Abstract
Multicellular spheroids (hereinafter referred to as spheroids) are 3D biological models. The metabolomic profiles inside spheroids provide crucial information reflecting the molecular phenotypes and microenvironment of cells. To study the influence of an anticancer drug on the spatially resolved metabolites, spheroids were cultured using HCT-116 colorectal cancer cells, treated with the anticancer drug Irinotecan under a series of time- and concentration-dependent conditions. The Single-probe mass spectrometry imaging (MSI) technique was utilized to conduct the experiments. The MSI data were analyzed using advanced data analysis methods to efficiently extract metabolomic information. Multivariate curve resolution alternating least square (MCR-ALS) was used to decompose each MS image into different components with grouped species. To improve the efficiency of data analysis, both supervised (Random Forest) and unsupervised (cluster large applications (CLARA)) machine learning (ML) methods were employed to cluster MS images according to their metabolomic features. Our results indicate that anticancer drug significantly affected the abundances of a variety of metabolites in different regions of spheroids. This integrated experiment and data analysis approach can facilitate the studies of metabolites in different types of 3D tumor models and tissues and potentially benefit the drug discovery, therapeutic resistance, and other biological research fields.
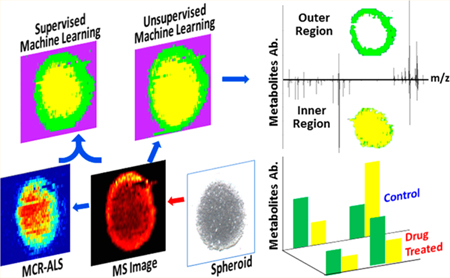
Graphical Abstract
Spheroids, the spherical aggregates of tumor cells, fill the gap between the simplified 2D cell culture models and very complex real tissues.1 Compared with common 2D-cultured cells, spheroids provide more vivid and cost-efficient models with a higher degree of relevance to clinical and biological applications.2 Particularly, the 3D-structured spheroids can mimic the microenvironment of cells with higher fidelity.3,4 For example, the gradients of nutrients, oxygen, and pH value result in different proliferation status of cells from the inside to the outside regions of spheroids.5 The spheroid has become an important platform for broader ranges of in vitro studies such as proteomics,6 drug screening,7 and metabolomics.8,9 Among these applications, metabolomics focuses on small molecules (e.g., M.W. < 1500 Da),10 with both endogenous and exogenous origins, in biological samples such as cells, tissues, and biofluids.11 The changes of metabolites can rapidly and directly reflect the state of biological systems affected by a variety of factors, including microenvironment perturbation, genetic mutation, kinetic activity of enzymes, and changes in metabolic reactions.12–14 Metabolomics studies utilizing spheroids have a broad influence on drug discovery, toxicology, and disease diagnosis.7,15
Current metabolomics studies of biological tissues are primarily carried out using lysates prepared from samples, and measurements are conducted using mass spectrometry (MS), which is usually coupled to liquid chromatograph (LC) or gas chromatograph (GC) separation techniques,16 or nuclear magnetic resonance (NMR), typically 1H NMR.17 However, because lysates need to be prepared from homogenized samples,18 the spatial distribution of metabolites, which is critical to understand the complex biological process and the pathophysiology, is inevitably lost.19,20 To obtain the spatially resolved metabolites, molecular imaging techniques, such as positron emission tomography (PET), magnetic resonance imaging (MRI), and MS imaging (MSI), have been developed. PET can locate tumor areas using certain target molecules (e.g., radiolabeled glucose (2-[18F]fluoro-2-deoxy-D-glucose (FDG)) owing to their accumulations in tumors.21 MRI can diagnose many types of cancers by visualizing certain metabolomic biomarkers.22,23 However, the broader applications of PET and MRI are largely limited by their relatively low coverage of molecular types.24 MS imaging (MSI), with high sensitivity and wide ranges of molecular coverage, is a powerful technique to visualize the distribution of metabolites on tissue slices.20 MSI has been applied to numerous metabolomics studies of plants,25 drugs,26 and diseases such as cancers.27,28 Among all developed MSI techniques, vacuum based sampling and ionization techniques, such as matrix assist laser desorption ionization MS (MALDI-MS) and secondary ion MS (SIMS), provide superior sensitivity and excellent spatial resolution,29 whereas ambient MSI techniques, such as desorption electrospray ionization (DESI)30 and laser ablation electrospray ionization (LAESI),31 require minimum sample preparation and allow for experiments to be conducted under convenient conditions.32 Particularly, the absence of matrix molecules in sample preparation enables ambient MSI techniques to effectively detect small molecules such as metabolites.33
In addition to a rapid development of MSI experimental techniques, advanced data analysis methods become increasingly important to effectively extract chemical information from a large amount of MSI data (e.g., several to hundred GB can be typically generated from MALDI MSI experiments34). Although the traditional methods, such as principle component analysis (PCA)35 and partial least squares discriminant analysis (PLS-DA),36 have been applied to the analysis of MSI data, they have some intrinsic limitations.4 For example, the negative values in the PCA and PLS-DA score plots have no physical meaning (i.e., ion intensities in mass spectra cannot be negative).37,38 In contrast, multivariate curve resolution (MCR-ALS), a multivariate data analysis method, has the advantages of not only decomposing the image data into major components but also extracting the ions contributing to each component.39,40 However, because intensive computing is needed during the MCR-ALS analysis, this method is less than ideal when analyzing large amounts of MSI data, particularly for samples possessing similar features such as slices obtained from the same tissue.
Machine learning (ML) methods, including unsupervised and supervised ML, are powerful tools for efficient MSI data processing.41,42 Unsupervised ML is utilized to cluster MS image data into different groups of molecules according to their similarities of MS profiles without any prior knowledge.38 Common unsupervised ML algorithms include hierarchical clustering, k-means, and cluster large applications (CLARA).34 These common ML methods have been employed for MSI data analysis. Hierarchical clustering is an algorithm to build the hierarchical cluster tree, and each branch is split and followed down to individual groups of data such as mass spectra in MS imaging data sets.43 K-means is a widely used method; however, it is sensitive to outliers. Thus small numbers of pixels (i.e., potential outliers) in MS image data might be unnecessarily grouped into clusters.34 CLARA is superior to other unsupervised ML methods for MSI data analysis due to its capability of optimizing the cluster numbers of MS image data.40,44 On the other hand, supervised ML methods, including Supporting Vector Machine (SVM) and Random Forest, can be employed to classify MS image data into several predefined groups after model training.45 SVM is used to find a hyperplane that can separates one or more classes based on their mass spectrum.46 Random Forest has less of an overfitting issue, and it is efficient in analyzing large data sets.47,48 Both unsupervised and supervised ML methods can facilitate the analysis of metabolites in MS imaging data.
Here we used the Single-probe MSI technique to measure the spatial distributions of metabolites in spheroids, which were cultured using HCT-116 colorectal cancer cells and treated with anticancer drug Irinotecan. The Single-probe is a multifunctional sampling and ionization device that has been applied into many research fields, including high resolution MSI,49,50 live single cell analysis,51–56 and the measurement of extracellular metabolites in live spheroids.9 MSI data were then analyzed using MCR-ALS, unsupervised ML (CLARA), and supervised ML (Random Forest) methods. MCR-ALS was used to decompose the MS images into major components with grouped molecules, whereas Random Forest and CLARA were utilized to efficiently classify the MS images into different regions in spheroids. In addition, we investigated the metabolites altered by anticancer drug treatment in different regions of spheroids. Our studies provided a combined MSI experiment and advanced data analysis to effectively analyze metabolomics and investigate the influence of microenvironment on metabolite change in tissues. This comprehensive method can potentially benefit the biomarker discovery and metabolomics studies.
EXPERIMENTAL SECTION
Chemicals and Materials.
Chemicals used in the experiments include reagents such as methanol, water, agarose (Sigma-Aldrich, St. Louis, MO, USA), and the anticancer drug Irinotecan (Thermo Scientific, Ward Hill, MA, USA). Materials needed to fabricate the Single-probe include fused silica capillary (O.D. 105 μm; I.D. 40 μm, Polymicro Technologies, Phoenix, AZ, USA) and dual-bore quartz tubing (O.D. 500 μm; I.D. 127 μm, Friedrich & Dimmock, Inc., Millville, NJ, USA). Reagents used to culture HCT-11 cells and spheroids (ATCC, Manassas, VA, USA) include McCoy’s 5A cell culture media, FBS (fetal bovine serum), and Pen Strep (Life Technologies, Grand Island, NY, USA).
Single-Probe Fabrication and Experimental Setup.
The detailed fabrication protocols are described in our previous work,50 and only the outlined procedures are provided here. The Single-probe (Figure S1) has three components: a laser-pulled dual-bore quartz needle, a fused silica capillary (solvent-providing capillary), and a nano-electrospray (nano-ESI) emitter. A Sutter P-2000 laser micropipette puller (Sutter Instrument, Novato, CA, USA) is used to prepare the dual-bore needle and nano-ESI emitter. A Single-probe is fabricated by embedding within a dual-bore quartz needle with one fused silica capillary and one nano-ESI emitter. The experiment setup is largely adopted from our previous MS single cell and MSI studies49,50 (Figure 1). To precisely control the movement of the tissue slice, the sample was attached to a XYZ-translational stage system (CONEX-MFACC, Newport Co., Irvine, CA, USA) controlled using a LabView software package.57 A digital microscope was placed next to the Single-probe to adjust the distance between the Single-probe tip and the tissue slice surface and to monitor the sampling process. MS spectra were collected using a Thermo LTQ XL mass spectrometer (Thermo Scientific, Waltham, MA, USA) with the following parameters: mass resolution 60,000 (m/Δm), 4.5 kV ionization voltage (positive ion mode), 1 microscan, 100 ms max injection time, and AGC on. The sampling solvent (i.e., 85% methanol/15% water (v/v)) was continuously delivered (flow rate 200 nL/min) by a syringe pump (PHD ULTRA, Harvard Apparatus, Holliston, MA, USA). The MS images were generated using MSI QuickView software.58
Figure 1.
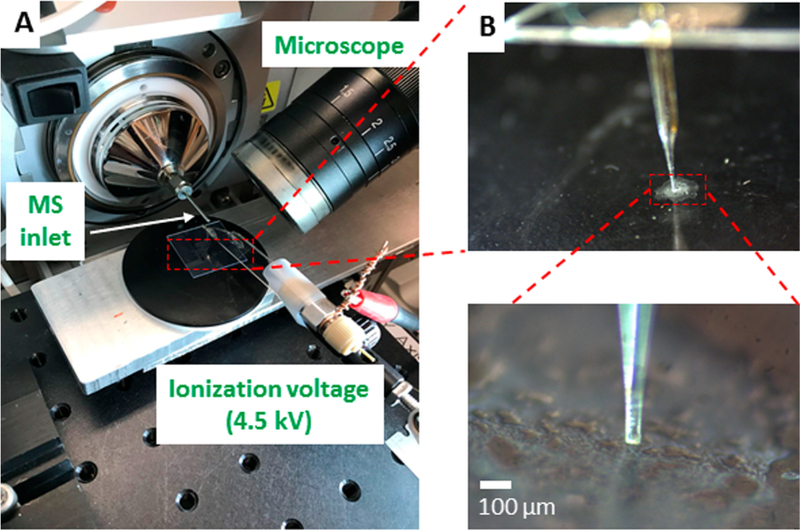
Single-probe MSI setup. (A) The major components of this setup, in which a microscope was used to monitor the MSI experimental process and an ionization voltage was applied on conductive union. (B) Zoomed-in pictures showing the Single-probe tip (inset) and the spheroid slice.
Spheroids Culture and Sectioning.
The colon carcinoma cell line HCT-116 was used to culture spheroids. Cells were maintained in McCoy’s 5A cell culture media containing 10% FBS and 1% Pen Strep. Cells were grown in an incubator (HERA cell, Heraeus, USA) under well controlled conditions (5% CO2 at 37 °C), and cell passage was performed very 2 days. Spheroids were cultured using the modified protocols based on previous publications.59,60 Briefly, 60 μL agarose gel (1.7% agarose in plain McCoy’s culture media) was used to coat wells in a U-bottom 96-well plate (VWR, Radnor, PA, USA), and about 10,000 HCT-116 cells were seeded into each agarose coated well. To improve the successful rate of spheroid culture, we used the U-bottom 96-well plates to promote the accumulation of cells during spheroid growth. Cells were incubated for 2−3 days to allow them aggregating into solid spheroids. Culture medium was changed every 2 days, and spheroids were harvested after being cultured for 10 days. Spheroids were treated using Irinotecan under a series of conditions (i.e., 5 μM for 1 h, 10 μM for 1 h, 20.6 μM for 1 h, 20.6 μM for 10 h, and 20.6 μM for 24 h) and rinsed twice using PBS (phosphate-buffered saline) to remove the drug residue on the spheroid surface. Spheroids were then embedded in 10% HMPC ((hydroxypropyl)methyl cellulose, Sigma-Aldrich St. Louis, MO) and frozen on dry ice. Depending on their sizes, spheroids were sectioned into 20 to 30 slices (∼15 μm thickness for each slice) using a cryotome (American Optical 845 Cryo-cut Mictorome, Southbridge, MA, USA) at −15 °C. To better represent the symmetric histological structures of spheroids (e.g., inner region and outer region), slices in the middle part of well-aligned spheroids (e.g., slice number ∼10 to ∼15) were selected and inspected using a microscope. The selected slices were attached onto plastic microscope slides (VWR, Radnor, PA, USA), dried in air, and stored at −80 °C before usage. The optical images of slides were taken using a PathScan Enabler IV histology slide scanner (Meyer Instruments, Houston, TX, USA).
Data Analysis.
Data Preprocessing.
Before conducting the MCR-ALS analysis, MSI data (.raw) need to be converted into an appropriate format that can be input into a home-built MATLAB processing platform. First, MSConvert (a tool in ProteoWizard) was used to convert the data format from .raw to .mzML, which was further converted into .imzML format using an imzML converter.61 Second, data preprocessing, including smoothing, noise removal, peak alignment, peak picking, and insensitivity normalization, was executed using a Bioinformatics Toolbox, a built-in function of MATLAB. Third, each set of MS imaging data was exported as a data matrix with high dimensionalities (Table S1). For example, the data matrix of the MS image of a control sample slice is composed of 7840 × 307, i.e., 7840 pixels (196 (scans/line) × 40 lines) with 307 aligned common ions, and each aligned peak stands for a dimension of the data set. The number of pixels (ranging from 6,000 to 10,000) of each MS image depends on the size and the spatial resolution of the MS image. A log-transformation (log 2) was applied to the data matrix produced from the preprocessing step to better represent features of low-intensity ions. The details of data preprocessing are provided in the Supporting Information.
MCR-ALS Analysis.
The multivariate curve resolution toolbox, developed by Tauler et al.,62 was utilized to group the ions with similar spatial distribution patterns in MS images. Singular value decomposition (SVD) was used to determine the number of major components, which usually represent the majority of variations (>80%) in the data matrix. The spatial distribution of each component was carried out using a user-friendly interface, and the mass spectrum of each component was generated using R (Supporting Information).
Unsupervised ML (CLARA).
CLARA was used as an unsupervised algorithm to analyze the MSI data sets. To generate data sets that are suitable for the CLARA model, t-SNE (t-Distributed Stochastic Neighbor Embedding) was used to reduce the high dimensionality of the data matrix generated from the preprocessing into 2D space (parameters are listed in the Supporting Information). CLARA (provided within “cluster” package63) was then used to analyze the data sets with 2D space, and the optimal numbers of clusters were determined using the average silhouette width. To illustrate the CLARA results according to the spatial distribution of ions in each group, their histological distributions were reconstructed using a home-built R program.
Supervised ML (Random Forest).
Random forest provided in R language (the package “randomForest”64) was used as a supervised ML algorithm to classify the MSI data sets. Based on MCR-ALS results, data were selected from each region (i.e., inner or outer) of the spheroid to serve as the training data and testing data. The training data were used to train the Random Forest model (details are provided in the Supporting Information), whereas the testing data were utilized to evaluate prediction accuracy (Table S2). Once the prediction accuracy was satisfactory (>90%), the optimized model was employed to predict the rest of the data set. To visualize overall results obtained from the supervised ML approach, the predicted spatial distribution features of the metabolites on spheroid slices were constructed using R language. (All packages used in ML models can be found at https://cran.r-project.org.)
RESULTS AND DISCUSSION
Mass Spectrometry Images of Spheroids.
Irinotecan is a common anticancer drug that has been widely used in clinic treatment of broad ranges of cancers (e.g., colorectal, pancreatic, and lung cancers) and fundamental research. This drug compound was selected to treat spheroids cultured using a colorectal cancer cell line (HCT-116). To investigate the influence of treatment time and concentration on the change of metabolites, experiments were conducted using spheroids in three different groups: control (no drug treatment), concentration-dependent treatment, and time-dependent treatment. In concentration-dependent experiments, the treatment time was fixed at 1 h, whereas three different concentrations (5.0, 10.0, and 20.6 μM) were selected. In the time-dependent experiment, the concentration was 20.6 μM, which is the IC50 of Irinotecan for HCT-116 spheroids,8 whereas the treatment time was varied (1, 10, and 24 h). MSI experiments were carried out using the Single-probe MSI technique, and MS images of selected ions were constructed (Figure 2). Because phosphatidylcholine (34:1) (PC(34:1)) is a very common and abundant lipid (with the ion intensity ∼107), its MS image was used to represent the shape of the spheroid slice. The MS images of this representative phospholipid ([PC(34:1) + H]+, Figure 2A) and the drug compound ([Irinotecan + H]+, Figure 2C) were shown along with the corresponding optical images of the spheroid slices (Figure 2B), indicating a generally good match of spatial features obtained from both techniques. It is worth noting that all spheroids were cultured under the same conditions and their slices were prepared using the same protocols; however, the shapes of slices can be slightly different, likely due to the variance in multiple factors such as the number of cells seeded for spheroid culture, orientation of spheroids in bedding material, and sectioning positions in spheroids. Interestingly, the morphology of spheroids was not obviously changed within our drug treatment time (24 h) as shown in Figure S2.
Figure 2.
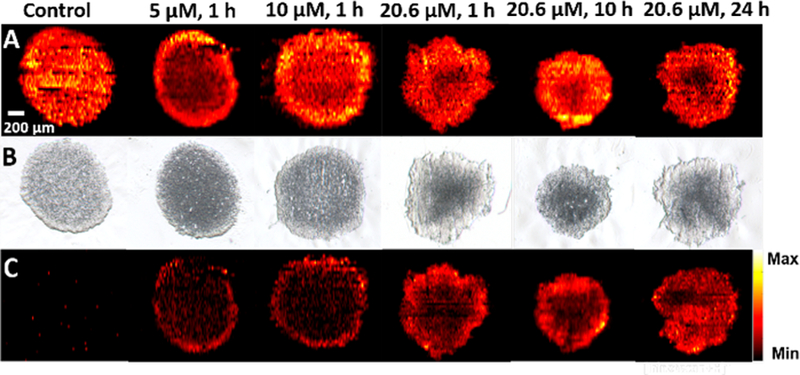
MS and optical images of spheroid slices. (A) A common ion [PC(34:1) + H]+ (m/z 760.5964) illustrating shapes of MS images of spheroid slices in the control and drug treatment groups. (B) Optical images of the corresponding spheroid slices. (C) MS images of [Irinotecan + H]+(m/z 587.2923) in drug treated spheroids.
As expected, Irinotecan was detected in spheroids subjected to the treatment using this drug compound but not observed in the control sample. In the concentration-dependent experiments (Figure 2C), Irinotecan was only detected in a thin layer of the spheroid for a 1 h treatment; the drug penetration depth was slightly increased as the concentration was increased from 5.0 to 20.6 μM. Alternatively, a contour plot (Figure S3) provides a more quantitative description of Irinotecan distribution of the same sample.
In the time-dependent experiments, this drug compound was observed in much deeper regions as the treatment time was increased. For example, Irinotecan mainly distributed in the outer region of the spheroid for 10 h treatment, whereas it was detected in the inner region after a 24 h treatment. The distribution patterns of Irinotecan can likely be attributed to the mechanisms of molecular diffusion in tissues. Due to the lack of developed blood vascular systems inside tumors, molecules (e.g., nutrients and drugs) penetrate tumors mainly through a molecular diffusion mechanism, which is primarily determined by drug concentration65 and other factors such as drug molecular weight, cell density in tumors, and cells’ microenvironment.66 Similar to tumors, the 3D structure of spheroids possesses histological heterogeneity due to a heterogeneous distribution of nutrients, oxygen, carbon dioxide, wastes, etc. For example, nutrients and oxygen can be absorbed more efficiently by the cells in the outer region than those in the inner region, whereas wastes and carbon dioxide are more abundant in the inner region than the outer region.2,67
The heterogeneous cell microenvironment inside spheroids can affect the cell metabolic pathways and result in metabolite changes in different regions. To illustrate the heterogeneity of a spatial distribution of molecules in spheroids, we constructed the MS images of a number of representative lipids (e.g., PC, phosphatidylethanolamine (PE), and phosphatidylglycerol (PG)) that are more abundant in the outer regions (Figure 3A). In addition, we investigated the influence of anticancer drug treatment on the spatial distribution of metabolites such as glucosylceramides [GlcCer(38:1) + H]+ (Figure 3B). Previous studies indicate that the upregulation of GlcCer in cancer cells is attributed to their hypoxia environment, in which the glucosylceramide synthase (GCS) possesses higher activities transferring ceramides to glucosylceramides.68,69 Our results (Figure 3B) indicate that GlcCer(38:1) has relatively higher abundances in the inner regions of spheroids, in which cells are in hypoxia environment. Compared with cells in the outer regions, Irinotecan treatment has less influence on the metabolism and microenvironment of cells in the inner regions.
Figure 3.
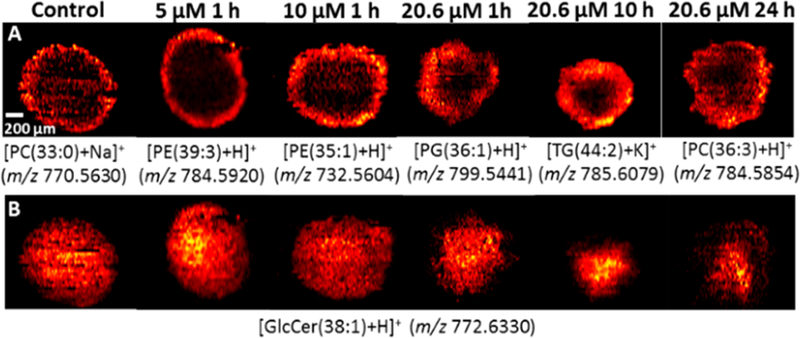
MS images of spheroid slices from control and drug treated samples. (A) MS images of representative metabolites primarily present in the outer region. (B) Influence of anticancer drug treatment on the spatial distribution of a selected metabolite GlcCer(38:1).
Multivariate Curve Resolution Analysis.
It is expected that there are large numbers of grouped metabolites possessing similar spatial distributions, whereas a manual selection approach is unlikely to be effective to acquire all metabolites in each group. For more comprehensive and efficient data analysis, MCR-ALS was employed to classify ions into different groups according to their spatial distribution features. The data generated from the preprocessing step (see Data Preprocessing) was directly analyzed using the MCR-ALS algorithm. The MSI data of drug treated samples (5 μM Irinotecan, 1 h) were decomposed into three major components, which represent the majority molecular information present in the MSI data, i.e., >80% of variance was explained (Figure S4). These three components (i.e., grouped ions) represent species in the outer region, the inner region, and the background, respectively (Figure 4). The alternative contour plots of Figure 4 were constructed based on the MCR analysis results (Figure S5). The top-ten abundant ions in each component were summarized in Table S3. Similarly, the MSI data obtained from spheroids under other treatment conditions were also decomposed into these three groups (Figure S6).
Figure 4.
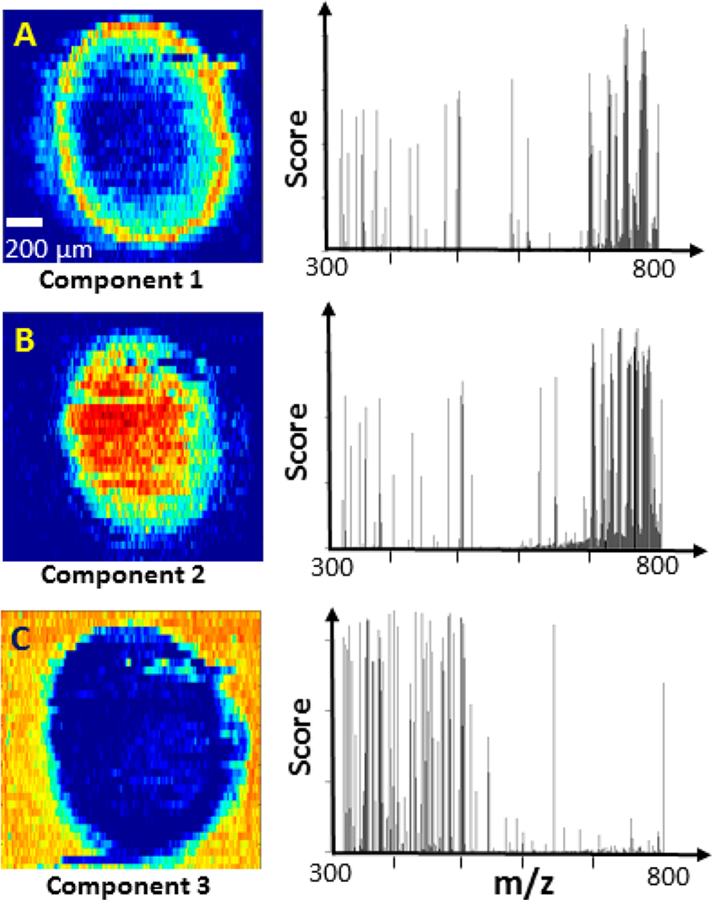
Results of MCR-ALS analysis of MSI data obtained from Irinotecan treated spheroid (5 μM, 1 h). (A) The spatial distribution patterns and the corresponding ions of (A) component 1 (outer region of spheroid), (B) component 2 (inner region of spheroid), and (C) component 3 (background).
The spatial distribution patterns of metabolites are likely related to the inherent heterogeneity of spheroids. Previous studies indicate that spheroids can be divided into two major regions: nonviable and viable regions.70 In the nonviable region, nutrients and oxygen are insufficient to sustain cells’ viability. Because cells in this region undergo necrosis and apoptosis due to the hypoxia microenvironment, the nonviable region can be further divided into quiescent layer and necrotic core.66 In contrast, cells in the viable region can acquire adequate nutrients and oxygen for proliferation. Due to the diffusion-limited transport of oxygen,2 the thickness of the viable region generally ranges from 150 to 200 μm, which is similar to the thickness of the outer region measured from MS images of spheroids in the present study (Figure 4A). Therefore, those two regions in spheroids obtained from MCR-ALS analysis (Figures 4A and 4B) are very likely to be the viable (outer) and nonviable (inner) regions, respectively. However, we were unable to differentiate the quiescent layer and necrotic core in the nonviable region in the current MSI studies. Similar results were obtained from spheroids in the control and other drug treated groups using higher drug concentrations and longer treatment times (Figure S6).
ML: CLARA and Random Forest.
For more efficient data analysis, the unsupervised ML was applied to classify the MSI data. First, t-SNE was used to reduce the high dimensionality of the preprocessed data matrix into a 2D space (Figure S7B) along with the optimal number (i.e., three) of clusters (Figure S7A): the same number of grouped ions obtained from MCR-ALS analysis. In fact, the 2D t-SNE shows a highly stable performance for dimensionality reduction, indicated by a narrow range (0.85−0.91) of the calculated Adjusted Rand Index (Figure S8). We also tested the 3D t-SNE and obtained a similar result as the 2D t-SNE (Figure S9). Second, we utilized CLARA, a common classification method primarily used for grouping large data sets with optimal numbers of clusters,44 to analyze the MSI data. Third, to clearly illustrate the spatial locations of clustered ions on a spheroid slice, we assigned a color to each cluster (i.e., color coding), and reconstructed MS images (Figure 5A) using these clustered species (Figure 5B), in which green, yellow, and purple represent the outer region, the inner region, and the background, respectively. As a popular method, PCA has been utilized for the analysis of MS images.4,35 We tested PCA for dimensionality reduction of our MSI data sets, and then performed CLARA. The results are not satisfactory: only two major components (i.e., the entire spheroid and the background) can be obtained, whereas the inner and outer regions of the spheroid cannot be distinguished (Figure S10).
Figure 5.
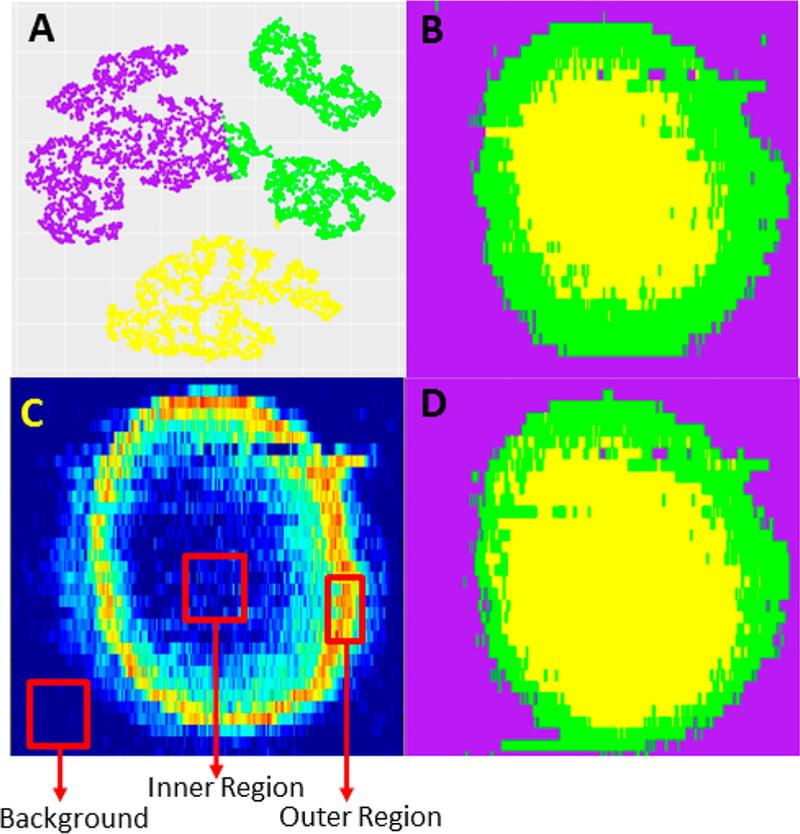
MSI data analysis using ML methods for an Irinotecan treated spheroid (5 μM, 1 h). (A) Classification of MSI data obtained from unsupervised ML (CLARA). (B) Three clustered MSI data from CLARA analysis were color-coded to reconstruct their spatial distributions representing the inner region (yellow), the outer region (green), and the background (purple), respectively. (C) The selection of the training data for supervised ML (Random Forest) was based on MCR-ALS results. (D) Color-coded spatial distributions obtained from Random Forest are similar to the CLARA results shown in part B.
Supervised ML (Random Forest) was also used to classify the MS images of spheroid slices. The selection of training data was based on the MCR-ALS results (Figure 5C, details are provided in Table S4), and the color-coded spatial distributions (Figure 5D) were reconstructed using the same approach as mentioned above. Similar to CLARA results, we were able to predict all MSI data and classify them into those three regions using Random Forest (Figure S11B). Interestingly, as shown in all six color-coded classification images generated using unsupervised (Figure S11A) and supervised (Figure S11B) ML methods, the thickness of the outer region of spheroids is not significantly affected by the drug treatment. This observation is similar to the results obtained from MCR-ALS analysis.
Because training data selection may affect the prediction accuracy of supervised ML, we compared the performance of MCR-ALS with the other two common methods: k-means and hierarchical clustering. Our results indicate the training data selection based on three different methods (i.e., MCR-ALS, k-means, and hierarchical clustering) resulted in very similar prediction of MSI data (Figure S12).
Changes of Metabolites inside Spheroids Induced by Irinotecan Treatment.
Anticancer drug treatment can change the microenvironment of cells, affect metabolomic pathways, and result in changes of metabolites. To obtain metabolites altered by Irinotecan inside spheroids, we generated two averaged mass spectra representing metabolites in the inner and outer regions of spheroids in both control and treatment groups. Specifically, according to results obtained from unsupervised and supervised ML models, mass spectra representing inner and outer regions, respectively, were averaged to generate these two mass spectra, and the intensity of each ion was then normalized to TIC (total ion current). By conducting t-test of species in the averaged spectra, metabolites with significantly changed abundances can be determined (details are provided in the Supporting Information).
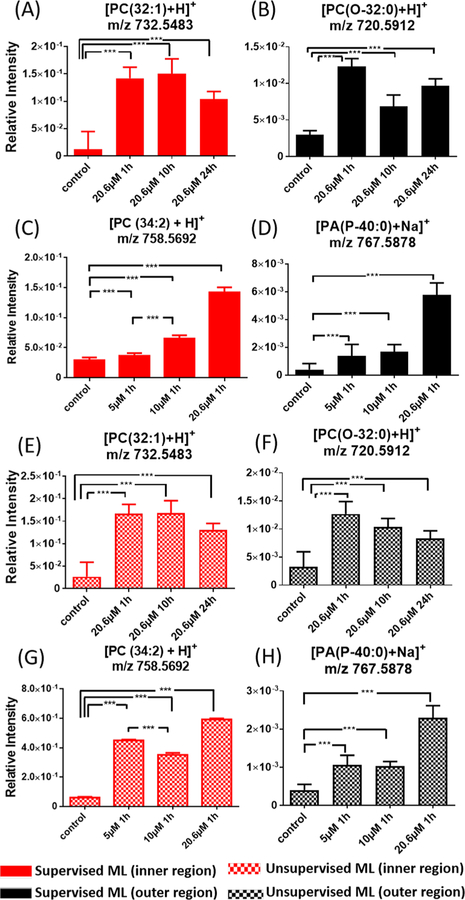
For the time-dependent experimental results, supervised ML analyses provided a number of metabolites with relatively higher abundances (i.e., upregulation) in the drug treated spheroids than those in the control sample. For example, the relative intensities of [PC(32:1) + H]+ (m/z 732.5483) and [PE(O-35:0) + H]+ (m/z 720.5908) in the inner and outer regions of the drug treated spheroids are about 10 and 3−4 times higher, respectively, than those in the same regions in the control (Figures 6A and 6B). Similarly, in the concentration-dependent experiments, a group of metabolites possess significantly different abundances in the outer and inner regions between the drug-treated and control samples. For example, the relative ion intensity of [PC(34:2) + H]+ (m/z 758.5685) is about five times higher in the inner region of the control than that in the treated spheroid (20.6 μM Irinotecan). Similar trends were obtained from unsupervised ML analysis (Figure 6G) with most metabolites upregulated due to the drug treatment. Because the comparison of ion intensities (Figure 6) was based on the grouped data (i.e., pixels in inner and outer regions) from the supervised and unsupervised ML methods (e.g., Figures 5B and D), minor changes of data grouping using difference approaches may lead to variances in relative ion intensities. For example, the supervised ML results (Figure 6C) indicate that the relative intensity of [PC(34:2) + H]+ in the outer region of the spheroid increased as the drug concentration was increased (from 5 μM to 10 μM), whereas this order was reversed in the unsupervised ML results (Figure 6G).
Figure 6.
Representative common metabolites in the inner and outer regions upregulated by Irinotecan treatment. Results were obtained from (A)−(D) supervised ML (Random Forest) and (E)−(H) unsupervised ML (CLARA) analyses. (*** p < 0.001).
All ions significantly altered by the drug treatment in the outer or inner region are summarized in Table S5. The identifications of all ions were carried out using tandem MS (MS/MS) measurements (Figure S13), and these spectra were compared with online database Metlin.71 To further confirm the metabolites’ structures, MSn analysis can be carried out using standard compounds for comparison. For example, the detection of [PC (34:1) + H]+ from a spheroid slice was confirmed by comparing its MS3 spectra with those obtained from the standard compound (Figure S14).
Large numbers of phospholipids were observed in our experiments. Phospholipids are structural building blocks of cell membranes. In addition, they have important biological functions (e.g., signaling, energy storage, and disease biomarker72) and play key roles in the cell motility, invasion, and metastasis.73,74 The composition of phospholipids can be affected by the extracellular stimuli such as growth factor, oncogene, and hypoxia conditions.75 In our study, the relative abundances of a series of phospholipids, including [PC(32:1) + H]+ (m/z 732.5483) and [PC(34:2) + H]+ (m/z 758.5692), were significantly higher in the Irinotecan treated groups compared with the control. Previous studies indicate that tumor cells can alter their metabolisms to reinforce drug resistance.76 For example, the nuclear receptor PXR (Pregnant X receptor) in cancer cells is activated upon exposure to Irinotecan, resulting in an overexpression of CYP3A4, which is an oxidizing enzyme for xenobiotics such as drug molecules. Overexpressed CYP3A4 further reduces the abundance of Irinotecan inside cells and leads to strengthened drug resistance.77 Meanwhile, the de novo lipogenesis is enhanced by the activated PXR to increase the level of phospholipids,78 which are also believed to promote the cell survival.79,80 Similarly, our previous studies of the extracellular species in spheroids indicate that a large number of phospholipids (e.g., PC, PE, and PA) are increased by Irinotecan treatment.9 Therefore, the increased levels of phospholipids are very likely attributed to the metabolomic response of cancer cells to the anticancer drug in the microenvironment inside spheroids.
CONCLUSION
In this study we cultured HCT-116 spheroids as tumor models and treated them using the anticancer drug Irinotecan. Experiments were carried out using the Single-probe MSI technique. Advanced data analysis methods, including MCR-ALS and ML, were employed to analyze spatially resolved metabolites on spheroid slices, particularly for those significantly regulated by the drug compound. The MCR-ALS algorithm was utilized to decompose each MS image data into three major components: the inner region, the outer region, and the background. Both unsupervised (Random Forest) and supervised (CLARA) ML algorithms were employed to classify each MS image, and similar results were obtained. We further compared the metabolites between the inner and outer regions for each set of MS image data obtained from spheroids in the control and drug treatment groups. We acquired the grouped species in both inner and outer regions that were significantly regulated by the anticancer drug. Compared with conventional methods based on bulk analysis, our method can potentially extract crucial metabolomic information from MSI data and provide spatially resolved metabolites and biomarkers reflecting the influence of drug treatment. The fully established methods may benefit the drug screening, therapeutic resistance, and biomarker discovery.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from National Institutes of Health (R01GM116116 and R21CA204706).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.9b00026.
Experimental details, supporting tables, and supporting figures (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Fennema E; Rivron N; Rouwkema J; van Blitterswijk C; de Boer J Trends Biotechnol 2013, 31 (2), 108–15. [DOI] [PubMed] [Google Scholar]
- (2).Lin RZ; Chang HY Biotechnol. J 2008, 3 (9−10), 1172–84. [DOI] [PubMed] [Google Scholar]
- (3).Hirschhaeuser F; Menne H; Dittfeld C; West J; Mueller-Klieser W; Kunz-Schughart LA J. Biotechnol 2010, 148 (1), 3–15. [DOI] [PubMed] [Google Scholar]
- (4).Weaver EM; Hummon AB; Keithley RB Anal. Methods 2015, 7 (17), 7208–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Minchinton AI; Tannock IF Nat. Rev. Cancer 2006, 6 (8), 583–92. [DOI] [PubMed] [Google Scholar]
- (6).Gaedtke L; Thoenes L; Culmsee C; Mayer B; Wagner EJ Proteome Res 2007, 6 (11), 4111–4118. [DOI] [PubMed] [Google Scholar]
- (7).Friedrich J; Seidel C; Ebner R; Kunz-Schughart LA Nat. Protoc 2009, 4 (3), 309–24. [DOI] [PubMed] [Google Scholar]
- (8).Liu X; Weaver EM; Hummon AB Anal. Chem 2013, 85 (13), 6295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sun M; Tian X; Yang Z Anal. Chem 2017, 89 (17), 9069–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zhang A; Sun H; Wang P; Han Y; Wang X Analyst 2012, 137 (2), 293–300. [DOI] [PubMed] [Google Scholar]
- (11).Bundy JG; Davey MP; Viant MR Metabolomics 2009, 5 (1), 3–21. [Google Scholar]
- (12).Sreekumar A; Poisson LM; Rajendiran TM; Khan AP; Cao Q; Yu J; Laxman B; Mehra R; Lonigro RJ; Li Y; Nyati MK; Ahsan A; Kalyana-Sundaram S; Han B; Cao X; Byun J; Omenn GS; Ghosh D; Pennathur S; Alexander DC; Berger A; Shuster JR; Wei JT; Varambally S; Beecher C; Chinnaiyan AM Nature 2009, 457 (7231), 910–4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- (13).Spratlin JL; Serkova NJ; Eckhardt SG Clin. Cancer Res 2009, 15 (2), 431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Griffin JL; Shockcor JP Nat. Rev. Cancer 2004, 4 (7), 551–61. [DOI] [PubMed] [Google Scholar]
- (15).Roux A; Lison D; Junot C; Heilier JF Clin. Biochem 2011, 44 (1), 119–35. [DOI] [PubMed] [Google Scholar]
- (16).Major HJ; Williams R; Wilson AJ; Wilson ID Rapid Commun. Mass Spectrom 2006, 20 (22), 3295–302. [DOI] [PubMed] [Google Scholar]
- (17).Weljie AM; Newton J; Mercier P; Carlson E; Slupsky CM Anal. Chem 2006, 78 (13), 4430–4442. [DOI] [PubMed] [Google Scholar]
- (18).Werner E; Croixmarie V; Umbdenstock T; Ezan E; Chaminade P; Tabet J-C; Junot C Anal. Chem 2008, 80 (13), 4918–4932. [DOI] [PubMed] [Google Scholar]
- (19).Lee DY; Bowen BP; Northen TR BioTechniques 2010, 49 (2), 557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Miura D; Fujimura Y; Wariishi HJ Proteomics 2012, 75 (16), 5052–60. [DOI] [PubMed] [Google Scholar]
- (21).Evans JD; Jethwa KR; Ost P; Williams S; Kwon ED; Lowe VJ; Davis BJ Pract. Radiat. Oncol 2018, 8 (1), 28–39. [DOI] [PubMed] [Google Scholar]
- (22).Beger RD Metabolites 2013, 3 (3), 552–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Haddadin IS; McIntosh A; Meisamy S; Corum C; Styczynski Snyder AL; Powell NJ; Nelson MT; Yee D; Garwood M; Bolan PJ NMR Biomed 2009, 22 (1), 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Judenhofer MS; Wehrl HF; Newport DF; Catana C; Siegel SB; Becker M; Thielscher A; Kneilling M; Lichy MP; Eichner M; Klingel K; Reischl G; Widmaier S; Rocken M; Nutt RE; Machulla HJ; Uludag K; Cherry SR; Claussen CD; Pichler BJ Nat. Med 2008, 14 (4), 459–65. [DOI] [PubMed] [Google Scholar]
- (25).Li Y; Shrestha B; Vertes A Anal. Chem 2008, 80 (2), 407–420. [DOI] [PubMed] [Google Scholar]
- (26).He J; Luo Z; Huang L; He J; Chen Y; Rong X; Jia S; Tang F; Wang X; Zhang R; Zhang J; Shi J; Abliz Z Anal. Chem 2015, 87 (10), 5372–9. [DOI] [PubMed] [Google Scholar]
- (27).Wang X; Han J; Hardie DB; Yang J; Pan J; Borchers CH Biochim. Biophys. Acta, Proteins Proteomics 2017, 1865 (7), 755–767. [DOI] [PubMed] [Google Scholar]
- (28).Kurreck A; Vandergrift LA; Fuss TL; Habbel P; Agar NYR; Cheng LL Prostate Cancer Prostatic Dis 2018, 21 (3), 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Amstalden van Hove ER; Smith DF; Heeren RM J. Chromatogr. A 2010, 1217 (25), 3946–54. [DOI] [PubMed] [Google Scholar]
- (30).Takats Z; Wiseman JM; Cooks RG J. Mass Spectrom 2005, 40 (10), 1261–75. [DOI] [PubMed] [Google Scholar]
- (31).Nemes P; Vertes A Anal. Chem 2007, 79 (21), 8098–8106. [DOI] [PubMed] [Google Scholar]
- (32).Wu C; Dill AL; Eberlin LS; Cooks RG; Ifa DR Mass Spectrom. Rev 2013, 32 (3), 218–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Knochenmuss R; Dubois F; Dale MJ; Zenobi R Rapid Commun. Mass Spectrom 1996, 10 (8), 871–877. [DOI] [PubMed] [Google Scholar]
- (34).McCombie G; Staab D; Stoeckli M; Knochenmuss R Anal. Chem 2005, 77 (19), 6118–24. [DOI] [PubMed] [Google Scholar]
- (35).Sodhi RN Analyst 2004, 129 (6), 483–7. [DOI] [PubMed] [Google Scholar]
- (36).Dill AL; Eberlin LS; Costa AB; Zheng C; Ifa DR; Cheng LA; Masterson TA; Koch MO; Vitek O; Cooks RG Chem. - Eur. J 2011, 17 (10), 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Alexandrov T; Becker M; Deininger SO; Ernst G; Wehder L; Grasmair M; von Eggeling F; Thiele H; Maass PJ Proteome Res 2010, 9 (12), 6535–6546. [DOI] [PubMed] [Google Scholar]
- (38).Alexandrov T BMC Bioinf 2012, 13 (Suppl 16), S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ruckebusch C; Blanchet L Anal. Chim. Acta 2013, 765, 28–36. [DOI] [PubMed] [Google Scholar]
- (40).Tian X; Zhang G; Shao Y; Yang Z Anal. Chim. Acta 2018, 1037, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Goodacre R; Vaidyanathan S; Dunn WB; Harrigan GG; Kell DB Trends Biotechnol 2004, 22 (5), 245–52. [DOI] [PubMed] [Google Scholar]
- (42).Inglese P; McKenzie JS; Mroz A; Kinross J; Veselkov K; Holmes E; Takats Z; Nicholson JK; Glen RC Chem. Sci 2017, 8 (5), 3500–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Deininger S-O; Ebert MP; Fütterer A; Gerhard M; Röcken C J. Proteome Res 2008, 7 (12), 5230–5236. [DOI] [PubMed] [Google Scholar]
- (44).Nagpal A; Jatain A; Gaur D 2013 IEEE Conference on Information and Communication Technologies (Ict 2013) 2013, 298–303. [Google Scholar]
- (45).Heylman C; Datta R; Sobrino A; George S; Gratton E PLoS One 2015, 10 (12), e0144572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Schwamborn K; Krieg RC; Reska M; Jakse G; Knuechel R; Wellmann A Int. J. Mol. Med 2007, 20 (2), 155–9. [PubMed] [Google Scholar]
- (47).Hanselmann M; Kothe U; Kirchner M; Renard BY; Amstalden ER; Glunde K; Heeren RMA; Hamprecht FA J. Proteome Res 2009, 8 (7), 3558–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Smith A; Piga I; Galli M; Stella M; Denti V; Del Puppo M; Magni F Int. J. Mol. Sci 2017, 18 (12), 2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Rao W; Pan N; Tian X; Yang ZJ Am. Soc. Mass Spectrom 2016, 27 (1), 124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Rao W; Pan N; Yang ZB J. Am. Soc. Mass Spectrom 2015, 26 (6), 986–993. [DOI] [PubMed] [Google Scholar]
- (51).Pan N; Rao W; Kothapalli NR; Liu R; Burgett AW; Yang Z Anal. Chem 2014, 86 (19), 9376–80. [DOI] [PubMed] [Google Scholar]
- (52).Pan N; Rao W; Standke SJ; Yang Z Anal. Chem 2016, 88 (13), 6812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Sun M; Yang Z; Wawrik B Front. Plant Sci 2018, 9, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Liu R; Zhang G; Yang Z Chem. Commun 2018, 54, 14100 [Google Scholar]
- (55).Sun M; Yang Z Anal. Chem 2019, 91 (3), 2384–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Standke SJ; Colby DH; Bensen RC; Burgett AWG; Yang Z Anal. Chem 2019, 91 (3), 1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lanekoff I; Heath BS; Liyu A; Thomas M; Carson JP; Laskin J Anal. Chem 2012, 84 (19), 8351–6. [DOI] [PubMed] [Google Scholar]
- (58).Thomas M; Heath BS; Laskin J; Li DS; Liu E; Hui K; Kuprat AP; van Dam KK; Carson JP 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Embc) 2012; pp 5545–5548. [DOI] [PubMed]
- (59).Li H; Hummon AB Anal. Chem 2011, 83 (22), 8794–801. [DOI] [PubMed] [Google Scholar]
- (60).Ahlf Wheatcraft DR; Liu X; Hummon AB J. Visualized Exp 2014, No. 94, e52313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Race AM; Styles IB; Bunch JJ Proteomics 2012, 75 (16), 5111–5112. [DOI] [PubMed] [Google Scholar]
- (62).Jaumot J; Gargallo R; de Juan A; Tauler R Chemom. Intell. Lab. Syst 2005, 76 (1), 101–110. [Google Scholar]
- (63).Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K R package version 2.0.7–1; 2018.
- (64).Liaw A; Wiener M R news 2002, 2 (3), 18–22. [Google Scholar]
- (65).Tredan O; Galmarini CM; Patel K; Tannock IF J. Natl. Cancer Inst 2007, 99 (19), 1441–54. [DOI] [PubMed] [Google Scholar]
- (66).Mehta G; Hsiao AY; Ingram M; Luker GD; Takayama SJ Controlled Release 2012, 164 (2), 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ward JP; King JR Math. Biosci 2003, 181 (2), 177–207. [DOI] [PubMed] [Google Scholar]
- (68).Bleicher RJ; Cabot MC Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2002, 1585 (2), 172–178. [DOI] [PubMed] [Google Scholar]
- (69).Testai FD; Kilkus JP; Berdyshev E; Gorshkova I; Natarajan V; Dawson GJ Neurochem 2014, 131 (4), 530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Alvarez-Perez J; Ballesteros P; Cerdan S MAGMA 2005, 18 (6), 293–301. [DOI] [PubMed] [Google Scholar]
- (71).Smith CA; O’Maille G; Want EJ; Qin C; Trauger SA; Brandon TR; Custodio DE; Abagyan R; Siuzdak G Ther. Drug Monit 2005, 27 (6), 747–751. [DOI] [PubMed] [Google Scholar]
- (72).Lordan R; Tsoupras A; Zabetakis I Molecules 2017, 22 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Cummings BS Biochem. Pharmacol 2007, 74 (7), 949–59. [DOI] [PubMed] [Google Scholar]
- (74).Inazu M Biopharm. Drug Dispos 2014, 35 (8), 431–49. [DOI] [PubMed] [Google Scholar]
- (75).Jung JH; Lee MY; Choi DY; Lee JW; You S; Lee KY; Kim J; Kim KP Proteomics 2015, 15 (4), 824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Tamada M; Nagano O; Tateyama S; Ohmura M; Yae T; Ishimoto T; Sugihara E; Onishi N; Yamamoto T; Yanagawa H; Suematsu M; Saya H Cancer Res 2012, 72 (6), 1438–48. [DOI] [PubMed] [Google Scholar]
- (77).Wang J; Hong F; Xue T; Xu P; Zhai Y Recent Advance In Colon Cancer 2017, 2, 2–36. [Google Scholar]
- (78).Moreau A; Vilarem MJ; Maurel P; Pascussi JM Mol. Pharmaceutics 2008, 5 (1), 35–41. [DOI] [PubMed] [Google Scholar]
- (79).Zhou Y; Bollu LR; Tozzi F; Ye X; Bhattacharya R; Gao G; Dupre E; Xia L; Lu J; Fan F; Bellister S; Ellis LM; Weihua Z Mol. Cancer Ther 2013, 12 (12), 2782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Menendez JA; Lupu R Nat. Rev. Cancer 2007, 7 (10), 763–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.