Abstract
The rapid photoperiodic response in Japanese quail is so precise that it allows neural analyses of how photoperiodic information is transduced into an endocrine response. After transfer from short [SD; 6L:18D (6:18 hr light/dark cycle)] to long (LD; 20L:4D) days, luteinizing hormone (LH) first rises 20 hr after dawn. Using Fos immunocytochemistry, we examined the basal tuberal hypothalamus (BtH) to determine the relationship between brain cell activation and the first endocrine changes. Two separate cell populations within the BtH expressed Fos-like immunoreactivity (FLI) by hour 18 of the first LD. Importantly, this activation occurred before the LH rise. Median eminence activation appeared within glial cells, whereas activated infundibular nucleus cells were neuronal, providing support to the view that gonadotropin-releasing hormone (GnRH) release can be controlled at the terminals by glia. The FLI induction parallels LH changes, suggesting that gene expression may be involved in events preceding photostimulation and is the earliest photoperiodically stimulated physiological change yet reported.
Additional experiments provided further support for this hypothesis. First, photoperiodically induced activation is not a result peculiar to castrates because intact birds displayed similar results. Second, the critical length of 14 hr of light had to be exceeded to cause both BtH activation and a LH rise 30 hr from dawn. Finally, valuable evidence of the response specificity was provided by using a unique property of the quail photoperiodic clock in which exposure to 10L:26D, but not 10L:14D, causes photoinduction. The 36 hr paradigm increased both plasma LH and BtH activation.
Keywords: Japanese quail, Coturnix coturnix japonica, photoperiodism, c-fos, glia, luteinizing hormone
The avian hypothalamus plays a critical integrating role in the behavioral neurobiology of birds. Not only does it co-ordinate daily patterns of activity, endocrine function, and other physiological processes, it is crucial for orchestrating seasonal patterns of reproduction, molt, and migration. The precise neural pathways by which day length is detected, measured, and transduced into an endocrine response have yet to be elucidated. Highly photoperiodic Japanese quail present an excellent model to study these mechanisms because their reproductive neuroendocrine axis is activated by a single long day (LD) (Nicholls et al., 1983). We demonstrated previously, using Fos immunocytochemistry, that photostimulation activates cells within the basal tuberal hypothalamus (BtH) by 30 hr after dawn of the first LD (Meddle and Follett, 1995a,b). Fos is a member of a family of immediate early genes (IEGs) that couple short-term signals received at the cell surface to long-term cellular phenotype alteration (Morgan and Curran, 1991). The chicken Fos antibody used in this study was first validated by Meddle and Follett (1995a) and has since been used to investigate avian brain regions activated after copulation (Meddle et al., 1997), hyperosmotic stimuli (Meddle, 1995; Sharp et al., 1995), incubation behavior (Sharp et al., 1996), vocal behavior (Kimpo and Doupe, 1997), and circadian manipulations (King and Follett, 1997).
In the present studies, we provide evidence that cells in the tuberal hypothalamus are specifically activated and involved in the photoperiodic response. First, we investigated whether the photoperiodically driven activation of Fos-like immunoreactivity (FLI) is a result peculiar to castrated birds. Second, we examined the precise time course of this activation during the first LD and its relationship to luteinizing hormone (LH) induction. Follett et al. (1977) demonstrated that all necessary photoperiodic information must be received by 14.7 hr from dawn. Indeed, melatonin administration shifts the photoinducible rhythm of quail, so that 12 hr of light can be interpreted as stimulatory (Juss et al., 1993). Gonadotropin-releasing hormone (GnRH) release after LD stimulation occurs 22 hr from dawn (Perera and Follett, 1992). This 7 hr time lag between light coinciding with the photoinducible rhythm and LH output is hypothesized to be a neural or neuroendocrine phenomenon and not caused by unresponsiveness of the pituitary gland (Davies and Bicknell, 1976). Third, we established the critical length of light required to induce both LH secretion and FLI in the BtH.
Ensuring that tuberal hypothalamus activation is caused by photoperiodic induction and is not a consequence of the bird being more active because the lights are on (i.e., increased food and water intake and visual stimuli) is not straightforward. However, substantial support would come if FLI is induced in situations unique to the photoperiodic response. We have taken advantage of a unique property of the quail’s photoperiodic machinery in the fourth experiment whereby 10 hr of light can be inductive if given in a 36 hr [10:26 hr light/dark cycle (10L:26D)] but not in a 24 hr cycle (10L:14D) (Follett et al., 1992; Juss et al., 1995).
Preliminary reports on parts of this paper were presented at the Physiological Society meeting in Cardiff, UK, in September 1995 (Meddle and Follett, 1995b), at conferences in Florida (May 1994) and Vienna (August 1994), and at a meeting of the British Neuroendocrine Group in Edinburgh (July 1996).
MATERIALS AND METHODS
Animals
Japanese quail (Coturnix coturnix japonica) were raised, gonadectomized, and pretreated as outlined in Meddle and Follett (1995a) so that each bird would respond with a photoperiodically driven rise in LH when exposed to a single LD (20L:4D). Intact males used in the first study were 10 weeks of age and had not been exposed previously to a long photoperiod. Blood samples were collected by wing venipuncture, and LH was measured by a micromodification of the radioimmunoassay originally devised by Follett et al. (1972). The results are expressed in terms of nanograms per milliliter against a chicken LH standard (fraction IRC2).
Experimental design
The effects of transfer from short to long days of intact male quail. This experiment followed a similar protocol to that outlined in Meddle and Follett (1995a) in which birds were transferred from a short nonstimulatory photoperiod (SD) (6L:18D) to LDs (20L:4D) and perfused at various time points thereafter using immunocytochemical detection (ICC) of Fos protein as a marker of cell activation. In total, 26 intact male quail (10 weeks old) were reared and maintained on SDs (8L:16D). Birds were perfused at varying times during the first 6 hr of a SD (n = 7) and on the second (n = 7; 30 hr relative to dawn of the first stimulatory LD), third (n = 6; 50 hr), and fifth (n= 6; 98 hr) LDs. The experiment was designed so that birds from each group were killed on the same calendar day. Blood samples were taken for LH measurement before treatment (prebleed) and immediately before perfusion (postbleed). Finally, both testes were removed after fixation and weighed.
Investigation into the effect of transfer of SDs to LDs during the first 30 hr of photostimulation in castrated quail. This series of experiments was designed to analyze when cell activation began within the infundibular complex during exposure to the first LD. A total of 79 quail were used in eight self-contained runs, each of which comprised 8–20 quail. Castrates were transferred from SDs (6L:18D) to one LD (20L:4D) and perfused for Fos ICC at the following hours after dawn of the LD: 1 (n = 5), 6 (n = 6), 16 (n = 5), 18 (n = 13), 20 (n = 7), 22 (n = 8), 24 (n = 16), and 30 (n = 10). Two groups of SD controls (6L:18D) were perfused (in the dark) at hours 18 (n = 4) and 24 (n = 5). Two blood samples were taken from each quail, one on the day preceding photoperiodic treatment and one immediately before perfusion. Some sections from each brain were also labeled with anti-glial fibrillary acidic protein (GFAP) antiserum that is a specific marker for astrocytes (glial cells).
The duration of the photophase: its effect on LH secretion and FLI within the tuberal hypothalamus at hour 30 from dawn. In this experiment quail were exposed to a day containing 6–24 hr of light, then returned to 6L:18D, and perfused 30 hr after the original dawn. The aim was to ascertain the critical length of light required to induce both a rise in LH secretion and Fos activation in the BtH. Forty-five castrated quail were used in five self-contained runs (nine birds each). The birds were held on SDs (6L:18D), divided into eight groups (n = 5 or 6), and exposed to a photophase containing 6, 10, 12, 14, 16, 18, 20, or 24 hr of light. Blood samples for plasma LH were taken from all birds the day before (prebleed) and immediately before perfusion (postbleed) after the photoperiodic treatment.
The effect on FLI and LH induction of exposing castrated quail to a resonance cycle of 10L:26D. The aim was to compare a 36 hr resonance paradigm (Nanda and Hamner, 1958) using a photophase of 10 hr of light per cycle with responses to that of a SD of 10L:14D. Measurements of plasma LH were taken as an indicator of photoinduction. The experiment was designed so that all 12 birds were killed on the same calendar day. Two weeks before the experiment, SD-castrated quail (8L:16D) were accustomed to 10L:14D and divided into two groups of six, and a prebleed was taken. One group remained on 10L:14D, whereas the second was subjected to three cycles of 10L:26D. All birds were killed between hours 4 and 9 from dawn (lights on 9:00 A.M.), and a postbleed was taken immediately before perfusion.
Immunocytochemical procedures and analysis
The detection of Fos protein in these birds was performed as documented previously (Meddle and Follett, 1995a; Meddle et al., 1997). Briefly, birds were anesthetized with an overdose of sodium pentobarbitone (250 mg/kg, i.m.) and perfused intracardially with 300 ml of modified Zamboni’s fixative (pH 7.2–7.4). Brains were post-fixed overnight, and 60 μm sections were cut through the hypothalamus on a vibratome and processed as free floating in PBS buffer, pH 7.4, in small petri dishes on an orbital shaker. Immunocytochemistry was performed using a rabbit polyclonal anti-chicken Fos antibody (code 9/3; gift of P. Sharp, Roslin Institute, Midlothian, UK) raised against the 20 amino acid sequence of the C-terminal of chicken Fos. This antibody has been thoroughly validated for use in immunocytochemical studies in quail (Meddle, 1995;Meddle and Follett, 1995a; Sharp et al., 1995). After endogenous peroxidase was blocked, sections were incubated in the Fos primary antibody at a concentration of 1:5000 at 4°C for ∼70 hr. The antibody–antigen complex was visualized with a solution of 0.025% diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) containing 0.03% H2O2 (Sigma) in Tris buffer, pH 7.4. Control sections incubated in the presence of 1 mg/ml synthetic peptide (against which the antibody was raised) or with replacement of the primary antiserum with the preimmune serum from the rabbit in which the antibody was raised resulted in no nuclear staining. For double-staining, the primary Fos antibody was visualized with nickel-intensified DAB using the above protocol, except that visualization was performed in a solution of DAB-nickel sulfate in 0.0175 m sodium acetate. After visualization, sections were incubated in a polyclonal rabbit anti-cow glial fibrillary acidic protein (GFAP; Dako, Glostrup, Denmark; Bucks. UK code Z334) antibody (1:1000) for 48 hr and visualized as detailed in Meddle and Follett (1995a) with DAB used as the second chromagen.
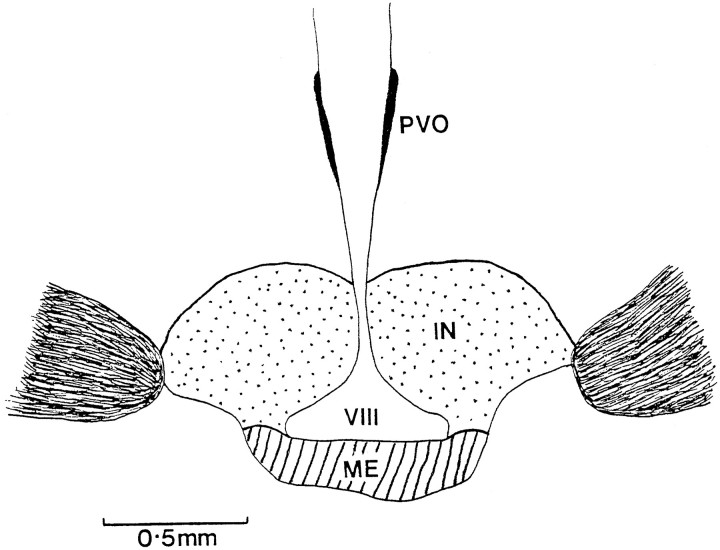
Sections were mounted serially on gelatin-coated slides, dehydrated, coverslipped in D.P.X. mountant, coded, and examined under the microscope at 200×. All positively stained FLI cell nuclei were counted within every section that contained the infundibular complex (see Meddle and Follett, 1995a). Typically, this equates to ∼20 sections per brain. The infundibular complex was divided into two regions (see Fig. 3) for analysis of the infundibular nucleus and median eminence populations. High-power oil immersion was used to identify double-labeled cells. The medial habenular was also analyzed to compare baseline FLI between treatments with control for differences in general brain activation. This brain region occurs in the same section as the infundibular complex and has been used as a control region in previous investigations (Meddle and Follett, 1995a). Statistical analyses of the numbers of activated nuclei and of plasma LH levels used standard t tests as well as one-factor ANOVA without repeated measures for the Fos data and with repeated measures for the LH results (in which serial blood samples were taken from the same bird). Fisher’s least significant difference test was appliedpost hoc.
Fig. 3.
Schematic coronal drawing of the anterior tuberal hypothalamus of a quail, showing the basal tuberal hypothalamus. It has been sectioned into two different areas labeled the infundibular nucleus (stippled) and the median eminence (hatched). IN, Infundibular nucleus;ME, median eminence; PVO, paraventricular organ; VIII, third ventricle.
RESULTS
FLI-labeled cells were widespread throughout the forebrain in all animals with density and distribution not varying obviously between treatment groups. Details have been discussed previously in depth (seeMeddle et al., 1997). Briefly, FLI was routinely observed in the hippocampus, hyperstriatum ventrale, septal region, habenular nucleus, and infundibular complex and within the mesencephalon, the intercollicularis nucleus, and the central gray region. Only the habenular and BtH were analyzed in this particular study because previous investigations have shown changes in BtH activation after photoperiodic manipulations (Meddle and Follett, 1995a).
The effect of photostimulation on LH secretion and FLI in the infundibular complex of intact male quail
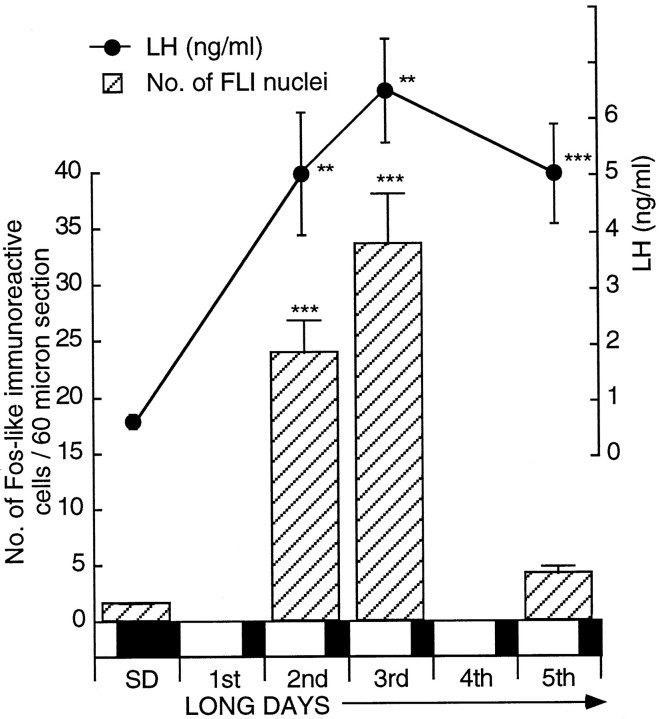
After transfer to LDs, intact male quail showed a significant increase in plasma LH early on the second day with absolute levels rising from 1.02 ± 0.23 ng/ml (n = 7) to 5.04 ± 1.09 ng/ml (n = 7) (± SEM) at hour 30 after 20L:4D (p < 0.01). LH levels remained high thereafter (Fig. 1). This was also reflected in testicular growth with the mass increasing fivefold from 30 to 150 mg after 4 LDs [one-factor ANOVA;F(3,22) = 9.89; p < 0.001]. LD stimulation resulted in an increase in the number of FLI-labeled cells within the tuberal hypothalamus, and the magnitude was comparable with that reported for castrated birds (Meddle and Follett, 1995a). A highly significant increase had occurred by early in the second LD (30 hr after dawn of the first LD; Fig. 1), the average number of activated cells per 60 μm section rising from 1.5 ± 0.1 (n = 7) to 23.9 ± 2.9 (n = 7) (unpaired t test, t = 7.81;p = 0.0001) to 33.6 ± 4.5 (n = 6) (unpaired t test, t = 7.83;p = 0.0001) after 2 long days. In gross terms, this corresponds to ∼30 activated cells within the infundibular complex per brain on SDs rising to 478 after 1 LD and to 672 after 2 LDs. The number of activated cells after 4 LDs (4.1 ± 0.7;n = 6) did not differ from that observed on SDs [ANOVA; F(3,22) = 35.29; p > 0.05] and was similar to that observed in castrates after 5 LDs (Meddle and Follett, 1995a). A one-factor ANOVA of all the results showed a highly significant effect of LD stimulation [F(3,22) = 35.29; p < 0.001] with significant increases after 1 and 2 LDs. The medial habenular nucleus served as the control region so that baseline FLI within the hypothalamus could be compared between treatment groups. FLI activation within the habenular averaged 81.7 ± 6.7 cells (per 60 μm section) on SDs, and a one-factor ANOVA showed this not to change via treatments [F(3,12) = 0.12; p = 0.959; data not shown].
Fig. 1.
Cell activation within the basal tuberal hypothalamus and changes in plasma LH secretion (mean ± SEM) in intact male quail after transfer from a SD to LDs. Activation was measured by the number of nuclei containing FLI (± SEM; per 60 μm section). Birds were sampled on SDs (n = 7) and on the 2nd (n = 7), 3rd (n = 6), and 5th (n = 6) LDs. The extent of FLI is highly significant compared with that of SDs [ANOVA;F(3,22) = 35.29; ***p < 0.001]. Changes in plasma LH secretion are shown above thehistogram. LH is significantly higher 30 hr after dawn of the first LD (** P < 0.01; i.e., early in the second LD). Overall, the data show a significant change over time [one-factor ANOVA; F(7,44)= 8.63; ***p = 0.001]. The bar beneath the diagram shows the SD and subsequent LDs (darkness is represented by the shaded areas; light is represented by the nonshaded areas).
The induction of LH and FLI in castrated quail within the basal tuberal hypothalamus throughout the first 16–30 hr from dawn of a single LD (20L:4D)
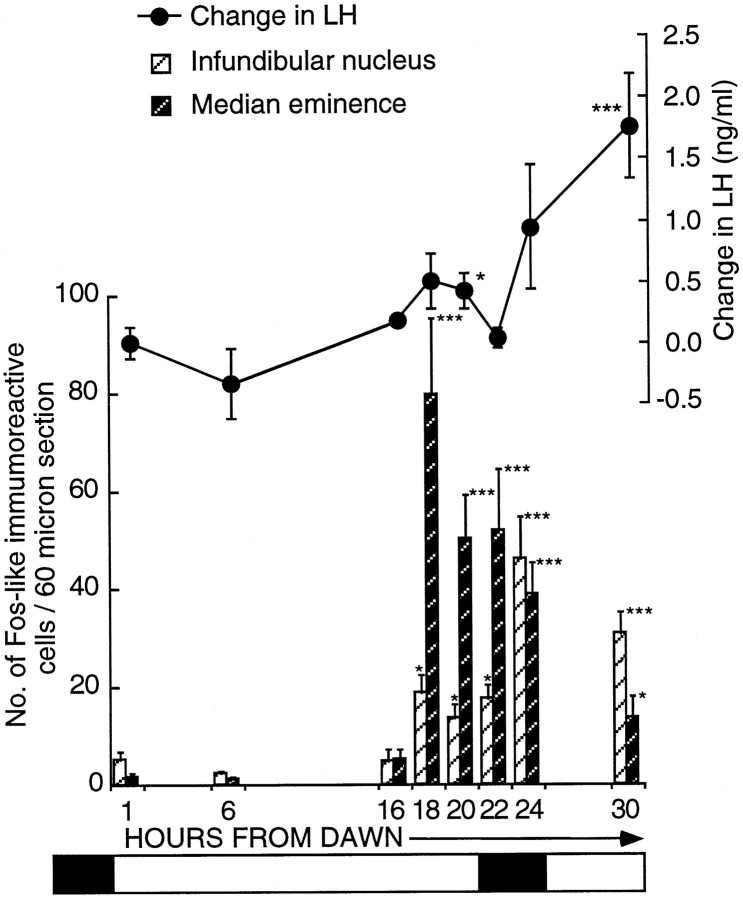
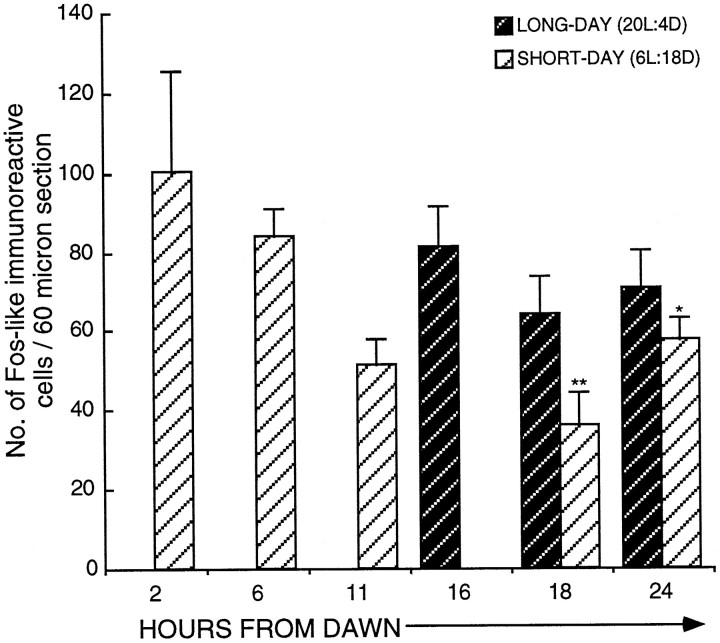
This series of experiments analyzed cell activation within the infundibular complex during the first LD by sampling across the first 30 hr and using plasma LH to track the time when photoperiodic induction first occurred. Figure 2 shows the LH changes (nanograms per milliliter ± SEM; postbleed − prebleed) and induction to have begun by hour 20. Absolute plasma LH levels of 0.92 ± 0.15 ng/ml (n = 11) on SDs increased to 2.0 ± 0.48 ng/ml (n = 16) by hour 24 and doubled again to 4.73 ± 2.29 ng/ml (n = 10) by hour 30. The change in plasma LH altered significantly over time [F(6,63) = 3.1; p = 0.01). The first increase in LH became significant at hour 20 [change in LH (ng/ml), 0.43 ± 0.14; n = 7; unpairedt test, t = −2.5; p < 0.02; n = 11] from dawn compared with SD levels. The changes at hours 22 (n = 8) and 24 (n = 16) failed to reach significance (unpaired t tests,t = −1.06; p > 0.5; andt = −1.82; p > 0.5; respectively).
Fig. 2.
Activation of FLI cells within the ME (dark hatched bars) and the infundibular nucleus (hatched bars) in quail transferred from SDs (6L:18D) to a single LD (20L:4D). Birds were killed at hours 1 (n = 5), 6 (n = 6), 16 (n = 5), 18 (n = 13), 20 (n = 7), 22 (n = 8), 24 (n = 16), and 30 (n = 10). FLI first becomes significantly increased (mean ± SEM) at hour 18 in both the infundibular nucleus [18.9 ± 3.7 (n = 13); p< 0.05] and the median eminence [79.7 ± 15.4 (n = 13); ***p < 0.001]. Changes in LH (mean ± SEM) are illustrated above thehistogram. The first significant rise in LH occurred at hour 20 (*p < 0.05) from dawn. The bar beneath the diagram represents the photoperiodic schedule (darkness is represented by the shaded areas; light is represented by the nonshaded areas.
When the brains were analyzed for Fos induction, it was apparent that the distribution of FLI activity differed from that observed in previous investigations. Cells were activated in the tuberal complex, but in addition a discrete population within the median eminence (ME) could be identified. In our earlier studies (Meddle and Follett, 1995a), the ME population was included in the infundibular complex. Activation, particularly early in the photoinduction, was widespread throughout the ME, but primarily it was concentrated within the ependymal layer and the area around the nerve terminals. Therefore for analysis, two areas were defined, first that encompassing only the infundibular nucleus (IN) and second that within the ME (Fig.3).
The following conclusions were made regarding the two areas (Fig. 2): first, the IN showed increased activation 18 hr after dawn [18.9 ± 3.7 cells per section (n = 13) compared with 5.1 ± 1.8 cells per section (n = 5) 1 hr from dawn (unpaired t test, t= −2.22;p < 0.05) and 2.3 ± 0.6 cells per section (n = 6) 6 hr after dawn (unpaired t test,t = −2.96; p < 0.01)]. FLI activity was still low at hour 16 [4.7 ± 2.3 counts per section (n = 5); p = 0.9154 compared with hour 1]. The level of activation was constant between hours 18 and 22 (17.8 ± 2.6 counts per section; n = 8) but rose again by hour 24 [46.1 ± 8.4 counts per section (n = 16); p < 0.03]. There was no further rise between hours 24 and 30 [30 hr, 31.1 ± 4.3 counts per section (n = 10); p = 0.194]. At hour 30, the numbers of activated cells was still much greater than at either hour 1 (unpaired t test, t = −4.11;p < 0.002) or hour 22 (unpaired t test,t = −2.46; p < 0.03). Second, there was a very marked increase in ME activation across the LD [one-factor ANOVA; F(7,62) = 7.46; p = 0.0001; Fig. 2]. Counts rose from SD levels of 5.1 ± 2.2 (n = 5) at hour 16 to 79.7 ± 15.4 (n = 13) at hour 18 (p < 0.01). This is a 16-fold increase and suggests there were at least 1500 activated cells within the entire ME. After the large increase at hour 18, activation dropped so that by hour 24, the number of cells was 38.9 ± 6.4 (n = 16; p = 0.014 compared with hour 18; Fig. 4C). A further fall occurred by hour 30 [13.7 ± 4.2 counts per section (n = 10);p < 0.002 compared with hour 18], but activation at hour 30 was still significantly higher than that in quail sampled at hour 6 (p < 0.05). In summary, cellular activation in both the ME and the IN precedes the rise in LH. The activation can be seen in the photomicrograph of a bird sampled 18 hr after dawn of the LD (Fig.4B).
Fig. 4.
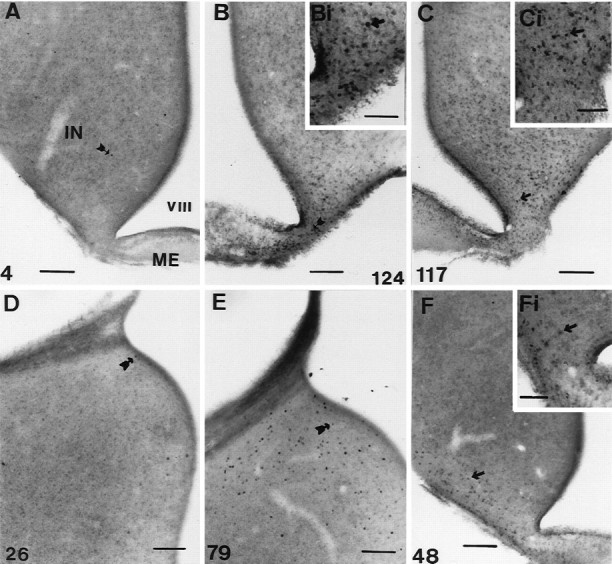
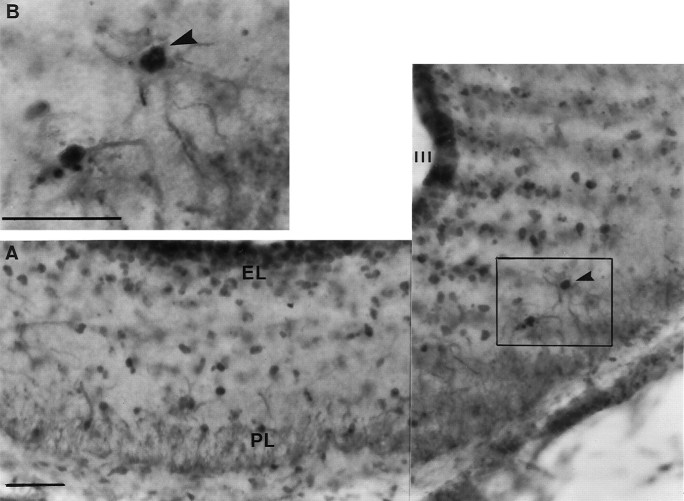
Photomicrographs showing examples of FLI activation within the tuberal hypothalamus and ME. A,B, Photomicrographs taken from quail sampled 18 hr after dawn. A is taken from a bird held on SDs (6L:18D), andB is from a bird photostimulated by 18 hr of light.C, Photomicrograph taken from a bird photostimulated by 24 hr of light, 24 hr after dawn. D, E, Photomicrographs taken from the medial habenular nucleus of quail under SDs (6L:18D) at hours 6 (D) and 18 (E) after dawn. F, Photomicrograph of a bird treated with three cycles of 10L:26D. The insetsBi, Ci, and Fi in theright corners of B, C, andF show magnified views, respectively. Thearrows in each micrograph point to a single FLI nucleus, and the number in the corner indicates the number of nuclei counted for that half of the particular section.IN, Infundibular nucleus; ME, median eminence; VIII, third ventricle. Scale bars:A–F, 100 μm; insets, 50 μm.
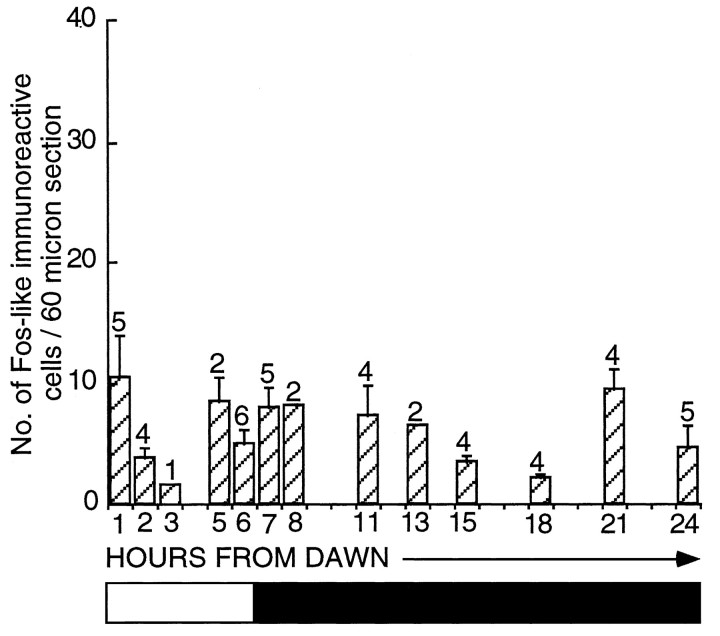
Given the abrupt increase in FLI within the hypothalamus at hour 18, it was important to show that this alteration was not a circadian (diurnal) change and did not occur under SDs. Birds held on 6L:18D were perfused at hours 18 (2.2 ± 0.4 counts per section;n = 4) and 24 (4.6 ± 1.8 counts per section;n = 5). These levels of activation are low and significantly less than seen in photostimulated birds (hour 18, unpaired t test, t = 2.44; p= 0.326; hour 24, unpaired t test, t = 3.97;p < 0.002). In other words, activation in the latter quarter of the LD does not seem to be a consequence of a diurnal rhythm. To explore this point further, we compared activated cell counts within the infundibular complex from all the various SD controls taken previously (Meddle and Follett, 1995a). The data are plotted to give a composite picture (Fig. 5). Although the sets of experiments were performed at different times (and some months apart), inferences can be drawn from these results because all immunocytochemical runs were standardized. There is no significant change in activity across the SD [one-factor ANOVA;F(12,35) = 1.69; p = 0.113], and the mean counts ranged only between 2 and 10 (compare Fig. 2). A photomicrograph taken from a SD bird sampled 18 hr after dawn is represented in Figure 4A.
Fig. 5.
Histogram representing the number of FLI-labeled cells per section (mean ± SEM) within the infundibular complex of castrated quail sampled at various hours throughout a SD (6L:18D). It is a composite graph of all SD control birds used in a previous study (Meddle and Follett, 1995a) in addition to the experiments presented here. There is no significant change in FLI across 24 hr [ANOVA;F(12,35) = 1.69; p > 0.05]. The bar beneath the diagramrepresents the photoperiodic schedule (the shaded areasrepresent darkness, and the nonshaded areas represent light).
An important question relates to the cellular identity (i.e., neurons or glial cells) of Fos activation in both the ME and IN. This was resolved in a separate experiment in which brains were labeled for glial cells (GFAP, brown cytoplasmic staining) in addition to FLI (black nuclear staining). The GFAP distribution was similar to that described previously for quail by Cameron-Curry et al. (1991). Immunoreactivity for GFAP was observed throughout the diencephalon with immunoreactive elements scattered throughout the entire infundibular complex and the ME. Double staining (both FLI and GFAP) was observed within the ME of birds taken from hours 18 to 30 after dawn of the first LD of photostimulation, and examples are illustrated (Fig.6). Figure 6A shows the ME of a bird sampled at hour 18 from dawn and double-labeled for both FLI and GFAP. The widespread colocalization argues that the majority of the FLI-labeled cells within the ME 18–24 hr after dawn of a LD are glial cells. No double-labeled cells were observed in the ME of SD birds.
Fig. 6.
FLI-labeled glia are widespread throughout the ME after photostimulation. A, The composite photomicrograph was taken at the level of the ME of a quail killed after 18 hr of light. B, A high power example is shown in theinset micrograph; an example of an activated glial cell (as demonstrated by FLI) within the ME is indicated by thearrowhead. The darker-labeled FLI nuclei can be located within the centers of the lighter cytoplasmic-stained glial cells.III, Third ventricle; EL, ependymal layer of the ME; PL, palisade layer of the ME. Scale bars, 25 μm.
As a test for the photoperiodic specificity of activation within the infundibular complex, the medial habenular nucleus (four from each group) was counted at hours 18 and 24 of a SD (6L:18D) and LD (20L:4D). Figure 7 illustrates that SD (35.8 ± 8.5 counts per section; n = 4) and LD (63.9 ± 9.6 counts per section; n = 4) birds sampled at hour 18 were not significantly different (p = 0.0712). Similarly, activation was not significantly different between birds on SDs (57.4 ± 5.6 counts per section; n = 4) and LDs (70.1 ± 9.6 counts per section; n = 4) when sampled at hour 24 (p = 0.299). The medial habenular nucleus was analyzed across the 24 hr period, and the results are shown in Figure 7. Unexpectedly, there was an effect of time of day on activation with a significant decrease occurring in the middle of the night [83.9 ± 7.1 counts per section (n = 4) at lights off decreasing to 35.8 ± 8.5 counts per section (n = 4) in the middle of the night; one-factor ANOVA;F(4,15) = 4.1; p < 0.02]. Photomicrographs of the medial habenular nucleus of SD quail sampled at these times are shown in Figure 4, D and E. There was also a decrease in activated cells just before dawn at hour 24, compared with 6 hr after dawn (p < 0.03). Birds sampled just before dawn (hour 24) and just after dawn (hour 2) were not significantly different from one another (p> 0.05).
Fig. 7.
FLI-labeled cells (mean ± SEM) within the medial habenular nucleus of quail sampled at various time points after dawn under a SD (6L:18D) and a LD (20L:4D). Four birds were sampled at each time point. There is a significant diurnal rhythm in FLI within the medial habenular under SD conditions [one-factor ANOVA;F(4,15) = 4.1; p < 0.02]. A significant threefold decrease occurs between hours 6 (day) and 18 (night) (**p < 0.01), whereas hour 24 had significantly less FLI than did hour 6 (*p < 0.05). The number of nuclei activated at hour 18 in SD and LD birds is not significantly different (p > 0.05).
The duration of the photophase: its effect on LH secretion and FLI within the tuberal hypothalamus at hour 30 from dawn
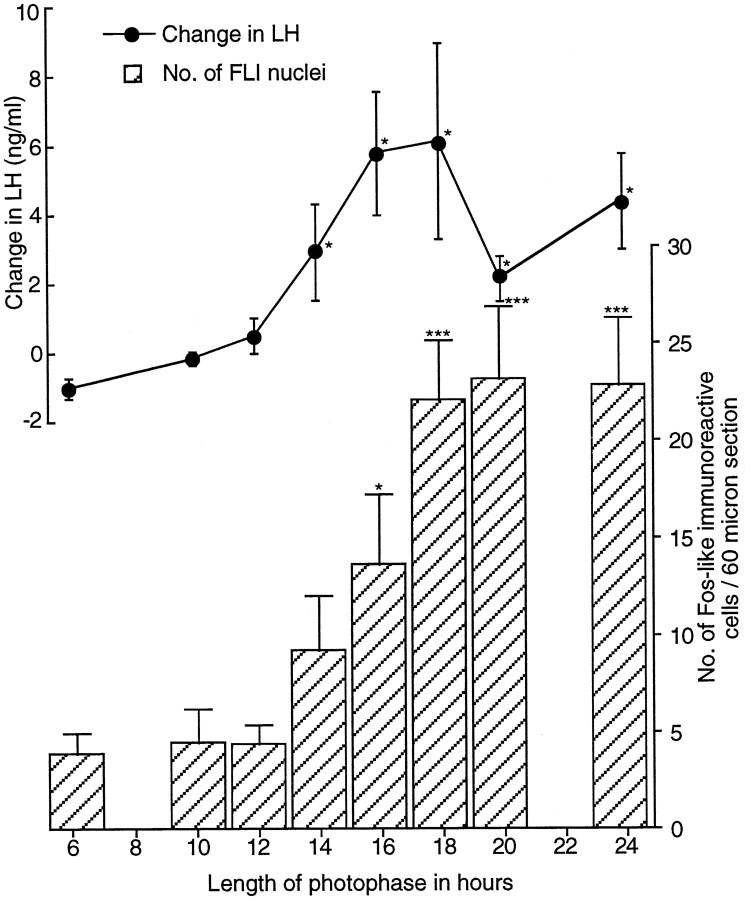
This experiment used a paradigm first used by Follett et al. (1977). Primed quail were exposed to a single day containing a varying number of hours of light (6–24), and the degree of photoinduction was measured 6 hr into the following SD (i.e., at hour 30). Birds exposed to light of 14 hr or longer were photostimulated, and their LH levels were significantly increased compared with those in birds given 6 hr of light (Fig. 8) (e.g., change in LH for 14 hr, 3.00 ± 1.39 ng/ml; n = 6; for 6 hr, −0.93 ± 0.29 ng/ml; n = 5; t = −2.53; p < 0.05). LH levels at hour 30 after a 14 hr day were similar to those after continuous light (24L:0D;p > 0.05) and were not different from any other day longer than 14 hr. The apparently lower levels in the change in LH secretion after 20 hr of light were not significantly different from that after 18 hr of light (unpaired t test,t = 1.24; p = 0.246; 20 vs 24 hr, unpaired t test, t = −1.46;p = 0.182).
Fig. 8.
The change in LH (ng/ml ± SEM) and in the number of activated cells within the infundibular complex measured 30 hr after dawn in castrated quail after exposure to various photoperiods, illustrating the relationship between these two events after photophase extension. Birds were sampled after 6 (n = 5), 10 (n = 6), 12 (n = 6), 14 (n = 6), 16 (n = 6), 18 (n = 6), 20 (n = 5), or 24 (n = 5) hr of light. As the photophase lengthens, there is a significant increase in the change in LH [one-factor ANOVA; F(7,37)= 3.36; p < 0.01]. The change is significant at hour 30 after photophases as short as 14 hr (3.0 ± 1.39 ng/ml;n = 6; *p < 0.05). Numbers of FLI (mean ± SEM) also increased significantly with an increase in photophase [one-factor ANOVA; F(7,37) = 9.65; ***p < 0.001]. The number of FLI cells is first significant at hour 30 after a photophase of 16 hr (13.6 ± 3.5; n = 6).
The photoinduction was paralleled in terms of FLI activated cells (Fig.8). Clearly, the length of the LD plays a significant role in determining BtH activation. The numbers of activated cells increased significantly [one-factor ANOVA; F(7,37) = 9.65; p < 0.001] with the length of the light phase (Fig. 8). Although cell activation was greater in the 14 hr light group than in quail exposed to 6, 10, or 12 hr, it just failed to reach significance (unpaired t test, t = −1.6;p = 0.143). A day of 16 hr (13.6 ± 3.5 counts per section; n = 6) was enough to cause a significant increase in FLI expression [6L:18D, 3.9 ± 1.1 counts per section (n = 6); unpaired t test, t= −2.4; p = 0.04]. The 16 hr group was not significantly different from the 18 hr group [22.1 ± 3.0 counts per section (n = 6); p = 0.101] or any of the longer photophase groups. Basal levels of FLI within the medial habenular nucleus in this experiment were comparable across treatment groups. Therefore, differences observed within the infundibular complex between treatments were not a consequence of differential generalized brain activation [one-factor ANOVA; F(2,15) = 0.532; p > 0.5]. Cell counts per section per brain were ∼80 ± 1.8 (n = 18). The data are not shown.
The effect on FLI and LH induction of exposing quail to a 10L:26D resonance cycle or to 10L:14D
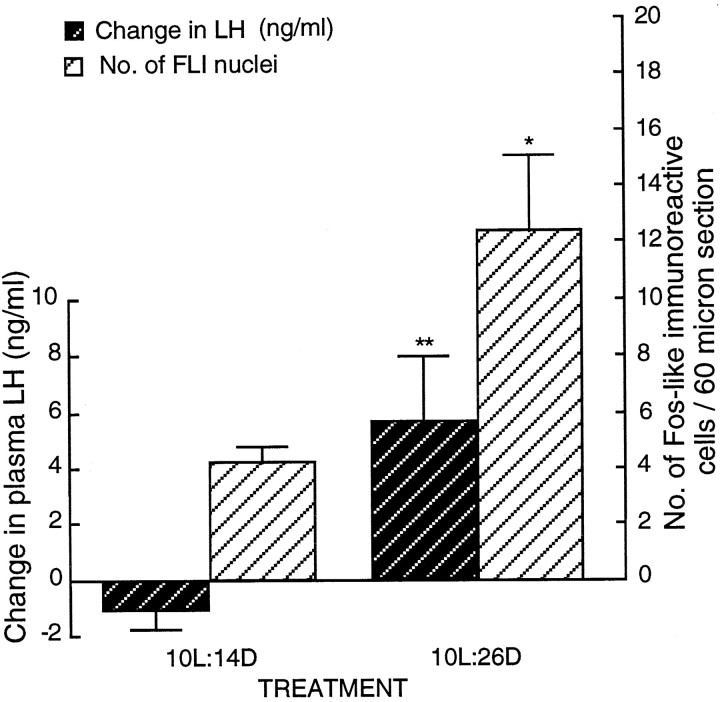
Exposure to three cycles of 10L:26D caused photoinduction and plasma LH to rise significantly from 2.03 ± 0.60 to 8.82 ± 1.56 ng/ml (n = 6; paired t test,t = −3.32; p < 0.01). Photoinduction did not occur in quail exposed to 10L:14D (prebleed levels of 4.16 ± 1.98 ng/ml vs postbleed levels of 3.12 ± 1.52 ng/ml;n = 6; paired t test, t = −1.74; p > 0.1). Postbleed levels in the 10L:14D group were significantly lower than the levels in those subjected to 10L:26D (p < 0.05). Importantly, the increase in LH secretion was mirrored by FLI activation (Fig.9; Fig. 4F, photomicrograph). FLI cell numbers within the infundibular complex were significantly increased after 10L:26D [12.4 ± 2.7 counts per section (n = 6)] compared with 10L:14D [4.3 ± 0.6 counts per section (n = 6); unpaired ttest, t = −2.94; p < 0.05]. To exclude the possibility that the increased activity within the infundibular complex under the 10L:26D paradigm was a consequence of generalized brain activation, we compared FLI within the medial habenular nucleus between treatments. There was no significant difference, counts ranging from 86.8 ± 3.9 (n = 4) on 10L:14D to 80.0 ± 5.9 (n = 4) on 10L:26D (unpaired t test, t = 0.97;p > 0.3). The data are not shown.
Fig. 9.
Changes in plasma LH levels and the number of FLI cells within the basal hypothalamus in castrated quail after resonance cycle treatment. Exposure to three cycles of 10L:26D was highly inductive in terms of LH secretion, with the change being from −1.04 (± 0.61 ng/ml; n = 6) to 5.66 (± 2.33 ng/ml;n = 6; **p < 0.01). The number of FLI cells was significantly increased after 10L:26D (unpairedt test, t = −2.94; *p < 0.05).
DISCUSSION
The results demonstrate that cells within the hypothalamus become activated at the time of photoinduction, before the first detectable rise in LH, and this seems to be the earliest photoperiodic effect yet reported in quail (or any other organism). A close relationship between activation of the BtH and the first stages of gonadotropin secretion is evident in several ways. First, the increase in LH and FLI occur late in the first LD. Second, photoperiodic induction builds up progressively over the first days of photostimulation in both intact and castrated quail (so the activation is not an aberration of castration), and this is reflected by sustained FLI induction. How LH release and FLI activation within the infundibular complex are related is still unclear, but the evidence obtained is indicative that they are causally related. The prolonged photostimulated cellular induction in the infundibulum may be a consequence of temporal staggering of Fos and Fos-related antigen expression (Sonnenberg et al., 1989), because the antibody used in our studies detects both (Meddle and Follett, 1995a). Activation within the IN is widespread and is difficult to compartmentalize anatomically, but it is possible that its longevity is a result of different cell populations becoming activated at different times. The increase in activity is not because of a diurnal rhythm because it is absent in SD birds killed at any time point throughout 24 hr. The infundibular complex is of paramount importance to the avian photoperiodic response. If lesioned, photoinduced gonadal growth is blocked (Sharp and Follett, 1969; Ohta and Homma, 1987), even if GnRH fibers remain intact (Juss, 1993). Putative deep brain photoreceptors have been located in this region (Saldanha et al., 1994), and implants of radioluminous paint (Oliver and Baylé, 1982) and light-conducting fibers (Yokoyama et al., 1978) to SD birds induce testicular growth.
The diurnal fluctuations in SD habenular activity have no bearing on the infundibular complex results. Activation within the habenular does not differ across 24 hr in LD birds or when compared with SD birds. The decrease in habenular activation is real, because the infundibular complex exhibits no such diurnal changes. FLI fluctuations in the rat habenular have been described by Chastrette et al. (1991). The habenular has been implicated in exploratory behavior (Sutherland, 1982) that in turn displays circadian variations (Rusak, 1981). The 40% variation in quail habenular activity is similar to that of the rat suprachiasmatic nucleus during 24 hr (Kononen et al., 1990), but further experiments are required to elucidate whether habenular activity is directly driven by the circadian system.
One aspect of the photoperiodic response is the time lag that exists between a LD being registered and the first LH rise. Follett et al. (1977) demonstrated that LH rose around hour 20 of the first LD as long as it exceeded 14.7 hr of light. Similar time lags are observed in White-crowned sparrows (Zonotrichia leucophrys gambelii) (Follett et al., 1975) and black-headed buntings (Emberiza melanocephala) (Kumar et al., 1996). Use of the first-day release system (i.e., exposure to a LD of varying light duration; Nicholls et al., 1983) induces an LH rise (30 hr from its onset) with a day containing 16 hr of light. This leads to IN activation, with the degree of activation a function of photophase duration. Once critical day length is exceeded, IN activation remains consistent. The best evidence yet to suggest involvement of these activated cells in part of the photoinductive process was provided by the final experiment, whereby quail were exposed to 10 hr of light (below the critical day length) in a resonance paradigm that leads to photoperiodic induction (Juss et al., 1995) compared with 10 hr of light in a 24 hr cycle.
The question arises as to the phenotype of these FLI-activated cells. The avian tuberal hypothalamus contains neurons and receptors of many different types, e.g., vasoactive intestinal polypeptide (Yamada et al., 1982), neuropeptide Y (Aste et al., 1991), aromatase (Balthazart et al., 1990), estrogen receptors (Gahr and Hutchison, 1992), extra retinal photoreceptors (Saldanha et al., 1994, 1995), and glia (Sharp, 1972; Cameron-Curry et al., 1991). It is assumed that IN activation must constitute a neuronal population because they were not GFAP immunopositive. Cells within the ME are exclusively glial (Sharp, 1972), and because these cells were activated, it suggests glial involvement before a LH rise.
Several mechanisms have been postulated to regulate hypothalamic GnRH release. The degree of glial ensheathment of the GnRH terminals has been shown to vary with endocrine status (Kozlowski and Coates, 1985;King et al., 1996). This is analogous to the ensheathment of vasopressin terminals by pituicytes in the pituitary (Tweedle, 1983). Already, the hypothalamus offers examples of the participation of glia on physiological structural plasticity, e.g., oxytocinergic neurons during dehydration and lactation (Theodosis and Poulain, 1984;Theodosis et al., 1986; Chapman et al., 1986). Even seasonal photoperiodic changes have been associated with hypothalamic structural morphological changes (Lee et al., 1995). Glia respond to increased neuronal activity by providing a buffering mechanism for potassium ions. Being sensitive to changes in extracellular potassium, they change shape exposing the neurosecretory nerve endings to allow GnRH release. Recent evidence suggests that glia may be involved in mechanisms of synaptic plasticity orchestrated by gonadal steroids (Montagnese et al., 1988; Olmos et al., 1989; Witkin et al., 1991;Garcia-Segura et al., 1994, 1996; King et al., 1996). Plastic changes appropriately relate to hormone demand, especially in controlling GnRH release (Garcia-Segura et al., 1994; Naftolin et al., 1996). King and Letourneau (1994) reported dramatic changes in the rat ME after castration, whereas Witkin et al. (1991) reported similar results after changes in the monkey gonadal steroid state. Kozlowski and Coates (1985) stated that it was rare to find rat GnRH terminals in direct contact with pituitary portal capillaries; instead glia ensheathment of GnRH fibers changed with reproductive state. Barres (1992) reviewed evidence that pubertal GnRH release is a direct consequence of estrogen acting on estrogen receptors containing glia (Langub and Watson, 1992), unlike GnRH neurons that are devoid of these receptors (Ojeda et al., 1990; Witkin et al., 1991). Glia cells have been shown to display morphological changes in response to noradrenaline (Van Calker and Hamprecht, 1980) and to have adrenergic receptors (Hosli and Hosli, 1982; Hosli et al., 1982) so that one mechanism could involve cAMP, because stimulation of cAMP causes glial retraction. Dopamine is also thought to control GnRH release at the ME and in the infundibular region in the chick (Fraley and Kuenzel, 1993) and hen (Contijoch et al., 1992). Serotonin (Kiss and Halasz, 1985) and catecholamine (Watanabe and Nakai, 1987) transmitter systems have also been shown to be located in the area of the ME in rodents and play a regulatory role in GnRH release via glia.
Mammalian GnRH neurons have been shown to express Fos during an ovulatory LH rise (Lee et al., 1992). However, we have not observed FLI activation in GnRH cells associated with the photoperiodically driven LH rise in quail. We know that Fos is not required for LH surge initiation in the sheep (Moenter et al., 1993), rodent (Berriman et al., 1992; Lee et al., 1992; Doan and Urbanski, 1994), and monkey (Witkin et al., 1994); rather it is associated with the synthetic requirements resulting from GnRH depletion. Instead, FLI activation within the terminals of the ME offers evidence to suggest that GnRH release is regulated at the terminals.
Mechanisms regulating GnRH secretion are still not defined. Although many neurotransmitters or neuromodulators can regulate synthesis, synaptic input to GnRH cells are poor (Witkin and Silverman, 1985). During photorefractoriness in the starling (Sturnus vulgaris), synaptic modification of GnRH cells change, consistent with an inhibitory input (Parry and Goldsmith, 1993). In addition, GnRH synthesis is reduced because GnRH levels and its precursor are significantly low (Parry et al., 1997). Once GnRH levels are replenished under SDs, we can now hypothesize that its release may be under the fine control of glial cells, allowing rapid release of stores once the photoperiod exceeds critical day length.
In summary, because glia have a close relationship with GnRH terminals and because they are activated before and during increases in LH, it is tempting to speculate that they may directly influence GnRH release. Recently Saldanha et al. (1995) reported that opsin immunopositive ME cells directly contact glia. Are these the same glia that ensheath GnRH terminals, providing a direct connection to the GnRH circuitry? Together these findings provide the exciting possibility that light could directly influence GnRH release via glial cells. The infundibular complex is an important site for photoperiodic regulation of reproduction, and its activation must be a critical part of the photoneuroendocrine machinery that ultimately leads to GnRH release. Photoinduction must at least involve three different processes from photoreception, daylight measurement, to the stimulation of GnRH release. Whether these activated cells are neuronal or glial, acting singularly or as populations, the integration of such activity must form some component of the photoneuroendocrine system after photostimulation in quail.
Footnotes
This investigation was performed in accordance with United Kingdom Home Office Regulations. We thank Dr. P. J. Sharp for the gift of the Fos antiserum.
Correspondence should be addressed to Dr. Simone L. Meddle, Department of Zoology, Box 351800, University of Washington, Seattle, WA 98195.
Prof. Follett’s present address: Vice-Chancellor, University of Warwick, Coventry CV4 7AL, UK.
REFERENCES
- 1.Aste N, Viglietti-Panzica C, Fasolo A, Andreone C, Vaudry H, Pelletier G, Panzica GC. Localization of neuropeptide Y-immunoreactive cells and fibres in the brain of the Japanese quail. Cell Tissue Res. 1991;265:219–230. doi: 10.1007/BF00398070. [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- 3.Barres BA. A role for glia in LHRH release. Curr Opin Neurobiol. 1992;2:645–647. doi: 10.1016/0960-9822(92)90111-m. [DOI] [PubMed] [Google Scholar]
- 4.Berriman SJ, Wade GN, Blaustein JD. Expression of Fos-like proteins in gonadotropin-releasing hormone neurons of Syrian hamsters: effects of estrous cycles and metabolic fuels. Endocrinology. 1992;131:2222–2227. doi: 10.1210/endo.131.5.1425420. [DOI] [PubMed] [Google Scholar]
- 5.Cameron-Curry P, Aste N, Viglietti-Panzica C, Panzica GC. Immunocytochemical distribution of glial fibrillary acidic protein in the central nervous system of the Japanese quail (Coturnix coturnix japonica). Anat Embryol (Berl) 1991;184:571–581. doi: 10.1007/BF00942579. [DOI] [PubMed] [Google Scholar]
- 6.Chapman DB, Theodosis DT, Montagnese C, Poulain DA, Morris JF. Osmotic stimulation causes structural plasticity of neurone–glial relationships of the oxytocin but not vasopressin secreting neurones in the hypothalamic supraoptic nucleus. Neuroscience. 1986;17:679–686. doi: 10.1016/0306-4522(86)90039-4. [DOI] [PubMed] [Google Scholar]
- 7.Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and night-time stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563:339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- 8.Contijoch AM, Gonzalez C, Singh HN, Malamed S, Troncoso S, Advis JP. Dopaminergic regulation of luteinizing hormone–releasing hormone release at the median eminence level: immunocytochemical and physiological evidence in hens. Neuroendocrinology. 1992;55:290–300. doi: 10.1159/000126128. [DOI] [PubMed] [Google Scholar]
- 9.Davies DT, Bicknell RJ. The effect of testosterone on the responsiveness of the quail’s pituitary to luteinizing hormone–releasing hormone (LH–RH) during photoperiodically induced testicular growth. Gen Comp Endocrinol. 1976;30:487–499. doi: 10.1016/0016-6480(76)90119-2. [DOI] [PubMed] [Google Scholar]
- 10.Doan A, Urbanski HF. Diurnal expression of fos in luteinizing hormone–releasing hormone neurons of Syrian hamsters. Biol Reprod. 1994;50:301–308. doi: 10.1095/biolreprod50.2.301. [DOI] [PubMed] [Google Scholar]
- 11.Follett BK, Scanes CG, Cunningham FJ. A radioimmunoassay for avian luteinizing hormone. J Endocrinol. 1972;52:359–378. [PubMed] [Google Scholar]
- 12.Follett BK, Farner DS, Mattocks PW. Luteinizing hormone in the plasma of White-crowned sparrows (Zonatrichia leucophrys gambelii) during artificial photostimulation. Gen Comp Endocrinol. 1975;26:126–134. doi: 10.1016/0016-6480(75)90223-3. [DOI] [PubMed] [Google Scholar]
- 13.Follett BK, Davies DT, Gledhill B. Photoperiodic control of reproduction in Japanese quail: changes in gonadotrophin secretion on the first day of induction and their pharmacological blockade. J Endocrinol. 1977;74:449–460. doi: 10.1677/joe.0.0740449. [DOI] [PubMed] [Google Scholar]
- 14.Follett BK, Kumar V, Juss TS. Circadian nature of the photoperiodic clock in Japanese quail. J Comp Physiol [A] 1992;171:533–540. doi: 10.1007/BF00194586. [DOI] [PubMed] [Google Scholar]
- 15.Fraley GS, Kuenzel WJ. Immunocytochemical and histochemical analyses of gonadotrophin releasing hormone, tyrosine hydroxylase, and cytochrome oxidase reactivity within the hypothalamus of chicks showing early sexual maturation. Histochemistry. 1993;99:221–229. doi: 10.1007/BF00269140. [DOI] [PubMed] [Google Scholar]
- 16.Gahr M, Hutchison JB. Behavioral action of estrogen in the male dove brain: area differences in co-distribution of aromatase activity and estrogen receptors are steroid dependent. Neuroendocrinology. 1992;56:74–84. doi: 10.1159/000126211. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Segura LM, Luquin S, Parducz A, Naftolin F. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity and glial ultrastructure in the rat neuroendocrine hypothalamus. Glia. 1994;10:59–69. doi: 10.1002/glia.440100108. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Segura LM, Fernandez-Galaz MC, Chowen JA, Torres-Aleman I, Naftolin F. Role of astroglia in gonadal hormone-dependent synaptic plasticity. Ital J Anat Embryol. 1996;101:107–108. doi: 10.1016/s0361-9230(97)00238-4. [DOI] [PubMed] [Google Scholar]
- 19.Hosli E, Hosli L. Evidence for the existence of α- and β- adrenoceptors on neurones and glial cells of cultured rat central nervous system–an autoradiographic study. Neuroscience. 1982;7:2873–2881. doi: 10.1016/0306-4522(82)90110-5. [DOI] [PubMed] [Google Scholar]
- 20.Hosli L, Hosli E, Zehntner R, Lehman R, Lutz TW. Evidence for the existence of a- and b-adrenoceptors on cultured glial cells–an electrophysiological study. Neuroscience. 1982;7:2867–2872. doi: 10.1016/0306-4522(82)90109-9. [DOI] [PubMed] [Google Scholar]
- 21.Juss TS. Neuroendocrine and neural changes associated with the photoperiodic control of reproduction. In: Sharp PJ, editor. Avian endocrinology. Society for Endocrinology; Bristol, UK: 1993. pp. 47–60. [Google Scholar]
- 22.Juss TS, Meddle SL, Servant RS, King VM. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnix japonica). Proc R Soc Lond [Biol] 1993;254:21–28. doi: 10.1098/rspb.1993.0121. [DOI] [PubMed] [Google Scholar]
- 23.Juss TS, King VM, Kumar V, Follett BK. Does an unusual entrainment of the circadian system under T36 hr photocycles reduce the critical daylength for photoperiodic induction in Japanese quail? J Biol Rhythms. 1995;10:17–32. doi: 10.1177/074873049501000102. [DOI] [PubMed] [Google Scholar]
- 24.Kimpo RR, Doupe AJ. FOS is induced by singing in distinct neuronal populations in a motor network. Neuron. 1997;18:315–325. doi: 10.1016/s0896-6273(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 25.King JC, Letourneau RJ. Luteinizing hormone–releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology. 1994;134:1340–1351. doi: 10.1210/endo.134.3.8119174. [DOI] [PubMed] [Google Scholar]
- 26.King JC, Ronsheim PM, Rubin BS. Confocal microscopy reveals dynamic remodelling of the LHRH terminals/tanycytic end-feet interactions in the median eminence of cycling female rats. Ital J Anat Embryol. 1996;101:112–113. [Google Scholar]
- 27.King VM, Follett BK. C-fos expression in the putative avian suprachiasmatic nucleus. J Comp Physiol [A] 1997;180:541–551. doi: 10.1007/s003590050071. [DOI] [PubMed] [Google Scholar]
- 28.Kiss J, Halasz B. Demonstration of serotoninergic axons terminating on luteinizing hormone–releasing hormone neurons in the preoptic area of the rat using a combination of immunocytochemistry and high resolution autoradiography. Neuroscience. 1985;14:69–78. doi: 10.1016/0306-4522(85)90164-2. [DOI] [PubMed] [Google Scholar]
- 29.Kononen J, Koistinaho J, Alho H. Circadian-rhythm in c-fos like immunoreactivity in the rat brain. Neurosci Lett. 1990;120:105–108. doi: 10.1016/0304-3940(90)90179-d. [DOI] [PubMed] [Google Scholar]
- 30.Kozlowski GP, Coates PW. Ependynoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res. 1985;242:301–311. doi: 10.1007/BF00214542. [DOI] [PubMed] [Google Scholar]
- 31.Kumar V, Jain N, Follett BK. The photoperiodic clock in blackheaded buntings (Emberiza melanocephala) is mediated by a self-sustaining circadian system. J Comp Physiol [A] 1996;179:59–64. doi: 10.1007/BF00193434. [DOI] [PubMed] [Google Scholar]
- 32.Langub MC, Jr, Watson RE., Jr Estrogen receptor-immunoreactive glia, endothelia and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology. 1992;130:364–372. doi: 10.1210/endo.130.1.1727710. [DOI] [PubMed] [Google Scholar]
- 33.Lee WS, Smith MS, Hoffman GE. C-fos activity identifies recruitment of luteinizing hormone releasing hormone neurones during the ascending phase of the proestrous luteinizing hormone surge. J Neuroendocrinol. 1992;4:161–166. doi: 10.1111/j.1365-2826.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee W, Watanabe M, Glass JD. Photoperiod affects the expression of neural cell adhesion molecule and polysialic acid in the hypothalamus of the siberian hamster. Brain Res. 1995;690:64–72. doi: 10.1016/0006-8993(95)00588-h. [DOI] [PubMed] [Google Scholar]
- 35.Meddle SL (1995) Photoperiodic control of reproduction in Japanese quail: the use of immediate early genes (c-fos) as a marker of cell activation. PhD thesis, University of Bristol.
- 36.Meddle SL, Follett BK. Photoperiodic activation of Fos-like immunoreactive protein in neurones within the tuberal hypothalamus of Japanese quail. J Comp Physiol [A] 1995a;176:79–89. doi: 10.1007/BF00197754. [DOI] [PubMed] [Google Scholar]
- 37.Meddle SL, Follett BK. Activation of the basal tuberal hypothalamus of castrated Japanese quail preceding photoperiodically driven luteinising hormone (LH) release. J Physiol (Lond) 1995b;489:172–173. [Google Scholar]
- 38.Meddle SL, King VM, Follett BK, Wingfield JW, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- 39.Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133:896–903. doi: 10.1210/endo.133.2.8344224. [DOI] [PubMed] [Google Scholar]
- 40.Montagnese C, Poulain DA, Vincent J-D, Theodosis DT. Synaptic and neuronal-glial plasticity in the adult oxytocinergic system in response to physiological stimuli. Brain Res Bull. 1988;20:681–692. doi: 10.1016/0361-9230(88)90078-0. [DOI] [PubMed] [Google Scholar]
- 41.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 42.Naftolin F, Mor G, Horvath TL, Luquins S, Fajer AB, Kohen F, Garcia-Segura LM. Synaptic remodelling in the arcurate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotrophin surge. Endocrinology. 1996;137:5576–5580. doi: 10.1210/endo.137.12.8940386. [DOI] [PubMed] [Google Scholar]
- 43.Nanda KK, Hamner KC. Studies on the nature of the endogenous rhythm affecting photoperiodic response of Biloxi Soybean. Bot Gaz (Chicago) 1958;120:14–25. [Google Scholar]
- 44.Nicholls TJ, Follett BK, Robinson JE. A photoperiodic response in gonadectomized Japanese quail exposed to a single long day. J Endocrinol. 1983;97:121–126. doi: 10.1677/joe.0.0970121. [DOI] [PubMed] [Google Scholar]
- 45.Ohta M, Homma K. Detection of neuronal connections to the infundibular complex by partial or complete hypothalamic deafferentation in male quail. Gen Comp Endocrinol. 1987;68:286–292. doi: 10.1016/0016-6480(87)90040-2. [DOI] [PubMed] [Google Scholar]
- 46.Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholtsiebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver J, Baylé JD. Brain photoreceptors for the photoinduced testicular response in birds. Experientia. 1982;38:1021–1029. doi: 10.1007/BF01955346. [DOI] [PubMed] [Google Scholar]
- 48.Olmos G, Naftolin F, Perez J, Tranque PA. Synaptic remodelling in the rat arcurate nucleus during the estrous cycle. Neuroscience. 1989;32:663–667. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- 49.Parry DM, Goldsmith AR. Ultrastructural evidence for changes in synaptic input to the hypothalamic luteinizing hormone-releasing hormone neurons in photosensitive and photorefractory starlings. J Neuroendocrinol. 1993;5:387–395. doi: 10.1111/j.1365-2826.1993.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 50.Parry DM, Goldsmith AR, Millar RP, Glennie LM. Immunocytochemical localisation of GnRH precursor in the hypothalamus of European starlings during sexual maturation and photorefractoriness. J Neuroendocrinol. 1997;9:235–243. doi: 10.1046/j.1365-2826.1997.00575.x. [DOI] [PubMed] [Google Scholar]
- 51.Perera AD, Follett BK. Photoperiodic induction in vitro: the dynamics of gonadotrophin-releasing hormone release from hypothalamic explants of the Japanese quail. Endocrinology. 1992;13:2898–2908. doi: 10.1210/endo.131.6.1446626. [DOI] [PubMed] [Google Scholar]
- 52.Rusak B. Vertebrate behavioral rhythms. In: Aschoff J, editor. Handbook of behavioral neurobiology. Plenum; New York: 1981. pp. 183–213. [Google Scholar]
- 53.Saldanha CJ, Leak RK, Silver R. Detection and transduction of daylength in birds. Psychoneuroendocrinology. 1994;19:641–656. doi: 10.1016/0306-4530(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 54.Saldanha CJ, Silverman AJ, Silver R. Deep brain photoreception; relation between opsin and GnRH expressing neurons. Soc Neurosci Abstr. 1995;21:46.3. [Google Scholar]
- 55.Sharp PJ. Tanycyte and vascular patterns in the basal hypothalamus of Coturnix quail with reference to their possible neuroendocrine significance. Z Zellforsch Mikrosk Anat. 1972;127:552–569. doi: 10.1007/BF00306871. [DOI] [PubMed] [Google Scholar]
- 56.Sharp PJ, Follett BK. The effect of hypothalamic lesions on gonadotrophin release in Japanese quail. Neuroendocrinology. 1969;5:205–218. doi: 10.1159/000121861. [DOI] [PubMed] [Google Scholar]
- 57.Sharp PJ, Li Q, Talbot RT, Barker P, Huskisson N, Lea RW. Identification of hypothalamic nuclei involved in osmoregulation using fos immunocytochemistry in the domestic hen (Gallus domesticus), Ring dove (Streptopelia risoria), Japanese quail (Coturnix japonica) and Zebra finch (Taenopygia guttata). Cell Tissue Res. 1995;282:351–361. [Google Scholar]
- 58.Sharp PJ, Li Q, Georgiou G, Lea RW. Expression of Fos-like immunoreactivity in the hypothalamus of the Ring dove (Streptopelia risoria) at the onset of incubation. J Neuroendocrinol. 1996;8:291–298. doi: 10.1046/j.1365-2826.1996.04586.x. [DOI] [PubMed] [Google Scholar]
- 59.Sonnenberg JL, Macgregor-Leon PF, Curran T, Morgan JI. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989;3:359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- 60.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Res. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 61.Theodosis DT, Poulain DA. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984;11:183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- 62.Theodosis DT, Chapman DB, Montagnese C, Poulain DA, Morris JF. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986;17:661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- 63.Tweedle CD. Ultrastructrual manifestations of increased hormone release in the neurohypophysis. In: Cross BA, Leng G, editors. Progress in brain research, Vol 60, The neurohypophysis: structure, function and control. Elsevier; Amsterdam: 1983. pp. 259–272. [DOI] [PubMed] [Google Scholar]
- 64.Van Calker D, Hamprecht B. Effects of neurohormones on glia cells. In: Federoff S, Hertz L, editors. Advances in cellular neurobiology, Vol 1. Academic; New York: 1980. pp. 31–67. [Google Scholar]
- 65.Watanabe T, Nakai Y. Electron microscopic cytochemistry of catecholaminergic innervation of LHRH neurons in the medial preoptic area of the rat. Arch Histol Jpn. 1987;50:103–112. doi: 10.1679/aohc.50.103. [DOI] [PubMed] [Google Scholar]
- 66.Witkin JW, Silverman AJ. Synaptology of luteinizing hormone-releasing hormone neurons in rat preoptic area. Peptides. 1985;6:263–271. doi: 10.1016/0196-9781(85)90050-6. [DOI] [PubMed] [Google Scholar]
- 67.Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- 68.Witkin JW, Xiao E, Popilskis S, Ferin M, Silverman A. Fos expression in the gonadotropin-releasing hormone (GnRH) neuron does not increase during the ovarian steroid-induced GnRH surge in the rhesus monkey. Endocrinology. 1994;135:956–961. doi: 10.1210/endo.135.3.8070392. [DOI] [PubMed] [Google Scholar]
- 69.Yamada S, Mikami S, Yanaihara N. Immunohistochemical localization of vasoactive intestinal polypeptide (VIP) containing neurons in the hypothalamus of the Japanese quail, Coturnix coturnix. Cell Tissue Res. 1982;226:13–26. doi: 10.1007/BF00217078. [DOI] [PubMed] [Google Scholar]
- 70.Yokoyama K, Oksche A, Darden TR, Farner DS. The sites of encephalic photoreception in photoperiodic induction of the growth of the testes in the white-crowned sparrow. Cell Tissue Res. 1978;189:441–467. doi: 10.1007/BF00209132. [DOI] [PubMed] [Google Scholar]