from Eli Lilly, consulting Sanofi, uncompensated consulting Novartis and Takeda. Dr. Navar has received research support from Amgen, Regeneron, Sanofi, and consulting/advisory board for Amgen and Sanofi. Dr. Stergiou has received research support from iHealth, InBody, Maisense, Microlife, and consulting for Maisense, Microlife, Omron. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Disclaimer: The views expressed in this report are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the Department of Health and Human Services. Dr. Rakotz is an employee of the American Medical Association. The findings and conclusions in this report are those of the author and do not necessarily represent the views of the American Medical Association.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults. J Am Coll Cardiol 2018;71:2199–2269.29146533 [Google Scholar]
- 2.Muntner P, Carey RM, Gidding S et al. Potential U.S. Population Impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol 2018;71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth J A short history of blood pressure measurement. Proc R Soc Med 1977;70:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu AL. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163:778–86. [DOI] [PubMed] [Google Scholar]
- 6.Pickering TG, Hall JE, Appel LJ et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005;45:142–61. [DOI] [PubMed] [Google Scholar]
- 7.Giorgini P, Weder AB, Jackson EA, Brook RD. A review of blood pressure measurement protocols among hypertension trials: implications for "evidence-based" clinical practice. J Am Soc Hypertens 2014;8:670–6. [DOI] [PubMed] [Google Scholar]
- 8.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med 2011;154:781–788. [DOI] [PubMed] [Google Scholar]
- 9.Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertens 2014;8:930–8. [DOI] [PubMed] [Google Scholar]
- 10.Alpert BS. 'Oscillometric': a type of device, not a type of measurement. Oh when will they ever learn? J Hypertens 2017;35:1717. [DOI] [PubMed] [Google Scholar]
- 11.Association for the Advancement of Medical Instrumentation. American National Standard Non-invasive Sphygmomanometers – pat 2: Clinical validation of automated measurement type ISO 81060-2/ANSI-AAMI. 2nd ed. Arlington, VA, 2013. [Google Scholar]
- 12.Ostchega Y, Prineas RJ, Nwankwo T, Zipf G. Assessing blood pressure accuracy of an aneroid sphygmomanometer in a national survey environment. Am J Hypertens 2011;24:322–7. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RH, Knaus VL, Bauer JH. Aneroid sphygmomanometers. An assessment of accuracy at a university hospital and clinics. Arch Intern Med 1991;151:1409–12. [DOI] [PubMed] [Google Scholar]
- 14.Quinn B, Forrest S, Hillebrenner M, Zuckerman B. Automated noninvasive blood pressure monitors: a Food and Drug Administration review perspective. Blood Press Monit 2017;22:182–183. [DOI] [PubMed] [Google Scholar]
- 15.Stergiou GS, Alpert B, Mieke S et al. A Universal Standard for the Validation of Blood Pressure Measuring Devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension 2018;71:368–374. [DOI] [PubMed] [Google Scholar]
- 16.Alpert BS. Can 'FDA-cleared' blood pressure devices be trusted? A call to action. Blood Press Monit 2017;22:179–181. [DOI] [PubMed] [Google Scholar]
- 17.Myers MG, Valdivieso M. Evaluation of an automated sphygmomanometer for use in the office setting. Blood Press Monit 2012;17:116–9. [DOI] [PubMed] [Google Scholar]
- 18.Graves JW, Nash C, Burger K, Bailey K, Sheps SG. Clinical decision-making in hypertension using an automated (BpTRU) measurement device. J Hum Hypertens 2003;17:823–7. [DOI] [PubMed] [Google Scholar]
- 19.Gustavsen PH, Hoegholm A, Bang LE, Kristensen KS. White coat hypertension is a cardiovascular risk factor: a 10-year follow-up study. J Hum Hypertens 2003;17:811–7. [DOI] [PubMed] [Google Scholar]
- 20.Burgess SE, MacLaughlin EJ, Smith PA, Salcido A, Benton TJ. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens 2011;5:484–8. [DOI] [PubMed] [Google Scholar]
- 21.Head GA, Mihailidou AS, Duggan KA et al. Definition of ambulatory blood pressure targets for diagnosis and treatment of hypertension in relation to clinic blood pressure: prospective cohort study. BMJ 2010;340:c1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J of Hypertens 2001;14:1263–9. [DOI] [PubMed] [Google Scholar]
- 23.Myers MG, Oh PI, Reeves RA, Joyner CD. Prevalence of white coat effect in treated hypertensive patients in the community. Am J of Hypertens 1995;8:591–7. [DOI] [PubMed] [Google Scholar]
- 24.Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009;27:280–6. [DOI] [PubMed] [Google Scholar]
- 25.Leung AA, Daskalopoulou SS, Dasgupta K et al. Hypertension Canada's 2017 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults. Can J Cardiol 2017;33:557–576. [DOI] [PubMed] [Google Scholar]
- 26.Stergiou G, Kollias A, Parati G, O'Brien E. Office Blood Pressure Measurement: The Weak Cornerstone of Hypertension Diagnosis. Hypertension 2018;71:813–815. [DOI] [PubMed] [Google Scholar]
- 27.Bauer F, Seibert FS, Rohn B et al. Attended Versus Unattended Blood Pressure Measurement in a Real Life Setting. Hypertension 2017;71:243–249. [DOI] [PubMed] [Google Scholar]
- 28.Johnson KC, Whelton PK, Cushman WC et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parati G, Stergiou G, O'Brien E et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014;32:1359–66. [DOI] [PubMed] [Google Scholar]
- 30.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med 2015;163:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgkinson J, Mant J, Martin U et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ 2011;342:d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stergiou GS, Skeva II, Baibas NM, Kalkana CB, Roussias LG, Mountokalakis TD. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens 2000;18:1745–51. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa J, Shimizu M, Sugiyama Edison E et al. Assessment of the reductions in nighttime blood pressure and dipping induced by antihypertensive medication using a home blood pressure monitor. J Hypertens 2014;32:82–9. [DOI] [PubMed] [Google Scholar]
- 34.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens 2016;10:224–234 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padwal RS, Townsend RR, Trudeau L, Hamilton PG, Gelfer M. Comparison of an in-pharmacy automated blood pressure kiosk to daytime ambulatory blood pressure in hypertensive subjects. J Am Soc Hypertens 2015;9:123–9. [DOI] [PubMed] [Google Scholar]
- 36.Alpert BS. Are kiosk blood pressure readings trustworthy? Blood Press Monit 2012;17:257–8. [DOI] [PubMed] [Google Scholar]
- 37.Albasri A, O'Sullivan JW, Roberts NW, Prinjha S, McManus RJ, Sheppard JP. A comparison of blood pressure in community pharmacies with ambulatory, home and general practitioner office readings: systematic review and meta-analysis. J Hypertens 2017;35:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kronish IM, Kent S, Moise N et al. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens 2017;11:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng C, Studdiford JS, Diamond JJ, Chambers CV. Primary care physician beliefs regarding usefulness of self-monitoring of blood pressure. Blood Press Monit 2003;8:249–54. [DOI] [PubMed] [Google Scholar]
- 40.Logan AG, Dunai A, McIsaac WJ, Irvine MJ, Tisler A. Attitudes of primary care physicians and their patients about home blood pressure monitoring in Ontario. J Hypertens 2008;26:446–52. [DOI] [PubMed] [Google Scholar]
- 41.Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol 2011;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz JE, Burg MM, Shimbo D et al. Clinic Blood Pressure Underestimates Ambulatory Blood Pressure in an Untreated Employer-Based US Population: Results From the Masked Hypertension Study. Circulation 2016;134:1794–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolan E, Stanton A, Thijs L et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005;46:156–61. [DOI] [PubMed] [Google Scholar]
- 44.Conen D, Aeschbacher S, Thijs L et al. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension 2014;64:1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mancia G, Fagard R, Narkiewicz K et al. 2013. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–219. [DOI] [PubMed] [Google Scholar]
- 46.Thomas SJ, Booth JN 3rd, Bromfield SG et al. Clinic and ambulatory blood pressure in a population-based sample of African Americans: the Jackson Heart Study. J Am Soc Hypertens 2017;11:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth JN 3rd, Diaz KM, Seals SR et al. Masked Hypertension and Cardiovascular Disease Events in a Prospective Cohort of Blacks: The Jackson Heart Study. Hypertension 2016;68:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palla M, Saber H, Konda S, Briasoulis A. Masked hypertension and cardiovascular outcomes: an updated systematic review and meta-analysis. Integr Blood Press Control 2018;11:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007;25:2193–8. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Huang W, Mai W et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens 2017;35:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muntner P, Booth JN 3rd, Shimbo D, Schwartz JE. Is white-coat hypertension associated with increased cardiovascular and mortality risk? J Hypertens 2016;34:1655–8. [DOI] [PubMed] [Google Scholar]
- 52.Mancia G, Bombelli M, Facchetti R et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension 2009;54:226–32. [DOI] [PubMed] [Google Scholar]
- 53.Ugajin T, Hozawa A, Ohkubo T et al. White-coat hypertension as a risk factor for the development of home hypertension: the Ohasama study. Arch Intern Med 2005;165:1541–6. [DOI] [PubMed] [Google Scholar]
- 54.Sheppard JP, Fletcher B, Gill P, Martin U, Roberts N, McManus RJ. Predictors of the Home-Clinic Blood Pressure Difference: A Systematic Review and Meta-Analysis. Am J Hypertens 2016;29:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang YC, Shimbo D, Muntner P, Moran AE, Krakoff LR, Schwartz JE. Prevalence of Masked Hypertension Among US Adults With Nonelevated Clinic Blood Pressure. Am J Epidemiol 2017;185:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA. Prevalence, Determinants, and Clinical Significance of Masked Hypertension in a Population-Based Sample of African Americans: The Jackson Heart Study. Am J Hypertens 2015;28:900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mallion JM, Genes N, Vaur L et al. Detection of masked hypertension by home blood pressure measurement: is the number of measurements an important issue? Blood Press Monit 2004;9:301–5. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Dov IZ, Ben-Arie L, Mekler J, Bursztyn M. Reproducibility of white-coat and masked hypertension in ambulatory BP monitoring. Int J Cardiol 2007;117:355–9. [DOI] [PubMed] [Google Scholar]
- 59.Viera AJ, Lin FC, Tuttle LA et al. Reproducibility of masked hypertension among adults 30 years or older. Blood Press Monit 2014;19:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urrea B, Misra S, Plante TB et al. Mobile Health Initiatives to Improve Outcomes in Primary Prevention of Cardiovascular Disease. Curr Treat Options Cardiovasc Med 2015;17:59. [DOI] [PubMed] [Google Scholar]
- 61.Kumar N, Khunger M, Gupta A, Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens 2015;9:130–6. [DOI] [PubMed] [Google Scholar]
- 62.Ding XR, Zhang YT, Liu J, Dai WX, Tsang HK. Continuous Cuffless Blood Pressure Estimation Using Pulse Transit Time and Photoplethysmogram Intensity Ratio. IEEE Trans Biomed Eng 2016;63:964–972. [DOI] [PubMed] [Google Scholar]
- 63.Plante TB, Urrea B, MacFarlane ZT et al. Validation of the Instant Blood Pressure Smartphone App. JAMA Intern Med 2016;176:700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theilade S, Joergensen C, Persson F, Lajer M, Rossing P. Ambulatory tonometric blood pressure measurements in patients with diabetes. Diabetes Technol Ther 2012;14:453–6. [DOI] [PubMed] [Google Scholar]
- 65.Rosner B, Polk BF. Predictive values of routine blood pressure measurements in screening for hypertension. Am J Epidemiol 1983;117:429–42. [DOI] [PubMed] [Google Scholar]
- 66.Cook NR, Rosner BA. Screening rules for determining blood pressure status in clinical trials. Application to the trials of hypertension prevention. Am J Epidemiol 1993;137:1341–52. [DOI] [PubMed] [Google Scholar]
- 67.Rosner B, Langford HG. Judging the effectiveness of antihypertensive therapy in an individual patient. J Clin Epidemiol 1991;44:831–8. [DOI] [PubMed] [Google Scholar]
- 68.Ettinger WH Jr., Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc 1994;42:1035–44. [DOI] [PubMed] [Google Scholar]
- 69.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA 1997;277:728–34. [PubMed] [Google Scholar]
- 70.den Ouden ME, Schuurmans MJ, Mueller-Schotte S, Bots ML, van der Schouw Y. Do subclinical vascular abnormalities precede impaired physical ability and ADL disability? Exp Gerontol 2014;58:1–7. [DOI] [PubMed] [Google Scholar]
- 71.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating G. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347–60. [DOI] [PubMed] [Google Scholar]
- 72.Grimm RH Jr., Margolis KL, Papademetriou VV et al. Baseline Characteristics of Participants in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2001;37:19–27. [DOI] [PubMed] [Google Scholar]
- 73.Picone DS, Schultz MG, Otahal P et al. Accuracy of Cuff-Measured Blood Pressure: Systematic Reviews and Meta-Analyses. J Am Coll Cardiol 2017;70:572–586. [DOI] [PubMed] [Google Scholar]
- 74.Gibbons CH, Schmidt P, Biaggioni I et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 2017;264:1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy AK, Jogendra MR, Rosendorff C. Blood pressure measurement in the geriatric population. Blood Press Monit 2014;19:59–63. [DOI] [PubMed] [Google Scholar]
- 76.Cavallini MC, Roman MJ, Blank SG, Pini R, Pickering TG, Devereux RB. Association of the auscultatory gap with vascular disease in hypertensive patients. Ann Intern Med 1996;124:877–83. [DOI] [PubMed] [Google Scholar]
- 77.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM, Investigators AS. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension 2011;57:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 1992;19:508–19. [DOI] [PubMed] [Google Scholar]
- 80.Juraschek SP, Daya N, Rawlings AM et al. Association of History of Dizziness and Long-term Adverse Outcomes With Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern Med 2017;177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Juraschek SP, Daya N, Appel LJ et al. Orthostatic Hypotension in Middle-Age and Risk of Falls. Am J Hypertens 2017;30:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Juraschek SP, Daya N, Appel LJ et al. Orthostatic Hypotension and Risk of Clinical and Subclinical Cardiovascular Disease in Middle-Aged Adults. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens 2016;34:351–8. [DOI] [PubMed] [Google Scholar]
- 84.Panel on Prevention of Falls in Older Persons AGS, British Geriatrics S. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 2011;59:148–57. [DOI] [PubMed] [Google Scholar]
- 85.Juraschek SP, Miller ER 3rd, Appel LJ. Orthostatic Hypotension and Symptoms in the AASK Trial. Am J Hypertens 2018;31:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fleg JL, Evans GW, Margolis KL et al. Orthostatic Hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial: Prevalence, Incidence, and Prognostic Significance. Hypertension 2016;68:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wright JT Jr., Williamson JD, Whelton PK et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berlowitz DR, Breaux-Shropshire T, Foy CG et al. Hypertension Treatment and Concern About Falling: Baseline Data from the Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc 2016;64:2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freeman R, Wieling W, Axelrod FB et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 90.Gabb GM, Mangoni AA, Anderson CS et al. Guideline for the diagnosis and management of hypertension in adults - 2016. Med J Aust 2016;205:85–9. [DOI] [PubMed] [Google Scholar]
- 91.Raiha I, Luutonen S, Piha J, Seppanen A, Toikka T, Sourander L. Prevalence, predisposing factors, and prognostic importance of postural hypotension. Arch Intern Med 1995;155:930–5. [DOI] [PubMed] [Google Scholar]
- 92.Maurer MS, Cohen S, Cheng H. The degree and timing of orthostatic blood pressure changes in relation to falls in nursing home residents. J Am Med Dir Assoc 2004;5:233–8. [DOI] [PubMed] [Google Scholar]
- 93.Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc 2011;59:655–65. [DOI] [PubMed] [Google Scholar]
- 94.Braam EA, Verbakel D, Adiyaman A, Thien T. Orthostatic hypotension: revision of the definition is needed. J Hypertens 2009;27:2119–20; author reply 2120. [DOI] [PubMed] [Google Scholar]
- 95.Fedorowski A, Hamrefors V, Sutton R et al. Do we need to evaluate diastolic blood pressure in patients with suspected orthostatic hypotension? Clin Auton Res 2017;27:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones DW. Body weight and blood pressure. Effects of weight reduction on hypertension. Am J Hypertens 1996;9:50s–54s. [DOI] [PubMed] [Google Scholar]
- 98.Ostchega Y, Hughes JP, Zhang G, Nwankwo T, Chiappa MM. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit 2013;18:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irving G, Holden J, Stevens R, McManus RJ. Which cuff should I use? Indirect blood pressure measurement for the diagnosis of hypertension in patients with obesity: a diagnostic accuracy review. BMJ Open 2016;6:e012429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kollias A, Stergiou GS. Automated measurement of office, home and ambulatory blood pressure in atrial fibrillation. Clin Exp Pharmacol Physiol 2014;41:9–15. [DOI] [PubMed] [Google Scholar]
- 101.Sykes D, Dewar R, Mohanaruban K et al. Measuring blood pressure in the elderly: does atrial fibrillation increase observer variability? BMJ 1990;300:162–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pagonas N, Schmidt S, Eysel J et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension 2013;62:579–84. [DOI] [PubMed] [Google Scholar]
- 103.Stergiou GS, Kollias A, Destounis A, Tzamouranis D. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens 2012;30:2074–82. [DOI] [PubMed] [Google Scholar]
- 104.Giantin V, Perissinotto E, Franchin A et al. Ambulatory blood pressure monitoring in elderly patients with chronic atrial fibrillation: is it absolutely contraindicated or a useful tool in clinical practice and research? Hypertens Res 2013;36:889–94. [DOI] [PubMed] [Google Scholar]
- 105.Domenech M, Berruezo A, Molina I, Mont L, Coca A. Nighttime ambulatory blood pressure is associated with atrial remodelling and neurohormonal activation in patients with idiopathic atrial fibrillation. Rev Esp Cardiol (Engl Ed) 2013;66:458–63. [DOI] [PubMed] [Google Scholar]
- 106.Lakhal K, Ehrmann S, Martin M et al. Blood pressure monitoring during arrhythmia: agreement between automated brachial cuff and intra-arterial measurements. Br J Anaesth 2015;115:540–9. [DOI] [PubMed] [Google Scholar]
- 107.O'Brien E, O'Malley K. Evaluation of blood pressure measuring devices with special reference to ambulatory systems. J Hypertens Suppl 1990;8:S133–9. [PubMed] [Google Scholar]
- 108.Bello NA, Woolley JJ, Cleary KL et al. Accuracy of Blood Pressure Measurement Devices in Pregnancy: A Systematic Review of Validation Studies. Hypertension 2018;71:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 110.Salazar MR, Espeche WG, Leiva Sisnieguez BC et al. Significance of masked and nocturnal hypertension in normotensive women coursing a high-risk pregnancy. J Hypertens 2016;34:2248–52. [DOI] [PubMed] [Google Scholar]
- 111.Hermida RC, Ayala DE. Prognostic value of office and ambulatory blood pressure measurements in pregnancy. Hypertension 2002;40:298–303. [DOI] [PubMed] [Google Scholar]
- 112.Fishman PA, Anderson ML, Cook AJ et al. Accuracy of blood pressure measurements reported in an electronic medical record during routine primary care visits. J Clin Hypertens 2011;13:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Green BB, Kaplan RC, Psaty BM. How do minor changes in the definition of blood pressure control affect the reported success of hypertension treatment? Am J Manag Care 2003;9:219–24. [PubMed] [Google Scholar]
- 114.Ahmad FS, Chan C, Rosenman MB et al. Validity of Cardiovascular Data From Electronic Sources: The Multi-Ethnic Study of Atherosclerosis and HealthLNK. Circulation 2017;136:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boonyasai RT, Carson KA, Marsteller JA et al. A bundled quality improvement program to standardize clinical blood pressure measurement in primary care. J Clin Hypertens 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chobanian AV, Bakris GL, Black HR et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 117.Campbell NR, Berbari AE, Cloutier L et al. Policy statement of the world hypertension league on noninvasive blood pressure measurement devices and blood pressure measurement in the clinical or community setting. J Clin Hypertens 2014;16:320–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: a systematic review. J Hypertens 2017;35:421–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.True Consortium. Recommended Standards for Assessing Blood Pressure in Human Research Where Blood Pressure or Hypertension Is a Major Focus. Kidney Int Rep 2017;2:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stergiou GS, Kollias A, Protogerou AD. Evidence on Blood Pressure Measurement Methodology and Clinical Implementation: Research Agenda for the 21st Century. J Am Coll Cardiol 2017;70:587–589. [DOI] [PubMed] [Google Scholar]
- 121.True Consortium. Recommended standards for assessing blood pressure in human research where blood pressure or hypertension is a major focus. J Clin Hypertens 2017;19:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011;342:d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burnier M, Gasser UE. End-digit preference in general practice: a comparison of the conventional auscultatory and electronic oscillometric methods. Blood Press 2008;17:104–9. [DOI] [PubMed] [Google Scholar]
- 124.Appel LJ, Miller ER 3rd, Charleston J. Improving the measurement of blood pressure: is it time for regulated standards? Ann Intern Med 2011;154:838–40. [DOI] [PubMed] [Google Scholar]
- 125.Frieden TR, Berwick DM. The "Million Hearts" initiative--preventing heart attacks and strokes. N Engl J Med 2011;365:e27. [DOI] [PubMed] [Google Scholar]
- 126.American Heart Association/American Medical Association. BP guideline. Top 5 Takeaways for Your Practice: Target BP, 2017. Available at: https://targetbp.org/. Accessed April 24, 2018. [Google Scholar]
- 127.World Health Orgainzation. Global Hearts Initiative. 2018. Available at: http://www.who.int/cardiovascular_diseases/global-hearts/en/. Accessed April 24, 2018.
- 128.Frieden TR, Jaffe MG. Saving 100 million lives by improving global treatment of hypertension and reducing cardiovascular disease risk factors. J Clin Hypertens 2018;20:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2015;162:192–204. [DOI] [PubMed] [Google Scholar]
- 130.Rakotz MK, Townsend RR, Yang J et al. Medical students and measuring blood pressure: Results from the American Medical Association Blood Pressure Check Challenge. J Clin Hypertens 2017;19:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.