Abstract
We retrospectively analyzed published studies to investigate historical trends in outcome of childhood absence epilepsy (CAE). We included patients based on onset of absence seizures in childhood, 3 Hz bilateral spike-wave discharges on EEG, and availability of seizure-free outcome data. The primary endpoint was seizure-freedom off medications by study publication year. We also analyzed relationships between seizure-freedom and 1. treatment medication, and 2. CAE diagnostic criteria. We included 29 studies published 1945–2013, encompassing 2,416 patients. Seizure-freedom off medications was higher for studies after 1985 versus before 1975 (82% versus 35%; p<0.001). Ethosuximide and valproate were used more commonly after 1985, and patients previously treated with ethosuximide or valproate had higher seizure-freedom off medications than those treated only with other medications (64% versus 32%; χ2>10; p<0.001). Although differences in diagnostic criteria for early vs. later studies did not reach statistical significance, later studies tended to use normal EEG background (p=0.09) and absence of comorbid disorders (p=0.09) as criteria more commonly. These findings demonstrate that seizure-freedom off medications has improved in published CAE studies after 1985. Our results are limited due to retrospective analysis. Further work is needed with prospective, controlled trials to establish factors leading to improved long-term prognosis in CAE.
Keywords: Childhood absence epilepsy, ethosuximide, valproate, epileptogenesis, prognosis
1. Introduction
Childhood absence epilepsy (CAE) is a relatively common disorder, characterized by brief episodes of unresponsiveness and 3–4 Hz spike-wave discharges on electroencephalography (EEG) (Guo et al., 2016). Outcome is variable, and although many children may outgrow CAE without sequelae, a substantial minority continue with seizures into adulthood or may have other long-term psychosocial dysfunction (Callenbach et al., 2009; Caplan et al., 2008; Wirrell et al., 1996). Diagnosis and treatment of CAE have changed substantially over the years and an intriguing question is whether long-term prognosis has changed as well. Introduction of effective medical treatments for CAE clearly reduced the incidence of seizures, but recent work from animal absence models and human epidemiology suggest that effective treatments might also have a disease-modifying effect, improving seizure-free prognosis off medications (Berg et al., 2014; Blumenfeld et al., 2008). In addition, diagnostic criteria for CAE have changed substantially over the years and the defining clinical characteristics in more recent studies could be associated with better prognosis. As a first step to investigate these questions, we retrospectively analyzed 29 studies published between 1945 and 2013 to evaluate any trends in long-term seizure outcome in CAE. In addition, to motivate future prospective studies we retrospectively examined changes in use of the effective medications ethosuximide and valproate in relation to seizure-free outcome, and changes in diagnostic criteria during the same time period.
2. Materials and Methods
Studies were identified by Pubmed search and by cross-referencing in reviews and articles. Criteria for study inclusion were: 1. that the patient population meet basic diagnostic criteria for CAE, specifically bilateral synchronous 3 Hz spike-wave discharges on EEG and absence seizures with onset in childhood, and 2. that seizure remission be reported within the study. Treatment groups of less than 5 patients were excluded from analysis.
For each study, we recorded the following: CAE diagnostic criteria, age of onset, number of patients, treatments used, number of patients trialed off medication (when documented), number seizure free on or off medication, length of follow-up, and dropout rate. Seizure freedom was defined as being completely without seizures, including both absence seizures and other seizure types, over the period of follow-up, which ranged from 8 months to 25 years. Methods of determining seizure freedom in most studies was based either explicitly or implicitly (when not specified) on parental and/or patient report, but a few studies also included EEG recording, in-office hyperventilation or direct observations. Patient treatment was classified as being either: 1. valproate or ethosuximide (with or without other medications) or 2. other medications without ethosuximide or valproate. Other medications included bromides, phenobarbital, trimethadione, acetazolamide, non-ethosuximide succinimides, primidone, paramethadione, mephobarbital, phenytoin, dexamphetamine, diazepam, oxazolidine, carbamazepine, chlorphenacemide, sulthiame, lamotrigine, topiramate, or levetiracetam. Data were analyzed by Pearson correlation, two-tailed t-test or chi-squared test as appropriate with significance threshold p<0.05.
3. Results
A total of 38 studies were identified between 1923 and 2013, 29 of which met criteria for inclusion (Table 1). The others were excluded because they only had presenting seizures other than typical absence seizures, did not have onset in childhood, did not include 3Hz spike-wave EEG as a diagnostic criteria, or did not include remission rates. Within the 29 included studies, 2416 subjects met our inclusion criteria of 3 Hz spike-wave on EEG and absence seizure onset in childhood. Of these, 691 were trialed off medications.
Table 1.
Studies used for analysis, including CAE diagnostic criteria, seizure-free outcomes, and dropout rates.
| Publ Yeara |
First Author |
N | Age at Onset, years |
EEG 3 Hz SWDe |
Nl EEG Backgrounde |
≥1 Sz per daye |
Sz Duration 5–30 se |
Abrupt Sz On/ Offsete |
No GTC or Other Szse |
No Abnl Motor Activitye |
No Other Disorders,f |
Seizure Free Allg |
Seizure Free On Medsg |
Seizure Free Off Medsg |
Dropout Rateh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1945 | Lennox | 40 | <20 | + | + | + | + | + | + | + | + | 28% | 28% | ||
| 1955 | Janz | 88 | 4 – 14 | + | − | − | − | − | − | − | − | 16% | |||
| 1957 | Paal | 38 | 3 – 9 | + | − | − | − | − | − | − | − | 36% | 36% | 12% | |
| 1962 | Holowach | 144 | 2 – 9 | + | − | + | + | + | + | + | − | 43% | 63% | 22% | |
| 1963 | Currier | 32 | 3 −17 (7.5)b | + | − | + | − | − | + | + | + | 38% | 38% | 0% | |
| 1963 | Hertoft | 50 | 2 – 12 (5.9)c | + | + | − | − | − | + | − | + | 38% | 38% | 12% | |
| 1965 | Gordon | 45 | 1 – 14 | + | − | − | − | − | + | − | − | 33% | 33% | 4% | |
| 1965 | Livingston | 117 | 2 – 15 | + | − | + | + | + | − | − | − | 78% | 78% | ||
| 1966 | Gibberd | 139 | 5 – 10 | + | − | − | + | + | − | + | − | 41% | 41% | ||
| 1976 | Sato | 48 | 2 – 13 (6.8)c | + | − | − | − | − | − | + | − | 35% | 35% | 8% | |
| 1977 | Ohtahara | 9 | 1 – 15 | + | − | − | − | − | − | − | − | 100% | 100% | ||
| 1978 | Okuma | 22 | childhood | + | − | − | − | − | − | + | − | 73% | 50% | ||
| 1979 | Annegers | 33 | + | − | − | − | − | − | − | − | 60% | 60% | 34% | ||
| 1980 | Kawai | 43 | childhood | + | − | − | − | − | + | + | − | 77% | 77% | 12% | |
| 1983 | Sato | 39 | 2 – 13 (6.8)c | + | − | − | − | − | + | + | − | 64% | |||
| 1983 | Losieau | 69 | 3 – 14 | + | − | − | − | − | + | + | + | 44% | 44% | ||
| 1984 | Wolf | 294 | 4 – 12 | + | − | − | − | − | − | − | − | 50–65% | 50–65% | ||
| 1985 | Dieterich | 184 | 8 – 15 (6.5)d | + | − | − | − | − | − | − | − | 44–96% | 44–96% | ||
| 1991 | Olsson | 56 | 3 – 11 | + | − | − | − | − | + | + | − | 82% | 82% | ||
| 1995 | Loiseau | 39 | 3 – 10 | + | − | + | − | − | + | − | − | 95% | 95% | 41% | |
| 1996 | Wirrell | 65 | 1 – 14 | + | + | + | − | − | + | − | + | 74% | 74% | 11% | |
| 2004 | Trinka | 64 | <10 (7)c | + | + | + | − | + | − | + | + | 60% | 20% | ||
| 2004 | Coppola | 29 | 3 – 13 (7.5)c | + | + | − | − | − | + | + | + | 53–68% | 53–68% | 32% | |
| 2005 | Grosso | 84 | pre-puberty | + | + | + | − | + | − | − | + | 90% | 90% | 15% | |
| 2008 | Verrotti | 21 | 5 – 13 (8.9)c | + | + | + | + | + | + | + | + | 67% | 67% | 52% | |
| 2008 | Holmes | 46 | 3 – 13 (7.3)c | + | − | − | − | − | + | − | + | 46% | 46% | 43% | |
| 2009 | Callenbach | 43 | 1 – 16 (5.4)c | + | + | + | + | + | + | + | + | 70–95% | 70–95% | 9% | |
| 2012 | Hwang | 89 | 4 – 9.8(6.4)c | + | + | + | + | + | + | + | + | 64–87% | 64–87% | 25% | |
| 2013 | Glauser | 446 | 2.5–13(7.4)b | + | + | + | + | + | + | + | + | 50–86% | 50–86% |
Full references included in Supplementary Table S1.
Median.
Mean.
Mode.
These criteria were used (+) or not used (−) for the definition of CAE in each study.
Disorders considered in different studies included psychiatric disease, autism, pervasive development disorders and structural brain lesions, as well as any cardiovascular, hepatic, renal, gynecologic, musculoskeletal, or endocrine disorders.
Seizure freedom was defined as being completely without seizures over the length of follow-up. Seizure Free All included outcome for all patients regardless of treatment; Seizure Free On Meds, outcome for patients while still on medications; Seizure Free Off meds, outcome for patients after medications were discontinued.
Dropout rate was defined as percentage of subjects initially in the study who were lost to follow-up.
Abbreviations: Publ, Publication. N, number of patients included. EEG, electroencephalogram. SWD, spike-wave discharge. Nl, normal. Sz, seizure. GTC, generalized tonic-clonic seizure. Abnl, abnormal.
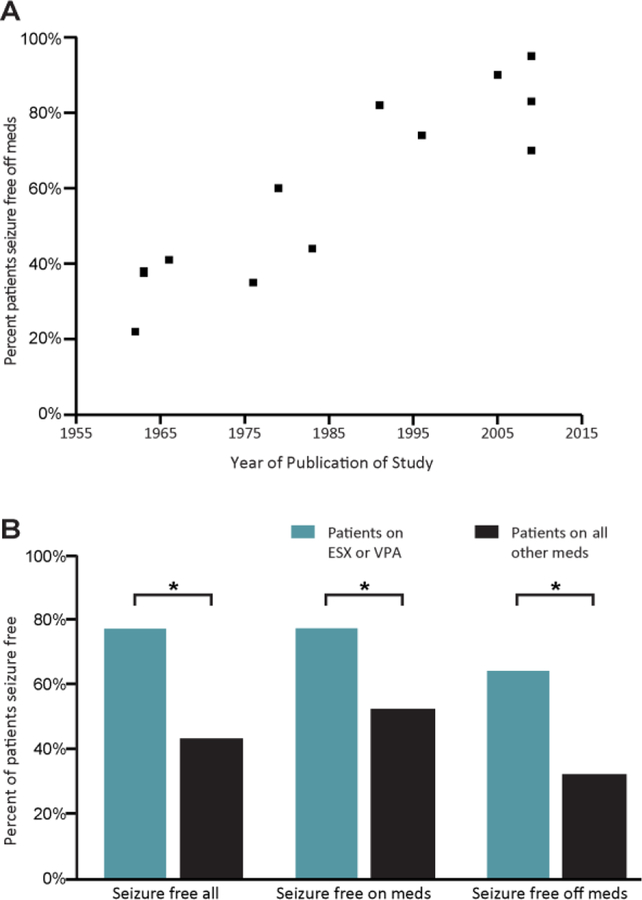
We observed a striking increase in the percentage of seizure freedom off medications in relation to the year of study publication (Figure 1A). Seizure freedom off medications was strongly correlated with year of publication (r=0.82, p<0.001). Overall seizure freedom (on or off medications) was also significantly correlated with year of publication (r=0.61, p<0.001), however seizure freedom on medications was not (r=0.39, p=0.149). In addition, to compare outcome from early vs. later time points, we divided the studies into two groups, before 1975 and after 1985. For the earlier group, the mean percentage of seizure freedom off medications was 35% (SD 9%) and for the later group it was significantly higher at 82% (SD 9%) (p < 0.001, two-tailed t-test).
Figure 1. Seizure-free outcome off medications has improved over time, and is better following treatment with ethosuximide or valproate.
A. Percent of patients experiencing seizure freedom off medications plotted with respect to year of study publication (Table 1). Studies published prior to 1975 had significantly lower rates of seizure freedom than those published after 1985 (p < 0.001, two-tailed t-test). Note: there are 2 studies presented that were published in 1963, with cure rates of 37.5% and 38%, therefore the datapoint at this timepoint consists of 2 overlapping datapoints.
B. Percent of subjects experiencing seizure freedom overall (Seizure free all), while on medication, and once off medication, in patients treated with ethosuximide (ESX) or valproate (VPA) versus all other medications. A significantly higher rate of seizure freedom was found in all three groups in patients treated with ESX or VPA compared to those treated with other medications. * p < 0.001, chi-squared analysis (see text).
To further investigate possible relationships between outcome and treatment, the total patient population across studies was divided into patients on ethosuximide or valproate versus other medications, and further subdivided into patients on versus trialed off medication (Figure 1B). The percentage of patients with seizure freedom on medication was significantly greater in patients on ethosuximide or valproate (77%, N= 801 of 1032) than on other medications (43%, N= 229 of 442) (χ2 > 10, p <0.001). The percentage of patients with seizure freedom off medication was also significantly greater in patients previously on ethosuximide or valproate (64%, N= 160 of 248) than on other medications (32%, N= 140 of 443) (χ2 > 10, p <0.001). Of note, although outcomes while taking ethosuximide or valproate were reported as early as 1965, outcomes once off ethosuximide or valproate were only reported for studies published after 1985.
Defining criteria for CAE varied somewhat across studies (Table 1). Although none differed significantly for early vs. later studies (Fisher’s exact test for criteria used in pre-1975 vs. post-1985 studies), there was a tendency for normal EEG background (p=0.09) and the absence of other disorders (p=0.09) to be used more commonly as criteria in later studies. The age of seizure onset varied but was most commonly 6–8 years. Dropout rate ranged from 0–52%, and there was no significant correlation between dropout rate and year of publication (r=0.20, p=0.44), nor did it appear related to seizure-free outcome (r=0.004, p=0.99) (Table 1). Length of follow-up tended to be somewhat longer in studies that reported outcome off medications (median 11.5 years; interquartile range 5–20 years) versus those that did not (median 5.0 years; interquartile range: 1–10 years), although this did not reach statistical significance (p=0.057).
4. Discussion
This retrospective study finds that the prognosis for seizure-freedom off medications in CAE as reported in the literature has improved markedly over time, with the greatest increase during the 1970s and 1980s. This improvement corresponds with the increasing use of effective medications, particularly ethosuximide and valproate, and also with gradual shifts in diagnostic criteria which, along with other unknown factors all may have contributed to improvements in reported outcome. These findings raise the intriguing possibility that effective medical treatment of CAE has had a disease-modifying benefit, although further investigation of these and other possible contributing factors is clearly needed using a prospective design. Even if the observed changes in published prognosis are due to changes in diagnostic criteria and not disease-modifying benefits, this is nevertheless an important historical fact that has not been reported before and should be quantified and investigated further.
Our results should be interpreted with all the appropriate cautions and limitations applicable to retrospective studies. In particular, we only required basic criteria for CAE diagnosis across all studies, namely 3 Hz spike-wave discharges and onset of absence seizures in childhood, and we did not fully control for changes in diagnostic criteria and the many other changes in medical practice and patient behavior that likely occurred during the study time period. Methods of determining seizure freedom were not available in all studies and in most cases were based on history obtained from the parents and/or patients, known to potentially lead to under-reporting of seizures.(Elger and Hoppe, 2018) Reliance on such methods alone was more common in earlier studies, so it is unlikely that use of more rigorous methods of determining seizure freedom would lead to a decrease in seizure free rates in later studies. Changes in study dropout rates could also potentially influence outcome if, for example patients with good outcome dropped out more often in earlier studies, however we did not observe any systematic change in dropout rates over time. Furthermore, the length of follow-up may have been too short to assess seizure freedom off medications in some studies. Seizure freedom on medications showed less improvement over time than seizure freedom off medications, likely because patients with continued seizures are more prone to remain on medications in follow-up, and possibly due to the heterogeneity of treatments. We did observe that patients treated with ethosuximide or valproate had better seizure control than treatment with other medications, in agreement with prior work (Glauser et al., 2013). Interestingly, we also observed that seizure freedom remained improved for those treated with ethosuximide or valproate even after the medications were stopped. This finding could possibly be explained by a prescription bias if for example, physicians might tend to prescribe these two medications to patients who inherently have a better prognosis. However, another recent study of CAE outcome found improved long-term seizure freedom (5 years after stopping medication) in children previously treated with ethosuximide even after controlling for variable clinical or electrographic features (Berg et al., 2014). It is possible that if the same criteria had been used throughout the years studied here outcomes might have remained equivalent, but this is impossible to determine with retrospective methods and it is nonetheless an important observation that reported prognosis has changed, whatever the cause.
Despite the obvious limitations of the present retrospective study, the possibility that effective treatment improves CAE long-term prognosis warrants further investigation. Recent work in experimental animal absence models has shown that early effective treatment with anti-absence medications has disease-modifying effects on epileptogenesis, improves long-term seizure freedom off medications, and also prevents disease-associated changes in brain structure, DNA methylation, ion channel expression and behavioral comorbidities (Blumenfeld et al., 2008; Dezsi et al., 2013; van Luijtelaar et al., 2013). Ongoing and future human CAE studies should prospectively investigate the possible relationship between different effective treatments and long-term seizure freedom as well as other comorbid features of CAE once medication has ceased (Berg et al., 2014; Glauser et al., 2013; Shinnar et al., 2017). In addition, future prospective studies should examine other clinical factors including age of onset, EEG characteristics, occurrence of other seizure types and additional features which may help identify subgroups most responsive to early intervention. As the genetic basis of CAE is better understood it may ultimately be possible to initiate treatment early enough to prevent both short-term and long-term morbidities and provide better outcomes for children with this disorder.
Supplementary Material
Highlights.
We performed a meta-analysis of historical trends in childhood absence epilepsy.
Improved prognosis in CAE is seen in studies after 1985 versus before 1975.
Ethosuximide and valproate were used more commonly after 1985.
Ethosuximide and valproate were associated with seizure freedom off medication.
5. Acknowledgements
We thank Claude G. Wasterlain for sharing his impression that long-term outcome in childhood absence epilepsy improved after the introduction of effective treatments. This work was supported by NIH R01 NS055829, the Loughridge Williams Foundation and the Betsy and Jonathan Blattmachr family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures of Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- Berg AT, Levy SR, Testa FM, Blumenfeld H, 2014. Long-term seizure remission in childhood absence epilepsy: Might initial treatment matter? Epilepsia 55, 551–557. 10.1111/epi.12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR, 2008. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 49, 400–9. 10.1111/j.1528-1167.2007.01458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callenbach PMC, Bouma PAD, Geerts AT, Arts WFM, Stroink H, Peeters EAJ, van Donselaar CA, Peters ACB, Brouwer OF, 2009. Long-term outcome of childhood absence epilepsy: Dutch Study of Epilepsy in Childhood. Epilepsy Res 83, 249–256. 10.1016/j.eplepsyres.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R, Shields WD, 2008. Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia 49, 1838–1846. 10.1111/j.1528-1167.2008.01680.x [DOI] [PubMed] [Google Scholar]
- Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O’Brien TJ, Jones NC, 2013. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia 54, 635–43. 10.1111/epi.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger CE, Hoppe C, 2018. Diagnostic challenges in epilepsy: seizure under-reporting and seizure detection. Lancet. Neurol 17, 279–288. 10.1016/S1474-4422(18)30038-3 [DOI] [PubMed] [Google Scholar]
- Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, Clark PO, Adamson PC, Childhood Absence Epilepsy Study Team, 2013. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: Initial monotherapy outcomes at 12 months. Epilepsia 54, 141–155. 10.1111/epi.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JN, Kim R, Chen Y, Negishi M, Jhun S, Weiss S, Ryu JH, Bai X, Xiao W, Feeney E, Rodriguez-Fernandez J, Mistry H, Crunelli V, Crowley MJ, Mayes LC, Constable RT, Blumenfeld H, 2016. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet. Neurol 15, 1336–1345. 10.1016/S1474-4422(16)30295-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar RC, Shinnar S, Cnaan A, Clark P, Dlugos D, Hirtz DG, Hu F, Liu C, Masur D, Weiss EF, Glauser TA, Childhood Absence Epilepsy Study Group, 2017. Pretreatment behavior and subsequent medication effects in childhood absence epilepsy. Neurology 89, 1698–1706. 10.1212/WNL.0000000000004514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luijtelaar G, Mishra AM, Edelbroek P, Coman D, Frankenmolen N, Schaapsmeerders P, Covolato G, Danielson N, Niermann H, Janeczko K, Kiemeneij A, Burinov J, Bashyal C, Coquillette M, Lüttjohann A, Hyder F, Blumenfeld H, van Rijn CM, 2013. Anti-epileptogenesis: Electrophysiology, diffusion tensor imaging and behavior in a genetic absence model. Neurobiol. Dis 60, 126–38. 10.1016/j.nbd.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirrell EC, Camfield CS, Camfield PR, Gordon KE, Dooley JM, 1996. Long-term prognosis of typical childhood absence epilepsy: remission or progression to juvenile myoclonic epilepsy. Neurology 47, 912–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.