Abstract
B-50/GAP-43 is a nervous tissue-specific protein, the expression of which is associated with axon growth and regeneration. Its overexpression in transgenic mice produces spontaneous axonal sprouting and enhances induced remodeling in several neuron populations (Aigner et al., 1995; Holtmaat et al., 1995). We examined the capacity of this protein to increase the regenerative potential of injured adult central axons, by inducing targeted B-50/GAP-43 overexpression in Purkinje cells, which normally show poor regenerative capabilities. Thus, transgenic mice were produced in which B-50/GAP-43 overexpression was driven by the Purkinje cell-specific L7 promoter. Uninjured transgenic Purkinje cells displayed normal morphology, indicating that transgene expression does not modify the normal phenotype of these neurons. By contrast, after axotomy numerous transgenic Purkinje cells exhibited profuse sprouting along the axon and at its severed end. Nevertheless, despite these growth phenomena, which never occurred in wild-type mice, the severed transgenic axons were not able to regenerate, either spontaneously or into embryonic neural or Schwann cell grafts placed into the lesion site. Finally, although only a moderate Purkinje cell loss occurred in wild-type cerebella after axotomy, a considerable number of injured transgenic neurons degenerated, but they could be partially rescued by the different transplants placed into the lesion site. Thus, B-50/GAP-43 overexpression substantially modifies Purkinje cell response to axotomy, by inducing growth processes and decreasing their resistance to injury. However, the presence of this protein is not sufficient to enable these neurons to accomplish a full program of axon regeneration.
Keywords: transgenic mice, axon growth-associated genes, L7, cerebellum, embryonic neural graft, Schwann cell transplantation
Although axon growth in the adult mammalian brain is primarily hampered by adverse environmental conditions, the success of regenerative processes is also dependent on the capability of injured neurons to express the intrinsic molecular machinery required for neurite elongation (for review, see Fawcett, 1992; Schwab and Bartholdi, 1996; Herdegen et al., 1997). The axon growth-associated gene program is thought to be inhibited in mature intact neurons, most likely through retrograde extrinsic influences (Skene, 1989, 1992; Smith and Skene, 1997). Nevertheless, several adult neuron populations upregulate growth-associated genes after axotomy, and this expression is related to their ability to regenerate their axons into growth-permissive territories (Campbell et al., 1991;Tetzlaff et al., 1991, 1994; Schaden et al., 1994).
The intrinsic regenerative response of adult neurons involves the coordinated expression of a specific set of mostly unidentified molecules, which are designated growth-associated proteins (Skene, 1989; Tetzlaff et al., 1994). Among them, the best characterized is B-50/GAP-43, which is expressed by most neuron populations during developmental axonogenetic and synaptogenetic processes, whereas in adulthood it is present only in restricted brain regions thought to be involved in functional plasticity (for review, see Skene, 1989;Benowitz and Routtenberg, 1997). B-50/GAP-43 has been related to terminal axon arbor remodeling (Caroni and Grandes, 1990; Mehta et al., 1993; Verzé et al., 1996). In addition, several adult neuron populations upregulate this protein after axotomy (Skene, 1989, 1992;Doster et al., 1991; Tetzlaff et al., 1991, 1994; Verhaagen et al., 1993; Schaden et al., 1994), and this expression can be enhanced by growth-promoting environmental cues (Hüll and Bähr, 1994;Robinson, 1994; Vaudano et al., 1995; Chong et al., 1996). Thus, although the precise role of B-50/GAP-43 in axon growth is still debated, its expression has been strictly associated with the plastic and regenerative potential of adult neurons. B-50/GAP-43 overexpression in transgenic mice enhances spontaneous or induced sprouting in both the peripheral nervous system and the CNS (Aigner et al., 1995;Holtmaat et al., 1995), and it potentiates the regeneration of crushed peripheral axons (Aigner et al., 1995). However, it is unknown whether B-50/GAP-43 overexpression can increase the regenerative capabilities of transsected axons in the adult brain.
Among central neurons, cerebellar Purkinje cells of adult rodents are most peculiar for their strong resistance to axotomy (Dusart and Sotelo, 1994) and their poor ability to regrow their axons even when confronted with a growth permissive environment (Rossi et al., 1995,1997; Bravin et al., 1997; Dusart et al., 1997). The failure of Purkinje cell axon regeneration is likely attributable to their inability to respond to injury by expressing the growth-associated gene program. Thus, neurite growth might be obtained by experimentally inducing the expression of growth-associated molecules in these neurons. To investigate this hypothesis, transgenic mice were produced in which B-50/GAP-43 overexpression was directed by the Purkinje cell-specific L7 promoter (Oberdick et al., 1990). In these mice we have examined the response of Purkinje cells to axotomy, and we have assessed whether they are able to regenerate their neurites either spontaneously or into embryonic neural or Schwann cell transplants.
Preliminary reports of this study have been published previously (Holtmaat et al., 1994; Buffo et al., 1996).
MATERIALS AND METHODS
Experimental animals. The study was performed on adult heterozygous transgenic FVB mice, using the wild-type (IFFA Credo, Lyon, France) as controls (body weight 30–40 gm at the time of lesion). The tissue for cerebellar and neocortical grafts was isolated from the brain of wild-type embryos of the same strain, and newborn [postnatal day (P) 0–P1] wild-type FVB pups were used as Schwann cells donors. All of the surgical procedures on adult mice were performed under deep general anesthesia obtained with an intraperitoneal injection of chloral hydrate (400 mg/kg). The experimental plan was designed according to the Italian law for care and use of experimental animals (DL116/92) and approved by the Italian Ministry of Health.
Construction of the L7-B-50/GAP-43 transgene and generation of transgenic mouse lines. To generate L7-B-50/GAP-43 hybrid transgene, the B-50/GAP-43 open reading frame (ORF) was inserted in a vector named L7ΔAUG, essentially as described by Smeyne et al. (1995). This vector includes 1 kb of the L7 promoter, the four exons and three introns, and 200 bp downstream of the TGA stop codon of the L7 gene, from which the normal and all potential downstream ATGs were removed (Smeyne et al., 1995). The B-50/GAP-43 ORF was inserted into the unique BamHI site in the fourth exon of the L7 gene (see Fig. 1A). To achieve this, the L7ΔAUG vector was digested with BamHI, and the 3′ and 5′ overhangs were converted to blunt ends with Klenow polymerase. The B-50/GAP-43 ORF was removed from pBluescript KS by digestion with AatII and KpnI (Holtmaat et al., 1995), also end-filled with Klenow polymerase and ligated in the BamHI-digested L7ΔAUG vector. The B-50/GAP-43 junctions were sequenced to determine whether the orientation of the insert was correct. The transgene was removed from the vector by digestion with EcoRI and HindIII. The resulting 3.7 kb fragment was gel-purified, dialyzed against sterile Millipore water, and used for injection of mouse zygotes essentially as described (Hogan et al., 1986). Embryo donors were FVB/N superovulated females. Injected embryos were implanted in the oviducts of day 1 pseudopregnant foster females. Forty-eight mice were born from these mothers, of which three had incorporated the transgene into tail-DNA. Two of these mice, which passed the transgene to their offspring, were used for breeding to generate transgenic lines (designated L1657 and L1658). Transgenic mice were identified by Southern blotting of genomic tail-DNA according to previously described methods (Laird et al., 1991; Holtmaat et al., 1995).
Fig. 1.
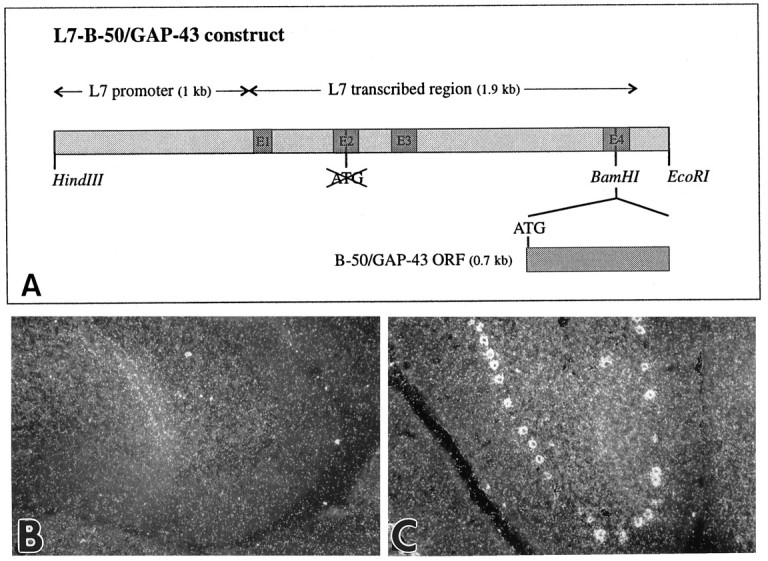
Directed expression of B-50/GAP-43 in cerebellar Purkinje cells of transgenic mice. A, Diagram of the construct used to generate L7-B-50/GAP-43 transgenic mice. The B-50/GAP-43 open reading frame (ORF) was inserted in the unique BamHI site of a vector containing 1 kb of the L7 promoter, the four exons and three introns, and 200 bp downstream of the TGA of the L7 gene. In this vector the endogenous ATGs of the L7 gene had been deleted, and the only translational start signal (ATG) was introduced with the B-50/GAP-43 ORF in the fourth exon of the L7 gene. Details of the cloning of this construct are given in Materials and Methods. B, C, Dark-field micrographs ofin situ hybridization of cerebella of wild-type (B) and transgenic (C) mice from line 1658 (L1658) with a radiolabeled B-50/GAP-43-antisense riboprobe. Cerebellar Purkinje cells do not normally express B-50/GAP-43 mRNA in adulthood (B). In contrast, the B-50/GAP-43 mRNA is abundantly present in these cells in transgenic mice (C).
In situ hybridization. The preparation of radiolabeled antisense B-50/GAP-43 RNA probe and the procedures used for in situ hybridization were performed as described previously (Verhaagen et al., 1990). Ten-micrometer-thick paraformaldehyde-fixed sections were mounted on poly-l-lysine-coated microscope slides. The sections were dried for 2 hr, post-fixed in 2% paraformaldehyde for 30 min, rinsed in PBS, and stored overnight in 70% ethanol. The sections were subsequently rinsed in PBS and in 2× SSC and acetylated in 0.25% acetic anhydride/0.1 m triethanolamine. After additional rinses in 2× SSC and PBS, sections were incubated in 0.1 mglycine/0.1 m Tris HCl, pH 8.0, for 30 min, rinsed briefly in 2× SSC, and dehydrated. Hybridization was performed at 62°C for 5 hr in hybridization solution (50% formamide, 10% dextran sulfate, 250 μg/ml denatured salmon sperm DNA, 1 mg/ml tRNA, 10 mmdithiothreitol, 4× SSC) containing [35S]αUTP radiolabeled B-50/GAP-43 probe. After hybridization, sections were rinsed in 2× SSC/50% formamide at 50°C followed by 0.1× SSC/20 mm β-mercaptoethanol at 62°C, dehydrated, dipped in NTB-2 liquid emulsion (Kodak), and exposed for 1 week.
Graft preparations. The preparation of embryonic tissue for grafting was performed according to previously described methods (Rossi et al., 1995). Briefly, under deep general anesthesia (see above), cesarean sections were performed on pregnant mice, and the embryos were extracted and placed in 0.12 m phosphate buffer with 0.6% glucose and immediately decapitated. The mother was killed by an overdose of anesthetic. The cerebellar primordium was dissected from 12- to 13-d-old embryos (mating day = E0), whereas neocortical tissue was taken from E17 embryos. The dissected specimens were kept in the same buffer at room temperature until transplantation.
Cell suspensions of sciatic nerves were prepared from cryoanesthetized newborn wild-type FVB pups, according to a modification of the protocol described by Brockes et al. (1979) (also see Bravin et al., 1997). Under sterile conditions the sciatic nerves were excised, desheathed, and collected in L-15 medium (Life Technologies, Paisley, UK) with penicillin–streptomycin (10,000 U/100 ml). The tissue was incubated in 0.1% collagenase (Sigma, St. Louis, MO) for 30 min at 37°C and then briefly in 0.25% trypsin (Sigma). After centrifugation (3 min at 1200 rpm) the medium was removed, and the pellet was resuspended in DMEM (Life Technologies) with 10% fetal calf serum (Life Technologies) and mechanically dissociated by means of a fire-polished Pasteur pipette. The yield from 20 mice was on the average of 2–3 × 106 cells, which were resuspended at a final concentration of 20,000–40,000 cells/μl for grafting.
Cerebellar lesions and grafting. Purkinje cell axons were transected according to a previously described approach (Dusart and Sotelo, 1994; Rossi et al., 1995). Briefly, the deeply anesthetized mice were placed in a stereotaxic frame, the occipital bone was exposed, and a small hole was drilled in its posterior aspect around the midline. A microknife made of a small piece of a razor blade (0.5 mm wide and 3–5 mm long) was introduced in the cerebellum from the right side of the vermis and then turned to the left. By this method, the axons running in the axial white matter of several cerebellar lobules are sectioned.
In the different sets of graft-recipient animals, transplantation procedures were performed immediately after lesioning. Morsels of embryonic cerebellar or neocortical tissues were pressure-injected into the lesion track by using a glass micropipette connected to a Hamilton syringe (Rossi et al., 1995). Sciatic nerve cell suspensions were injected by means of a glass micropipette (tip diameter 200 μm) connected to a PV800 Pneumatic Picopump (WPI, New Haven, CT). The frequency and duration of pressure pulses were adjusted to inject 2 μl of suspension (e.g., 40,000–80,000 cells) during 10 min, and the pipette was gradually retracted to distribute grafted cells along the lesion track (Brook et al., 1994; Bravin et al., 1997). The pipette was left in situ for an additional 5 min to avoid an excessive leakage of the grafted cells from the cerebellar surface. Thereafter, the skin was sutured, and the animals were returned to their cages and given free access to food and water. Survival times after the lesion/graft ranged from 24 hr to 60 d (Table1). In addition, three intact transgenic and three intact wild-type mice were processed as controls.
Table 1.
Number of lesioned wild-type and transgenic animals
| Survival time | Axotomy | Cerebellar graft | Neocortical graft | Schwann cells graft | ||||
|---|---|---|---|---|---|---|---|---|
| Wild type | Transgenic | Wild type | Transgenic | Wild type | Transgenic | Wild type | Transgenic | |
| 24 hr | 1 | |||||||
| 3 d | 1 | 1 | 1 | 1 | 1 | |||
| 7 d | 2 | 2 | 3 | 1 | ||||
| 14 d | 2 | 2 | 1 | 1 | 1 | 2 | ||
| 21 d | 1 | 1 | 3 | |||||
| 30 d | 4 | 3 | 2 | 6 | 2 | 4 | 2 | 5 |
| 60 d | 3 | 6 | 2 | 6 | 2 | 3 | 2 | 4 |
The number of mice considered after the different experimental manipulations is reported. In addition, three intact wild-type and three intact transgenic mice were processed as controls.
Histological procedures. At different post-lesion survival times, the mice were anesthetized and transcardially perfused with 500 ml of 4% paraformaldehyde in 0.12 m phosphate buffer, pH 7.2. The brains were immediately dissected and kept in fixative overnight at 4°C. After post-fixation, all the brains were transferred to 30% sucrose in phosphate buffer until they sank. The cerebella were cut by means of a freezing microtome in several series of 30-μm-thick sagittal sections. The sections were collected in PBS and incubated for 30 min in 0.3% H2O2 in PBS to quench endogenous peroxidase. Subsequently, the sections were incubated with different primary antibodies and diluted in PBS with 0.25% Triton X-100 and 0.2% normal serum overnight at room temperature or at 4°C. Purkinje cells were stained by antibodies to calbindin D-28K (monoclonal, 1:3000; Swant, Bellinzona, Switzerland) and L7 (polyclonal, 1:6000) (Oberdick et al., 1988), and in transgenic mice by anti-B-50/GAP-43 affinity-purified rabbit immunoglobulin (8920b1, 1:600) (Oestreicher et al., 1983; Ulenkate et al., 1993). In some instances, to visualize Schwann cells a monoclonal antibody to P75 low-affinity NGF receptor (monoclonal 192-IgG, 1:1000; gift from Dr. A. Cattaneo, Scuola Internazionale Superiore di Studi Avanzati, Trieste, Italy) was used. In these cases post-fixation lasted only 2 hr. Immunohistochemical staining was performed according to the avidin–biotin–peroxidase method (Vectastain, ABC elite kit, Vector, Burlingame, CA) using diaminobenzidine as chromogen. The reacted sections were mounted on chrome-alum gelatinized slides, air-dried, dehydrated, and coverslipped. Some of the sections were also counterstained by thionine or propidium iodide (30 min incubation at 37°C in a 0.04% solution in Tris buffer 0.05 m, pH 7.4). The histological preparations were examined by bright-field or Nomarsky interference contrast using a Zeiss Axiophot light microscope.
Quantitative analysis of Purkinje cell degeneration after axotomy or transplantation. To evaluate the degeneration of Purkinje cells in wild-type and transgenic cerebella after the different experimental manipulations, we estimated the number of Purkinje cells/millimeter of Purkinje cell layer. To this aim, we selected lobuli III and IV–V of the anterior lobe, which were most frequently affected by our lesion/grafting experiments. The analyzed animals were divided into six experimental sets (Table 2): intact wild type (n = 3) and transgenic (n = 3), axotomized wild-type (n = 3) and transgenic (n = 5), and axotomized/graft wild-type (n = 3) and transgenic (n = 3). Regarding the treated animals, the analysis was restricted to those killed at 2 months survival time. Among axotomized mice, we selected those lobuli that were completely transsected close to their proximal end through several adjacent sections, and only sections far from the lateral edges of the lesion were considered. Similarly, for animals receiving a graft, only those cases in which the completely transected lobuli directly abutted the tissue placed into the lesion track were chosen. These criteria restricted the number of cases that could be sampled. Thus, because similar results were obtained with different types of transplant (Table 2), these were pooled together. Finally, because careful check of Nissl- or propidium iodide-counterstained sections showed that all surviving Purkinje cells in the affected folia retained strong immunoreactivity for the applied antibodies (see Fig.7A), cell counts were performed on anti-L7 immunolabeled sections, which allowed the best detection of Purkinje cell perikarya.
Table 2.
Number of Purkinje cells/millimeter in the mice selected for the quantitative analysis
| Wild type | Transgenic | ||||
|---|---|---|---|---|---|
| WT1 | Intact | 50.6 | T1 | Intact | 53.2 |
| WT2 | Intact | 49.5 | T2 | Intact | 51 |
| WT3 | Intact | 48.7 | T3 | Intact | 49.8 |
| WA1 | Axotomy | 37.6 | TA1 | Axotomy | 25.6 |
| WA2 | Axotomy | 35.8 | TA2 | Axotomy | 30.3 |
| WA3 | Axotomy | 41.7 | TA3 | Axotomy | 20 |
| TA4 | Axotomy | 27 | |||
| TA5 | Axotomy | 29.9 | |||
| WG1 | Graft (cerebellum) | 40.3 | TG1 | Graft (Schwann) | 37.5 |
| WG2 | Graft (cerebellum) | 36.5 | TG2 | Graft (cerebellum) | 35.6 |
| WG3 | Graft (Schwann) | 36.7 | TG3 | Graft (Schwann) | 38.1 |
For each examined wild-type and transgenic animal (indicated by the acronym on the left of each column) is reported the applied treatment (center of each column; the type of transplant is given in parentheses for the relevant cases) and the number of Purkinje cells/millimeter of Purkinje cell layer length. Data from the different groups have been pooled to produce the histogram of Figure7B.
Fig. 7.
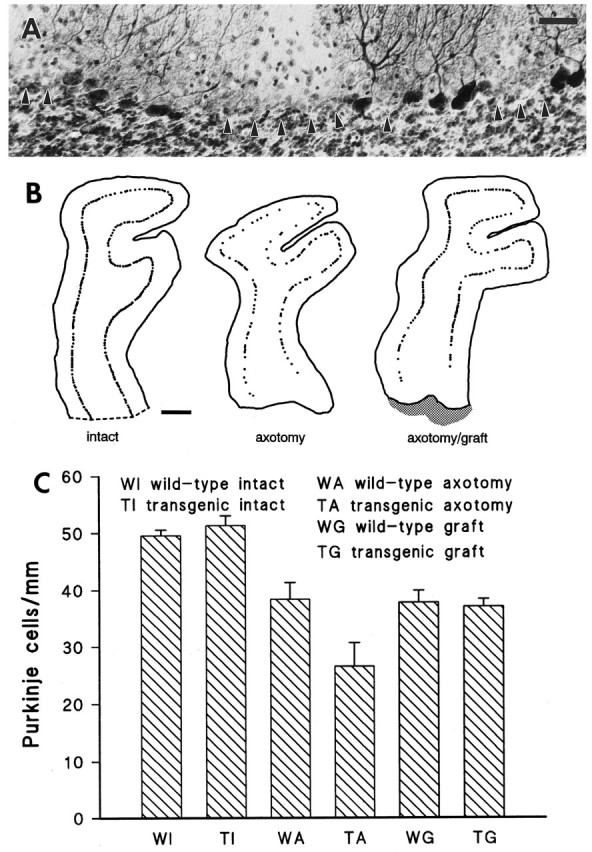
Purkinje cell loss in the injured wild-type and transgenic cerebella. A transected transgenic cerebellar lobule, immunolabeled by anti-L7 antibodies and counterstained by thionine, is displayed in A. Arrowheads point to areas where Purkinje cells have degenerated. Note that all surviving neurons are immunolabeled and no immunonegative thionine-stained Purkinje cell perikarya remain. B shows three representative camera lucida reconstructions of lobuli IV–V from transgenic mouse cerebella. Two months after axotomy (center) the number of Purkinje cells is considerably decreased compared with control (left). By contrast, when a graft (shaded area on the right: axotomy/graft) is placed in the lesion track, the injured cells are partially rescued. Quantitative estimations of the number of Purkinje cells/millimeter of Purkinje cell layer are shown in the histogram C. Intact wild-type and transgenic mice have similar values, indicating that no spontaneous cell loss occurs in transgenic animals. By contrast, after axotomy the number of Purkinje cells/millimeter is reduced in both animal sets, although a statistically more severe cell loss is observed in transgenic animals. Finally, when a graft is placed into the lesion site, both wild-type and transgenic mice show similar values that are not statistically different from that obtained from axotomized wild-type cerebella, indicating that axotomy-induced Purkinje cell loss in transgenic mice can be prevented by graft-derived trophic support. Scale bars: A, 40 μm; B, 200 μm.
For each animal, two or three sections were selected according to these criteria. On such sections the outline of the Purkinje cell layer and the position of all Purkinje cell somata in lobuli III and IV–V were reproduced by means of a camera lucida at 200× magnification (see Fig.7B). On the obtained drawings, the length of the Purkinje cell layer and the number of Purkinje cell bodies were calculated by means of a magnetic tablet using the Sigma-Scan software (Jandel Scientific, Corte Madera, CA). The numbers of Purkinje cells/millimeter obtained from the animals of each set were pooled to yield the final values. Statistical analysis was performed by means of Student’st test.
RESULTS
The L7 promoter directs expression of B-50/GAP-43 to cerebellar Purkinje cells
In both transgenic lines (L1657 and L1658) obtained with the L7-B-50/GAP-43 construct (Fig.1A), in situhybridization with a B-50/GAP-43 riboprobe on cerebella of 3-month-old transgenic mice revealed the expression of B-50/GAP-43 in Purkinje cells, whereas the same neurons of wild-type mice showed no expression of B-50/GAP-43 mRNA (Fig. 1B,C). Offspring of L1658, the line that displayed the highest expression levels (Fig.1C), were used for all experiments described here.
The response of wild-type mouse Purkinje cells to axotomy and to growth-permissive grafts
The peculiar morphological modifications that affect axotomized Purkinje cells, and especially their neurites, have been thoroughly described in previous studies (Ramón y Cajal, 1928; Dusart and Sotelo, 1994). Our observations of injured Purkinje cells in wild-type mice are fully consistent with these reports. Severed Purkinje cell axons displayed prominent torpedoes along their initial course through the granular layer, whereas recurrent collateral branches appeared hypertrophied and formed typical arciform fibers. The corticofugal axon branches running along the axial white matter of transected lobuli appeared thinner than their intact counterparts and ended with round-shaped terminal clubs apposed to the injury track or were slightly retracted (see Fig. 5G). These morphological modifications remained unchanged for the whole examined period, 2 months after injury (also see Rossi et al., 1995), and the transsected fibers never displayed the spontaneous sprouting observed in the rat several months after axotomy (Dusart and Sotelo, 1994).
Fig. 5.
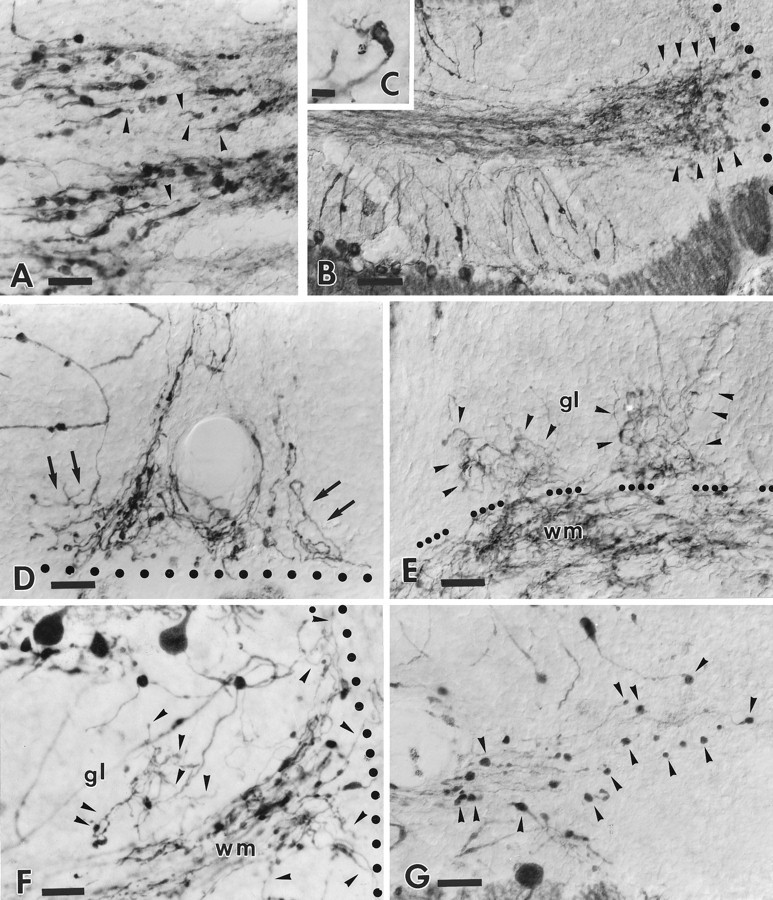
Morphological modifications of the distal portion of the transgenic Purkinje cell axons after axotomy. Ashows the transected tips of transgenic Purkinje axons 24 hr after injury (lesion site is beyond the right edge of the picture).Arrowheads indicate fine processes that emanate from the terminal clubs. One month after injury (B) a dense plexus of newly formed sprouts (arrowheads) has developed in the vicinity of the lesion track (dotted line). The inset C shows one such transected axon ending in a terminal club from which several sprouts originate. The structural features and tortuous courses taken by these terminal sprouts (arrows) can be better appreciated inD. Note that despite the profuse growth the newly formed processes do not elongate across the injury site (dotted line). Another terminal plexus is shown in E. In this case the newly formed processes are extended in the white matter (wm) and also (as indicated byarrowheads) in the adjacent granular layer (gl) (dots point to the granular layer–white matter border). Anti-calbindin immunostaining in transgenic cerebella (F) also depicts numerous sprouts (arrowheads) abutting the lesion site (dotted line) or elongating in the nearby granular layer (gl). By contrast, in wild-type animals (G) (anti-L7 immunostaining) the transected axons remain close to the lesion site (just beyond the right edge of the picture), but they terminate with round-shaped terminal clubs (some are indicated by arrowheads). Survival times: 24 hr (A); 30 d (B, C, E, G); 60 d (D, F). Scale bars: A, D, E, F, G, 30 μm; B, 60 μm; C, 8 μm.
This picture did not change when embryonic cerebellum, neocortex, or dissociated neonatal sciatic nerve cells were grafted into the lesion track. Consistent with previous in vivo (Rossi et al., 1995, 1997; Bravin et al., 1997) and in vitroexperiments (Dusart et al., 1997), none of these grafts, which are known to promote the vigorous growth of other injured cerebellar axons, ever induced any sprouting or regeneration of Purkinje cell neurites (see Fig. 6B).
Fig. 6.
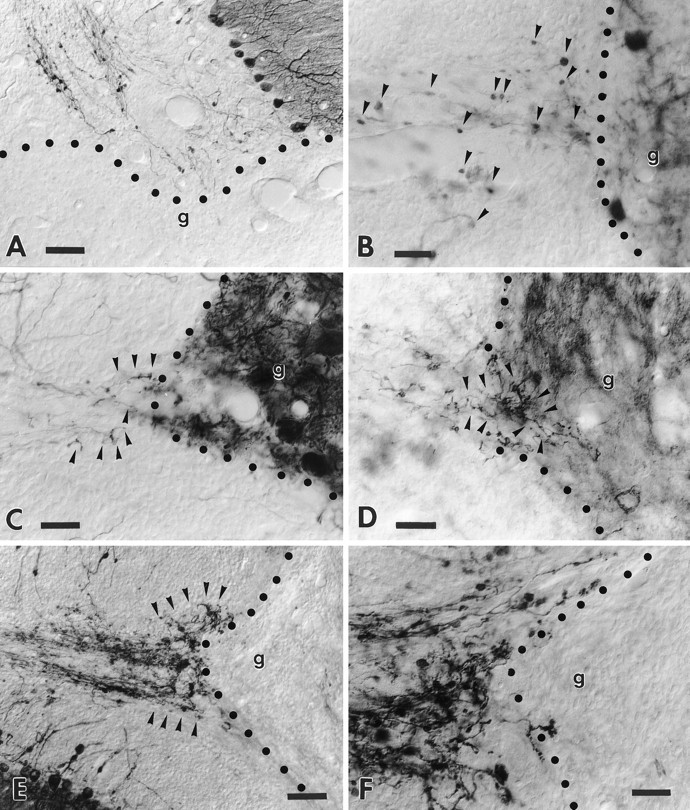
Transgenic Purkinje cell axons are unable to regenerate into growth-permissive transplants. A shows a transected folium from a transgenic cerebellum facing a large embryonic neocortical transplant. Note the anti-L7-immunolabeled axons that end at the graft–host interface. The high-magnification picture (B) shows anti-calbindin-immunolabeled wild-type Purkinje cell axons that terminate with round-shaped terminal clubs (arrowheads) close to the edge of an embryonic cerebellar transplant, highlighted by the presence of grafted Purkinje cells. By contrast, the micrograph C shows a similar situation in a transgenic animal also stained by anti-calbindin antibodies: several thin sprouts (arrowheads) emanate from the transected host axons. Anti-B-50/GAP-43 immunolabeling of the adjacent section (D) shows several sprouts (arrowheads) that elongate for a short distance into the transplant. Note, however, that these sprouts do not show the morphology of terminal varicose branches. The micrographE displays a prominent plexus (arrowheads) of anti-B-50/GAP-43-immunolabeled sprouts abutting a Schwann cell graft. The higher-magnification pictureF shows the typical morphology of the newly formed sprouts that abruptly stop at the graft–host border. In all picturesg indicates the graft, whereas the dotted line highlights the graft–host interface. Survival times: 30 d (A); 60 d (B–F). Scale bars: A, E, 60 μm; B, C, D, F, 30 μm.
B-50/GAP-43 expression in intact and injured wild-type mouse Purkinje cells
In most neuron populations B-50/GAP-43 expression is developmentally regulated, but it can be reinduced during adulthood in response to injury (for references, see Benowitz and Routtenberg, 1997). Uninjured Purkinje cells do not show B-50/GAP-43 expression either during development (Oestreicher and Gispen, 1986; Console-Bram et al., 1996) or in adulthood (Oestreicher and Gispen, 1986; Meberg and Routtenberg, 1991; Kruger et al., 1993; Baürle et al., 1994;Console-Bram et al., 1996). However, it is not known whether B-50/GAP-43 can be upregulated in adult Purkinje cells after axon injury or transplantation of growth-promoting tissues.
In our wild-type cerebella, B-50/GAP-43 immunolabeling faithfully reproduced previously reported distribution patterns (Oestreicher and Gispen, 1986; Baürle et al., 1994): the molecular layer displayed an intense punctate staining, whereas all Purkinje cell perikarya were virtually negative (Fig.2A,B). Faintly labeled axons were scattered across the granular layer or gathered in bundles along the axial white matter of cortical folia (Fig.2A). In addition, sparse, dimly labeled varicose fibers were present in the deep nuclei (Fig. 2C). After injury, the same labeling pattern was substantially maintained in the transected lobuli, and Purkinje cells always remained negative (Fig.2D). Furthermore, labeled axons completely disappeared from the axial white matter and granular layer of these lobuli, showing that the corticofugal Purkinje cell axons did not contain this protein (Fig. 2D). An almost identical picture was observed when the lesioned lobuli were directly apposed to embryonic neural or Schwann cell transplants (Fig.2E). Altogether, these observations corroborate the notion that B-50/GAP-43 is not normally present in adult Purkinje cells, and they show that its expression cannot be induced by axon injury or transplant-derived cues.
Fig. 2.
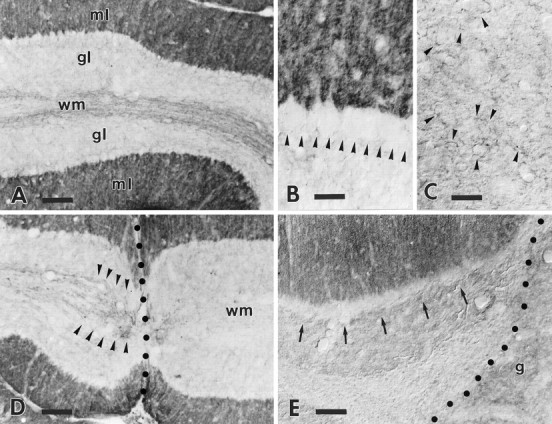
B-50/GAP-43 expression in intact and injured wild-type cerebella. The survey micrograph A shows the anti-B-50/GAP-43 labeling pattern in the wild-type cerebellum. The molecular layer (ml) shows an intense punctate labeling, whereas the granular layer (gl) is almost completely unlabeled. The row of virtually immunonegative Purkinje cell somata at the granular–molecular layer interface is highlighted by the contrast with the strongly labeled molecular layer. Note the presence of several dimly labeled axons running along the axial white matter (wm) of this folium. The higher magnification in B shows the absence of staining in Purkinje cell perikarya (arrowheads). Labeling in the deep nuclei (C) is restricted to sparse varicose branches (some are indicated by arrowheads) displaying a faint immunoreactivity. The labeling pattern in the cortex is substantially unaltered in the transected folia (D): Purkinje cells remain immunonegative. Note that all immunoreactive axons (arrowheads) stop abruptly at the lesion site (dotted line), and no labeled profiles remain in the white matter (wm) on the other side of the injury. A similar picture is observed when the transected folia face a transplant (E), a cerebellar graft (g) in this case: arrows point to the immunonegative Purkinje cell somata (dotted lineindicates the graft–host border). Survival times: 21 d (D), 60 d (E). Scale bars: A, D, 90 μm; B, C, 30 μm;E, 60 μm.
Cerebellar phenotype and B-50/GAP-43 expression in intact transgenic mice
The transgenic mouse cerebella displayed a substantially normal anatomy, with a typical foliation pattern and cortical layering (Fig. 3A). All different neuron populations were present and correctly positioned in the different cortical layers or in the deep nuclei. Also the pattern of expression of B-50/GAP-43 was similar to that of wild-type mice, except for the intense immunolabeling of Purkinje cells (Fig.3A–C). In these neurons, B-50/GAP-43 immunoreactivity was restricted to the perikaryal cytoplasm and the initial segment of the primary dendritic trunk (Fig. 3B,C). In addition, the whole axon was intensely stained up to the terminal boutons in the deep nuclei (Fig. 3H) or in the cortical ganglionic plexuses (Fig. 3C).
Fig. 3.
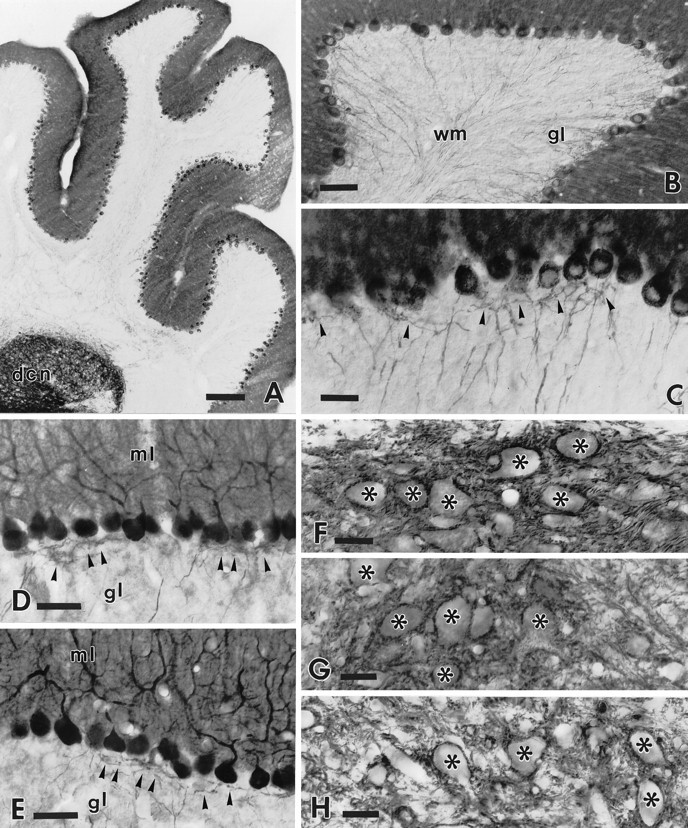
Cerebellar and Purkinje cell phenotype in the adult intact transgenic cerebella. The low-power micrograph (A) shows the general morphology of an intact transgenic cerebellum stained by anti-B-50/GAP-43 antibodies. The labeling pattern is substantially similar to that of wild-type mice except for the strong staining of Purkinje cell perikarya and neurites. Note also the intense staining of the deep cerebellar nuclei (dcn) attributable to the dense terminal meshwork of Purkinje cell axons. Anti-B-50/GAP-43 immunolabeling of Purkinje cells is shown in B. Note the typical morphology and course of immunoreactive Purkinje cell axons across the granular layer (gl) toward the white matter (wm). The higher magnification picture (C) shows the thin axons emerging from the basal pole of Purkinje cell perikarya and the fine infraganglionic recurrent terminal plexus (arrowheads). D andE show anti-L7 immunolabeling of the cerebellar cortex in wild-type (D) and transgenic (E) mice. Transgenic Purkinje cells display the typical structure and orientation of dendritic trees in the molecular layer (ml). In addition, the infraganglionic recurrent axonal plexuses (indicated by arrowheads in both pictures) in the granular layer (gl) show a similar morphology and extension. MicrographsF–H show the terminal distribution of Purkinje axons in the deep cerebellar nuclei in wild-type (F; anti-L7 labeling) and transgenic (G; anti-L7 labeling) mice;H shows anti-B-50/GAP-43 staining. An almost identical labeling pattern is observed in both wild-type and transgenic animals with the typical clustering of Purkinje axon terminals surrounding the perikarya of unlabeled deep nuclear neurons (indicated byasterisks). Note also the very similar pattern of immunoreactivity obtained by anti-L7 and anti-B-50/GAP-43 antibodies in transgenic cerebella (G, H). Scale bars:A, 200 μm; B, 60 μm; D, E, 50 μm; C, F–H, 30 μm.
Because B-50/GAP-43 overexpression induces spontaneous sprouting of uninjured motorneurons, hippocampal mossy fibers (Aigner et al., 1995), and primary olfactory neurons (Holtmaat et al., 1995), we first examined the intact transgenic Purkinje cells to assess whether they also showed abnormal features in their morphology or connectivity. Purkinje cell dendritic trees, stained by anti-L7 or anti-calbindin immunolabeling, displayed normal structure and orientation (Fig.3D,E). Thin neurites, visualized by these and by anti-B-50/GAP-43 antibodies, emerged from the basal pole of Purkinje cell somata and run straight through the granular layer toward the white matter, where they gathered in bundles (Fig. 3B,C,E). Fine recurrent collaterals arose in the granular layer and spread in a loose terminal meshwork of varicose branches just below or above the Purkinje cell layer (Fig. 3C–E). This terminal ganglionic plexus was well developed, although it was not more extended or dense than in wild-type cerebella (compare Fig. 3, D andE).
The terminal distribution of anti-L7 immunolabeled Purkinje cell axons in the deep cerebellar and vestibular nuclei of transgenic mice was similar to that observed in wild-type individuals and matched previous descriptions in rodents (De Zeeuw and Berrebi, 1995). The deep cerebellar nuclei were completely covered by the terminal branches of Purkinje cell axons, which displayed an intense anti-B-50/GAP-43 immunoreactivity (Fig. 3F,H; compare also Fig. 3Hwith Fig. 2C). Their synaptic boutons typically enveloped the perikarya and proximal dendrites of their target neurons (De Camilli et al., 1984). Thus, the terminal pattern and distribution of Purkinje cell axons in the cortical ganglionic plexuses or in the deep cerebellar and vestibular nuclei was similar to that of wild-type animals, indicating that, at least at this level of analysis, B-50/GAP-43 overexpression does not induce any clear abnormality in the Purkinje cell phenotype.
Response of transgenic mouse Purkinje cells to axotomy
The basic morphological changes induced by axon injury in transgenic Purkinje cells were essentially similar to those observed in wild-type cerebella (Fig.4A). Nevertheless, in addition to these modifications, the overexpression of B-50/GAP-43 was associated with some remarkable axonal remodelling.
Fig. 4.
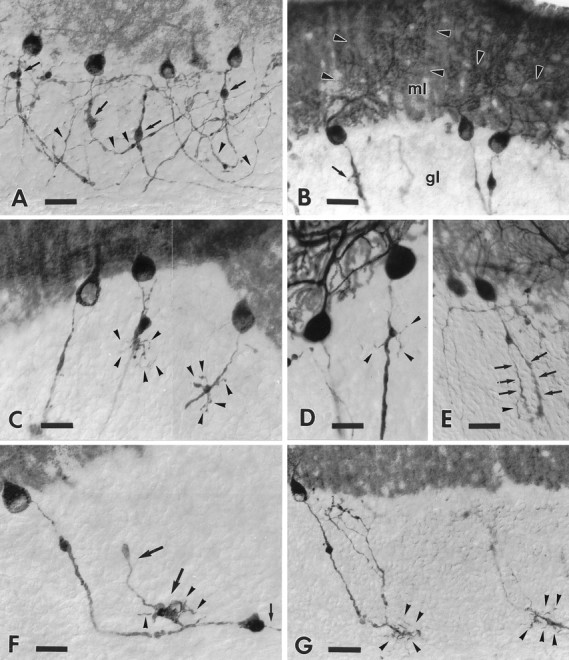
Morphological modifications of the initial portion of the transgenic Purkinje cell axons after axotomy. Ashows the general morphology of anti-B-50/GAP-43-immunostained transected Purkinje axons in the cerebellar cortex. Note the prominent torpedoes (arrows) and the thickened arciform fibers (arrowheads). The dendritic labeling (arrowheads) shown by some injured Purkinje cells is shown in B; arrow points to a fine sprout emitted by a thickened Purkinje axon in the granular layer (gl); ml, molecular layer. The compound micrograph (C) shows two different optical sections from an anti-B-50/GAP-43-immunolabeled transgenic cerebellum. Arrowheads point to several sprouts bearing rare varicosities, budding from the torpedoes of the injured axons. Anti-L7-immunolabeled transgenic Purkinje cells are shown inD and E. Arrowheads inD indicate thin sprouts emerging from a tiny torpedo, whereas arrows in E point to longer and thicker, newly formed processes, one of which emits a fine ramification (arrowhead). F shows an injured Purkinje axon stained by anti-B-50/GAP-43 antibodies. The thickened portion of this neurite ends with a large club from which a thinned corticofugal branch (small arrow) emanates. The large arrows point to a thick recurrent branch of this axon that also bears a large varicosity studded with several short sprouts (arrowheads). Two additional transected Purkinje axons, stained by anti-B-50/GAP-43 antibodies, are shown in G. Note the numerous thin sprouts (arrowheads) emerging from the initial portion of the corticofugal branch. Survival times: 30 d (A, D, G); 14 d (B); 60 d (C, E, F). Scale bars: A, B, E, G, 30 μm; C, D, 20 μm; F, 25 μm.
Injured Purkinje cells maintained a strong anti-B-50/GAP-43 immunolabeling up to the longest survival times examined, indicating that the expression of the transgene was not affected by the lesion and that B-50/GAP-43 expression remained high after axotomy. However, some subtle changes in the cellular distribution of the protein occurred: anti-B-50/GAP-43 immunostaining was particularly intense in axonal torpedoes, in the hypertrophied arciform fibers, and in the terminal axon segment heading toward the lesion site, whereas it became fainter in the remaining portion of the neurite running along the axial white matter of the injured folia. In addition, starting from a few weeks after lesion, a number of injured Purkinje cells displayed a clear dendritic labeling (Fig. 4B).
Axotomized transgenic Purkinje cells exhibited a profuse sprouting, which was first observed 24 hr after injury and occurred in the majority of the affected neurites from 1 month after the lesion onward (Figs. 4A–G,5A–G). Most frequently, several thin spikes arose from the torpedoes or the thickened arciform axons and radiated through the granular layer (Fig.4B–G). The majority of these sprouts were unbranched and bore rare varicosities or tiny terminal boutons (Fig.4C,D,F), but some longer and thicker processes with a few ramifications were also encountered (Fig.4E–G).
At the same time, remarkable remodeling also occurred at the distalmost portion of the corticofugal axon branch heading toward the lesion site (Fig. 5A–G). Twenty-four hours after lesion, thin processes, a few micrometers long, emanated from the terminal clubs of the severed axons (Fig. 5A). The number and length of these sprouts progressively increased during the following days, and they gradually developed an extensive network of tightly packed intermingled processes (Fig. 5B–E). This terminal network covered several hundred micrometers of the folial white matter abutting the injury site (Fig. 5B,D–F). Although many of these sprouts also elongated for short distances along the lesion track, none of them ever succeeded in growing across the injury site (Fig.5D,F). By contrast, numerous newly formed processes invaded the adjacent granular layer up to the most superficial portions (Fig. 5E,F), but they did not display morphological features reminiscent of terminal Purkinje axon branches in the recurrent infraganglionic plexus.
The sprouts usually bud from those axon segments in which anti-B-50/GAP-43 immunolabeling was most intense, and they were always strongly stained by this antibody, whereas they were less extensively labeled by the other Purkinje cell-specific antisera, suggesting that lower amounts of calbindin or L7 protein were present in the thinnest processes. However, the morphological features displayed by transgenic axons immunolabeled by the latter antisera were definitely different from those of their wild-type counterparts (compare Fig. 5,F and G), thus ruling out the possibility that similar morphological modifications could not be visualized in wild-type neurons because of their lack of anti-B-50/GAP-43 immunolabeling. In addition, the observation that no corticofugal B-50/GAP-43-immunoreactive axons exist in wild-type animals (Fig.2D), together with the morphological features observed in anti-calbindin or anti-L7 immunolabeled transgenic cerebella (Fig. 5F), clearly shows that the newly formed terminal plexus was made exclusively of sprouting Purkinje cell axons. Thus, B-50/GAP-43 overexpression remarkably modifies the response of Purkinje cells to axon injury by inducing a profuse sprouting, which occurs both along the neurites and at their severed ends.
Response of transgenic mouse Purkinje cells to growth-permissive grafts
Our observations on intact and injured transgenic Purkinje cells indicate that although B-50/GAP-43 overexpression does not alter the normal structure and connectivity of these neurons, it nonetheless induces a profuse sprouting in response to axotomy. Thus, to assess whether these growth phenomena correspond to an enhanced regenerative capacity of transgenic Purkinje cell axons, we grafted embryonic cerebellar or neocortical tissue or freshly dissociated sciatic nerve cells into the lesion site.
The different types of transplant survived into the injured cerebella and developed typical morphological features, as reported previously (Rossi et al., 1995, 1997; Bravin et al., 1997). In many instances the grafted tissues filled up the lesion cavity and directly abutted the axial white matter of severed cortical lobuli (Fig.6A–F). The injured Purkinje cells located in these cortical areas developed the same structural changes as their counterparts in lesioned cerebella that did not receive a graft: numerous thin sprouts emanated from torpedoes and arciform axons, whereas extensive terminal networks developed from the corticofugal branches close to the injury site. Nevertheless, the severed axons were never able to regenerate into the grafts. In most instances the emerging processes were arrested at the host–graft interface (Fig. 6A,E,F). Sometimes they appeared to penetrate into the transplant for short distances, but they never exceeded the normal intermingling occurring between graft and host elements at the injury site (Fig. 6D). In addition, even in these last cases, the outgrowing processes never displayed morphological features suggestive of terminal axon branches. Thus, although B-50/GAP-43 overexpressing Purkinje cells undergo growth phenomena in response to axon injury, they remain unable to regenerate their axons into the transplants.
Purkinje cell death after injury in wild-type and transgenic mice
Among central adult neurons, Purkinje cells are known to be strongly resistant to axotomy both in vivo (Dusart and Sotelo, 1994) and in vitro (Dusart et al., 1997). Indeed, the qualitative examination of our injured wild-type mice did not reveal any overt Purkinje cell loss in the sectioned folia. By contrast, when long-time injured transgenic cerebella labeled with any of the applied antibodies were considered, the number of Purkinje cells in the affected cortical regions appeared to be consistently reduced (Fig. 7A,B). Careful analysis of the same sections after Nissl or propidium iodide counterstaining clearly showed that this phenomenon was not caused by a loss of immunoreactivity but had to be attributed to neuron degeneration (Fig.7A).
To quantify this phenomenon, we calculated the number of Purkinje cell bodies per millimeter of Purkinje cell layer length in lobuli III and IV–V of the anterior lobe (Table 2, Fig. 7B,C). The same number of Purkinje cells/millimeter was counted in intact wild-type and transgenic animals (49.6 ± 0.9 and 51.3 ± 1.7 SD, respectively), indicating that cell death did not occur spontaneously in uninjured transgenic cerebella. By contrast, when the same estimations were made on injured cerebella 2 months after lesion, 38.4 (± 3 SD) Purkinje cells/millimeter were counted in wild-type animals, whereas in transgenic mice they were only 26.6 (± 4.1 SD). Both values were significantly different from their relevant control (Student’s t tests; p = 0.004 andp < 0.001 for wild-type and transgenic mice, respectively), and they were also statistically different from each other (Student’s t test; p = 0.005). When the number of Purkinje cells/millimeter was estimated in transected lobuli apposed to a transplant, the value calculated in wild-type animals (37.8 ± 2.1 SD) was not statistically different from that obtained from their counterparts that received no graft. Most surprisingly, however, a very similar result (37.1 ± 1.3 SD) was obtained from transgenic mouse lobuli facing a graft. In addition, this value turned out to be statistically higher than that obtained from injured transgenic cerebella that did not receive a graft (Student’st test; p = 0.006). Thus, although transgenic Purkinje cells seem to be more sensitive to axotomy than wild-type ones, they can be partially rescued by the transplanted tissues.
DISCUSSION
To assess whether the intrinsic regenerative potential of adult Purkinje cells can be increased by experimentally inducing the expression of growth-associated genes, we have examined transgenic mice in which B-50/GAP-43 overexpression is driven by the Purkinje cell-specific L7 promoter (Oberdick et al., 1990). Our results show (1) that B-50/GAP-43 overexpression in intact mice does not induce major abnormalities in Purkinje cell structure and connectivity; (2) that transgenic Purkinje cells respond to axotomy by profuse axonal sprouting; (3) that transgenic Purkinje cells nevertheless remain unable to regenerate their axons into growth-permissive transplants; and (4) finally, that although a moderate Purkinje cell loss occurs in injured wild-type cerebella, a higher number of transgenic neurons degenerates after axotomy, but they can be partially rescued by the different tissues grafted into the lesion site. Thus, although B-50/GAP-43 overexpression modifies the response of Purkinje cells to axotomy by eliciting growth phenomena and by affecting their resistance to injury, it is not sufficient to induce the regeneration of the injured axons.
B-50/GAP-43 overexpression does not modify the intact Purkinje cell phenotype
Although uninjured transgenic Purkinje cells displayed normal structural features, in our material subtle morphological changes of their axons in the deep cerebellar nuclei might have been missed because of the tightly packed terminal network. However, the normal morphology of cortical ganglionic plexuses and the typical distribution of Purkinje axons in their target nuclei support the conclusion that B-50/GAP-43 overexpression does not induce major modifications of uninjured Purkinje cell phenotype. Indeed, L7 expression in wild-type mice starts only after the completion of most Purkinje cell axonogenetic processes (Oberdick et al., 1988). In addition, B-50/GAP-43 expression in transgenic Purkinje cells has been observed only after the third postnatal week (J. Verhaagen and A. Holtmaat, unpublished observations), indicating that transgene expression cannot interfere significantly with Purkinje cell development.
Spontaneous sprouting or altered patterns of terminal axon distribution, however, have been observed in several adult brain regions from other transgenic mice overexpressing B-50/GAP-43 (Aigner et al., 1995; Holtmaat et al., 1995). This discrepancy between transgenic Purkinje cells and other neuron types overexpressing B-50/GAP-43 is likely attributable to population-specific differences in the constitutive propensity to axon growth and plasticity. Indeed, although the remarkable plastic properties of wild-type primary olfactory neurons, hippocampal mossy fibers, and motorneurons are well established, similar evidence regarding Purkinje cells is scanty (Baürle et al., 1992). Thus, intrinsic properties or environmental constraints that normally act to stabilize neuritic growth may prevent the development of B-50/GAP-43-induced morphogenic remodeling in intact transgenic Purkinje cells. Alternatively, the intact cerebellar environment might lack specific cues needed to elicit B-50/GAP-43-mediated growth processes. In this context, it is worth mentioning that the growth-promoting action of B-50/GAP-43 in transfected neuroblastoma cells is only evident in the presence of agents promoting neuronal differentiation (Morton and Buss, 1992). Furthermore, Holtmaat et al. (1995) noted that the spontaneous sprouting of B-50/GAP-43 overexpressing primary olfactory neurons was largely restricted to the olfactory glomeruli, their natural target territory. This also suggests that the neuronal microenvironment contributes significantly to the B-50/GAP-43-induced sprouting response.
Transgenic Purkinje cell axons sprout in response to axotomy but remain unable to regenerate into growth-permissive grafts
Our experiments were primarily aimed at assessing whether B-50/GAP-43 overexpression modifies the response to axotomy and increases the capacity of adult injured Purkinje axons to regenerate into growth-permissive grafts known to induce the regeneration of adult olivocerebellar fibers in vivo (Rossi et al., 1995, 1997;Bravin et al., 1997) and immature Purkinje cell neurites in vitro (Dusart et al., 1997).
Regenerative attempts reminiscent of the sprouting observed in our transgenic cerebella occur several months after the lesion at the severed tips of adult rat Purkinje cell axons abutting the lesion site (Dusart and Sotelo, 1994). In these animals, however, no sprouting from torpedoes or arciform axons has been reported. Furthermore, no such growth phenomena were ever present in our age-matched injured wild-type mice. Thus, although wild-type Purkinje cells may be able to produce some axonal remodeling at times long after axotomy, these phenomena are greatly amplified and accelerated by B-50/GAP-43 overexpression.
Axonal sprouts emanated from precise segments of transgenic Purkinje neurites—the torpedoes, the thickened arciform fibers, and the severed tips—that were also characterized by a particularly intense anti-B-50/GAP-43 immunolabeling. Ultrastructural examination of the intracellular distribution of B-50/GAP-43 in neurons (Burry et al., 1992; van Lookeren Campagne et al., 1992) or transfected non-neuronal cells (Verhaagen et al., 1994) consistently indicate that this protein is targeted and enriched in actively growing processes. Thus, it is likely that Purkinje axon sprouting was specifically triggered at sites where a critical concentration of B-50/GAP-43 was accumulating as a result of a likely impaired axoplasmic flow, and this might partially account for the absence of spontaneous outgrowth in the intact neurons. Alternatively, however, it is possible that accumulation of the protein was enhanced at specific sites as a result of ongoing sprouting.
The newly formed sprouts ranged from short spikes to longer ramified processes, but they never displayed the distinctive morphological features of terminal axon branches in contact with a target. Rather, they were similar to the filopodia-like processes budding from B-50/GAP-43-overexpressing non-neuronal cells (Zuber et al., 1989;Yankner et al., 1990; Widmer and Caroni, 1993; Verhaagen et al., 1994). This observation indicates that B-50/GAP-43-induced Purkinje cell sprouts are unable to establish new synaptic connections. Similarly, the outgrowing processes were never able to penetrate into growth-permissive transplants. This fact may be attributed to an insufficient level of B-50/GAP-43 expression or to the presence of nonpermissive glial scars at the graft–host interface. However, both possibilities are unlikely, because other cerebellar axons, characterized by a less intense B50/GAP-43 immunolabeling than transgenic Purkinje neurites, vigorously regenerate across the lesion site into the same transplants (Rossi et al., 1995, 1997; Bravin et al., 1997). Thus, it is more likely that the failure of transgenic Purkinje axon regeneration is caused by the lack of other intrinsic determinants.
Although B-50/GAP-43 upregulation is strictly associated with plastic and regenerative potential of adult central axons (Doster et al., 1991; Tetzlaff et al., 1991, 1994; Schaden et al., 1994), the precise role played by this protein in axon growth and even its strict requirement are still debated (Strittmatter et al., 1995; Benowitz and Routtenberg, 1997). Our results show that B-50/GAP-43 overexpression alone is not sufficient to obtain true axon regeneration, including neurite elongation, pathfinding, target recognition, and synapse formation. The accomplishment of this multistep process likely requires the coordinate expression and interaction of several intrinsic and extrinsic determinants. Although we do not know whether B-50/GAP-43 overexpression in Purkinje cells is able to drive the expression of other growth-associated genes, it is clear that it is not sufficient to enable transgenic neurons to interpret growth-promoting environmental cues or to connect with possible targets encountered by the outgrowing sprouts.
Transgenic Purkinje cells degenerate after axotomy, but they can be rescued by the transplanted tissues
An unexpected finding was the degeneration of numerous axotomized Purkinje cells in the transgenic cerebella. Previous studies have shown the strong resistance of these neurons to axon injury (Dusart and Sotelo, 1994; Dusart et al., 1997). In our wild-type animals, only 25% of Purkinje cells degenerate 2 months after injury, and the same number is lost in the cerebella that received a graft, suggesting that these cells die because of direct damage induced by the lesion procedure by as yet unknown mechanisms. In contrast, the loss of an additional 25% of injured Purkinje cells in transgenic cerebella clearly shows that these neurons are more sensitive to axotomy than their wild-type counterparts. Most interestingly, however, these neurons can be partially rescued if a graft is placed in the lesion site. Thus, although transgenic Purkinje cells are not able to regenerate their axon into the transplants, they are nonetheless sensitive to the trophic action exerted by the grafted cells.
The reason for this enhanced sensitivity of transgenic Purkinje cells to axotomy remains to be elucidated. However, it is tempting to speculate that cellular changes induced by B-50/GAP-43 overexpression, and especially growth phenomena, might be associated with Purkinje cell degeneration. Indeed, spontaneous neuronal death has been observed in other B-50/GAP-43-overexpressing transgenic mouse lines (Aigner et al., 1995). In addition, in several neuronal systems injury conditions leading to stronger B-50/GAP-43 expression and regenerative processes are also associated with a more severe neuronal degeneration (Richardson et al., 1982; Misantone et al., 1984; Villegas-Perez et al., 1988; Bray and Aguayo, 1989; Doster et al., 1991; Tetzlaff et al., 1991, 1994; Herdegen et al., 1997). This suggests that some relationship may link regenerative processes and degenerative phenomena in injured neurons. According to this view, B-50/GAP-43 overexpression by inducing growth processes in Purkinje cell axons might also trigger as yet unknown cellular modifications that eventually lead to cell death. This view is also supported by the trophic effect exerted by the grafts, which is reminiscent of the rescue of axotomized neurons by similar transplants observed in other systems (Villegas-Perez et al., 1988; Barron et al., 1989; Tetzlaff et al., 1994). Our results, however, indicate that retrograde trophic effects and growth-promoting actions may be dissociated phenomena (also see Chen et al., 1997).
In conclusion, although B-50/GAP-43 overexpression modifies the response of Purkinje cells to axotomy and potentiates their growth capabilities, it is not sufficient to enable the transgenic neurons to carry out a complete regeneration program. Thus, further essays aimed at obtaining axon regeneration by enhancing the intrinsic properties of injured neurons should be designed to control the coordinate expression of a larger set of growth-associated genes.
Footnotes
This work was supported by grants from Associazione Italiana Sclerosi Multipla, Ministero dell’Università e della Ricerca Scientifica e Tecnologica, Consiglio Nazionale delle Ricerche, and European Community Biotechnology Programme (ERBBIO4-CT96-0774). We thank Mrs. L. Milano for technical help and Miss G. Milano for secretarial assistance.
Correspondence should be addressed to Ferdinando Rossi, Department of Neuroscience, University of Turin, Corso Raffaello 30, I-10125 Turin, Italy.
REFERENCES
- 1.Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 2.Barron KD. Neuronal responses to axotomy: consequences and possibilities for rescue from permanent atrophy or cell death. In: Seil FJ, editor. Neural regeneration and transplantation. Alan Liss; New York: 1989. pp. 79–99. [Google Scholar]
- 3.Baürle J, Grover BG, Grüsser-Cornehls U. Plasticity of GABAergic terminals in Deiters nucleus of Weaver mutant and normal mice: a quantitative light microscopic study. Brain Res. 1992;591:305–318. doi: 10.1016/0006-8993(92)91712-n. [DOI] [PubMed] [Google Scholar]
- 4.Baürle J, Oestreicher AB, Gispen WH, Grüsser-Cornehls U. Lesion-specific pattern of immunocytochemical distribution of growth associated protein B-50 (GAP-43) in the cerebellum of weaver and PCD-mutant mice: lack of B-50 involvement in neuroplasticity of Purkinje cell terminals? J Neurosci Res. 1994;38:327–335. doi: 10.1002/jnr.490380311. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 6.Bravin M, Savio T, Strata P, Rossi F (1997) Olivocerebellar axon regeneration and target reinnervation following dissociated Schwann cell grafts in surgically injured cerebella of adult rats. Eur J Neurosci, in press. [DOI] [PubMed]
- 7.Bray GM, Aguayo AJ. Exploring the capacity of CNS neurons to survive injury, regrow axons, and form synapses in adult mammals. In: Seil FJ, editor. Neural regeneration and transplantation. Alan Liss; New York: 1989. pp. 67–78. [Google Scholar]
- 8.Brockes JP, Fields KL, Raff MC. Studies on cultured Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 9.Brook GA, Lawrence JM, Shah B, Raisman G. Extrusion transplantation of Schwann cells into the adult rat thalamus induces directional host axon growth. Exp Neurol. 1994;126:31–43. doi: 10.1006/exnr.1994.1040. [DOI] [PubMed] [Google Scholar]
- 10.Buffo A, Holtmaat AJGD, Savio T, Oestreicher AB, Gispen WH, Verhaagen J, Strata P, Rossi F. B-50/GAP-43 over-expression in transgenic mouse Purkinje cells is not sufficient to induce axon regeneration. Soc Neurosci Abstr. 1996;22:1018. [Google Scholar]
- 11.Burry RW, Lah JJ, Hayes M. GAP-43 distribution is correlated with development of growth cones and presynaptic terminals. J Neurocytol. 1992;21:413–425. doi: 10.1007/BF01191506. [DOI] [PubMed] [Google Scholar]
- 12.Campbell G, Anderson PN, Turmaine M, Lieberman AR. GAP-43 in the axons of mammalian CNS neurons regenerating into peripheral nerve grafts. Exp Brain Res. 1991;87:67–74. doi: 10.1007/BF00228507. [DOI] [PubMed] [Google Scholar]
- 13.Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen DF, Schneider GE, Martinou J-C, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 15.Chong MS, Woolf CJ, Turmaine M, Emson PC, Anderson PN. Intrinsic versus extrinsic factors in determining the regeneration of central processes of rat dorsal root ganglion neurons: the influence of a peripheral nerve graft. J Comp Neurol. 1996;370:97–104. doi: 10.1002/(SICI)1096-9861(19960617)370:1<97::AID-CNE9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Console-Bram LM, Fitzpatrick-McElligott SG, McElligott JG. Distribution of GAP-43 mRNA in the immature and adult cerebellum: a role for GAP-43 in cerebellar development and neuroplasticity. Dev Brain Res. 1996;95:97–106. doi: 10.1016/0165-3806(96)00079-x. [DOI] [PubMed] [Google Scholar]
- 17.De Camilli P, Miller PE, Levitt P, Walter U, Greengard P. Anatomy of cerebellar Purkinje cells in the rat determined by a specific immunohistochemical marker. Neuroscience. 1984;11:761–817. doi: 10.1016/0306-4522(84)90193-3. [DOI] [PubMed] [Google Scholar]
- 18.De Zeuuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 19.Doster KS, Lozano AM, Aguayo AJ, Willard MB. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991;6:635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- 20.Dusart I, Sotelo C. Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: degenerative changes and regenerative attempts of severed axons. J Comp Neurol. 1994;347:211–232. doi: 10.1002/cne.903470206. [DOI] [PubMed] [Google Scholar]
- 21.Dusart I, Airaksinen MS, Sotelo C. Purkinje cell survival and axonal regeneration are age dependent: an in vitro study. J Neurosci. 1997;17:3710–3726. doi: 10.1523/JNEUROSCI.17-10-03710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fawcett JW. Intrinsic neuronal determinants of regeneration. Trends Neurosci. 1992;15:5–8. doi: 10.1016/0166-2236(92)90338-9. [DOI] [PubMed] [Google Scholar]
- 23.Herdegen T, Skene JHP, Bähr M. The c-Jun transcription factor: bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–230. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 24.Hogan BLM, Costantini F, Lacy E. Manipulation of the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1986. [Google Scholar]
- 25.Holtmaat AJGD, Dijkhuizen, van der Lugt N, Berns ATM, Veerbeck JS, Margolis FL, Oberdick J, Oestreicher AB, Gispen WH, Verhaagen J. Directed expression of B-50 (GAP-43) to cerebellar Purkinje cells and mature olfactory neurons in transgenic mice. Soc Neurosci Abstr. 1994;20:661. [Google Scholar]
- 26.Holtmaat AJGD, Dijkhuizen PA, Oestreicher AB, Romijn HJ, Van der Lugt NMT, Berns A, Margolis FL, Gispen WH, Verhaagen J. Directed expression of the growth-associated protein B50/GAP-43 to olfactory neurons in transgenic mice results in changes in axon morphology and extraglomerular fiber growth. J Neurosci. 1995;15:7953–7965. doi: 10.1523/JNEUROSCI.15-12-07953.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hüll M, Bähr M. Regulation of immediate-early gene expression in retinal ganglion cells following axotomy and during regeneration through a peripheral nerve graft. J Neurobiol. 1994;25:92–105. doi: 10.1002/neu.480250109. [DOI] [PubMed] [Google Scholar]
- 28.Kruger L, Bendotti C, Rivolta R, Samanin R. Distribution of GAP-43 in the adult rat brain. J Comp Neurol. 1993;333:417–434. doi: 10.1002/cne.903330308. [DOI] [PubMed] [Google Scholar]
- 29.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meberg PI, Routtenberg A. Selective expression of protein F1/(GAP-43) mRNA in pyramidal but not granule cells of the hippocampus. Neuroscience. 1991;45:721–733. doi: 10.1016/0306-4522(91)90284-u. [DOI] [PubMed] [Google Scholar]
- 31.Mehta A, Reynold ML, Woolf CJ. Partial denervation of the medial gastrocnemius muscle results in growth-associated protein 43 immunoreactivity in sprouting axons and Schwann cells. Neuroscience. 1993;57:433–442. doi: 10.1016/0306-4522(93)90075-q. [DOI] [PubMed] [Google Scholar]
- 32.Misantone LJ, Gershenbaum M, Murray M. Viability of retinal ganglion cells after optic nerve crush in adult rats. J Neurocytol. 1984;13:449–465. doi: 10.1007/BF01148334. [DOI] [PubMed] [Google Scholar]
- 33.Morton JA, Buss TN. Accelerated differentiation in response to retinoic acid after retrovirally mediated gene transfer of GAP-43 into mouse neuroblastoma cells. Eur J Neurosci. 1992;4:910–916. doi: 10.1111/j.1460-9568.1992.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 34.Oberdick J, Levinthal F, Levinthal C. A Purkinje cell differentiation marker shows a partial DNA sequence homology to the cellular sis/PDGF2 gene. Neuron. 1988;1:367–376. doi: 10.1016/0896-6273(88)90186-9. [DOI] [PubMed] [Google Scholar]
- 35.Oberdick J, Smeyne RJ, Mann JR, Zackson S, Morgan JI. A promoter that drives transgene expression in cerebellar Purkinje cells and retinal bipolar neurons. Science. 1990;248:223–226. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- 36.Oestreicher AB, Gispen WH. Comparison of the immunocytochemical distribution of the phosphoprotein B-50 in the cerebellum and hippocampus of immature and adult rat brain. Brain Res. 1986;375:267–279. doi: 10.1016/0006-8993(86)90747-x. [DOI] [PubMed] [Google Scholar]
- 37.Oestreicher AB, Van Dongen CJ, Zwies H, Gispen WH. Affinity purified anti-B50 protein antibody: interference with the function of the phosphoprotein B50 in synaptic plasma membranes. J Neurochem. 1983;41:331–340. doi: 10.1111/j.1471-4159.1983.tb04747.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramón y Cajal S (1928) Degeneration and regeneration of the nervous system. Reprint (De Felipe J, Jones EJ, eds; May R, translator). Oxford: Oxford UP, 1991.
- 39.Richardson PM, Issa VM, Shemie S. Regeneration and retrograde degeneration in the rat optic nerve. J Neurocytol. 1982;11:949–966. doi: 10.1007/BF01148310. [DOI] [PubMed] [Google Scholar]
- 40.Robinson GA. Immediate early gene expression in axotomized and regenerating retinal ganglion cells of the adult rat. Mol Brain Res. 1994;24:43–54. doi: 10.1016/0169-328x(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 41.Rossi F, Jankovsky A, Sotelo C. Differential regenerative response of Purkinje cell and inferior olivary axons confronted with embryonic grafts: environmental cues versus intrinsic neuronal determinants. J Comp Neurol. 1995;359:663–677. doi: 10.1002/cne.903590412. [DOI] [PubMed] [Google Scholar]
- 42.Rossi F, Bravin M, Buffo A, Fronte M, Savio T, Strata P. Intrinsic properties and environmental factors in the regeneration of adult cerebellar axons. In: de Zeeuw CI, Voogd J, Strata P, editors. The cerebellum: from structure to control. Elsevier; Amsterdam: 1997. pp. 301–314. [DOI] [PubMed] [Google Scholar]
- 43.Schaden H, Stuermer CAO, Bähr M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J Neurobiol. 1994;25:1570–1578. doi: 10.1002/neu.480251209. [DOI] [PubMed] [Google Scholar]
- 44.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 45.Skene JHP. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- 46.Skene JHP. Retrograde pathways controlling expression of a major growth cone component in the adult CNS. In: Letourneau PC, Kater SB, Macagno ER, editors. The nerve growth cone. Raven; New York: 1992. pp. 463–475. [Google Scholar]
- 47.Smeyne RJ, Chu T, Lewin A, Bian F, Cuisman S, Kunsch C, Lira SA, Oberdick J. Local control of granule cell generation by cerebellar Purkinje cells. Mol Cell Neurosci. 1995;6:1230–1251. doi: 10.1006/mcne.1995.1019. [DOI] [PubMed] [Google Scholar]
- 48.Smith DS, Skene JHP. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strittmatter SM, Fankhauser C, Huang PL, Mahimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- 50.Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression of cytoskeletal proteins and GAP-43. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tetzlaff W, Kobayashi NR, Giehl KMG, Tsui BJ, Cassar SL, Bedard AM. Response of rubrospinal and corticospinal neurons to injury and neurotrophins. In: Seil FJ, editor. Neural regeneration, Vol 103. Elsevier; Amsterdam: 1994. pp. 271–286. [DOI] [PubMed] [Google Scholar]
- 52.Ulenkate HJLM, Verhaagen J, Plantiga LC, Merccken M, Veldman H, Jennekens FGI, Gispen WH, Oestreicher AB. Upregulation of B50/GAP-43 in Schwann cells at denervated motor end plates and in motoneurones after rat facial nerve crush. Restor Neurol Neurosci. 1993;6:35–47. doi: 10.3233/RNN-1993-6104. [DOI] [PubMed] [Google Scholar]
- 53.van Lookeren Campagne M, Dotti CG, Jap Tjoen San ERA, Verkley AJ, Gispen WH, Oestreicher AB. B-50/GAP-43 localization in polarized hippocampal neurones in vitro: an ultrastructural quantitative study. Neuroscience. 1992;50:35–52. doi: 10.1016/0306-4522(92)90380-k. [DOI] [PubMed] [Google Scholar]
- 54.Vaudano E, Campbell G, Anderson PM, Davies AP, Woolhead C, Schreyer DJ, Lieberman AR. The effects of a lesion or a peripheral nerve graft on GAP-43 upregulation in the adult rat brain: an in situ hybridization and immunocytochemical study. J Neurosci. 1995;15:3594–3611. doi: 10.1523/JNEUROSCI.15-05-03594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall YS, Gispen WH, Margolis FL. Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP-43 and the olfactory marker protein. J Neurosci Res. 1990;26:31–44. doi: 10.1002/jnr.490260105. [DOI] [PubMed] [Google Scholar]
- 56.Verhaagen J, Zhang Y, Hamers FPT, Gispen WH. Elevated expression of B-50 (GAP-43)-mRNA in a subpopulation of olfactory bulb mitral cells following axotomy. J Neurosci Res. 1993;35:162–169. doi: 10.1002/jnr.490350206. [DOI] [PubMed] [Google Scholar]
- 57.Verhaagen J, Hermens WTJMC, Oestreicher AB, Gispen WH, Rabkin SD, Pfaff DW, Kaplitt MG. Expression of the growth-associated protein B-50/GAP-43 via a defective herpes-simplex virus vector results in profound morphological changes in non-neuronal cells. Mol Brain Res. 1994;26:26–36. doi: 10.1016/0169-328x(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 58.Verzè L, Buffo A, Rossi F, Oestreicher AB, Gispen WH, Strata P. Increase of B-50/GAP-43 immunoreactivity in uninjured muscle nerves of mdx mice. Neuroscience. 1996;70:807–815. doi: 10.1016/s0306-4522(96)83017-x. [DOI] [PubMed] [Google Scholar]
- 59.Villegas-Perez MP, Vidal-Sanz M, Bray GM, Aguayo AJ. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widmer F, Caroni P. Phosphorylation-site mutagenesis of the growth-associated protein GAP-43 modulates its effects on cell spreading and morphology. J Cell Biol. 1993;120:503–512. doi: 10.1083/jcb.120.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yankner BA, Benowitz LI, Villa-Komaroff L, Neve RL. Transfection of PC12 cells with GAP-43 gene: effects on neurite outgrowth and regeneration. Mol Brain Res. 1990;7:39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- 62.Zuber MX, Goodman DW, Karns LR, Fishman MC. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989;244:1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]


