Abstract
Background
Ovarian cancer has the highest mortality rate among all female genital tumors because of its insidious onset and drug resistance. Hypoxia-inducible factor 1α (HIF-1α), one of the best-studied oncogenes, plays an important part in tumor adaptation to microenvironmental hypoxia and was found to be overexpressed in several malignancies, including ovarian cancer. Previous studies found that the effect of HIF-1α on cancers may be correlated with autophagy and some signaling pathways, such as PI3K/AKT/mTOR, in several tumors. However, the function and potential mechanism have not been clearly defined.
Material/Methods
The expression of HIF-1α in ovarian cancer tissues were detected by immunohistochemistry. HIF-1α was knocked down by siRNA transfection. Cell viability was examined by CCK8 and colony formation assay. Apoptosis and autophagy were detected with flow cytometry, transmission electron microscopy, and laser scanning confocal microscopy, respectively. The proteins related to autophagy and PI3K/AKT/mTOR were detected through Western blot analysis.
Results
HIF-1α was expressed at higher levels in epithelial or metastatic ovarian cancer tissue than in normal fallopian tube tissue. When HIF-1α was knocked down by siRNA in A2780 and SKOV3 cells, the viability of ovarian cancer cells was weakened, but the apoptosis and autophagy were strengthened. Accordingly, autophagosome formation increased and the expression of autophagy-related proteins LC3 and P62 increased in HIF-1α knockdown cells. The PI3K/Akt/mTOR signaling pathway was also found to be inactivated in HIF-1α knockdown cells.
Conclusions
These findings show that knockdown of HIF-1α promoted autophagy and inhibited the PI3K/AKT/mTOR signaling pathway in ovarian cancer cells.
MeSH Keywords: Autophagy; Hypoxia-Inducible Factor 1, alpha Subunit; Ovarian Neoplasms; Phosphatidylinositol 3-Kinases; Signal Transduction; TOR Serine-Threonine Kinases
Background
The hypoxia-inducible factor 1α (HIF-1α), which is one of the best-studied oncogenes, plays a critical role in tumor adaptation to microenvironmental hypoxia, and is an appealing chemotherapeutic target. It has been proved that the hypoxic environment in solid tumors is the key to the development of tumor growth, invasion, metastasis, angiogenesis, and chemotherapy resistance. In recent years, many studies have shown that HIF-1 is highly expressed in solid tumors, demonstrating that hypoxia can make tumor cells become more unstable and increase tumor invasiveness and resistance to chemotherapy [1]. HIF-1 contains 2 subunits – α and β – and there are 3 subtypes – HIF-1α, HIF-2α, and HIF-3α. HIF-1α is the only oxygen-regulating subunit and determines the transcription activity of HIF-1. The degradation of HIF-1α can be inhibited by the anoxic environment; therefore, the concentration of HIF-1α indicates hypoxia in the tissue. HIF-1α is a transcriptional factor that regulates tumor cell survival, proliferation, angiogenesis, invasion, apoptosis, and autophagy [2]. Therefore, the downregulation of HIF-1α in ovarian cancer cells inhibits the growth of tumors and increases the sensitivity of ovarian cancer chemotherapy, but the mechanism is not clear.
Autophagy is an evolutionarily conserved process involving degradation of components in cytoplasm, such as damaged organelles and proteins [3]. It is a process of self-digestion that plays an important role in homeostasis, development, tumorigenesis, and infection. Autophagosomes form during the autophagy progress, which is realized in 2 conjugated protein pathways. One is the autophagy-related gene Atg12-Atg5 conjugation system and the other is the microtubule-associated protein light chain 3 beta (LC3)-lipid phosphatidylethanolamine (PE) conjugation system [4]. Autophagy is a protective mechanism by which cells adapt to stress conditions in the microenvironment, such as hypoxia [5]. Defective autophagy has been shown to be correlated with malignant tumors and poor prognosis of human cancers, while other studies showed that enhancing autophagy promotes the survival of tumor cells and reduces the sensitivity of chemotherapy drugs [6]. Reducing autophagy can, to some extent, increase the sensitivity of resistant ovarian cancer to chemotherapy and improve the prognosis.
In tumor cells, autophagy is regulated by a variety of signaling pathways, of which the PI3K/AKT/mTOR pathway is the most classic. It is the central regulatory mechanism, which promotes the growth and proliferation of tumor cells and also inhibits autophagy. mTOR is a receptor that senses ATP and amino acid changes in cells and is an important kinase regulating cell apoptosis and autophagy. Under stress conditions such as hypoxia and starvation, autophagy can be activated by mTOR to provide energy and nutrition for cell survival. Ding et al. found that inhibiting the PI3K/AKT/mTOR pathway in SKOV3 and OVCAR4 cells can induce autophagy [7]. In advanced ovarian cancer, activation of autophagy can promote the survival of tumor cells, resulting in reduced sensitivity of cancer cells to chemotherapy [8]. Therefore, in the present study, we investigated whether HIF-1α, as an oxygen regulator in the tumor microenvironment, can regulate autophagy and the PI3K/AKT/mTOR signaling pathway and affect the biological behavior of ovarian cancer cells.
Material and Methods
Patients and tissue collection
The tissue samples for the study were collected from 87 patients with ovarian cancer who had surgeries in the Department of Gynecology, Renmin Hospital, Wuhan University during January 2016 to October 2018 (the permission number for clinical ethics study: 2019K-K001). All cases of ovarian cancer were confirmed as epithelial ovarian malignancy (58 cases of serous adenocarcinoma, 19 cases of mucous adenocarcinoma, and 10 cases of endometrioid adenocarcinoma) by at least 2 pathologists. None of the patients underwent chemotherapy or radiotherapy before surgery. Focal cancer tissue, metastatic tissue, and normal fallopian tube tissue from the other side as ovarian cancer as control were obtained during the surgery and prepared as paraffin sections for immunohistochemistry.
Immunohistochemistry
Surgical specimens were obtained and cut into pieces 1×1×0.5 cm, then fixed in 4% paraformaldehyde at 4°C for 24 h. The fixed tissues were washed with PBS buffer solution (soaked 3 times for 30 min each time) and then dehydrated with ethanol in a 4-degree gradient. The tissue was immersed twice in liquid paraffin wax (2 h each time), and then cut into slices after the wax solidified completely. Slides were treated with poly-lysine to avoid stripping, and then heated in an oven at 58–60°C for 30–60 min. Endogenous enzyme activity was quenched by incubation in 50% ethanol solution containing 3% H2O2 for 30 min. The sections were sequentially blocked with protein block for 30 min and in bovine serum albumin for 30 min. Then, the sections were incubated with primary antibodies against HIF-1α (1: 1000; CST) overnight at 4°C. After washing in PBS, the sections were incubated with peroxidase-labeled anti-rabbit IgG (1: 500; CST) for 30 min. All slides were incubated with DAB-Substrate (CST) and stained in hematoxylin before being dehydrated and mounted. The optical density of representative images was analyzed with IPP software (Image-Pro Plus6.0).
Cell culture
The human ovarian cancer cell lines A2780 and SKOV3 were obtained from the State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). A2780 and SKOV3 cells were cultured and generated, and then subcultured in logarithmic phase in 1×106/ml suspension, with 95% of living cells stained by Trypan Blue.
Cell transfection assay
We obtained HIF-1α-specific siRNA and scrambled negative control siRNA for cell transfection from Shanghai GenePharma (China). The siRNA sequences were: HIF-1α siRNA (sense, 5′-GCUGGAGACAAUCAUAUTT-3′; antisense, 5′-AUAUGAUUGUGUCUCCAGCTT-3′), and scrambled negative control siRNA (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense, 5′-ACGUGACACGUUCGGAGAATT-3′). A2780 and SKOV3 cells (2×105 cells/well) were seeded in 24-well plates and grown to 30% confluence. Cells were divided into 3 groups: a control group, an si-control group (transfected with negative control siRNA), and an si-HIF-1α group (transfected with HIF-1α siRNA). The transfection mixture was replaced after 6 h with DMEM/F-12 with 20% FBS. Then, the cells were incubated for another 24 h and subjected to Western blot analysis and GFP-LC3 adenoviral vector transfection.
Western blot analysis
Total protein was extracted from tumor cells after siRNA transfection. Protein samples were separated in 10–15% SDS-polyacrylamide gels and electrophoretically transferred to polyvinylidene difluoride membrane. The membrane was blotted with 5% non-fat milk, washed, and then probed with antibodies of HIF-1α, P62, LC3, bcl-2, Bax, caspase-3, p-PI3K, PI3K, p-AKT, AKT, p-mTOR, mTOR, and GAPDH (all antibodies were purchased from CST) overnight at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (KPL) after being washed and visualized. The experiment was repeated 3 times. Then, the membranes were scanned and analyzed, and the gray value was calculated by FC AlphaEase software.
Cell Counting Kit-8 (CCK8) assay
First, 3 groups of A2780 and SKOV3 cells (control, si-control, and si-HIF-1α group) were seeded at the density of 5×104/well in 96-well microtiter plates and incubated in a 37°C 5% CO2 incubator overnight. Then, we put 10 μl CCK8 in each well and incubated for another 4 h and assessed the OD level at 450 nm. Cell viability was detected at 12 h, 24 h, 48 h, and 72 h.
Colony formation assay
Logarithmically grown cells in each group were digested with 0.25% trypsin and blown into single cells, and the cells were suspended in DMEM medium with 10% FBS serum. Each group of cells was inoculated into the culture dish with gradient density of 500 cells per dish and then incubated at 37°C. We terminated the culture when visible clones appeared. After washing with PBS twice, the cells were fixed with 1: 3 acetic acid/methanol and then dyed with Giemsa for 10–30 min. The staining solution was washed off slowly with running water and air-dried. Colonies were counted visually. The colony-forming efficiency was calculated as the number of clones/number of inoculated cells ×100%.
EdU cell proliferation detection
Three groups of A2780 and SKOV3 cells (control, si-control, and si-HIF-1α group) were seeded at a density of 5×104/well in 96-well microtiter plates. We prepared 50 μM EdU medium by diluting the EdU solution at 1000: 1 with complete cell culture medium (CST). Each well was incubated with 100 μL 50 μM EdU medium for 2 h, and then the medium was discarded. Then, we washed cells 1–2 times with PBS for 5 min each time. Each well was incubated at room temperature with 50 μL cell fixative (PBS containing 4% paraformaldehyde) for 30 min, and then the fixative was discarded. We added 50 μL 2 mg/mL of glycine per well, incubated it for 5 min in a decolorizing shaker, then discarded the glycine solution. Subsequently, we incubated cells with 100 μL penetrant (0.5% tritonx-100 PBS) for 10 min after washing with PBS. The cells were stained with 1X Apollo stain and then with 1× Hoechst33342. Images were taken with a fluorescence microscope, and the cell proliferation rate was calculated.
Cytofluorometric apoptosis
A2780 and SKOV3 cells treated by siRNA (2×106) were seeded into each well of a 6-well plate. After washing with PBS twice, cells were labeled with 5 μl Annexin V-FITC and 10 μl propidium iodide (50 μg/ml) in the dark at 4°C for 15 min, and detected in a FACS Calibur device (Becton Dickinson Immunocytometry Systems).
Transmission electron microscopy (TEM)
The A2780 and SKOV3 cells were prepared into single-cell suspension, centrifugated at 1500–2000 RPM for 10 min, and the supernatant was discarded. We slowly added 0.5% glutaraldehyde and then allowed the mixture to stand for 15–30 min at 4°C. We slowly added 3% glutaraldehyde fixative solution along the pipe wall after centrifugation at 10 000 rpm for 15 min. Cells were collected and autophagy vesicles were observed by transmission electron microscopy.
Autophagy detection with mRFP-GFP-LC3 adenoviral vector
Cells were seeded on coverslips in 24-well plates after transfection. mRFP-GFP-LC3 adenoviral vectors were purchased from Beyotime Biotechnology, China. mRFP-GFP-LC3 adenoviral infection was performed according to the manufacturer’s instructions. A2780 and SKOV3 cells in all 3 groups (control group, si-control group, and si-HIF-1α group) were washed 3 times with ice-cold PBS and fixed with 4% paraformaldehyde for 15 min at room temperature after being treated. The cap plate was fixed on the slide after the cells were washed 3 times with PBS. Then, the expression of GFP and mRFP was observed using a laser scanning confocal microscope (Olympus America, Inc). GFP and mRFP were used to label and track LC3, and the weakening of GFP indicated the fusion of lysosomes and autophagosomes to form autolysosomes. As the GFP fluorescence protein is sensitive to acid, only red fluorescence can be detected when the GFP fluorescence is quenched after the fusion of autophagosomes and lysosomes. The level of autophagy was measured by counting the number of red dots and yellow dots after being merged.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). SPSS13.0 software was used for statistical analysis. Mean values of the experimental groups were compared by t test and chi-square test, and P<0.05 was considered statistically significant. Western blot results were analyzed with Kruskal-Wallis test using Quantity One software. Experiments were repeated in triplicate, with similar results each time, and the figures show representative experimental results.
Results
HIF-1α protein in ovarian cancer tissue
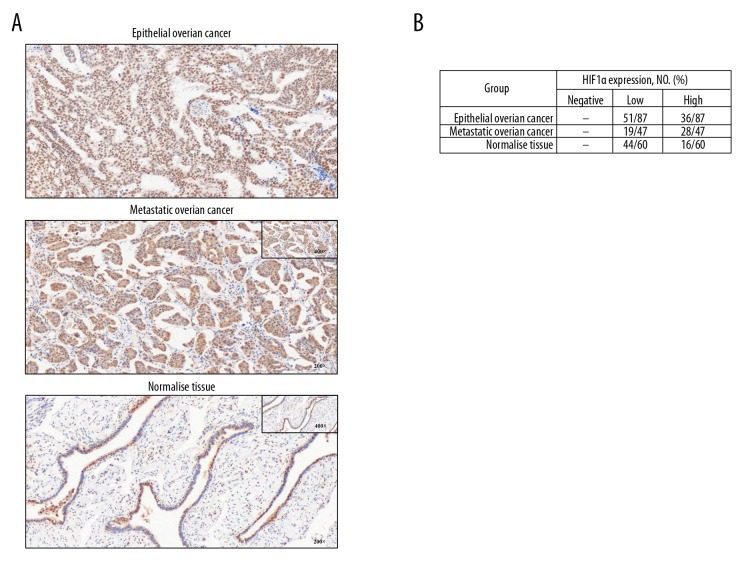
Positive staining of HIF-1α showed brown-yellow in nuclei. Immunohistochemical results shows that HIF-1α protein in epithelial ovarian cancer tissue and metastatic ovarian cancer tissue was higher than that in the normal fallopian tubes (Figure 1A). The positive rates of high expression of HIF-1α in ovarian cancer tissues and metastases tissues were both higher than in the normal tissue group (Figure 1B).
Figure 1.
The expression levels of HIF-1α protein were higher in ovarian cancer tissue and metastatic ovarian cancer tissue than in normal tissue. (A) Representative immunohistochemical images of HIF-1α protein localization in ovarian cancer tissue, metastatic tissue, and normal tissue (from a patient with serous adenocarcinoma). Photographs were taken at magnification 200×. (B) The positive rate of HIF-1α high expression in all ovarian cancer tissues and metastases tissues was calculated, and they were both higher than in the normal tissue group.
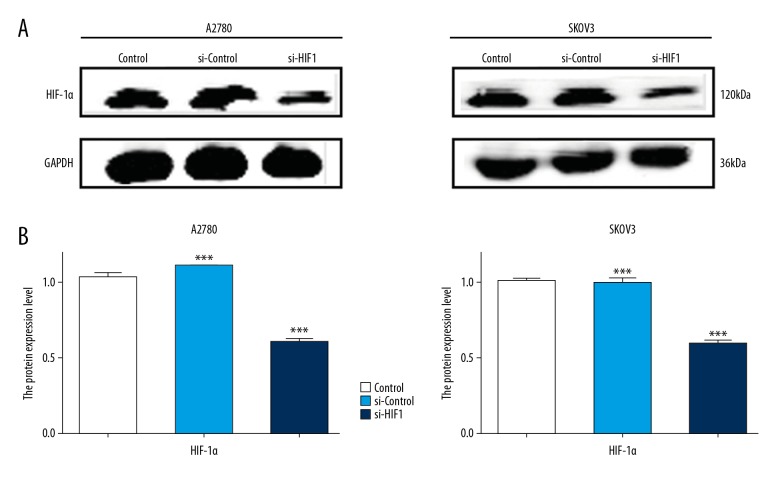
HIF-1α was knocked down after transfection with siRNA in a2780 and SKOV3 cells
Both A2780 and SKOV3 cells were transfected with siRNA, and the level of HIF-1α protein was detected using Western blot assay. It was found that the expression of HIF-1α was not significantly different between the control group and the si-control group. Expression of HIF-1α was significantly decreased in the si-HIF-1α group compared with that in the si-control group in A2780 and SKOV3 cells, which indicated that the siRNA transfection successfully established a microenvironment with low HIF-1α protein levels in the 2 cell lines (Figure 2)
Figure 2.
The knockdown effect of HIF-1α siRNA was detected by Western blot analysis. (A) A2780 and SKOV3 cells were transfected with HIF-1α siRNA and scrambled negative control siRNA, and the level of HIF-1α protein were detected by Western blot. (B) The quantitative comparison of the difference of expression of HIF-1α in each group. Total protein levels were normalized to GAPDH levels. The data are presented as the means ±SD from at least 3 independent experiments (* p<0.05; ** p<0.01; *** p<0.001 by the Kruskal-Wallis test).
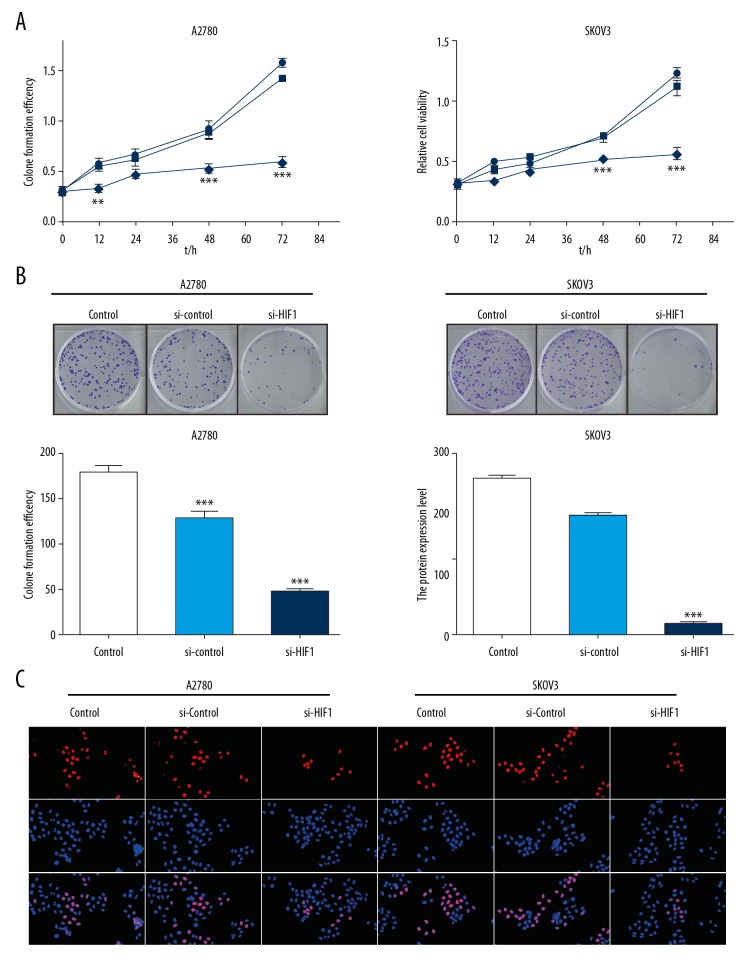
Knockdown of HIF-1α inhibited viability of ovarian cancer cells
From the above results, we confirmed that the cells showed low HIF-1α expression after siRNA transfection. Assessment of cell activity via CCK8 assay showed that the OD value, reflecting cell activity, was lower in the si-HIF-1α group than in the si-control group and control group, indicating that inhibition of HIF-1α in ovarian cancer cells could inhibit the growth activity of tumor cells in a time-dependent way. The longer the cells incubated in the environment of low HIF-1α protein, the stronger the inhibition of tumor cell growth (Figure 3A), and the colony formation assay showed the same result. There were significantly fewer cell colonies in the si-HIF-1α group than in the other 2 groups, suggesting that tumor cell growth was restricted after HIF-1α was inhibited in both A2780 and SKOV3 cell lines (Figure 3B). The EdU assay result indicated that the proportion of cells in S phase was significantly reduced, indicating that cell proliferation was inhibited in the si-HIF-1α group in A2780 and SKOV3 cell lines (Figure 3C).
Figure 3.
Knockdown of HIF-1α inhibited viability of ovarian cancer cells. (A) A2780 and SKOV3 cells were transfected with HIF-1α siRNA and scrambled negative control siRNA, and the viability of cells were detected by CCK8 method. The vertical axis represents cell viability, and the horizontal axis represents the duration of the culture. (B) Photographs of viable colonies of A2780 and SKOV3 cells in the HIF-1α siRNA-transfected group, negative control siRNA-transfected group, and the control group, stained with crystal violet. Cell density indicates cell viability. (C) EdU assay: Cells stained red are in the S phase, while the blue staining represents the nucleus, and the overlap represents the proportion of proliferating cells. There were significantly fewer HIF-1α siRNA-transfected cells in S phase.
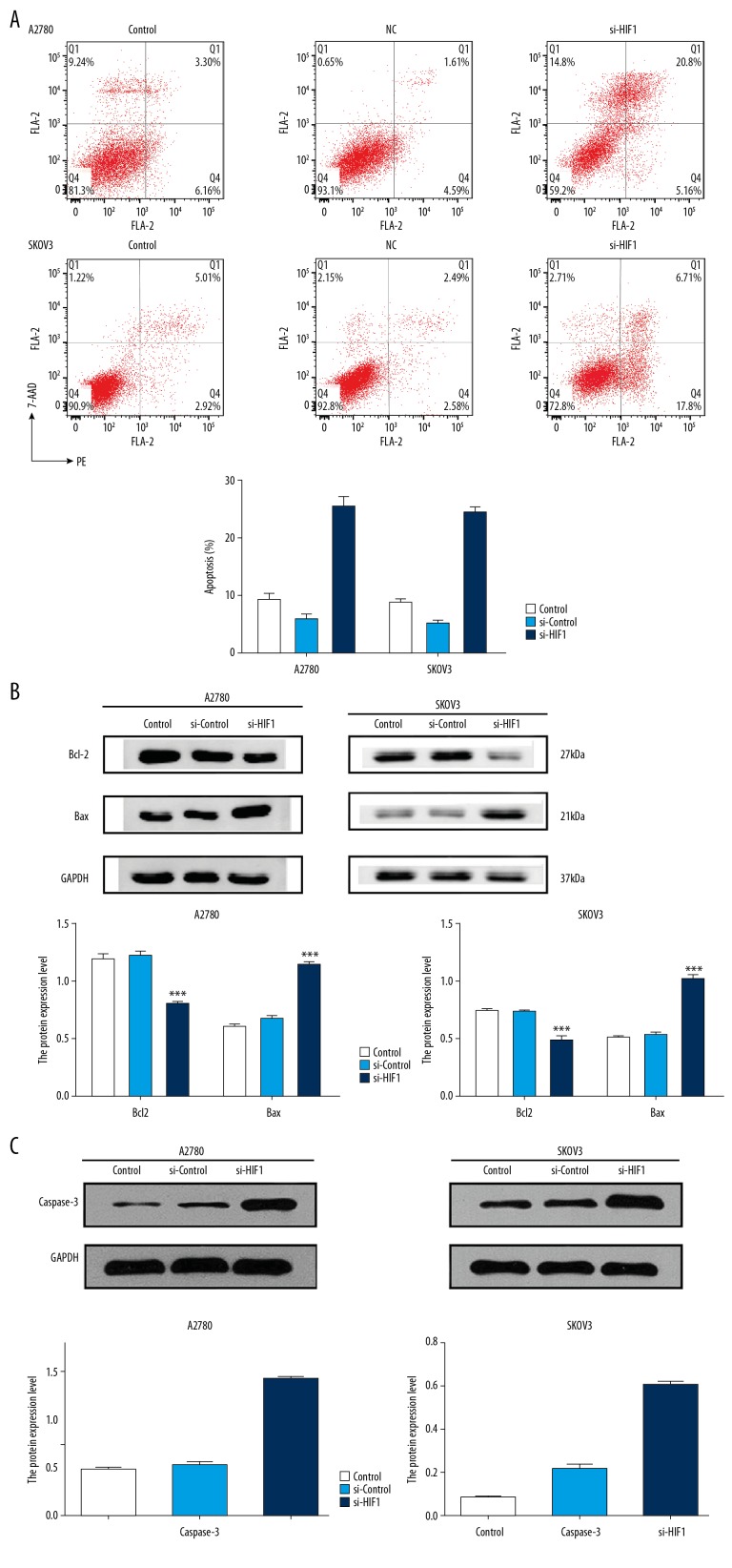
Apoptosis was promoted after HIF-1α knockdown in ovarian cancer cells
To explore the mechanism by which HIF-1α influenced viability of ovarian cancer cells, we detected the mode of cell death in the cell lines transfected with siRNA. The results from flow cytometry showed that the apoptosis rate in the A2780 cell line in the si-HIF-1α group (25.96%) was significantly higher than in the si-control group (6.2%) and control group (9.46%). Similar results were observed in the SKOV3 cell lines; the apoptosis rate in the si-HIF-1α group (24.51%) was significantly higher than that in the si-control group (5.07%) and control group (7.93%) (Figure 4A). Moreover, Western blot analysis showed that the expression level of bcl-2 was downregulated, while the expression level of Bax and caspase-3 proteins were upregulated (Figures 4B, 4C).
Figure 4.
Knockdown of HIF-1α promoted apoptosis in ovarian cancer cells. (A) The transfected cells and control cells were assayed for apoptosis by V/PI staining. Four quadrants in the image referred to different cells. LL – lower left quadrant refereed to the living cells. LR – lower right quadrant refereed to the early apoptosis cells. UR – right upper quadrant refereed to the late apoptosis cells. UL – left upper quadrant referred to the necrotic cells. Therefore, the total apoptosis rate was LR+UR. The data were presented as the means ±SD from at least 3 independent experiments. (B) Bax proteins was upregulated, and bcl-2 protein was downregulated after transfection. (* p<0.05; ** p<0.01; *** p<0.001 by the Kruskal-Wallis test). (C) Caspase-3 proteins was upregulated after transfection in A2710 and SKOV3 cells (* p<0.05; ** p<0.01; *** p<0.001 by the Kruskal-Wallis test).
The results showed that cell apoptosis increased with HIF-1α knockdown, and HIF-1α affected the viability of tumor cells by interfering with cell apoptosis.
Autophagy was inhibited in A2780 and SKOV3 cells while HIF-1α was knocked down
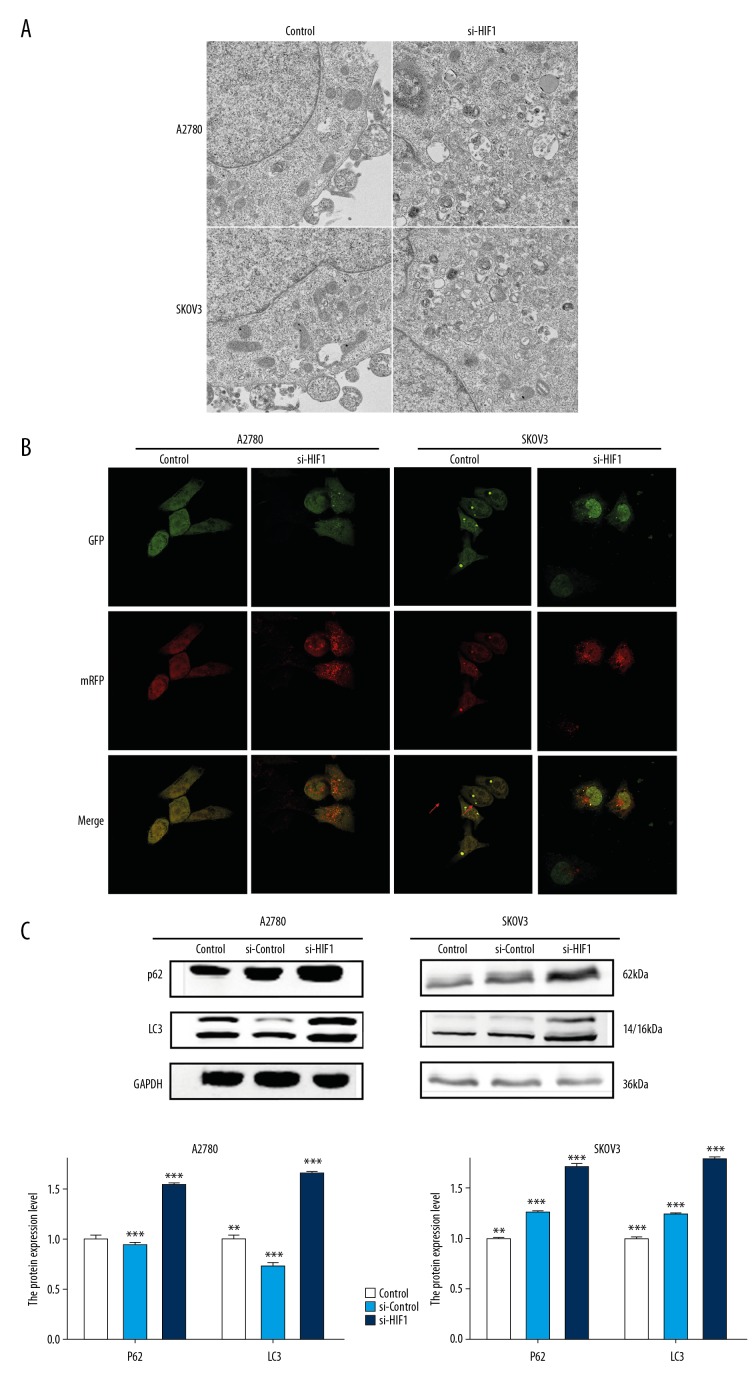
First, to confirm whether autophagy is affected by HIF-1α in ovarian cancer cells, we conducted a series of experiment. Formation of autophagic vacuoles (AV) in the cytoplasm is a typical manifestation of autophagy. In the present study, we used transmission electron microscopy to observe cells and found that numerous AV were formed in the cancer cells transfected with si-HIF-1α, and cytoplasm and organelles (e.g., mitochondria/endoplasmic reticulum) were wrapped in the double-layer membrane. However, few AV were observed in the si-control group and the control group (Figure 5A)
Figure 5.
Autophagy detection in A2780 and SKOV3 cells. (A) Representative images of transmission electron microscopy performed on A2780 and SKOV3 cells receiving HIF-1α siRNA transfection treatment as described in the Materials and Methods section. The red arrows point to autophagosomes. (B) Detection of Mrfp-GFP-LC3 puncta indicative of autophagy in ovarian cancer cells transfected with scrambled control siRNA or HIF-1α-specific siRNA. Photographs were taken at 1600×. In the merged picture, the yellow dots represent the level of autophagosomes and the red dots represent the level of autolysosomes. (C) Autophagy-associated proteins P62 and LC3 were detected by Western blot. Total protein levels were normalized to GAPDH levels. Data are presented as the means ±SD from at least 3 independent experiments (* p<0.05; ** p<0.01; *** p<0.001 by Kruskal-Wallis test).
Second, laser scanning confocal microscopy remains one of the most accurate methods for detection of autophagy. The autophagy flux was totally reflected by mRFP-GFP-LC3 assay. The results showed that numerous cytoplasmic autophagosomes (yellow dots in the merge group) were present after transfection with HIF-1α-specific siRNA. However, few autophagosomes were observed in the si-control group, especially in the A2780 cell lines. And we also found there were more red dots, which represent the autolysosome, in the si-HIF-1α group in both cell lines (Figure 5B).
Third, LC3 and p62 protein, which are important autophagy marker proteins, were assessed by Western blot in A2780 and SKOV3 cells transfected with scrambled control siRNA or HIF-1α-specific siRNA. As shown in Figure 5C, LC3 and p62 protein were expressed at higher levels in the si-HIF-1α group compared with the si-control group (P<0.05), while no difference was observed between the si-control group and the control group (P>0.05) (Figure 5C)
The above results show that autophagy was significantly inhibited in ovarian cancer cells after knockdown of HIF-1α.
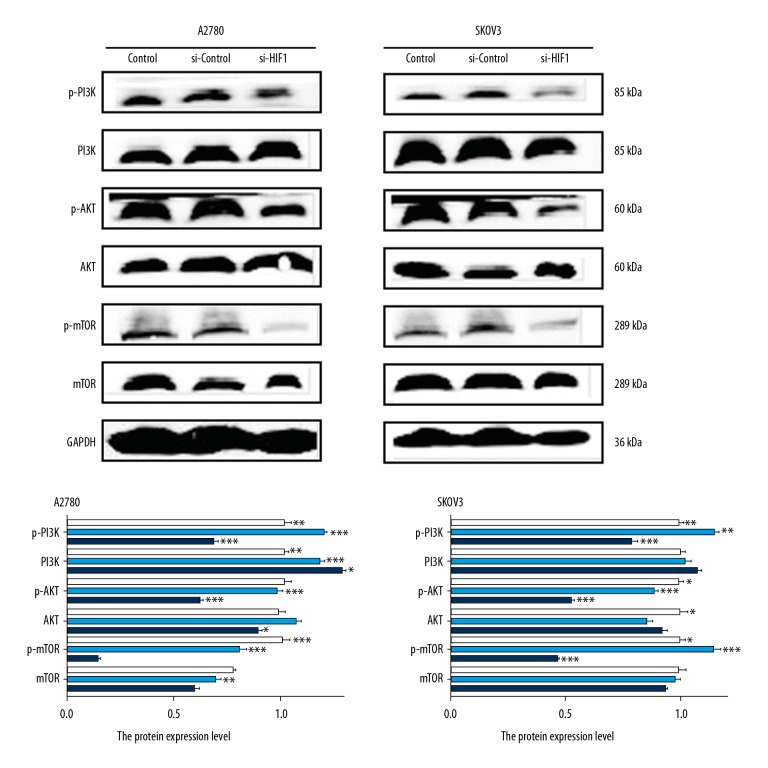
PI3K/AKT/mTOR signaling was inhibited after HIF-1α knockdown
Abnormal expression of PI3K/AKT/mTOR signals is known to be a key regulator of autophagy. Inhibition of PI3K/AKT/mTOR can reverse apoptosis resistance and significantly enhance apoptosis. In this study, PI3K/AKT/mTOR proteins and their phosphorylation forms, p-PI3K/p-AKT/p-mTOR, were detected by Western blot in A2780 and SKOV3 cells transfected with scrambled control siRNA or HIF-1α-specific siRNA. We found that the expressions of p-PI3K/p-AKT/p-mTOR were significantly inhibited in the si-HIF-1α group, while the expression of PI3K/AKT/mTOR proteins showed no significant difference compared with the si-control group (Figure 6).
Figure 6.
The level of expression of PI3K/AKT/mTOR in the transfected cells were detected by Western blot. A2780 and SKOV3 cells were transfected with HIF-1α siRNA and scrambled negative control siRNA, and the level of HIF-1α PI3K/AKT/mTOR proteins and their phosphorylation form protein were detected by Western blot. The quantitative comparison of the difference in expression of PI3K/AKT/mTOR in each group. Total protein levels were normalized to GAPDH levels. Data are presented as the means ±SD from at least 3 independent experiments (* p<0.05; ** p<0.01; *** p<0.001 by Kruskal-Wallis test).
Discussion
Ovarian cancer is one of the 3 most common malignancies of the female reproductive system and is the leading cause of reproductive cancer deaths in women [9]. The 5-year survival rate of ovarian cancer patients is less than 40% because the cancer usually occurs insidiously and develops rapidly [10]. In recent years, there have been many studies on the relevant tumorigenesis and development mechanisms, among of which the changed tumor microenvironment has attracted wide attention. HIF-1α is considered as a transcriptional factor which regulates cell survival, proliferation, and angiogenesis because it plays a central role in the regulation of hypoxia, mediates most of the hypoxia stress responses, and makes tumor cells become resistant to drugs. Thus, HIF-1α has become a therapeutic target to reverse the drug resistance of tumors [11]. It was found that the positive rate and positive intensity of HIF-1α progressively increased in benign, borderline, malignant epithelial ovarian tumors [12], which suggests that the level of HIF-1α is related to the degree of malignancy of epithelial ovarian tumors. In this study, the expression of HIF-1α in ovarian cancer sections was first detected by immunohistochemistry, showing that the expression of HIF-1α in ovarian cancer tissue was higher than that in paracancerous tissue and normal fallopian tube tissue, which confirmed the high expression of HIF-1α in ovarian cancer and the existence of a hypoxic microenvironment.
To determine the role of HIF-1α in ovarian cancer, a cell environment with HIF-1α knockdown was successfully established using siRNA transfection. Our CCK8 assay and colony formation assay experiments confirmed that the viability of ovarian cancer cells was obviously inhibited while HIF-1α was knocked down by siRNA. EdU is a thymidine nucleoside analogue, and its acetylene group is rare in natural compounds. It can be inserted into the DNA molecules being copied during cell proliferation and can effectively detect the percentage of cells in S phase. In the present study, the red staining represents the S phase cells; this staining was obviously weaker in the HIF-1α knockdown cells, showing that proliferation was inhibited. These results suggest that HIF-1α promotes the viability of ovarian cancer cells.
In therapy of advanced ovarian cancer, chemotherapy is still an important method, so how to improve the sensitivity of cancer cells to chemotherapy is the key of the treatment, and the ultimate goal of improving the sensitivity of cells is to increase the mortality of tumor cells. There are many ways to induce cell death, the most important of which are apoptosis and autophagy [7]. Apoptosis and autophagy are known as cell programmed death I and II, and although these 2 kinds of death are different in many ways, research has shown they may be linked. Some important regulatory molecules, such as p53 gene, the PI3K/AKT signaling pathway, and interaction between bcl-2 and Beclin 1, can control both apoptosis and autophagy [13]. In some special conditions, certain signal lead to simultaneous activation of autophagy and apoptosis [14]. Studies have suggested that autophagy, as a protective mechanism in tumor cells, occurs before apoptosis, and autophagy could become the main form of cell death in cells with defective apoptosis when apoptosis is prevented [15]. In addition, through the detection of apoptosis-related proteins, we found that the expression of bcl-2 was downregulated and the expression of Bax and caspase-3 were increased. Bcl-2 family proteins are important regulatory factors in the process of apoptosis. Bcl-2 is the first member of the bcl-2 family and the most studied anti-apoptotic protein. Caspase-3 is the most important terminal splicing enzyme in the process of apoptosis. The imbalance between apoptotic protein Bax and anti-apoptotic protein bcl-2 triggers the release of cytochrome c from mitochondria to cytosol, thereby activating the caspase-dependent apoptosis cascade [16]. In this study, flow cytometry assay and detection of apoptosis proteins showed that apoptosis was enhanced while HIF-1α was knocked down, suggesting that HIF-1α affects the viability of tumor cells by interfering with cell apoptosis.
Autophagy is important in maintaining cellular homeostasis, and defective autophagy has been reported to be involved in numerous human pathophysiological processes, including cancer, myopathy, and some other diseases [17]. Other studies found that enhanced autophagy in tumor cells promotes their repair, thus increasing drug resistance. Hypoxia, a common stress condition in the cellular microenvironment, can trigger activation of autophagy [18]. Autophagy is a complex cellular process that can be divided into 4 processes: induction, nucleation, expansion, and completion of the isolation membrane. Lysosomes degrade autophagosomes containing organelles and proteins in the cytoplasm as autophagosomes form. Studies in yeast have found that autophagy is regulated by a group of highly evolutionarily-conserved genes. At first, by studying the molecular mechanism of yeast as a model, a series of discovered regulatory genes were uniformly named “Atg”, and their translation products were called “autophagy-related proteins”, such as LC3, p62, P53, and Nrf2 [19]. In the present study, Western blot analysis showed that the expression levels of LC3 and P62 in cells were all higher than in the hypoxia cells. In the process of mammalian autophagy formation, LC3 protein is a homolog of yeast Apg8/Aut7p, which is involved in the modification process of Atg12 binding and LC3 protein, and plays a crucial role [20]. LC3 has become a specific marker protein for autophagy formation. P62 is also an important autophagy marker protein and is involved in protein aggregation and damaged organelles transport. P62, located at the site of autophagic lysosomes, can bind to LC3 or interact directly with LC3 and be degraded in the subsequent process, therefore, it can reflect the activity of autophagic lysosomes [21]. All the above results suggest that the enhancement of the hypoxic environment in cells can increase autophagy.
The formation of autophagosomes is a very important step during autophagy. When autophagy is activated, the isolated membrane takes in part of the cytoplasm to form autophagosomes, whose outer membranes fuse with the outer membranes of lysosomes, eventually leading to autophagosome degradation [22]. Microscopic observation of autophagosomes is also a reliable way to assess the level of autophagy in cells. In the present study, observation of autophagy in ovarian cancer cells by laser scanning confocal microscopy and transmission electron microscopy showed that the more autophagosomes form in cells with lower HIF-1α expression.
Through the above experiments, we confirmed that when hypoxia was inhibited in cells, the growth activity of ovarian cancer cells was weakened, and apoptosis and autophagy were enhanced, but the mechanism is remains unclear.
There are many signaling pathways associated with autophagy in cells [23], of which the most classic is the PI3K/AKT/mTOR pathway. This pathway regulates a series of important biological activities in cells, including cell proliferation, apoptosis, invasion, migration, and autophagy, which are all possible factors causing development of malignant tumors [24]. Activation of the PI3K/AKT/mTOR pathway has been found in cervical cancer, ovarian cancer, and endometrial cancer [25]. PI3K is a member of the lipid kinase family, and it can be divided into classes I, II, and III. PI3KC I is involved in cell proliferation, insulin signal transduction, immune function, and inflammation. PI3KC II is involved in membrane transport regulation. PI3KC III is composed of the Vps34 complex and plays an important role in autophagy [26]. AKT is a downstream signaling molecule of PI3K, which is activated by phosphorylated phosphatidylinositol family members on the cell membrane [25]. Phosphorylated AKT is activated and regulates multiple downstream targets, including mTOR. mTOR (mammalian target of rapamycin) is an atypical serine/threonine protein kinase that can sense nutrition, energy, and growth factors and other extracellular signals [27]. mTOR also can participate in gene transcription, protein translation, ribosome synthesis, cell growth, and programmed cell death [28]. The PI3K/AKT/mTOR signal pathway can affect autophagy in different ways. AKT phosphorylates Beclin1 at Ser295 and 234 sites in a state independent of mTOR to inhibit autophagy [24]. PI3KC III is involved in formation of the autophagosome membrane after binding to Beclin1 at some sites [29]. mTOR is involved in the initiation of autophagy and can inhibit autophagy after being activated [30]. Therefore, we assessed PI3K/Akt/mTOR and p-PI3K/Akt/mTOR protein expression in the A2780 and SKOV3 cells. The results showed that P-PI3K, P-AKT, and P-mTOR were all downregulated, while HIF-1α was knocked down with siRNA compared with the si-control group. Expression of unphosphorylated PI3K/Akt/mTOR was not affected by HIF-1α levels. These results suggest that HIF-1α in ovarian cancer cells promote activation of the PI3K/Akt/mTOR signaling pathway. According to previous studies, as a classical pathway regulating autophagy, activation of the PI3K/Akt/mTOR signaling pathway can down-regulate autophagy [31], and in this study we confirmed that HIF-1α activates the PI3K/Akt/mTOR signaling pathway, thus suggesting that HIF-1α regulates autophagy via the PI3K/Akt/mTOR signaling pathway. However, further research is needed to confirm this.
The present study has certain limitations: (1) Lack of animal experiments; (2) Patient data were not collected and no correlation analysis of clinical/pathology factors was performed; (3) The level of detection was at the protein level (Western blot analysis), and there was no experimental detection at the gene level (PCR); (4) No further exploration of the relationship between the PI3K signaling pathway and autophagy was performed. Thus, further research is needed to provide additional insight into these matters.
Conclusions
We demonstrated that HIF-1α was expressed at higher levels in ovarian cancer tissue than in paracancerous or normal fallopian tube tissue. When HIF-1α was knocked down by siRNA transfection in A2780 and SKOV3 cells, it was found that the viability of ovarian cancer cells was weakened and apoptosis and autophagy were promoted. In HIF-1α knockdown cells, the formation of autophagosomes increased, as did the expression of autophagy-related proteins LC3 and P62. The PI3K/Akt/mTOR signaling pathway was also found to be inactivated in the HIF-1α specific siRNA-transfected cells. Taken together, these findings reinforce the view that knockdown of HIF-1α promotes autophagy and inhibits the PI3K/AKT/mTOR signaling pathway in ovarian cancer.
Acknowledgements
My deepest gratitude goes first and foremost to Professor Li Hong, my supervisor, for her constant encouragement and guidance. In addition, I would like to thank my colleagues, especially Danhua Lu and Likun Gao, for their help with experiments, which contributed greatly to completion of this study.
Footnotes
Source of support: Departmental sources
References
- 1.Gomez-Roman N, Sahasrabudhe NM, McGregor F, et al. Hypoxia-inducible factor 1 alpha is required for the tumourigenic and aggressive phenotype associated with Rab25 expression in ovarian cancer. Oncotarget. 2016;7(16):22650–64. doi: 10.18632/oncotarget.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunfei W, Whitney SG, Rebecca AP, et al. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res. 2015;21(2):448–59. doi: 10.1158/1078-0432.CCR-14-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haiqi L, Guangliang L, Leiming L, et al. Regulation and function of mitophagy in development and cancer. Autophagy. 2013;9(11):1720–36. doi: 10.4161/auto.26550. [DOI] [PubMed] [Google Scholar]
- 4.Ohsumi Y, Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol. 2004;15(2):231–36. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Zhan L, Zhang Y, Wang W, et al. Autophagy as an emerging therapy target for ovarian carcinoma. Oncotarget. 2016;7(50):83476–87. doi: 10.18632/oncotarget.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36(12):1619–30. doi: 10.1038/onc.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.YongHui D, Zhiwei Z, ChunFang H, et al. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther. 2015;9:425–64. doi: 10.2147/DDDT.S74062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bast RJ. Molecular approaches to personalizing management of ovarian cancer. Ann Oncol. 2011;22(Suppl 8):viii5–15. doi: 10.1093/annonc/mdr516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 10.Nasioudis D, Sisti G, Kanninen TT, et al. Epidemiology and outcomes of squamous ovarian carcinoma; A population-based study. Gynecol Oncol. 2016;141:128–33. doi: 10.1016/j.ygyno.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Deben C, Deschoolmeester V, DeWaele J, et al. Hypoxia-induced cisplatin resistance in non-small cell lung cancer cells is mediated by HIF-1 alpha and mutant p53 and can be overcome by induction of oxidative stress. Cancers. 2018;10(4):721–32. doi: 10.3390/cancers10040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain I, Waheed S, Ahmad KA, et al. Scutellaria baicalensis targets the hypoxia-inducible factor-1 and enhances cisplatin efficacy in ovarian cancer. J Cell Biochem. 2018;119:7515–24. doi: 10.1002/jcb.27063. [DOI] [PubMed] [Google Scholar]
- 13.Seiji M, Hiromasa K, Ryoko T, Tomoyuki S. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137(1):173–79. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Gong W, Yang ZY, et al. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 2017;22(4):544–57. doi: 10.1007/s10495-016-1334-2. [DOI] [PubMed] [Google Scholar]
- 15.Fitzwalter BE, Towers CG, Sullivan KD, et al. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev Cell. 2018;44:555–59. doi: 10.1016/j.devcel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsay D, Matthew H, Paul K, et al. Autophagy inhibition enhances sunitinib efficacy in clear cell ovarian carcinoma. Mol Cancer Res. 2017;15(3):250–58. doi: 10.1158/1541-7786.MCR-16-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huimei W, Zifeng J, Peishan D, et al. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci Rep. 2015;5:12291. doi: 10.1038/srep12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007;581(11):2156–61. doi: 10.1016/j.febslet.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 20.Liuyu X, Xiyu Z, Yinuo L, et al. Neferine induces autophagy of human ovarian cancer cells via p38 MAPK/JNK activation. Tumour Biol. 2016;37(7):8721–29. doi: 10.1007/s13277-015-4737-8. [DOI] [PubMed] [Google Scholar]
- 21.LingJie B, Melba CJ, ZhenBo Z, et al. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int J Clin Exp Pathol. 2014;7(4):1502–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nut Rev Mol Cell Biol. 2018;19:349–64. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 23.Nestor P, Raquel O, Anna F, et al. Modulation of autophagy by sorafenib: Effects on treatment response. Front Pharmacol. 2016;7:151. doi: 10.3389/fphar.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He WD, Cheng Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 2018;97:607–15. doi: 10.1016/j.biopha.2017.10.152. [DOI] [PubMed] [Google Scholar]
- 25.Wang SS, Zhu MY, Wang QY, et al. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018;9:1027–39. doi: 10.1038/s41419-018-1036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal S, John S, Sapra L, et al. Targeted disruption of PI3K/Akt/mTOR signaling pathway, via PI3K inhibitors, promotes growth inhibitory effects in oral cancer cells. Cancer Chemother Pharmacol. 2019;83(3):451–61. doi: 10.1007/s00280-018-3746-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim YC, Guan KL. mTOR: A pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husseinzadeh N, Husseinzadeh HD. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: A critical review. Gynecol Oncol. 2014;133(2):375–81. doi: 10.1016/j.ygyno.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 29.McKnight NC, Zhenyu Y. Beclin 1, an essential component and master regulator of PI3K-III in health and disease. Curr Pathobiol Rep. 2013;1(4):231–38. doi: 10.1007/s40139-013-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Brink M. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim Biophys Acta. 2016;1863(7 Pt B):1894–903. doi: 10.1016/j.bbamcr.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36:1619–30. doi: 10.1038/onc.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]