Abstract
Gene therapy is an ideal choice to cure many inborn errors of metabolism of the liver. Ex-vivo, lentiviral vectors have been used successfully in the treatment of many hematopoietic diseases in humans, as their use offers stable transgene expression due to the vector’s ability to integrate into the host genome. This method demonstrates the application of ex vivo gene therapy of hepatocytes to a large animal model of hereditary tyrosinemia type I. This process consists of 1) isolation of primary hepatocytes from the autologous donor/recipient animal, 2) ex vivo gene delivery via hepatocyte transduction with a lentiviral vector, and 3) autologous transplant of corrected hepatocytes via portal vein injection. Success of the method generally relies upon efficient and sterile removal of the liver resection, careful handling of the excised specimen for isolation of viable hepatocytes sufficient for re-engrafting, high-percentage transduction of the isolated cells, and aseptic surgical procedures throughout to prevent infection. Technical failure at any of these steps will result in low yield of viable transduced hepatocytes for autologous transplant or infection of the donor/recipient animal. The pig model of human type 1 hereditary tyrosinemia (HT-1) chosen for this approach is uniquely amenable to such a method, as even a small percentage of engraftment of corrected cells will lead to repopulation of the liver with healthy cells based on a powerful selective advantage over native-diseased hepatocytes. Although this growth selection will not be true for all indications, this approach is a foundation for expansion into other indications and allows for manipulation of this environment to address additional diseases, both within the liver and beyond, while controlling for exposure to viral vector and opportunity for off-target toxicity and tumorigenicity.
Keywords: Medicine, Issue 141, Gene Therapy, Lentiviral Vector, Hepatocyte Isolation, Pig Surgical model, Autologous Transplantation, Inborn Errors of Metabolism
Introduction
Inborn errors of metabolism of the liver are a family of genetic diseases that collectively affect as many as 1 in 800 live births1. Many of these diseases are single gene defects2 and can be functionally cured by introducing a single corrected copy of the affected gene into a sufficient number of hepatocytes3. The actual percentage of hepatocytes that needs to be corrected varies by the disease4 and is largely dependent on the nature of the protein it encodes, for example, excreted proteins versus cytoplasmic. In most cases, efficacy of any treatment for metabolic disease is easily assayed through the presence of biomarkers often available in the circulation.
HT-1 is an inborn error of metabolism of the liver that results from a defect in fumarylacetoacetate hydrolase (FAH)5, the last enzymatic step in tyrosine metabolism6. FAH deficiency leads to the build up of toxic metabolites in the liver that can cause acute liver failure and death or in the chronic form of the disease can cause cirrhosis and hepatocellular carcinoma. The disease is clinically managed by administration of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), a small molecule inhibitor of an enzyme upstream of FAH in tyrosine metabolism. The disease provides an ideal environment in which to test gene therapy methods, as successful correction of even a small number of hepatocytes will eventually result in the repopulation of the entire liver with corrected cells in both small and large animal models7,8. This occurs because corrected cells have a profound survival advantage over uncorrected cells due to the accumulation of toxic metabolites in the latter. The loss of uncorrected hepatocytes allows for selective expansion of corrected hepatocytes consistent with the regenerative capacity of the liver. Treatment can be easily followed by measuring the decrease in circulating tyrosine and succinylacetone levels following transplantation.
In order to justify the invasive nature of the procedure, which includes a partial hepatectomy, the goal of this approach must be a durable cure. Therefore, replication incompetent lentiviral vectors are used because they will stably integrate into the hepatocyte genome9. ensuring delivery of the corrected gene to all daughter cells as the liver grows and expands to replace the rapid loss of uncorrected cells. This is advantageous over adeno associated viral (AAV) vectors, which primarily exist as episomes that can only be passed to a single daughter cell during mitosis10 thereby losing any effect of the therapy in a matter of weeks.
Although a growing body of literature supports the safety of lentivirus11, concerns over genotoxic events are mitigated by limiting the transduction of host cells to a controlled in vitro environment. Free vector is never systemically introduced to the host when this method is performed, limiting exposure to the hepatocytes that will be re-introduced with autologous transplant via the portal vein.
This report describes the method of the surgical and ex vivo procedures used to isolate hepatocytes for gene therapy ex vivo and subsequent autologous transplantation12 for the treatment of the HT-1 pig8. The full process includes 1) a partial hepatectomy that serves as a source of hepatocytes and a growth stimulus for the host’s liver, 2) isolation of hepatocytes from the excised liver followed by ex vivo gene correction, and finally 3) reintroduction of the corrected hepatocytes back into the host. The method described is applicable to all large animal models with some modification, but only the FAH-deficient pig13 will have the advantage of the selective environment for corrected hepatocytes.
Protocol
All animal procedures were performed in accordance with institutional guidelines and were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) prior to study conduct. Procedures described here were performed on male and female large white farm pigs (50% Landrace/50% Large White genetic background) up to 3 months of age that are deemed healthy and suitable for surgery. Animals are socially housed unless considered incompatible by institutional veterinary care staff. Animals are fed appropriate levels of chow twice daily and observed for clinical signs by care staff at least once daily.
1. Preparation for Surgery
Administer 5 mg/kg telazol and 2 mg/kg xylazine intramuscularly to induce sedation. Administer buprenorphine SR subcutaneously at a dose of 0.18 mg/kg for post-operative analgesia (anticipated analgesia from this dose will be up to 72 h). Manually check the animal for responses to verify sedation.
Once sedated, insert an intravenous catheter into a peripheral ear vein for pharmacologic access.
- Administer intravenous 25 mg/kg cefazolin and intramuscular 5 mg/kg of ceftiofur pre-operatively.
- Administer normal saline intravenously to keep blood pressure and heart rate within normal physiologic limits throughout the procedure.
Place an orogastric tube to decompress the stomach. Perform endotracheal intubation with an appropriately sized endotracheal tube, verify proper placement by compressing the chest manually to detect exhalation, and then mechanically ventilate the animal with 1–3% isoflurane.
Place electrocardiogram (ECG) leads, blood pressure cuff, temperature probe, and pulse-oximeter to monitor and record vital signs. Place the animal in a supine position, scrub the abdomen from rib cage to pelvis with betadine, and drape sterilely.
- Ventilate subject with 1–3% isoflurane during the procedure to maintain a surgical plane of anesthesia. Continue to monitor vital signs for changes and adjust isoflurane accordingly to maintain heart rate at pre-incision levels If blood pressure drops dramatically, reduce isoflurane and initiate institutionally-approved agents to restore vitality, such as intravenous epinephrine.
- Record heart rate, respiration rate, blood pressure, and body temperature on a surgical procedure record to track and maintain animal welfare as per institution-specific policy to ensure compliance with large animal welfare standards.
2. Laparoscopic Partial Hepatectomy
Perform initial port site entry using an open Hassen technique cephalad to the umbilicus. When peritoneum is visualized, safely introduce a 12 mm trocar into the abdominal cavity. Pass a 5 mm, 30°, scope through this port.
- Insufflate the abdomen with CO2 to 15 mmHg. Under direct visualization with the laparoscopic camera, place two additional 5 mm ports triangulating on the left lateral lobe of the liver. Exact placement of the ports will depend on size and anatomy of the animal.
- At this point, place the laparoscope in one of the 5 mm ports.
- Identify the left lateral segment of the liver at the point of the major fissure of left lobe. This fissure separates the medical and left lateral segments. Secure the portal structures along with the hepatic vein along this fissure using a surgical stapler (see Table of Materials). Transect the parenchyma through this fissure using sequential firings from the stapler using 60, 45, or 30 mm long vascular loads.
- When parenchymal resection is complete, assess the remnant liver to ensure adequate hemostasis. Control any bleeding from the parenchyma with cautery or suture.
Retrieve the liver section using endoscopic graspers and an endoscopic tissue retrieval bag.
Remove the ports and close the 12 mm port incision in three layers with interrupted size 0 suture for the midline fascia, a running 2–0 suture for the deep dermal layer and a running 4–0 suture for the subcuticular layer. The 5 mm ports may be closed in one layer with 2–0.
Place a sterile dressing on the incisions (see Table of Materials).
When cells will be ready in less than 4 hours, maintain the animals under general anesthesia post-operatively until the time of autotransplantation.
Alternatively, if cell manipulations require longer periods (such as to allow formation of hepatocyte spheroids or longer transduction/selection periods based on individual applications of this method) allow the animal to recover from anesthesia and repeat the anesthetic induction steps prior to autotransplantation when the cells are ready.
Materials
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) | Yecuris | 20–0027 | |
| 12 mm Trocar | Covidien | B12STS | |
| 5 mm Trocar | Covidien | B5SHF | |
| Endo Surgical Stapler 60 | Covidien | EGIA60AMT | |
| Endo Surgical Stapler 45 | Covidien | EGIA45AVM | |
| Endo Surgical Stapler 30 | Covidien | SIG30AVM | |
| Endo catch bag | Covidien | 173050G | |
| 0 PDS | Ethicon | Z340H | |
| 2–0 Vicryl | Ethicon | J459H | |
| 4–0 Vicryl | Ethicon | J426H | |
| Dermabond | Ethicon | DNX12 | Sterile Dressing |
| Williams’-E Powder | Gibco | ME16060P1 | |
| NaHCO3 | Sigma Aldrich | S8875–1KG | |
| HEPES | Fisher | BP310–1 | |
| Pen/Strep | Gibco | 15140–122 | |
| Fetal Bovine Serum | Corning | 35–011-CV | |
| NaCl (g/L) | Sigma Aldrich | S1679–1KG | |
| KCl (g/L) | Sigma Aldrich | P3911–500G | |
| EGTA (g/L) | Oakwood Chemical | 45172 | |
| N-acetyl-L-cysteine | Oakwood Chemical | 3631 | |
| (N-A-C, g/L) | Sigma Aldrich | A9165–100G | |
| CaCl2 2H2O (g/L) | Sigma Aldrich | 223506–500G | |
| Collagenase D (mg/mL) | Crescent Chemical | 17456.2 | |
| Dulbecco's modified eagle medium | Corning | 15–013-CV | |
| (DMEM) | |||
| Dexamethasone | Fresenius Kabi | NDC6337 | |
| Epidermal Growth Factor | Gibco | PHG0314 |
3. Hepatocyte Isolation
- Connect a peristaltic pump with perfusion set to deliver warm dispersion solutions (maintained in 43 °C water bath) to catheters to be placed in the large exposed vessels of the liver section, such that solution temperature is 38 °C upon introduction to the tissue. All tubing, equipment, and solutions must be maintained sterile to ensure the cells are suitable for transplantation.
- Prime the pump and tubing with Per II up to a stopcock positioned to switch between Per I and Per II delivery to the tissue. Switch the stopcock to the Per I position and prime the rest of the tubing set, filling an in-line bubble trap completely to prevent introducing air bubbles into the tissue.
Prepare a clean transverse cut perpendicular to the hepatic circulation to reveal cross sections of portal vein branches and hepatic veins for catheterization.
- Catheterize the available exposed veins with a snug-fitting catheter to avoid leakage of the perfusate. Cannulate at least 1 portal and 1 hepatic vein to ensure adequate perfusion of the tissue.
- Ensure that the catheter(s) are primed/free of air before placing the catheter into a vein.
Perfuse with warm Per I at 100 mL/min, moving the outlet tube from vein to vein every 30 seconds to 1 min. Cycle through all available veins for 15–20 min. During Per I infusion, set an evacuation tube to empty the procedure tray into a waste container under vacuum.
- Switch to Per II at 100 mL/min, cycling through the veins as with Per I, for a total of 30 min. During Per II, use a return pump to recycle Per II back to the reservoir for re-administration to conserve preparation of the active enzyme. Set the return pump to less than the outflow pump (i.e., 94 mL/min), being careful not to return excessive air to the source bottle.
- If the liver capsule breaks, this time can be reduced.
- If the liver is not fully digested (does not remain dimpled with gentle focal pressure) an additional 5 min of perfusion can be performed.
Occasionally (approximately every 5 minutes) document surface temperature of the bubble trap and/or liver to ensure the hepatocytes are not getting cold. If the liver is getting cold (<35 °C) increase the heat of the water bath to restore the liver temperature closer to 38 °C. The liver should blanche as the perfusion proceeds.
Remove liver to sterile plate or Petri dish for transport to cell culture hood. Cover sterile field with a sterile drape to maintain integrity during the hepatocyte processing.
Expose the liver section from sterile drape and submerge the liver with cold hepatocyte wash media (HWM). Disrupt the capsule by dragging scissors across the top. Wearing sterile gloves, gently massage the liver to release the hepatocytes into the medium.
- Filter the HWM containing hepatocytes through sterile gauze into large (~200 mL) centrifuge bottles. Wash the hepatocytes through the following procedure in triplicate, combining the cell pellet from the multiple bottles after the first resuspension (as applicable):
- Centrifuge at 50 × g, 5 min, 4 °C.
- Aspirate and resuspend in 150 mL of HWM.
- Leave the pellet in HWM after the 3rd wash.
Count cell concentration using a hemocytometer or other device, as available. These cells are now ready for ex vivo manipulation of interest, including gene therapy, cell sorting, or other specialization prior to autotransplantation. Final volume of HWM can be adjusted to target a specific concentration (cells/mL).
4. Hepatocyte Transduction
Resuspend hepatocytes in media (Dulbecco’s modified eagle medium [DMEM], 10% fetal bovine serum [FBS], Penicillin-Streptomycin, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], dexamethasone, epidermal growth factor [EGF], and NTBC) at 1–2 million cells per mL in a conical tube on ice.
Thaw lentiviral vector at room temperature and add to conical tube on ice at target 20 multiplicity of infection (MOI) to bind the cells. Rotate cells at 4 °C at 10 rpm for 90 min to bind the viral vector to the cell surface.
Transfer tubes to 37 °C for 30–40 min. Invert to mix every 5 minutes to facilitate the vector transducing the bound cells.
Centrifuge cells at 50 × g at 4 °C for 4 min. Resuspend cell pellet in saline at desired concentration on ice. Repeat this wash to ensure removal of any unbound vector from the preparation.
-
If possible, plate sufficient aliquots of cells (i.e., 500,000 cells per well in a 6 well cell culture plate) to check for viability, adhesion, transduction efficiency, etc.
NOTE: It is often not possible to assess these parameters prior to autotransplantation, especially for same-day procedures. For example, the procedure described herein employed an untagged Fah cDNA under the control of the alpha-1 antitrypsin liver-specific promoter, which was not readily assayable in the hepatocytes prior to transplantation. However, these plated cells can be assayed subsequent to the autotransplantation as an indicator of the success of transduction (i.e., via Western blotting) or a predictor of the general success of the procedure.
5. Hepatocyte Transplantation
Identify the portal vein via ultrasound using a 2 to 5 MHz transducer. Direct an 18 G, 5 inch needle towards the main portal vein proximal to its bifurcation.
- Begin slow manual infusion of up 1 × 109 hepatocytes (approximately 10 g) using a syringe. Monitor portal pressures during transplantation using an infusion catheter.
- If portal pressures increase more than 8 mmHg above baseline, pause infusion and allow the pressure to return to baseline levels.
- If portal pressures do not return to baseline after pausing infusion for five minutes, discontinue the infusion.
After transplantation and catheter removal, use the ultrasound to evaluate for the presence of thrombotic events and forward flow in the portal vein.
6. Postoperative Recovery and Maintenance
Move subject to a proper recovery area and observe until the animal is fully ambulatory, monitoring heart rate, oxygen saturation and body temperature every 15–30 min. Maintain body temperature with warm blankets or an air-heated wrap, as needed.
Administer 1 mg/kg omeprazole by mouth daily (through the end of the study) to reduce the chances of gastric ulceration that can occur with bouts of inappetence.
Postoperatively, the animal is monitored closely by lab and veterinary staff and typically does not require additional pain medication separate from the pre-operative buprenorphine dose. If signs of distress/pain are observed by qualified veterinary care staff (incision guarding, inappetence, lethargy), anti-inflammatory agents (i.e., Carprofen) may be administered.
7. Recipes
See Table 1 for recipes used in this protocol.
Table 1:
Recipes for Solutions Used in Hepatocyte Isolation from Liver Sections.
| Reagent | HWM | Reagent | Per I (10x) | Per II (10x) |
|---|---|---|---|---|
| Williams’-E Powder (g/L) | 10.8 | NaCl (g/L) | 83 | 39 |
| NaHCO3 (g/L) | 2.2 | KCl (g/L) | 5 | 5 |
| HEPES (g/L) | 2.6 | HEPES (g/L) | 24 | 240 |
| Pen/Strep (100x, mL/L) | 10 | EGTA (g/L) | 9.5 | - |
| Fetal Bovine Serum (mL/L) | 100 | N-acetyl-L-cysteine | 8 | 8 |
| pH | 7.3 | (N-A-C, g/L) | ||
| Nitroglycerin (mL/L) | 5 | 5 | ||
| CaCl2 2H2O (g/L) | - | 7 | ||
| Collagenase D (mg/mL) | - | 0.2 | ||
| PH | 7.4 | 7.6 |
Representative Results
The liver resection and autologous transplantation are represented schematically in Figure 1. In a representative cohort of 5 pigs that underwent hepatic resection, most had yields of >1 × 109 hepatocytes with approximately 80% viability (Table 2), providing plenty of cells for any type of desired manipulations, including gene therapy. Subsequent culture of the non-transplanted portion of prepared hepatocytes from each of those 5 pigs showed good viability and adhesion, with typical hepatocyte morphology 46 hours after transduction and initial plating (Figure 2). These results are representative of a successful preparation, while poor results would be indicated with low numbers of cells, viability, or minimal adherence of cells in a monolayer.
Figure 1: Hepatectomy and Autologous Transplantation.
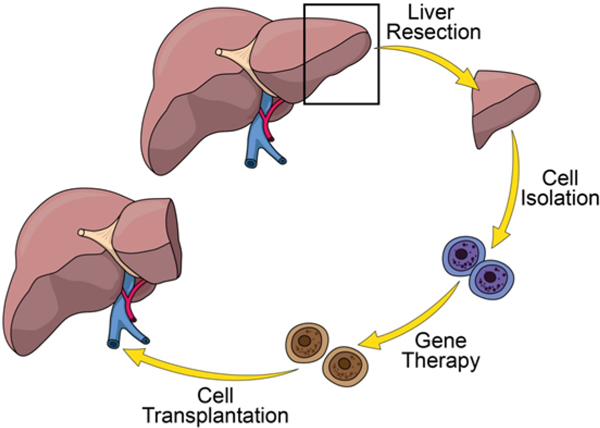
(Clockwise from top) A partial liver resection is performed on the subject to provide a source of hepatocytes and stimulate liver regeneration. Hepatocytes are isolated from the resected liver (blue cells), transduced ex vivo with the lentiviral vector containing the transgene of interest (brown cells), and then the transduced cells are autologously transplanted back to the source animal via portal vein injection. Please click here to view a larger version of this figure.
Table 2:
Representative hepatocyte results from primary isolations from 5 pigs.
| Pig | Total Cells (x 106) | Live Cells (x 106) | Viability (%) |
|---|---|---|---|
| 1 | 1,381 | 1,160 | 84 |
| 2 | 1,000 | 770 | 77 |
| 3 | 1,213 | 995 | 82 |
| 4 | 789 | 671 | 85 |
| 5 | 1318 | 975 | 74 |
| Average | 1,140 | 914 | 80 |
| St Dev | 244 | 194 | 5 |
Figure 2: Primary Pig Hepatocytes from Liver Sections.

Hepatocytes were cultured from liver resections from 5 different pigs, transduced with lentiviral vector, and allowed to grow for 48 h to demonstrate morphology, viability, purity, and adhesion to culture dishes. These in vitro qualitative assessments serve as surrogate indicators for the likelihood of successful engraftment in vivo for each preparation. Please click here to view a larger version of this figure.
A liver biopsy prior to treatment of the Fah−/− pig shows no expression of FAH via immunohistochemistry (Figure 3). Initial engraftment will vary by the amount of cells reintroduced during transplantation. Transduction frequencies of porcine hepatocytes in vitro using lentivirus at an MOI of 10 TU/hepatocyte is typically 70–100%. The engrafted cells will clonally expand in the Fah−/− liver until the entire liver is repopulated by the corrected cells. Representative biopsies at 2, 6, and 12 months demonstrate a timeline of FAH-positive cell expansion, which is typically complete at 12 months after transplantation (Figure 3). Even a low percentage of engraftment would be expected to eventually repopulate the liver, although actual initial engraftment rates were not evaluated in this experiment.
Figure 3: Corrected Hepatocytes Repopulate the Fah−/− Pig Liver.
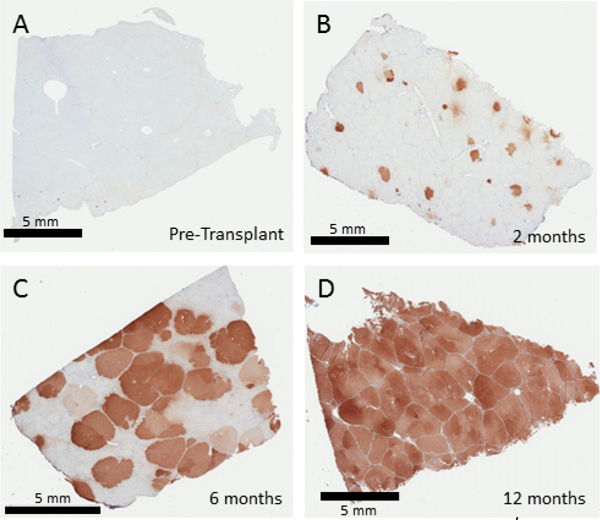
FAH immunohistochemistry from liver biopsies 0, 2, 6 and 12 months after ex vivo gene therapy of autologous hepatocytes. Untreated Fah−/− pigs show no FAH-positive cells in the liver (A). Two months after transplantation (B), individual foci of FAH positive hepatocytes are seen, which then undergo expansion to replace 50–60% of the liver at 6 months (C) followed by complete replacement, seen at 12 months (D). Please click here to view a larger version of this figure.
Pigs are carefully monitored post-transplantation for weight gain, as failure to thrive is a sign that not enough corrected hepatocytes are present. In this case animals are cycled back on NTBC as needed until the corrected hepatocyte population is sufficient to allow complete weaning from the drug. Evaluation of circulating biomarkers provides easy access to follow the therapy. Once an animal has achieved about 20% repopulation of corrected hepatocytes in the liver, tyrosine and succinylacetone levels will normalize when compared to wild-type animals (Figure 4A). Furthermore, the under-treated or untreated Fah−/− pig demonstrates similar fibrotic liver changes seen in affected humans, which can be followed by Masson’s trichrome staining of serial biopsies8. Surrogate markers of ongoing liver injury can be evaluated by assaying circulating liver enzymes, such as aspartate aminotransferase and alkaline phosphatase. While uncorrected animals show significant elevation in both parameters compared to wild type animals, ex vivo gene therapy returns these serum values to normal (Figure 4B and 4C). Lastly, general liver metabolic health is disrupted in untreated Fah−/− pigs, as indicated by elevations in circulating ammonia. Repopulation of the liver with corrected cells restores wild-type levels of ammonia (Figure 4D).
Figure 4: Serum Biochemistry at 10 months post ex vivo gene therapy in Fah−/− Pigs.
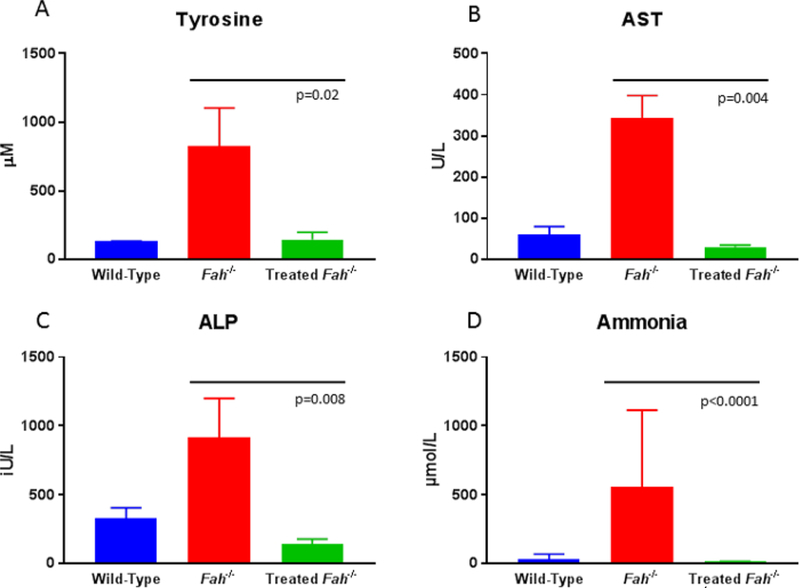
10 months after therapy, tyrosine, aspartate aminotransferase14, alkaline phosphatase (ALP) and ammonia levels are significantly lower than untreated FAH deficient animals and are indistinguishable from wild-type levels. Data were analyzed for significance using the Mann-Whitney U test (p values as presented). Please click here to view a larger version of this figure.
Discussion
This report describes an ex vivo autologous gene therapy approach to cure a porcine model of HT-1. It involves a partial hepatectomy, followed by ex vivo hepatocyte isolation and transduction of isolated hepatocytes with lenti virus carrying the corrective transgene. Corrected autologous hepatocytes are then transplanted back to the FAH deficient animal through the portal vein8. Although the method described is applicable to all large animal models with some modification, the FAH-deficient pig has the unique advantage of a highly selective environment for corrected cells13,15. This method is an effective cure for HT-1, as dosed animals show NTBC independent growth with normalization of biochemically measured metabolites and inflammatory liver biomarkers, prevention of cirrhosis and HCC and the complete reversal of early fibrosis seen with NTBC cycling. Furthermore, a more recent study involving extensive histological analysis has demonstrated no detectable uncorrected cells, fibrosis, cirrhosis or tumorigenicity 3 years post therapy (manuscript in review). However, the utility of the FAH-null background can serve as a tool to allow evaluation of a wide array of interrogations in hepatocyte physiology and disease indications beyond HT-1.
Relative success of the procedure can be evaluated by the number of cycles of NTBC required before the subject can be completely weaned from this protective drug. In numerous iterations with this model and in other small animal models of HT-1, approximately 20% correction is required before NTBC independent growth is achieved. More cycling with NTBC is indicative of poor initial engraftment/viability, advanced liver disease at the time of initial engraftment, or poor transgene expression, although other factors may also play a role. Biochemical markers of liver health (AST, ALT, and ammonia) are readily available and offer insight into the extent of corrected hepatocyte expansion, but ultimate verification of phenotypic cure is best proven by the normalization of serum tyrosine and succinylacetone and histologic confirmation of FAH-expressing hepatocytes in the absence of fibrosis. This is achieved when the liver has been repopulated with corrected hepatocytes.
The efficacy of the procedure relies most heavily upon the integrity of the hepatocytes, which must be maintained sterile and viable throughout the entire isolation, transduction and transplantation process. Reintroduction of non-viable or uncorrected hepatocytes will not rescue the phenotype, and an unsuccessful procedure could take months of subject observation to verify.
In this model, good transgene expression of the functional FAH enzyme is a minimum requirement for liver repopulation. Lentivirus is only one way to deliver an effective copy of the transgene. This method will allow the possibility for the use of other delivery systems including nonviral systems and those aimed at editing the specific genomic defect. Ultimately, this model will allow for a vast array of possibilities, including the correction of other defects in the FAH deficient bioreactor. So long as the transplanted cell expresses a functional FAH enzyme, any other modification of the cells ex vivo would also be propagated. This would allow any of the inborn errors of metabolism of the liver to be corrected in the FAH−/− background. As the expansion of corrected hepatocytes ultimately repopulates the liver, relevant numbers of hepatocytes for any disease indication can be achieved, which underscores the value of this model and procedure as a basic science and preclinical therapy model.
Acknowledgements
The authors thank Duane Meixner for expertise in performing the portal vein injection, Steve Krage, Joanne Pederson, and Lori Hillin for support during the surgical procedures. This work was supported by the Children’s Hospital of Minnesota Foundation and Regenerative Medicine Minnesota. R.D.H. was funded through an NIH K01 DK106056 award and a Mayo Clinic Center for Regenerative Medicine Career Development Award.
Footnotes
Disclosures
The authors have nothing to disclose.
Video Link
The video component of this article can be found at https://www.jove.com/video/58399/
References
- 1.Mak CM, Lee HC, Chan AY, & Lam CW Inborn errors of metabolism and expanded newborn screening: review and update. Critical Reviews in Clinical Laboratory Sciences. 50 (6), 142–162, (2013). [DOI] [PubMed] [Google Scholar]
- 2.Hansen K, & Horslen S Metabolic liver disease in children. Liver Transplantation. 14 (5), 713–733, (2008). [DOI] [PubMed] [Google Scholar]
- 3.Schneller JL, Lee CM, Bao G, & Venditti CP Genome editing for inborn errors of metabolism: advancing towards the clinic. BMC Medicine. 15 (1), 43, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunetti-Pierri N Gene therapy for inborn errors of liver metabolism: progress towards clinical applications. Italian Journal of Pediatrics. 34 (1), 2, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson DF et al. Efficient TALEN-mediated gene knockout in livestock. Proceedings of the National Academy of Sciences of the United States of America. 109 (43), 17382–17387, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindblad B, Lindstedt S, & Steen G On the enzymic defects in hereditary tyrosinemia. Proceedings of the National Academy of Sciences of the United States of America. 74 (10), 4641–4645, (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickey RD et al. Noninvasive 3-dimensional imaging of liver regeneration in a mouse model of hereditary tyrosinemia type 1 using the sodium iodide symporter gene. Liver Transplantation. 21 (4), 442–453, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickey RD et al. Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1. Science Translational Medicine. 8 (349), 349ra399, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naldini L et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 272 (5259), 263–267, (1996). [DOI] [PubMed] [Google Scholar]
- 10.Bouard D, Alazard-Dany D, & Cosset FL Viral vectors: from virology to transgene expression. British Journal of Pharmacology. 157 (2), 153–165, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakuma T, Barry MA, & Ikeda Y Lentiviral vectors: basic to translational. Biochemical Journal. 443 (3), 603–618, (2012). [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury JR et al. Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science. 254 (5039), 1802–1805, (1991). [DOI] [PubMed] [Google Scholar]
- 13.Elgilani F et al. Chronic Phenotype Characterization of a Large-Animal Model of Hereditary Tyrosinemia Type 1. The American Journal of Pathology. 187 (1), 33–41, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patyshakuliyeva A et al. Carbohydrate utilization and metabolism is highly differentiated in Agaricus bisporus. BMC Genomics. 14, 663, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey RD et al. Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Research. 13 (1), 144–153, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


