Abstract
Objective:
To develop a Vaccine Confidence Index (VCI) that is capable of detecting variations in parental confidence towards childhood immunizations centered on trust and concern issues that impact vaccine confidence.
Methods:
We used a web-based national poll of 893 parents of children <7 years in 2016 to assess the measures created for the Emory VCI (EVCI). EVCI measures were developed using constructs related to vaccine confidence identified by the U.S. National Vaccine Advisory Committee (i.e., “Information Environment”, “Trust”, “Healthcare Provider”, “Attitudes and Beliefs”, and “Social Norms”). Reliability for EVCI was assessed using Cronbach’s alpha. Using the variables related to each of the constructs, we calculated an overall EVCI score that was then assessed against self-reported childhood vaccine receipt using chi-square and the Cochrane-Armitage trend tests.
Results:
Respondents’ EVCI scores could range from 0 to 24, and the full range of values was observed in this sample (Mean = 17.5 (SD 4.8)). EVCI scores were significantly different (p ≤ 0.006 for all comparisons) between parents who indicated their child(ren) received routinely recommended vaccines compared with parents who indicated they had delayed or declined recommended immunizations. There was also a significant, consistent association between higher EVCI scores and greater reported vaccine receipt.
Conclusions:
We developed EVCI to reliably measure parental vaccine confidence, with individuals’ scores linked to parental vaccine-related attitudes, intentions, and behaviors. As such, EVCI may be a useful tool for future monitoring of both population and individual confidence in childhood immunization.
Keywords: Vaccine confidence, Vaccine measurement, Vaccine index, Immunization coverage, Immunization assessment
1. Introduction
Childhood vaccination coverage in the United States (U.S.) has been consistently high in recent years [1–3]. However, prior research has identified early vaccine delay and/or refusal that may leave young children susceptible to infectious diseases [4,5]. Recent findings indicate variability in parental attitudes may link to their confidence in vaccines, perceived immunization need, social norms, and message influences [6–9]. Although national childhood vaccination coverage remains at a level sufficient to mitigate sustained large-scale outbreaks, decreases in confidence in vaccination may lead to drops in childhood immunization rates that could threaten the herd immunity built up by public health efforts over the last few decades [10–14]. The ongoing burden of vaccine preventable diseases (VPDs) is a reminder of the continued importance of vaccination and the need to be attuned to parental confidence in recommended childhood immunization [15–17].
A 2015 report by the U.S. National Vaccine Advisory Committee (NVAC) defined vaccine confidence as “the trust that parents or health-care providers have (1) in the immunizations recommended by the Advisory Committee on Immunization Practices (ACIP), (2) in the provider(s) who administer(s) vaccines, and (3) in the processes that lead to vaccine licensure and the recommended vaccination schedule” [18]. The same report identified a set of domains often found to be correlates of vaccine confidence and recommended the creation of an index that would enable reliable and helpful regular measurement of confidence at a population and/or individual level [18]. The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) Working Group on Vaccine Hesitancy and others have articulated that confidence, convenience (access), and complacency are core domains associated with hesitance and immunization decision-making [19–21].
Since 2010, there have been cross-disciplinary and collaborative efforts in the measurement of vaccine confidence and the development of indices [19,21–23]. Public trust in healthcare and institutions such as government, non-governmental organizations (NGOs), media, and business have already resulted in proprietary “trust-in-systems” indices widely used by decision-makers in business, government, and other economic sectors [24]. Within the vaccine confidence field, a confidence scale was developed using questions from the 2010 National Immunization Survey (NIS) – Teen and it was evaluated using data from the 2011 NIS. This study indicated that both an eight-item and four-item measure held promise for measuring parental vaccine confidence with an adolescent population, but those questions have not been included on more recent iterations of this survey [25].
Another recently developed and utilized scale, the Parental Attitudes about Childhood Vaccines (PACV) survey, measured vaccine attitude and belief-related constructs related to vaccine hesitancy among parents of children 19–35 months of age (e.g., Overall, how hesitant about childhood shots would you consider yourself to be?) [26–29]. Yet, vaccine confidence is not the same as vaccine hesitancy; as confidence increases, hesitancy presumably decreases. Vaccine confidence encompasses components of trust, faith, and a lack of worry and concern about vaccine- and health-related outcomes, whereas vaccine hesitancy is associated with reluctance, doubt, and willingness to undertake a recommended action. Our findings have previously found support for the inverse relationship between vaccine hesitancy and confidence [29], and highlighted the need to develop indices aligned with NVAC recommendations. Arguably, a vaccine confidence index (VCI) should go beyond measuring traditional attitudes about vaccines (e.g., safety, effectiveness) and include a broader assessment of trust and confidence in the vaccination system. Just as trust in physicians is a key component of confidence in physicians and medical care [30], trust in the major institutions involved in the immunization system is a key component of vaccine confidence.
We conducted this study to initiate development and evaluation of a set of comprehensive and efficient measures of the influences or levers that could help predict the acceptance or receipt of immunization reported by U.S. parents. Global efforts are underway to measure vaccine confidence, trust, and hesitancy, and this study contributes to the broader collaboration within the field [19,21,22,25–29,31,32]. In this case, it does so by uniquely focusing on the trust and confidence that U.S. parents place in people and processes around vaccination. The Emory VCI (EVCI) measures were developed to offer potential research, clinical, and surveillance tools for assessment of parental vaccine confidence as well as to identify shifts in attitudes and behaviors.
2. Methods
2.1. Study design and sample
During the fall of 2016, we assessed the draft EVCI using a survey with U.S. parents drawn from market research panels aggregated by Qualtrics (Provo, Utah). Qualtrics employs methodologies to create an overall panel that approximates the adult U.S. population; further details are available in published materials [33]. Our sampling frame included English speaking, non-institutionalized parents/guardians aged ≥18 years living in the U.S. with child(ren) aged <7 years. Panel[notdef]members meeting inclusion criteria were randomly selected to receive an email invitation to participate.
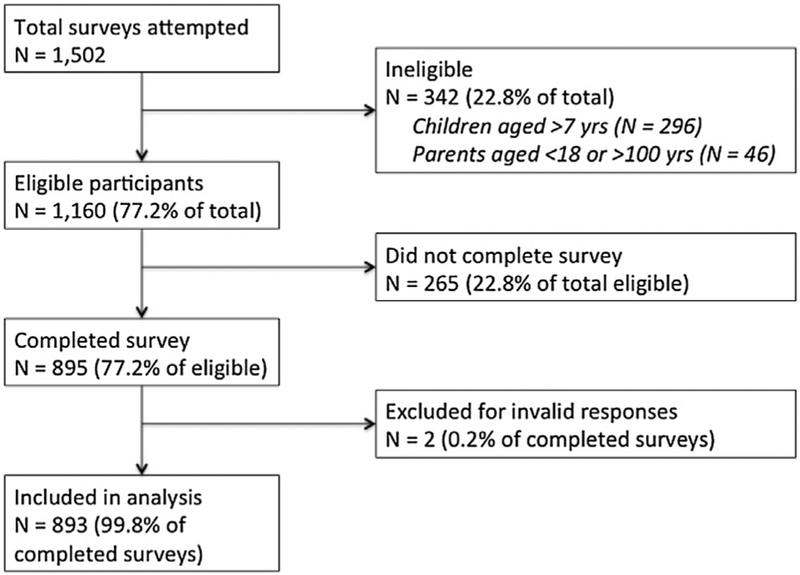
Participants received a $0.50 cash-equivalent incentive for participation in the survey (N = 114 survey items). The survey was comprehensive and included items beyond those assessed in this study, including community opinion leadership, personal values, media consumption, and provider relationship issues. Our overall response rate was 59.5% (893 valid responses/1502 attempts). Participation is described in Fig. 1. This study was reviewed by the Emory University Institutional Review Board and determined to be exempt from human subjects’ research.
Fig. 1.
Participant flow diagram.
2.2. Measurement
2.2.1. Endpoint assessment
The survey was designed to assess confidence-related constructs and the childhood vaccination decisions of parents of children <7 years, as these children are primarily affected by the Centers for Disease Control and Prevention’s (CDC) recommended immunization schedule for infants and children, established by the Advisory Committee on Immunization Practices (ACIP) and school entry immunization policies [34,35]. To assess vaccination decisions, we asked respondents if their youngest child had received all doses of recommended vaccines, specifically: Hepatitis B (Hep B), Rotavirus (RV), Diphtheria, Tetanus, and acellular Pertussis (DTaP), Haemophilus influenzae type b (Hib), Pneumococcal (PCV), Inactivated Poliovirus (IPV), Influenza (Flu), Measles, Mumps, Rubella (MMR), Varicella (chickenpox), and Hepatitis A (Hep A). Although many children likely received specific vaccines in combination, we separately assessed them as described; the survey did not include combination vaccine formulations (e.g., DTaPHib-IPV, MMR-V).
2.2.2. Independent variables
Participant sociodemographic data (e.g., insurance type, child age) are reflected in Table 2. Using the NVAC definition of “vaccine confidence” as a framework, we developed a matrix that enabled us to classify 30 individual survey items with regard to the NVAC definition of vaccine confidence and the five key component influences that NVAC identified as associated with confidence. Thus, survey items were classified as they corresponded to the constructs “Information Environment”, “Trust”, “Healthcare Provider”, “Attitudes and Beliefs”, and “Social Norms” (Supplementary Table 1). Because items had different response options, we recoded each item to a four-level outcome for consistent comparison (Supplementary Table 2). The four levels were: (1) Lowest agreement/Lowest trust/Strongest disagreement (score = 0); (2) Don’t know/Don’t Use/Neutral (score = 1); (3) Moderate agreement/Moderate trust (score = 2); and (4) Strongest agreement/Highest trust (score = 3).
Table 2.
Sociodemographic characteristics of survey respondents.
| N | % | ||
|---|---|---|---|
| Parent gender | Female | 732 | 82.0 |
| Male | 161 | 18.0 | |
| Race | White | 726 | 81.3 |
| Black/African-American | 106 | 11.9 | |
| American Indian | 27 | 3.0 | |
| Asian | 40 | 1.5 | |
| Native Hawaiian/Pacific Islander | 3 | 0.3 | |
| Other | 25 | 2.8 | |
| Ethnicity | Hispanic | 109 | 12.2 |
| Not Hispanic | 784 | 87.8 | |
| Number of children under 18 in the household | 1 | 384 | 43.0 |
| 2 | 313 | 35.1 | |
| 3 | 132 | 14.8 | |
| 4 | 47 | 5.3 | |
| 5 or more | 17 | 1.9 | |
| Parental health insurance | Private insurance plan | 443 | 49.6 |
| Medicare | 100 | 11.2 | |
| Medicaid | 258 | 28.9 | |
| Veterans health insurance | 22 | 2.4 | |
| Other government health care program | 69 | 7.7 | |
| No health insurance | 62 | 6.9 | |
| Don’t know | 9 | 1.0 | |
| Highest level of education completed by parent | Less than high school graduate | 26 | 2.9 |
| High school graduate/GED | 170 | 19.0 | |
| Some college but no degree | 188 | 21.1 | |
| Technical/vocational or Associate’s degree | 165 | 18.5 | |
| Bachelor’s degree | 237 | 26.5 | |
| Graduate degree | 107 | 12.0 | |
| Region of residence | Midwest | 197 | 22.1 |
| Northeast | 179 | 20.0 | |
| South | 366 | 41.0 | |
| West | 143 | 16.0 | |
| US Territory | 8 | 0.9 | |
| Parent age (years) | Mean [standard deviation] | 31.3 [6.7] | |
| Median [Q1, Q3] | 30 [26, 35] | ||
| Child’s age (years) | Mean [standard deviation] | 2.8 [1.7] | |
| Median [Q1, Q3] | 2.8 [1.4, 4.3] |
2.3. Statistical analysis
2.3.1. Exploratory factor analyses
We grouped the individual survey items using factor analysis. Alpha coefficients were computed within the individual factors and across the full group of variables. This process enabled our analytic team to determine which items or variables could be removed to increase alpha or minimally reduce alpha while increasing parsimony. After reducing the number of EVCI items from 30 to 17 based on alpha coefficients, we computed the coefficient of variation based on the mean and variation for each variable to identify variables with the highest variability, and retained nine variables in the matrix with a coefficient of variation greater than 25%.
Among the remaining nine variables, we compared the mean variable-specific score between parents who reported their child did or did not receive each vaccine, and we retained six variables with a between-group difference of 0.25 for assessment of at least 6 of the 9 vaccines. When comparing the final variable list to the NVAC vaccine confidence definition, it was noted that no construct related to healthcare providers remained in the reduced scale. To ensure that the resulting EVCI remained aligned with the NVAC vaccine confidence definition, we retained the variables addressing confidence in healthcare providers, resulting in a final 8-item index.
Respondents’ overall EVCI score was calculated by summing scores across each collapsed variable, and could range from 0 (i.e., no confidence) to 24 (i.e., complete confidence). To help assess the EVCI’s validity, we used ANOVA to compare differences in mean summary scores by self-reported vaccine receipt, excluding responses from parents who reported “Don’t Know.” Overall scores were also stratified into a three-level categorical variable: 0–12 (corresponding to an average of 0–1.5 on all EVCI questions and reflecting little or no confidence), 13–20 (corresponding to an average of greater than 1.5–2.5 on all EVCI questions and reflecting moderate confidence), and 21–24 (corresponding to an average of greater than 2.5–3 on all EVCI questions and reflecting high confidence).
2.3.2. Internal consistency
Initially, 30 items representing five constructs were included in the EVCI (alpha = 0.926); the initial reduction of the EVCI to 17 items resulted in an index (alpha = 0.918). Additional items were removed following assessment of coefficients of variation (which resulted in retaining nine variables with alpha = 0.855) and mean differences across self-reported vaccination receipt (which resulted in retaining six variables with alpha = 0.826). The final eight core item index resulted in alpha = 0.857 (Table 1).
Table 1.
Emory Vaccine Confidence Index (EVCI) (N = 8 items) and associated vaccine confidence domains.
| Aspect of vaccine confidence domain addressed | EVCI question stem | EVCI question | Answer options |
|---|---|---|---|
| Processes that lead to vaccine licensure and the recommended vaccination schedule | Please rate your level of trust in each of the following items | 1. Scientists involved in developing and testing new vaccines 2. Federal government agencies responsible for monitoring the safety of recommended childhood vaccines 3. Centers for Disease Control and Prevention (CDC), the federal government agency that makes recommendations about who should get licensed vaccines 4. Food & Drug Administration (FDA), the federal government agency that licenses vaccines |
No Trust Little Trust Moderate Trust Mostly Trust Complete Trust Don’t Know Don’t Use |
| The immunizations recommended by the ACIP | Please indicate your level of confidence in each item below about childhood vaccines for children aged 0 to 6 years | 5. Vaccines recommended for young children are safe | I don’t know Not Confident at all Somewhat confident Confident Mostly Confident Very Confident |
| Please indicate how strongly you agree with the following statements | 6. It is important for everyone to get the recommended vaccines for their child(ren) | Strongly disagree Disagree Neutral Agree Strongly Agree |
|
| The provider(s) who administer(s) vaccines | Please indicate your level of confidence in each item below about childhood vaccines for children aged 0–6 years | 7. My doctor or nurse is a trustworthy source for vaccine information 8. My doctor or nurse has my child(ren)’s best interest in mind when making vaccine recommendations |
I don’t know Not Confident at all Somewhat confident Confident Mostly Confident Very Confident |
2.3.3. Predictive validity testing
Predictive validity was assessed by testing the association among the EVCI scores with self-reported vaccination responses. Self-reported childhood vaccine receipt was compared across this categorical vaccine confidence score using the Fisher’s exact test as well as the Cochrane-Armitage test for trend. For each vaccine, we conducted logistic regression to estimate the increase in odds of reporting that a child received the vaccine for a one-point increase in EVCI summary score, adjusted for available demographic characteristics (parental age, race, ethnicity, income, education, number of children in the household, and rural/suburban/urban residence location).
3. Results
One goal was to create a confidence index that was understandable, time efficient, and usable in different settings (e.g., surveillance, clinical, research). On average, participants spent 8.1 min completing the 114 item survey (inclusive of sociodemographics), with a mean time of 4.3 s per question. Accounting for any extra time needed (e.g., clinician distraction), the final proposed eight-item scale should take less than one minute to complete; it has a grade 7.7 reading level according to the Flesch-Kincaid readability test, and has a Flesch reading ease score of 61.4.
3.1. Sociodemographic characteristics
Respondent demographics are shown in Table 2. The majority of respondents were female, between ages 25–44 years, non-Hispanic white, and had completed some college or more.
3.2. EVCI score and completeness of vaccination
EVCI scores in this sample ranged the entire scale from 0 to 24 (Mean = 17.5 (SD = 4.8); median 18). Children of parents in the lowest vaccine confidence category (EVCI score 0–12) had the lowest level of reported coverage for the routinely recommended vaccines. In addition, reported uptake of each vaccine increased with increasing EVCI category (Table 3). For each vaccine, the Cochrane-Armitage test for trend estimated statistically significant increases in vaccine receipt with higher EVCI score category. Further, for each one-point increase in EVCI score, the odds of parental report of their child receiving a vaccine increased by approximately 10–15% (Table 4). The results from these fully adjusted models were similar to those from unadjusted logistic regression models. Finally, a table highlights resulting EVCI items compared to other vaccine trust measures (Table 5).
Table 3.
Percentage of parents in each EVCI level who reported their child received the vaccine.
| 0–12 (Low) (N = 118) |
13–20 (Medium) (N = 531) |
21–24(High) (N = 244) |
Chi–square p-value |
Cochran-Armitage Trend Test p-value | |
|---|---|---|---|---|---|
| DTaP | 81.8 | 94.3 | 96.6 | <0.0001 | <0.0001 |
| Polio | 79.0 | 92.8 | 94.4 | <0.0001 | <0.0001 |
| Hepatitis A | 74.3 | 87.3 | 87.1 | 0.0027 | 0.0047 |
| Hepatitis B | 80.4 | 91.0 | 91.5 | 0.0037 | 0.0038 |
| Haemophilus influenzae type b | 71.3 | 86.9 | 91.3 | <0.0001 | <0.0001 |
| Rotavirus | 73.1 | 90.6 | 91.4 | <0.0001 | <0.0001 |
| MMR | 78.4 | 90.3 | 93.6 | <0.0001 | 0.0003 |
| Pneumococcal | 61.7 | 77.2 | 83.0 | 0.0002 | <0.0001 |
| Varicella | 64.0 | 83.1 | 87.6 | <0.0001 | <0.0001 |
Table 4.
Odds ratio of increased likelihood of parental report that child received vaccination for each 1 point increase in EVCI.
| Odds ratio (95% CI)* |
Unadjusted odds ratio (95% CI) |
|
|---|---|---|
| DTaP | 1.14 (1.09, 1.20) | 1.14 (1.09, 1.19) |
| Polio | 1.12 (1.07, 1.17) | 1.12 (1.07, 1.17) |
| Hepatitis A | 1.09 (1.05, 1.14) | 1.08 (1.04, 1.12) |
| Hepatitis B | 1.11 (1.06, 1.16) | 1.08 (1.03, 1.12) |
| Haemophilus influenzae type b | 1.13 (1.08, 1.18) | 1.11 (1.06, 1.15) |
| Rotavirus | 1.15 (1.10, 1.20) | 1.12 (1.07, 1.16) |
| MMR | 1.10 (1.06, 1.15) | 1.10 (1.06, 1.15) |
| Pneumococcal | 1.10 (1.06, 1.14) | 1.09 (1.05, 1.13) |
| Varicella | 1.10 (1.06, 1.14) | 1.10 (1.06, 1.14) |
Adjusted for parent’s gender, race, ethnicity, education, income, parent’s age, number of children in the household, and number of children in the household.
Table 5.
Survey component similarity across published indexes.
| Confidence/Hesitancy Measure | EVCI | PACV | VCP | VCS |
|---|---|---|---|---|
| Emory Vaccine Confidence Index (EVCI) | ||||
| Vaccines recommended for children are safe | × | ▲ | × | × |
| My doctor/nurse is a reliable source of trustworthy vaccine information | × | ▲ | ▲ | |
| My doctor/nurse has my child(ren)’s best health interest in mind when making vaccine recommendations | × | × | ||
| It is important for everyone to get the recommended vaccines for their child(ren) | × | ▲ | × | ▲ |
| Trust in: Food & Drug Administration (FDA), the federal government agency that licenses vaccines | × | |||
| Trust in: Centers for Disease Control and Prevention (CDC), the federal government agency that makes recommendations about who should get licensed vaccines | × | |||
| Trust in: Federal government agencies responsible for monitoring the safety of recommended childhood vaccines | × | |||
| Trust in: Scientists involved in developing and testing new vaccines | × | |||
| Parent Attitudes About Childhood Vaccines (PACV) [1–4] | ||||
| Have you ever delayed having your child get a shot for reasons other than illness or allergy? | × | |||
| Have you ever decided not to have your child get a shot for reasons other than illness or allergy? | × | |||
| How sure are you that following the recommended shot schedule is a good idea for your child? | ▲ | × | ▲ | |
| Do you agree with the following statement? It is my role as a parent to question shots | × | |||
| If you had another infant today, would you want him/her to get all the recommended shots? | ▲ | × | ||
| How concerned are you that your child might have a serious side effect from a shot? | ▲ | × | ▲ | × |
| How concerned are you that any one of the childhood shots might not be safe? | ▲ | × | ▲ | ▲ |
| How concerned are you that a shot might not prevent the disease? | × | ▲ | ▲ | |
| Do you know of anyone who has had a bad reaction to a shot? | × | |||
| Which of the following statements reflect your general attitude and trust towards vaccines? | × | |||
| The only reason I have my child get shots is so that they can enter day-care or school | × | |||
| I am able to openly discuss my concerns about shots with my child’s doctor | ▲ | × | ▲ | |
| All things considered, how much do you trust your child’s doctor? | ▲ | × | ||
| Vaccine Confidence Project (VCP) [5–7] | ||||
| Vaccines are important for children to have | × | × | ▲ | |
| Overall I think vaccines are safe | × | ▲ | × | × |
| Overall I think vaccines are effective | × | ▲ | ||
| Vaccines are compatible with my religious beliefs | × | |||
| Vaccination Confidence Scale (VCS) [8–10] | ||||
| Vaccines are necessary to protect the health of teenagers | ▲ | ▲ | × | |
| Vaccines do a good job in preventing the diseases they are intended to prevent | ▲ | ▲ | × | |
| Vaccines are safe | × | ▲ | × | × |
| If I do not vaccinate my teenager, he/she may get a disease such as meningitis and cause other teenagers or adults also to get the disease | × | |||
| Teenagers receive too many vaccines | × | |||
| If I vaccinate my teenager, he/she may have serious side effects | ▲ | ▲ | ▲ | × |
| In general medical professionals in charge of vaccinations have my teenager’s best interest at heart | ▲ | ▲ | × | |
| I have a good relationship with my teenager’s health care provider | ▲ | ▲ | × | |
× Although wording or populations (e.g. children vs. teenagers) may differ between surveys, factors measured and how they are measured (e.g. considering a vaccine safe vs. considering a vaccine not safe) are consistent.
▴ Similar measure was used in the vaccine confidence/hesitancy scale, but scope or how they are measured (e.g. considering a vaccine safe vs. considering a vaccine not safe)are different enough to be considered separate measures.
4. Discussion
This study supports NVAC’s recommendation for the development of a vaccine confidence index anchored in the decision-making factors the committee identified as important to vaccine acceptance [18]. Recent findings indicate that even among parents of children who are up-to-date on immunizations, broad concerns still exist about pediatric immunizations [6]. Thus, having trust in those who produce vaccines, provide vaccine oversight, and offer vaccine recommendations is important for engendering and sustaining public confidence [36]. Our index provides an early set of options towards an efficient, yet comprehensive, set of items that assess trust in the processes that lead to vaccine licensure, advisory committee recommendations and trust in oversight entities, and in those who recommend vaccines and schedules [18]. Our tool links parental confidence to vaccine uptake that, in turn, will aid in the development and provision of effective education and outreach. By using the set of EVCI tools, there is greater potential to identify varying confidence and more effectively address challenges such as vaccine hesitancy and lower immunization coverage in the future.
In this study, we developed a parsimonious, relevant eight-item index that was able to assess vaccine confidence with a highly acceptable internal validity score. This index can be understood by individuals with a middle school level of education, and takes only around one minute for parents to complete. The reduction in alpha for our final eight-item index, pared from the original 30 questions, was small and suggests the strength of the final items included is nearly matched to the expanded set of items.
This study advances the NVAC recommendation for research to create a vaccine confidence index with factors associated with vaccine confidence (e.g., trust in healthcare providers, institutions)[18]. Our index also included questions related to domains identified by previous literature such as trust, vaccine safety, immunization behavior, and information seeking [22,26,37]. While none of the initial “Information Environment” variables were retained in the final EVCI, the inclusion of two variables addressing the importance of health care providers in the immunization recommendation process underscores prior research indicating that healthcare providers are the most trusted source of vaccine information [38–41]. The next step in this research process will be to assess accuracy of self-reported immunization compared vs. record (e.g., registry, provider medical records).
In response to parental concerns in the U.S. on vaccine topics like vaccine ingredients and simultaneous delivery of multiple vaccines, we included several questions to specifically assess levels of trust in federal and scientific institutions involved in the vaccine licensure and recommendation process [42]. Recent indices have been constructed and validated in populations with very young children (under 3 years of age) or adolescents [25,28,31]. In this study, we sought to measure vaccine confidence for parents of children within a wider range of ages, who would have been eligible for early childhood as well as kindergarten vaccinations. In doing so, we hoped to provide a more comprehensive snapshot of parental confidence levels. Our work also builds upon previous international findings that identified differences in parents’ attitudes towards immunization needs and types of vaccines (childhood immunizations, seasonal and pandemic influenza, HPV vaccination, travel vaccination), indicating the need to consider vaccinations both as a group and individually [43].
The categories of vaccine confidence scores we identified correspond well with parent-reported vaccine receipt of all routinely recommended childhood vaccines, with percent of children vaccinated increasing in a stepwise fashion for each increasing confidence category. In our survey, we collected additional sociodemographic variables to be used in future analyses to meet the challenges of gauging the overall level of U.S. parental vaccine confidence, and also to identify clusters of parents with lower confidence, where they live, and how they seek healthcare for their children. By incorporating the sociodemographic variables, reports of individual vaccinations, and vaccine confidence scores, these analyses will have the potential to provide more comprehensive explanation for the adherence to childhood vaccination recommendations in the U.S.
The consistency of increased odds of self-reported vaccine receipt with increasing EVCI score, combined with similar trends in vaccine receipt across categories of EVCI, indicate the robustness of this scale in identifying vaccine confidence factors associated with vaccine receipt. Even with the limitation of parental self-report of childhood vaccination status, this consistency supports, at a minimum, the potential for greater awareness of vaccination status among parents with greater confidence in vaccines. Additionally, while the results from the adjusted and unadjusted logistic regression models were similar, adjustment for parental sociodemographics did lead, in some cases, to slightly increased odds ratios. This is in line with prior studies identifying the association of these sociodemographics with vaccine uptake [2,44–46]. While we cannot attribute all non-vaccination to lower vaccine confidence, it is possible that parents with higher EVCI scores may be more willing to seek vaccination, even in the face of logistic barriers, than those with lower EVCI scores.
Historically, there have been many reasons for low vaccine receipt or under vaccination, including lack of access to vaccination services (e.g., transportation difficulty, lack of a medical home) and the complexity of the childhood vaccine schedule [47–49]. Although the Vaccines for Children program has greatly decreased disparities in coverage among historically underserved populations, other barriers to full vaccination, such as perceptions of risk associated with vaccination and concerns about too many vaccines being administered, persist [36,50–52]. These factors, combined with the substantial size of the U.S. annual birth cohort, can influence changes in population-level immunity [14,51,53].
Although a recommendation from a clinician remains the factor most associated with vaccine uptake, not all vaccination visits allow for the time necessary for clear recommendations or in-depth conversations with parents [38,54]. In these instances, other factors influencing vaccine acceptance may increase in importance in shaping a parent’s confidence in vaccines [36,38]. Use of EVCI could provide utility for research, surveillance, and clinical needs. Further development of this instrument may be also useful to providers and policymakers to help inform continued vaccination recommendations and promotion. While vaccine coverage in the U.S. is high, it has been estimated that more than 1 in 8 parents are “fence sitters” when it comes to vaccination [37]. This population of parents may be susceptible to small perturbations in vaccine confidence, and a shift to non-vaccination among these parents could substantially decrease national vaccine coverage levels.
5. Limitations
Several sources of bias limit the ability of self-reported vaccination decisions to represent actual vaccination behavior, including recall, response, and social desirability bias. Because parents of children aged 0–7 were included in our analysis, not all vaccinations included in our analysis are age-appropriate for all children (e.g., MMR, varicella, and Hepatitis A are not scheduled until 12 months of age) [55]. We recognize that parents may not be aware of the specific vaccines to be administered at specific well-child appointments; thus, this form of recall bias may have been an issue. Additionally, we did not include brand names of vaccines, which may have negatively affected recall of some vaccines for which we typically see higher uptake (e.g., pneumococcal conjugate vaccine). We also did not directly assess systemic differences between participant demographics and that of the broader parent population of children <7 years. The study population has a higher education attainment level than most U.S. parents and is English-speaking; both factors may contribute to sampling bias. Expansion of EVCI to broader populations (e.g., pregnant women, men who have sex with men) will provide a means to mitigate these limitations moving forward.
6. Conclusions
We developed and undertook an initial assessment of vaccine confidence measures that are reliable and provide an efficient tool to measure shifts in parents’ attitudes and perceptions. This endeavor emphasizes a positive approach (predictors of vaccine acceptance) to measurement rather than a negative one (predictors of hesitancy). Further analyses of these data may provide insight on populations at risk for incomplete vaccination. Future development of the EVCI tools with immunization status validation will support public health practices to sustain high levels of early childhood immunization.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the parents and guardians who responded to our poll, the staff of Qualtrics for their support during the implementation of this study, and Chelsea S. Lutz, MPH, CPH for her critical review of the manuscript.
Source of funding
This research was supported in part by a grant from the National Vaccine Program Office (1VSRNV00000). Dr. Bednarczyk is supported by a grant (K01AI106961) from the National Institute for Allergy and Infectious Diseases, National Institutes of Health. We are grateful to Dr. Vialla Hartfield-Mendez and the staff of the Emory University Center for Faculty Development and Excellence for their support. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Department of Health and Human Services.
Footnotes
Conflicts of interest
There are no conflicts of interest by the authors.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2018.09.043.
References
- [1].Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19–35 months - United States, 2013. MMWR Morb Mortal Wkly Rep 2014;63(34):741–8. [PMC free article] [PubMed] [Google Scholar]
- [2].Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Dietz V. Vaccination coverage among children aged 19–35 months – United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65(39):1065–71. [DOI] [PubMed] [Google Scholar]
- [3].Seither R Vaccination coverage for selected vaccines, exemption rates, and provisional enrollment among children in Kindergarten—United States, 2016–17 School Year. MMWR Morb Mortal Wkly Rep 2017;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nadeau JA, Bednarczyk RA, Masawi MR, et al. Vaccinating my way—Use of alternative vaccination schedules in New York state. J Pediat 2015;166(1). 151–156.e151. [DOI] [PubMed] [Google Scholar]
- [5].Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics 2012;130(1):32–8. [DOI] [PubMed] [Google Scholar]
- [6].Kennedy A, LaVail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff 2011;30(6):1151–9. [DOI] [PubMed] [Google Scholar]
- [7].Frew PM, Fisher AK, Basket MM, et al. Changes in childhood immunization decisions in the United States: Results from 2012 & 2014 National Parental Surveys. Vaccine 2016;34(46):5689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chung Y, Schamel J, Fisher A, Frew PM. Influences on immunization decision-making among US parents of young children. Mater Child Health J December 2017;21(12):2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frew PM, Chung Y, Fisher AK, Schamel J, Basket MM. Parental experiences with vaccine information statements: Implications for timing, delivery, and parent-provider immunization communication. Vaccine 2016;34(48):5840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol 2008;168(12):1389–96. [DOI] [PubMed] [Google Scholar]
- [11].Glanz JM, McClure DL, Magid DJ, et al. Parental refusal of pertussis vaccination is associated with an increased risk of pertussis infection in children. Pediatrics Jun 2009;123(6):1446–51. [DOI] [PubMed] [Google Scholar]
- [12].Glanz JM, McClure DL, Magid DJ, Daley MF, France EK, Hambidge SJ. Parental refusal of varicella vaccination and the associated risk of varicella infection in children. Arch Pediatr Adolesc Med January 2010;164(1):66–70. [DOI] [PubMed] [Google Scholar]
- [13].Bisgard KM, Rhodes P, Connelly BL, et al. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998–2001. Pediatrics August 2005;116(2):e285–94. [DOI] [PubMed] [Google Scholar]
- [14].Bednarczyk RA, Orenstein WA, Omer SB. Estimating the number of measles-susceptible children and adolescents in the United States using data from the national immunization survey-teen (NIS-Teen). Am J Epidemiol 2016;184 (2):148–56 [DOI] [PubMed] [Google Scholar]
- [15].Measles - United States, January 1-August 24, 2013. MMWR Morb Mortal Wkly Rep September 13 2013; 62(36): p. 741–43. [PMC free article] [PubMed] [Google Scholar]
- [16].Winter K, Glaser C, Watt J, Harriman K. Pertussis epidemic - california, 2014. MMWR Morb Mortal Wkly Rep 2014;63(48):1129–32. [PMC free article] [PubMed] [Google Scholar]
- [17].Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA 2016;315(11):1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Orenstein WA, Gellin BG, Beigi RH, et al. Assessing the state of vaccine confidence in the United States: recommendations from the national vaccine advisory committee. Public Health Rep 2015;130(6):573–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Larson HJ, Schulz WS, Tucker JD, Smith DM. Measuring vaccine confidence: introducing a global vaccine confidence index. PLoS currents 2015:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine 2015;33(34):4161–4. [DOI] [PubMed] [Google Scholar]
- [21].Larson HJ, Jarrett C, Schulz WS, et al. Measuring vaccine hesitancy: the development of a survey tool. Vaccine 2015;33(34):4165–75. [DOI] [PubMed] [Google Scholar]
- [22].Gilkey MB, Magnus BE, Reiter PL, McRee AL, Dempsey AF, Brewer NT. The vaccination confidence scale: a brief measure of parents’ vaccination beliefs. Vaccine 2014;32(47):6259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Prashanth GP. Measurement of vaccine confidence using media surveillance system. Lancet Infect Dis Mar 2014;14(3):187–8. [DOI] [PubMed] [Google Scholar]
- [24].Edelman. 2018 Edelman Trust Barometer; 2018. [Google Scholar]
- [25].Gilkey MB, McRee AL, Magnus BE, Reiter PL, Dempsey AF, Brewer NT. Vaccination confidence and parental refusal/delay of early childhood vaccines. PLoS ONE 2016;11(7):e0159087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Opel DJ, Mangione-Smith R, Taylor JA, et al. Development of a survey to identify vaccine-hesitant parents: the parent attitudes about childhood vaccines survey. Human Vaccines Apr 2011;7(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Opel DJ, Taylor JA, Mangione-Smith R, et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine 2011;29(38):6598–605. [DOI] [PubMed] [Google Scholar]
- [28].Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediat Nov 2013;167(11):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oladejo O, Allen K, Amin A, Frew PM, Bednarczyk RA, Omer SB. Comparative analysis of the Parent Attitudes about Childhood Vaccines (PACV) short scale and the five categories of vaccine acceptance identified by Gust et al. Vaccine 2016;34(41):4964–8. [DOI] [PubMed] [Google Scholar]
- [30].Pearson SD, Raeke LH. Patients’ trust in physicians: many theories, few measures, and little data. J Gen Intern Med 2000;15(7):509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gilkey MB, Reiter PL, Magnus BE, McRee AL, Dempsey AF, Brewer NT. Validation of the vaccination confidence scale: a brief measure to identify parents at risk for refusing adolescent vaccines. Acad Pediat 2016;16(1): 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Larson HJ, de Figueiredo A, Xiahong Z, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMed October 2016;12:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].ESOMAR 28: 28 Questions to Help Research Buyers of Online Panels; 2014. <http://success.qualtrics.com/rs/qualtrics/images/ESOMAR%2028%202014.pdf>.
- [34].Orenstein WA, Hinman AR. The immunization system in the United States -the role of school immunization laws. Vaccine 1999;17(Suppl 3):S19–24. [DOI] [PubMed] [Google Scholar]
- [35].CDC. Recommended immunizations for children from birth through 6 years old; 2015. <http://www.cdc.gov/vaccines/parents/downloads/parent-ver-sch-0-6yrs.pdf> [accessed 28.01.16].
- [36].Smith MJ. Promoting vaccine confidence. Infect Dis Clin North Am Dec 2015;29(4):759–69. [DOI] [PubMed] [Google Scholar]
- [37].Gust D, Brown C, Sheedy K, Hibbs B, Weaver D, Nowak G. Immunization attitudes and beliefs among parents: beyond a dichotomous perspective. Am J Health Behav 2005;29(1):81–92. [DOI] [PubMed] [Google Scholar]
- [38].Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine 2016;34(52):6700–6. [DOI] [PubMed] [Google Scholar]
- [39].Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. Association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics Nov 2006;118(5):e1287–92. [DOI] [PubMed] [Google Scholar]
- [40].Connors JT, Slotwinski KL, Hodges EA. Provider-parent communication when discussing vaccines: a systematic review. J Pediatr NursMar 2017;33:10–5. [DOI] [PubMed] [Google Scholar]
- [41].Williams SE. What are the factors that contribute to parental vaccinehesitancy and what can we do about it? Human Vaccines Immunotherapeut 2014;10(9):2584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sobo EJ, Huhn A, Sannwald A, Thurman L. Information curation among vaccine cautious parents: Web 2.0, pinterest thinking, and pediatric vaccination choice. Med Anthropol 2016;35(6):529–46. [DOI] [PubMed] [Google Scholar]
- [43].Velan B, Boyko V, Lerner-Geva L, Ziv A, Yagar Y, Kaplan G. Individualism, acceptance and differentiation as attitude traits in the public’s response to vaccination. Human Vaccines Immunotherapeut 2012;8(9):1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen W, Elam-Evans LD, Hill HA, Yankey D. Employment and socioeconomic factors associated with children’s up-to-date vaccination status. Clin Pediatr 2017;56(4):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weinberg M, Dietz S, Potter R, Swanson R, Miller C, McFadden J. Vaccine shot-limiting: estimating the prevalence, indicators, and impact on vaccination status - Michigan, 2012. Vaccine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smith PJ, Chu SY, Barker LE. Children who have received no vaccines: who are they and where do they live? Pediatrics 2004;114(1):187–95. [DOI] [PubMed] [Google Scholar]
- [47].Hinman AR. What will it take to fully protect all American children with vaccines? Am J Dis Children (1960) 1991;145(5):559–62. [DOI] [PubMed] [Google Scholar]
- [48].Copeland VC. Immunization among African American children: implications for social work. Health Soc Work 1996;21(2):105–14. [DOI] [PubMed] [Google Scholar]
- [49].Smith PJ, Molinari NA, Rodewald LE. Underinsurance and pediatric immunization delivery in the United States. Pediatrics 2009;124(Suppl 5): S507–14. [DOI] [PubMed] [Google Scholar]
- [50].Niederhauser VP. Measuring parental barriers to childhood immunizations: the development and validation of the searching for hardships and obstacles to shots (SHOTS) instrument. J Nurs Meas 2010;18(1):26–35. [DOI] [PubMed] [Google Scholar]
- [51].Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine 2015;33(Suppl 4):D66–71. [DOI] [PubMed] [Google Scholar]
- [52].Walsh B, Doherty E, O’Neill C. Since the start of the vaccines for children program, uptake has increased, and most disparities have decreased. Health Affairs (Project Hope) 2016;35(2):356–64. [DOI] [PubMed] [Google Scholar]
- [53].Childs L, Bednarczyk RA. Estimating pertussis susceptibility among 0–23-month-old children in the United States. Using national immunization survey (NIS) 2013. Pediatr Infect Dis J 2017. [DOI] [PubMed] [Google Scholar]
- [54].Kempe A, O’Leary ST, Kennedy A, et al. Physician response to parental requests to spread out the recommended vaccine schedule. Pediatrics 2015;135(4):666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Robinson CL. Advisory committee on immunization practices recommended immunization schedules for persons aged 0 through 18 years-United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65(4):86–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.