Abstract
A crucial challenge in efforts to link psychological disorders to neural systems, with the aim of developing biologically informed conceptions of such disorders, is the problem of method variance (Campbell & Fiske, 1959). Since even measures of the same construct in differing domains correlate only moderately, it is unsurprising that large sample studies of diagnostic biomarkers yield only modest associations. To address this challenge, a construct-network approach is proposed in which psychometric operationalizations of key neurobehavioral constructs serve as anchors for identifying neural indicators of psychopathology-relevant dispositions, and as vehicles for bridging between domains of clinical problems and neurophysiology. An empirical illustration is provided for the construct of inhibition–disinhibition, which is of central relevance to problems entailing deficient impulse control. Findings demonstrate that: (1) a well-designed psychometric index of trait disinhibition effectively predicts externalizing problems of multiple types, (2) this psychometric measure of disinhibition shows reliable brain response correlates, and (3) psychometric and brain-response indicators can be combined to form a joint psychoneurometric factor that predicts effectively across clinical and physiological domains. As a methodology for bridging between clinical problems and neural systems, the construct-network approach provides a concrete means by which existing conceptions of psychological disorders can accommodate and be reshaped by neurobiological insights.
Keywords: psychoneurometric assessment, neurobehavioral construct, externalizing psychopathology, disinhibition, Research Domain Criteria
In connection with upcoming revisions to the major diagnostic classifications systems in use worldwide—the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM–IV; American Psychiatric Association, 2000) and the International Classification of Diseases, 10th revision (ICD-10; World Health Organization, 2004)—calls have intensified for more neurobiologically based approaches to conceptualizing, studying, and treating psychological disorders (Hyman, 2007; Insel & Scolnick, 2006). However, salient challenges exist to understanding behavioral pathology in neuroscientific terms. One is that psychological disorders manifest in diverse ways clinically (phenotypically) and they show frequent overlap (comorbidity). Another is that clinical phenotypes operationalized through interview- or self-report show only modest covariation with physiological indices of neural systems or processes, due to method variance (Campbell & Fiske, 1959). Yet another challenge consists of the often unknown psychometric properties of individual-differences variance in dependent measures from experimental (including cognitive and affective neuroscience) tasks (Cronbach, 1957; Vul, Harris, Winkelman, & Pashler, 2009).
The National Institute of Mental Health’s Research Domain Criteria (RDoC; Inselet al., 2010; Sanislow et al., 2010) initiative was launched with the aim of moving the field toward new conceptions of psychological disorders that are more amenable to a neurobiological analysis. The initiative calls for a shift away from the study of traditional diagnostic entities (as defined in DSM or ICD) toward a focus on dimensional constructs reflecting core psychological processes presumed to have a clear basis in neural systems, in domains of negative affect, positive affect, cognition, social processes, and regulatory systems. Constructs within these domains are to be studied using differing units of observation, ranging from genes to brain circuits and physiology to units consisting of self-report or behavioral variables. Additionally, the the RDoC approach emphasizes (1) continuing study of clinical groups, selected for symptomatic features or intersection with the construct of interest rather than on the basis of traditional diagnoses, and (2) consideration of the role of development in relations between basic constructs and clinical problems.
The aim of the current paper is to describe a systematic construct-network approach to bridging between clinical problems as indexed by standard assessment methods (clinical interview or self-report) and activity in neural systems as indexed by brain response measures. Consistent with RDoC, our proposed approach focuses on neurobehavioral trait constructs (i.e., dispositions with direct referents in both neurobiology and behavior) that connect with clinical problems, as opposed to traditional diagnoses. However, we encourage a focus on dispositional variables that (a) show up as dimensions in structural analyses of clinical problems or symptoms (i.e., reflecting their broad clinical relevance), and (b) exhibit reliable neurophysiological correlates (i.e., supporting their measurability in the brain response domain). For example, as discussed below, the construct of inhibition[H11002] disinhibition (represented in RDoC by the construct of “effortful control” within the Cognitive Systems domain) can be operationalized as the broad “externalizing” factor that emerges from structural analyses of impulse-related problems and traits in children and adults that in turn predicts anomalies in brain potential response. Convenient and effective measures of disinihibition-proneness defined in this way can serve as valuable referents for neurobiologically oriented studies.
Also consistent with RDoC, our proposed approach focuses on use of multiple measurement approaches, within a developmental framework, to elucidate the genetic and neural bases of target dispositions and their role in normal and abnormal behavior over time as a function of experience. Additionally, our approach calls for a strategic blending of experimental and correlational methods (what Cronbach [1957, 1975] referred to as “the two disciplines of scientific psychology”) as a basis for identifying biologically based “person” characteristics of relevance to clinical problems. Identifying and understanding clinically relevant traits is viewed as a “back and forth” process between the methods of smaller-N experimentation and larger-N correlational analysis (so as to clarify the psychological meaning of laboratory task variables in terms of their convergent and discriminant validity), and between the measurement domains of clinical evaluation and physiological assessment (so as to enable psychological conceptions of trait constructs to be shaped by physiological data). Ideas about the nature of a trait construct and how to measure it are considered provisional and subject to modification based on data (Cronbach & Meehl, 1955)—including data collected specifically for purposes of construct refinement (Tellegen & Waller, 2008).
We begin with a discussion of how dispositional constructs inferred from structural models of psychopathology can serve as referents for linking clinical problems with neural systems. We then summarize key points from classic construct-network theory and discuss how this perspective can be applied to the challenge of conceptualizing mental health problems in dimensional terms that link more clearly to neural systems. As an illustration, we discuss how impulse control (externalizing) disorders can be organized around a construct of inhibition–disinhibition, and demonstrate empirically how a psychometric operationalization of inhibition–disinhibition can serve as a bridge between clinical symptoms and brain response variables. We close with a section that discusses broader applications of the proposed construct-network approach beyond the specific “bridging” application highlighted in this review, and that considers potential implications of the approach for treatment perspectives and methods.
Conceptual Background and Description of the Construct-Network Approach
Immovable Roadblocks Call for New Roads: Reconceptualizing Psychopathology to Facilitate Linkages With Neurobiology
As discussed in papers by the RDoC group (Morris & Cuthbert, 2012; Sanislow et al., 2010), mental disorders as currently conceptualized in DSM–IV are not well-suited to neurobiological analysis. For one thing, conditions such as conduct disorder, antisocial personality, alcoholism, depression, and social phobia do not represent classic “disease” entities, analogous to discrete physical diseases whose observable symptoms can be traced to some coherent underlying biological disturbance. Instead, mental disorders of most types appear to represent extreme points along continua of normal-to-abnormal functioning, with extent of underlying polygenic liability and accumulation of adverse experience determining presence (vs. absence) and severity of maladjustment (Cicchetti & Rogosh, 1996; Gottesman, 1991; Zuckerman, 1999). A further problem is that mental disorders are defined in the DSM in terms of subsets of symptoms from among larger criterion sets. The fact that differing individuals can meet criteria for a given disorder in alternative ways results in heterogeneity that complicates linkages of disorders to measures in other domains. Additionally, diagnostic comorbidity across categories poses a different type of problem for establishing biological linkages, in that biological anomalies identified for a particular disorder of interest may in fact reflect features in common with other disorders rather than features specific to that disorder.
Consistent with RDoC, our view is that these limitations can be addressed by allowing conceptions of mental disorders to shift in response to insights gained from research on individual difference constructs with direct referents in neurobiology and behavior (i.e., neurobehavioral trait conceptions; Collins & Depue, 1992; Depue & Iacono, 1989; see also Allport, 1937; Eysenck, 1967; Gray, 1987; Tellegen, 1985). An example of such a construct is inhibition–disinihibition, which bridges between brain and cognitive-performance measures of frontal-executive function and problems involving deficient impulse control—including conduct problems in childhood, persistent antisocial-aggressive behavior in adult-hood, and alcohol and drug problems at varying ages (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Patrick et al., 2006; Young et al., 2009). Given that a trait dimension of inhibition–disinhibition can be conceived of in differing ways (e.g., so as to reflect impulsive-aggressive tendencies, shyness vs. social assertiveness, clarity vs. “looseness” of thought, etc.), we suggest that conceptions corresponding to factors from structural models of distinct problem domains can serve as effective bridging constructs for these domains—particularly when the factors in question have known neurobiological or neurocognitive correlates.
In the case of inhibition–disinhibition as it pertains to problems of impulse control, the general disinhibitory (“externalizing”) factor that emerges from structural analyses of problems of this type and affiliated personality variables (Krueger et al., 2002; Krueger, Markon, Patrick, Benning, & Kramer, 2007) can serve as a referent for linking this problem domain to physiological indices of hypothesized neural circuits. As an empirical illustration of the construct-network approach, the latter sections of this paper describe our efforts to develop a psychometric-trait measure of this general factor and identify neurophysiological correlates that relate in turn to clinical problems assessed via interview. This work demonstrates how the construct of inhibition–disinhibition operationalized as general externalizing proneness can serve as a bridge between clinical problems and neurophysiological indicators.
As a foundation for the empirical illustration that follows, we describe in the next section how concepts from classic assessment theory can be used to address the challenge of bridging between clinical and neurophysiological domains. In doing so, we highlight the need for multivariate correlational studies in conjunction with lab experimental studies, and discuss how an iterative approach to measurement and validation (cf. Tellegen & Waller, 2008) can serve as a vehicle for construct refinement.
Classic Assessment Theory: Construct Networks and Measurement Domains
In their classic treatise on construct validity in psychological assessment, Cronbach and Meehl (1955; see also: Loevinger, 1957; MacCorquodale & Meehl, 1948; Meehl, 1959; Tellegen, 1991) articulated a conceptual framework for the enterprise of quantifying psychological phenomena through differing methods of measurement. Crucial to their model is the distinction between hypothetical constructs and observed variables. A psychological construct is defined as “some postulated attribute of people, assumed to be reflected in test performance,” such as intelligence, moral integrity, or fearfulness. Observed variables are quantified empirical entities, such as scores on self-rating or performance tests (e.g., mentally challenging verbal or cognitive tasks), overt behavioral measures (e.g., resisting vs. succumbing to lab-analog temptations), or indices of physiological activity or response (e.g., enhanced electro dermal or startle reactivity during aversive as compared to neutral cuing). While selected for quantification because of their presumed relevance to constructs of interest (e.g., intellect, moral restraint, and fearfulness, respectively, in the foregoing examples), the observed variables are not equivalent to the target constructs, but rather serve as partial, imperfect indicators of those constructs. Further, from a perspective of scientific realism, it is also important to distinguish between conceptions of attributes (i.e., constructs) and actual attributes—that is, between theoretical constructions and the real-world attributes those constructions are intended to capture (Meehl, 1959).
If constructs themselves are hypothetical (i.e., exist in the theoretical-conceptual realm as opposed to the empirical-observable realm), how is it possible to gauge the effectiveness of differing observable variables as indicators of target constructs? To address this crucial question, Cronbach and Meehl introduced the notion of a construct network, or “nomological network”: “Scientifically speaking, to ‘make clear what something is” means to set forth the laws in which it occurs. We shall refer to the interlocking system of laws which constitute a theory as a nomological network.”(p. 290). That is, the psychological meaning of an observed variable (observable) and its relevance to a construct of interest is revealed through its empirical relations (both convergent and discriminant) with other observables. From this perspective, an observed variable qualifies as more versus less indicative of a target construct to the extent it interrelates more or less closely with other observables known to cohere in ways that accord with the hypothesized meaning and properties of the construct (i.e., consistent with the theory of the construct). As a corollary, Cronbach and Meehl noted that ideas about the nature and scope of the construct itself, and perspectives as to the best available methods for operationalizing it, can (and often do) undergo revision as knowledge regarding the interrelations among key observables in the nomological network progresses. Cronbach and Meehl referred to this process of construct refinement through iterative delineation of observable-observable relations as bootstrapping.
An additional important point in this model is that because constructs of interest transcend specific operationalizations, observed variables from differing domains of measurement can and should be used as indicators, to flesh out the nomological network of the construct. As an example, candidate indicators of a construct of dispositional fearfulness might include ratings of experiential distress in relation to aversive objects and events (domain of self-report), signs of overt discomfort when interacting with unfamiliar people (domain of behavior), and enhancement of the startle reflex during processing of unpleasant versus neutral cues (domain of physiology). Further, multiple observed variables from each of several domains of measurement (e.g., clinical symptoms, selfreport or performance indices, physiological response measures) can be used as indicators of a hypothetical construct (e.g., inhibition–disinhibition, or dispositional fear)—in which case, latent-variable approximations of the construct, reflecting systematic variance in common among differing observed variables, can be specified. The advantage of latent variables over individual manifest indicators is that they provide for more precise quantification of characteristics within a measurement domain. However, it bears noting that the latent variables are not equivalent to the target construct. As with the manifest indicators that serve to demarcate them, the latent variables are only approximations of the target construct whose psychological meaning, like that of the manifest indicators, must be inferred from observed relations (convergent and discriminant) with other variables situated within the network of the construct.
An important constraint on convergence among putative measures of a common construct derived from differing domains of measurement is the issue of method variance (Campbell & Fiske, 1959). Because systematic sources of influence contribute to the observed variance in indicators within a particular domain, comparably valid indices of a construct tend to covary more strongly with one another when operationalized in the same as compared to differing domains of measurement. For example, two self-report-based measures with established validity as indices of fearfulness will generally exhibit stronger relations with one another than either will with an overt-behavioral or physiological measure of comparable validity—that is, reliable measures of the same construct from the same domain are expected to correlate strongly (.6–8 range), whereas measures from differing domains are expected to correlate only moderately (.3–5), with correlations for measures of only somewhat related constructs expected to be even lower. Examples of domain-specific sources of variance include response biases (e.g., yay-saying vs. nay-saying) in the domain of self-report, general activity level in the domain of behavior, and skull thickness or scalp conductivity in the domain of brain electrophysiology (EEG/ERP). Campbell and Fiske incorporated these points into their concept of a multitrait–multimethod matrix—a structured arrangement of variable–variable associations in which convergence of observed indicators around a hypothesized construct of interest can be distinguished inferentially from convergence attributable to domain of measurement.
Construct-Network Approach to Connecting Clinical Problems With Neural Systems
The construct-network model provides a strategic framework for bridging between domains of clinical problems (e.g., social maladjustment, dysphoria, harmful substance use, impulsive-aggressive acts) and neural circuits/processes. The key is to recognize that problem behaviors and brain response measures (or other physiological variables presumed to be driven at some level by brain processes) are observed variables, from separate domains of measurement, rather than constructs. Viewed in this way, the task of linking problems with neural systems becomes one of establishing a construct network in which relations among observables within and across these two domains are mapped out with reference to psychologically meaningful bridging constructs.
Regarding clinical variables to focus on, current diagnostic conceptions are limited in ways discussed earlier, but nonetheless represent useful referents for evaluating the clinical relevance of new approaches to conceptualization and measurement. However, we also concur with the RDoC group’s position (cf. Sanislow et al., 2010) that dimensions of variability underlying problematic tendencies are likely to prove more useful as referents for a construct-oriented analysis than diagnostic categories per se. Along this line, a considerable amount is known about relations among symptoms associated with common problems of clinical concern. For example, structural models have been delineated for impulse control (externalizing) disorders (Krueger et al., 2002, 2007), mood/anxiety (internalizing) disorders (Brown, Chorpita, & Barlow, 1998; Clark & Watson, 1991; Mineka, Watson, & Clark, 1998; Sellbom, Ben-Porath, & Bagby, 2008), and personality disorders (Clark, 1993; Krueger, Derringer, Markon, Watson, & Skodol, 2012; Livesley & Jackson, 2009), and systematic efforts have been made to examine external correlates of dimensions from these models in order to elucidate their psychological meaning. While improved approaches to characterizing and organizing clinical symptoms might well be developed outside the current DSM framework, it seems likely that dimensions such as disinhibition (externalizing proneness), fearfulness, negative affectivity or distress, cognitive disorganization, aberrant perceptions, and dysphoria delineated by analyses of DSM symptoms will also emerge in analyses of alternative criterion sets—since the features encompassed by these dimensions are pervasive in problems of clinical concern. Thus, dimensions of existing models that can be conceptualized in neurobehavioral trait terms provide effective referents for the type of construct-oriented bridging effort proposed here.
In contrast with the extensive work that has been done to characterize relations among clinical symptom variables and their associations with personality variables, relatively little is known about inter-relations among differing physiological (including brain response) indicators of mental disorders, and minimal work has been done to clarify the psychological basis of known covariation. A major reason is that physiological indicators of psychological processes are typically developed through small-N experimental research whereas measures of clinical problems or personality dispositions are typically developed through larger-N correlational studies. As Cronbach (1957) noted many years ago, multivariate correlational work is crucial for establishing the convergent and discriminant validity of response measures derived from experimental tasks. Notably, systematic work of this kind has been undertaken with respect to behavioral response measures from laboratory tasks (e.g., Carter & Barch, 2007; Durbin, Hayden, Klein, & Olino, 2007; Kochanska, 1997; Miyake, Emerson, & Friedman, 2000). However, systematic efforts to develop and validate coherent sets of physiological measures for indexing psychological characteristics of relevance to clinical problems have not been undertaken.
Here, we propose a methodology for incorporating physiological indicators into the assessment of psychological characteristics that predict clinical problems. The approach is grounded in classic psychometric theory, but includes a number of novel features: (1) a focus on trait dimensions from structural models of psychopathology as points of reference for bridging between clinical and neurobiological domains—that is, trait dimensions like inhibition–disinhibition or fearfulness that can be conceptualized in neurobiological terms (Patrick, Durbin, & Moser, 2012) and that have known physiological correlates (Nelson, Patrick, & Bernat, 2011; Vaidyanathan, Patrick, & Bernat, 2009); (2) an emphasis on the systematic mapping of physiological indicators to well-designed psychometric measures of trait dimensions (which in turn predict behavioral symptoms) so as to ensure linkage of physiology to clinical problems; (3) a focus on covariance among differing physiological indicators as a core element of the measurement strategy—in particular, the variance in common among sets of physiological indicators that overlaps in turn with the psychometric index of the target trait; and (4) an emphasis on allowing psychological conceptions of target constructs to be reshaped by accumulating knowledge of physiological indicators (and properties of tasks in which they are embedded) that cohere with the psychometric index of the target trait—as a strategic implementation of what Cronbach and Meehl (1955) referred to as bootstrapping. The goal of the proposed strategy is not simply to assemble sets of indicators from differing measurement domains that cohere loosely around a broad psychological trait conception that persists unaltered, but to move toward a revised conception of the original psychologically-based trait construct that reflects the nexus of physiological indicators with self-report based (or, by extension, behavioral) indicators of the trait. That is, the conception of the trait itself shifts as knowledge is gained about reliable points of intersection between self-report (and/or behavioral) operationalizations of the trait and physiological operationalizations of the trait.
In the remainder of this paper, we use empirical findings from our recent work on the externalizing spectrum model (Krueger et al., 2007) to illustrate how observables in the clinical and physiological domains can be linked using this construct-network approach. We present evidence for the effectiveness of an efficient self-report measure of trait disinhibition (reflecting the general factor of the externalizing spectrum model) for predicting multiple clinical symptom variables assessed via interview, and describe progress in identifying multiple brain event-related potential (ERP) correlates of scores on this measure. We demonstrate how a latent variable index of disinhibition can be assembled jointly from self-report psychometric and physiological indicators to serve as a bridge between (i.e., provide for effective prediction across) clinical and physiological domains.
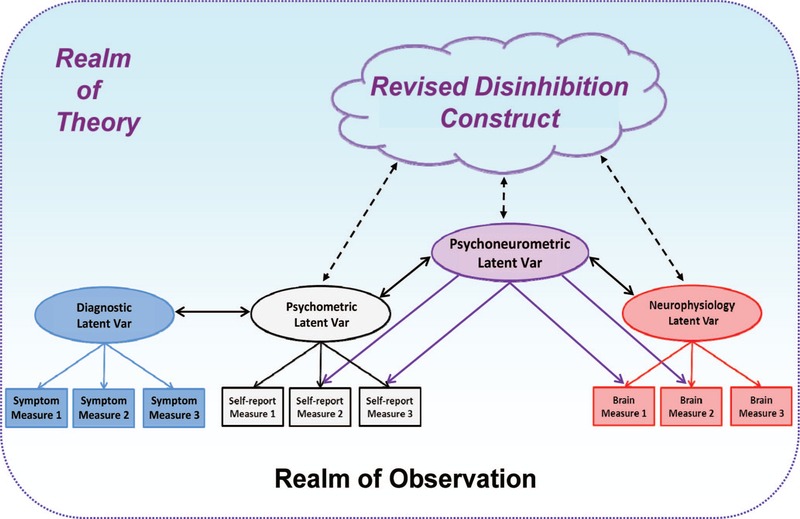
As a conceptual illustration of this approach, Figure 1 depicts a construct network for a hypothesized neurobehavioral trait construct of disinhibition that serves as an interface between measurement domains of clinical symptoms, self-report attributes, and neurophysiology. The Figure depicts how indicators of a self-report-based Psychometric latent variable (factor) within the network, which demonstrates close empirical relations with a counterpart Diagnostic factor defined by symptom indicators, can be combined with selected Neurophysiological indicators to form a joint Psychoneurometric factor. This Psychoneurometric factor represents a dimension of variability situated between perceived psychological attributes and neurophysiology, and serves to bridge observed variables in the domain of clinical symptoms with those in the domain of neurophysiology—predicting effectively to measures in each. Psychologically, the meaning of this Psychoneurometric factor will differ from the meanings of counterpart factors in one or the other domain—and will need to be clarified through evaluation of convergent and discriminant relations with other observables in the network. Through this process, the theoretical construction of the trait can shift from a more diffuse psychological conception to a more focused psychophysiological conception (i.e., representing as effectively as possible the interface between psychological and neurophysiological indicators and the network of observed relations surrounding this interface).
Figure 1.
Illustration of a construct network for a neurobehavioral construct (cloud-like shape) including observable measures (denoted by squares) as indicators of latent variables (ovals) in the domains of clinical symptoms, self-report, and neurophysiology. Var = Variable. The space surrounding the network of interconnected shapes is shaded to denote the interface between observation and theory, via the latent variables. A close empirical association is depicted between a latent Psychometric variable operationalized by self-report indicators and a latent Diagnostic variable operationalized by symptom indicators. The figure also depicts how a joint Psychoneurometric variable, operationalized using indicators from both self-report and brain response domains, can serve to bridge between clinical symptoms and neurophysiology. In the process, the theoretical conception of the trait shifts from a more diffuse multidomain (“three-systems”; cf. Lang, 1968) construct to a more focused psychophysiological construct.
Empirical Illustration of the Construct-Network Approach: Bridging Diagnostic and Physiological Domains in Assessment of Externalizing Psychopathology
Psychological Conceptualization and Measurement of Trait Inhibition–Disinhibition
Working from the longstanding idea of a trait-dispositional component to disinhibitory problems (e.g., Gorenstein & Newman, 1980; Gottfredson & Hirschi, 1990; Iacono et al., 1999; Tellegen, 1985; Sher & Trull, 1994), and research documenting systematic comorbidity among disruptive/antisocial disorders and substance problems (Achenbach & Edelbrock, 1978; Krueger, 1999), Krueger et al. (2002) demonstrated the presence of a highly heritable “externalizing” factor accounting for observed covariance among symptoms of child conduct disorder, adult antisocial behavior, alcohol dependence, and drug dependence, along with scores on a self-report measure of disinhibitory personality. Krueger et al. (2007) extended this work by operationalizing a comprehensive measurement model for organizing and assessing the spectrum of disinhibitory traits and behaviors, in the form of an assessment instrument, the Externalizing Spectrum Inventory (ESI). Developed using an iterative approach to item formulation and refinement, the ESI includes 23 unidimensional first-order scales designed to index differing facets of this domain, including: varying forms of impulsiveness, irresponsibility, blame externalization, differing types of aggression (physical, relational, and destructive), deficient empathy, rebelliousness, excitement seeking, and alcohol, drug, and marijuana use/problems. Confirmatory factor analyses of these 23 facet scales supported a structural model in which all scales loaded substantially (.45 or higher) on a broad externalizing factor reflecting general disinhibitory tendencies, with residual variance in selected subscales loading additionally on one of two subordinate factors reflecting callous-aggression and substance abuse.1
Findings from the ESI work are consistent with the idea that a common dispositional factor undergirds the spectrum of impulse control problems and affiliated traits and behaviors. In addition, the results of the ESI modeling work indicate that this general disinhibitory propensity intersects with two coherent problem domains, one involving callous-aggressive tendencies and the other proneness toward excessive substance use. These facets of disinhibition can be viewed as distinct behavioral expressions of externalizing proneness, attributable in part (i.e., more so for some individuals than others) to deficient inhibitory control, but also reflecting influences separate from externalizing proneness.
Trait Variations in Inhibition–Disinhibition: Relevance to Externalizing Problems
Our recent work has examined diagnostic correlates of general disinhibitory tendencies as indexed by overall scores on abbreviated screening versions of the ESI,2 which correlate very highly (–.9) with scores on the full (415-item) ESI and its general disinhibition factor. Venables and Patrick (2012) utilized a sample of incarcerated male prisoners (N = 162) to evaluate the validity of scores derived from a 159-item version of the ESI that provides coverage of the general externalizing factor (disinhibition) along with separable callous-aggression and substance abuse sub factors. Table 1 (upper section) shows correlations in the range of .29 to .57 between total scores on the ESI and interview-assessed symptoms of DSM–IV disinhibitory disorders. Within this sample, the correlation of ESI scores with scores on a general factor reflecting covariance among symptoms of these differing disinhibitory disorders was notably higher (r = .66) than with any single disorder variable, providing evidence that the ESI as a whole indexes a general underlying dispositional propensity toward impulse control problems.3
Table 1.
Correlations Between Externalizing Spectrum Inventory (ESI) Scores and Interview-Assessed Symptoms of DSM-IV Externalizing (EXT) and Internalizing (INT) Disorders
| Sample/Symptom count variable | r with ESI Score |
|---|---|
| Prisoners (N = 162)a | |
| EXT: | |
| Adult antisocial behavior | .54 |
| Conduct disorder | .42 |
| Drug dependence | .57 |
| Alcohol dependence | .30 |
| Nicotine use disorder | .29 |
| Community (N = 476)b | |
| EXT: | |
| Adult antisocial behavior | .70 |
| Conduct disorder | .49 |
| Drug dependence | .65 |
| Drug abuse | .67 |
| Alcohol dependence | .65 |
| Alcohol abuse | .62 |
| Borderline personality disorder | .47 |
| Histrionic personality disorder | .29 |
| Histrionic personality disorder | .23 |
| INT: | |
| Major depression | .28 |
| Generalized anxiety | .12 |
| Specific phobia | .06 |
| Specific phobia | −.01 |
| Pre-selected community (N = 90)b(c) | |
| EXT: | |
| Adult antisocial behavior | .70 (.59) |
| Conduct disorder | .55 (.50) |
| Drug dependence | .67 (.56) |
| Drug abuse | .57 (.47) |
| Alcohol dependence | .53 (.44) |
| Alcohol abuse | .59 (.56) |
| Borderline personality disorder | .61 (.55) |
| Histrionic personality disorder | .29 (.19) |
| Histrionic personality disorder | .37 (.31) |
| INT: | |
| Major depression | .09 (.19) |
| Generalized anxiety | .25 (.23) |
| Specific phobia | −.09 (−.02) |
| Specific phobia | −.15 (−.06) |
Note. Bolded font entries are significant at p < .005.
159-item ESI Total score.
100-item ESI Total score.
20-item ESI Disinhibition score.
The middle section of Table 1 shows results from a separate mixed-gender sample (N = 476) consisting of adult twins recruited from the community who were administered a 100-item version of the ESI and interviewed to assess for clinical problems. Consistent with prediction, ESI scores showed robust positive correlations with symptoms of substance disorders, child and adult antisocial behavior, and other Cluster B personality disorders as assessed by clinical interview. Associations were notably higher for child and adult antisocial behavior, substance-related problems, and borderline personality symptoms than for symptoms of narcissistic or histrionic personality disorder, indicating that these latter personality disorders intersect with disinhibitory proneness, but to a lesser degree than with antisocial behavior or substance abuse. The middle portion of Table 1 also shows rs for representative internalizing disorders, only one of which (depression) evinced a significant positive correlation with ESI-100 scores. Using data for this sample, we also modeled an externalizing diagnostic factor from multiple symptom indicators (i.e., adult antisocial behavior, conduct disorder, alcohol abuse/dependence, and drug abuse/dependence) and found that scores on this externalizing diagnostic factor were related almost to the level of unity (.92) with ESI total scores. The results for this sample provide further compelling evidence for the robustness (and in general, selectivity) of associations between scores on the ESI-100 and DSM-defined disinhibitory psychopathology.
Lastly, we examined phenotypic associations between scores on the ESI-100 and symptoms of clinical and personality conditions in a separate sample of adult community participants (N = 90) preselected to overrepresent very high or low scores on the ESI. Table 1 (lower section) presents validity coefficients for the ESI-100 in this sample along with (in parentheses) coefficients for a 20-item Disinhibition scale (DIS-20) consisting of items without direct reference to aggression- or substance-related behaviors. Coefficients are also shown for internalizing disorders. Results from this cross-validation sample again demonstrate robust and selective associations for ESI scores with Cluster B personality disorder (in particular, antisocial and borderline) symptoms along with substance-related disorders.
Neurobiological Bases and Physiological Correlates of Inhibition–Disinhibition
What brain systems/processes contribute to the general proneness to impulse control problems reflected in the broad factor of the externalizing spectrum model? Several lines of evidence point to anterior brain structures, including the prefrontal cortex (PFC) and anterior cingulate cortex (ACC), as playing crucial roles (Barkley, 1997; Blumer & Benson, 1975; Damasio, Tranel, & Damasio, 1990; Morgan & Lilienfeld, 2000; Peterson & Pihl, 1990). The PFC in particular is theorized to be important for “top-down” processing; that is, the guidance of behavior by internal goal representations across novel or dynamic situations, in which reliance on immediate stimulus cues alone is likely to produce undesired outcomes (Miller & Cohen, 2001). Subdivisions of the PFC appear to play differing roles in the guidance of behavior, with dorsolateral PFC particularly important for active processes that involve top-down (“cognitive”) control of behavioral responses (cf. Petrides, 2000), and ventromedial and orbitofrontal regions playing a greater role in anticipation of affective consequences of behavior (Bechara, Damasio, Tranel, & Damasio, 1997; Wagar & Thagard, 2004), unlearning of stimulus-reward associations (Dias, Robbins, & Roberts, 1996; Rolls, 2000), and the regulation of emotion (Damasio et al., 1990; Davidson, Putnam, & Larson, 2000).
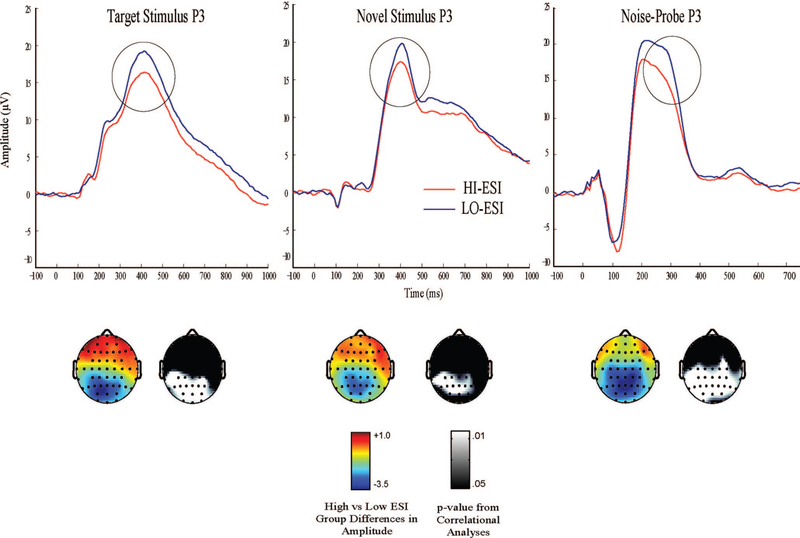
Whereas our understanding of “executive control” circuits in the brain has advanced dramatically through basic neuroscience research, existing knowledge regarding neural mechanisms and correlates of disinhibition-related traits remains quite underdeveloped. The best established physiological indicator of disinhibition proneness is reduced amplitude of the P300 (P3) brain potential response. Reduced P3 amplitude has been observed in relation to various specific impulse control problems (Iacono, Carlson, Malone, & McGue, 2002), and recent research has established reduced P3 as an indicator of the broad externalizing factor that these disorders share (Patrick et al., 2006). The relationship between P3 and externalizing proneness is illustrated in Table 2 and Figure 2 by data from a subset (n = 393) of the large community sample for which relations between ESI scores and externalizing disorder diagnoses were presented in Table 1. Table 2 shows correlations between continuous ESI scores and amplitude of three variants of P3 response at the midline parietal (Pz) scalp site in this sample: P3 responses to target and novel stimuli in a visual oddball task, and P3 response to loud noises occurring without warning during a picture-viewing task. Consistent with prior results for large-N studies (Hicks et al., 2007; Patrick et al., 2006), and as expected for indicators of presumably related but nonidentical constructs (Campbell & Fiske, 1959), the observed associations in each case are modest but robust. Also shown are correlations for another self-report trait measure, the Aggression subscale of the Multidimensional Personality Questionnaire-brief form (Patrick, Curtin, & Tellegen, 2002), which past research has shown to correlate robustly with externalizing proneness (Krueger, 1999; Patrick, Kramer, Markon, & Krueger, 2013) and also (negatively) with oddball P3 response (Venables, Patrick, Hall, & Bernat, 2011). Figure 2 shows average ERP waveform plots for stimuli of these three types in participants falling within the lowest and highest quartiles of scores on the ESI within this study sample.
Table 2.
Correlations Between Self-Report (ESI, MPQ-Aggression) and Brain Response (P3) Indices of Disinhibition Proneness in Adults From the Community (n = 393)
Note. ESI = total score on a 100-item version of the Externalizing Spectrum Inventory (cf. Hall et al., 2007); MPQ-Aggression = Aggression subscale of the Multidimensional Personality Questionnaire-brief form (Patrick et al., 2002). Numeric entries are Pearson correlations.
p < .01.
Figure 2.
Average event-related potential waveforms depicting amplitude of P3 response at electrode site Pz to stimuli of differing types in subgroups of participants scoring in the lowest (blue line) and highest (red line) quartiles on a 100-item version of the Externalizing Spectrum Inventory (ESI) in a mixed-gender sample of 393 adults from the community. Left and middle plots: P3 to target and novel stimuli in a three-stimulus variant of a rotated-heads visual oddball task (cf. Patrick et al., 2006) in which target stimuli consisted of schematic “heads” calling for a discriminative response and novel stimuli consisted of affective and neutral picture stimuli requiring no response. Right plot: P3 to noise-probe stimuli (50-ms, 105-db broadband noise bursts) occurring without warning during viewing of neutral scenes in a standard picture-startle task (cf. Lang, Bradley, & Cuthbert, 1990). Color topographic maps below each waveform plot depict the relative magnitude and directionality of group differences (low minus high externalizing) at differing scalp sites for each P3 response measure; adjacent monochrome topographic maps depict, for the participant sample as a whole, the significance of the correlation between continuous ESI externalizing scores and amplitude of response at differing scalp sites for each P3 measure.
Although valuable as a highly reliable indicator of externalizing proneness that appears to meet criteria for an endophenotype (Gould & Gottesman, 2006; Gilmore, Malone, & Iacono, 2010; Gould & Gottesman, 2006), the finding of reduced P3 response provides limited clues as to neural systems relevant to disinhibition. Stronger insights stand to be gained from brain response measures with clearer functional meaning and better-defined neural sources. One such measure is the error-related negativity (ERN), an ERP response that occurs following errors in performance and that is known to arise from the ACC (Agam et al., 2011; Miltner, Braun, & Coles, 1997). The ACC is theorized to invoke the control functions of the PFC as needed to support task performance, either by detecting errors as they occur (Gehring, Coles, Meyers, & Donchin, 1995; Scheffers, Coles, Bernstein, Gehring, & Donchin, 1996), by monitoring conflict among competing response tendencies (Carter et al., 1998), or by estimating error likelihood at the time a response is called for (Brown & Braver, 2005). Following up on prior demonstrations of reduced ERN in relation to disinhibitory personality traits (Dikman & Allen, 2000; Pailing & Segalowitz, 2004), Hall, Bernat, & Patrick (2007) reported evidence of reduced ERN response following performance errors in a flanker discrimination task for individuals high in externalizing proneness as indexed by the ESI. Reduced ERN is also associated with disinhibitory problems in children (Albrecht et al., 2008; van Meel, Heslenfeld, Oosterlaan, & Sergeant, 2007), suggesting some developmental continuity to this measure as an indicator of disinhibitory tendencies.
Bridging Externalizing Disorder Diagnoses and Brain Response Measures: Operationalizing Disinhibition as a Joint Trait-Neurophysiology Factor
Following from the foregoing demonstrations of strong associations for ESI externalizing scores with externalizing disorder diagnoses (see Table 1) and modest but robust relations between ESI scores and brain ERP measures (Table 2 and Figure 2), we utilized available diagnostic, psychometric, and ERP response measures for the aforementioned adult community sample (n = 393) to (a) evaluate interrelations among differing ERP measures and between ERP measures and diagnostic variables, and (b) determine whether we could improve prediction between the domains of neurophysiology (brain ERPs) and clinical problems (externalizing disorder symptoms) through specification of a psychoneurometric disinhibition factor jointly demarcated by self-report indicators and brain response indicators.
The foundation for this analytic approach was laid by a prior study (Nelson et al., 2011) that undertook analyses of relations among multiple ERP indicators of externalizing proneness recorded from 88 undergraduate participants in three different task procedures: an ERN flanker task, a choice-feedback task (cf. Gehring & Willoughby, 2002), and a three-stimulus oddball P3 task. The 100-item ESI was administered to index externalizing proneness. The ERP indicators included one measure from the choice-feedback task (i.e., amplitude of P3 response to gain/loss feedback cues that occurred following choices) and two from the flanker task (i.e., amplitude of P3 response to flanker target stimuli; amplitude of ERN response following incorrect responses to flanker stimuli), along with two others from the oddball task (i.e., amplitude of P3 response to infrequent target and novel stimuli). The three ERP variables from the choice-feedback and flanker tasks were interrelated with one another (rs = .24 to .27), and each showed a significant negative correlation with ESI externalizing scores (rs = –.24 to–37).
When these three ERP measures were entered together into a factor analysis, a single common factor emerged that accounted for appreciable variance in each individual measure. Scores on this common factor predicted scores on the two oddball P3 measures to a high degree (rs~7), and scores on the ESI to a moderate degree (r ~4). Notably, when these same three ERP indicators were entered together with ESI externalizing scores into a follow-up factor analysis, a single dominant factor again emerged, on which ESI-100 scores loaded comparably (r = –.60) with the three ERP measures (.44 to .60). The single common factor emerging from this analysis was interpreted as a predominantly neurophysiological (ERP-based) externalizing factor on which the self-report ESI measure also loaded.
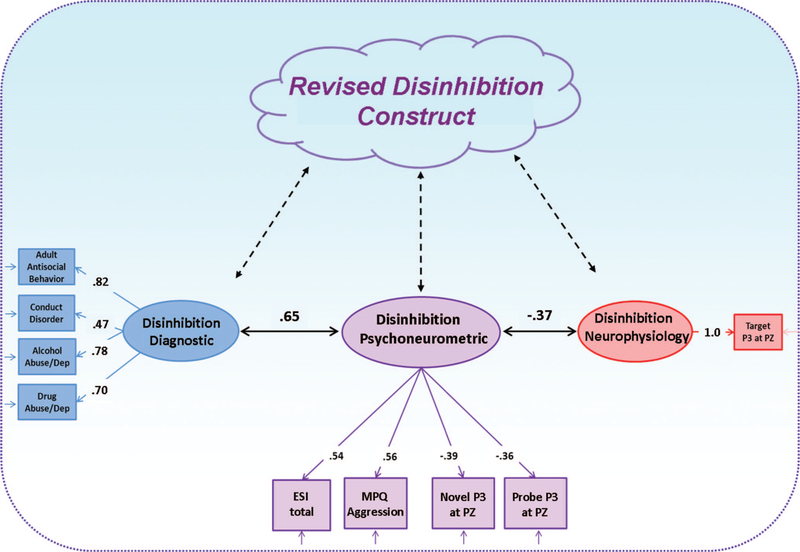
We extended this analysis using data from our large adult community sample (n = 393) to further evaluate the possibility of operationalizing a joint psychoneurometric factor and to evaluate its predictive utility across measurement domains. ESI scores and MPQ-Aggression scores were used as self-report indicators of this cross-domain factor. Two of the three ERP response variables available for this sample, the P3 to novel stimuli from the oddball task and the P3 to noise-probe stimuli from the picture-viewing task, were selected as neurophysiological indicators. The other ERP variable, target stimulus P3 from the oddball task, was used as a criterion in validation analyses (cf. Nelson et al., 2011) to permit evaluation of the effectiveness of the psychoneurometric factor in predicting brain response as well as clinical symptom criteria. A principal-axis factor analysis of these four indicators revealed the presence of a single common factor,4 on which all indicators loaded to an appreciable degree. Loadings for the two self-report indicators were modestly higher than for the two ERP variables (see Figure 3), indicating somewhat greater representation of the self-report domain in the common factor than the neurophysiological domain.
Figure 3.
Conceptual-empirical depiction of results from an analysis directed at operationalizing a joint Psychoneurometric factor to serve as a bridge between observables in domains of diagnosis (externalizing symptomatology) and neurophysiology (brain response). The lower portion of the figure (comprising circles, squares, and solid arrows) depicts relations among observed variables from differing domains and latent variables (factors) derived from those variables within a sample of 393 adults. The upper part of the figure (cloud shape and dashed arrows) depicts hypothesized links of observed and latent variables to a neurobehavioral construct of inhibition –disinhibition, reformulated to reflect the interface between psychological and neurophysiological indicators.
Table 3 shows correlations between scores on this psychoneurometric (DIS/ERP) factor and criterion variables in the domain of diagnoses (externalizing symptom variables, in particular) and neurophysiology (brain response). Correlations with individual externalizing symptom variables are in the range of .4 to .6; the correlation with scores on an externalizing diagnostic factor derived from a factor analysis of symptom counts for alcohol abuse/dependence and drug abuse/dependence (i.e., mean of standardized abuse and dependence scores) along with conduct disorder and adult antisocial behavior is .65; the corresponding r with an internalizing diagnostic factor reflecting covariance among symptoms of major depression, generalized anxiety disorder, social phobia, and specific phobia was only .15.5 Correlations of individual externalizing symptom variables with the criterion measure of brain response, target stimulus P3, were low to modest (–.07 to –.20; M = .13). By contrast, the psychoneurometric factor predicted quite well to this brain response criterion measure (r = –37). Harkening back to Figure 1, Figure 3 depicts graphically how the psychoneurometric factor operationalized this way functions as a predictive bridge between observables in the domains of psychopathology (externalizing symptomatology) and neurophysiology (brain response) within a nomological network structured around the neurobehavioral construct of inhibition–disinhibition. Two things that may have constrained prediction to brain response in this example were that (1) the psychoneurometric factor was defined less strongly by ERP indicators than by self-report trait indicators (loadings = –.36/–.39 vs. .54/.56), and (2) the brain response criterion consisted of just a single ERP indicator. In follow-up work, described next, we found prediction to be stronger when a factor defined equally by ERP and self-report trait indicators was used to predict scores on a composite brain criterion.
Table 3.
Correlations of Individual ERP Indicators and Psychoneurometric (DIS/ERP) Factor Scores With ERP and Diagnostic Criterion Variables (n = 393)
| Criterion variables | Target P3 | Novel P3 | Probe P3 | DIS/ERP Factor |
|---|---|---|---|---|
| DSM-IV Symptom variables | ||||
| Axis-I: | ||||
| Alcohol abuse | −.16* | −.14* | −.17* | .49* |
| Alcohol dependence | −.16* | −.06 | −.13* | .47* |
| Drug abuse | −.11 | −.09 | −.12 | .47*. |
| Drug dependence | −.09 | −10 | −.07 | .43* |
| Axis-II: | ||||
| Conduct disorder | −.07 | −.11 | −.01 | .44* |
| Adult antisocial behavior | −.20* | −.18* | −.16* | .60* |
| Borderline personality disorder | −.09 | −.10 | −.09 | .41* |
| Diagnostic factors: | ||||
| Internalizing factor | −.19* | −.16* | −.19* | .65* |
| Internalizing factor | .00 | .01 | −.01 | .15* |
| ERP Response variable | ||||
| Target P3 amplitude | — | .55** | .29** | −.37* |
Note. DIS/ERP Factor = scores on factor reflecting variance in common among two self-report measures of disinhibition proneness (total score on a 100-item version of the Externalizing Spectrum Inventory; Aggression subscale of the Multidimensional Personality Questionnaire-brief form) and two event-related potential measures (Novel P3 = amplitude of P3 response to novel pictures in a three-stimulus visual oddball task; Probe P3 = amplitude of P3 response to unwarned noise probes during a picture-viewing task). Target P3 = amplitude of P3 response to target stimuli in a three-stimulus visual oddball task. Axis I = DSM-IV clinical disorder diagnoses; Axis II = DSM-IV personality disorder diagnoses. Externalizing factor = scores on a factor reflecting variance in common among diagnostic symptom counts for alcohol abuse/dependence, drug abuse/dependence, conduct disorder, and adult antisocial behavior. Internalizing factor = scores on a factor reflecting variance in common among diagnostic symptom counts for major depression, generalized anxiety disorder, social phobia, and specific phobia. Bolded font entries are significant at p < .001.
p < .01.
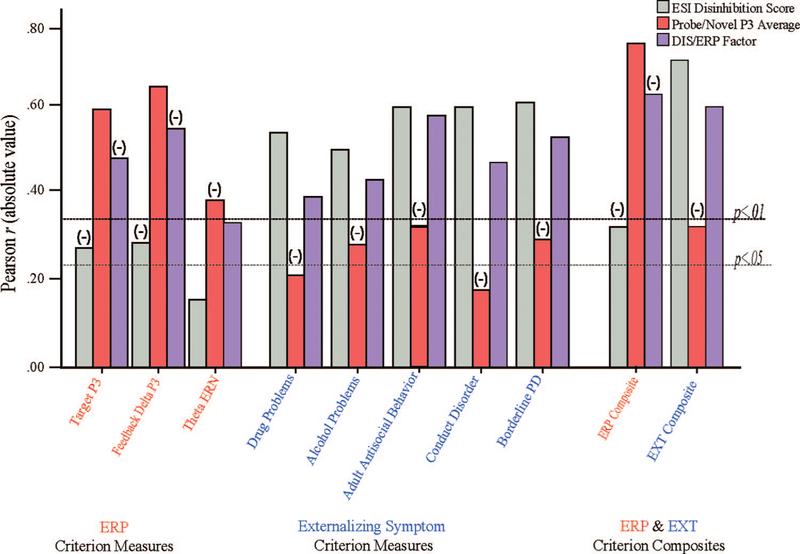
To confirm its effectiveness, we cross-validated this operational approach in a separate dataset that included scores for the four indicators of the cross-domain factor along with externalizing symptom criteria and a broader array of ERP criterion measures. Data were from a subset of participants (n = 60) from the adult sample represented in the lower portion of Table 1 who were tested in a lab physiological protocol that included visual oddball, choice-feedback, and flanker discrimination tasks. Participants were administered the 100-item version of the ESI along with a smaller subset of ESI items required to compute scores on a scale-based index of general disinhibitory tendencies consisting of 20 items. This 20-item Disinhibition (ESI-DIS) scale was used in place of overall ESI-100 scores because it is faster to administer and does not contain items referencing aggression or substance use/problems and thus provides a more trait-based index of disinhibition exhibiting less direct overlap with externalizing diagnostic criteria. Scores on the psychoneurometric factor were computed using a unit-weighting approach (i.e., raw values for each of the four indicators [ESI-DIS, MPQ-Aggression, Novel Stimulus P3, Noise-Probe P3] were standardized and then summed).
Figure 4 depicts correlations between scores on this four-indicator DIS/ERP factor (purple bars) and criterion variables consisting of additional ERP variables (target P3 from the oddball task; feedback stimulus P3 from the choice task; and response ERN from the flanker task; left bars), externalizing disorder symptom variables (middle bars), and composites reflecting unit-weighted aggregates of ERP criterion variables and diagnostic criterion variables (right bars). Also depicted, for comparison purposes, are rs for the ESI-DIS scale indicator alone (gray bars) and the mean of the two ERP indicators with the various ERP and diagnostic criterion variables. It can be seen from the figure that the psychoneurometric factor predicts criterion variables in the diagnostic and brain response domains to comparable robust degrees; the correlations for this factor with ERP composite scores and diagnostic composite scores (gray bars in right group) both exceed .6. By contrast, ESI-Disinhibition (DIS) scores alone (gray bars) predict criterion variables in the diagnostic domain very effectively but criteria in the brain response domain only modestly, whereas ERP indicators alone (red bars) predict criterion variables in the brain response domain very effectively but criteria in the diagnostic domain only modestly.
Figure 4.
Correlations between scores on a four-indicator Psychoneurometric (DIS/ERP) factor (purple bars) and criterion variables consisting of ERP variables (target stimulus P3 from an oddball task; feedback stimulus P3 from a choice-feedback task; and response-locked ERN from a flanker task; left group of bars), externalizing disorder symptom variables (middle group of bars), and composites reflecting unit-weighted aggregates of ERP criterion variables and diagnostic criterion variables (right group of bars). Also depicted are correlations for the ESI Disinhibition scale indicator of the DIS/ERP factor alone (gray bars) and the mean of the two ERP indicators alone with the various ERP and diagnostic criterion variables. (−) above bar indicates a negative correlation coefficient for the variable indicated.
Conclusions and Future Directions
Research aimed at clarifying the role of neurobiological systems and processes in clinical disorders has been identified as a high priority by authorities in the mental health field. Toward this end, we propose a construct-network approach that is compatible with the RDoC initiative in terms of its focus on psychological constructs with biological referents rather than on putatively discrete disorders, yet distinct in terms of its emphasis on larger-N validation work to clarify the psychological (convergent/discriminant) meaning of individual-differences variance in lab experimental measures, and its emphasis on allowing psychological conceptions of target constructs to be reshaped through a process of bootstrapping. The current paper describes a specific psychoneurometric implementation of the construct-network approach, directed at establishing new constructs reflecting the interface between psychological conceptions of clinically relevant traits (as operationalized by self-report) and conceptions of neural systems/processes (as operationalized by brain response measures). The final two sections below provide additional perspective on the construct-network approach by considering how the tightly focused psychoneurometric implementation fits within a multilevel analysis of core psychological processes (cf. Anderson, 1998; Cacioppo & Berntson, 1992; Sanislow et al., 2010) and the utility of the approach for prevention and treatment.
Broader Application of the Construct-Network Approach
While the focus of the current paper is on applying the construct-network approach to the specific problem of bridging between psychological and neurobiological conceptions, it is important to emphasize that components of the reliable variance in self-report or behavioral indicators of interest are not excluded from further investigation simply because they do not cohere with available physiological indicators. Indeed, it is expected that there will be variance in self-report or behavioral measures of constructs like inhibition–disinhibition or fearfulness that do not cohere with physiological variables. These distinct sources of variance can become the focus of separate construct-network mapping efforts designed to clarify their distinct psychological meaning (in terms of convergent and discriminant relations with other variables from the same or other, nonphysiological domains) and potential relevance to clinical problems. The issue of relevance to clinical problems is important because there may be aspects of self-report or behavioral measures that lack known biological correlates but have clear implications for treatment efficacy or long-term prognosis. Examples include symptoms of self- or identity disturbance in psychotic spectrum disorders and borderline personality, respectively. Notably, the use of distinct symptom-oriented variables as targets for research and clinical intervention represents a prominent focus of the RDoC initiative (Sanislow et al., 2010).
Clinical Feasibility and Utility
The clinical feasibility and utility of joint psychometric-neurophysiological measures will need to be established through further systematic research spanning nonclinical community and clinical populations. Although physiological assessments are not routinely used in applied clinical settings at this time, as technology advances and expands, the inclusion of assessments of this type in routine clinical practice is likely to become more common (cf. Thomas, Aldao, & De Los Reyes, 2012). Operating from a construct-network approach, costlier and less available methods such as fMRI can be used to validate and refine clinical assessments based on more widely available methods such as EEG/ERP or autonomic psychophysiology. Work directed at establishing norms for psychoneurometric variables of the sort described here would provide a basis for evaluating their sensitivity and specificity for identifying individuals experiencing clinical problems currently, or who are at risk for developing such problems. From the perspective of the preceding section, other assessments focusing on domain-specific aspects of problem tendencies could provide a useful supplement to psychoneurometric assessments.
The construct-network approach also has important treatment implications. In particular, it provides a framework for generating and testing more targeted interventions for addressing underlying brain processing differences directly, in contrast to relying on extant treatment packages for diffuse disorder categories (e.g., behavioral or motivational therapy for antisocial personality disorder). The framework provided here is transdiagnostic (Nolen-Hoeksema & Watkins, 2011), shifting the focus from disorders to traits, and thus has applications to treatment across disorders. Furthermore, consistent with practice in many current clinical settings, our approach calls for use of multiple measures of physiology and behavior in treatment planning and as indicators of outcome. Because of the dimensional approach taken, such interventions could be preventative, for those at risk, as well as ameliorative for clinical patients with active impairments. Future studies should include other clinically relevant variables aside from diagnostic symptoms, for example, measures indexing course of illness, motivation for and response to treatment, and potential for self-harm. Inclusion of such variables will be important for establishing the practical utility of the proposed construct-network approach with treatment-seeking individuals.
Acknowledgments
Preparation of this article was supported by Grants MH65137, MH072850, and MH089727 from the National Institute of Mental Health.
Footnotes
The model as reported in Krueger et al. (2007) was expressed as a bi factor (hierarchical) model in which the general disinhibitory factor (on which all lower-order scale indicators loaded) was parameterized as independent of the two sub factors reflecting aggressive and addictive tendencies (on which differing subsets of scales loaded). The fit of this model (based on a quantitative index of comparative fit, the Bayesian Information Criterion) was superior to that for a higher-order model, in which the sub factors were specified as correlated with and subordinate to the general factor. However, a case could be made for preferring the alternative higher-order model on rationale grounds, given that all lower-order scales loaded prominently on the general factor, with none loading appreciably more on either sub factor. Our perspective is that the two models provide complementary perspectives on the structure of the scale indicators, with the higher-order model highlighting the general interdependency of existing scale indicators, and the bifactor model highlighting the possibility (evaluable through research directed at developing “purer” scale indicators of the sub factors) that separable constructs are embedded within a putatively unitary domain.
The two abbreviated ESI versions discussed in this section were developed in the process of constructing the full ESI, to provide for more efficient assessment of overall externalizing proneness (100-item version; cf. Hall, Bernat, & Patrick, 2007) and estimation of scores on the three higher-order ESI factors (159-item version; cf. Venables & Patrick, 2012). More recently, we (Patrick, Kramer, Krueger, & Markon, 2013) have developed a 160-item brief ESI that yields scores on the 23 ESI facet constructs, along with scores on the ESI higher-order factors in the form of factor score estimates or item-based scale scores (18 –20 items/scale); this newer brief form is recommended for research screening use because it includes more comprehensive coverage than the earlier 100- or 159-item versions. Copies of the full ESI and any of these shorter screening versions can be obtained from the first author upon request.
A limitation of the version of the ESI used in this study is that 67 of its 159 items are directly indicative of physical/destructive aggression or alcohol/drug use. However, scores on a “purer” index of trait disinhibition that omitted ESI items directly indicative of aggressiveness or alcohol/drug use also showed robust associations with diagnostic variables, albeit some-what reduced from those for total ESI scores (range of rs = .22 to .47).
The presence of a one-factor solution was indicated both by visual inspection of the scree plot (i.e., magnitude of first eigenvalue was 1.63, with all others <1) and by parallel analysis (Horn, 1965), a technique for determining the number of factors to retain by comparing actual eigenvalues for the sample data with eigenvalues estimated on the basis of randomly generated data (in the current instance, 100 random samples).
It will be important in future research to further evaluate the discriminant validity of a psychoneurometric index of disinhibition operationalized in this way, for example, by evaluating its effectiveness in differentiating between problems involving externalizing tendencies and problems entailing psychotic symptoms (i.e., in light of evidence for reductions in ERP response in individuals diagnosed with schizophrenia; Ford, 1999; Foti, Kotov, Bromet, & Hajcak, 2012). The proposed construct-network approach addresses the issue of diagnostic specificity of physiological indicators in a novel way, through systematic delineation of their relations with other physiological variables (including neuroimaging-based measures that can help to clarify relevant anatomic sources) and distinguishable dimensions of clinical problems in a multivariate analytic framework. The construct mapping process provides a means for establishing whether externalizing-related variance in brain potential measures such as P300 or ERN overlaps with or is separate from psychosis-related variance in these measures (i.e., whether effects for such measures reflect circuits/processes in common between externalizing and psychotic problems, or circuits/processes that differ between the two). This perspective highlights the idea that the stable “person” variance in electro cortical measures from lab tasks can reflect differing sources of influence (i.e., distinguishable psychological characteristics, associated with separable brain processes). As the differing sources of influence are clarified, efforts can be directed toward developing tasks/measures with greater specificity for each.
Contributor Information
Lindsay D. Nelson, Department of Neurology, Medical College of Wisconsin;
Brian M. Hicks, University of Michigan
Mark D. Kramer, Minneapolis Veterans Affairs Health Care System and Center for Chronic Disease Outcomes Research, Minneapolis, MN.
References
- Achenbach TM, & Edelbrock CS (1978). The classification of child psychopathology: A review and analysis of empirical efforts. Psychological Bulletin, 85, 1275–1301. doi: 10.1037/0033-2909.85.6.1275 [DOI] [PubMed] [Google Scholar]
- Agam Y, Hämäläinen MS, Leeb AKC, Dyckman KA, Friedman JS, Isom M,…Manoach DS (2011). Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. PNAS: Proceedings of the National Academy of Sciences, USA, 108, 17556–17561. doi: 10.1073/pnas.1103475108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M,…Banaschewski T (2008). Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: Evidence for an endopheno type. Biological Psychiatry, 64, 615–625. doi: 10.1016/j.biopsych.2007.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport GW (1937). Personality: A psychological interpretation. New York, NY: Holt, Rinehart, & Winston. [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed, text rev.). Washington, DC: Author. [Google Scholar]
- Anderson NB (1998). Levels of analysis in health science: A framework for integrating socio nbehavioral and biomedical research. Annals of the New York Academy of Sciences, 840, 563–576. doi: 10.1111/j.1749-6632.1998.tb09595.x [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121, 65–94. doi: 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, & Damasio AR (1997). Deciding advantageously before knowing the advantageous strategy. Science, 275, 1293–1295. doi: 10.1126/science.275.5304.1293 [DOI] [PubMed] [Google Scholar]
- Blumer D, & Benson DF (1975). Personality changes with frontal and temporal lobe lesions In Benson DF & Blumer D (Eds.), Psychiatric aspects of neurological disease (pp. 151–169). New York, NY: Grune & Stratton. [Google Scholar]
- Brown JW, & Braver TS (2005). Learned predictions of error likelihood in the anterior cingulate cortex. Science, 307, 1118–1121. doi: 10.1126/science.1105783 [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, & Barlow DH (1998). Structural relationships among dimensions of the DSM–IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology, 107, 179–192. doi: 10.1037/0021-843X.107.2.179 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, & Berntson GG (1992). Social psychological contributions to Decade of the Brain: Doctrine of multi-level analysis. American Psychologist, 47, 1019–1028. doi: 10.1037/0003-066X.47.8.1019 [DOI] [PubMed] [Google Scholar]
- Campbell DT, & Fiske DW (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin, 56, 81–105. doi: 10.1037/h0046016 [DOI] [PubMed] [Google Scholar]
- Carter CS, & Barch DM (2007). Cognitive neuroscience-based approaches to measuring and improving treatment on cognition in schizophrenia: The CNTRICS initiative. Schizophrenia Bulletin, 33, 1131–1137. doi: 10.1093/schbul/sbm081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll DN, & Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280, 747–749. doi: 10.1126/science.280.5364.747 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8, 597–600. doi: 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Clark LA (1993). Schedule for Nonadaptive and Adaptive Personality (SNAP): Manual for administration, scoring, and interpretation. Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100, 316–336. doi: 10.1037/0021-843X.100.3.316 [DOI] [PubMed] [Google Scholar]
- Collins PF, & Depue RA (1992). A neurobehavioral systems approach to developmental psychopathology: Implications for disorders of affect In Cicchetti D & Toth SL (Eds.), Developmental perspectives on depression. Rochester symposium on developmental psychopathology (pp. 29–101). Rochester, NY: University of Rochester Press. [Google Scholar]
- Cronbach LJ (1957). The two disciplines of scientific psychology. American Psychologist, 12, 671–684. doi: 10.1037/h0043943 [DOI] [Google Scholar]
- Cronbach LJ (1975). Beyond the two disciplines of scientific psychology. American Psychologist, 30, 116–127. doi: 10.1037/h0076829 [DOI] [Google Scholar]
- Cronbach LJ, & Meehl PE (1955). Construct validity in psychological tests. Psychological Bulletin, 52, 281–302. doi: 10.1037/h0040957 [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, & Damasio H (1990). Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research, 41, 81–94. doi: 10.1016/0166-4328(90)90144-4 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, & Larson CL (2000). Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence, Science, 289, 591–594. doi: 10.1126/science.289.5479.591 [DOI] [PubMed] [Google Scholar]
- Depue RA, & Iacono WG (1989). Neurobehavioral aspects of affective disorders. Annual Review of Psychology, 40, 457–492. doi: 10.1146/annurev.ps.40.020189.002325 [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, & Roberts AC (1996). Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380, 69–72. doi: 10.1038/380069a0 [DOI] [PubMed] [Google Scholar]
- Dikman ZV, & Allen JJ (2000). Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology, 37, 43–54. doi: 10.1111/1469-8986.3710043 [DOI] [PubMed] [Google Scholar]
- Durbin CE, Hayden EP, Klein DN, & Olino TM (2007). Stability of laboratory-assessed temperamental emotionality traits from ages 3 to7. Emotion, 7, 388–399. doi: 10.1037/1528-3542.7.2.388 [DOI] [PubMed] [Google Scholar]
- Eysenck HJ (1967). The biological basis of personality. Springfield, IL: Charles C Thomas. [Google Scholar]
- Ford JM (1999). Schizophrenia: The broken P300 and beyond. Psychophysiology, 36, 667–682. doi: 10.1111/1469-8986.3660667 [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet E, & Hajcak G (2012). Beyond the broken error-related negativity: Functional and diagnostic correlates of error processing in psychosis. Biological Psychiatry, 71, 864–872. doi: 10.1016/j.biopsych.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Coles MGH, Meyer DE, & Donchin E (1995). A brain potential manifestation of error-related processing In Karmos G,Molnar M, Csepe V, Czigler I, & Desmedt JE (Eds.), Perspectives of event-related potentials research (pp. 267–272). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Gehring WJ, & Willoughby AR (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295, 2279– 2282. doi: 10.1126/science.1066893 [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, & Iacono WG (2010). Brain electrophysiological endophenotypes for externalizing psychopathology: A multivariate approach. Behavior Genetics, 40, 186–200. doi: 10.1007/s10519-010-9343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein EE, & Newman JP (1980). Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review, 87, 301–315. doi: 10.1037/0033-295X.87.3.301 [DOI] [PubMed] [Google Scholar]
- Gottesman II (1991). Schizophrenia genesis: The origins of madness.New York, NY: Freeman. [Google Scholar]
- Gottfredson MR, & Hirschi T (1990). A general theory of crime. Palo Alto, CA: Stanford University Press. [Google Scholar]
- Gould TD, & Gottesman II (2006). Psychiatric endophenotypes and the development of valid animal models. Genes, Brain, & Behavior, 5, 113–119. doi: 10.1111/j.1601-183X.2005.00186.x [DOI] [PubMed] [Google Scholar]
- Gray JA (1987). The psychology of fear and stress, 2nd ed. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Hall JR, Bernat EM, & Patrick CJ (2007). Externalizing psychopathology and the error-related negativity. Psychological Science, 18, 326–333. doi: 10.1111/j.1467-9280.2007.01899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bernat EM, Malone SM, Iacono WG, Patrick CJ, Krueger RF, & McGue M (2007). Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology, 44, 98–105. doi: 10.1111/j.1469-8986.2006.00471.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL (1965). A rationale and test for the number of factors in factor analysis. Psychometrika, 30, 179–185. doi: 10.1007/BF02289447 [DOI] [PubMed] [Google Scholar]
- Hyman SE (2007). Can neuroscience be integrated into the DSM-V? Nature Reviews: Neuroscience, 8, 725–732. doi: 10.1038/nrn2218 [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, & McGue M (2002). P3 event-related potential amplitude and risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry, 59, 750–757. doi: 10.1001/archpsyc.59.8.750 [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, & McGue M (1999). Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology, 11, 869–900. doi: 10.1017/S0954579499002369 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K,…Wang P (2010). Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Insel TR, & Scolnick EM (2006). Cure therapeutics and strategic prevention: Raising the bar for mental health research. Molecular Ps chiatry, 11, 11–17. doi: 10.1038/sj.mp.4001777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G (1997). Multiple pathways to conscience for children with different temperaments: From toddlerhood to age 5. Developmental Psychology, 33, 228–240. doi: 10.1037/0012-1649.33.2.228 [DOI] [PubMed] [Google Scholar]
- Krueger RF (1999). The structure of common mental disorders. Archives of General Psychiatry, 56, 921–926. doi: 10.1001/archpsyc.56.10.921 [DOI] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon KE, Watson D, & Skodol AE (2012). Initial construction of a maladaptive personality trait model and inventory for DSM-5. Psychological Medicine, 42, 1879–1890. doi: 10.1017/S0033291711002674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, & McGue M (2002). Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology, 111, 411–424. doi: 10.1037/0021-843X.111.3.411 [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, & Kramer MD (2007). Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology, 116, 645–666. doi: 10.1037/0021-843X.116.4.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ (1968). Fear reduction and fear behavior: Problems in treating a construct In Shlien JM (Ed.), Research in psychotherapy (Vol. 3, pp. 90–102). Washington, DC: American Psychological Association. doi: 10.1037/10546-004 [DOI] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BM (1990). Emotion, attention, and the startle reflex. Psychological Review, 97, 377–395. doi: 10.1037/0033-295X.97.3.377 [DOI] [PubMed] [Google Scholar]
- Livesley WJ, & Jackson DN (2009). Manual for the Dimensional Assessment of Personality Pathology—Basic Questionnaire. Port Huron, MI: Sigma Press. [Google Scholar]
- Loevinger J (1957). Objective tests as instruments in psychological theory. Psychological Reports, 3, 635–694. Retrieved from http://www.amsciepub.com [Google Scholar]
- MacCorquodale K, & Meehl PE (1948). On a distinction between hypothetical constructs and intervening variables. Psychological Review, 55, 95–107. doi: 10.1037/h0056029 [DOI] [PubMed] [Google Scholar]
- Meehl PE (1959). Some ruminations on the validation of clinical procedures. Canadian Journal of Psychology/Revue Canadienne de Psychologie, 13, 102–128. doi: 10.1037/h0083769 [DOI] [Google Scholar]
- Miller EK, & Cohen JD (2001). Integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, & Coles MGH (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection Journal of Cognitive Neuroscience, 9, 788–798. doi: 10.1162/jocn.1997.9.6.788 [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, & Clark LA (1998). Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology, 49, 377–412. doi: 10.1146/annurev.psych.49.1.377 [DOI] [PubMed] [Google Scholar]
- Miyake A, Emerson MJ, & Friedman NP (2000). Assessment of executive functions in clinical settings: Problems and recommendations. Seminars in Speech and Language, 21, 0169–0183. doi: 10.1055/s-2000-7563 [DOI] [PubMed] [Google Scholar]
- Morgan AB, & Lilienfeld SO (2000). A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review, 20, 113–136. doi: 10.1016/S0272-7358(98)00096-8 [DOI] [PubMed] [Google Scholar]
- Morris SE, & Cuthbert BN (2012). Research Domain Criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience, 14, 29–37. Retrieved from http://www.dialogues-cns.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, & Bernat EM (2011). Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology, 48, 64–72. doi: 10.1111/j.1469-8986.2010.01047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Watkins ER (2011). A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science, 6, 589–609. doi: 10.1177/1745691611419672 [DOI] [PubMed] [Google Scholar]
- Pailing PE, & Segalowitz SJ (2004). The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology, 41, 84–95. doi: 10.1111/1469-8986.00124 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, &McGue MK (2006). P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology, 43, 84–92. doi: 10.1111/j.1469-8986.2006.00376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, & Tellegen A (2002). Development and validation of a brief form of the multi-dimensional personality question-naire. Psychological Assessment, 14, 150–163. doi: 10.1037/1040-3590.14.2.150 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Durbin CE, & Moser JS (2012). Conceptualizing proneness to antisocial deviance in neurobehavioral terms. Development and Psychopathology, 24, 1047–1071. doi: 10.1017/S0954579412000533 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Kramer MD, Krueger RF, & Markon KE (2013). Optimizing efficiency of psychopathology assessment through quantitative modeling: Development of a brief form of the Externalizing Spectrum Inventory. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, & Pihl RO (1990). Information processing, neuropsychological function, and the inherited predisposition to alcoholism. Neuropsychology Review, 1, 343–369. doi: 10.1007/BF01109029 [DOI] [PubMed] [Google Scholar]
- Petrides M (2000). Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. The Journal of Neuroscience, 20, 7496–7503. Retrieved from http://www.jneurosci.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2000). The orbitofrontal cortex and reward. Cerebral Cortex, 10, 284–294. doi: 10.1093/cercor/10.3.284 [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK,…Cuthbert BN (2010). Developing constructs for psychopathology research: Research Domain Criteria. Journal of Abnormal Psychology, 119, 631–639. doi: 10.1037/a0020909 [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MGH, Bernstein P, Gehring WJ, &Donchin E, (1996). Event-related potentials and error-related processing: An analysis of incorrect responses to go and no-go stimuli. Psychophysiology, 33, 42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x [DOI] [PubMed] [Google Scholar]
- Sellbom M, Ben-Porath YS, & Bagby RM (2008). On the hierarchical structure of mood and anxiety disorders: Confirmatory evidence and an elaborated model of temperament markers. Journal of Abnormal Psychology, 117, 576–590. doi: 10.1037/a0012536 [DOI] [PubMed] [Google Scholar]
- Sher KJ, & Trull TJ (1994). Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. Journal of Abnormal Psychology, 103, 92–102. doi: 10.1037/0021-843X.103.1.92 [DOI] [PubMed] [Google Scholar]
- Tellegen A (1985). Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report In Tuma AH &Maser JD (Eds.), Anxiety and the anxiety disorders (pp. 681–706). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Tellegen A (1991). Personality traits: Issues of definition, evidence, and assessment In Grove WM & Cicchetti D (Eds.), Thinking clearly about psychology: Essays in honor of Paul E. Meehl, Vol. 2: Personality and psychopathology (pp. 10–32). Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- Tellegen A, & Waller NG (2008). Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire In Boyle GJ, Matthews G, & Saklofske DH (Eds.), Handbook of personality theory and testing: Personality measurement and assessment (Vol. II, pp. 261–292). London, UK: Sage. doi: 10.4135/9781849200479.n13 [DOI] [Google Scholar]
- Thomas SA, Aldao A, & De Los Reyes A (2012). Implementing clinically feasible psychophysiological measures in evidence-based assessments of adolescent social anxiety. Professional Psychology: Research and Practice, 43, 510–519. doi: 10.1037/a0029183 [DOI] [Google Scholar]
- Vaidyanathan U, Patrick CJ, & Bernat EM (2009). Startle reflex potentiation during aversive picture viewing as an index of trait fear. Psychophysiology, 46, 75–85. doi: 10.1111/j.1469-8986.2008.00751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, & Sergeant JA (2007). Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research, 151, 211– 220. doi: 10.1016/j.psychres.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Venables NC, & Patrick CJ (2012). Validity of the externalizing spectrum inventory in a criminal offender sample: Relations with disinhibitory psychopathology, personality, and psychopathic features. Psychological Assessment, 24, 88–100. doi: 10.1037/a0024703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ, Hall JR, & Bernat EM (2011). Clarifying relations between dispositional aggression and brain potential response: Overlapping and distinct contributions of impulsivity and stress reactivity. Biological Psychology, 86, 279–288. doi: 10.1016/j.biopsycho.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, & Pashler H (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science, 4, 274–290. doi: 10.1111/j.1745-6924.2009.01125.x [DOI] [PubMed] [Google Scholar]
- Wagar BM, & Thagard P (2004). Spiking Phineas Gage: A neurocomputational theory of cognitive-affective integration in decision-making. Psychological Review, 111, 67–79. doi: 10.1037/0033-295X.111.1.67 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2004). International statistical classification of diseases and health related problems. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118, 117–130. doi: 10.1037/a0014657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M (1999). Vulnerability to psychopathology: A biosocial model. Washington, DC: American Psychological Association. doi: 10.1037/10316-000 [DOI] [Google Scholar]