Abstract
Background:
Smoking reduction treatment is a promising approach to increase abstinence amongst smokers initially unwilling to quit. However, little is known about which reduction treatment elements increase quit attempts and the uptake of cessation treatment amongst such smokers.
Methods:
This study is a secondary analysis of a 4-factor randomized factorial experiment conducted amongst primary care patients (N=517) presenting for regular healthcare visits in Southern Wisconsin who were unwilling to quit smoking but willing to cut down. We evaluated the main and interactive effects of Motivation-phase intervention components on whether participants: 1) made a quit attempt (intentional abstinence ≥ 24 hours) by 6- and 26- weeks post-study enrollment and, 2) used cessation treatment. We also evaluated the relations of quit attempts with abstinence. The four intervention components evaluated were: 1) Nicotine Patch vs. None; 2) Nicotine Gum vs. None; 3) Motivational Interviewing (MI) vs. None; and 4) Behavioral Reduction Counseling (BR) vs. None. Intervention components were administered over 6 weeks, with an option to repeat treatment; participants could request cessation treatment at any point.
Results:
Nicotine gum significantly increased the likelihood of making a quit attempt by 6 weeks (23% vs. 15% without gum; p<.05). Conversely, nicotine patch reduced quit attempts when used with BR. Patch also discouraged use of cessation treatment (15.8% vs. 23% without patch; p<.05). Aided vs. unaided quit attempts produced abstinence in 42% vs. 10% of participants, respectively.
Conclusion:
Nicotine gum is a promising Motivation-phase intervention that may spur quit attempts amongst smokers initially unwilling to quit.
Keywords: primary care, quit attempts, smoking reduction, chronic care smoking treatment, comparative effectiveness, nicotine replacement therapy
1. Introduction
Healthcare visits offer a prime opportunity for smokers to quit smoking (Curry et al., 2008) and for primary care clinicians to deliver effective smoking treatment to them (Eisenberg et al., 2008; Wu et al., 2006). However, the vast majority of healthcare visits do not result in smokers engaging in evidence-based treatment for their smoking (e.g., Bentz et al., 2006; Papadakis et al., 2014; Rigotti et al., 2011). One reason for this is that healthcare systems typically offer only cessation treatment. That is, they tend to offer treatment only to smokers already motivated to make aided quit attempts and offer little or nothing to patients who are not ready to quit―a group comprising the majority of smokers at any given clinic visit (70–90%; (Piper et al., 2013; Wewers et al., 2003).
Reduction treatment has shown promise for smokers who are initially unwilling to quit. Components of reduction treatment typically include nicotine replacement therapy (NRT; e.g., gum or patch) and brief counseling such as behavioral reduction counseling (see Moore et al., 2009) or motivational counseling (Carpenter et al., 2004). First, reduction treatment appears to engage a meaningfully larger group of primary care patients in smoking treatment than would otherwise enter it; the offer of reduction treatment increases the proportion of primary care patients entering treatment by about one-third (Cook et al., 2016). Second, evidence suggests that reduction treatment decreases smoking heaviness amongst smokers initially unwilling to quit and increases the likelihood that they will ultimately become abstinent (Cook et al., 2016; Moore et al., 2009). For instance, in one meta-analysis (Moore et al., 2009), reduction treatment doubled the 6-month abstinence rate relative to placebo (from 3 to 6%).
Reduction treatment studies have focused primarily on abstinence as the key outcome (Asfar et al., 2011; Cook et al., 2016; Moore et al., 2009). While abstinence from tobacco is clearly the ultimate clinical goal, more proximal goals are also important, such as the ability of reduction treatment to motivate quit attempts; i.e., to convert the smoker who is unwilling to make a quit attempt into one who is willing. The Phase-Based Model is a treatment model that identifies different phases of the smoking treatment process (i.e., Motivation, Preparation, Cessation, Maintenance, and Relapse Recovery), each with its own challenges, opportunities, and phase-relevant outcomes (Baker et al., 2011). Such challenges and opportunities can be addressed with different types of intervention components to achieve phase-specific goals. The Motivation phase is characterized, in part, by an unwillingness to make a quit attempt (although this can vacillate: Baker et al., 2011). Motivation phase treatments are focused on increasing quit attempts and the success of those attempts. Quit attempts are an important treatment target since they represent an increase in motivational activation necessary for change (Baker et al., 2011; Prochaska and DiClemente, 1983). While it is true that most quit attempts ultimately fail (Babb et al., 2017), they may spur later quit attempts and result in learning that makes these later attempts more successful (Petersen et al., 2017). This research is aimed at not only identifying Motivation-phase intervention components that increase quit attempts, but also evaluating the clinical significance of the resulting attempts: i.e., their association with abstinence.
This research sought to identify intervention components that significantly increased quit attempts and engagement in evidence-based cessation treatment in an effort to develop an optimal Motivation-phase treatment for smokers unwilling to quit. Four reduction intervention components (Nicotine Patch, Nicotine Gum, Behavioral Reduction [BR] Counseling, and Motivational Interviewing [MI]) were selected for this research based upon substantive and empirical bases. Nicotine patch and gum each have the capacity to: 1) reduce smoking (Moore et al., 2009; Shiffman et al., 2009) and thereby blunt dependence and withdrawal symptoms (Baker et al., 2012a; Baker et al., 2012b); 2) attenuate smoking-environment and smoking-reward contingencies (e.g., Schuz and Ferguson, 2015) and 3) enhance confidence in the ability to make a quit attempt by ameliorating withdrawal symptoms and providing pre-quit use practice (McCarthy et al., 2008). MI was selected to enhance intrinsic motivation and the personal relevance of reducing or quitting (Heckman et al., 2010; Lai et al., 2010). Finally, BR counseling was intended to reduce cigarette consumption and exposure to cigarette smoking cues (e.g., through increased exposure to smoke-free environments), and to encourage the development of coping skills that could increase quitting self-efficacy (Bolt et al., 2009; Broms et al., 2008).
This research used data from a 4-factor randomized factorial experiment by Cook et al. (2016). That study examined the main and interactive effects of four Motivation phase intervention components (Nicotine Gum, Nicotine Patch, Behavioral Reduction Counseling, and Motivational Interviewing) on smoking heaviness and abstinence amongst patients presenting to primary care visits who were initially willing to cut down their smoking but not make a quit attempt. Cook et al. (2016) found that behavioral reduction counseling improved 12-week abstinence rates, and that nicotine gum, when used without MI, increased abstinence after participants made a subsequent aided quit attempt.
The present research aimed to identify the intervention components that are effective amongst smokers initially unwilling to quit in increasing: 1) quit attempts (abstinence ≥ 24 hours) at 6 and 26-weeks post-study enrollment (research question 1; RQ1), and 2) use of evidence-based cessation treatment (RQ2). This study also explored sociodemographic, smoking, and person factors that might be associated with making a quit attempt and engaging in evidence-based cessation treatment. Finally, we examined the relation between making a quit attempt and achieving point-prevalence abstinence at 26 weeks in the context of Motivation-phase intervention (RQ3). Further, we examined whether the quit attempt-abstinence relation differed in those who did and did not use cessation treatment. We did so to evaluate the clinical significance of quit attempts as they occurred in different treatment contexts.
2. Methods
2.1. Procedure
This is a secondary analysis of data from a factorial screening experiment (Cook et al., 2016). From June 2010 to October 2013, participants were recruited from 11 primary care clinics in two healthcare systems in southern Wisconsin. Patients who were identified as smokers in the electronic health record were invited by medical assistants to participate in a research program to help them either quit or reduce their smoking1. Interested patients were electronically referred to the research office. During the screening call, smokers who expressed an interest in smoking reduction versus quitting within the next 30 days were invited to enroll in a research program for smoking reduction. Inclusionary criteria were: 18 years of age or older; smoked at least 5 cigarettes per day during the past 6 months; no interest in quitting smoking within the next 30 days but willing to reduce; able to read, write, and speak in English; agreed to complete study assessments; planned to remain within the geographic region for at least 6 months; not taking varenicline or bupropion; agreed to use only study smoking medications during the course of the study; no medical contraindications to Nicotine Replacement Therapy (NRT) use; and, for women with childbearing potential, agreed to use approved contraceptives. Eligible patients were invited to return to their primary care clinic to learn more about the study, provide written informed consent, complete baseline assessments, and receive the intervention component(s) to which they had been randomized.
Reduction interventions were administered over a 6-week treatment period. At the end of this period, participants had the option to repeat the same treatment for an additional 6 weeks. During any point in the 6-month study period, participants had the option to enter cessation treatment, and they received explicit invitations to engage in cessation treatment at the end of each 6-week round of reduction treatment. Cessation treatment was identical for all participants and consisted of 8 weeks of nicotine patch + gum and two brief telephone counseling sessions.
2.2. Intervention Factors
Participants were randomly assigned to 1 of 16 possible combinations of the four intervention components: 1) Nicotine Patch versus None; 2) Nicotine Gum versus None; 3) Behavioral Reduction counseling (BR) versus None; and 4) Motivational Interviewing (MI) strategies versus None (Cook et al., 2016) (see Supplement 1).
Nicotine Patch versus no Patch.
Participants in the Nicotine Patch condition were instructed to use 14-mg nicotine patches for the 6-week intervention period. The other half of participants did not receive nicotine patches. We used low dose nicotine replacement (both patch and gum) because participants would be smoking while using the medication; standard cessation NRT dosing might increase the risk of adverse events related to nicotine toxicity.
Nicotine Gum versus No Gum.
Participants in the Nicotine Gum condition were instructed to use 2-mg nicotine gum (up to 9/day, 1 piece every 1–2 hours) for the 6-week intervention period. The other half of participants did not receive nicotine gum.
MI versus No MI.
Participants in the MI condition received an initial 20-minute in-person counseling session followed by 3 bi-weekly, 10-minute counseling phone calls during the 6-week intervention period. MI counseling sessions, developed for this study using established treatment guides for MI (Miller and Rollnick, 2002; Rollnick et al., 1999), focused on helping smokers overcome ambivalence and build intrinsic motivation to quit smoking. The other half of participants did not receive MI counseling.
BR versus no BR.
Participants in the BR condition received an initial 20-minute, in-person counseling session followed by six weekly 10-minute counseling calls. Sessions focused on helping participants set smoking reduction goals and develop reduction strategies (e.g., delaying smoking, choosing to abstain in certain places). Participants were also asked to record daily smoking patterns, which care managers used to track successes and challenges. The other half of participants did not receive BR counseling.
2.3. Assessments
At baseline, participants completed assessments of demographic information (i.e., age, gender, race, education, and income), self-reported history of being diagnosed and/or treated for depression or anxiety, smoking history (i.e., average cigarettes per day, time since most recent quit attempt, number of serious past quit attempts, and home smoking ban), motivation to quit (“How motivated are you to quit smoking?” [1–10 scale]), cessation self-efficacy (“How confident are you that you can quit smoking successfully in the next month?” [1–10 scale]), tobacco dependence (Fagerström Test of Nicotine Dependence [FTND] (Heatherton et al., 1991); and the Wisconsin Inventory of Smoking Dependence Motives [WISDM] (Piper et al., 2004). The WISDM comprises two subscales measuring primary dependence motives (PDM; motives tied to heavy, automatic smoking, craving, and tolerance) and secondary dependence motives (SDM; smoking for instrumental reasons or in response to environmental cues). As in prior research (Baker et al., 2012b; Piper et al., 2008), standardized PDM and SDM scores were derived from univariate regressions of PDM on SDM (and vice versa) to partial shared variance from each. Baseline alcohol use was also assessed; alcohol consumption frequency was coded as rare/never (1 time/year or never), occasional (2 times/week to 3 times/year), or frequent (daily to 3 times/week). Alcohol consumption quantity was coded as light/none (0–2 drinks/occasion), moderate (3–6 drinks/occasion), or heavy (7–25 drinks/occasion).
2.4. Outcome Measures
Participants completed weekly phone assessments during the 6-week treatment period and follow-up phone assessments at 12 and 26 weeks. During these assessments, participants were asked if they had abstained from smoking for the purpose of quitting within a specified time period (e.g., the past week), and for how many hours they abstained. Participants who reported intentional abstinence for more than 24 hours at the 6-week assessment call and at the 26-week follow-up call were identified as having made a quit attempt by 6 and 26 weeks, respectively (RQ1). Engagement in evidence-based cessation treatment during a quit attempt, or “phase-shifting” from Motivation to Cessation-phase treatment, was indexed by attending an initial cessation treatment appointment (i.e., making an aided quit attempt) (RQ2). Seven-day point-prevalence abstinence was assessed 26-weeks after study enrollment (RQ3).
2.5. Analytic Plan
Analyses were conducted using SPSS version 22.0 (IBM Corporation, 2013). We used logistic regression analysis to examine the main and interactive effects of intervention components using effect coding (1 = on, −1 = off) on: 1) making a quit attempt by end-of-treatment (6 weeks) and by end-of-study (26 weeks) (RQ1), and 2) engaging in cessation treatment (1 [engaged in cessation treatment] and 0 [did not engage in cessation treatment]) by end-of-study (26 weeks) (RQ2). We analyzed the 0–6 week time period because that is when all participants were engaged in their assigned Motivation phase treatment; we analyzed quit attempts across 0–26 weeks in order to capture the net impact of such treatment across time.
Participants who did not provide outcome information were assumed to have not made a quit attempt (i.e., missing = no quit attempt). The percentage of participants with missing quit attempt data decreased from 25% at Week 6 to 8% at Week 26 due to follow-up efforts. Whether participants repeated reduction treatment (1 = yes, 0 = no) was included in the Week 26 models as a covariate. Next, univariable logistic regression analysis identified demographic factors and smoking characteristics associated with making at least one quit attempt by Weeks 6 and 26. Finally, multivariable logistic regression analysis identified a best-fitting model predicting quit attempts, controlling for intervention components and repetition of intervention components. Using the backward model-building procedure proposed by Hosmer and Lemeshow (2000), all univariate predictors with p < .25 were included in the initial multivariable model. Then, each non-significant predictor was backward-eliminated until a best-fitting model was determined. Finally, we determined 26-week point-prevalence abstinence rates for those who did and did not make aided quit attempts (RQ3).
3. Results
3.1. Participants
Of 517 participants enrolled in the study, 63.4% were women. The majority of participants were white (91.1%), 4.8% were African American, and 1% were Hispanic. The majority earned greater than a high school diploma (61.6%). Participants’ mean age was 47 years (SD=14.4) and they smoked an average of 17.5 cigarettes per day (SD=7.8). Finally, 54% of participants made an intentional quit attempt during participation in the study, and 19% opted to use cessation treatment provided by the study.
3.2. Quit Attempts
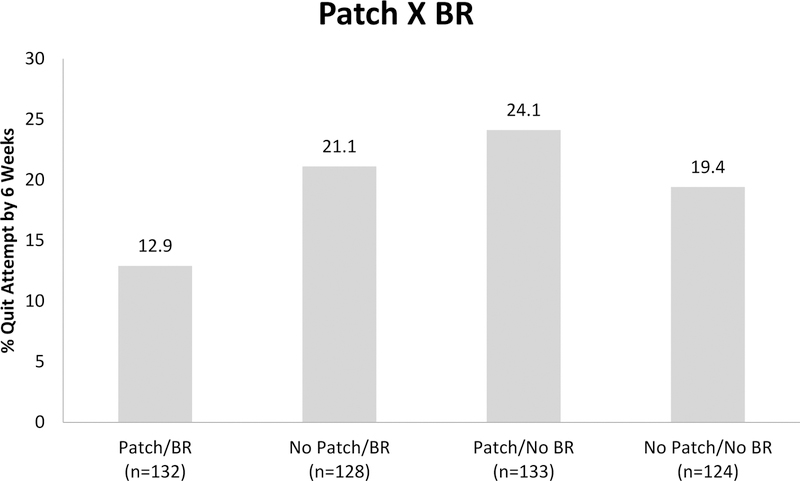
Table 1 shows results for the main and interactive effects of intervention components on making a quit attempt by Weeks 6 and 26 (RQ1). Nicotine Gum significantly increased the likelihood of making a quit attempt by 6 weeks (23% of those receiving gum versus 15% of those not receiving gum). There was also a statistically significant two-way interaction of Patch BR. As shown in Figure 1, those receiving Patch and no BR were most likely to make a quit attempt (24.1%) followed by BR and no Patch (21.1%), no Patch and no BR (19.4%), and both Patch and BR (12.9%). There were no statistically significant main effects or interaction effects predicting quit attempts by week 262.
Table 1.
Logistic Regression Analyses of Reduction Treatment Predicting Quit Attempts by Week 6 and 26.
| Reduction Treatment (N = 517) | 6 Weeks OR (95%CI) | 26 Weeks OR (95%CI) |
|---|---|---|
| Patch | .94 (.74, 1.18) | .93 (.77, 1.11) |
| Gum | 1.35 (1.07, 1.72)* | .88 (.74, 1.05) |
| Behavioral Reduction | .84 (.67, 1.07) | .88 (.74, 1.05) |
| Motivational Interviewing | 1.21 (.96, 1.54) | .99 (.83, 1.18) |
| Patch X Gum | .96 (.76 1.22) | .90 (.75, 1.07) |
| Patch X BR | .78 (.61, .99)* | .86 (.72, 1.02) |
| Patch X MI | .96 (.76, 1.22) | .96 (.80, 1.14) |
| Gum X BR | .97 (.77, 1.23) | 1.03 (.86, 1.22) |
| Gum X MI | .97 (.76, 1.22) | 1.11 (.93, 1.33) |
| BR X MI | 1.22 (.97, 1.55) | 1.04 (.87, 1.24) |
| Patch X Gum X BR | 1.12 (.89, 1.42) | .97 (.82, 1.16) |
| Patch X Gum X MI | 1.04 (.82, 1.31) | .94 (.79, 1.12) |
| Patch X BR X MI | 1.06 (.83, 1.34) | 1.04 (.88, 1.25) |
| Gum X BR X MI | 1.06 (.84, 1.35) | 1.11 (.93, 1.32) |
| Patch X Gum X BR X MI | .99 (.78, 1.26) | 1.13 (.95, 1.35) |
| Repeated Treatment | --- | .81 (.48, 1.35) |
Note:
p < .05
BR = Behavioral reduction counseling; MI = Motivational Interviewing.
Figure 1.
Bar graph showing a two-way interaction between two reduction treatments, Patch and BR, in the regression model predicting making a quit attempt by 6-weeks post-study enrollment (N=517).
Table 2 shows results for the univariable and best-fitting multivariable prediction models for the 6-week quit attempt outcome. Results from univariable models show that the following demographic characteristics increased the likelihood of making a quit attempt: younger age, male gender, non-white race, and lower income (<$25,000/year versus ≥$25,000/year). Smoking-related factors associated with making a quit attempt by Week 6 included having lower nicotine dependence (both WISDM Primary Dependence Motives [PDM] and FTND), smoking fewer cigarettes per day, having higher cessation self-efficacy, and having a home smoking ban. Additionally, consuming a moderate amount of alcohol compared to both light/none and heavy quantities predicted a higher likelihood of making a quit attempt by Week 6. After controlling for the effects of intervention components, the final best-fitting multivariable model revealed that male gender, earning less than $25,000 per year, smoking fewer cigarettes per day, and having a lower FTND score were associated with making a quit attempt by Week 6.
Table 2.
Univariable and Multivariable Logistic Regression Analyses Predicting Making a Quit Attempt by Week 6.
| Variable | No Attempt (n=417) | Quit Attempt (n=100) | Univariable OR (95%CI) | Final Model OR (95% CI) |
|---|---|---|---|---|
| Age- M (SD) | 48.08 (14.09) | 42.70 (14.64) | .97 (.96, .99)* | |
| Gender- n (%) | ||||
| Female (n= 328)† | 276 (84.1) | 52 (15.9) | ||
| Male (n= 189) | 141 (74.6) | 48 (25.4) | 1.81(1.16, 2.81)* | 1.84(1.11, 3.07)* |
| Race- n (%) | ||||
| Non-white (n=46)† | 29 (63.0) | 17 (37.0) | ||
| White (n=471) | 388 (82.4) | 83 (17.6) | .37 (.19, .70)* | |
| Education- n (%) | ||||
| High School or < (n=198)† | 160 (80.8) | 38 (19.2) | ||
| > High School (=317) | 255 (80.4) | 62 (19.6) | 1.02 (.65, 1.61) | |
| Income- n (%) | ||||
| <$25,000 (n=145)† | 105 (72.4) | 40 (27.6) | ||
| ≥ $25,000 (n=334) | 280 (83.8) | 54 (16.2) | .51 (.32, .81)* | .47 (.28, .78)** |
| Anxiety History- n (%) | ||||
| No (n=397) † | 322 (81.1) | 75 (18.9) | ||
| Yes (n=119) | 94 (79.0) | 25 (21.0) | 1.14 (.69, 1.90) | |
| Depression History- n (%) | ||||
| No (n=318)† | 260 (81.8) | 58 (18.2) | ||
| Yes (n=198) | 156 (78.8) | 42 (21.2) | 1.21 (.77, 1.88) | |
| Alcohol Frequency- n (%) | ||||
| No/light use (n=117) | 92 (78.6) | 25 (21.4) | 1.13 (.66, 1.93) | |
| Moderate use (n=257)† | 207 (80.5) | 50 (19.5) | ||
| Heavy use (n=132) | 108 (81.8) | 24 (18.2) | .76 (.54, 1.58) | |
| Alcohol Quantity- n (%) | ||||
| No/light use (n=267) | 219 (82.0) | 48 (18.0) | .44 (.21, .92)* | |
| Moderate use (n=39)† | 26 (66.7) | 13 (33.3) | ||
| Heavy use (n=211) | 172 (81.5) | 39 (18.5) | .45 (.21, .96)* | |
| Cigs per day- M (SD) | 18.35 (8.00) | 13.40 (6.82) | .90 (.87, .94)** | .94 (.89, .98)** |
| Quit Motivation- M (SD) | 6.02 (2.27) | 6.26 (2.35) | 1.05 (.95, 1.15) | |
| Quit Self-Efficacy - M (SD) | 3.34 (2.04) | 3.91 (2.57) | 1.12 (1.02, 1.24)* | |
| PDM-M (SD) | 0.08 (0.97) | −0.32 (1.06) | .67 (.53, .83)*** | |
| SDM- M (SD) | −0.01 (1.01) | 0.02 (0.95) | 1.03 (.83, 1.28) | |
| FTND- M (SD) | 5.03 (1.96) | 3.86 (2.11) | .71 (.63, .80)** | .84 (.72, .98)* |
| Past quit attempts- M (SD) | 2.71 (2.39) | 2.47 (2.33) | .96 (.87, 1.06) | |
| Time since last quit- n (%) | ||||
| Within past year (n=100)† | 76 (76.0) | 24 (24.0) | ||
| Within 5 yrs (n=206) | 168 (81.6) | 38 (18.4) | .72 (.40, 1.28) | |
| > 5 yrs or never (n=193) | 161 (83.4) | 32 (16.6) | .63 (.35, 1.14)‡ | |
| Home smoking ban- n (%) | ||||
| No (n=247)† | 211 (85.4) | 36 (14.6) | ||
| Yes (n=246) | 203 (76.0) | 64 (24.0) | 1.85 (1.18, 2.90)* |
Note:
Indicates reference category (coded 0)
Indicates p≤.25
p < .05
p <.01
p<.001
Final model adjusted for main effects of reduction treatments received; Final Model represents the best-fitting multivariable model; PDM = Primary Dependence Motives standardized residuals controlling for SDM; SDM = Secondary Dependence Motives standardized residuals controlling for SDM; FTND = Fagerström Test for Nicotine Dependence.
Results for univariable and multivariable regression models of person variables predicting quit attempts by Week 26 are shown in Table 3. Consistent with models predicting quit attempts by 6 weeks, the following demographic variables were associated with occurrence of any quit attempt: younger age, a more than high school education, non-White race, and lower income (<$25,000/year versus ≥ $25,000/year). The following smoking variables were also associated with quit attempts by Week 26: smoking fewer cigarettes per day, greater baseline motivation to quit, greater cessation self-efficacy, lower Primary Dependence Motives, lower FTND score, having a home smoking ban, and having made a quit attempt in the last year versus more than 5 years ago or never. For alcohol quantity, moderate versus light/none and moderate versus heavy consumption predicted quit attempts by 26 weeks. The best-fitting multivariable model, controlling for the effects of intervention components and for repeating reduction treatment3, revealed the following statistically significant predictors of making a quit attempt by 26 weeks: non-White race, drinking a moderate amount of alcohol compared to light/no alcohol, higher cessation self-efficacy, lower FTND score, and having made a quit attempt within the past year versus within the past 1–5 years, or versus more than 5 years ago/never.
Table 3.
Univariable and Multivariable Logistic Regressions Predicting Making a Quit Attempt by Week 26.
| Variable | No Attempt (n=238) | Quit Attempt (n=279) | Univariable OR (95%CI) | Final Model OR(95% CI) |
|---|---|---|---|---|
| Age- M (SD) | 48.34 (14.71) | 45.78 (14.08) | .99 (.98, 1.00)* | |
| Gender- n (%) | ||||
| Female (n= 328)† | 161 (49.1) | 167 (50.9) | ||
| Male (n= 189) | 77 (40.7) | 112 (59.3) | 1.40 (.98, 2.01)‡ | |
| Race- n (%) | ||||
| Non-white (n=46)† | 7 (15.2) | 39 (84.8) | ||
| White (n=471) | 231 (49.0) | 240 (51.0) | .19 (.08, .43)*** | .19 (.08, .46)*** |
| Education- n (%) | ||||
| High School or < (n=198)† | 103 (52.0) | 95 (48.0) | ||
| > High School (=317) | 134 (42.3) | 183 (57.7) | 1.48 (1.04, 2.12)* | |
| Income- n (%) | ||||
| <$25,000 (n=145)† | 55 (37.9) | 90 (62.1) | ||
| ≥ $25,000 (n=334) | 163 (48.8) | 171 (51.2) | .64 (.43, .96)* | |
| Anxiety History- n (%) | ||||
| No (n=397)† | 181 (45.6) | 216 (54.4) | ||
| Yes (n=119) | 57 (47.9) | 62 (52.1) | .91 (.61, 1.37) | |
| Depression History- n (%) | ||||
| No (n=318)† | 151 (47.5) | 167 (52.5) | ||
| Yes (n=198) | 87 (43.9) | 111 (56.1) | 1.15 (.81, 1.65) | |
| Alcohol Frequency- n (%) | ||||
| No/light use (n=117) | 52 (44.4) | 65 (55.6) | 1.05 (.67, 1.62) | |
| Moderate use (n=257)† | 117 (45.5) | 140 (54.5) | ||
| Heavy use (n=132) | 61 (46.2) | 71 (53.8) | .97 (.64, 1.48) | |
| Alcohol Quantity- n (%) | ||||
| No/light use (n=267) | 137 (51.3) | 130 (48.7) | .33 (.15, .70)** | .31 (.13, .73)** |
| Moderate use (n=39)† | 10 (25.6) | 29 (74.4) | ||
| Heavy use (n=211) | 91 (43.1) | 120 (56.9) | .46 (.21, .98)* | |
| Cigs per day- M (SD) | 19.24 (8.30) | 15.63 (7.35) | .94 (.92, .96)*** | |
| Quit Motivation- M (SD) | 5.64 (2.25) | 6.40 (2.27) | 1.15 (1.07, 1.25)*** | |
| Quit Self-Efficacy- M (SD) | 2.89 (1.70) | 3.85 (2.36) | 1.22 (1.12, 1.33)*** | 1.24 (1.13, 1.37)*** |
| PDM- M (SD) | .015 (0.96) | −0.12 (1.02) | .76 (.63, .91)** | |
| SDM- M (SD) | 0.02 (1.03) | −0.01 (0.98) | .97 (.82, 1.16) | |
| FTND- M (SD) | 5.24 (1.88) | 4.32 (2.10) | .79 (.72, .86)*** | .80 (.73, .89)*** |
| Past quit attempts- M (SD) | 2.56 (2.52) | 2.75 (2.28) | 1.03 (.96, 1.11) | |
| Time since last quit- n (%) | ||||
| Within past year (n=100)† | 34 (34.0) | 66 (66.0) | ||
| Within 5 yrs (n=206) | 89 (43.2) | 117 (56.8) | .68 (.41, 1.11)‡ | .55 (.32, .94)* |
| > 5 yrs or never (n=193) | 107 (55.4) | 86 (44.6) | .41 (.25, .68)** | .33 (.19, .58)*** |
| Home smoking ban- n (%) | ||||
| No (n=247)† | 129 (52.2) | 118 (47.8) | ||
| Yes (n=267) | 108 (40.4) | 159 (59.6) | 1.61 (1.14, 2.28)** |
Note:
Indicates reference category (coded 0)
Indicates p≤.25
p < .05
p < .01
p < .001
Final model adjusted for main effects of reduction treatment and whether reduction treatment was repeated; Final Model represents the best-fitting multivariable model; PDM = Primary Dependence Motives; SDM = Secondary Dependence Motives; FTND = Fagerström Test for Nicotine Dependence.
3.3. Use of Evidence-Based Cessation Treatment
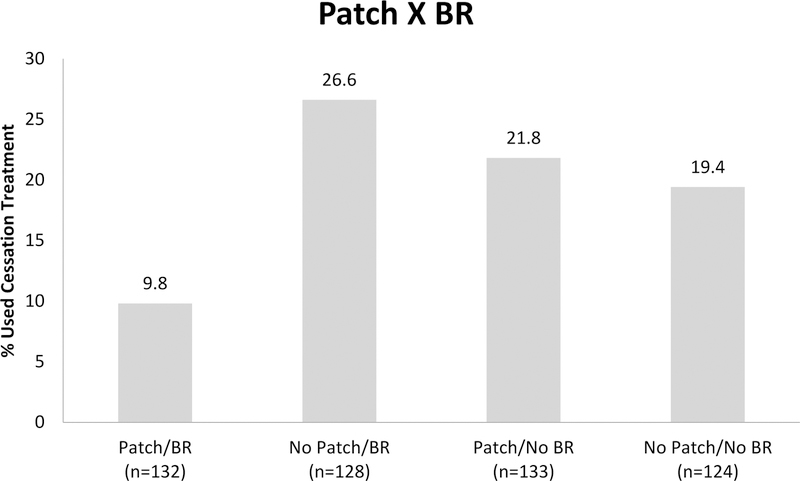
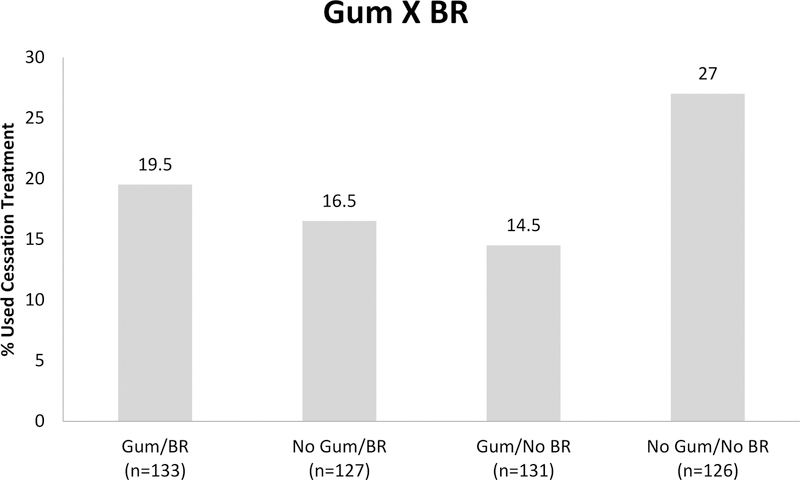
Table 4 shows results for the main and interactive effects of intervention components on using evidence-based cessation treatment provided by study by Week 26 (RQ2). There was one statistically significant main effect showing that use of Patch was associated with a lower likelihood of making an aided quit attempt (15.8% with patch vs 23% without patch). There were also two statistically significant two-way interactions: Patch × BR and Gum × BR. Figure 2 shows those receiving BR and no Patch were most likely to make an aided quit attempt (26.6%), followed by Patch and no BR (21.8%), no Patch and no BR (19.4%), and lastly, both Patch and BR (9.8%). For the Gum × BR interaction (see Figure 3), those with no Gum and no BR were most likely to engage in cessation treatment (27%), followed by Gum and BR (19.5%), then no Gum and BR (16.5%) and Gum and no BR (14.5%).
Table 4.
Logistic Regression Analysis of Reduction Treatment Predicting Use of Evidence-based Cessation Treatment by Week 26 (n=100).
| Reduction Treatment | Aided Attempt OR (95%CI) |
|---|---|
| Patch | .76 (.59, .98)* |
| Gum | .91 (.71, 1.18) |
| Behavioral Reduction | .87 (.68, 1.12) |
| Motivational Interviewing | .98 (.76, 1.26) |
| Patch X Gum | 1.06 (.83, 1.37) |
| Patch X BR | .69 (.54, .89)** |
| Patch X MI | 1.09 (.85, 1.40) |
| Gum X BR | 1.34 (1.04, 1.72)* |
| Gum X MI | .97 (.75, 1.24) |
| BR X MI | 1.27 (.99, 1.63) |
| Patch X Gum X BR | 1.10 (.85, 1.41) |
| Patch X Gum X MI | .91 (.71, 1.17) |
| Patch X BR X MI | 1.11 (.86, 1.43) |
| Gum X BR X MI | .89 (.69, 1.14) |
| Patch X Gum X BR X MI | .96 (.74, 1.23) |
| Repeated Treatment | .75 (.36, 1.54) |
Note:
p<.05
p<.01
BR = Behavioral reduction counseling; MI = Motivational interviewing.
Figure 2.
Bar graph showing a two-way interaction between two reduction treatments, Patch and BR, in the regression model predicting engagement in cessation treatment by end-of-study (26-weeks) (N=517).
Figure 3.
Bar graph showing a two-way interaction between two reduction treatments, Gum and BR, in the regression model predicting engagement in cessation treatment by end-of-study (26-weeks) (N=517).
3.4. Evaluation of the Relation between Cessation Treatment Use, Quit Attempts, and Abstinence
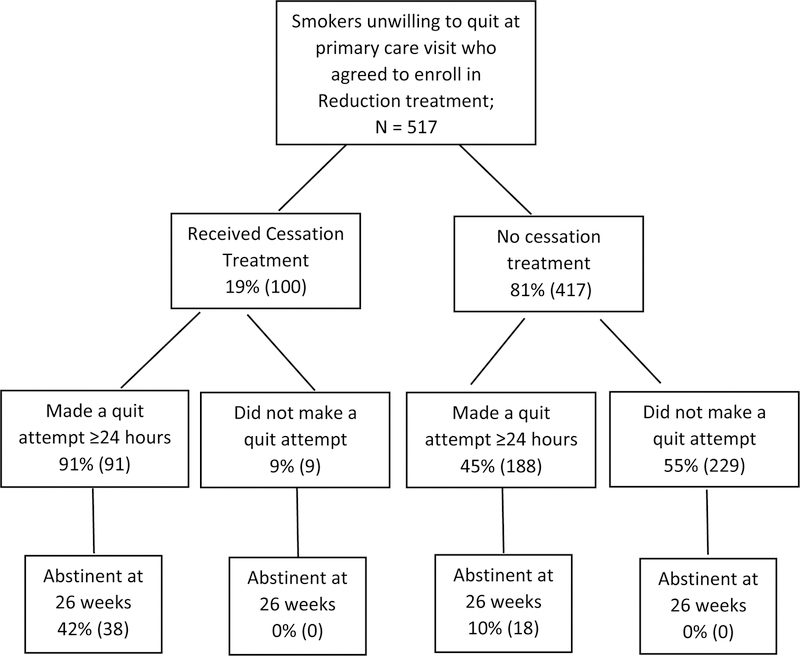
We also explored relations between making a quit attempt and 7-day point-prevalence abstinence at 26 weeks (RQ3: see Figure 4). We did so both for those who did and did not use cessation treatment offered by the study. Of those who opted to use cessation treatment, 91% made a quit attempt. Amongst those who did not use cessation treatment, 45% made a quit attempt. The 26-week abstinence rates for those who did and did not use cessation treatment were 42% and 10%, respectively (p<.001).
Figure 4.
Flow chart illustrating use of cessation treatment, making a quit attempt within 26 weeks post study enrollment, and 7-day point prevalence abstinence at 26-week follow-up.
4. Discussion
The primary goal of this research was to identify treatment factors that motivated primary care patients initially unwilling to quit to make a quit attempt. Results indicated that nicotine Gum, relative to no Gum, increased the likelihood of making a quit attempt by the end-of-treatment (6-weeks). Interestingly, a prior report with these participants (Cook et al., 2016) revealed that the Gum factor participated in statistical interactions suggesting that Motivation-phase treatment combinations containing gum were particularly effective in enhancing success in later aided cessation attempts. Thus, nicotine gum appeared to spur quit attempts only while smokers were using it. It is possible that extending the duration of nicotine gum beyond 6 weeks might produce greater, cumulative effects. Previous trials amongst smokers unwilling to quit have demonstrated the effectiveness of NRT on abstinence and smoking reduction when delivered for 6 to 12 months (Moore et al., 2009).
In the current analyses, no other treatment factors yielded main effects on quit attempts by 6 or 26 weeks. However, a statistically significant two-way interaction between Patch and BR revealed that smokers receiving both Patch and BR counseling made relatively few quit attempts by Week 6, indicating that these treatments may be more effective when delivered separately. It is unclear why receipt of both components suppressed quit attempts. An advantage of the factorial experiment is that it can reveal such interactions; the causes of such interactions remain unclear (Baker, 2017; Baker et al., 2017).
We also investigated whether particular intervention components increased participants’ decision to use cessation treatment. The only significant main effect indicated that Patch, relative to no Patch, decreased the likelihood of using cessation treatment. In addition, interaction effects revealed meaningful differences in component effects depending on the levels of other components. BR counseling, depending upon components with which it was paired, produced relatively high rates of engagement in cessation treatment. For instance, BR produced relatively high rates of aided quit attempts when not paired with Patch (26.6%) and yielded lower rates when paired with Patch (9.8%).
It is possible that nicotine patch discouraged engagement in cessation treatment, which consisted of combination NRT (patch + gum) and cessation counseling, because participants receiving the patch as part of their Motivation-phase treatment believed that they were already receiving sufficient treatment for quitting. Similarly, nicotine gum, which effectively increased quit attempts in general, failed to increase use of cessation treatment. On the other hand, BR counseling, when not paired with nicotine patch, yielded relatively high rates of aided quit attempts (Figure 2), perhaps because it motivated participants to access pharmacotherapy to aid with quitting. Thus, it is possible that Motivation-phase pharmacotherapy replaces or substitutes for formal cessation treatment in the eyes of smokers, while BR intervention does not. Moreover, these results suggest that if counseling only is used as a Motivation-phase treatment, it should be accompanied by ready access to pharmacotherapy should the patient decide to make a quit attempt.
Identifying Motivation-phase intervention components that spur patients to transition to Cessation-phase treatment appears important since aided quitters were more likely than unaided quitters to make a quit attempt (91% vs. 41%, respectively) and achieve abstinence (42% vs. 10%, respectively, achieved point-prevalence abstinence at 26 weeks). The relatively high abstinence rate amongst those who used cessation treatment compares favorably with abstinence rates obtained from other Motivation-phase interventions (7–20%) (Asfar et al., 2011; Glasgow et al., 2009; Moore et al., 2009), none of which provided a cessation intervention when smokers became ready to quit. In sum, there was some evidence that quit attempts in the context of Motivation-phase treatment possessed clinical significance (i.e., were associated with eventual abstinence to a meaningful degree), especially amongst individuals who participated in cessation treatment. It is tempting to speculate that the use of cessation treatment increased the success of the quit attempts. However, it may be that those smokers most motivated to quit were the ones who chose to enter cessation treatment.
Finally, we examined how sociodemographic, psychological/behavioral, and smoking factors related to making a quit attempt by 6 and 26 weeks. Results were fairly consistent with prior literature showing that smokers are more likely to make quit attempts when there are reasons to believe they are likely to succeed; i.e., they are less dependent, smoke less, control their drinking, and have greater confidence that they can quit. Indeed, these same factors do, in fact, predict greater likelihood of quitting successfully (Gwaltney et al., 2009; Japuntich et al., 2011; Jardin and Carpenter, 2012; Smith et al., 2016). Further, there was evidence that underserved populations (minority, low income) were especially likely to make quit attempts. It is possible that such smokers previously had little access or exposure to smoking treatment (Piper et al., 2010) and thus saw the additional support provided by treatment as affording them an especially good opportunity to try quitting. The finding that underserved smokers were especially likely to make quit attempts supports the notion that Motivation-phase treatment might be especially helpful for smokers who might not otherwise enter cessation treatment or benefit from it (Petersen et al., 2017).
This research has limitations that should be considered. First, it is possible that additional effects might have been detected if intervention components had been administered over a longer duration or at different intensities (Moore et al., 2009). Thus, failure to find a significant effect for an intervention component in this research does not per se confirm the null hypothesis. These results indicate that future research is needed to examine the optimal timing, intensity, and duration of Motivation-phase treatments. Second, this paper addresses an a priori, but secondary outcome (quit attempts), entailing analyses in addition to those directed at the primary aim (abstinence: Cook et al., 2016). This may have increased experimentwise error. Finally, findings from smokers willing to cut-down on their smoking but not quit may not generalize to smokers not willing to cut down or engage in a research study.
5. Conclusion
Growing evidence suggests that reduction treatment expands the reach of tobacco treatment by engaging smokers who might not otherwise enter cessation treatment (Petersen et al., 2017). Reduction treatment has also been shown to decrease smoking heaviness and increase abstinence (Ali et al., 2018; Cook et al., 2016; Moore et al., 2009). This study extends prior research by showing that nicotine gum per se increased the likelihood of making a quit attempt by end-of-treatment (6 weeks). On the other hand, the nicotine patch decreased the likelihood of using cessation treatment and diminished the effectiveness of counseling designed to encourage smoking reduction and quit attempts. In this same sample, the patch has also been found to decrease the likelihood of abstinence amongst smokers initially unwilling to quit (Cook et al., 2016). The patch is an effective Cessation-phase intervention, but these findings suggest the need to experimentally evaluate intervention components for each phase of smoking treatment (per the Phase-Based Model) (Baker et al., 2011). This research also suggests that Motivation-phase interventions may be effective in increasing quit attempts amongst underserved smokers who typically have less access to cessation treatment. Finally, this study provided smokers with easy access to evidence-based cessation treatment; results showed that smokers who used such cessation treatment during their quit attempts were especially likely to quit successfully.
Supplementary Material
Highlights.
Nicotine gum compared to no gum increased quit attempts by 6 weeks.
Patch, when used with behavioral reduction counseling, reduced quit attempts.
Aided quit attempts were more likely to produce abstinence than unaided attempts.
Acknowledgments
Role of Funding Source
This research was supported by grants 9P50CA143188, 1P01CA180945, and 1K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. This material is based upon work carried out in part while Dr. Engle was a postdoctoral fellow in the Advanced Fellowship in Addiction Treatment at the United States Department of Veterans Affairs and is supported by the Office of Academic Affiliations, Department of Veterans Affairs. Dr. Cook is also supported by Merit Review Award 101CX00056 from the United States Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Early during the course of the trial, participants were offered reduction treatment only after indicating that they were not interested in making a quit attempt within the next 30 days. Procedures were revised to offer patients the choice of a track: one for smokers ready to quit (cessation) or one for smokers not interested in quitting but interested in cutting down (reduction/motivation).
In these analyses we assumed that those with missing data had not made a quit attempt, an assumption that could have biased the results. Therefore, we conducted sensitivity analyses using only participants who were ascertained at all study visits up to 6 weeks (n=371) or who were ascertained at the operative 26-week follow-up (n=468). These analyses yielded the same pattern of effects as those reported.
Results of the multivariable models controlling for the effects of the intervention components and repeating treatment were nearly identical to results when models did not control for these.
References
- Ali A, Kaplan CM, Derefinko KJ, & Klesges RC (2018). Smoking cessation for smokers not ready to quit: meta-analysis and cost-effectiveness analysis. Am J Prev Med, 55(2), 253–262. doi: 10.1016/j.amepre.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfar T, Ebbert JO, Klesges RC, & Relyea GE (2011). Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav, 36(7), 764–768. doi: 10.1016/j.addbeh.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, & Jamal A (2017). Quitting smoking among adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep, 65(52), 1457–1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- Baker TB (2017). The 2016 Ferno Award address: three things. Nicotine Tob Res doi: 10.1093/ntr/ntx039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Breslau N, Covey L, & Shiffman S (2012a). DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction, 107(2), 263–275. doi: 10.1111/j.1360-0443.2011.03657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, … Fiore MC (2011). New methods for tobacco dependence treatment research. Ann Behav Med, 41(2), 192–207. doi: 10.1007/s12160-010-9252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, Loh WY, & Bolt D (2012b). Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. J Abnorm Psychol, 121(4), 909–921. doi: 10.1037/a0027889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Smith SS, Bolt DM, Loh WY, Mermelstein R, Fiore MC, … Collins LM (2017). Implementing clinical research using factorial designs: a primer. Behav Ther, 48(4), 567–580. doi: 10.1016/j.beth.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, & McAfee T (2006). The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med, 30(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, & Baker TB (2009). The Wisconsin Predicting Patients’ Relapse questionnaire. Nicotine Tob Res, 11(5), 481–492. doi: 10.1093/ntr/ntp030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Korhonen T, & Kaprio J (2008). Smoking reduction predicts cessation: longitudinal evidence from the Finnish adult twin cohort. Nicotine Tob Res, 10(3), 423–427. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, & Callas PW (2004). Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol, 72(3), 371–381. doi: 10.1037/0022-006X.72.3.371 [DOI] [PubMed] [Google Scholar]
- Cook JW, Collins LM, Fiore MC, Smith SS, Fraser D, Bolt DM, … Mermelstein R (2016). Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction, 111(1), 117–128. doi: 10.1111/add.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SJ, Keller PA, Orleans CT, & Fiore MC (2008). The role of health care systems in increased tobacco cessation. Annu Rev Public Health, 29, 411–428. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, … Pilote L (2008). Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ, 179(2), 135–144. doi: 10.1503/cmaj.070256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, … Wewers ME (2008). Treating tobacco use and dependence: 2008 update Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service. [Google Scholar]
- Glasgow RE, Gaglio B, Estabrooks PA, Marcus AC, Ritzwoller DP, Smith TL, … France EK (2009). Long-term results of a smoking reduction program. Med Care, 47(1), 115–120. doi: 10.1097/MLR.0b013e31817e18d1 [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, & Shiffman S (2009). Self-efficacy and smoking cessation: a meta-analysis. Psychol Addict Behav, 23(1), 56–66. doi: 10.1037/a0013529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Egleston BL, & Hofmann MT (2010). Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control, 19(5), 410–416. doi: 10.1136/tc.2009.033175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, & Lemeshow S (2000). Applied Logistic Regression (2nd ed.). New York: John Wiley & Sons, Inc. [Google Scholar]
- IBM Corporation. (2013). IBM SPSS Statistics for Windows, Version 22.0 Armonk, NY: IBM Corporation. [Google Scholar]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, & Baker TB (2011). The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol, 79(1), 34–42. doi: 10.1037/a0022154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin BF, & Carpenter MJ (2012). Predictors of quit attempts and abstinence among smokers not currently interested in quitting. Nicotine Tob Res, 14(10), 1197–1204. doi: 10.1093/ntr/nts015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai DT, Cahill K, Qin Y, & Tang JL (2010). Motivational interviewing for smoking cessation. Cochrane Database Syst Rev(1), CD006936. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, & Baker TB (2008). Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction, 103(9), 1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational interviewing: Preparing people for change (2nd ed.) New York: Guilford Press. [Google Scholar]
- Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, & Barton P (2009). Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ, 338, b1024. doi: 10.1136/bmj.b1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis S, Gharib M, Hambleton J, Reid RD, Assi R, & Pipe AL (2014). Delivering evidence-based smoking cessation treatment in primary care practice: experience of Ontario family health teams. Canadian Family Physician, 60(7), e362–371. [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Mermelstein R, Berg KM, Baker TB, Smith SS, Jorenby D, … Cook JW (2017). Offering smoking treatment to primary care patients in two Wisconsin healthcare systems: Who chooses smoking reduction versus cessation? Prev Med, 105, 332–336. doi: 10.1016/j.ypmed.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Baker TB, Mermelstein R, Collins LM, Fraser DL, Jorenby DE, … Fiore MC (2013). Recruiting and engaging smokers in treatment in a primary care setting: developing a chronic care model implemented through a modified electronic health record. Translational behavioral medicine, 3(3), 253–263. doi: 10.1007/s13142-012-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Bolt DM, Kim SY, Japuntich SJ, Smith SS, Niederdeppe J, … Baker TB (2008). Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives. J Abnorm Psychol, 117(4), 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, & Loh WY (2010). Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res, 12(6), 647–657. doi: 10.1093/ntr/ntq067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, & Baker TB (2004). A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). J Consult Clin Psychol, 72(2), 139–154. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, & DiClemente CC (1983). Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol, 51(3), 390–395. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Bitton A, Kelley JK, Hoeppner BB, Levy DE, & Mort E (2011). Offering population-based tobacco treatment in a healthcare setting: a randomized controlled trial. Am J Prev Med, 41(5), 498–503. doi: 10.1016/j.amepre.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Mason P, & Butler C (1999). Health behavior change: A guide for practitioners Edinburgh: Churchill Livingstone. [Google Scholar]
- Schuz N, & Ferguson SG (2015). Australian smokers’ and nonsmokers’ exposure to antismoking warnings in day-to-day life: a pilot study. Nicotine Tob Res, 17(7), 876–881. doi: 10.1093/ntr/ntu253 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, & Strahs KR (2009). Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. Am J Prev Med, 36(2), 96–104 e101. doi: 10.1016/j.amepre.2008.09.039 [DOI] [PubMed] [Google Scholar]
- Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, & McKee SA (2016). Sex/gender differences in smoking cessation: A review. Prev Med, 92, 135–140. doi: 10.1016/j.ypmed.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers ME, Stillman FA, Hartman AM, & Shopland DR (2003). Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med, 36(6), 710–720. [DOI] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, & Mills EJ (2006). Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health, 6, 300. doi: 10.1186/1471-2458-6-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.