Abstract
Osteogenesis imperfecta (OI) is a rare congenital bone disorder with underlying Type 1 collagen defect, in which patients are prone to fractures. The disease is associated with increased spinal curvature, short stature, loose joints, and poor muscle tone, all contributing to difficulty in identifying landmarks and hindering successful subarachnoid block. We report an interesting case of a successful ultrasonogram (USG)-guided subarachnoid block given in an adolescent girl with OI for fixation of femur fracture. This report underlines the importance USG in managing difficult neuraxial blocks.
Keywords: Osteogenesis imperfecta, subarachnoid block, ultrasound guided
INTRODUCTION
Osteogenesis imperfecta (OI), also known as ’brittle bone disease’, is a congenital connective tissue disorder which is caused by a mutation of the collagen Type I COLIA 1 and COLIA 2 genes.[1] According to Sillence classification, Type I and Type IV are autosomal dominant with mild-to-moderate orthopaedic and hearing defects. Type II and Type III are autosomal recessive with Type II being lethal in utero and Type III being the most severe survivable form.[2] The clinical manifestations include seizures, blue sclera, dental abnormalities, hearing defects, bleeding disorders, kyphoscoliosis, and cardiorespiratory abnormalities like mitral valve prolapse.[3] Such patients with OI may present to us for anaesthetic management for different surgeries including orthopaedic procedures. Difficult airway with neck abnormalities, restrictive lung disease, cardiovascular anomalies, and positioning problems may complicate a general anaesthetic administration. Spine abnormalities may preclude an easy neuraxial blockade. Hence, we used ultrasonogram (USG) to overcome difficulties in identifying the intrathecal space and avoiding the potential dangers of general anaesthesia.
CASE REPORT
A 14-year-old female patient, a known case of OI Type I, came with a closed fracture of the shaft of right femur after a history of fall at home. She was a known case of OI with history of earlier femur and tibia fracture, which were operated uneventfully under general anaesthesia. On general examination, patient weighed 40 kg with short stature of 140 cm, barrel-shaped chest, kyphoscoliosis, and was bedridden. Multisystem examination was normal except kyphoscoliosis. On airway assessment, she had poor dentition, short neck, and Mallampati class II. Her investigations revealed haemoglobin of 13.4 g%, white blood cell count of 10,000, platelet count 2.49 lacs, blood sugar 87 mg/dl, blood urea 17 mg/dl, serum creatinine 0.9 mg/dl, serum potassium 3.4 meq/l, serum sodium 137 meq/l, and serum calcium 8.7 meq/l. Preoperative coagulation profile was normal. 2D echo revealed no valvular abnormalities and a normal ejection fraction (60%). Pulmonary function tests revealed a moderate degree of restrictive lung disease.
The patient was accepted for surgery as American Society of Anesthesiologists physical status III for fixation of femur. The coagulation parameters were normal. Hence in presence of restrictive lung disease and increased chances of difficult intubation, the plan of anaesthesia decided was intrathecal regional anaesthetic technique using USG guidance. Pre-operative preparation included attaching monitors including ECG, Non invasive blood pressure (NIBP), SpO2, skin temperature monitoring and securing 22 G intravenous access in both upper limbs. Wide bore access was difficult to establish. Patient was preloaded with 500 ml Ringer's lactate. Her baseline blood pressure was 114/84 mmHg, heart rate was 72 beats/min, and SpO2 on room air was 98%. Patient was given sitting position with adequate care including padding of pressure points considering brittle bones. Sterile precautions were followed and the patients back was painted and prepared. Linear USG probe was selected and covered by sterile plastic drapes. Due to the kyphoscoliosis, there was extreme difficulty in finding the subarachnoid space as the intervertebral spaces were unequal in length and the tissue planes were distorted, but once clearly visualised with ultrasound [Figure 1], using out-of-plane approach, 25-G Quincke spinal needle was inserted and 2.5 ml of bupivacaine heavy 0.5% was given in L3–L4. Patient was immediately placed in the supine position and the level of block was assessed using pinprick and motor block with modified Bromage scale. Sensory and motor block were achieved up to T8 level. The surgery lasted for 2 h and 15 min, in which vital parameters were maintained and the recovery was uneventful and adequate.
Figure 1.
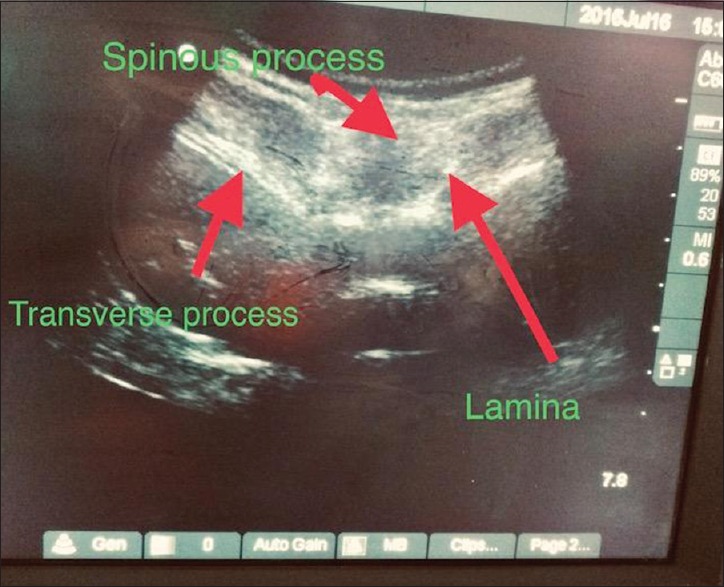
Ultrasound-guided visualisation of subarachnoid space: linear probe in L3–L4 intervertebral space
DISCUSSION
The term OI, which means ’imperfect bone formation’, is one of the most common skeletal dysplasias, affecting approximately 6–7 per 100,000 people and occurring in approximately 1 in 20,000 births.[4] It appears to affect males and females equally and has no partiality for a particular race.[4] OI is classified as either congenita meaning that fractures are present at or prior to birth or tarda based on the presence of fractures after birth.[5] The common feature of all cases is a gene mutation that leads to either defective collagen formation or a reduced collagen formation.[4] Due to defects in its structure, connective tissues are weakened, causing bones to be brittle and to fracture following trivial trauma.[4]
There are multiple anaesthetic concerns in a case of OI. Several aspects of patient care have to be supervised by a vigilant anaesthesiologist. Careful positioning of a patient with OI is of utmost importance to prevent fractures.[6] The table should be padded with careful consideration to pressure points. Joint laxity can lead to dislocation, implying that care should be taken to avoid overextension during positioning for surgical exposure. If available, a moulding mattress is preferred, as these patients may be unable to lay flat and supine.[7] Overextension of the cervical spine can lead to odonto-axial dislocation or fracture and thus must be avoided.[8] Other methods for maintaining cervical spine immobility during intubation include the use of an intubating laryngeal mask airway or, in emergency cases, the use of a stylet. A short neck, protruding mandible, and presence of a pigeon chest will make visualisation of the glottis difficult and should be anticipated. The optimal patient position can be achieved by carefully placing a blanket beneath the upper back to help restore anatomical relationships. We padded the patient properly to avoid any injuries. Direct laryngoscopy should be performed with minimal disturbance to the mucosa, as bleeding and bruising occur easily.[9] Respiratory complications secondary to chest deformity and scoliosis lead to limitations in thoracic function and make it the principal cause of death in most patients with OI.[5] Severe thoracic deformity will cause a reduced vital capacity, decreased chest wall compliance, and hypoxemia due to ventilation/perfusion mismatch.[10] A diagnosis of severe dysfunction is made when vital capacity falls below 15 ml/kg (normal, greater than 70 ml/kg).[11] Positive pressure ventilation will worsen the already present ventilation-perfusion mismatch in patients with OI and may necessitate an increase in the inspired oxygen content and/or positive end-expiratory pressure.[12] Hence, we planned to go for regional anaesthesia as kyphoscoliosis and thoracic cage deformity is prevalent in the OI population.[13] Performing regional blocks may be difficult or impossible because of orthopaedic and anatomical anomalies in patients with OI.[6] Platelet dysfunction is a concern for the anaesthesia provider performing spinal blocks and epidurals. The platelet level should be checked and platelet transfusions administered if necessary. Coagulopathies are of concern also, so tests such as prothrombin time, partial thromboplastin time, and thrombin time should be performed to evaluate primary haemostasis.[12] In our case, we had normal coagulation parameters. We utilised ultrasound as a guide for an entry into subarachnoid space in light of difficulty in identifying landmarks. The ideal technique in these patients is probably spinal anaesthesia as it avoids the need of tracheal intubation and reduces the risk of malignant hyperthermia. Hence, we preferred spinal anaesthesia, as the patient was undergoing lower limb surgery.[3] Utilising ultrasound for regional anaesthesia has been used in the past and can be utilised in patients with difficult anatomy.[14] This is reported for its rarity and a successful use of ultrasound in identifying subarachnoid space in difficult times.
CONCLUSION
We conclude that USG guidance is ideal to overcome the technical difficulties of identification of subarachnoid space in patients with OI. Successful regional technique may avoid general anaesthesia and its associated dangerous complications in such cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bhandari G, Shahi KS, Bhadoria P, Bhalotra AR, Sandhya OD, Arya M. Osteogenesis imperfecta: No place for imperfect anaesthesiologist. Indian J Anaesth. 2008;52:577–80. [Google Scholar]
- 2.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–16. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta D, Purohit A. Spinal anesthesia in osteogenesis imperfecta. Anaesth Pain Intensive Care. 2016;20:86–8. [Google Scholar]
- 4.Oakley I, Reece L. Anesthetic implications for the patient with osteogenesis imperfecta. AANA J. 2010;78:47–53. [PubMed] [Google Scholar]
- 5.Widmann RF, Bitan FD, Laplaza FJ, Burke SW, DiMaio MF, Schneider R. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine. 1999;24:1673–8. doi: 10.1097/00007632-199908150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Libman RH. Anesthetic considerations for the patient with osteogenesis imperfecta. Clin Orthop Relat Res. 1981;159:123–5. [PubMed] [Google Scholar]
- 7.Rodrigo C. Anesthesia for maxillary and mandibular osteotomies in osteogenesis imperfecta. Anesth Prog. 1995;42:17–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Maya D, Nayyar BM, Patra P. Anaesthetic management of a case of OIwith associated bronchial asthma for repair of corneal perforation. Indian J Anaesth. 2006;50:223–5. [Google Scholar]
- 9.Karabiyik L, Parpuco M, Kurtipek O. Total intravenous anaesthesia and the use of an intubating laryngeal mask airway in a patient with osteogenesis imperfecta. Acta Anaesthesiol Scand. 2002;46:618–21. doi: 10.1034/j.1399-6576.2002.460525.x. [DOI] [PubMed] [Google Scholar]
- 10.Cho E, Dayan SS, Marx GF. Anaesthesia in a parturient with osteogenesis imperfecta. Br J Anaesth. 1992;68:422–3. doi: 10.1093/bja/68.4.422. [DOI] [PubMed] [Google Scholar]
- 11.Ryan JF. Pediatric anesthesia. In: Morgan GE, Mikhail MS, Murray MJ, editors. Clinical Anesthesiology. 4th ed. New York, NY: McGraw-Hill; 2006. pp. 922–50. [Google Scholar]
- 12.Mercer M. Anaesthesia for the patient with respiratory disease. Update Anaesth. 2000;12:1–3. [Google Scholar]
- 13.Papneja C, Tandon MS, Singh D, Ganjoo P. Anaesthetic management in a patient of OI with basilar invagination. Internet J Anesthesiol. 2006:10–12. [Google Scholar]
- 14.Lee PJ, Tang R, Sawka A, Krebs C, Vaghadia H. Brief report: Real-time ultrasound-guided spinal anesthesia using Taylor's approach. Anesth Analg. 2011;112:1236–8. doi: 10.1213/ANE.0b013e31820ec53c. [DOI] [PubMed] [Google Scholar]


