Abstract
Heart failure (HF) is a functional lack of myocardial performance due to a loss of molecular control over increases in calcium and ROS, resulting in proteolytic degradative advances and cardiac remodeling. Mitochondria are the molecular powerhouse of cells, shifting the sphere of cardiomyocyte stability and performance. Functional mitochondria rely on the molecular abilities of safety factors such as TFAM to maintain physiological parameters. Mitochondrial transcription factor A (TFAM) creates a mitochondrial nucleoid structure around mtDNA, protecting it from mutation, inhibiting NFAT (ROS activator/hypertrophic stimulator), and transcriptionally activates Serca2a to decrease calcium mishandling. Calpainl and MMP9 are proteolytic degratory factors that play a major role in cardiomyocyte decline in HF. Current literature depicts major decreases in TFAM as HF progresses. We aim to assess TFAM function against Calpainl and MMP9 proteolytic activity and its role in cardiac remodeling. To this date, no publication has surfaced describing the effects of aortic banding (AB) as a surgical HF model in TFAM-TG mice. HF models were created via AB in TFAM transgenic (TFAM-TG) and C57BLJ-6 (WT) mice. Eight weeks post AB, functional analysis revealed a successful banding procedure, resulting in cardiac hypertrophy as observed via echocardiography. Pulse wave and color doppler show increased aortic flow rates as well as turbulent flow at the banding site. Preliminary results of cardiac tissue immuno-histochemistry of HF-control mice show decreased TFAM and compensatory increases in Serca2a fluorescent expression, along with increased Calpainl and MMP9 expression. Protein, RNA, and IHC analysis will further assess TFAM-TG results post-banding. Echocardiography shows more cardiac stability and functionality in HF-induced TFAM-TG mice than the control counterpart. These findings complement our published in vitro results. Overall, this suggests that TFAM has molecular therapeutic potential to reduce protease expression.
Keywords: Heart failure, TFAM transgenic, Calpainl, MMP9, Serca2a, NFAT
Introduction
Hypertensive heart disease and heart failure (HF) are the leading causes of death in the United States [1]. HF is composed of activated cell death pathways and mechanisms of degradation [2]. The culmination of Ca2+ mishandling and ROS-driven degradation within cardiomyocytes leads to a functional decline of the myocardium and hypertrophic expansion. HF or the inefficiency of the heart to pump blood has a high fatality rate 5 years post onset [1]. Mitochondrial factors such as TFAM play a vital role in inhibiting cardiac remodeling factors, which lead to cardiac hypertrophy and HF [3]. Throughout our work, we observed that TFAM overexpression resulted in cellular/functional recovery by reducing cardiac remodeling.
HF has reduced TFAM expression, increased oxidative stress, Ca2+ mishandling, and protease expression leading to cardiomyocyte functional decline [4]. Previous studies involved in TFAM knockdown have resulted in reduced mitochondrial biogenesis, dilated cardiomyopathy, and neonatal death [5]. TFAM’s inhibitory function to mitigate molecular abnormalities has previously resulted in cardioprotective effects when overexpressed [3]. A mechanistic analysis of TFAM’s protective role in pathological cardiac remodeling gives further insight into both TFAM’s inhibitory effect on the NFAT-MMP9 pathway and regulatory effect on the SERCA2a-Calpainl pathway.
Calpainl is responsible for the degradation of contractile proteins, initiation of mitochondrial-driven apop-tosis, and decrease of Ca2+ transporters in HF [6–8]. HF patients exhibit marked increases in calpains [9]. Within the Ca2+–driven catabolic pathway, Ca2+ activates and increases the expression of calpain proteases and the Ca2+–calcineu-rin-NFAT cascade that promotes pathological hypertrophy and activates NADPH oxidases to increase ROS production [10].
NFAT is a pathological hypertrophic gene regulator and inducer of ROS via activation of NADPH oxidases [10, 11]. NFAT increases in cardiac remodeling and hypertrophy [12]. Cytoplasmic Ca2+ increases maladaptive factors NFAT4 and Calpainl.
MMP9 is a known pathological indicator of HF, and its expression is highly up-regulated in the pathological myocardium [13]. In alignment with this logic, the erasure of MMP9 evokes opposite effects: targeted deletion of MMP9 prevents cardiac dysfunction [14]. Early-stage aortic banding (AB) results in inhibition of MMP9, but sustained mechanical overload causes de-compensatory HF, leading to increased MMP9 expression and cardiac fibrosis in end-stage HF. As aforementioned, activation of MMP9 increases extracellular matrix turnover in the failing heart, resulting in increased apoptotic signaling in pressure overload HF. Mitochondrial dysfunction induces ROS production, activating MMPs in cardiomyocytes [15]. Superoxides create stress, potentially mutating and damaging mitochondrial DNA.
TFAM physically wraps around mitochondrial DNA, forming a mitochondrial nucleoid; it is essential to mitochondrial biogenesis. One transcriptional function of TFAM is regulation over factors such as NFAT and SERCA2a, which provides insight into reducing Ca2+ mishandling and ROS production [16, 17]. Within TFAM overexpression models, it is observed that proteolytic enzyme MMP9 is reduced in Mi-induced HF, along with functional morphologies [18]. Contrastingly, our previous study shows that CRISPR-CAS9 knockdown of TFAM induces the activation of proteases MMP9 and Calpainl, as well as pathological hypertrophic stimulator and ROS-producer NFAT4 [19].
The usage of animal models to study cardiac hypertrophy and HF is vital to further elucidation of pathophysiological mechanisms. Rockman et al. [20] developed the murine TAC procedure, also known as AB. TAC narrows the transverse aorta, causing both a pressure increase in the left ventricular chamber and the resultant force required for blood ejection. Increased resistance within this model causes a pressure overload, resulting in severe hypertension and HF.
The purpose of this study is to investigate the role of TFAM overexpression in cardiac remodeling within the TAC HF model. We hypothesized that TFAM overexpression would reduce molecular remodeling factors and associated morphologies. We examined whether TFAM overexpression would reduce molecular hypertrophic (NFAT) and proteolytic factors (MMP9 & Calpainl) involved in adverse remodeling. Our results affirm that TFAM overexpression in TAC HF models reduces cardiac hypertrophy, molecular hypertrophic factors, and proteolytic agents.
Materials and methods
Animal models and experimental design
Wild-type mice (WT, C57/BL6/J) were purchased from the Jackson Laboratory (Bar Harbor, ME). TFAM transgenic mice were procured from Cyagen Biosciences (Santa Clara, CA, USA) and housed in the animal care facility at the University of Louisville with access to standard chow and water. The AB surgical procedure was performed in mice of 8–10 weeks of age with starting weights of 22–26 g. The groups were further divided into WT Sham, WT TAC, TFAM Sham, and TFAM TAC. Animals were euthanized according to National Institute of Health Guidelines for animal research and were reviewed and approved by the Institute of Animal Care and use committee of the University of Louisville (IACUC #16684 & 14028).
Genotyping
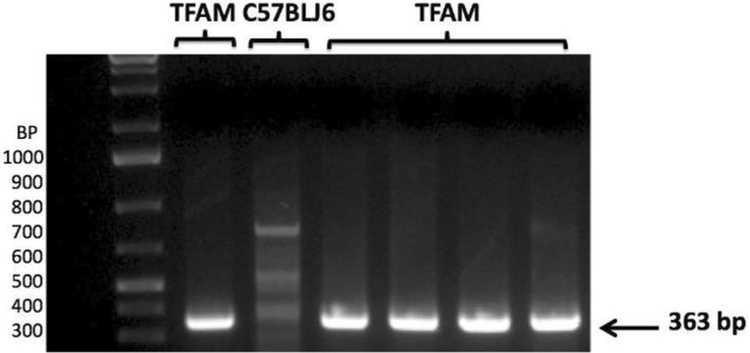
The TFAM transgenic mice background was confirmed according to the Cyagen Bioscience protocol for genotyping. Samples from the tails of the mice were collected for DNA extraction. DNA was amplified with TFAM Primer sets and run on PCR. The PCR product samples were run on a 2% agarose gel containing [Acetic Acid, Tris Base, EDTA, pH 8.4] with ethidium bromide. Gel images were recorded on a Bio-Rad gel documentation system (Bio-Rad, Hercules, CA). The transgenic TFAM gene yields a product size of 363 bp in a 2% agarose gel and is observed in the BioRad ChemiDoc.
Antibodies and reagents
All primary antibodies were purchased from Abeam (Cambridge, United Kingdom). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Fluorescent secondary antibodies and lipofectamine 2000 reagent were purchased from Life Technologies (Carlsbad, California, USA).
Abeam primary antibodies used are as follows: Calpainl (ab28258), Serca2 (ab2861), NFAT4 (ab83832), TFAM (ab 131607) and MMP9 (ab38898).
Western blot analysis
Post 48-h treatment period, cardiac tissue protein was isolated using protein extraction buffer (RIPA lysis buffer, protease inhibitor cocktail and PMSF). Lysates were spun in extraction buffer for 12 h and then centrifuged at 12,000 × g for 15 min. Supernatant was transferred to new tubes and protein concentrations were analyzed via Bradford protein estimation assay. Samples were run on a 10/12% sodium dodecylsulfate (SDS)-polyacrylamide gel with Tris-glycine SDS buffer. The gel was transferred electrophoretically overnight onto a PVDF membrane at 4 °C. The membrane was blocked with a 5% milk solution for 1 h. Primary antibodies were diluted at a concentration 1:1000 in TBST and incubated on the membrane overnight. All membranes were washed in TBST solution four times and then incubated with secondary HRP conjugated antibody solution for 1 h at room temperature. Four TBST washing steps followed before membranes were developed using a chemilumines-cent substrate in a BioRad Chemidoc (Hercules, Calif.). Band intensity was determined using densitometry analysis. Beta-actin was used to normalize protein loading.
Immunohistochemistry
Post cell treatment, media were aspirated from each well of the eight well chamber slide and cells were rinsed with PBS. Cells were fixed at room temperature in 4% paraformaldehyde solution for 20 min and washed three times with PBS. Blocking was performed with a mixed solution of 2% bovine-serum-albumin (BSA) and 0.025% Triton X-100. Cells were permeabilized for 1 h. All primary antibodies were incubated in the chamber slide at a concentration of 1:100 overnight at 4 °C. The wells were aspirated, washed three times and incubated for 90 min with the secondary antibody (1:200) at room temp. The chamber slides were washed three times more, and DAPI was added at 1:10,000 for 10 min. This analysis was observed via Confocal microscopy.
Blood pressure
Non-invasive tail cuff method was used to measure blood pressure in conscious mice (CODA, Kent Scientific, Tor-rington CT). Animals were allowed time to acclimate to chambers and warming pad prior to data recordings. BP was recorded under standard conditions.
Ultrasound
Through Vevo 2100 imaging system, cardiac and aortic data were collected in pre-surgical and post-surgical animal models. Post epilation, the animal is placed supine on a warm platform (37 °C), under isofiurane anesthesia and fixed. Using a MS550D (22–25 mHz) transducer, the thoracic cavity was imaged. Aortic arch velocity and cardiographie function were assessed in pulse wave and color Doppler modes.
Dihydroethidium (DHE) super-oxide stain
Intra-cardial injection of DHE (Thermo-Fisher Scientific, D1168) was applied to mouse myocardium before preserving the tissue. Imaging performed within two hours of application and DHE was diluted in DMSO.
Aortic banding surgical procedure (frans-aortic constriction (TAC))
The TAC-banding procedure is an intra-thoracic surgical procedure (performed by Dr. George H. Kunkel) to induce cardiac hypertrophy 6–8 weeks post surgery and heart failure in mice 8–10 weeks post surgery. A midline ventral incision is made and the thoracic cavity is exposed above the third rib, rib-retractors are placed and a portion of the ascending aorta (between the innominate artery and left carotid artery) is isolated. Ligature is placed around the vessel to restrict flow (assisted by a 271/2 g needle). Sutures are used to close incisions.
Cryosectioning
Cardiac tissue was preserved in plastic tissue embedding molds (polysciences) containing tissue freezing media (Triangle Biosciences, Durham, NC). Tissues were stored at −80 °C. Sections of 8 μm were created using a cryocut (leica CM 1850). Cryosections were placed on superfrost plus (lysine coated) microscope slides and stored at − 80 °C for further use in immuno-histochemistry.
Statistical analysis
Data are expressed as means ± SE. Statistical analysis was performed by Graphpad Prism software using a one-way ANOVA followed by a Bonferroni comparison test. P< 0.05 was considered statistically significant. The statistical significance is relevant to an N of 5 per group.
Results
Verification of aortic banding procedure
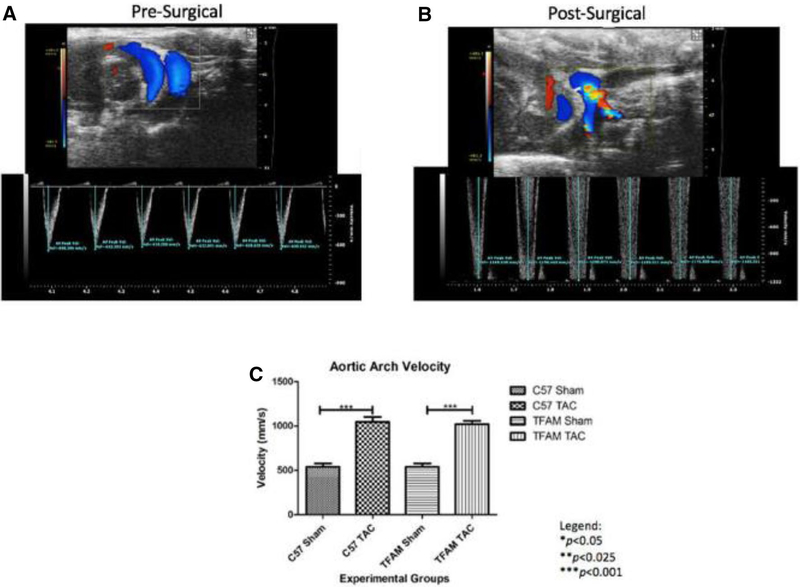
Through genotyping PCR, we confirmed Cyagen Biosciences TFAM transgenic mouse protocol to ensure pups are genetically modified with TFAM overexpression. TFAM gene is overexpressed at 363 base pairs (Fig. 1). This intra-thoracic surgical procedure known as TAC was performed to induce cardiac hypertrophy/HF in 8-week old mice. The AB procedure is verified via ultrasound, at which high aortic arch velocity, as well as turbulence was observed at the transverse aorta, comparing WT and TFAM sham groups to the aortic banded counterpart (P< 0.001) (Fig. 2).
Fig. 1.
Genotyping for TFAM gene in TFAM transgenic and C57BLJ6 mice. As per Cyagen Biosciences protocol, primers for TFAM gene expression are identified at 363 bp in a 2% agarose gel and analyzed via BioRad ChemiDoc
Fig. 2.
a The top, an ultrasound-echocardiographic snapshot, displays blood flow through the aorta of a particular pre-surgical mouse performed with a Vevo 2100 ultrasound pulse wave and color doppler analysis. As indicated by the blue coloration, the blood flow is rhythmically non-turbulent. Below, the respective real-time graph of AAV versus time displays a consistent AAV average value of 636.14 mm/s. b The ultrasound-echocardiographic snapshot shows blood flow through the aorta performed with a Vevo 2100 ultrasound pulse wave and color doppler analysis. As indicated by the blue-red-yellow amal gamation, the blood flow is rhythmically turbulent. The respective real-time graph of AAV versus time displays an increased AAV average value of 1184.10 mm/s from its pre-surgical value seen in 14A. c This graph displays the AAV corresponding to the specified experimental groups and the data were graphed for an N of 6 using SEM for significance. The substantial increase in aortic velocity from WT-Sham group to the WT-TAC group and the substantial increase from TFAM Sham group to the TFAM TAC group verify that the surgery worked. Values are expressed as ±SEM. N=6. ***p<0.001
Effect of aortic banding on cardiac function
TFAM overexpression reduced cardiac hypertrophy
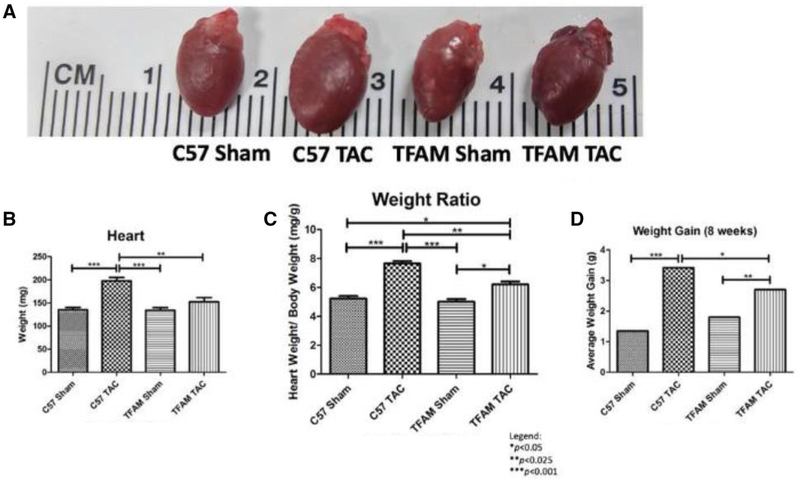
Cardiac hypertrophy was shown in three ways, a visual comparison of the hearts, heart weights, and heart weight/body weight ratio. Figure 3a shows a pictographic image of WT and TFAM-TG mice subjected to AB and visually comparing the hearts. This image shows a significant visual difference is found when comparing the TAC and sham groups and the WT TAC to the TFAM-TG TAC model. Heart weight is significantly increased in the WT TAC group compared to the WT sham, TFAM sham, and TFAM TAC group (Fig. 3b). The heart weight/body weight (Hw/Bw) ratio was also significantly increased in both the WT TAC and TFAM TAC groups. The WT TAC group had significantly increased Hw/Bw ratio when compared to the WT sham, TFAM sham, and the TFAM TAC (Fig. 3c). Additionally, a weight gain is observed within the banded animals. The WT TAC gained approximately 2 g more than the sham group and 1 g more than the TFAM-TAC group (Fig. 3d).
Fig. 3.
a The image displays the cardiac hypertrophy state after 8 weeks correlating to the respective experimental groups. Using visual confirmation, significantly enlarged WT heart due to aortic banding surgical procedure was observed when compared to the sham. Additionally, TFAM-TG reduced hypertrophic expansion compared to the TFAM-TG control, b This graph displays heart mass as assessed by micro-calibrated weight scale. The values correspond to the specified experimental groups after the course of 8 weeks. The TFAM TAC heart maintained a significantly lower mass when compared to the WT TAC. c This graph depicts the heart weight-to-body weight ratio that correspond to the specified experimental groups after the course of 8 weeks. The TFAM TAC heart maintained a significantly lower ratio when compared to the WT TAC. d This graph shows overall weight gain of the corresponding to the specified groups after the course of 8 weeks. This was performed by deducting the initial and final mouse weights allowed for an assessment of weight gain and variation among controls and transgenic animals. Furthermore, the TFAM TAC mice maintained a significantly lower mass weight gain compared to the WT TAC mice. Values are expressed as ±SEM. N=5; *P<0.05, **P< 0.025, ***P<0.001
Analysis of echocardiography and blood pressure results
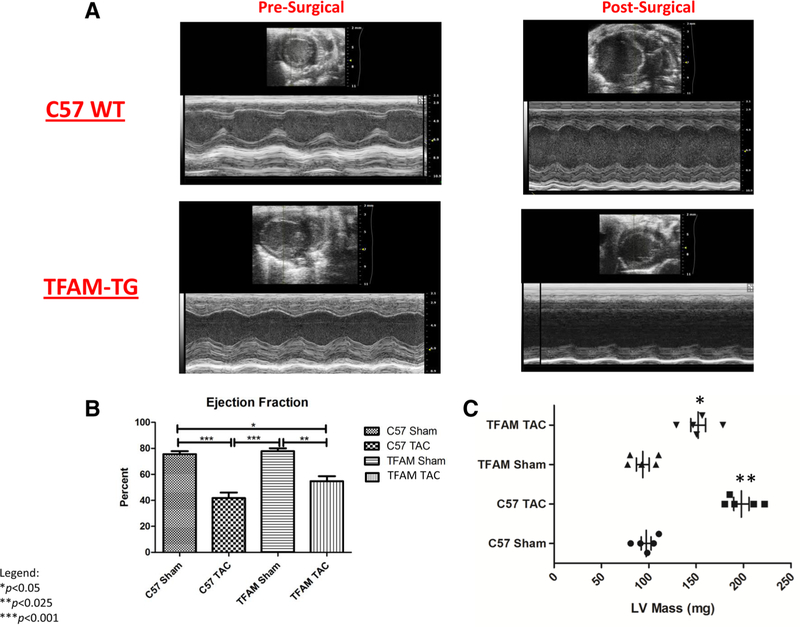
Echocardiography was performed to verify the banding procedure and to analyze size and flow variation between sham and TAC animals. Blood pressure was analyzed via the tail cuff method to assess hemodynamic changes due to banding. Echocardiography and blood pressure analysis show significant differences between the aortic banded and sham animals. Ejection fraction (EF) of both TFAM-TG and WT banded groups showed a significant reduction in comparison to respective shams (Fig. 4a, b). Changes in left ventricle (LV) mass post banding were also observed; significantly increased LV mass was found within the WT TAC and TFAM TAC groups when compared to the Sham groups. Additionally, a significant increase in LV mass was found in the WT TAC group when compared to the TFAM TAC group. Cardiac muscle hypertrophy is a compensatory response to pressure overload (Fig. 4c). Within Table 1, we observe that many significant functional changes occur during pressure overload HF, as is observed when comparing the Sham and TAC groups. Functional differences are also observed between the WT surgical model and the TFAM-TG model; such differences include increased fractional shortening, LV volume in systole, LV mass, LV volume in systole had a significant increase in volume in the WT TAC compared to the WT sham (Table 1). Variations exist between the TFAM transgenic and its wildtype counterpart suggesting that known and unknown mechanisms of mitochondrial protection are inhibiting functional decline in the failing myocardium.
Fig. 4.
a These images (i-iv) captured by echocardiographic-ultra-sound corresponding to the specified experimental groups and data taken after 8 weeks. The specified values taken from these images correspond to the values discussed in Table 1. b This graph displays the EF corresponding to the specified experimental groups and the data were taken after 8 weeks. The significant decrease in EF from WT Sham group to the WT TAC group and the significant decrease from TFAM Sham group to the TFAM TAC group verify that the aortic banding induced HF. Values are expressed for an N of 6 using SEM for significance as ±SEM. N=6. *P< 0.05, **P< 0.025, ***P<0.001. c This graph displays the LV mass corresponding to the specified experimental groups and the data were taken after 8 weeks. The significant increase in LV mass from WT Sham group to the WT TAC group and the significant increase from TFAM Sham group to the TFAM TAC group verify that the aortic banding induced HF. Values are expressed for an N of 6 using SEM for significance as ±SEM. N=6. *P<0.05, **P<0.025
Table 1.
Echocardiographic measurements of TFAM-TG mice at baseline and 8 weeks after sham/TAC surgery
| Baseline | 8-week | |||||||
|---|---|---|---|---|---|---|---|---|
| N=6 | N=7 | N=6 | N=6 | N=6 | N=5 | N=6 | N=5 | |
| Groups | C57 Sham | C57 TAC | TFAM Sham | TFAM TAC | C57 Sham | C57 TAC | TFAM Sham | TFAM TAC |
| IVSd (mm) | 0.65 ± 0.03 | 0.60 ± 0.04 | 0.62 ± 0.02 | 0.61 ± 0.03 | 0.60 ± 0.02 | 0.88 ± 0.02***,### | 0.64 ± 0.02 | 0.79 ± 0.03***,### |
| LVDd (mm) | 3.49 ± 0.15 | 3.57 ± 0.22 | 3.6 ± 0.07 | 3.53 ± 0.14 | 3.58 ± 0.05 | 4.13 ± 0.09*,# | 3.7 ± 0.08 | 3.97 ± 0.12*,# |
| LVPWd (mm) | 0.63 ± 0.02 | 0.67 ± 0.03 | 0.61 ± 0.04 | 0.65 ± 0.01 | 0.64 ± 0.07 | 0.97 ± 0.05**,## | 0.65 ± 0.01 | 0.88 ± 0.09**,## |
| LVDs (mm) | 1.74 ± 0.11 | 1.81 ± 0.08 | 1.85 ± 0.08 | 1.80 ± 0.10 | 1.91 ± 0.15 | 2.98 ± 0.15***,### | 1.94 ± 0.09 | 2.45 ± 0.07***,### |
| LV Vold (ml) | 58.2 ± 5.32 | 61.4 ± 3.88 | 60.9 ± 5.14 | 65.3 ± 3.21 | 63.24 ± 4.06 | 88.6 ± 4.35*,# | 62.05 ± 3.54 | 72.4 ± 3.86*,#,$ |
| LV Vols (ml) | 9.12 ± 0.88 | 10.24 ± 1.56 | 10.12 ± 1.04 | 11.48 ± 2.56 | 10.81 ± 2.09 | 66.54 ± 5.63***,### | 11.12 ± 2.08 | 48.39 ± 4.11**,###,$ |
| EF (%) | 81.7 ± 2.01 | 80.1 ± 1.35 | 82.4 ± 1.21 | 82.8 ± 1.19 | 81.6 ± 1.83 | 48.4 ± 3.69***,### | 80.2 ± 1.85 | 53.1 ± 4.81***,### |
| FS (%) | 48.2 ± 1.58 | 49.4 ± 1.08 | 48.6 ± 1.32 | 49.6 ± 1.78 | 49.5 ± 0.82 | 24.2 ± 1.84***,### | 48.4 ± 0.9 | 28.32 ± 4.64***,### |
| LV mass (mg) | 75.5 ± 4.62 | 78.4 ± 5.51 | 82.4 ± 3.8 | 84.2 ± 4.28 | 81.6 ± 5.6 | 191.6 ± 16.9***,### | 83 ± 4.84 | 164 ± 13.67***,###,$ |
| HR (beats/min) | 640 ± 27 | 690 ± 11.4 | 670 ± 33 | 648 ± 23 | 694 ± 15 | 701 ± 11 | 686 ± 10 | 691 ± 18 |
The table above depicts the echocardiographic measurements after 8 weeks of aortic banding surgery. The groups above signify the following: (1) IVSd, intra-ventricular septal diameter, (2) LVDd, Left ventricular dimension in diastole, (3) LVPWd, left ventricular posterior wall diameter, (4) LVDs, left ventricular dimension in systole, (5) LV Vold, left ventricular volume in diastole, (6) LV Vols, left ventricular volume in systole, (7) EF, ejection fraction, (8) FS, fractional shortening, (9) LV, left ventricular, (10) HR, heart rate
P< 0.001,
P< 0.025,
P<0.05 versus baseline in the same group
P< 0.001,
P< 0.025,
P< 0.05 versus sham in the same group at the same time point
P<0.001,
P<0.025,
P<0.05 versus TAC-operated WT mice. Values are expressed as ±SEM
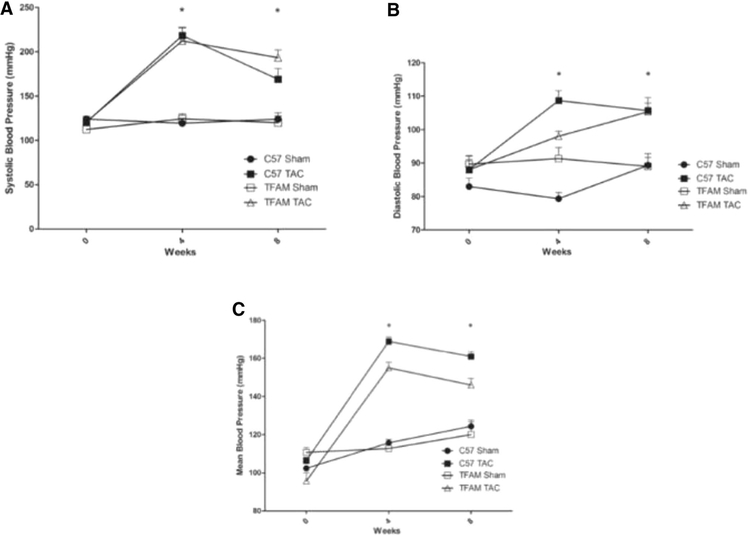
The Physiological functional assessment of hemodynamic blood pressure measurements was observed at three-time points: 0, 4, 8 weeks, via CODA tail cuff blood pressure system. Time point 0 was a pre-surgical assessment and is used as a baseline. At 4 weeks, severe hypertension is observed in both TAC groups and is significantly higher than the sham group (Fig. 5). Between 4 and 8 weeks, the WT group maintains a trending decline in systolic blood pressure, this functional change is associated with cardiac remodeling allowing for the heart to adapt to the pressure overload through hypertrophic expansion (Fig. 5a). The TFAM TAC group maintains a higher systolic blood pressure than the WT TAC group in the 4- to 8-week time point; therefore, a functional difference remains between the WT and TFAM TAC animals. Diastolic blood pressure was assessed and revealed a significant difference between the WT TAC and TFAM TAC groups and their sham counterparts at both 4- and 8-week time points (Fig. 5b). Mean blood pressure assessment showed a significant difference between the WT TAC and TFAM TAC groups and their sham counterparts (Fig. 5c).
Fig. 5.
a This graph displays the Systolic Blood Pressure measured by the CODA (Tail-cuff) blood pressure system. The values correspond to the specified experimental groups throughout the course of 8 weeks. The significant decline occurs after 4 weeks. Values are expressed as ±SEM. N=5; *P<0.05, **P< 0.025, ***P< 0.001. b This graph displays the Diastolic Blood Pressure measured by the CODA (Tail-cuff) blood pressure system. The values correspond to the specified experimental groups throughout the course of 8 weeks. The significant decline occurs after 4 weeks. Values are expressed as ± SEM. N = 5; * P < 0.05, ** P < 0.025, *** P < 0.001. c This graphdisplays the Mean Blood Pressure measured by the CODA (Tail-cuff) blood pressure system. The values correspond to the specified experimental groups throughout the course of 8 weeks. The significant decline occurs after 4 weeks. Values are expressed as ± SEM. N = 5; *P < 0.05, **P < 0.025, ***P < 0.001
The effect of TFAM overexpression on the NFAT-MMP9 pathway
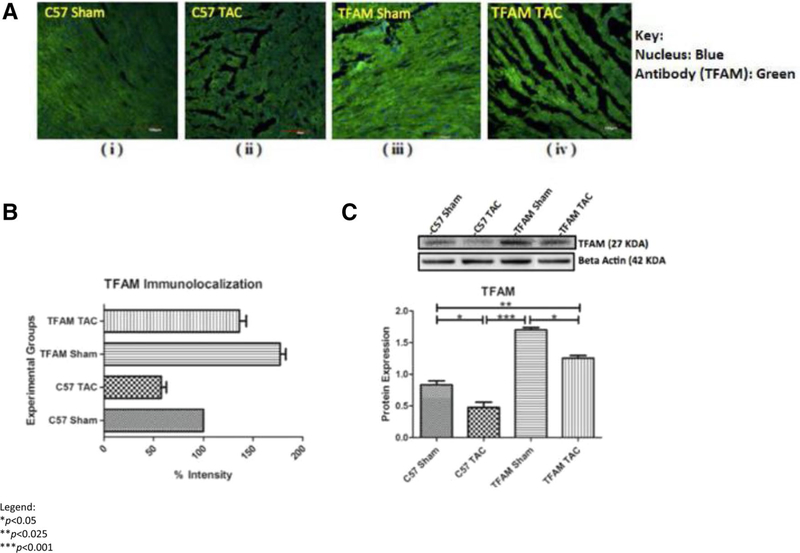
TFAM efficacy in transgenic models was confirmed via western blotting and immunohistochemistry for TFAM. Western blotting and IHC were used to analyze differences in protein expression amongst factors of cardiac remodeling involving protease MMP9. Fluorescent intensity was observed via confocal microscopy (Fig. 6a). Concluding that the TFAM sham had significantly increased TFAM fluorescent intensity (Fig. 6b). Western blotting confirmed a significant increase in TFAM protein expression in both TFAM sham and TFAM TAC models. There was a significant decrease in TFAM protein expression in the WT TAC model (Fig. 6c).
Fig. 6.
a These confocal microscopy and IHC images (i-iv) provide intra-cellular visualizations of TFAM within the corresponding experimental groups. TFAM is identified in green fluorescence and nuclei are identified by blue fluorescence, b This graph exhibits the fluorescent intensity of TFAM 8 weeks after TAC and was provided by the data collected from the Image J software. Each fluorescent intensity corresponds to TFAM’s presence within the specific experimental groups. With respect to TFAM fluorescent intensity, the TFAM overexpression Sham group was significantly greater relative to the WT Sham group. Also, the TFAM TAC group was shown to be significantly greater in intensity of TFAM relative to the WT TAC group, c This graph displays TFAM proteins expression 8 weeks after the aortic banding surgery. Lysates were analyzed by western blotting for TFAM protein expression. The TFAM overexpression Sham group was significantly greater in protein expression of TFAM relative to the WT Sham group. The TFAM overexpression TAC group was significantly greater in protein expression of TFAM relative to the WT TAC group. Both TAC groups were significantly decreased in TFAM protein expression when compared to their Sham counterparts. The bands were quantified using densitometry and the data were graphed for an N of 5 using SEM for significance. Values are expressed as ±SEM. N=5. *P<0.05, **P<0.025, ***P< 0.001
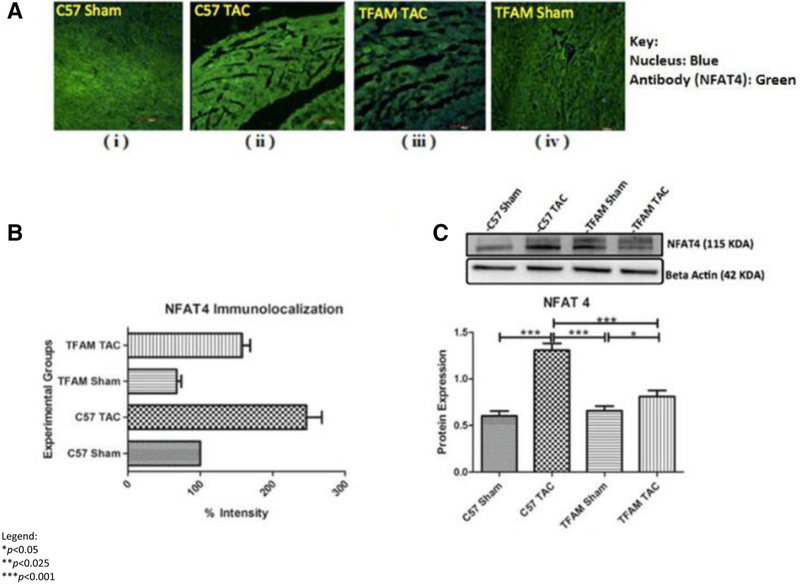
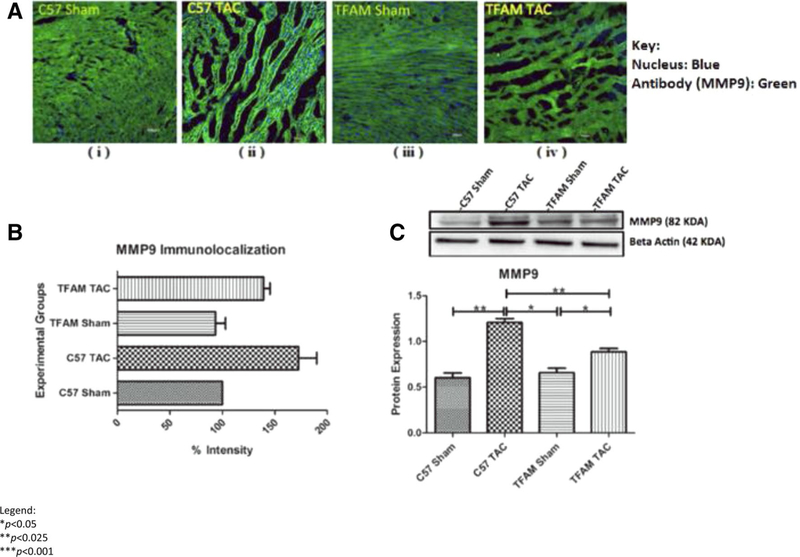
Determining if TFAM overexpression affected NFAT4, we observe NFAT4 fluorescent intensity and Western blotting. The WT TAC group had significantly increased NFAT4 expression compared to all groups (Fig. 7a, b). The TFAM TAC group had significantly higher NFAT4 protein expression in comparison to its sham (Fig. 7c). MMP9 fluorescent intensity is increased in the WT TAC model in comparison to the WT sham, TFAM sham, and TFAM TAC (Fig. 8a, b). Western blotting analysis revealed a significant increase in MMP9 protein expression (Fig. 8c). TFAM overexpression plays a role in the NFAT-ROS-MMP9 pathway.
Fig. 7.
a These confocal microscopy and IHC images (i–iv) provide intra-cellular visualizations of NFAT4 within the corresponding experimental groups. TFAM is identified in green fluorescence and nuclei are identified by blue fluorescence. b This graph exhibits the fluorescent intensity of NFAT4 8 weeks after TAC and was provided by the data collected from the Image J software. Each fluorescent intensity corresponds to NFAT4’s presence within the specific experimental groups. With respect to NFAT4 fluorescent intensity, the TFAM TAC group was shown to be significantly lower in intensity of NFAT4 relative to the WT TAC group. c This graph displays NFAT4 proteins expression 8 weeks after the aortic banding surgery. Lysates were analyzed by western blotting for NFAT4 protein expression. The TFAM overexpression TAC group was significantly lower in protein expression of NFAT4 relative to the WT TAC group. Both TAC groups were significantly increased in NFAT4 protein expression when compared to their Sham counterparts. The bands were quantified using densitometry and the data were graphed for an N of 5 using SEM for significance. Values are expressed as ± SEM. N = 5. *P < 0.05, ***P < 0.001
Fig. 8.
a These confocal microscopy and IHC images (i-iv) provide intra-cellular visualizations of MMP9 within the corresponding experimental groups. MMP9 is identified in green fluorescence and nuclei are identified by blue fluorescence, b This graph exhibits the fluorescent intensity of MMP9 8 weeks after TAC and was provided by the data collected from the Image J software. Each fluorescent intensity corresponds to MMP9’s presence within the specific experimental groups. With respect to MMP9 fluorescent intensity, the TFAM TAC group was shown to be significantly lower in intensity of MMP9 relative to the WT TAC group, c This graph displays MMP9 proteins expression 8 weeks after the aortic banding surgery. Lysates were analyzed by western blotting for MMP9 protein expression. The TFAM overexpression TAC group was significantly lower in protein expression of MMP9 relative to the WT TAC group. Both TAC groups were significantly increased in MMP9 protein expression when compared to their Sham counterparts. The bands were quantified using densitometry and the data were graphed for an N of 5 using SEM for significance. Values are expressed as ±SEM. N=5. *P<0.05, **P<0.025
The effect of TFAM overexpression on the SERCA2a-Calpainl pathway
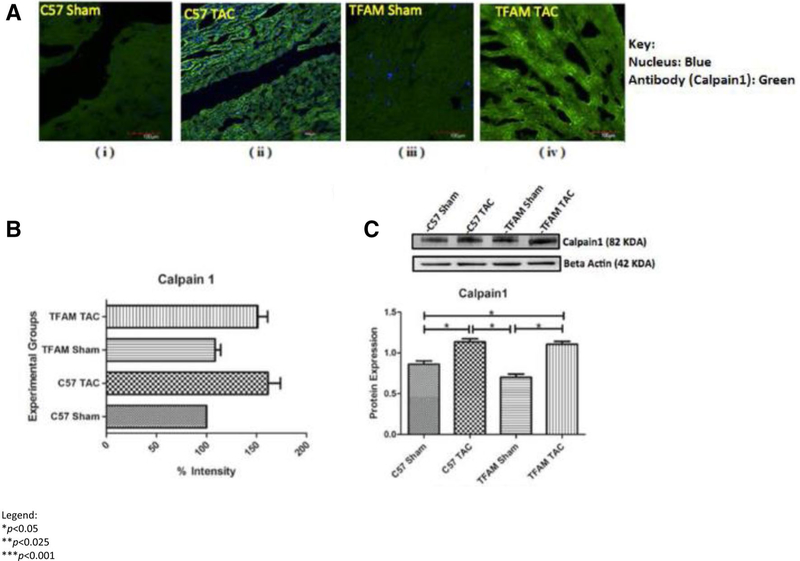
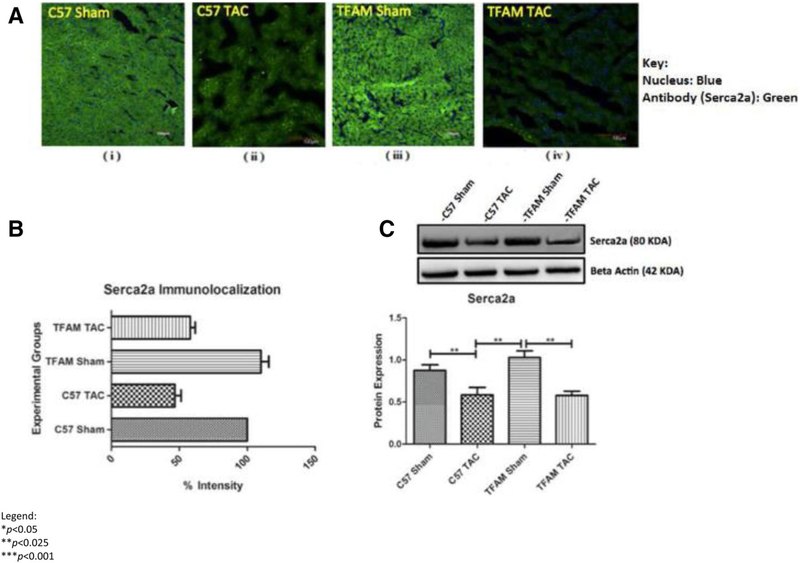
Western blotting and fluorescent analysis were also used to analyze the Serca2a-Calpainl pathway. This analysis of protein expression from cardiac tissue of mice subjected to AB and sham controls shows that SERCA2a is significantly decreased in pressure overload HF. Through Western blotting and immunohistochemistry, the SER-CA2a-Calpainl pathway was observed for protein and fluorescent expression. Fluorescent intensity for Calpainl was viewed via confocal microscopy (Fig. 9). Both aortic banded groups WT and TFAM-TG had significantly decreased SERCA2a expression. This was observed in western blotting and IHC (Fig. 10a–c).
Fig. 9.
a These confocal microscopy and IHC images (i-iv) provide intra-cellular visualizations of Calpainl within the corresponding experimental groups. Calpainl is identified in green fluorescence and nuclei are identified by blue fluorescence, b This graph exhibits the fluorescent intensity of Calpainl 8 weeks after TAC and was provided by the data collected from the Image J software. Each fluorescent intensity corresponds to Calpainl’s presence within the specific experimental groups. With respect to Calpainl fluorescent intensity, the TFAM TAC group was shown to be significantly greater in intensity of TFAM relative to the WT TAC group, c This graph displays Calpainl proteins expression 8 weeks after the aortic banding surgery. Lysates were analyzed by western blotting for Calpainl protein expression. The TFAM overexpression TAC group was significantly greater in protein expression of Calpainl relative to the WT TAC group. The bands were quantified using densitometry and the data were graphed for an N of 5 using SEM for significance. Values are expressed as ±SEM. N=5. *P<0.05, **P< 0.025, ***P< 0.001
Fig. 10.
a These confocal microscopy and IHC images (i–iv) provide intra-cellular visualizations of SERCA2a within the corresponding experimental groups. SERCA2a is identified in green fluorescence and nuclei are identified by blue fluorescence. b This graph exhibits the fluorescent intensity of SERCA2a 8 weeks after TAC and was provided by the data collected from the Image J software. Each fluorescent intensity corresponds to SERCA2a’s presence within the specific experimental groups. With respect to SERCA2a fluorescent intensity, the TFAM overexpression Sham group was significantly greater relative to the WT Sham group. Also, the TFAM TAC group was shown to be significantly greater in intensity of TFAM relative to the WT TAC group. c This graph displays SERCA2a proteins expression 8 weeks after the aortic banding surgery. Lysates were analyzed by western blotting for SERCA2a protein expression. The TFAM overexpression Sham group was significantly greater in protein expression of SERCA2a relative to the WT Sham group. The TFAM overexpression TAC group was significantly greater in protein expression of SERCA2a relative to the WT TAC group. Both TAC groups were significantly decreased in SERCA2a protein expression when compared to their Sham counterparts. The bands were quantified using densitometry and the data were graphed for an N of 5 using SEM for significance. Values are expressed as ± SEM. N = 5. *P < 0.05, **P < 0.025, ***P < 0.001
Analyzing if TFAM overexpression affected Calpainl, we observed Calpainl fluorescent intensity and protein expression. The WT TAC and TFAM-TG group had significantly increased Calpainl protein expression as observed through western blotting. Fluorescent intensity was also as equally increased in IHC. TFAM may play a role in physiological SERCA2a expression but this activity is not observed in our study (Fig. 10a–c). Our original hypothesis regarding TFAM activation of SERCA2a and reduction of Calpainl was not found.
Discussion
We demonstrate for the first time that TFAM maintains an inhibitory relationship with the NFAT4-MMP9 proteolytic pathway in TFAM transgenic mice subjected to aortic-banding-induced HF. We also found a mechanistic inconsistency in the SERCA2a-Calpainl pathway in banded TFAM transgenic mice. This finding varied in respect to the literature and our initial proposed mechanism. These findings have been expressed in our previous in vitro findings of TFAM reduction, and this in vivo study of TFAM overexpression.
In HF models in which TFAM is decreased, NFAT4 induces ROS production and activates hypertrophic gene expression. NFAT activates pathological cardiac hypertrophy, which we confirmed in the WT-TAC models. In TFAM reduction, there is a significant increase in heart weight/body weight ratio in comparison to the control groups. Furthermore, we observed a significant weight gain, which we deemed a result of edema, in the WT-TAC model compared to the WT Sham. Other papers have found similar results in HF models [21,22].
Our past, in vitro experimentation shows that significantly decreasing TFAM induces ROS-activated MMP9. Exogenous treatment of 127 micromolar H2o2 to HL-1 cardiomyocytes significantly increased MMP9 protein expression [19]. In AV fistula volume-overload (VO) HF, TFAM overexpression suppressed MMP up-regulation and limited mitochondrial oxidative stress [22].
In TFAM knockdown such as found in the failing heart of control animals (Fig. 8), excess ROS in the cytoplasm induces MMP9, which we and other groups have found increased [23, 24]. MMPs are collagenases and MMP9 excessively degrades collagen but in cardiac remodeling collagen turnover is faster than elastin, leading to a stiffening myocardium [15]. This interplay is a major factor in hypertrophy, remodeling and HF.
Increased NFAT4 stimulates a cardiac remodeling cascade inducing ROS production to increase MMP9, which degrades the extracellular matrix of cardiomyocytes. MMP9 is a distinguishing factor in pathological cardiac remodeling [15]. This observation correlates with known studies showing that increased activation of MMPs is observed in TFAM ablation and oxidative stress models [5, 25].
As was expected, TFAM overexpression initiated a chain reaction of decreased NFAT and ROS-activated MMP9. This in vivo experimentation showed that TFAM overexpression models maintained significantly increased TFAM protein expression post AB-induced HF (Fig. 6). In the TFAM overexpression model, NFAT4 is reduced (Fig. 7).
Through TFAM’s inhibition of NFAT, we conclude that AB in TFAM-TG mice prevented hypertrophy. In TFAM overexpression, we note a significant decrease in NFAT4, which correlates to our visual confirmation of reduced hypertrophy in the TFAM-TG banded group. Through analysis of heart weight/body weight ratio, we observe a reduction in cardiac hypertrophy with TFAM overexpression (Fig. 5). Our results are similar to the current literature in AVF-induced HF models, TFAM overexpression reduced protease activity and cardiac hypertrophy [22]. Studies show that TFAM overexpression attenuates cardiac dilation and dysfunction in myocardial infarction-induced HF models [18].
Oxidized proteins are prevalent in HF models, TFAM and mitochondrial repair enzymes serve to reduce oxidation when overexpressed. Overexpression of mitochondrial repair factors in TAC induced HF, decreased left ventricular remodeling [26]. Our study shows that the banded TFAM transgenic mice had reduced cardiac functional pathologies.
The inhibitory activity of TFAM to reduce NFAT4 pathological hypertrophy and ROS-driven MMP9 may delay the myocardium remodeling state, before becoming end-stage HF. TFAM overexpression protected the aortic banded myocardium from cardiac hypertrophy. The LV mass of the WTTAC was significantly heavier than the TFAM-TAC, noting that both were still significantly greater than the controls. This suggests that TFAM overexpression reduces the hypertrophic and cardiac remodeling process resulting in reduced LV mass (Fig. 4).
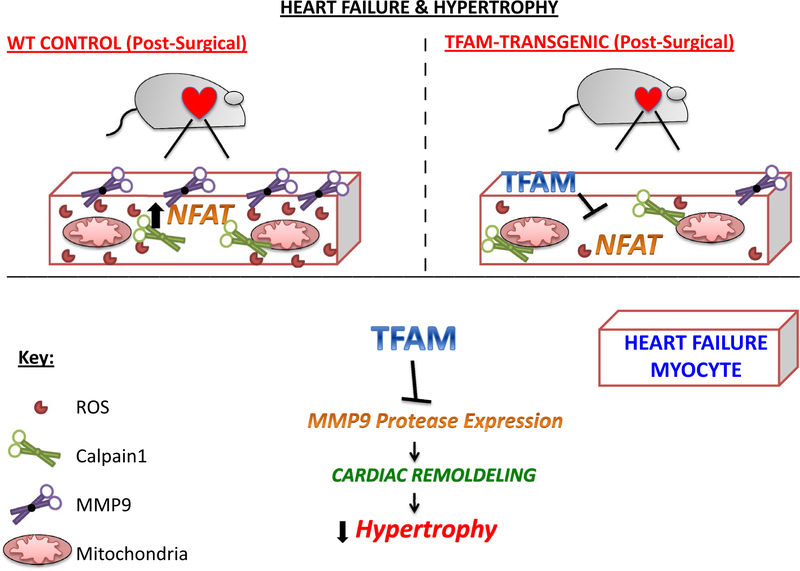
This mechanistic pathway NFAT4-MMP9 induces cardiac hypertrophy and remodeling. Cardiac remodeling includes both intra-cellular and extracellular remodeling factors that induce significant molecular and morphological changes to cardiomyocyte structure and function. Remodeling encompasses degradation of the extracellular matrix (ECM), pathological hypertrophy, fibrosis, and resultant organ dysfunction. This study provides further insight into TFAM’s therapeutic potential in TFAM knockdown/overexpression, oxidative stress, and AB induced HF. Through this work, we have shown that TFAM overexpression has the potential to reduce cardiac remodeling factors MMP9 and NFAT4 (Fig. 11). Supplemental evidence of superoxide staining suggests that ROS is reduced within TFAM-TG mice subjected to AB (Supplemental Fig. 1), which is supported by earlier works [10, 22]. Analysis of functional and molecular data within this work may give insight into the TFAM-NFAT-MMP9 pathway.
Fig. 11.
The diagram above depicts the figurative comparison of WT control and TFAM overexpression cardiomyocytes from AB induced mice. It shows the positive conclusion of reduced cardiac remodeling by decreasing MMP9 expression and shows TFAM overexpression inhibits NFAT activation. In the WT model NFAT stimulates ROS production, which activates ROS-driven MMP9 expression. MMP9 activation is a noted pathological marker for cardiac remodeling
The TFAM-SERCA2a-Calpainl pathway reveals a potential avenue for further exploration. Our work suggests that there is an anomaly regarding TFAM’s interaction with SERCA2a. Following the proposed mechanistic pathway, we found that SERCA2a protein expression was as equally significantly reduced in both TFAM transgenic and control TAC groups (Fig. 10). This finding correlates with reduced SERCA2a expression in HF models [27]. Aortic banded animals had significantly increased Calpainl protein expression compared to control animals, which may be a downstream effect of reduced SERCA2a levels (Fig. 9).
SERCA2a has a major functional role in physiological Ca2+ regulation and cardiomyocyte physiology. The Wata-nabe group found that exogenous overexpression of TFAM increased transcriptional activation of SERCA2a [16]. As a result, we hypothesized that TFAM overexpression would heighten SERCA2a protein expression, due to an increase in SERCA2a transcriptional activation. We presumed that this prominent expression of SERCA2a proteins would then drive more cytoplasmic Ca2+ into the SR, reducing Ca2+- activated calpain proteases. However, our results, although consistent, did not reflect this mechanism in our in vivo experiments. In retrospect, we can explain these unexpected discoveries as potential proteolytic degradation of SERCA2a and epigenetic inhibition of SERCA2a gene transcription. We are further analyzing the role of inflammatory interactions with SERCA2a in HF.
Conclusion
Evidence suggests that TFAM overexpression plays a vital role in the physiological and pathological myocardium. Through TFAM overexpression, we studied the potential treatment capabilities of TFAM by examining its molecular and functional effects on the cardiac remodeling.
As hypothesized, TFAM overexpression reduces pathological cardiac remodeling and associated factors in HF. Molecular insight into the remodeling cascade shows reduced protease and hypertrophic expression in TFAM overexpression models. Molecular techniques, including western blotting and IHC, reveal that TFAM overexpression reduces the NFAT4-MMP9 cardiac remodeling cascade. However, our initial hypothesis, regarding TFAM activation of SERCA2a and reduction of Calpainl, was not confirmed. Many regulatory elements may be involved in this anomaly.
This study provides mechanistic support of TFAM’s potential to reduce regulatory factors involved in cardiac pathologies. Therefore, TFAM overexpression treatment is a possible cardio-therapeutic approach to reducing MMP9-induced pathological remodeling.
Supplementary Material
Acknowledgements
This study was supported by the following grants from the US National Institute of Health; NIH F31 Grant 1F31HL132527–01 to GHK, and NIH grant HL 74185 to SCT. Thank you to Savanna C. Roberts of the University of Louisville English Department for editing this work.
Footnotes
This work is part of a Ph.D. thesis dissertation and was presented at the 2017 Experimental Biology Conference.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/sll010-018-3459-9) contains supplementary material, which is available to authorized users.
Conflict of interest The authors claim that there is no conflict of interest associated with this work.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS et al. (2016) Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133:447–454 [DOI] [PubMed] [Google Scholar]
- 2.Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC (2014) Dysregulation of Mfn2 and Drp-1 proteins in heart failure. Can J Physiol Pharmacol 92:583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunkel GH, Chaturvedi P, Tyagi SC (2015) Epigenetic revival of a dead cardiomyocyte through mitochondrial interventions. Bio-mol Concepts 2016 ‘Mitochondrial pathways to cardiac recovery: TFAM’. Heart Fail Rev 21:499–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauritzen KH, Kleppa L, Aronsen JM et al. (2015) Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol 309:H434–H449 [DOI] [PubMed] [Google Scholar]
- 5.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236 [DOI] [PubMed] [Google Scholar]
- 6.Patterson C, Portbury A, Schisler JC, Willis MS (2011) Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ Res 109:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Won DJ, Krajewski S, Gottlieb RA (2002) Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277:29181–29186 [DOI] [PubMed] [Google Scholar]
- 8.Wanichawan P, Hafver TL, Hodne K, Aronsen JM, Ida et al. (2014) Molecular basis of calpain cleavage and inactivation of the sodium-calcium exchanger 1 in heart failure. J Biol Chem 289:33984–33998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang DC, Ma ST, Tan Y et al. (2010) Imbalance of matrix metal-loproteinases/tissue inhibitor of metalloproteinase-1 and loss of fibronectin expression in patients with congestive heart failure. Cardiology 116:133–141 [DOI] [PubMed] [Google Scholar]
- 10.Williams CR, Gooch JL (2014) Calcineurin abeta regulates NADPH oxidase (Nox) expression and activity via nuclear factor of activated T cells (NFAT) in response to high glucose. J Biol Chem 289:4896–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang TH, Chen MC, Chang JP et al. (2016) Exploring regulatory mechanisms of atrial myocyte hypertrophy of mitral regurgitation through gene expression profiling analysis: role of NFAT in cardiac hypertrophy. PLoS ONE ll:e0166791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houser SR, Molkentin JD (2008) Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sei Signal l:pe31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujumdar VS, Smiley LM, Tyagi SC (2001) Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol 79:277–286 [DOI] [PubMed] [Google Scholar]
- 14.Prathipati P, Metreveli N, Nandi SS, Tyagi SC, Mishra PK (2016) Ablation of matrix metalloproteinase-9 prevents cardiomyocytes contractile dysfunction in diabetics. Front Physiol 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra PK, Givvimani S, Chavali V, Tyagi SC (2013) Cardiac matrix: a clue for future therapy. Biochim Biophys Acta 1832:2271–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe A, Arai M, Koitabashi N et al. (2011) Mitochondrial transcription factors TFAM and TFB2M regulate Serca2 gene transcription. Cardiovasc Res 90:57–67 [DOI] [PubMed] [Google Scholar]
- 17.Fujino T, Ide T, Yoshida M et al. (2012) Recombinant mitochondrial transcription factor A protein inhibits nuclear factor of activated T cells signaling and attenuates pathological hypertrophy of cardiac myocytes. Mitochondrion 12:449–458 [DOI] [PubMed] [Google Scholar]
- 18.Ikeuchi M, Matsusaka H, Kang D et al. (2005) O ver expression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112:683–690 [DOI] [PubMed] [Google Scholar]
- 19.Kunkel GH, Chaturvedi P, Theilen N, Nair R, Tyagi SC (2017) Mechanism of TFAM mediated cardiomyocyte protection. Can J Physiol Pharmacol 96(2): 173–181 [DOI] [PubMed] [Google Scholar]
- 20.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chein KR (1991) Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sei USA 88:8277–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeraveedu PT, Sanada S, Okuda K, Fu HY et al. (2017) Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem Pharmacol 138:73–80 [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Ide T, Fujino T et al. (2015) Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardio-protection associated with limited mitochondrial oxidative stress. PLoS ONE 10:e0119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Givvimani S, Tyagi N, Sen U et al. (2010) MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch Physiol Biochem 116:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox MJ, Sood HS, Hunt MJ, Chandler D, Henegar JR, Aru JM, Tyagi SC (2002) Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol 282:H1197–H1205 [DOI] [PubMed] [Google Scholar]
- 25.Lamparter S, Maisch B (2000) Significance of matrix metallopro-teinases in cardiovascular diseases. Z Kardiol 89:949–957 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Wang Q, Watson LJ, Jones SP, Epstein PN (2011) Cardiac overexpression of 8-oxoguanine DNA glycosylase 1 protects mitochondrial DNA and reduces cardiac fibrosis following transaortic constriction. Am J Physiol Heart Circ Physiol 301 :H2073–H2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS (2009) Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol 2:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.