Abstract
The AAA+ ATPase, Thorase, plays a critical role in controlling synaptic plasticity, learning and memory through regulating the expression of surface α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPAR). Here, by sequencing a cohort of schizophrenia patients we identify Thorase rare variants (TRV) that cause defects in glutamatergic signaling through impairment in AMPAR internalization, recycling and function. Internalization of AMPAR is significantly reduced in neurons expressing TRVs, leading to increased surface expression of the AMPAR subunit GluA2 and enhanced synaptic transmission. Furthermore, these defects lead to mouse behavioral deficits in schizophrenia-like models and importantly these impairments are rescued by the competitive AMPAR antagonist, perampanel. These findings suggest that Thorase could be an important mediator of symptoms associated with schizophrenia-like behaviors, and support the FDA approved AMPAR antagonist perampanel as a potential treatment strategy for patients with compromised AMPAR-mediated glutamatergic neurotransmission.
SUMMARY
Rare Thorase variants increase AMPAR surface expression resulting in schizophrenialike behavior that is normalized by the antagonist, perampanel.
INTRODUCTION
ATAD1 encodes a protein we recently identified as Thorase, an AAA+ ATPase, that plays a critical role in regulating the surface expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Thorase controls AMPA receptor endocytosis and internalization of AMPA receptors via disassembly of the AMPA receptor/glutamate receptor interacting protein 1 (GRIP1) complex to regulate synaptic plasticity, learning and memory (1). Altered glutamate signaling has recently been implicated as an underlying genetic contributor to schizophrenia (2–7).
The gene encoding Thorase, ATAD1, is located on chromosome 10q23.31 (89,512,875 – 89,552512 bp) in a region where our group has previously reported a strong linkage peak for schizophrenia in an Ashkenazi Jewish (AJ) population (8). This region has been replicated in other independent studies (9) and also linked with bipolar disorder (10). For this reason we hypothesized that a good strategy to increase the probability of identifying functional variants in the ATAD1 gene would be to explore variants found in schizophrenic patients. To do this we utilized our cohort of 712 schizophrenia cases and 649 controls of Ashkenazi Jewish origin.
RESULTS
We obtained bidirectional sequence of the ATAD1 coding exons in all cases and controls and found three rare coding variants, all missense changes, in five individuals (Table S1 and fig. S1A). The variants R9H and D221H were present only in schizophrenia cases, R9H in a female and the D221H in a male. Another variant E290K was present in two male schizophrenic cases and one female control. Two of these variants, R9H and E290K are seen with frequencies 8.4 × 10−7, 0 and 0.0001.1 ×10−4 in the exac database, which includes exomes of largely unscreened individuals. While results on such small sample sizes do not provide proof that these variants increase risk for psychiatric disease, we considered that our strategy increases the odds that they are functional and proceeded to examine their potential impact on the function of ATAD1.
Thorase mutants have no defects in ATPase activity
The ATPase activity of Thorase wild type and variants (R9H, D221H and E290K) was evaluated using purified recombinant His6 C-terminal tagged proteins (fig. S1). Interestingly, Thorase E290K is not recognized by the anti-Thorase antibody, suggesting that the E290K variant in Thorase alters the epitope (fig. S1, B and C). Circular dichroism (CD) analyses suggest that there is no significant change in the structure of the R9H variant (fig. S1D). However, both D221H and E290K show significant conformational changes. Both ATP binding (fig. S1E) and ATP hydrolysis (fig. S1, G and H) assays suggest that none of the variants affects ATPase activity.
Thorase D221H and E290K have defects in disassembly
Most AAA+ ATPase family members form oligomers, which are critical in assembling and disassembling protein complexes (11). To determine whether Thorase oligomerizes upon ATP binding, glutaraldehyde cross-linking of purified Thorase was examined in the presence of either ADP or ATP. The majority of ADP bound Thorase are dimers, whereas ATP bound Thorase forms large oligomeric complexes of molecular weights greater than 250 kDa (Fig. 1A). Upon ATP hydrolysis, the large Thorase oligomeric complexes disassemble into dimers. Thorase wild type and variants all form large oligomeric complexes. However, Thorase D221H and E290K variants show defects in disassembly (Fig. 1B). Since Thorase regulates AMPA receptor (AMPAR) trafficking, the interaction of the Thorase variants with the AMPAR subunit GluA2, and glutamate receptor interacting protein (GRIP1) were evaluated in neurons (Fig. 1, C and D) and by recombinant protein pull downs (fig. S2, A to F). Thorase R9H binds to GluA2 and GRIP1 similar to wild type however, D221H and E290K display defective binding. In the presence of ATP, Thorase wild type and R9H disassemble GRIP1 from GluA2, whereas Thorase D221H and E290K disassembly is significantly impaired (Fig. 1, E to G and fig. S2, G to J).
Figure 1. Thorase variants have defects in Thorase oligomer and GluA2-GRIP1 complex disassembly.
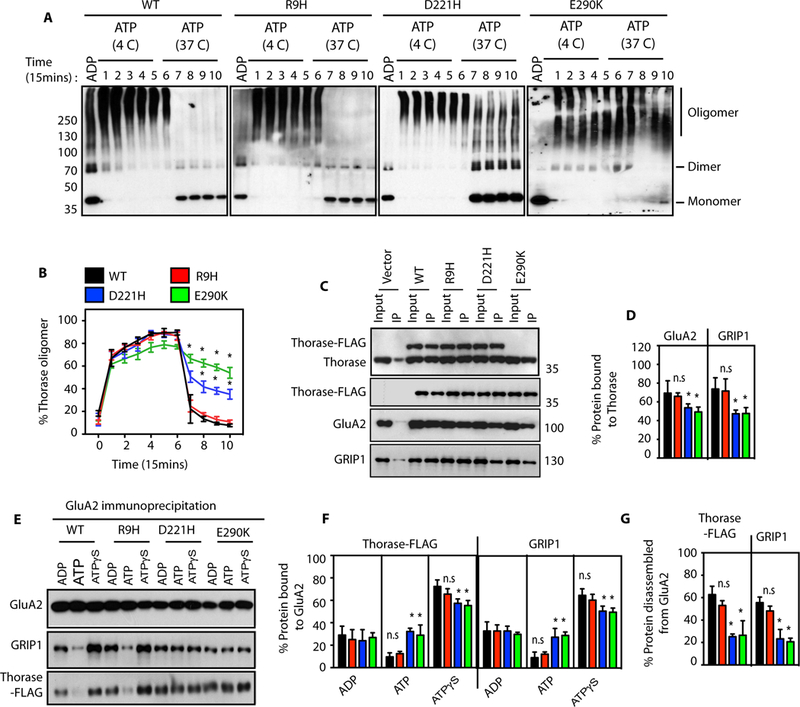
(A) Immunoblot analyses of Thorase wild type (WT) and variants oligomer formation. The samples were cross-linked by glutaraldehyde in the presence of 1 mM ADP or 1 mM ATP. The ATP treated samples were incubated at 4 °C (4 C) for Thorase oligomer formation and then at 37 °C (37 C) for ATP hydrolysis to disassembly Thorase oligomer. Samples were collected every 15 min over 150 min (lanes 1–10) during the incubation for cross-linking. (B) Graphical representation of the percentages of the oligomer states of Thorase at different times. (C) Immunoblot analyses of FLAG tagged Thorase immunoprecipitation (IP) from Thorase-heterozygous cortical neurons expressing FLAG tagged Thorase WT or variants in the presence of different nucleotides. The samples were resolved on 10% SDS-PAGE and immunoblotted with anti-Thorase, anti-FLAG-HRP, anti-GluA2 and anti-GRIP1 antibodies. (D) Normalized percent bound GluA2 and GRIP1 in the Thorase-FLAG IP samples for (C). (E) Immunoblot analyses of GluA2 IP from Thorase-heterozygous cortical neurons expressing FLAG tagged Thorase WT or variants in the presence of different nucleotides. The samples were separated on 10% SDS-PAGE and immunoblotted with anti-Flag, anti-GluA2 and anti-GRIP1 antibodies. (F) Normalized percent bound Thorase and GRIP1 in the GluA2 IP samples for (E). (G) The percent of Thorase and GRIP1 disassembled from the GluA2 complex upon ATP hydrolysis in (E). (mean ± standard error of the mean [SEM], n = 3, **p < 0.05, *p < 0.10, n.s. p > 0.10, Holm-Sidak post-hoc test compared with WT, Power: 1-β err prob = 0.8–1.0).
Schizophrenia-linked mutations in Thorase cause reduced endocytosis of GluA2
Several lines of evidence from neurodevelopmental, neuropathological, genetic, and behavioral pharmacological data indicate that AMPAR-mediated neurotransmission is compromised in patients with schizophrenia (12). Altered levels of AMPAR subunit, GluA2 in the prefrontal cortex (PFC) (3, 5) as well as the nucleus accumbens (4) have been reported in schizophrenia cases. We therefore examined the surface expression of GluA2 in neurons expressing the Thorase variants. There are increased levels of surface expression of GluA2 in Thorase D221H or E290K expressing neurons as compared to Thorase wild type or R9H expressing neurons (Fig. 2 A to C). This was further confirmed by surface protein bis(sulfosuccinimidyl)-suberate (BS3) crosslinking (Fig. 2, D and E) and biotinylation assays (fig. S3A). In response to N-Methyl-D-aspartate (NMDA) and glycine, there is a significant internalization of surface GluA2 in Thorase wild type neurons, while neurons expressing all three Thorase variants display significantly less internalization of surface GluA2.
Figure 2. Variants in Thorase cause reduced endocytosis and impaired trafficking of GluA2.
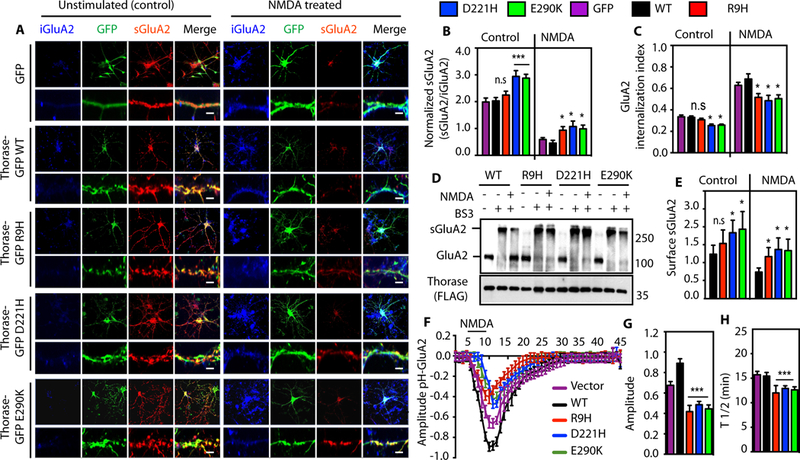
(A) Representative images of unstimulated or NMDA-induced endocytosis of GluA2 in hippocampal neurons expressing GFP, Thorase-GFP wild type (WT) or variants. Lower panels are high-resolution images with scale bar = 5 μm. (B) Quantification of the ratio of surface GluA2 (sGluA2) to internalized GluA2 (iGluA2) for (A). (C) GluA2 internalization index shown in (A-B) as measured as the ratio of iGluA2 to the total GluA2 (iGluA2 plus sGluA2) fluorescence intensities. (D) Immunoblot analyses of BS3-crosslinking of sGluA2 in Thorase-heterozygous cortical neurons expressing Thorase-FLAG WT or variants. The samples were separated on 4–12% gradient SDS-PAGE and immunoblotted with anti-GluA2 and anti-FLAG. (E) The optical densitometry quantification of sGluA2 for (D) (mean ± standard error of the mean [SEM], n = 3, **p < 0.05, *p < 0.10, n.s p > 0.10, ANOVA with Holm-Sidak post-hoc test when compared with WT, Power: 1-β err prob = 0.8 – 1.0). (F) Time trace of surface pH-GluA2 fluorescence change in neurons in response to NMDA treatment. (G) Maximum amplitudes of pH-GluA2 fluorescence intensity changes to NMDA stimulation. (H) Average recycling half-time (T½, the time taken from maximum endocytosis to 50% recycling) (mean ± SEM, n = 7, **p < 0.05, n.s p > 0.10, ANOVA with Holm-Sidak posthoc test when compared with WT, Power: 1-β err prob = 1.0).
To further evaluate the effects of the variants in Thorase on AMPAR trafficking pH-sensitive GFP (pHluorin) fused at the N-terminal extracellular domain of GluA2 was used to examine AMPAR distribution at the extracellular surface as previously described (1, 13). In Thorase variant neurons, NMDA stimulation leads to a significantly reduced internalization of GluA2, and recovery is significantly faster than in wild type neurons (Fig. 2, F to H and fig. S3B). These results suggest the identified variants in Thorase disrupt AMPAR internalization and trafficking. Recently, we showed that Thorase is also part of the mitochondrial protein quality control system and thereby plays an important role in maintaining mitochondrial integrity (14). The results of the function of mitochondria assessed in cells expressing the Thorase variants suggest that the variants have no significant effects on mitochondrial function (fig. S3, C and D).
Increased frequency and amplitude of sEPSCs and mEPSCs with schizophrenia-linked mutations in Thorase
To study the physiologic and behavioral consequences of the schizophrenia-linked Thorase variants, adenoviruses (AAV2) expressing GFP or FLAG tagged Thorase wild type or variants were delivered into postnatal day two Thorase heterozygous mice by intracerebroventricular injection (15, 16).Immunostaining of brain sections from mice expressing AAV2-Thorase-FLAG indicate expression of wild type or variant Thorase-FLAG throughout the mature brain (fig. S4, A to B). Immunoblot analysis reveals similar expression levels of wild type or variant Thorase-FLAG compared to endogenous Thorase (fig. S4, C and D). Several lines of clinical evidence suggest that behavioral core symptoms like reduced social drive or deficits in learning and memory, commonly observed in psychiatric disorders such as schizophrenia and autism, arise from a dysfunctional prefrontal cortex (PFC) (17, 18). We therefore performed ex vivo electrophysiology from mice in deep-layer prelimbic (PL) pyramidal neurons within the murine PFC due to evolutionary homology with the human PFC (19). The Thorase variants do not alter intrinsic excitability of PL pyramidal neurons (fig. S5, A to C). However, Thorase variant mice show an increase in both frequency and amplitude of spontaneous glutamate-mediated excitatory post-synaptic currents (sEPSCs) (Fig. 3, A to E) but sEPSCs rise and decay times do not differ between groups (fig. S5, D and E). We observe an increase in both amplitude and frequency of glutamate-mediated miniature excitatory post-synaptic currents (mEPSCs) from the D221H and E290K Thorase variant mice (Fig. 3, F to J). No changes are detected on the level of rise and decay times analysis of all groups (fig. S5, F and G).
Figure 3. Increased frequency and amplitude of sEPSCs and mEPSCs with schizophrenia-linked variants in Thorase.
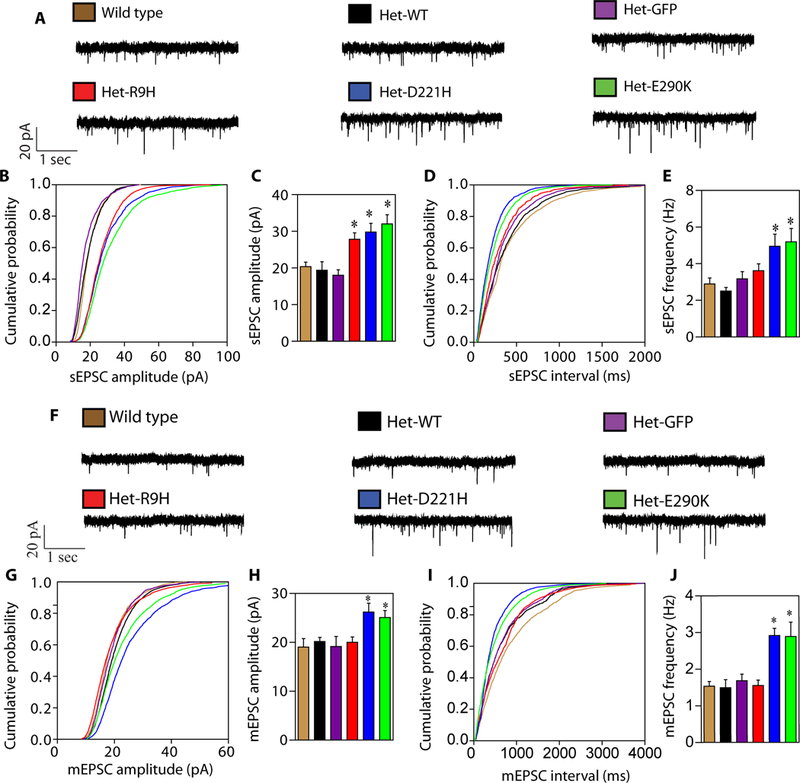
(A) Representative traces of sEPSCs recorded in control and experimental groups. (B-E) Mean cumulative probability distributions for sEPSC amplitude (B) and frequency (D) for cells recorded in the prelimbic portion of the mPFC from deep layers pyramidal neurons in brain slices from controls and Thorase variant mice. Mean sEPSC amplitude (C) and frequency (E) (mean ± SEM, n ≥ 5, **p < 0.05, n.s p > 0.10, ANOVA with Holm-Sidak post-hoc test when compared with wild type, Power: 1-β err prob = 0.8–1.0). (F) Representative traces of mEPSCs recorded in control and experimental groups. (G-J) Mean cumulative probability distributions for mEPSC amplitude (G) and frequency (I) for cells recorded in the prelimbic portion of the mPFC from deep layers pyramidal neurons in brain slices from controls and Thorase variant mice. Mean mEPSC amplitude (H) and frequency (J). (mean ± SEM, n ≥ 4, **p < 0.05, n.s p > 0.10, ANOVA with Holm-Sidak post-hoc test when compared with wild type, Power: 1-β err prob = 0.8).
The increase in mEPSC amplitude and frequency in the pyramidal neurons expressing D221H and E290K Thorase variants may suggest a more efficient unitary conductance (20) or a higher density of AMPARs at the synapses (21) compared to control neurons. The latter scenario is consistent with biochemical and immunostaining data, suggesting an increased surface expression of AMPARs in those neurons. Increased glutamate-mediated sEPSCs observed in all three Thorase variants might suggest a dysfunctional organization of glutamatergic synaptic transmission, which becomes evident when action potential dependent synaptic transmission is intact. The observed condition can be due to a cell autonomous adaptation or via a more broad network effect. Mechanistically, given that all three variants display a defect in NMDA receptor-mediated endocytosis of AMPARs it is plausible to speculate that the observed increased in amplitude and frequency of sEPSCs is a direct consequence of this aberrant cellular mechanism. The alterations of synaptic plasticity in the Thorase variants were further confirmed by increased long-term potentiation (LTP) and elimination of long-term depression (LTD) (fig. S6, A to F). Overall, these electrophysiological results strongly support the notion that a single point mutation occurring in the Thorase protein is capable of affecting the recycling rate of AMPARs at synapses, thus affecting excitatory synaptic transmission by playing a critical role in regulating the surface expression of AMPARs.
Behavioral changes and psychostimulant hypersensitivity in schizophrenia Thorase mutant mice
To determine whether the identified variants in Thorase lead to behavioral changes, a battery of behavioral tasks thought to reflect schizophrenia-related behaviors in mice was performed. Several studies have shown that the disruption of NMDA receptors with NMDA-receptor antagonists impairs cognitive function in healthy subjects and intensifies psychotic symptoms in schizophrenic patients (22, 23). NMDA receptors have been shown to modulate surface expression of AMPA receptors (1, 13). We therefore used the well known psychostimulant, a non-competitive NMDA-receptor antagonist, MK-801 (22, 23) to evaluate the sensitivity of the Thorase mutant mice to this psychostimulant. Thorase variant mice have deficits in exploration in a novel environment (Fig. 4, A to D and fig. S7, A to C) (24, 25) and enhanced locomotor response to psychostimulant, MK-801 (Fig. 4, A and B) compared to wild type or control mice. The Thorase variant mice are also hypersensitive to the psychostimulant, amphetamine (fig. S7D). These results are consistent with clinical observations that patients suffering schizophrenia are more sensitive to psychostimulants (22, 23).
Figure 4. Perampanel restores behavior deficits in Thorase variant mice.
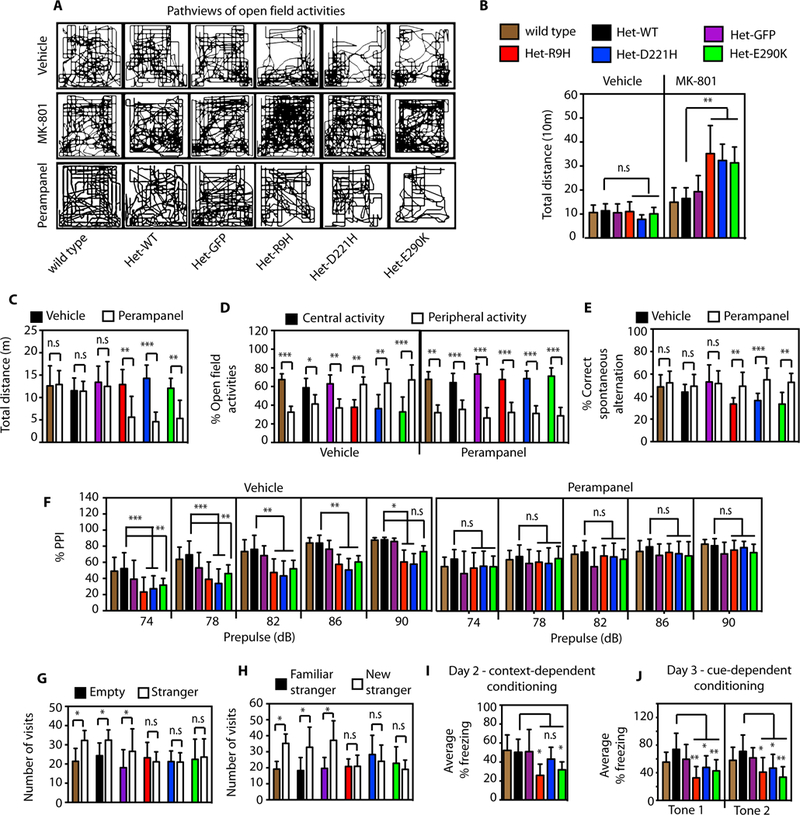
(A-D) Open field assessments in wild type and Thorase variant mice. (A) Representative pathview images of the open field activities of the mice treated with vehicle or MK-801 or perampanel showing characteristics of the patterns of locomotor activity. (B) Graphical representation of the total distance travelled by mice in the open field after vehicle or MK-801 treatment. (C) Graphical representation of the total distance travelled by mice in the open field after vehicle or perampanel treatment (mean ± SEM, n = 10, ***p < 0.01, **p < 0.05, *p < 0.1, n.s p > 0.10, ANOVA with Holm-Sidak post-hoc test when compared with wild type, Power: 1-β err prob = 0.8–1.0). (D) Percent central and peripheral activities in the open field of mice treated with vehicle or perampanel. (E) Graphical representation of percent correct spontaneous alteration of mice in the Y-maze spatial working memory tests after vehicle or perampanel treatment. (F) Prepulse inhibition (PPI) in the acoustic startle response test. The graphs represent the percent PPI at different prepulse intensity after vehicle or perampanel treatment (mean ± SEM, n = 7–10, ***p < 0.01, **p < 0.05, *p < 0.1, n.s p > 0.10, ANOVA with Holm-Sidak post-hoc test when compared with wild type, Power: 1-β err prob = 0.9 – 1.0). (G) Social interactions assessment of wild type and Thorase variant mice. Graph represents number of mice visits to chamber containing stranger mouse versus empty chamber. (H) Evaluation of social memory of controls and Thorase variant mice. Graph represents number of mice visits to chamber containing familiar stranger mouse versus chamber containing new stranger mouse. (I-J) Trace fear conditioning to assess contextual memory and associative learning in controls and Thorase variant mice. (I) Mean percent freezing of mice during the context test to assess contextual memory. (J) Mean percent freezing of mice during the cue test. Graphical representation of the average freezing time for the first 2 tones is shown. (mean ± standard error of the mean [SEM] n = 12, ***p < 0.005, **p < 0.01, *p < 0.05, Two-way ANOVA with Tukey-Kramer post-hoc test, compared with wild type, Power: 1-β err prob = 1.0).
Thorase mutant mice have deficits in memory and prepulse inhibition
Assessment of spatial working memory using spontaneous alteration in a Y-maze continuous trials procedure (Fig. 4E and fig. S7, E to F) indicates that variants in Thorase lead to deficits in both spatial working and spatial recognition memory. Several studies have shown that patients with psychiatric disorders such as schizophrenia exhibit deficits in prepulse inhibition (PPI) of the acoustic startle and similar deficits can be measured in mice (26, 27). Thorase variant mice exhibit significant deficits in PPI (Fig. 4F and fig. S7, G and H), compared to wild type or control mice. Thus, variants in Thorase impair PPI in the mice.
Impaired social behavior in Thorase mutant mice
Since deficits in social interactions are key negative symptoms of schizophrenia, the social behavior of the Thorase variant mice was examined. Wild type, or control mice made significantly more visits and spend significantly more time with stranger mice compared to Thorase variants (Fig. 4G and fig. S7I). In the social novelty preference test, wild type, or control mice make significantly more visits and spend more time with novel unfamiliar stranger mouse compared to the already explored familiar stranger (Fig 4H and fig.S7, J and K). In contrast, Thorase variant mice spend equal time and make equal visits to familiar and novel unfamiliar stranger mice. Taken together these data reveal deficits in social behaviors in the mice expressing variants of Thorase.
Thorase mutant mice have deficits in long term memory and associative learning
Associative learning in Thorase variant mice was assessed by performing trace fear conditioning. No significant difference in the mean percent freezing between the various groups of mice during training was observed (fig. S7L), suggesting that the hearing of Thorase variant mice and response to shock are not impaired. However, there is significantly less freezing during the contextual memory test in the Thorase R9H and E290K variant mice and a trend towards less freezing in the Thorase D221H mice compared to the control mice (Fig. 4I and fig. S7M). In addition, all Thorase variant mice display a significantly lower average percent freezing for the first two tones compared to control mice (Fig. 4J and fig. S7N). Taken together these results indicate Thorase variant mice have deficits in both context- and cue-dependent fear conditioning, suggesting impaired associative learning and memory. A summary of the biochemistry, physiology and behavior results are listed in table S2.
Perampanel restores behavior deficits in Thorase mutant mice
Psychosis is normally treated with antipsychotic medications that are only partially effective and most patients do not resume normal activities after hospitalization (12). Allosteric modulation of proteins linked to schizophrenia has been suggested as the best approach for the successful development of new drugs for effective treatments. Current treatments emphasis is now shifting to addressing the cognitive and negative symptoms of schizophrenia using allosteric modulators (12). We therefore evaluated the effects of the FDA approved AMPA receptor antagonist, perampanel on the Thorase variant mice displaying schizophrenia-like behaviors. The effects of perampanel (0.5 mg/kg) on locomotor activities in the open field test, spatial working memory in Y-maze test and PPI were evaluated. The open field results show that Thorase variant mice are more sensitive to perampanel than control mice but treatment normalizes the open field activities in the variant mice (Fig. 4, A to D). Treatment with perampanel normalizes deficits in exploration in a novel environment (Fig. 4D) and restores spatial working memory in Thorase variant mice (Fig. 4E). The PPI deficits in Thorase variant mice are prevented by perampanel treatment (Fig. 4F). Perampanel is also able to restore some MK-801 induced behavior deficits in the mice (fig. S8, A to E).
DISCUSSION
The deletion of chromosome 10q23.31, containing ATAD1 in a patient was associated with Bannayan-Riley-Ruvalcaba and Cowden syndromes with a history of macrosomia at birth, persistent macrocephaly, subcutaneous lipomas, gastrointestinal polyps, developmental delay and learning disabilities (28, 29). Although some of the patient’s symptoms were linked to the deletion of PTEN (29) (also located on 10q23.31) the authors could not explain other symptoms observed in the patient. The patient’s symptoms such as developmental delay and learning disabilities are consistent with symptoms observed in ATAD1 (Thorase) knockout mice (1). Moreover, the patient’s mother had a history of two first trimester miscarriages, which is also consistent with Thorase heterozygous pregnant mice carrying Thorase knockout pups. Most Thorase knockout pups die before birth. We therefore suggest that the deletion of Thorase in this patient accounts for some of the symptoms linked to the deletion of 10q23.31 and may also be an underlying cause of disease in the Ashkenazi Jewish schizophrenia patients identified. It is therefore appropriate to consider ATAD1 as a candidate for human psychiatric disorders such as schizophrenia.
Our results suggest that altered AMPA receptor-mediated synaptic activities in mice expressing schizophrenia-linked Thorase variants is due to dysfunctional AMPARs trafficking at the synapses. The elevated surface expression of AMPARs in the PFC in the Thorase variant mice accounts for the synaptic abnormalities in these neurons that are linked to the behavior deficits. Such abnormal behaviors are consistent with clinical findings that behavioral symptoms commonly observed in psychiatric disorders such as schizophrenia arises from a dysfunctional PFC (17, 18). Thus, the inability of Thorase variants to properly regulate AMPARs trafficking may be the cause of schizophrenia in the patients carrying these variants. Inhibition of the excess AMPARs at surface by perampanel restores synaptic activities to normal and hence, normal behavior in these mice. Thus, this study shows that the use of perampanel to modulate surface expression of AMPARs could offer an important therapeutic opportunity for a variety of neuronal disorders associated with AMPARs mediated neurotransmission.
MATERIAL AND METHODS
Study design
Purity of recombinant proteins used to determine the biochemical activities of Thorase variants identified in the schizophrenia cases were assessed by SDS-PAGE coomassie staining and also probed with Thorase antibody. Neuron cultures expressing GFP were used as controls for cultures expressing wild type or Thorase variants. A battery of well established behavioral tests that reflect schizophrenia-related behaviors in mice models (22, 23) were used to assess the behavior of mice expressing Thorase variants. For all tests the effect size was first calculated on the basis of preliminary experiments and then used in power analysis by the software G* Power to determine sample size by given error probability and power (Table S3). Mice were toe clipped for identification, however they we were randomly (and blinded) selected and then assigned numbers during behavioral tasks. A blinded observer assessed the outcomes and all data collected were included in the final analysis. Imaging and analysis of signal intensity were performed by automated software as indicated in the experimental procedures.
All antibodies were acquired commercially:
Thorase (mAb, Neuromab, RRID: AB_2564836), GluA2-N/C (mAb, Millipore-Chemicon, RRID: AB_2113875 and RRID: AB_2247874) and GRIP1 (pAb, Millipore-Chemicon, RRID: AB_11210079). Where mAb and pAb are monoclonal and polyclonal antibodies, respectively. Anti-GST-HRP (RRID:AB_771429), anti-His6 antibody (RRID:AB_771435) and actin-HRP were purchased from GE healthcare (Amersham) and anti-FLAG-HRP from SIGMA. Perampanel was provided by Eisai Co., Ltd. (Tokyo, Japan). Dizocilpine (MK-801) and N-Methyl-D-aspartate (NMDA) were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.).
Drugs preparations and treatments
Perampanel was reconstituted to 0.4 mg/ml (in sterile 0.1 M HCl in saline) (30), and MK-801 to 1 mg/ml in sterile saline. Drugs or vehicle (sterile 0.1M HCl in saline) were administered by intraperitoneal injections in equal volumes.
Animals
Thorase heterozygous (Thorase +/−) mice were obtained by crossing heterozygous (Thorase +/−) mice (1). Animal experiments were performed in compliance with the regulations of the Animal Ethical Committee of the Johns Hopkins University Animal Use and Care Committee. Mouse behavioral testing occurred in the Johns Hopkins University School of Medicine Behavior Core using protocols approved by and are standard protocols found in the behavior core manual.
Sequencing of ATAD1 coding exons
To identify Thorase (ATAD1) variants associated with schizophrenia cases of Ashkenazi Jewish origin we obtained bidirectional sequence of the ATAD1 coding exons in 712 schizophrenia cases and 649 controls of Ashkenazi Jewish origin as previously described (31). The functional activities of all three coding variants, R9H, D221H and E290K identified in schizophrenia cases were determined.
ATPase activity assay
ATP binding of the variants was determined by UV light-induced cross-linking of radiolabeled [α-P32]ATP and glutaraldehyde chemical cross-linking was used to evaluate oligomerization upon ATP binding as described by Babst et al (33). Structural and conformational changes were examined using circular dichroism. Details described in extended experimental procedures (Supplementary materials).
Evaluation of Thorase mutants interaction with GluR2 and GRIP1
For the in vitro Thorase-GluR2-GRIP1 interactions assays, purified GST-GluR2 and GST-GRIP1 recombinant proteins bound to glutathione beads were incubated with purified His6-tagged Thorase proteins. In a separate experiment GST-Thorase recombinant proteins bound to glutathione beads were incubated with purified Glu2 C-terminal fragment (GluR2C) and GRIP1 PDZ 4 /5 domains fragment. The GST beads were extensively washed to evaluate Thorase-GluR2-GRIP1 interactions. Immunoprecipitation of Thorase-FLAG in the presence of from primary cortical cultures expressing the Thorase variants was used to determine Thorase-GluR2-GRIP1 interactions in neurons. Details of procedures are described in extended experimental procedures (Supplementary materials)
Disassembly and endocytosis of GluR2/GRIP1 complex assays
The ability of Thorase variants to interact with and disassembly the GluR2-GRIP1 complex was evaluated by Immunoprecipitation of GluR2 in the presence of ADP, ATP or ATPγS from primary cortical cultures expressing the Thorase variants as previously described(1). An antibody feeding internalization assay for endocytosis of surface GluR2 receptors and GluR2 surface proteins crosslinking were used to evaluate GluR2 surface expression in the presence or absence of NMDA. pHluorin tagged GluR2 fusion protein was used to analyze the membrane trafficking of GluR2 after NMDAR activation in primary hippocampal cultures expressing the Thorase variants. Detail description in extended experimental procedures (Supplementary materials)
Electrophysiology
Schizophrenia-linked Thorase mutants were expressed in mice brain delivering AAV2 viruses carrying Thorase-FLAG wild type or schizophrenia-linked variants into newborn Thorase heterozygous mice by intracerebroventricular injection (Supplementary materials). To determine whether intrinsic excitability in neurons is altered in mice expressing schizophrenia-linked Thorase variants ex vivo electrophysiology was performed in the prelimbic pyramidal neurons portion of the medial prefrontal cortex. Details of procedures are described in extended experimental procedures (Supplementary materials).
Behavioral experiments
Behavioral experiments associated with schizophrenia were performed using 6–8 months old mice. The tests were performed in the following order: open field, y-maze, social interaction, PPI, trace fear conditioning MK-801 and perampanel challenge tests. Details of procedures for individual behavior tests are described in extended experimental procedures (Supplementary materials).
Data analyses and statistics
All experiments were repeated at least three times and quantitative data are presented as the mean ± standard error of the mean (SEM) performed by GraphPad prism6 software (Instat, GraphPad Software). Statistical significance was assessed by one-way or two-way ANOVA. The significant differences were identified by post-hoc analysis using the Holm-Sidak post-hoc test for multiple comparisons. Assessments were considered significant with a p < 0.05 and non-significant with a p > 0.05. Power analysis and sample size calculation for all experiments were determined using G*Power 3.1 and SigmaStat statistics software. Power was calculated as 1-β, assuming α = 0.05.
Supplementary Material
Acknowledgments:
We thank Eisai Co., Ltd. (Tokyo, Japan) for generously providing perampanel as noted in the experimental procedure.
Funding: This work was supported by grants from the National Institutes of Health (NIH) DA000266 to T.M.D. and V.L.D, the Simon’s Foundation Autism Research Initiative to T.M.D.
Footnotes
SUPPLEMENTARY MATERIALS
Detail experimental procedures, extended data display items, and statistical analyses (Table S3) are available in the online version of the paper.
Notes: T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases.
REFERENCES
- 1.Zhang J et al. , The AAA+ ATPase Thorase regulates AMPA receptor-dependent synaptic plasticity and behavior. Cell 145, 284–299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH, Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 32, 1888–1902 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Beneyto M, Meador-Woodruff JH, Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse 60, 585–598 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Cantrup R, Sathanantham K, Rushlow WJ, Rajakumar N, Chronic hyperdopaminergic activity of schizophrenia is associated with increased DeltaFosB levels and cdk-5 signaling in the nucleus accumbens. Neuroscience 222, 124–135 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Corti C et al. , Altered levels of glutamatergic receptors and Na+/K+ ATPase-alpha1 in the prefrontal cortex of subjects with schizophrenia. Schizophrenia research 128, 7–14 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Orozco IJ, Koppensteiner P, Ninan I, Arancio O, The schizophrenia susceptibility gene DTNBP1 modulates AMPAR synaptic transmission and plasticity in the hippocampus of juvenile DBA/2J mice. Molecular and cellular neurosciences 58, 76–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucholski J et al. , Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophrenia research 146, 177–183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallin MD et al. , Genomewide linkage scan for schizophrenia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 10q22. American journal of human genetics 73, 601–611 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraone SV et al. , Genome scan of Han Chinese schizophrenia families from Taiwan: confirmation of linkage to 10q22.3. The American journal of psychiatry 163, 1760–1766 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Venken T et al. , Chromosome 10q harbors a susceptibility locus for bipolar disorder in Ashkenazi Jewish families. Molecular psychiatry 13, 442–450 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Fujiki Y, Miyata N, Matsumoto N, Tamura S, Dynamic and functional assembly of the AAA peroxins, Pex1p and Pex6p, and their membrane receptor Pex26p involved in shuttling of the PTS1 receptor Pex5p in peroxisome biogenesis. Biochemical Society transactions 36, 109–113 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Menniti FS et al. , Allosteric modulators for the treatment of schizophrenia: targeting glutamatergic networks. Current topics in medicinal chemistry 13, 26–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DT, Huganir RL, PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 13903–13908 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC et al. , Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. The EMBO journal 33, 1548–1564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glascock JJ et al. , Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. Journal of visualized experiments : JoVE, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer K et al. , Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Molecular therapy : the journal of the American Society of Gene Therapy 23, 477–487 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schubert D, Martens GJ, Kolk SM, Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Molecular psychiatry 20, 795–809 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yizhar O et al. , Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balleine BW, O’Doherty JP, Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35, 48–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benke TA, Luthi A, Isaac JT, Collingridge GL, Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393, 793–797 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Tarusawa E et al. , Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. The Journal of neuroscience: the official journal of the Society for Neuroscience 29, 12896–12908 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C, Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neuroscience and biobehavioral reviews 32, 1014–1023 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Blot K, Bai J, Otani S, The effect of non-competitive NMDA receptor antagonist MK-801 on neuronal activity in rodent prefrontal cortex: an animal model for cognitive symptoms of schizophrenia. Journal of physiology, Paris 107, 448–451 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Zhan Y, Theta frequency prefrontal-hippocampal driving relationship during free exploration in mice. Neuroscience 300, 554–565 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Arias N, Mendez M, Arias J, The recognition of a novel-object in a novel context leads to hippocampal and parahippocampal c-Fos involvement. Behavioural brain research 292, 44–49 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Braff DL, Geyer MA, Swerdlow NR, Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156, 234–258 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Koch M, Clinical relevance of animal models of schizophrenia. Supplements to Clinical neurophysiology 62, 113–120 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Balciuniene J et al. , Recurrent 10q22-q23 deletions: a genomic disorder on 10q associated with cognitive and behavioral abnormalities. American journal of human genetics 80, 938–947 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arch EM et al. , Deletion of PTEN in a patient with Bannayan-Riley-Ruvalcaba syndrome suggests allelism with Cowden disease. American journal of medical genetics 71, 489–493 (1997). [PubMed] [Google Scholar]
- 30.Hanada T et al. , Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52, 1331–1340 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Clarke L et al. , The 1000 Genomes Project: data management and community access. Nature methods 9, 459–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger C et al. , Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10, 302–317 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Babst M, Wendland B, Estepa EJ, Emr SD, The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. The EMBO journal 17, 2982–2993 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd JD et al. , Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper O et al. , Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Science translational medicine 4, 141ra190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen P, Heimberg M, Ducray AD, Widmer HR, Meyer M, Expression of trefoil factor 1 in the developing and adult rat ventral mesencephalon. PloS one 8, e76592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawley JN, Designing mouse behavioral tasks relevant to autistic-like behaviors. Mental retardation and developmental disabilities research reviews 10, 248–258 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Pletnikov MV et al. , Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Molecular psychiatry 13, 173–186, 115 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


