Abstract
Background and Aims:
Ultrasound guided transversus abdominis plane block is an efficacious abdominal field block. The aim was to determine the effect of dexamethasone to 0.375% ropivacaine on the analgesic duration of TAP block in patients undergoing lower segment cesarean section (LSCS).
Methods:
A single-blinded randomised control study was conducted on 90 patients, who were divided in two groups of 45 each. Group R received 0.375% ropivacaine (25 ml) with normal saline (1 ml) each side and group D received 0.375% ropivacaine (25 ml) with dexamethasone 4 mg (1 ml) each side in transversus abdominis plane block after lower segment cesarean section. Primary objective was to compare time to first rescue analgesia and secondary objectives to compare the total amount of analgesia required in first 24 h postoperatively, visual analog scale scores for somatic and visceral pain and incidence of nausea and vomiting, between the two groups. Student's t test, Chi-square, or Fisher's exact test were performed using SPSS 17.0.
Results:
Time to first rescue analgesia was significantly less in group R (11.62 ± 3.80 h) compared to group D (19.04 ± 4.13 h) (P < 0.001). Total tramadol consumed in 24 h was significantly higher in group R (86.67 ± 30.55 mg) than group D (35.56 ± 39.54 mg) (P < 0.001). Visual analog scale scores for both somatic and visceral pain were significantly higher in group R than group D at 8 h, 12 h, and 24 h postoperatively.
Conclusion:
Addition of dexamethasone to ropivacaine in transversus abdominis plane block significantly prolongs the duration of postoperative analgesia.
Keywords: Cesarean section, dexamethasone, ropivacaine, transversus abdominis plane block, ultrasonography
INTRODUCTION
Lower segment cesarean section (LSCS) may result in significant postoperative pain, which can negatively impact ambulation, breastfeeding, and even maternal bonding. Pain after LSCS has a somatic component arising from nociceptors within the surgical wound and a visceral component originating from the uterine incision. The transversus abdominis plane (TAP) block, a relatively new regional anaesthesia technique, first described by Rafi[1] in 2001, anaesthetises the parietal peritoneum and the skin and muscles of anterolateral abdominal wall, including lower thoracic (T7-T12) and upper lumbar (L1-L3) nerve roots. An injection of local anaesthetic (LA) is made in the fascial plane between the internal oblique and transversus abdominis muscle at the anterior axillary line. The analgesic efficacy of the block[1] has been established in several lower abdominal surgeries[2,3] including parturients undergoing LSCS.[2] Advantages include technical simplicity, high analgesic effectiveness, opioid sparing, long duration,[2] and minimal side effects. Furthermore, the introduction of ultrasound facilitates accurate placement of block needle as well as real-time deposition of local anaesthetic, thereby increasing both the margin of safety and block quality. Ropivacaine is longer acting than lignocaine and has a documented better safety profile than bupivacaine. It is common practice nowadays to use ropivacaine for nerve blocks and fascial plane blocks. Dexamethasone is highly potent, long-acting glucocorticoid with analgesic, antiemetic, and anti-inflammatory properties, and when used as adjuvant in peripheral nerve blocks, increases the duration of analgesia[4] with minimal side effects and has an added advantage in decreasing postoperative nausea and vomiting (PONV).[5]
The aim of this study was to measure the effect of adding dexamethasone to ropivacaine on the duration and quality of analgesia in ultrasound-guided TAP block in patients undergoing LSCS.
METHODS
This prospective randomised, single-blinded, comparative study was conducted from October 2015 to September 2016 after approval from the local ethical committee. Ninety patients aged 18 years or older with American Society of Anesthesiologists (ASA) physical Status II undergoing elective LSCS under spinal anaesthesia were included in the study. The exclusion criteria included patient's refusal, emergency LSCS, patients with allergy to paracetamol, diclofenac, tramadol, hypersensitivity to LA, history of bleeding disorder, use of anticoagulants, chronic pain, PONV, motion sickness, diabetes or gestational diabetes, infection at the site of TAP block, any concomitant surgery other than LSCS, and patients requiring general anaesthesia for LSCS. Patients were randomly allocated by computer-generated numbers into two groups of 45 each. TAP block was given bilaterally in all patients. Group R received 25 ml of 0.375% ropivacaine with 1ml of normal saline on each side and group D received 25 ml of 0.375% ropivacaine with dexamethasone 4 mg (1 ml) each side. Patients were explained in detail about the study being undertaken, and informed written consent to participate was taken. Patients did not know about the group allocation at any time of the study. Demographic record and pertinent information (age, weight, height, body mass index [BMI], ASA grade, diabetes and cardiovascular disease) were obtained from all the patients. All patients were educated preoperatively about how to use and record the visual analog scale (VAS) for both somatic and visceral pain: somatic pain (VAS-S)- defined as superficial pain felt in the wound and visceral pain (VAS- V)- defined as deep and generalised pain. Two 100 mm scales were provided for reporting superficial and deep pain separately at specified time intervals, zero being no pain and 100 being the worst imaginable pain. Upon arrival in the operation theater, baseline heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and oxygen saturation (SpO2) were recorded and monitored throughout the surgery. Peripheral vascular access was established using 18-gauge intravenous cannula. All patients were administered 20 ml/kg of crystalloid fluid (Ringer lactate) before administering spinal anaesthesia. Under strict asepsis, spinal anaesthesia was administered in sitting position, using 1.8 ml of 0.5% bupivacaine heavy and 0.4 ml of fentanyl (20 mcg) (total 2.2 ml) at the level of L3-4 using a 27G “Quinke” spinal needle. The patient was made to lie supine and a 15-degree wedge was placed under the right buttock. Supplemental oxygen at 4 l/min was administered to all patients via aface mask until the end of the operation. Sensory block level was checked every 2 min using cold ethyl chloride sprays, and the surgery was commenced once an adequate block level (T7) had reached. At the end of surgical closure of the wound, TAP block was given bilaterally under full aseptic precautions. Needle entry site was identified between the iliac crest and the costal margin in the anterior axillary line. A 38 mm linear probe (SonoSite M-Turbo, Fujifilm USA) was placed transversely on the skin after part and probe preparation. SonoPlex Stim 22G, 80 mm needle was inserted under ultrasound guidance in an in-plane technique to position the tip of the needle between the internal oblique and transversus abdominis. One ml of sterile water was injected prior to LA injection to confirm the correct position of the needle tip. Distribution of LA was observed on ultrasound as a hypoechoic enlargement between the fascial planes as a real-time image [Figure 1]. All patients in both the groups received 1 gm paracetamol intravenously at the end of block, as a part of multimodal analgesia. HR, SBP, DBP, SpO2, VAS-S (somatic pain), VAS-V (visceral pain), and nausea scores were recorded at 1st, 2nd, 4th, 8th, 12th, and 24th postoperative hours. Rescue analgesia of 50 mg tramadol in 100 ml normal saline over 20 min was given either on patient's request or if VAS-S/VAS-V was >44 mm. This could be repeated in no less than 4 h. In case of reported nausea or episode of vomiting within 24 h, ondansetron 4 mg intravenously was given. Patients were assessed for and data recorded as time to first rescue analgesia- described as time interval between the end of block performance and first demand by patient for rescue analgesia, total amount of rescue analgesia required- described as total dose of tramadol required in 24 h since the end of block performance. Somatic and visceral pain scores were recorded separately at mentioned time intervals using VAS score[6] as no pain (0–4 mm), mild pain (5–44 mm), moderate pain (45–74 mm), or severe pain (75–100 mm); nausea scores using a categorical system (none-0, mild-1, moderate-2, and severe-3) or an any episode of vomiting and any other block or drug-related adverse effect.
Figure 1.
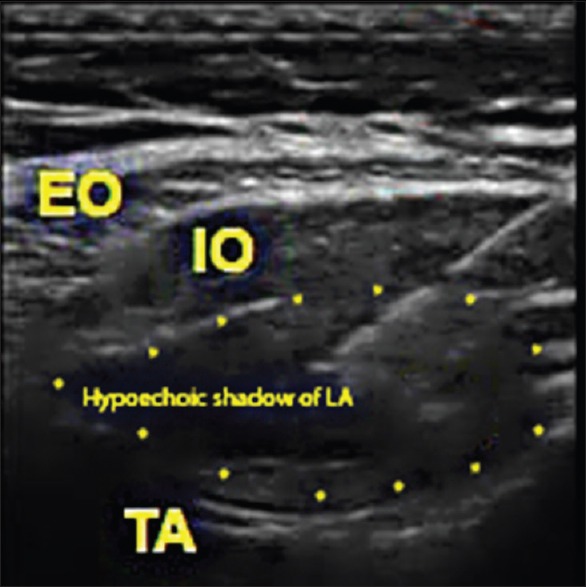
Sonoanatomy of TAP block with spread of LA. EO–External oblique, IO–Internal oblique, TA–Transversus abdominis
The primary objective was to compare the time to first rescue analgesia between the two groups. The secondary objectives were to compare the total amount of rescue analgesia required in first 24 h postoperatively, VAS scores for somatic and visceral pain and incidence of nausea and vomiting between the two groups.
A power analysis according to time for rescue analgesia in a study done by Akkaya et al.[7] reported standard deviation (SD) of 4.8 and 7.8 h in two groups. Using these values and to be able to detect a difference of minimum 4 h between these groups with power of 80%, sample size of 41 in each group was recommended. Because there may be dropouts due to technical failure or patient refusal to participate, 45 cases were included in each group. Statistical testing was conducted using statistical package for social science system version SPSS 17.0. Continuous variables are presented as mean ± SD and categorical variables as absolute numbers and percentage. The comparison of normally distributed continuous variables between the groups was performed using Student's t test. Nominal categorical data between the groups were compared using Chi-square test or Fisher's exact test as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Both groups were comparable in terms of age, weight, height, BMI, duration of surgery, and baseline vitals [Table 1]. Time to first rescue analgesia was significantly shorter in group R (11.62 ± 3.80 h) than the group D (19.04 ± 4.20 h) (P value <0.001) [Table 2]. The total tramadol requirement in postoperative period was significantly higher in group R (86.67 ± 30.55 mg) than the group D (35.56 ± 39.54 mg) (P value <0.001) [Table 2]. In group R, 16 patients (35.6%) required 50 mg of tramadol, 25 patients (55.6%) consumed 100 mg of tramadol, and 4 patients (8.9%) required 150 mg of tramadol as total analgesia in 24 h. While in group D, 18 patients (40%) had good pain relief with the block only and did not require any additional analgesia in 24 h postoperatively, 22 patients (48.9%) required only 50 mg of tramadol, and 5 patients (11%) required 100 mg of tramadol as total analgesia required in 24 h. VAS scores for both somatic and visceral pain were significantly higher in patients of group R than group D at 8 h (P < 0.001), 12 h (P < 0.001), and 24 h (P < 0.001) [Graphs 1 and 2]. The incidence of nausea was more in group R as compared to group D at 8 h, 12 h, and 24 h (P = 0.138) but was statistically significant only at 8 h (P = 0.004) and 12 h (P = 0.003) [Table 3]. No episode of vomiting and block related complications were observed in postoperative period in any patient of either group. HR, SBP, and DBP at 8 h, 12 h, and 24 h postoperatively were significantly higher in group R than group D patients (P < 0.05).
Table 1.
Baseline vitals
| Group R (n=45) (Mean±SD) | Group D (n=45) (Mean±SD) | P | |
|---|---|---|---|
| Age | 30.20±3.15 | 29.76±3.21 | 0.509 |
| Weight | 69.16±7.72 | 67.28±4.13 | 0.154 |
| Height | 159.96±3.87 | 161.29±4.24 | 0.123 |
| BMI | 27.08±3.72 | 25.83±2.15 | 0.053 |
| Duration of surgery | 47.56±7.20 | 45.44±5.62 | 0.125 |
| Baseline HR | 79.09±3.27 | 79.89±3.43 | 0.261 |
| Baseline SBP | 126.71±5.78 | 127.93±3.76 | 0.237 |
| Baseline DBP | 78.29±4.20 | 78.98±4.10 | 0.433 |
| Baseline SpO2 | 99.33±0.83 | 99.20±0.79 | 0.435 |
P<0.05, statistically significant SpO2–Peripheral capillary oxygen saturation
Table 2.
Time to first rescue analgesia and total tramadol consumption in 24 h
| Group R (n=45) Mean±SD |
Group D (n=27) Mean±SD |
P | |
|---|---|---|---|
| Time to first rescue analgesia | 11.62±3.80 | 19.04±4.20 | <0.001 |
| Postoperative total tramadol required | 86.67±30.55 | 35.56±39.54 | <0.001 |
P<0.05, statistically significant
Graph 1.
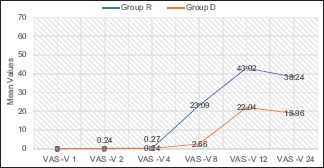
VAS scores for somatic pain at 1, 2,4, 8, 12, and 24 h postoperatively
Graph 2.
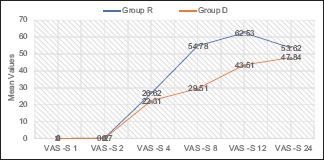
VAS scores for visceral pain at 1, 2, 4, 8, 12, and 24 h postoperatively
Table 3.
Nausea scores
| Nausea scores | Group R (n=45) Frequency |
Group D (n=45) Frequency |
P | |
|---|---|---|---|---|
| At 1 h | 0 | 45 | 45 | - |
| At 2 h | 0 | 45 | 45 | - |
| At 4 h | 0 | 45 | 45 | - |
| At 8 h | 0 | 32 | 43 | 0.004 |
| 1 | 4 | 2 | ||
| 2 | 9 | 0 | ||
| 3 | 0 | 0 | ||
| At 12 h | 0 | 35 | 43 | 0.003 |
| 1 | 9 | 2 | ||
| 2 | 1 | 0 | ||
| 3 | 0 | 0 | ||
| At 24 h | 0 | 39 | 44 | 0.138 |
| 1 | 5 | 1 | ||
| 2 | 1 | 0 | ||
| 3 | 0 | 0 | ||
DISCUSSION
Dexamethasone has been used as an adjuvant to LA in peripheral nerve blocks since long, but comprehensive research of literature revealed no study where dexamethasone has been used to augment the analgesic efficacy of TAP block with 0.375% ropivacaine in patients undergoing LSCS. Hence, this study was planned to evaluate if the addition of dexamethasone to ropivacaine improves analgesic quality and duration of TAP block.
Dexamethasone exerts its analgesic action by inhibiting transmission and neural discharge in nociceptive C fibers. Time to first rescue analgesia was significantly lower in group R (11.62 ± 3.80 h) than group D (19.04 ± 4.13 h) (P value <0.001). In a study,[7] it was observed similarly that time to request additional analgesics was significantly higher in dexamethasone group than levobupivacaine group (13 ± 7.8 h vs. 6.1 ± 4.8 h, P value 0.001) when dexamethasone was added to levobupivacaine in ultrasound-guided TAP block. A study[8] found that dexamethasone prolonged analgesia in interscalene block using ropivacaine [11.8 (9.7-13.8) vs. 22.2 (18.0-28.6) h] and bupivacaine [14.8 (11.8-18.1) vs. 22.4 (20.5-29.3) h] and also established that dexamethasone prolonged analgesia more with ropivacaine than with bupivacaine. A study[9] found that the addition of 4–8 mg of dexamethasone to LA in brachial plexus block reduced the onset of time and significantly prolonged the duration of analgesia. In this study, the average duration of analgesia was 12-24 h with dexamethasone, but only 4 h with LA and adrenaline. A study[10] found that dexamethasone given with mepivacaine had longer duration of analgesia of 332 min vs. 228 min in the control group during supraclavicular brachial plexus block. In a study,[11] dexamethasone added to bupivacaine microcapsules by subcutaneous infiltration produced a significant increase in analgesia time. Therefore, results of the study are consistent with previous studies, whereby addition of dexamethasone to LA reduces the time to first rescue analgesia, although most studies were performed with peripheral nerve block rather than fascial plane block.
Total tramadol required postoperatively was significantly higher in group R (86.67 ± 30.55 mg) than group D (35.56 ± 39.54 mg) (P value < 0.001). A study[7] also found that total postoperative tramadol requirement was higher in group L (92.9 ± 36 mg) than group D (50.0 ± 35 mg), (P = 0.001). A study found that the postoperative opioid consumption was reduced in the perineural dexamethsone group as compared to control.[12]
The differences in VAS S and VAS V scores of both the groups were insignificant at time points of 1 h, 2 h, and 4 h postoperatively. This was probably owing to the effect of spinal anaesthesia given in both the groups, which is expected to provide pain relief for up to 4 h per se. Both VAS S and VAS V scores were significantly higher in group R than group D at 8 h, 12 h, and 24 h postoperatively, thus suggesting that addition of dexamethasone to ropivacaine in TAP block significantly reduced pain from both somatic and visceral components. Similarly, a study[13] found that dexamethasone - bupivacaine in TAP block had significantly lower postoperative pain scores than bupivacaine in patients undergoing radical cystectomy. VAS S score was maximum at 12 h in group R compared to 24 h in group D, suggesting that group R patients experienced somatic pain significantly earlier compared to group D. VAS V was significantly higher in group R than group D at 8 h, 12 h, and 24 h postoperatively [Graph 2]; this positive effect of dexamethasone (8 mg) on visceral pain may be explained by the low but significant analgesic effect following systemic absorption. In the study,[7] it was found that Numeric Rating Scale scores for somatic and visceral pain were significantly lower in dexamethasone group.
At postoperative 1 h, 2 h, and 4 h, none of the patients in either group had nausea that could be because of minimal to no pain owing to the effect of spinal anaesthesia. In group R, the incidence of nausea (28.9% at 8 h, 22.2% at 12 h, and 13.3% at 24 h) was significantly more compared to group D (4.4% at 8 h, 4.4% at 12 h, and 2.2% at 24 h. This highlights the antiemetic effect of dexamethasone (8 mg) through direct central action at the solitary tract nucleus, interaction with the neurotransmitter serotonin and receptor proteins tachykinin NK1 and NK2, and alpha adrenaline maintaining the normal physiological functions of organs and systems, regulation of the hypothalamic-pituitary-adrenalaxis,[14] following its systemic absorption. Reduced nausea scores were also probably owing to the better pain relief and reduced tramadol consumption in group D. This finding was consistent with a study,[15] where dexamethasone has significantly decreased the incidence of PONV. There was no episode of vomiting observed in patients of either group. This may be explained by administration of 4 mg ondansetron intravenously immediately in case of reported nausea by the patient. Postoperative analgesia provided by TAP block in both the groups could also account for the decreased incidence of vomiting in both the groups.
No block or drug-related side effects such as trauma to surrounding viscera, haematoma, or LA toxicity were reported in both the groups. This suggests that the use of ultrasound enables better visualization of the abdominal structure, real-time visualisation of the needle, and spread of LA, thereby decreasing the chance of block failure and increasing the accuracy and safety of block. A study in 2013 also concluded that perineural administration of dexamethasone with LA was not associated with any adverse events.[16]
The study was not without limitations. The analgesic efficacy of TAP block has been demonstrated for up to 48 h in previous studies, whereas in this study patients were assessed for 24 h. The time to regression of spinal anaesthesia is different in different individuals that could have added to the analgesic efficacy of TAP block in the first four hours postoperatively. Some potential complications of dexamethasone such as delayed wound healing, hyperglycaemia, and adrenal suppression were not evaluated. However, previous studies have demonstrated that a single small dose of dexamethasone is not associated with significant side effects. It has been realised that in future studies monitoring of pain scores must be at more frequent intervals after 8 h postoperatively for better assessment of postoperative pain.
CONCLUSION
Use of dexamethasone along with 0.375% ropivacaine prolongs the analgesic duration of TAP block in patients undergoing LSCS and also has added benefits of opioid sparing and anti-emetic effects, thereby results in better postoperative recovery and improved maternal satisfaction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rafi AN. Abdominal field block: A new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, et al. The analgesic efficacy of transversus abdominis plane block after caesarean delivery: Arandomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 3.Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;8:007705. doi: 10.1002/14651858.CD007705.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SB, Saied NN, Bowens C, Jr, Mercaldo ND, Schildcrout JS, Malchow RJ. Duration of upper and lower extremity peripheral nerve blockade is prolongssed with dexamethasone when added to ropivacaine: Aretrospective database analysis. Pain Med. 2013;14:1239–47. doi: 10.1111/pme.12150. [DOI] [PubMed] [Google Scholar]
- 5.De Oliveira GS, Jr, Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: An updated meta-analysis of randomized controlled trials. Anesth Analg. 2013;116:58–74. doi: 10.1213/ANE.0b013e31826f0a0a. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: Areanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–14. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 7.Akkaya A, Yildiz I, Tekelioglu UY, Demirhan A, Bayir H, Ozlu T, et al. Dexamethasone added to levobupivacaine in ultrasound-guided tranversus abdominis plain block increased the duration of postoperative analgesia after caesarean section: A randomized, double blind, controlled trial. Eur Rev Med Pharmacol Sci. 2014;18:717–22. [PubMed] [Google Scholar]
- 8.Cummings KC, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–53. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha BR, Maharjan SK, Tabedar S. Supraclavicular brachial plexus block with and without dexamethasone: Acomparative study. Kathmandu Univ Med J. 2003;1:158–60. [PubMed] [Google Scholar]
- 10.Parrington S, O’Donnell D, Chan VW, Brown-Shreves D, Subramanyam R, Qu M, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010;35:422–6. doi: 10.1097/AAP.0b013e3181e85eb9. [DOI] [PubMed] [Google Scholar]
- 11.Holte K, Werner MU, Lacouture PG, Kehlet H. Dexamethasone prolongs local analgesia after subcutaneous infiltration of bupivacaine microcapsules in human volunteers. Anesthesiology. 2002;96:1331–5. doi: 10.1097/00000542-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 12.De Oliveira GS, Jr, Castro Alves LJ, Nader A, Kendall MC, Rahangdale R, McCarthy RJ. Perineural dexamethasone to improve postoperative analgesia with peripheral nerve blocks: Ameta-analysis of randomized controlled trials. Pain Res Treat. 2014;179029:1–9. doi: 10.1155/2014/179029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdalla W. Ultrasound-guided transversus abdominis plane block for radical cystectomy with and without dexamethasone: Aprospective, double-blinded controlled trial. Ain Shams J Anesthesiol. 2014;7:539–44. [Google Scholar]
- 14.Chu CC, Hsing CH, Shieh JP, Chien CC, Ho CM, Wang JJ, et al. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol. 2014;722:48–54. doi: 10.1016/j.ejphar.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2015;32:751–8. doi: 10.1097/EJA.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: Asystematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112:427–39. doi: 10.1093/bja/aet417. [DOI] [PubMed] [Google Scholar]


