Abstract
During an agonistic encounter, subordinate male hamsters display defensive and submissive postures and show increased secretion of glucocorticoids, whereas dominant males do not. To determine whether specific neuronal pathways are activated during the behavioral and neuroendocrine responses of subordinate males, expression of c-fos mRNA within the brains of subordinate males was compared with the pattern in dominant males after fighting. After 1 week of handling, pairs of hamsters were either swapped between cages (handled control males), or were allowed to interact for 30 min [dominant (DOM) males and subordinate (SUB) males]. A second group of control animals that received no handling or social stimulation (unhandled control males) were also included. After testing, all animals were killed by decapitation, their brains were removed for c-fos in situ hybridization, and trunk blood was collected for analysis of plasma cortisol and corticosterone levels. Exposure of males to their partner’s cage for 30 min resulted in increased expression of c-fos mRNA in multiple brain regions. In addition, fighting increased c-fosexpression in the medial amygdaloid nucleus of both DOM and SUB males as well as having more selective effects. In DOM males, c-fos expression was elevated within the supraoptic nucleus of the hypothalamus. In SUB males, c-fosexpression increased within a multitude of brain areas, including cingulate cortex, lateral septum, bed nucleus of the stria terminalis, medial preoptic area, several hypothalamic nuclei, central amygdaloid nucleus, amygdalohippocampal area, dorsal periaqueductal gray, dorsal raphe, cuneiform nucleus, and locus coeruleus. These findings are discussed in relation to neurocircuits associated with behavioral arousal and stress.
Keywords: c-fos, mapping activation, agonistic behavior, aggression, stress, fear, arousal, defense, hamster
Social interactions are critical to the survival of most animal species. However, not all interchanges that occur between or among individuals can be viewed as positive or equitable. Individuals who face such adverse conditions are likely to show emotional and physiological signs of stress and, in the case of humans, to exhibit increased rates of psychiatric disorders, disease, and even death (Blanchard et al., 1993; Kaplan et al., 1995; Lemieux and Coe, 1995; Gil-Rivas et al., 1996). The present study provides a “map” of areas within the hamster brain that are active during the loss of a social encounter.
In the hamster, fighting between males results in the selective activation of the hypothalamo-pituitary-adrenal (HPA) axis. After an acute agonistic encounter, the subordinate or socially defeated male hamster shows increased secretion of adrenocorticotropin, β-endorphin, cortisol, and corticosterone, whereas the dominant male does not (Huhman et al., 1990, 1991). These findings suggest that “losing” an agonistic encounter is stressful, whereas the act of aggression may be less so.
To date, only a limited number of studies have addressed the patterns of brain activity associated with aggressive encounters (Morton et al., 1984; Joppa et al., 1995; Kollack-Walker and Newman, 1995; Potegal et al., 1996a,b), with only two of these studies directly comparing the patterns of neuronal activity associated with winning or losing. In mice, aggressive males show enhanced metabolic activity within the habenula and locus coeruleus and reduced levels in the septum and dorsal central gray when compared with defensive animals (Morton et al., 1984). In hamsters, comparison of the number of Fos-immunoreactive neurons in dominant and subordinate males revealed no significance differences (Kollack-Walker and Newman, 1995). However, detection of significant differences in glucocorticoid secretion between dominant and subordinate male hamsters is dependent on the use of procedures that habituate males to handling and to exposure to novel environments (Huhman et al., 1991). These procedures were not incorporated into the study on Fos immunostaining patterns after fighting. Thus, an inability to detect significant differences between dominant and subordinate males may have resulted from the effect of novelty as a mild stressor.
The purpose of this study was to determine whether brain areas are selectively activated in subordinate males after fighting with the addition of procedures that habituate all males to handling and to the novelty of another male’s cage and odors. The pattern of neuronal activation associated with this handling procedure was determined by analyzing the expression of c-fos mRNA within the brains of handled and unhandled control males. The pattern of neuronal activation associated with social defeat was determined by analyzing the expression of c-fos mRNA within the brains of subordinate and dominant males after fighting and within control males after handling. We hypothesized that brain regions previously implicated in stress (for review, see Cullinan et al., 1995b) would be selectively activated in subordinate males. In addition, we expected subordinate males to possess activation within brain regions involved in fight or flight reactions (for review, see Graeff, 1994) or anxiety (for review, see Kuhar, 1986; Graeff, 1994).
MATERIALS AND METHODS
Behavioral testing. Twenty-four males were singly housed for at least 1 week. During this period of isolation, 18 animals were handled daily for 10–15 min by exposing one male to the odors and home cage environment of another male. “Cage-swapping” took place between the same pairs of males throughout the isolation period, and each dyad was selected randomly, being matched only by weight. The remaining six males were left alone during the handling pretests.
On the day of the experiment, six pairs of males with previous handling experience were allowed to interact; one male in each pair was randomly selected to be the intruder and was placed into the home cage of its partner at the start of aggression testing. These 12 males were retrospectively divided into dominant (DOM) and subordinate (SUB) groups based on the behavior shown during the test. The remaining six handled animals were simply swapped as before as controls (HC). The six unhandled controls (UHC) received no overt simulation. All tests were conducted within the first 4 hr of the animals’ dark period under dim illumination. The tests were 30 min in length and were videotaped for subsequent behavioral analyses.
Immediately after testing, each animal was rapidly transported to another room (within 1–2 min) and decapitated. Trunk blood was collected in heparinized vials on ice and stored at −20°C until samples could be analyzed for plasma levels of cortisol and corticosterone. Brains were removed, frozen in isopentane (−30 to −50°C), and then stored at −80°C until the tissue was processed for c-fos mRNA in situ hybridization histochemistry.
Radioimmunoassays. Cortisol was assayed using a kit obtained from Diagnostic Products Corp. (Los Angeles, CA; Coat-A-Count cortisol radioimmunoassay). The antiserum is highly specific for cortisol, with very low cross-reactivities to other compounds (e.g., <1% cross-reactivity to corticosterone). The minimal detectable dose of this assay is ∼0.2 μg/dl. Intra-assay and interassay variations were <10%.
Corticosterone was assayed using a highly specific antibody developed in our laboratory and characterized by Dr. D. L. Helmreich (Mental Health Research Institute, University of Michigan). Cross-reactivities to related compounds (including cortisol) were <3%. Intra-assay and interassay variations were <10%.
In situ hybridization histochemistry. Each brain was sectioned on a cryostat at 10 μm, and a series of sections were mounted on poly-l-lysine-coated slides. Sections were taken at ∼200 μm intervals, except at the level of the paraventricular nucleus (PVN), in which the sections were collected at 100 μm intervals. Slides were fixed in 4% paraformaldehyde in 0.1m sodium phosphate buffer, pH 7.4, for 1 hr at room temperature. The sections were then deproteinated with proteinase K (0.1 μg/ml) for 5 min at 37°C, rinsed in distilled water, and treated for 10 min in 0.1 m triethanolamine containing 0.25% acetic anhydride. The slides were then rinsed in distilled water and dehydrated in a series of alcohols.
35S-labeled cRNA probes were generated for c-fosfrom cDNA subclones in transcription vectors using standard in vitro transcription methods. The rat c-fos cDNA clone (courtesy of Dr. T. Curran, Roche) was subcloned in pGem 3Z and cut with HindIII to yield a 680 nucleotide cRNA probe. Probe was labeled in a transcription reaction that included 1 μg of linearized plasmid DNA, 1 μl each of the guanosine, cytosine, and adenosine nucleotide bases (10 mm concentration), 1 μl of T7 polymerase, 1 μl of RNase inhibitor, 5 μl of transcription buffer, and 7.5 μl of 35S-uridine triphosphate (uracil nucleotide base). The reaction was incubated at 37°C for 2–4 hr, and the probe was then separated from free nucleotides over a Sephadex G50 column. Probes were diluted in hybridization buffer to yield ∼2.0 × 106 dpm/70 μl. The hybridization buffer consisted of 50% formamide, 3× SSC, 50 mm sodium phosphate buffer, pH 7.4, 1× Denhardt’s solution, and 0.1 mg/ml yeast tRNA. Diluted probe (70 μl) was applied to each slide and coverslipped. Slides were placed in sealed plastic boxes lined with moistened filter paper (50% formamide) and were allowed to incubate overnight at 55°C. Coverslips were then removed, and slides were rinsed several times in 2× SSC. Slides were incubated in RNase A (200 μg/ml) for 60 min at 37°C and washed successively in 2× SSC and 1× SSC at room temperature for 5–10 min each, followed by incubating sections in 0.5× SSC at 60°C for 1 hr. Sections were then placed in fresh 0.5× SSC at room temperature for 5 min and dehydrated in a series of alcohols. All slides were exposed to Kodak (Rochester, NY) BioMax MR x-ray film for 1 week, with specific brain areas from each animal processed on the same film (e.g., septal region of all animals were processed on one film). The slides were subsequently dipped in Kodak NTB2 emulsion and stored in light-tight boxes for 8 weeks at 4°C. The emulsion-dipped slides were then developed in Kodak D-19 developer, counterstained with cresyl violet, dehydrated in a graded series of alcohols, cleared in xylene, and coverslipped with Permount.
During hybridization, several sections were either pretreated with RNase A (200 μg/ml at 37°C for 60 min) or hybridized with the sense c-fos mRNA probe to determine whether the cRNA probe reacted nonspecifically with tissue components other than mRNA in hamster brain.
Data analysis. As a way to standardize optical density measurements, a template or outline was developed for each brain nucleus or subnucleus based on the shape and size of the region. The location and relative size of each template are illustrated in Figure1; the sections used in this illustration have been modified from the rat brain atlas of Paxinos and Watson (1997). Using these templates, optical density measurements were taken for each brain region from the left and right sides of the brain (where possible) or from rostral–caudal sections spaced ∼200 μm apart. The optical density values were corrected for background, multiplied by the area sampled to produce an integrated optical density measurement, and then averaged to produce one data point for each brain region in each animal. These data points were averaged per group and compared statistically. Optical density measurements were quantified from x-ray film using National Institutes of Health Image software, with specific location of signal confirmed from emulsion-dipped slides.
Fig. 1.
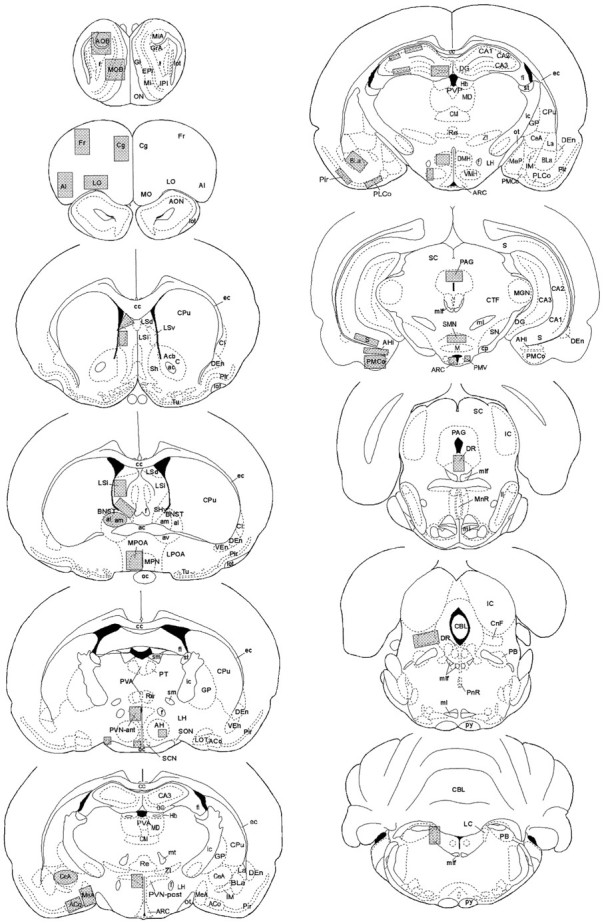
Location of templates used to sample optical density measurements within specific brain regions in each animal. See for definitions of abbreviations on this and subsequent figures.
To determine the effect of handling, the mean level of c-fosmRNA within specific brain regions was compared between UHC and HC groups using a Student’s t test (p< 0.05). The effect of behavior was determined by comparing the mean level of c-fos mRNA within the same brain regions among HC, DOM, and SUB groups using a one-factor ANOVA (p< 0.05), with post hoc comparisons made between groups using the Tukey–Kramer multiple comparison (MC) test (p < 0.05). The Student’s t test and ANOVA require that the variance be comparable between or among the groups being compared. Although this requirement was met for many brain regions, it was not true of others, as determined by Bartlett’s test of the homogeneity of variance. If the variance differed across the groups, the data were first log-transformed, and then the appropriate test run. In most cases, log transformation resulted in equivalent variance among the groups being tested. However, in a couple of brain regions, the variance remained different. In this case, the Mann–Whitney nonparametric test (p < 0.05) was used for UHC–HC group comparisons, and the Kruskal–Wallis nonparametric ANOVA test (p < 0.05) was used for HC–DOM–SUB comparisons, with post hoc between-group comparisons made using the Mann– Whitney test (p < 0.015). All statistical comparisons were made using Instat 2.01 software.
RESULTS
Behavioral observations
Aggressive behavior was observed between each home cage male and intruder pair. The home cage male was dominant in two of the six behavioral tests, whereas the intruder was dominant in the remaining four tests. Dominant or subordinate status was determined quite rapidly on pairing the two males (19 sec on average), and once established, the relationship remained constant throughout the entire 30 min test. Dominant status was given to the male that chased and attacked the other male, which responded by fleeing and showing a variety of defensive responses (for review, see Lerwill and Makings, 1971). In addition to defensive postures and escape reactions, each SUB animal was extremely vigilant of the DOM male’s location throughout the 30 min test. Furthermore, on completion of testing, all SUB males showed high reactivity to handling by the experimenter.
Glucocorticoid secretion
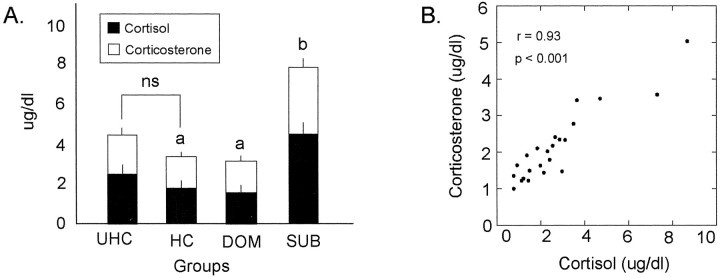
The ANOVA revealed significant differences in glucocorticoid secretion among HC, DOM, and SUB groups (cortisol, F(2,15)= 8.415; p < 0.01; corticosterone, F(2,15)= 11.209; p < 0.01) (Fig.2A). Pairwise comparisons (Tukey–Kramer MC Test, p < 0.05) showed that cortisol and corticosterone were significantly elevated in SUB males compared with the DOM and HC groups. A highly significant, positive correlation was observed between the levels of cortisol and corticosterone in all animals studied (Fig. 2B). No differences were observed in glucocorticoid secretion between the UHC and HC groups (cortisol, t(10) = 1.307;p > 0.05; corticosterone, t(10)= 1.005; p > 0.05).
Fig. 2.
Level of plasma cortisol and corticosterone within UHC, HC, DOM, and SUB groups (A) and correlation between the levels of cortisol and corticosterone for each male in all groups (B).
c-fos mRNA expression
Figure 3 illustrates the distribution of c-fos mRNA within select brain regions of UHC, HC, DOM, and SUB males. In general, low levels of c-fosmRNA were observed in the brains of UHC males. Placing a male in his partner’s cage on day 8 resulted in a significant elevation in c-fos expression throughout the neuraxis when compared with UHC group. In addition, allowing a pair of males to interact in an agonistic encounter produced significantly higher numbers of activated cells within several brain nuclei above that observed in HC males, the most prominent increases occurring in SUB males. These differences in activation patterns are believed to reflect the transient expression of the c-fos proto-oncogene in hamster brain as pretreating sections with RNase A or hybridizing sections with sense strand probe resulted in an autoradiographic signal not different from background (data not shown).
Fig. 3.
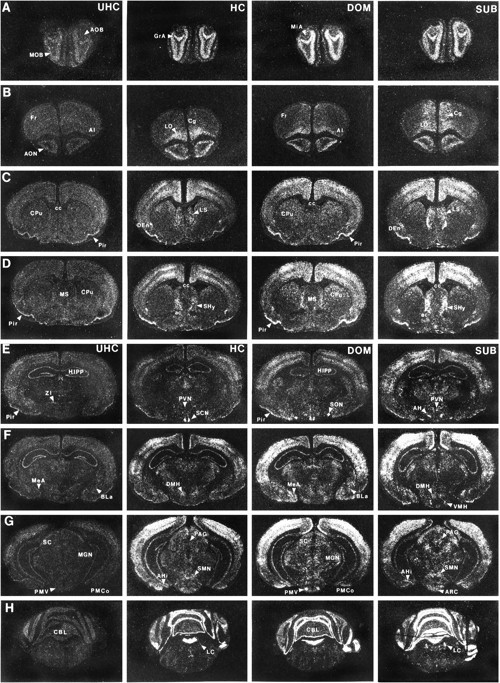
Photomicrographs illustrating the distribution of c-fos mRNA within select brain regions from animals in UHC, HC, DOM, and SUB groups.
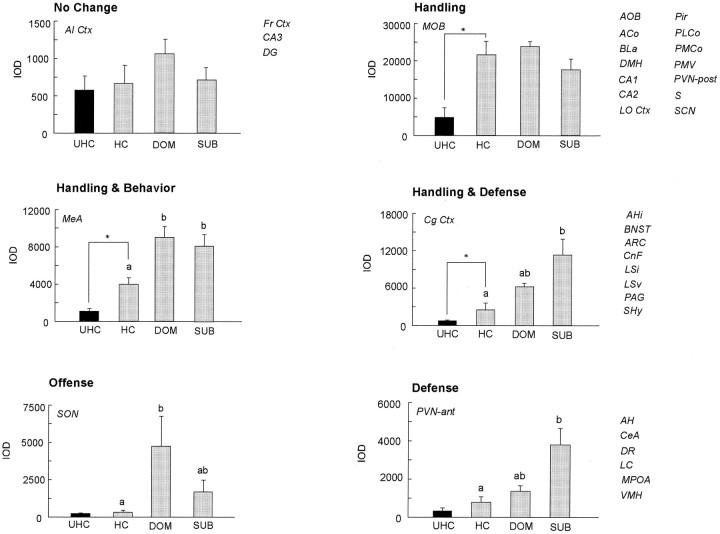
The integrated optical density measurements for each brain area in UHC, HC, DOM, and SUB groups are provided in Table1. Based on statistical comparisons between UHC and HC males (a measure of the effect of handling) and among HC, DOM, and SUB males (a measure of the effect of fighting), six distinct patterns of neuronal activation were noted: no change, handling, handling and behavior, handling and defense, defense, and offense (Fig. 4). Although most of these categories are self-explanatory, defense and offense patterns indicate that the greatest increase occurred in the SUB and DOM groups, respectively. However, in many instances, an increase in activation can be seen in both groups, with only a difference in the magnitude of activation.
Table 1.
Mean integrated optical density measurements per brain area in UHC, HC, DOM, and SUB groups
| Nucleus | UHC | HC | DOM | SUB |
|---|---|---|---|---|
| Forebrain | ||||
| AI cortex | 566 ± 188 | 652 ± 240 | 1046 ± 188 | 700 ± 164 |
| AOB | 5074 ± 2800* | 17988 ± 2442 | 26059 ± 4234 | 22379 ± 2471 |
| BNST | 116 ± 35* | 349 ± 108a | 670 ± 98a | 1987 ± 530b |
| Cg cortex | 751 ± 129* | 2542 ± 1112a | 6313 ± 544b | 11486 ± 2600c |
| Fr cortex | 720 ± 235 | 1044 ± 526 | 535 ± 282 | 219 ± 37 |
| LO cortex | 2127 ± 889* | 15732 ± 3756 | 16531 ± 2998 | 21024 ± 3264 |
| MOB | 4856 ± 2574* | 21492 ± 3696 | 23595 ± 1297 | 17505 ± 2743 |
| MPOA | 568 ± 117 | 978 ± 201a | 1504 ± 302a,b | 3276 ± 856b |
| Pir | 1197 ± 499* | 5197 ± 1009 | 3528 ± 495 | 3712 ± 677 |
| Amygdala | ||||
| ACo | 789 ± 290* | 2592 ± 470 | 3709 ± 837 | 2606 ± 651 |
| AHi | 259 ± 108* | 2078 ± 771a | 2934 ± 829a,b | 5424 ± 546b |
| BLa | 986 ± 494* | 3083 ± 672 | 2841 ± 570 | 3132 ± 412 |
| CeA | 216 ± 47* | 583 ± 171a | 883 ± 306a | 2158 ± 453b |
| MeA | 1023 ± 285* | 3940 ± 674a | 8901 ± 1144b | 8060 ± 1222b |
| PLCo | 1014 ± 505* | 3865 ± 1012 | 4523 ± 583 | 5583 ± 1116 |
| PMCo | 657 ± 303* | 7236 ± 1282 | 5024 ± 1241 | 6394 ± 645 |
| Hippocampus | ||||
| HF-CA1 | 245 ± 173* | 1303 ± 353 | 1075 ± 253 | 1873 ± 604 |
| HF-CA2 | 158 ± 106* | 556 ± 121 | 208 ± 92 | 464 ± 151 |
| HF-CA3 | 330 ± 219 | 980 ± 238 | 701 ± 170 | 625 ± 256 |
| HF-DG | 862 ± 578 | 1676 ± 407 | 1020 ± 283 | 760 ± 249 |
| S-ventral | 273 ± 131* | 1551 ± 225 | 788 ± 81 | 1443 ± 356 |
| Septum | ||||
| LSd | 612 ± 400* | 7581 ± 1776a,b | 2175 ± 763a | 11975 ± 1842b |
| LSi | 490 ± 247* | 5226 ± 1746a | 3843 ± 1336a | 20576 ± 2842b |
| LSv | 800 ± 672* | 10255 ± 2068a | 4479 ± 2133a | 25528 ± 3667b |
| SHy | 1138 ± 366* | 6327 ± 2246a | 6992 ± 1325a | 27861 ± 7445b |
| Hypothalamus | ||||
| AH | 183 ± 73 | 365 ± 70a | 2097 ± 517b | 5160 ± 971c |
| ARC | 291 ± 126* | 942 ± 233a | 649 ± 228a | 3314 ± 849b |
| DMH | 1248 ± 580* | 5673 ± 1150 | 3132 ± 1087 | 8512 ± 2344 |
| PMV | 240 ± 115* | 2303 ± 833 | 3512 ± 1442 | 4231 ± 996 |
| PVN-anterior | 325 ± 152 | 789 ± 283a | 1349 ± 279a,b | 3796 ± 880b |
| PVN-posterior | 459 ± 140* | 1579 ± 452 | 2722 ± 559 | 2822 ± 399 |
| SCN | 1251 ± 784* | 5302 ± 1497 | 5814 ± 1394 | 3383 ± 1187 |
| SMN | 1862 ± 868* | 5605 ± 1033a,b | 3628 ± 1833a | 15795 ± 3864b |
| SON | 83 ± 28 | 124 ± 34a | 4171 ± 1620b | 2064 ± 1011a,b |
| VMH-lateral | 405 ± 245 | 550 ± 172a | 2811 ± 991b | 6095 ± 759c |
| Midbrain | ||||
| DR | 332 ± 166 | 613 ± 230a | 1223 ± 112a,b | 1534 ± 304b |
| CFN | 172 ± 45* | 451 ± 97a | 2361 ± 346b | 8414 ± 1126c |
| PAG-dorsal | 252 ± 85* | 715 ± 202a | 4081 ± 1314b | 9397 ± 672c |
| Hindbrain | ||||
| LC | 123 ± 49 | 137 ± 19a | 1451 ± 524b | 4602 ± 1015c |
The statistical relationship among HC–DOM–SUB groups is shown in columns three through five.
a–cIn brain regions showing an overall group effect, the letters indicate the result of pairwise comparisons among HC, DOM, and SUB groups, with a significant difference in integrated optical density measurements between any two groups indicated by different letters.
Significant differences between UHC and HC groups.
Fig. 4.
Six distinct patterns of activation observed after handling and/or fighting (offense or defense). No Changesimply means that c-fos labeling in a given nucleus or subnucleus was not altered after handling or fighting (UHC = HC and HC = DOM = SUB). The Handling pattern indicates that c-fos expression increased within a specific brain region after handling alone (HC > UHC), and no further increases were apparent after fighting (HC = DOM = SUB). Handling & Behavior refers to increased activation after handling (HC > UHC) and after fighting, with the values for a given nucleus or subnucleus being equivalent between the DOM and SUB groups (DOM= SUB > HC). Handling & Defenseindicates increased expression of c-fos mRNA after handling (HC > UHC) and after defensive responding (SUB > HC) by the SUB males. In this category, the levels of activation for a given nucleus or subnucleus in DOM males were intermediate between HC and SUB levels (SUB = DOM and DOM = HC or SUB > DOM > HC). The Offense pattern simply refers to brain regions showing the greatest increases in c-foslabeling after aggressive responses (UHC = HC and DOM > HC), with the level from the SUB group intermediate between HC and DOM values (DOM = SUB and SUB = HC). Likewise, theDefense pattern refers to the brain areas possessing the greatest increases in c-fos labeling present after defensive responses (UHC = HC and SUB > HC), again with level from the DOM group often intermediate to those of the HC and SUB animals (HC = DOM and DOM = SUB or SUB > DOM > HC). All brain regions showing a similar pattern of activation are listed to the right of each graph.
No change
Although many brain regions showed an increase in c-fosmRNA after handling and/or fighting, the agranular insular (AI) and frontal (Fr) cortices were two areas that showed no change after either experimental procedure. A similar result was obtained for the CA3 and dentate gyrus (DG) of the hippocampus, although signal could be detected within these two areas in some males of the HC, DOM, and SUB groups.
Handling
After handling, c-fos expression increased within the accessory (AOB) and main (MOB) olfactory bulbs with grains present over cells within mitral cell layers. In addition, elevated levels of c-fos mRNA were observed in the lateral orbital cortex (LO), the amygdala [anterior (ACo), posteromedial (PMCo), and posterolateral (PLCo) cortical nuclei, basolateral nucleus (BLa)], the piriform cortex (Pir), the hippocampus [subfields CA1 and CA2 and ventral subiculum (S)], and within several nuclei of the hypothalamus, including the suprachiasmatic nucleus (SCN), the posterior aspect of the paraventricular nucleus (PVN-post), the dorsomedial nucleus (DMH), and the ventral premammillary nucleus (PMV). In all of these brain areas, c-fos expression increased after handling alone, with no further increases after fighting [e.g., BLa (Fig.5), DMH (Fig.6), hippocampal subfields CA1 and CA2 (Fig. 7), ventral subiculum (Fig.8), and posterior PVN (Fig.9)].
Fig. 5.
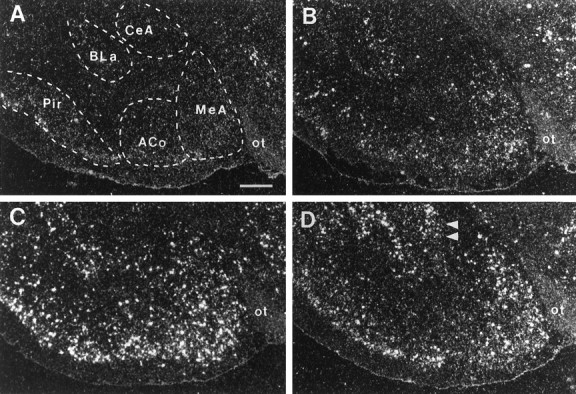
Photomicrographs illustrating the distribution of c-fos mRNA within the amygdala in UHC (A), HC (B), DOM (C), and SUB (D) groups. Scale bar, 500 μm. Note the selective increase in c-fos mRNA within the lateral portion of CeA in SUB group (D, double arrowheads).
Fig. 6.
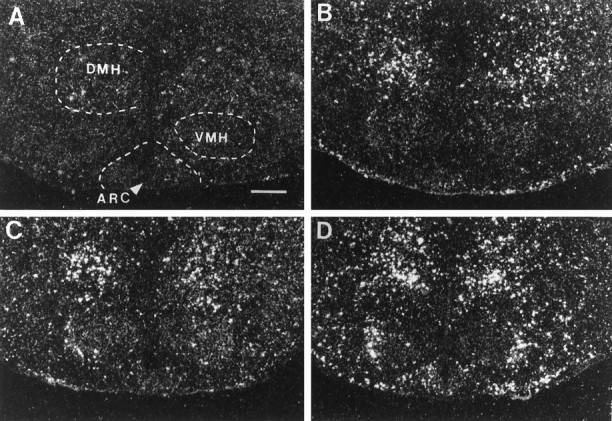
Photomicrographs illustrating the distribution of c-fos mRNA within the hypothalamus at the level of the DMH and VMH in UHC (A), HC (B), DOM (C), and SUB (D) groups. Scale bar, 500 μm.
Fig. 7.
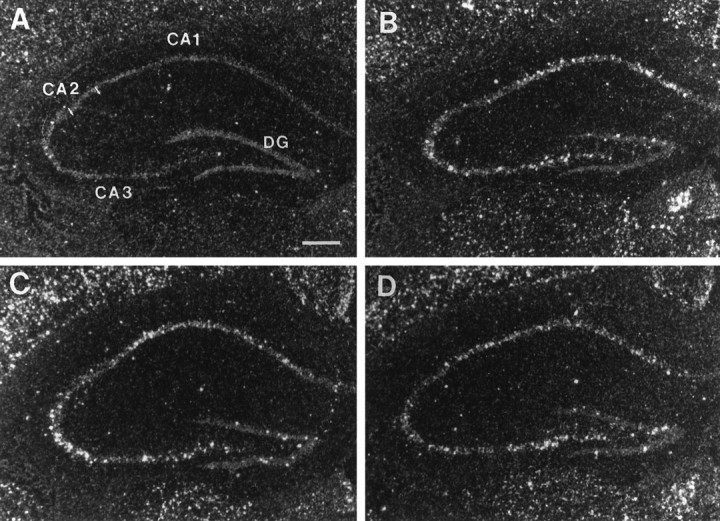
Photomicrographs illustrating the distribution of c-fos mRNA within the dorsal hippocampus in the UHC (A), HC (B), DOM (C), and SUB (D) groups. Scale bar, 500 μm.
Fig. 8.
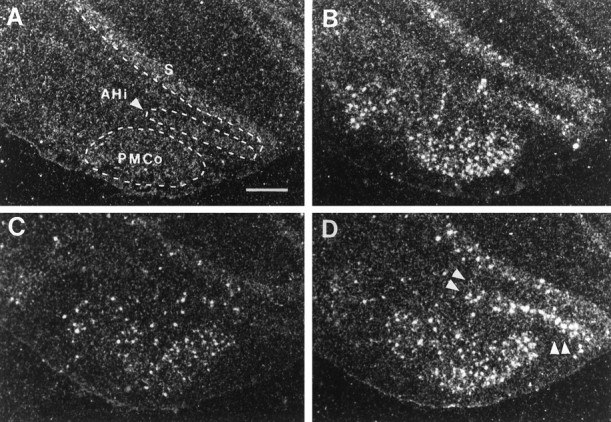
Photomicrographs illustrating the distribution of c-fos mRNA within the ventral subiculum, AHi and PMCo in UHC (A), HC (B), DOM (C), and SUB (D) groups. Scale bar, 500 μm. Note the selective increase in c-fos mRNA within the AHi of the SUB group (D, double arrowheads).
Fig. 9.
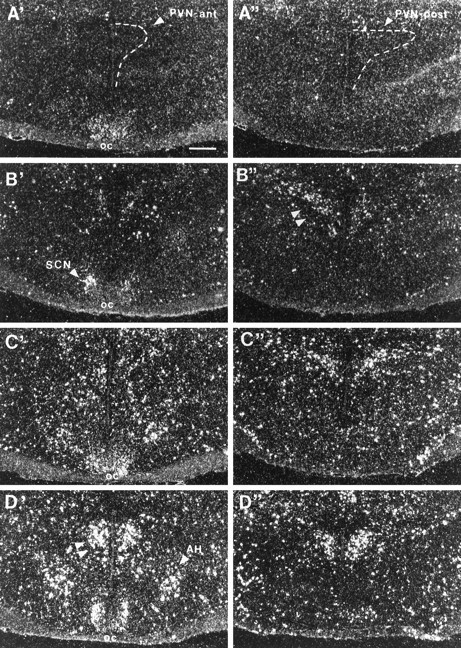
Photomicrographs illustrating the distribution of c-fos mRNA within the hypothalamus at the level of the anterior (left column, A′–D′) and posterior (right column, A"–D") subdivisions of the PVN in UHC (A), HC (B), DOM (C), and SUB (D) groups. Scale bar, 500 μm. Note the increase in expression of c-fos mRNA within posterior PVN of the HC group (B", double arrowheads) and within the anterior PVN of the SUB group (D′, double arrowheads).
Handling and behavior
The anterior subdivisions of the medial nucleus of the amygdala (MeA) showed increased c-fos labeling after handling, with further increases after fighting. The induction of c-fosmRNA by fighting was comparable between DOM and SUB males (Fig. 5).
Handling and defense
A number of brain regions with increased c-fosexpression after handling also showed further elevations after defense. These areas included the cingulate cortex (Cg), the ventral (LSv) and intermediate (LSi) subdivisions of the lateral septum, the septohypothalamic nucleus (SHy), the anterior subdivisions of the bed nucleus of the stria terminalis (BNST), the amygdalohippocampal area (AHi), the arcuate nucleus (ARC) of the hypothalamus, the dorsal periaqueductal gray (PAG), and the cuneiform nucleus (CnF) [Fig. 3; some areas shown at higher power: ARC (Fig. 6); AHi (Fig. 8); and SHy and BNST (Fig. 10)].
Fig. 10.
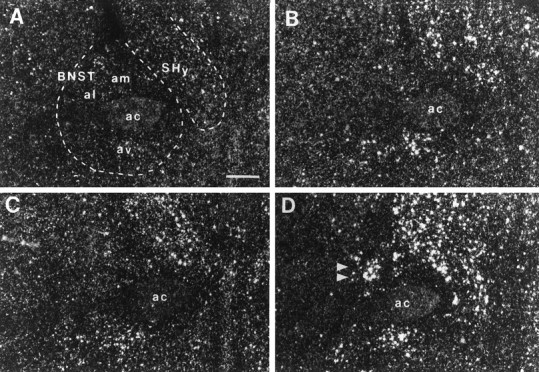
Photomicrographs illustrating the distribution of c-fos mRNA within the anterior subdivisions of the BNST in UHC (A), HC (B), DOM (C), and SUB (D) groups. Scale bar, 500 μm. Note the selective increase in c-fos mRNA within the lateral aspect of the BNST of the SUB group (D, double arrowheads).
Offense
The supraoptic nucleus of the hypothalamus (SON) was the only brain region analyzed in this study that possessed the greatest increases after the display of offense (although circuits involved in offensive aggression were not the main focus of this study).
Defense
After defense, c-fos mRNA expression increased within the central nucleus of the amygdala (CeA), medial preoptic area (MPOA), anterior (AH), and ventromedial (VMH) hypothalamic nuclei, dorsal raphe (DR) and locus coeruleus (LC). In the PVN, induction of c-fos mRNA after defense was observed within the more anterior and intermediate aspects of this nucleus, whereas the handling alone elevated signal within the posterior PVN (Fig. 9). Interestingly, c-fos-positive cells in the SUB males were localized laterally within CeA (Fig. 5), BNST (Fig. 10), and VMH (Fig. 6).
In addition, in several areas selectively activated after defense, the level of c-fos mRNA tended to be lower in DOM males in comparison with both the HC and SUB groups. This trend reached significance for the dorsal subdivision of the lateral septum (LSd) and the supramammillary nucleus (SMN), in which SUB males had a significantly greater level of activation than DOM animals, whereas the HC group had values that were intermediate (Figs. 3C,D,G,11).
Fig. 11.
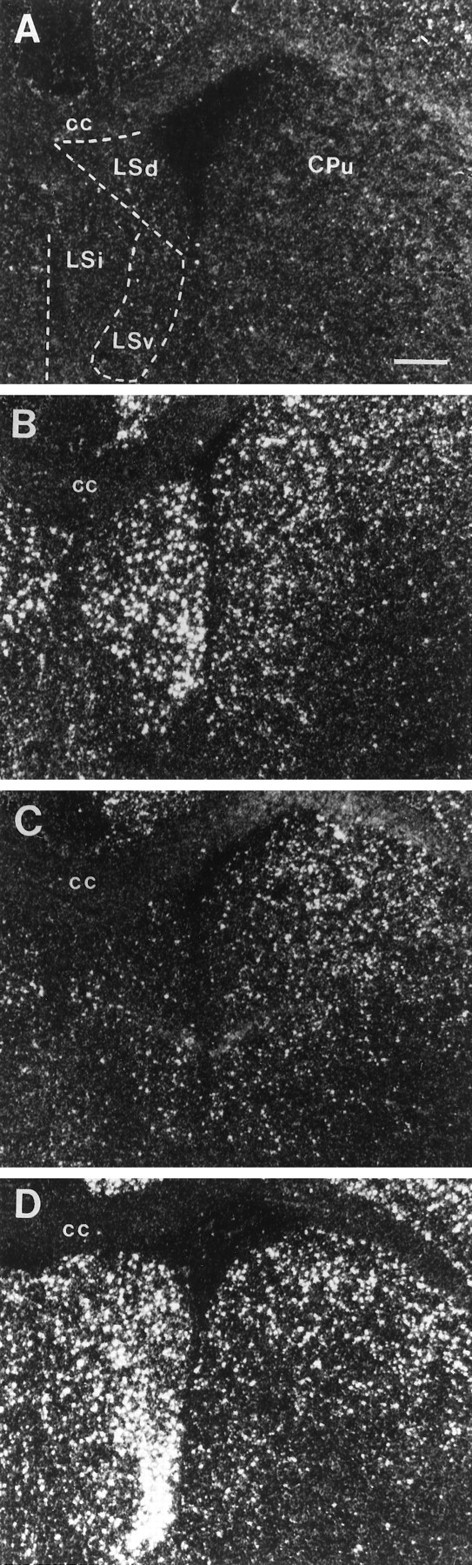
Photomicrographs illustrating the distribution of c-fos mRNA within the rostral subdivisions of the LS in UHC (A), HC (B), DOM (C) and SUB (D) groups. Scale bar, 500 μm. Note the low levels of c-fosexpression within LSd, LSi, and LSv of the DOM male compared with the levels present in the HC and SUB animals; this activation profile contrasts with c-fos labeling in the adjacent CPu (striatum), which appeared elevated in all three groups relative to UHC males.
DISCUSSION
Subordinate male hamsters possessed an increase in the frequency and intensity of activation of previously identified stress- and defense-related brain structures compared with dominant and handled control groups, and this pattern of neuronal activation was accompanied by elevations in plasma cortisol and corticosterone. In addition, increased levels of c-fos mRNA were observed within numerous brain areas after handling, in the SON after offense, and in MeA after either offense or defense. These findings underscore the stressful nature of “losing” a social encounter and provide a dynamic view of the neuronal activity that underlies the behavioral and physiological concomitants of social defeat.
As we discuss the specific findings revealed by this experiment, it is important to bear in mind that c-fos expression may not provide a complete map of all neurons activated during a given stimulus (Robertson et al., 1989). Consequently, although induction of c-fos mRNA provides positive identification of brain areas activated during fighting, the absence of such labeling does not mean the lack of involvement. Additional studies with other “activation” markers may be necessary to uncover the entire brain activation pattern associated with social defeat.
Repetitive exposure to partner’s cage
The purpose of placing each male in his partner’s cage daily was to reduce the effects of handling and exposure to another male’s odors on the responsiveness of the HPA axis. This goal was met, because plasma glucocorticoids and c-fos expression within the PVN were not significantly different between UHC and HC males.
Although the HC group did not show a stress response, these males did possess activation in numerous brain areas. Some of the most striking changes occurred in brain regions that process chemosensory cues including the mitral and granule cell layers of the AOB and MOB, and many of their central targets: amygdaloid nuclei (MeA, ACo, AHi, PLCo, and PMCo) and Pir cortex. Additional brain regions showed increased activation after handling including: Cg and LO cortices, lateral septum, anterior subdivisions of BNST, BLa, several hypothalamic nuclei (ARC, DMH, PMV, posterior PVN, and SCN), hippocampus, dorsal PAG, and cuneiform nucleus. It is likely that the specific functions mediated by activation within these various brain regions vary; however, these data indicate that behavioral arousal (e.g., locomotion and the investigation of conspecific odors by HC males) is associated with increased activation throughout the brain.
Acute exposure to intermale aggression
Fighting increased the activation of neurons within a number of brain regions. The MeA showed an equivalent level of c-fos expression in DOM and SUB males above an initial response to handling. The MeA has been implicated in a variety of behavioral responses [e.g., mating (Lehman et al., 1980; Lehman and Winans, 1982; Kollack-Walker and Newman, 1995), aggression (Shibata et al., 1982; Luiten et al., 1985; Kollack-Walker and Newman, 1995;Potegal et al., 1996b), and affiliative behavior (Kirkpatrick et al., 1994)], and induction of c-fos mRNA after fighting is consistent with a role of this brain region in behavioral arousal (De Jonge et al., 1992; Kollack-Walker and Newman, 1995; Potegal et al., 1996b) and social memory (Bolhuis et al., 1984; Vochteloo and Koolhaas, 1987). The SON showed the greatest increase in DOM males. The significance of activation within this nucleus to offense or to related processes is presently unknown. Finally, a multitude of brain regions showed increased activation after defense. The relevance of this neuronal activation pattern in SUB males to the neuroendocrine and behavioral responses of stress is discussed below.
c-fos expression in PVN and glucocorticoid secretion
In comparison to the HC and DOM groups, SUB males showed significant elevations in circulating levels of cortisol and corticosterone at the end of a 30 min agonistic encounter. This rise in circulating glucocorticoids was accompanied by increased expression of c-fos mRNA within neurons of the paraventricular nucleus of the hypothalamus (PVN), a critical node in regulation of the HPA axis to stress (for review, see Herman et al., 1996).
Although SUB males showed the greatest increases in c-fosexpression within the anterior region of the PVN, the DOM group had values that were intermediate. This finding suggests the possibility that some or all of the DOM males may have been stressed during the agonistic encounter, with an initial rise in plasma glucocorticoids that had declined to baseline by the end of testing. Indeed, studies in rats (Schuurman, 1980), mice (Bronson and Eleftheriou, 1964; Leshner, 1980), and swine (Fernandez et al., 1994) have reported elevated glucocorticoids in both the dominant and subordinate animals, with the subordinate males showing a larger response, as evidenced by higher peak levels and a slower decline to baseline.
Stress neurocircuits: neuroendocrine regulation
In addition to activation of the PVN, SUB males possessed a selective increase in c-fos labeling in brain regions previously implicated in stress regulation: Cg cortex, lateral septum, BNST, MPOA, CeA, hypothalamus (AH and ARC), and LC. This is one of the first studies to demonstrate that social stress activates neurons within distinct neurocircuits throughout the brain (also see Morton et al., 1984). The resultant pattern of neuronal activity parallels, in large part, the induction of immediate early genes after a variety of acute, nonsocial stressors (for review, see Cullinan et al., 1995a, and their illustrations).
The role of these various brain regions in regulation of the HPA axis has been the focus of several recent reviews and will be discussed here only briefly in light of our current findings. The activation of neurons within the CeA, anterior BNST, anterior MPOA, LS, DR, and LC is consistent with numerous studies showing that stimulation of these brain regions can increase levels of plasma glucocorticoids or adrenocorticotropin hormone (ACTH), which is released from corticotropes in the anterior pituitary, leading to the synthesis and release of adrenal steroids (for review, see Cullinan et al., 1995b;Herman et al., 1996). The role of several of these brain regions in facilitating adrenocortical activity is further supported by lesion studies showing that damage to cells within several of these areas can block or reduce glucocorticoid secretion in response to stress (for review, see Cullinan et al., 1995b; Herman et al., 1996).
The MeA has also been shown to stimulate glucocorticoid secretion (Dunn and Whitener, 1986). However, c-fos expression in this nucleus was elevated to comparable levels in DOM and SUB males, although cortisol and corticosterone were elevated only in the SUB group. This finding suggests that c-fos-positive neurons within the medial amygdala during fighting may not reflect excitation of the HPA axis but, rather, some other process, such as coupling sensory cues with behavioral arousal or social memory. Alternatively, activation within the medial amygdala could reflect an increased propensity for glucocorticoid secretion that is differentially regulated in DOM and SUB males at more central levels (e.g., blocked or inhibited in DOM males and stimulated in SUB males).
In addition to excitatory regulation, activation of neurons within the Cg cortex and ARC of SUB males is consistent with previous reports showing that these two brain regions play an inhibitory role in regulation of the HPA axis. Lesions of the ARC increase basal glucocorticoid levels, and enhance adrenocortical activity in response to stress (for review, see Cullinan et al., 1995b; Herman et al., 1996). Lesions of the cingulate cortex (medial prefrontal cortex) also result in prolongation of ACTH and glucocorticoid release during exposure to stress (Diorio et al., 1993). The placement of steroid implants within the medial prefrontal cortex can reduce glucocorticoid secretion in response to stress, implicating this brain region in glucocorticoid negative feedback (Diorio et al., 1993).
Surprisingly, two brain regions shown to play a role in stress responsivity were not activated in SUB males in the present study. The hippocampus (CA1–3 and subiculum) has been implicated in the mechanism of glucocorticoid negative feedback (for review, see Jacobson and Sapolsky, 1991; Herman et al., 1995). The DMH has been implicated in mediating cardiovascular responses to stress (De Novellis et al., 1995;Stotz-Potter et al., 1996b), as well as a possible role in stress-induced release of ACTH (Stotz-Potter et al., 1996a). Both brain regions showed a significant increase in c-fosexpression after handling with no further increases after fighting. Although one previous study did show a striking increase in c-fos expression within the hippocampus and DMH after stress (Cullinan et al., 1995a), the stressed animals used in that study were compared with unhandled control males, possibly missing an important effect of handling. This handling-induced activation must reflect processes present in aroused and stressed animals alike, such as activation of the autonomic nervous system to regulate heart rate (DMH) or processing of spatial cues associated with learning and memory (hippocampus).
Stress neurocircuits: behavioral regulation
In addition to elevated levels of adrenal steroids, stressed animals show changes in behavior, such as freezing or flight, reactions that presumably reflect a state of increased fear or anxiety during stress (Maestripieri et al., 1991; Heinrichs et al., 1992). Of interest, most brain regions activated in SUB males and implicated in excitatory regulation of the HPA axis have also been shown to play a facilitatory role in stress-induced behavior: CeA (Jellestad et al., 1986), BNST (Shaikh et al., 1986; Casada and Dafny, 1991), MPOA (Gonzalez et al., 1996), lateral septum (Pesold and Treit, 1992), DR (for review, see Graeff, 1994), and LC (for review, see Bremner et al., 1996). Furthermore, the pattern of activation after defeat mirrors the distribution of Fos protein after electrical stimulation of brain regions that elicit aversion and defense (Silveira et al., 1993, 1995) or after behavioral or conditioning paradigms associated with increased anxiety or fear (Silveira et al., 1994; Beck and Fibiger, 1995). Thus, it is plausible that the neurocircuits activated in SUB males mediate both the neuroendocrine and behavioral responses to the stress of defeat.
The expression of c-fos mRNA within the LS of SUB males may reflect most closely an increased state of anxiety or fear. This conclusion is based on previous reports that increased anxiety or fear can inhibit offensive aggression (Blanchard et al., 1984; Maestripieri et al., 1991) and the observation that DOM males in the present study had the lowest levels of c-fos expression within the dorsal and, to a lesser degree, the intermediate subdivisions of the LS. The lateral septum has been implicated in behavioral inhibition, a process considered equivalent to anxiety (for review, see Graeff, 1994). The relatively sparse labeling of c-fos-positive cells within the lateral septum of DOM males may reflect a state of low anxiety and may be critical for the display of offense by these animals. Of interest, lesions to the septal region of hamsters increase aggression and social contacts (Sodetz and Bunnell, 1970; Potegal et al., 1981), a finding consistent with the proposed function of this brain region in behavioral inhibition.
In addition to neural circuits classically associated with stress, we found increased expression of c-fos mRNA within the ventrolateral aspect of the AH, lateral VMH, SMN, AHi, dorsal PAG, and CnF in SUB males. Although the functional significance of activation within several of these brain regions is less clear, activation within the AH, VMH, and dorsal PAG play an important role in mediating defensive behavior (for review, see Siegel and Pott, 1988). In the hamster, lesions of the AH increase the likelihood that females will show aggression against other females (Hammond and Rowe, 1976), which implies the presence of a neural circuit in the anterior hypothalamic area that either inhibits aggression or stimulates a competing response like defense. Moreover, administration of norepinephrine into the MPOA–anterior hypothalamic region of dominant female hamsters results in a loss of their dominance and the display of submissive behaviors (Harmon et al., 1995). This latter study suggests a possible functional relationship between the activation observed within the AH and LC of SUB males. Interestingly, the unit activity of neurons within the LC of cats increases with the display of a defense reaction in response to threatening stimuli (Levine et al., 1990).
The ARC also displayed induction of c-fos mRNA selectively within SUB males. The ARC has been implicated in mediating the opioid form of stress-induced analgesia (Kelsey et al., 1987), a physiological response that may be critical for the display of defensive responses in situations that would normally elicit adaptive, more vegetative responses (e.g., licking a limb on injury). Opioid-mediated analgesia has been observed after defeat in mice (Miczek et al., 1982; Rodgers and Hendrie, 1983) and rats (Rodgers et al., 1983). The presence of analgesia in defeated male hamsters has not been reported.
Conclusions
Losing an agonistic encounter is clearly more stressful than winning. Our data provide evidence for the activation of specific neural circuits that may underlie the autonomic, neuroendocrine, and behavioral responses of socially defeated males. These findings lay the foundation for future experiments to identify the anatomical relationship among activated brain regions, the molecules involved in these specific brain circuits, and the regulation of these circuits after different types of stress.
The following abbreviations are used: ac, anterior commissure; Acb, nucleus accumbens; ACo, anterior cortical nucleus of the amygdala; AH, anterior hypothalamic nucleus; AHi, amygdalohippocampal area; AI, agranular insular cortex; AOB, accessory olfactory bulb;AON, anterior olfactory nucleus; ARC, arcuate nucleus of the hypothalamus; BNST, bed nucleus of the stria terminalis; BNSTam, anteromedial subdivision, BNST;ANSTal, anterolateral subdivision, BNST; BNSTav, anteroventral subdivision, BNST; BLa, basolateral nucleus of the amygdala, anterior; C, core, Acb; CA1–3, field CA1–3 of Ammon’s horn, HIPP; CBL, cerebellum;cc, corpus callosum; CeA, central nucleus of the amygdala; Cg, cingulate cortex; Cl, claustrum,CM, central medial thalamic nucleus; CnF, cuneiform nucleus; cp, cerebral peduncle; CPu, caudate putamen (striatum); CTF, central tegmental field;DEn, dorsal endopiriform nucleus; DG, dentate gyrus, HIPP; DMH, dorsomedial nucleus of the hypothalamus;DR, dorsal raphe; ec, external capsule;EPl, external plexiform layer of the olfactory bulb;f, fornix; fi, fimbria of the hippocampus;Fr, frontal cortex; Gl, glomerular layer of the olfactory bulb; GP, globus pallidus; GrA, granule cell layer, AOB; HIPP, hippocampus; Hb, habenula;ic, internal capsule; IC, inferior colliculus;IM, intercalated amygdaloid nucleus; IPl, internal plexiform layer of the olfactory bulb; La, lateral nucleus of the amygdala; LC, locus coeruleus; LH, lateral hypothalamus; LO, lateral orbital cortex;LOT, nucleus of the lateral olfactory tract; lot, lateral olfactory tract; LPOA, lateral preoptic area;LS, lateral septum; LSd, dorsal subdivision, LS;LSi, intermediate subdivision, LS; LSv, ventral subdivision, LS; M, mammillary nucleus; MD, mediodorsal thalamic nucleus; Me, medial nucleus of the amygdala; MeA, anterior subdivisions, Me; MeP, posterior subdivisions, Me; Mi, mitral cell layer, MOB;MiA, mitral cell layer, AOB; MGN, medial geniculate nucleus; ml, medial lemniscus; mlf, medial longitudinal fasciculus; MO, medial orbital cortex;MOB, main olfactory bulb; MPOA, medial preoptic area; MS, medial septum; mt, mammillothalamic tract; oc, optic chiasm; ot, optic tract;PAG, periaqueductal gray; PB, parabrachial nucleus; Pir, piriform cortex; PLCo, posterolateral nucleus of the amygdala; PMCo, posteromedial nucleus of the amygdala; PMV, ventral premammillary nucleus;PnR, pontine raphe nucleus; PT, paratenial thalamic nucleus; PVA, paraventricular thalamic nucleus, anterior; PVN, paraventricular nucleus of the hypothalamus;PVN-ant, PVN, anterior; PVN-post, PVN, posterior;PVP, paraventricular thalamic nucleus, posterior;py, pyramidal tract; S, subiculum; SC, superior colliculus; SCN, suprachiasmatic nucleus;Sh, shell, Acb; SHy, septohypothalamic nucleus;SMN, supramammillary nucleus; SN, substantia nigra; sm, stria medullaris; SON, supraoptic nucleus of the hypothalamus; st, stria terminalis;Re, reuniens thalamic nucleus; Tu, olfactory tubercle; VEn, ventral endopiriform nucleus; VMH, ventromedial nucleus of the hypothalamus; ZI, zona incerta.
Footnotes
This work was supported by Grant P30-HD-18258 to the Morphology Core of the National Institutes of Health National Institute of Child Health and Human Development Center for the Study of Reproduction at the University of Michigan, and National Institutes of Health Training Grants T32 DA07268-04 and T32 DK07245-20 to S.K.W. and Grant P01 MH42251 to S.J.W. We thank Jim Stewart for assistance with the killing of animals.
Correspondence should be addressed to Dr. Sara Kollack-Walker, Mental Health Research Institute, University of Michigan, 205 Zina Pitcher Place, Ann Arbor, MI 48109-0720.
REFERENCES
- 1.Beck CHM, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard RJ, Kleinschmidt CK, Flannelly KJ, Blanchard DC. Fear and aggression in the rat. Aggressive Behav. 1984;10:309–315. [Google Scholar]
- 4.Bolhuis JJ, FitzGerald RE, Kijk DJ, Koolhaas JM. The corticomedial amygdala and learning in an agonistic situation in the rat. Physiol Behav. 1984;32:575–579. doi: 10.1016/0031-9384(84)90311-1. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Bronson FH, Eleftheriou BE. Chronic physiological effects of fighting in mice. Gen Comp Endocrinol. 1964;4:9–14. doi: 10.1016/0016-6480(64)90033-4. [DOI] [PubMed] [Google Scholar]
- 7.Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- 8.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995a;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 9.Cullinan WE, Herman JP, Helmreich DL, Watson SJ. A neuroanatomy of stress. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiological and clinical consequences of stress: from normal adaptation to PTSD. Raven; New York: 1995b. pp. 3–26. [Google Scholar]
- 10.De Jonge FH, Oldenburger WP, Louwerse AL, van de Poll NE. Changes in male copulatory behavior after sexual exciting stimuli: effects of medial amygdala lesions. Physiol Behav. 1992;52:327–332. doi: 10.1016/0031-9384(92)90279-b. [DOI] [PubMed] [Google Scholar]
- 11.De Novellis V, Stotz-Potter E, Morin SM, Rossi F, DiMicco JA. Hypothalamic sites mediating cardiovascular effects of microinjected bicuculline and excitatory amino acids in rats. Am J Physiol. 1995;269:R131–R140. doi: 10.1152/ajpregu.1995.269.1.R131. [DOI] [PubMed] [Google Scholar]
- 12.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology. 1986;42:211–217. doi: 10.1159/000124442. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez X, Meunier-Salaun M-C, Mormede P. Agonistic behavior, plasma stress hormones, and metabolites in response to dyadic encounters in domestic pigs: interrelationships and effect of dominance status. Physiol Behav. 1994;56:841–847. doi: 10.1016/0031-9384(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 15.Gil-Rivas V, Fiorentine R, Anglin MD. Sexual abuse, physical abuse, and post-traumatic stress disorder among women participating in outpatient drug abuse treatment. J Psychoact Drugs. 1996;28:95–102. doi: 10.1080/02791072.1996.10471718. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 17.Graeff FG. Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz J Med Biol Res. 1994;27:811–829. [PubMed] [Google Scholar]
- 18.Hammond MA, Rowe FA. Medial preoptic and anterior hypothalamic lesions: influences on aggressive behavior in female hamsters. Physiol Behav. 1976;17:507–513. doi: 10.1016/0031-9384(76)90115-3. [DOI] [PubMed] [Google Scholar]
- 19.Harmon AC, Huhman KL, Moore TO, Albers HE. Microinjection of norepinephrine (NE) into the medial preoptic-anterior hypothalamus (MPOA-AH) regulates agonistic behavior in female Syrian hamsters. Soc Neurosci Abstr. 1995;21:2091. [Google Scholar]
- 20.Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- 21.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 22.Herman JP, Prewitt CM-F, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 23.Huhman KL, Bunnell BN, Mougey EH, Meyerhoff JL. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiol Behav. 1990;47:949–956. doi: 10.1016/0031-9384(90)90023-w. [DOI] [PubMed] [Google Scholar]
- 24.Huhman KL, Moore TO, Ferris CF, Mougey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Horm Behav. 1991;25:206–216. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenal axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 26.Jellestad FK, Markowska A, Bakke HK, Walther B. Behavioral effects after ibotenic acid, 6-OHDA and electrolytic lesions in the central amygdala nucleus of the rat. Physiol Behav. 1986;37:855–862. [PubMed] [Google Scholar]
- 27.Joppa MA, Meisel RL, Garber MA. c-Fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience. 1995;68:783–792. doi: 10.1016/0306-4522(95)00179-m. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan ML, Asnis GM, Lipschitz DS, Chorney P. Suicidal behavior and abuse in psychiatric outpatients. Comp Psychiatry. 1995;36:229–235. doi: 10.1016/0010-440x(95)90087-c. [DOI] [PubMed] [Google Scholar]
- 29.Kelsey JE, Hoerman WA, Kimball LD, Radack LS, Carter MV. Arcuate nucleus lesions reduce opioid stress-induced analgesia (SIA) and enhance non-opioid SIA in rats. Brain Res. 1986;382:278–290. doi: 10.1016/0006-8993(86)91337-5. [DOI] [PubMed] [Google Scholar]
- 30.Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole: Behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- 31.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- 32.Kuhar MJ. Neuroanatomical substrates of anxiety: a brief survey. Trends Neurosci. 1986;9:307–311. [Google Scholar]
- 33.Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240:27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- 34.Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- 35.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Lerwill CJ, Makings P. The agonistic behaviour of the golden hamster Mesocricetus auratus (Waterhouse). Anim Behav. 1971;19:714–721. [Google Scholar]
- 37.Leshner AI. The interaction of experience and neuroendocrine factors in determining behavioral adaptations to aggression. Prog Brain Res. 1980;53:427–438. doi: 10.1016/S0079-6123(08)60081-3. [DOI] [PubMed] [Google Scholar]
- 38.Levine ES, Litto WJ, Jacobs BL. Activity of the cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res. 1990;531:189–195. doi: 10.1016/0006-8993(90)90773-5. [DOI] [PubMed] [Google Scholar]
- 39.Luiten PGM, Koolhaas JM, de Boer S, Koopmans SJ. The cortico-medial amygdala in the central nervous system organization of agonistic behavior. Brain Res. 1985;332:283–297. doi: 10.1016/0006-8993(85)90597-9. [DOI] [PubMed] [Google Scholar]
- 40.Maestripieri D, Badiani A, Puglisi-Allegra S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav Neurosci. 1991;105:663–668. doi: 10.1037//0735-7044.105.5.663. [DOI] [PubMed] [Google Scholar]
- 41.Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- 42.Morton B, Blanchard RJ, Lee E, Hanohano D, Cabebe L, Blanchard DC. The use of [14C]-2-deoxyglucose to detect regional brain activities associated with aggressive and defensive behavior. In: Flannelly KJ, Blanchard RJ, Blanchard DC, editors. Biological perspectives on aggression. Liss; New York: 1984. pp. 295–304. [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- 44.Pesold C, Treit D. Excitotoxic lesions of the septum produce anxiolytic effects in the elevated plus-maze and the shock-probe burying tests. Physiol Behav. 1992;52:37–47. doi: 10.1016/0031-9384(92)90431-z. [DOI] [PubMed] [Google Scholar]
- 45.Potegal M, Blau A, Glusman M. Effects of anteroventral septal lesions on intraspecific aggression in male hamsters. Physiol Behav. 1981;26:407–412. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- 46.Potegal M, Ferris CF, Hebert M, Meyerhoff J, Skaredoff L. Attack priming in female Syrian golden hamsters is associated with a c-fos-coupled process within the corticomedial amygdala. Neuroscience. 1996a;75:869–880. doi: 10.1016/0306-4522(96)00236-9. [DOI] [PubMed] [Google Scholar]
- 47.Potegal M, Hebert M, DeCoster M, Meyerhoff JL. Brief, high-frequency stimulation of the corticomedial amygdala induces a delayed and prolonged increase of aggressiveness in male Syrian golden hamsters. Behav Neurosci. 1996b;110:401–412. doi: 10.1037//0735-7044.110.2.401. [DOI] [PubMed] [Google Scholar]
- 48.Robertson HA, Peterson MR, Murphy K, Robertson GS. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotation behavior. Brain Res. 1989;503:346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- 49.Rodgers RJ, Hendrie CA. Social conflict activates status-dependent endogenous analgesia or hyperalgesic mechanisms in male mice: effects of naloxone on nociception and behavior. Physiol Behav. 1983;30:775–780. doi: 10.1016/0031-9384(83)90177-4. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers RJ, Hendrie CA, Waters AJ. Naloxone partially antagonizes post-encounter analgesia and enhances defensive responding in male rats exposed to attack from lactating conspecifics. Physiol Behav. 1983;30:781–786. doi: 10.1016/0031-9384(83)90178-6. [DOI] [PubMed] [Google Scholar]
- 51.Schuurman T. Hormonal correlates of agonistic behavior in adult male rats. Prog Brain Res. 1980;53:415–420. doi: 10.1016/S0079-6123(08)60079-5. [DOI] [PubMed] [Google Scholar]
- 52.Shaikh MB, Brutus M, Siegel HE, Siegel A. Regulation of feline aggression by the bed nucleus of the stria terminalis. Brain Res Bull. 1986;16:179–182. doi: 10.1016/0361-9230(86)90031-6. [DOI] [PubMed] [Google Scholar]
- 53.Shibata S, Yamamoto T, Ueki S. Differential effects of medial, central and basolateral amygdaloid lesions on four models of experimentally-induced aggression in rats. Physiol Behav. 1982;28:289–294. doi: 10.1016/0031-9384(82)90077-4. [DOI] [PubMed] [Google Scholar]
- 54.Siegel A, Pott CB. Neural substrates of aggression and flight in the cat. Prog Neurobiol. 1988;31:261–283. doi: 10.1016/0301-0082(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 55.Silveira MCL, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res. 1993;56:115–118. doi: 10.1016/0166-4328(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 56.Silveira MCL, Graeff FG, Sandner G. Regional distribution of Fos-like immunoreactivity in the rat brain after exposure to fear-inducing stimuli. Braz J Med Biol Res. 1994;27:1077–1081. [PubMed] [Google Scholar]
- 57.Silveira MCL, Sandner G, Di Scala G, Graeff FG. C-fos immunoreactivity in the brain following electrical or chemical stimulation of the medial hypothalamus of freely moving rats. Brain Res. 1995;674:265–274. doi: 10.1016/0006-8993(94)01451-m. [DOI] [PubMed] [Google Scholar]
- 58.Sodetz FJ, Bunnell BN. Septal ablation and the social behavior of the golden hamster. Physiol Behav. 1970;5:79–88. doi: 10.1016/0031-9384(70)90017-x. [DOI] [PubMed] [Google Scholar]
- 59.Stotz-Potter EH, Morin SM, DiMicco A. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996a;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- 60.Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996b;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vochteloo JD, Koolhaas JM. Medial amygdala lesions in male rats reduce aggressive behavior: interference with experience. Physiol Behav. 1987;41:99–102. doi: 10.1016/0031-9384(87)90137-5. [DOI] [PubMed] [Google Scholar]