Abstract
Exposure of rat hippocampal slices to low concentrations of the muscarinic agonist carbachol (CCh) has been shown to produce a slow onset long-term potentiation (LTP) of reactivity to afferent stimulation in CA1 neurons. Although this potentiation shares a number of properties with tetanic LTP, muscarinic LTP (LTPm) is independent of activation of the NMDA receptor. We now demonstrate that low levels of hydrogen peroxide (H2O2) cause hippocampal slices to lose the ability to express LTPm. This powerful effect of H2O2 is selective in that it does not affect the reactivity of hippocampal neurons to higher concentrations of CCh. In fact, H2O2 also blocks induction of a slow onset, non-NMDA-dependent tetanic LTP (NN-LTP). The functional relevance of this action of H2O2 is exemplified by the fact that the hippocampus of aged rats, which produces higher levels of endogenous H2O2 than that of young rats, lacks LTPm and expresses a markedly reduced NN-LTP. In aged rats, the lack of LTPm contrasts with an apparently normal muscarinic suppression of the EPSP slope induced by higher concentrations of CCh. When hippocampal slices from aged animals are treated with catalase, an enzyme that breaks down H2O2, LTPm is restored, and NN-LTP is enhanced. Thus, our study proposes a unique and novel age-dependent peroxide regulation of LTPm in the brain and provides a link between the cholinergic system, aging, and memory functions.
Keywords: hippocampus, carbachol, aging, LTP, peroxides, catalase
Long-term potentiation (LTP), a long-lasting increase in the efficacy of synaptic transmission (Bliss and Lømo, 1973; Bliss and Collingridge, 1993), can be induced in pyramidal cells of area CA1 in the hippocampal slice by a brief, high-frequency tetanic stimulation of the Schaffer collateral and commissural fibers. This manner of LTP induction is dependent on the activation of NMDA receptors. LTP can also be induced in a non-NMDA-dependent manner by exposure of the slice to compounds that transiently increase cellular excitability (Aniksztejn and Ben-Ari, 1991; Bortolotto and Collingridge, 1993) or by repeated high-frequency tetani delivered in the presence of an NMDA antagonist (Grover and Teyler, 1990). LTP has been proposed as a cellular model of neuronal plasticity and is therefore used to study processes underlying plastic changes associated with learning and memory (Doyere and Laroche, 1992;Bliss and Collingridge, 1993).
Forebrain cholinergic neurons have been associated with cognitive functions of the brain (Buresova et al., 1964; Bartus et al., 1982). Cholinergic deficits have been found in aged, demented patients, and cholinergic drugs have been used to modulate memory in young rats (Bartus et al., 1982; Rossor, 1982; Coyle et al., 1983; Lebrun et al., 1990; Molchan et al., 1992). The search for the physiological actions of ACh may be of major importance for understanding the role of ACh in cognitive functions (Bartus et al., 1982; Segal, 1982a,b; Madison et al., 1987; Markram and Segal, 1992). Thus far, approximately a dozen effects of ACh on central neurons have been reported, but none is intuitively related in any selective manner to LTP or to neuronal plasticity associated with cognitive functions of the brain.
We have discovered recently a gradually developing, long-lasting potentiation induced by exposure of a hippocampal slice to a low concentration (0.5 μm) of carbachol (CCh) (Auerbach and Segal, 1994). This muscarinic LTP (LTPm) is mediated by an M2 muscarinic receptor and is independent of the activation of an NMDA receptor (Auerbach and Segal, 1996).
Among several agents that block the late onset of LTP, hydrogen peroxide (H2O2) (Pellmar et al., 1991) is generated naturally in living cells, and has also been shown to interact with kinases and phosphatases involved in LTP. In the present study we replicated the results of Pellmar et al. (1991). In addition, we found that LTPm is totally lacking in slices prepared from aged rats and is blocked by low levels of exogenously H2O2 added to slices from young rats. This is a unique age dependency of LTPm induction, which is correlated with an increased rate of peroxide production in aged hippocampal slices. We thus establish a link between aging, cholinergic functions, and oxygen radicals in the brain.
MATERIALS AND METHODS
Hippocampal slices (350 μm) were prepared from adult male Wistar rats obtained from a local breeding colony, and recordings were performed as described previously (Auerbach and Segal, 1996). Young rats were 7–10 weeks old, and aged rats were 24–30 months old. Experiments were performed in a submerged slice chamber, heated to 30–32°C, and superfused at a rate of 2.5 ml/min with standard artificial CSF (ACSF), containing (in mm): 124 NaCl, 4 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 2 MgSO4, and 10 glucose, pH 7.4. The ACSF was saturated with a 95% O2/5% CO2 gas mixture (flow rate, 0.4 l/min). A bipolar stainless steel stimulating electrode was placed in stratum radiatum of CA1. Test stimulation was delivered every 30 sec, 50 μsec pulse duration, with intensity adjusted such that evoked responses were approximately half-maximal. The extracellular recording electrode, containing 2 m NaCl (2–4 MΩ), was placed in stratum radiatum of the CA1 region for recording of EPSPs. Drugs were applied via the superfusion medium beginning 10 min after the establishment of a stable baseline response. A drug-naive hippocampal slice was used in each experiment. Tetanic LTP was induced by delivering a 100 Hz, 1 sec train through the stimulation electrode at twice the test stimulus intensity. Non-NMDA LTP (NN-LTP) was induced by delivering three 1 sec trains of 200 Hz, separated by 3 min inter-train intervals in the presence of 25 μm NMDA receptor antagonist dl-2-amino-5-phosphonovaleric acid (2-APV).
Stock solutions were prepared in distilled water and stored at −20°C. All working solutions were diluted in ACSF immediately before use. All drugs were obtained from Research Biochemicals (Natick, MA) except 2-APV, H2O2, and catalase, which were obtained from Sigma. Signals were amplified with an Axoclamp-2A amplifier and digitized with a DMA interface (Axon Instruments, Foster City, CA). Data were collected, stored, and analyzed on an IBM-compatible computer (Asyst 3.1; Asyst Software Tech., Inc., Rochester, NY). Data were pooled and normalized with respect to the steady baseline values (baseline = 1.00) and expressed as the mean ± SEM.
Endogenous hydrogen peroxide levels were determined using a variation of the method described by Cathcart et al. (1983). Briefly, 2,7-dichlorofluorescein (DCF) was freshly prepared and incubated with slices at a final concentration of 2–5 μm. Varying concentrations of H2O2 were added to blanks to construct a standard curve. The DCF and slices or known concentrations of H2O2 were incubated for 30–60 min at 37°C. The medium was then removed, and its fluorescence was measured with a standard fluorescein filter set. Calibration curves were constructed for measurements in the presence and absence of slices and both for young and aged slices. A linear relation between H2O2 concentration and fluorescence was established.
RESULTS
CCh (0.5 μm), perfused for a standard duration of 20 min at 30–32°C, produced a slow onset, long-lasting potentiation of the slope of the population EPSP, recorded in stratum radiatum of the hippocampal area CA1. The EPSP slope increased to ∼60% above baseline seen here (Fig.1A) and previously (Auerbach and Segal, 1994, 1996).
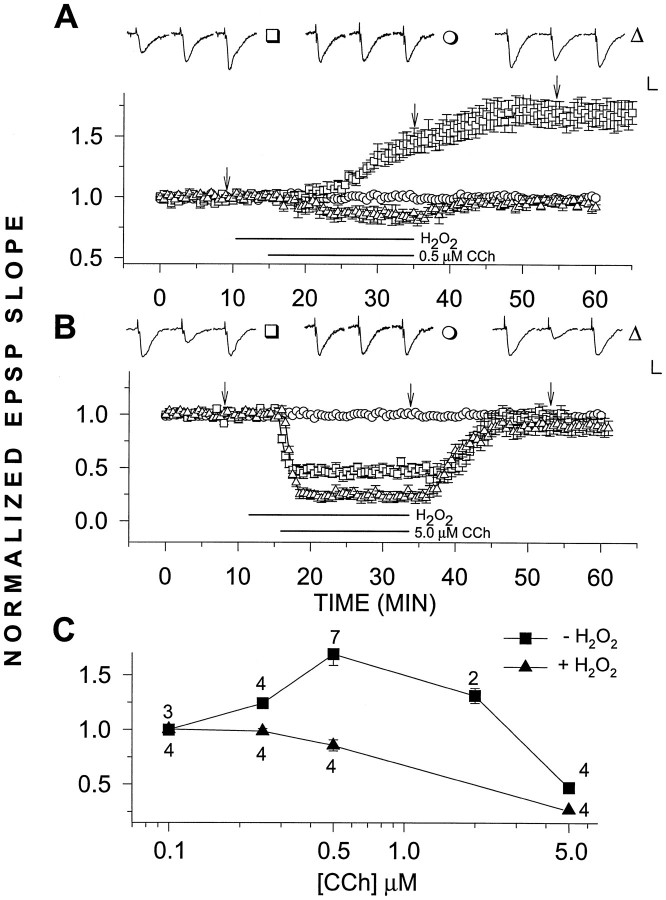
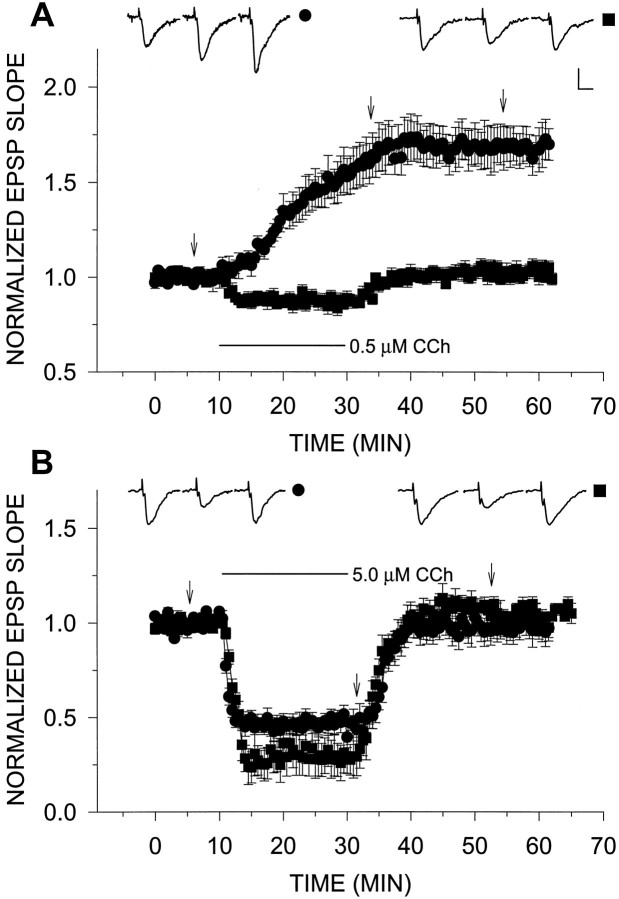
Fig. 1.
A, H2O2selectively blocks LTPm. H2O2 (29 μm) applied for 25 min had no observable effect (n = 4) (○). However, when applied 5 min before and together with CCh for an additional 20 min, H2O2 blocked the induction of LTPm(n = 4) (▵). This can be compared with the control case in which 0.5 μm CCh alone was added to the slices for 20 min, resulting in a potentiation of 69.3 ± 10.2% above baseline (n = 5) (□). B,H2O2 did not block the depression of the EPSP slope induced by 5.0 μm CCh. As above, H2O2 alone had no observable effect (n = 4) (○). CCh (5.0 μm) alone reduced the EPSP slope values to 47.4 ± 3.3% of baseline (n = 4) (□), whereas addition of 29 μm H2O2 with 5.0 μmCCh increased the CCh-induced depression, reducing the EPSP slope to 27.2 ± 4.4% of baseline values (n = 4) (▵). C, Dose–response relations for increasing concentrations of CCh in H2O2 clearly shows that although LTPm was abolished, the depressing effects of CCh were present. In this and the following graphs, representative EPSPs are presented above the grouped data. Each EPSP triplet is from one treatment, as identified by the symbol to theright of the triplet. Each individual EPSP within the triplet was sampled at a time corresponding to thearrows or lower case letters in the graph. Calibration, 0.5 mV, 5 msec. Error bars represent SEM, when larger than the symbol. The bars under the graphs designate the duration of drug application.
We studied the actions of H2O2 on LTPm by applying 0.0001% (29 μm) H2O2 via the perfusion medium before and together with varying concentrations of CCh. Addition of H2O2 by itself for 25 min followed by washout with normal medium did not affect the EPSP slope in recorded slices (n = 4; Fig. 1). However, LTPm produced by 0.5 μm CCh was completely eliminated by H2O2 (Fig. 1A). In fact, instead of potentiation, a slight depression of the EPSP slope was seen on addition of 0.5 μm CCh in the presence of H2O2. This effect was selective in that H2O2 alone did not affect the EPSP slope, nor did it block the depression of the EPSP slope induced by higher concentrations (5.0 μm) of CCh (Fig.1B). Actually, this depression was slightly enhanced when 5.0 μm CCh was added in the presence of H2O2. In addition, H2O2together with 0.1 μm CCh had no effect on the EPSP slope, indicating that H2O2 did not act simply to sensitize the slice to CCh and change its dose–response characteristics (Fig. 1C).
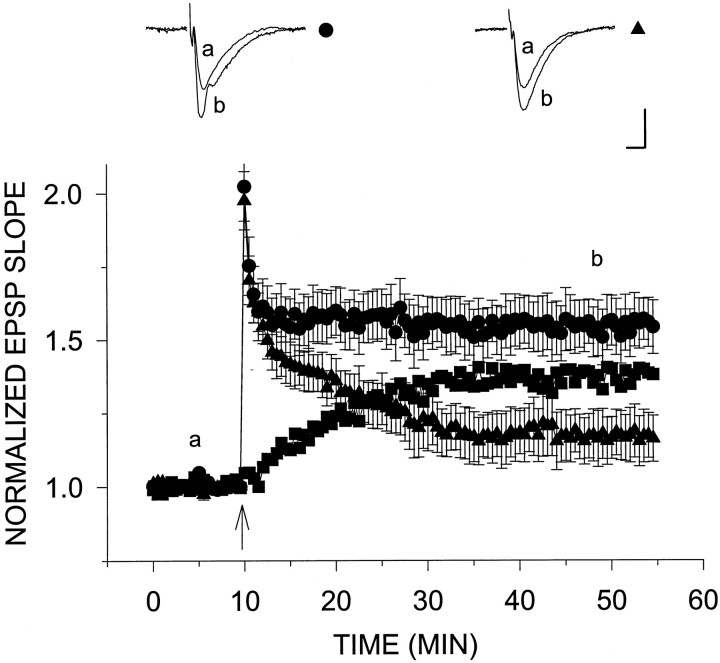
Previously, H2O2 has been shown to reduce the late phase of tetanic LTP (Pellmar et al., 1991). Here, we confirm this result using 29 μm H2O2, a concentration lower than that previously used by Pellmar et al. (1991). H2O2 (29 μm) was applied via the perfusion medium beginning 10 min before tetanus and continued until the end of the experiment. This treatment caused a significant reduction in the amount of potentiation induced by a 100 Hz, 1 sec tetanus, compared with the control condition in the absence of H2O2 (Fig. 2). H2O2 had no effect on baseline responses or on levels of post-tetanic potentiation reached immediately after tetanus. Potentiation in the slices exposed to H2O2decayed faster after tetanus and reached stable potentiation plateau levels 17.4 ± 8.5% (n = 8) above baseline, which was well below those of the control slices (54.5 ± 8.9%;n = 12). When the potentiation in the presence of H2O2 was subtracted from the control group, it appeared that H2O2 blocked a slow onset LTP.
Fig. 2.
H2O2 reduces the slow component of tetanic LTP. A single tetanus was applied at twice the test intensity 10 min after establishment of stable baseline (arrowhead) to slices from young rats in the presence (▴) and absence (•) of H2O2. H2O2 had no effect on the amount of post-tetanic potentiation immediately after the tetanus. However, H2O2 caused an increased decay in this potentiation, and in its presence the EPSP slope leveled off at 17.4 ± 8.5% above baseline (n = 8) (▴) compared with levels 54.5 ± 8.9% above baseline (n = 12) (•) without H2O2. Subtracting tetanic LTP in the presence of H2O2 from that in its absence yields the middle curve in the graph (▪). Calibration, 0.5 mV, 5 msec.
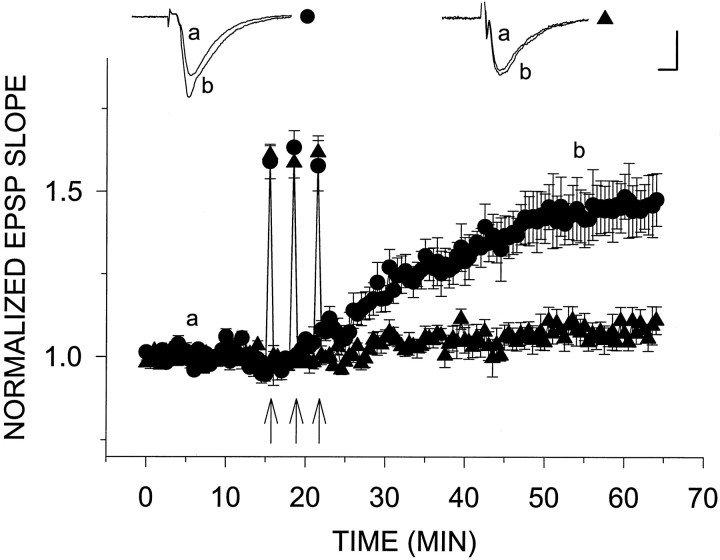
A non-NMDA receptor-mediated LTP can be induced in area CA1 of the hippocampus in the presence of the NMDA antagonist 2-APV by application of repeated strong tetani (200 Hz) to the Schaffer collaterals (Grover and Teyler, 1990; Çavus and Teyler, 1996) (Fig.3). This type of LTP has been attributed to Ca2+ influx through voltage-gated calcium channels. It is different from NMDA-mediated LTP in that it develops slowly over a period of 20–30 min after tetanus. We induced NN-LTP in the presence of 25 μm 2-APV by applying three successive tetani (at a frequency of 200 Hz) 3 min apart. Under these conditions, a slow onset potentiation of 43 ± 7% above baseline was recorded (n = 4). Identical to its effects on LTPm, 29 μmH2O2 added to the perfusion medium starting 10 min before the first of three tetani virtually eliminated NN-LTP (EPSP slope potentiation 6.2 ± 3.4% above baseline; n= 4; Fig. 3). These results indicate that LTPm and slow onset NN-LTP share a common mechanism, sensitive to a low concentration of H2O2.
Fig. 3.
H2O2 blocks NN-LTP. On induction of NN-LTP by three consecutive 200 Hz, 1 sec tetani (arrows), the EPSP slope reached a level of 43.3 ± 7.3% above baseline (n = 4) (•). The same induction protocol for NN-LTP in the presence of 29 μmH2O2 caused only a very slight increase in the EPSP slope (6.2 ± 3.4%; n = 4) (▴). Theinsets above the plots are representative EPSPs sampled at the times indicated by letters(a) and (b) in the graph. Calibration, 0.5 mV, 5 msec.
H2O2 is normally produced in cells from superoxides (*O2−), and its concentration is controlled through a balance between production by CuZn-superoxide dismutase and breakdown by peroxidases (De Haan et al., 1995). An imbalance in the synthetic machinery of H2O2 leads to an increase in ambient H2O2 in the aged brains (De Haan et al., 1995). We next searched for a change in H2O2 between slices from young and aged brains. A marked increase in the rate of H2O2 production in slices from aged rats was found. At two different exposure times after incubation of slices with a fluorescent dye sensitive to H2O2, the average rate of H2O2 production was 17.5 ± 0.80 nmol/hr per slice in aged slices (n = 8) compared with 3.53 ± 0.60 nmol/hr per slice in the young slices (n = 8).
We compared electrophysiological properties of slices taken from young and aged rats. The baseline slopes of the EPSPs were somewhat slower in the aged rats compared with the young ones [0.475 ± 0.036 V/sec (n = 10) versus 0.611 ± 0.097 V/s (n = 9) in the aged versus young slices, respectively;p >0.05, two-way t test]. We consistently used a stimulation intensity that produced half-maximal responses in the control conditions and that allowed the slices of both groups to undergo potentiation to about the same level (seen in the responses to tetanic stimulation; see Fig. 6).
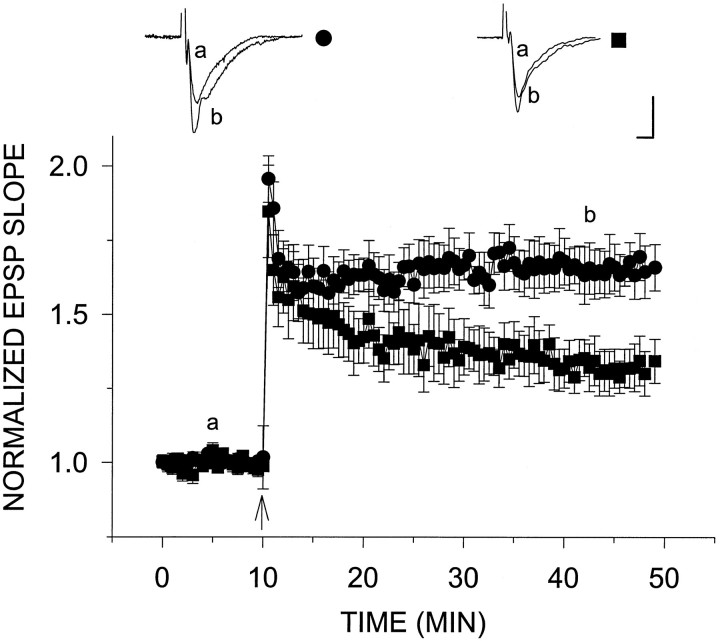
Fig. 6.
Impairment of tetanic LTP in slices from aged animals. A single tetanus was applied at twice the test intensity 10 min after establishment of stable baseline (arrowhead) to slices from aged (▪) versus young (•) rats. The amount of post-tetanic potentiation in both groups of slices was similar. Immediately after the tetanus, EPSP slopes from aged slices reached levels 84.7 ± 15.6% (n = 6) (▪) above baseline compared with levels 95.6 ± 7.8% above baseline in young slices (n = 7) (•). However, 30 min after tetanus, plateau potentiation levels reached in aged slices (34.3 ± 7.4%) were substantially less than those in young slices (65.9 ± 7.8%). Calibration, 0.5 mV, 5 msec.
We then examined whether LTPm can be induced in aged rat hippocampal slices shown previously to have enhanced production of H2O2 relative to young controls. CCh (0.5 μm)-induced LTPm was completely absent in four of seven slices prepared from aged rats; in one of seven slices CCh produced a small, lasting depression of the EPSP slope (to 89.7% of baseline levels), and in two of seven slices a slight potentiation (12.7 ± 2.8% above baseline) was seen. On average, 0.5 μm CCh caused an initial depression followed by a recovery to baseline levels in aged slices (Fig.4A). This loss of LTPm was selective in that no age-dependent reduction in the depression of the EPSP slope caused by 5.0 μm CCh was seen (Fig. 4B). The disappearance of LTPmcannot reflect the moderate reduction in cholinergic receptor density known to occur with aging (Bartus et al. 1982); however, it may result from an age-related change in second messengers activated by CCh to produce LTPm (Auerbach and Segal, 1994).
Fig. 4.
Slices from aged animals lack LTPm but express cholinergic depression of EPSP slope. A,Addition of 0.5 μm CCh to slices from aged rats resulted in a slight initial depression of the EPSP slope, which recovered to baseline levels once the drug was removed from the medium (n = 7) (▪). This was compared with LTPm in young slices shown here in which 0.5 μm CCh potentiated the EPSP slope to 69.3 ± 10.2% above baseline (n = 5) (•). B, CCh (5.0 μm) reduced the EPSP slope in aged slices to 29.4 ± 9.3% of baseline values (n = 4) (▪) compared with a reduction of 47.4 ± 3.3% in young slices (n = 4) (•). The insets above the plots are representative EPSPs from single experiments sampled at times indicated by the arrows. Calibration, 0.5 mV, 5 msec.
Parenthetically, the depression caused by high CCh (5.0 μm) in aged slices (to 29.4 ± 9.3% of baseline) (Fig. 4B) was similar to the depression caused by high CCh in normal slices in presence of H2O2(27.2 ± 4.4%; Fig. 1B). This is contrasted with a depression of only 47.4 ± 3.3% caused by the high CCh (5.0 μm) in young slices in the absence of H2O2 (Fig. 1B). These results suggest that both the enhanced CCh (5.0 μm)-induced depression and the absence of LTPm may reflect the higher endogenous levels of H2O2 in aged slices compared with young slices.
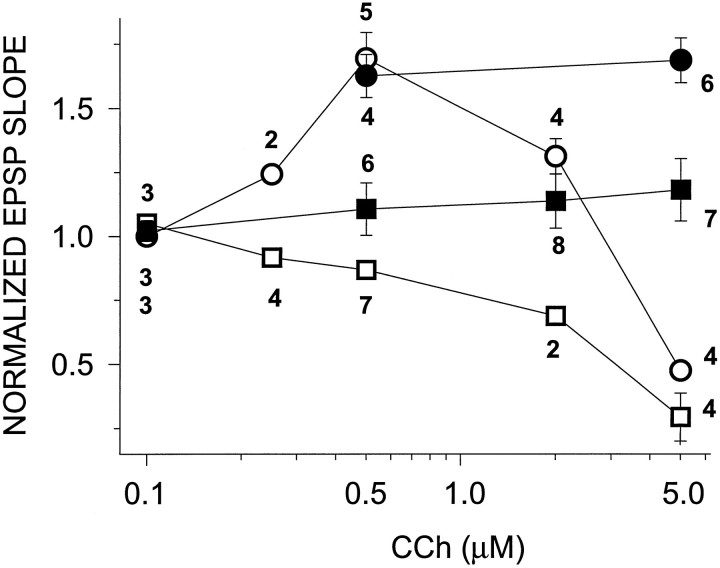
The dose–response profile for CCh effects in slices from aged versus young animals shows that LTPm was completely lacking in aged slices and that this was not simply a result of a shift in the dose–response curve (Fig. 5). Furthermore, we have shown previously that LTPm can be induced in young slices by a high concentration of CCh (5.0 μm) if the M3 muscarinic receptor, which mediates the muscarinic depression of the EPSP slope, and GABAergic inhibition are blocked (Auerbach and Segal, 1996). Here we show that in young slices, 5.0 μm CCh applied in the presence of 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) (an M3 antagonist) and picrotoxin (a GABA antagonist) induced potentiation of 68.6 ± 8.7% above baseline (n = 6). This level of potentiation is indistinguishable from that induced by 0.5 μm CCh alone in slices from young rats (69.3 ± 10.2%; n = 5; Fig. 5). However, in slices from aged animals, 5.0 μm CCh in the presence of 4-DAMP and picrotoxin potentiated the EPSP slope to only 18.1 ± 12.2% above baseline (n = 7). This is significantly less than the potentiation seen in young slices under the same conditions, indicating that the lack of LTPm in aged slices is not attributed to enhanced GABAergic inhibition. The CCh dose–response curve of CCh in aged slices is similar to that of young slices in the presence of H2O2 (Fig. 1C).
Fig. 5.
Dose–response curve of effects of CCh on slices from young (○, •) and old (□, ▪) rats. CCh had a biphasic concentration effect on young slices, i.e., potentiation at low concentrations and depression at high concentrations. Old slices showed only the depression at high CCh concentrations (empty symbols). The potentiating effects of CCh were completely lacking at all concentrations tested. Slices were also tested in the presence of 4-DAMP and picrotoxin (filled symbols). Whereas this treatment revealed LTPminduction at high CCh concentration (5.0 μm) in young slices (68.6 ± 8.7%) (•), only a slight potentiation was seen in aged slices (18.1 ± 12.2%) (▪). The numbersunderneath the symbols in the graph correspond to the number of slices tested for each condition.
Tetanic LTP induced by a single 100 Hz, 1 sec tetanus was compared between the two age groups. Immediately after the tetanus, levels of post-tetanic potentiation reached by both the young and aged groups were virtually the same. However, there was a difference in the amount of LTP induced in slices prepared from aged versus young rats (Fig.6). The EPSP slope measured in young slices reached a steady plateau level of 65.9 ± 7.8% above baseline (n = 7) 30 min after tetanus. This is compared with the EPSP slope in old slices, which decayed continuously after tetanus and, in fact, did not reach plateau but reached 34.3 ± 7.4% above baseline (n = 6) 30 min after LTP induction.
The possibility that the difference in late LTP between the young and the aged slices is attributable to NN-LTP was examined. Slices from aged animals had an apparently lower level of expression of NN-LTP, evoked by three trains of tetanic stimulation in the presence of 2-APV, than young slices. Aged slices reached a stable potentiated plateau of 32 ± 8% above baseline (n = 8), whereas young slices reached potentiated levels of 43 ± 7% (n= 4; p > 0.05, nonsignificant difference; data not shown).
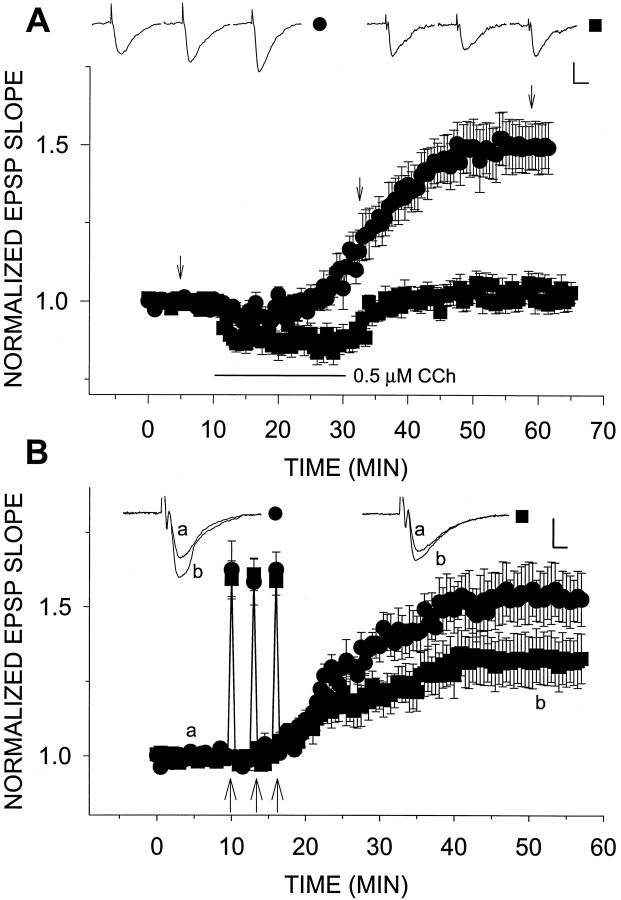
In aged rats the decrease in tetanic and NN-LTP and the complete absence of LTPm can be attributed to cumulative damage stemming from chronic exposure to H2O2, to some other age-related degenerative process, or to enhanced ambient [H2O2]. In the latter case, one should be able to counteract the accumulation of H2O2 by the administration of exogenous peroxidase. Superfusion with 100 U/ml catalase for 25 min had no effect on the EPSP slope recorded in slices from aged (n = 3; data not shown) and young (n = 3; see Fig. 8) animals. In addition, in all slices tested, treatment with 100 U/ml catalase for >1 hr and up to 6 hr did not affect their basal reactivity to afferent stimulation. However, on addition of 0.5 μm CCh to catalase-treated, aged slices, LTPm was restored (potentiation of 48.6 ± 8.0%;n = 8) (Fig.7A). In addition, treatment of aged slices with catalase was sufficient to increase NN-LTP (Fig.7B). Catalase elevated NN-LTP in aged slices from 32.2 ± 8.4% (n = 8) to 58.9 ± 8.9% (n = 6). When these steady-state potentiation plateau levels were compared across experiments and between groups, this difference was found to be significant (p <0.05,t test). However, catalase failed to increase levels of 100 Hz, 1 sec tetanic LTP (n = 4; data not shown).
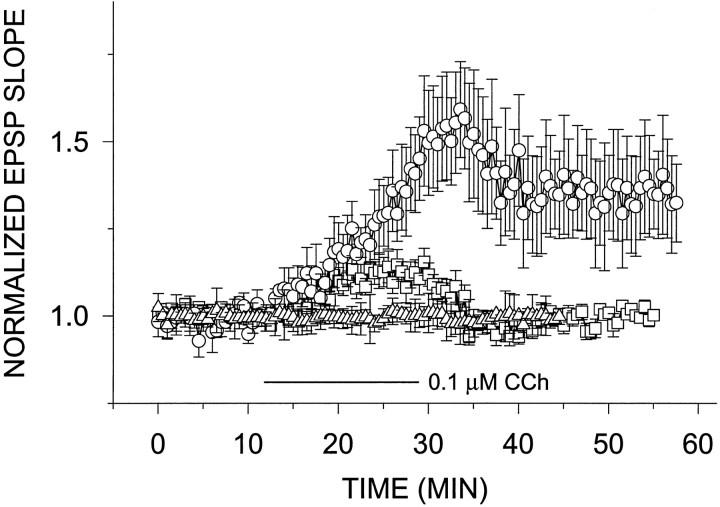
Fig. 8.
Catalase lowers the threshold for LTPminduction in slices from young rats. Slices treated with 25 U/ml catalase showed a slight potentiation (12.1 ± 3.9% above baseline) of the EPSP slope after exposure to 0.1 μm CCh (n = 6) (□). Slices treated with 100 U/ml showed a stable, long-lasting potentiation 35.3 ± 14.3% above baseline (n = 4) (○). Catalase (100 U/ml) alone added to young slices in the superfusion 10 min after the establishment of a steady baseline had no effect on evoked response (n= 3) (▵).
Fig. 7.
Catalase restores LTP in aged slices.A, LTPm is returned to aged slices by treatment with catalase. Slices were incubated in 100 U/ml catalase 1 hr before and continuously throughout the recording session. Under these conditions, 0.5 μm CCh induced LTPm to a level 48.6 ± 8.0% above baseline (n = 8) (•). This was compared with the lack of effect of 0.5 μm CCh in aged slices in the absence of catalase (n = 7) (▪). B, NN-LTP was elevated by catalase-treated aged slices. The treatment was the same as in A. The potentiation in aged slices enabled by catalase increased from 32.2 ± 8.4% (n = 8) (▪) to 58.9 ± 8.9% (n = 6) (•).
To test whether endogenously produced H2O2 can also affect LTPm in the young slices, we superfused slices from young rats with 25 U/ml (n = 4; data not shown) or 100 U/ml catalase (n = 3; Fig.8) for 25 min and found no effect on the EPSP slope. Slices were also incubated in either catalase concentration for at least 1 hr in the incubation chamber. Slices were then transferred to the recording chamber and perfused with catalase-containing ACSF. After 10 min of baseline recording, 0.1 μm CCh was added to the perfusion medium for 20 min. Under these conditions, the slices treated with 25 U/ml catalase showed a slight reversible potentiation of the EPSP slope, reaching 12.1 ± 3.9% above baseline after exposure to 0.1 μm CCh (n = 6). Slices treated with 100 U/ml showed a stable, long-lasting potentiation, 35.3 ± 14.3% above baseline (n = 4; Fig. 8). Slices treated with either concentration of catalase for >1 hr and up to 6 hr showed no effect on their basal reactivity to afferent stimulation. By itself, 0.1 μm CCh did not produce LTPm (Fig.1C).
DISCUSSION
The present studies demonstrate that low concentrations of H2O2 can decrease levels of tetanic and NN-LTP, as well as completely block the formation of LTPm resulting from exposure to CCh. H2O2 is formed in normal brain tissue, and its ambient concentration in neurons is kept low primarily by a fast breakdown by glutathione peroxidase (in neurons) or catalase (in many other cell types). H2O2 can, however, reach high levels in brains of aged animals, owing to a change in the balance between its production and breakdown. The production of H2O2 is catalyzed by the enzyme CuZn-superoxide dismutase, which is a ubiquitous enzyme. In fact, levels of H2O2 need not be very high to have an effect. We observe a total suppression of LTPm with added H2O2 in the micromolar concentration range, which is 20 times lower than doses used by others to observe effects on LTP in the hippocampus (Pellmar et al., 1991). These micromolar concentrations can indeed be reached in the aged brain, as shown previously (Sohal et al., 1994) and as we have also shown here. It is thus possible that overproduction of H2O2may underlie the inability of aged brains to express LTPm. If this is the case, then an acute enhancement of the breakdown of H2O2 with catalase should reverse the loss of LTPm in aged slices, and indeed it does. Although catalase is not likely to cross the cell membrane, H2O2flows freely into and out of the cell, and its breakdown by catalase should certainly shift the equilibrium to reduce [H2O2] inside the cell.
The inability of slices from aged animals to produce LTPmcorrelates with deficits in the maintenance of tetanic LTP, as shown here and previously (Barnes, 1994), and deficits in spatial memory seen in aged rats (Buresova et al., 1964; Bartus et al., 1982). LTPm is produced within a narrow dose range of CCh and is likely to be attributed to a change in postsynaptic reactivity to AMPA (Auerbach and Segal, 1996). Also, the inhibitory action of CCh on evoked synaptic responses and other cholinergic effects (Segal, 1982b) are still present in aged brains. These two findings together predict that an attempt to restore muscarinic functions by enhancing the presence of acetylcholine in the synapse (e.g., by blocking ACh esterase) will not be an effective treatment for age-related memory dysfunctions. Instead, a more promising strategy may involve enhancing the ability of the postsynaptic cell of the aged brain to alter oxidative metabolism. The present results also predict that the oxidative damage seen in aged brains may not be irreversible, and readjustment of the oxidative balance may restore cholinergic and perhaps other functions.
The assumed ability of endogenous H2O2 to block the expression of LTP in aged rats is not the only case in which H2O2 interferes with neuronal plasticity. We have used recently transgenic mice, made to overexpress the enzyme CuZn-superoxide dismutase (CuZn-SOD), which results in overproduction of H2O2. These mice are also deficient in learning abilities and in expression of sustained LTP. As in the present study, LTP in the CuZn-SOD mice is enhanced by the addition of catalase, which downregulates the level of H2O2(Gahtan et al., 1997). In fact, catalase can also reduce the threshold for LTPm in our young slices, indicating that continuous production of H2O2 may regulate LTPm also in the young slice.
One major unresolved issue is what the cellular mechanisms affected by the low concentration of H2O2 are. Peroxides affect an array of cellular functions, with a major target being protein phosphatases. At higher concentrations (in the submillimolar range), H2O2 blocks the activity of tyrosine phosphatases, thus enhancing activity of tyrosine kinases (Nakamura et al., 1993; Whisler et al., 1995). These kinases and phosphatases play important roles in regulating K+ and other ion selective channels (Levitan, 1994; Holmes et al., 1996). The lack of effect of H2O2 on the shape or size of the EPSPs recorded in the present studies, as well as the lack of obvious hyperexcitability in the slices, indicate that potassium channels are not the prime target of either H2O2 or CCh in our case. We have evidence to indicate that a serine and threonine kinase (Auerbach and Segal, 1994) is critically involved in the generation of LTPm. However, if H2O2 blocks phosphatases, it should enhance rather than suppress LTPm. At any rate, the reported effects of H2O2 on tyrosine phophatase are exerted at a higher concentration than that needed to block LTPm, and so it is likely that the effect seen here is not mediated by an interaction with protein phosphatases. Another possible site of action is protein kinase C (PKC), which is reported to be activated by H2O2 (Whisler et al. 1995). Here again, it is not clear what a persistent calcium-independent activation of PKC will do to kinase-dependent processes related to the muscarinic receptor. Another possible site of action of H2O2 is perhaps more related to the muscarinic receptor; it has been reported that H2O2 blocks cholinergic stimulation of AP-1 transcription factor (Li et al., 1996), an action that seems to involve phosphoinositide hydrolysis. The muscarinic activation of AP-1 could have been an attractive hypothesis with regard to the site of action of CCh for the expression of LTPm, except that it seems to involve M1 rather than M2 receptor, which is the one believed to underlie LTPm(Auerbach and Segal, 1996). Thus, the site of action of H2O2 for its effect on LTPm is yet unknown.
One clue to the possible site of action of H2O2 is the location of the muscarinic M2 receptor assumed to be activated to produce LTPm. Although a high concentration of M2 receptors is found on somata of stratum oriens and alveus interneurons, an electron microscopic analysis reveals many M2 immunoreactive dendritic profiles, indicating that these receptors may actually reside on the pyramidal neurons that are directly involved in the production of LTPm (Rouse et al. 1997). This confirms earlier observations suggesting that other M2 responses are present in hippocampal pyramidal neurons (Madison et al. 1987). Thus, the location of the M2 receptor does not provide a clue as to the molecular mechanism associated with the effect of H2O2 on LTPm.
The similarities between NN-LTP and LTPm in their independence of the activation of the NMDA receptor and the slow time course indicate that they share mechanisms of initiation with the voltage-dependent calcium current (VDCC)-LTP described elsewhere (Çavus and Teyler, 1996). In that study, it is suggested that VDCC-LTP is mediated by a tyrosine kinase, whereas the NMDA-LTP is mediated by a serine and threonine kinase. We were able to verify their results, in that genistein, a tyrosine kinase inhibitor, blocks NN-LTP as well as LTPm (J. M. Auerbach and M. Segal, unpublished observations). The effect of H2O2on the slow component of the tetanic LTP, as well as the total suppression of NN-LTP and LTPm, indicates that H2O2 may indeed act via a tyrosine kinase. On the other hand, LTPm is blocked by H7, a general serine and threonine kinase inhibitor (Auerbach and Segal, 1994), so the correlation between the two kinase inhibitors and the LTP types involved may not be straightforward.
One issue, which can be useful in zooming in on the site of action of H2O2, is the degree of its selectivity; does H2O2 affect the muscarinic receptor, a second messenger associated with this receptor, or some downstream process shared by a number of stimuli that evoke LTP? Clearly the inhibitory presynaptic muscarinic action is not reduced by H2O2. On the other hand, a slow onset LTP evoked by repeated tetanic stimulations is also blocked by H2O2. This indicates that H2O2 affects a common final path shared by a number of plasticity-producing stimuli. Whatever the exact mechanism, these studies provide a direct link between the cholinergic system, aging, neuronal plasticity, and oxidative metabolism in the brain.
Footnotes
We thank Drs. Y. Groner and Y. Zick for critical discussions.
Correspondence should be addressed to Menahem Segal, Department of Neurobiology, The Weizmann Institute, Rehovot 76100, Israel.
Dr. Auerbach’s present address: Laboratory of Molecular Biology, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20897-4092.
REFERENCES
- 1.Aniksztejn L, Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991;349:67–69. doi: 10.1038/349067a0. [DOI] [PubMed] [Google Scholar]
- 2.Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol. 1994;72:2034–2040. doi: 10.1152/jn.1994.72.4.2034. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach JM, Segal M. Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Physiol (Lond) 1996;492:479–493. doi: 10.1113/jphysiol.1996.sp021323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 5.Bartus RT, Reginald L, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 8.Bortolotto ZA, Collingridge GL. Characterisation of LTP induced by the activation of glutamate metabotropic receptors in area CA1 of the hippocampus. Neuropharmacology. 1993;32:1–9. doi: 10.1016/0028-3908(93)90123-k. [DOI] [PubMed] [Google Scholar]
- 9.Buresova O, Bures J, Bohdanecky Z, Weiss T. The effect of atropine on learning, extinction, retention and retrieval in rats. Psychopharmacology. 1964;5:255–263. doi: 10.1007/BF02341258. [DOI] [PubMed] [Google Scholar]
- 10.Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 11.Çavus I, Teyler TJ. Two forms of long-term potentiation in area CA1 activate different signal transduction cascades. J Neurophysiol. 1996;76:3038–3047. doi: 10.1152/jn.1996.76.5.3038. [DOI] [PubMed] [Google Scholar]
- 12.Coyle JT, Price DL, Delong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 13.De Haan JB, Crstiano F, Iannello RC, Kola I. Cu/Zn-superoxide dismutase and glutathione peroxidase during aging. Biochem Mol Biol Int. 1995;35:1281–1297. [PubMed] [Google Scholar]
- 14.Doyere V, Laroche S. Linear relationship between the maintenance of hippocampal long-term potentiation and retention of an associative memory. Hippocampus. 1992;2:39–48. doi: 10.1002/hipo.450020106. [DOI] [PubMed] [Google Scholar]
- 15.Gahtan E, Auerbach JM, Groner Y, Segal M (1997) Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci, in press. [DOI] [PubMed]
- 16.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 17.Holmes TC, Fadool DA, Levitan IB. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci. 1996;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebrun C, Durkin TP, Marighetto A, Jaffard R. A comparison of the working memory performances of young and aged mice combined with parallel measures of testing and drug-induced activations of septo-hippocampal and noncortical cholinergic neurones. Neurobiol Aging. 1990;11:515–521. doi: 10.1016/0197-4580(90)90112-d. [DOI] [PubMed] [Google Scholar]
- 19.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Song L, Jope RS. Cholinergic stimulation of AP-1 and NFkB transcription factors is differentially sensitive to oxidative stress in SH-SY5Y neuroblastoma: relationship to phosphoinositide hydrolysis. J Neurosci. 1996;16:5914–5922. doi: 10.1523/JNEUROSCI.16-19-05914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markram H, Segal M. The inositol 1,4,5-triphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol (Lond) 1992;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molchan SE, Martinez RA, Hill JL, Weingartner HJ, Thompson K, Vitiello B, Sunderland T. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Rev. 1992;17:215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Hori T, Sato N, Sugie K, Kawakami T, Yodoi J. Redox regulation of a src family protein tyrosine kinase p56lck in T cells. Oncogene. 1993;8:3133–3139. [PubMed] [Google Scholar]
- 25.Pellmar TC, Hollinden GE, Sarvey JM. Free radicals accelerate the decay of long-term potentiation in field CA1 of guinea-pig hippocampus. Neuroscience. 1991;44:353–359. doi: 10.1016/0306-4522(91)90060-2. [DOI] [PubMed] [Google Scholar]
- 26.Rossor MN. Neurotransmitters and CNS disease: dementia. Lancet. 1982;ii:1200–1203. doi: 10.1016/s0140-6736(82)91212-0. [DOI] [PubMed] [Google Scholar]
- 27.Rouse ST, Thomas TM, Levey AI. Muscarinic acetylcholine receptor subtype M2: diverse functional implications of differential synaptic localization. Life Sci. 1997;60:1031–1038. doi: 10.1016/s0024-3205(97)00044-1. [DOI] [PubMed] [Google Scholar]
- 28.Segal M. Multiple actions of acetylcholine at a muscarinic receptor in the rat hippocampal slice. Brain Res. 1982a;246:77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- 29.Segal M. Changes in neurotransmitter actions in the aged rat hippocampus. Neurobiol Aging. 1982b;3:121–124. doi: 10.1016/0197-4580(82)90007-0. [DOI] [PubMed] [Google Scholar]
- 30.Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 31.Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatase in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]