Abstract
Background
Dressings are part of the routine postoperative management of people after transtibial amputation. Two types of dressings are commonly used; soft dressings (e.g. elastic bandages, crepe bandages) and rigid dressings (e.g. non‐removable rigid dressings, removable rigid dressings, immediate postoperative protheses). Soft dressings are the conventional dressing choice as they are cheap and easy to apply, while rigid dressings are costly, more time consuming to apply and require skilled personnel to apply the dressings. However, rigid dressings have been suggested to result in faster wound healing due to the hard exterior providing a greater degree of compression to the stump.
Objectives
To assess the benefits and harms of rigid dressings versus soft dressings for treating transtibial amputations.
Search methods
In December 2018 we searched the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Ovid Embase, EBSCO CINAHL Plus, Ovid AMED and PEDro to identify relevant trials. To identify further published, unpublished and ongoing studies, we also searched clinical trial registries, the grey literature, the reference lists of relevant studies and reviews identified in prior searches. We used the Cited Reference Search facility on ThomsonReuters Web of Science and contacted relevant individuals and organisations. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that enrolled people with transtibial amputations. There were no restrictions on the age of participants and reasons for amputation. Trials that compared the effectiveness of rigid dressings with soft dressings were the main focus of this review.
Data collection and analysis
Two review authors independently screened titles, abstracts and full‐text publications for eligible studies. Two review authors also independently extracted data on study characteristics and outcomes, and performed risk of bias and GRADE assessments.
Main results
We included nine RCTs and quasi‐RCTs involving 436 participants (441 limbs). All studies recruited participants from acute and/or rehabilitation hospitals from seven different countries (the USA, Australia, Indonesia, Thailand, Canada, France and the UK). In all but one study, it was clearly stated that amputations were secondary to vascular conditions.
Primary outcomes
Wound healing
We are uncertain whether rigid dressings decrease the time to wound healing compared with soft dressings (MD ‐25.60 days; 95% CI ‐49.08 to ‐2.12; one study, 56 participants); very low‐certainty evidence, downgraded twice for very high risk of bias and once for serious imprecision. It is not clear whether rigid dressings increase the proportion of wounds healed compared with soft dressings (RR 1.14; 95% CI 0.74 to 1.76; one study, 51 participants); very low‐certainty evidence, downgraded twice for very high risk of bias and twice for very serious imprecision.
Adverse events
It is not clear whether rigid dressings increase the proportion of skin‐related adverse events compared with soft dressings (RR 0.65; 95% CI 0.32 to 1.32; I2 = 0%; six studies, 336 participants (340 limbs)); very low‐certainty evidence, downgraded twice for very high risk of bias and once for serious imprecision.
It is not clear whether rigid dressings increase the proportion of non skin‐related adverse events compared with soft dressings (RR 1.09; 95% CI 0.60 to 1.99; I2 = 0%; six studies, 342 participants (346 limbs)); very low‐certainty evidence, downgraded twice for very high risk of bias and once for serious imprecision. In addition, we are uncertain whether rigid dressings decrease the time to no pain compared with soft dressings (MD ‐0.35 weeks; 95% CI ‐2.11 to 1.41; one study of 23 participants); very low‐certainty evidence, downgraded twice for very high risk of bias and twice for very serious imprecision.
Secondary outcomes
We are uncertain whether rigid dressings decrease the time to walking compared with soft dressings (MD ‐3 days; 95% CI ‐9.96 to 3.96; one study, 56 participants); very low‐certainty evidence, downgraded twice for very high risk of bias and twice for very serious imprecision. We are also uncertain whether rigid dressings decrease the length of hospital stay compared with soft dressings (MD ‐30.10 days; 95% CI ‐49.82 to ‐10.38; one study, 56 participants); very low‐certainty evidence, downgraded twice for very high risk of bias and once for serious imprecision. It is also not clear whether rigid dressings decrease the time to readiness for prosthetic prescription and swelling compared with soft dressings, as results are based on very low‐certainty evidence, downgraded twice for very high risk of bias and once/twice for serious/very serious imprecision. None of the studies reported outcomes on patient comfort, quality of life and cost.
Authors' conclusions
We are uncertain of the benefits and harms of rigid dressings compared with soft dressings for people undergoing transtibial amputation due to limited and very low‐certainty evidence. It is not clear if rigid dressings are superior to soft dressings for improving outcomes related to wound healing, adverse events, prosthetic prescription, walking function, length of hospital stay and swelling. Clinicians should exercise clinical judgement as to which type of dressing they use, and consider the pros and cons of each for patients (e.g. patients with high risk of falling may benefit from the protection offered by a rigid dressing, and patients with poor skin integrity may have less risk of skin breakdown from a soft dressing).
Plain language summary
Rigid versus soft dressings for transtibial (below the knee) amputations
What is the aim of this review?
The aim of this review was to determine whether rigid dressings are more effective than soft dressings in helping the wound to heal following transtibial (below the knee) amputations. Researchers from Cochrane searched for all relevant studies (randomised controlled trials (RCTs) and quasi‐randomised controlled trials) to answer this question and found nine relevant studies.
Key messages
The certainty of evidence for all outcomes was very low because the results could not rule in or rule out important benefits or harms, and because the design and reporting of the studies was not of a high standard. Therefore, we cannot be certain if the use of rigid dressings leads to better or worse patient outcomes compared with soft dressings.
What was studied in the review?
We studied the effects of rigid dressings such as plaster casts or fibreglass dressings on outcomes including wound healing, adverse events, prescription of prosthetics, physical function, length of hospital stay, patient comfort, quality of life, cost and swelling in people following transtibial amputations. Rigid dressings were compared with soft dressings such as gauze or elastic bandages in all included studies.
What are the main results of the review?
We included results from nine RCTs and quasi‐RCTs involving 436 participants (441 limbs) in this review. Participants were recruited from acute and/or rehabilitation hospitals from seven different countries. Sample sizes of studies ranged from 15 to 154, while the average age of participants ranged from 54 to 75. More than half of all participants had diabetes and other co‐morbidities (e.g. anaemia, smoking history, hypertension, cardiac disease). Amputations were all secondary to vascular conditions (e.g. peripheral artery disease) although the cause of amputation was not always specified.
We are uncertain whether rigid dressings lead to more wounds healed, fewer adverse events, faster recovery time for pain and wound healing, walking and prosthetic prescription, greater reduction in swelling, and a shorter hospital stay, compared with soft dressings. We are unsure about these results because all studies had very severe methodological limitations, and most results were based on a small number of studies (i.e. one to three studies of 21 to 65 participants).
How up to date is this review?
We searched for studies that had been published up to December 2018.
Summary of findings
Summary of findings for the main comparison. Rigid dressings compared with soft dressings for transtibial amputations.
| Rigid dressings compared with soft dressings for transtibial amputations | |||||||
| Patient or population: people who had undergone transtibial amputations Setting: acute and rehabilitation hospitals Intervention: rigid dressings Comparison: soft dressings | |||||||
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with soft dressings | Risk with rigid dressings | ||||||
| Wound healing | Time from amputation to wound healing (days) | The mean time from amputation to wound healing was 96.8 days | MD 25.60 lower (49.08 lower to 2.12 lower) | ‐ | 56 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | We have very little confidence that rigid dressings speed up wound healing. The true effect is likely to be substantially different from the estimate of effect. |
| Proportion of wounds healed (measured at 21 days) | Study population | RR 1.14 (0.74 to 1.76) | 51 (1 RCT) | ⊕⊝⊝⊝ Very low 3 4 | We are uncertain if rigid dressings increase or decrease the proportion of wounds healed. The true effect is likely to be substantially different from the estimate of effect. | ||
| 58 per 100 | 67 per 100 (43 to 100) | ||||||
| Adverse events | Proportion of skin‐related adverse events ‐ all types (measured at 21 days and unknown time points) | Study population | RR 0.65 (0.32 to 1.32) | 336 (6 RCTs) | ⊕⊝⊝⊝ Very low 5 6 | We are uncertain if rigid dressings increase or decrease the proportion of skin‐related adverse events. The true effect is likely to be substantially different from the estimate of effect. | |
| 10 per 100 | 7 per 100 (3 to 13) | ||||||
| Proportion of non skin‐related adverse events ‐ all types (measured at 30 days, within 18 months and unknown time points) | Study population | RR 1.09 (0.60 to 1.99) | 342 (6 RCTs) | ⊕⊝⊝⊝ Very low 5 6 | We are uncertain if rigid dressings increase or decrease the proportion of non skin‐related adverse events. The true effect is likely to be substantially different from the estimate of effect. | ||
| 11 per 100 | 12 per 100 (6 to 21) | ||||||
| Time from amputation to no pain (weeks) | The mean time from amputation to no pain was 5.18 weeks | MD 0.35 lower (2.11 lower to 1.41 higher) | ‐ | 23 (1 RCT) | ⊕⊝⊝⊝ Very low 4 7 | We are uncertain if rigid dressings result in absence of pain in a shorter or longer time frame post‐op. The true effect is likely to be substantially different from the estimate of effect. | |
| Physical function ‐ Time from amputation to walking (days) | The mean time from amputation to walking was 33.3 days | MD 3 lower (9.96 lower to 3.96 higher) | ‐ | 56 (1 RCT) | ⊕⊝⊝⊝ Very low 2 7 | We are uncertain if rigid dressings result in walking in a shorter or longer time frame post‐op. The true effect is likely to be substantially different from the estimate of effect. | |
| Length of hospital stay (days) | The mean length of hospital stay was 129.9 days | MD 30.10 lower (49.82 lower to 10.38 lower) | ‐ | 56 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | We have very little confidence that rigid dressings reduce the length of hospital stay. The true effect is likely to be substantially different from the estimate of effect. | |
| Patient comfort | No data reported | ||||||
| Quality of life | No data reported | ||||||
| Cost | No data reported | ||||||
| aThe risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded by one level due to optimal information size criterion not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004).
2 Downgraded by two levels due to very high risk of bias for blinding of outcome assessor and selective reporting, and unclear risk of bias for random sequence generation and concealed allocation.
3 Downgraded by two levels due to optimal information size criterion not met for dichotomous outcome (i.e. at least 190 patients from a sample size power calculation based on an absolute target difference of 20%, two‐sided alpha of 0.05 and power of 80%), and 95% CI crossing no effect.
4 Downgraded by two levels due to very high risk of bias for incomplete outcome data and selective reporting, and unclear risk of bias for random sequence generation, concealed allocation and blinding of outcome assessor.
5 Downgraded by one level due to 95% CI crossing no effect.
6 Downgraded by two levels due to all 6 studies scoring very high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting.
7 Downgraded by two levels due to optimal information size criterion not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004), and 95% CI crossing no effect.
Background
Description of the condition
Lower limb amputation can result from non‐traumatic causes (e.g. dysvascular disease, malignancy and congenital deficiencies) or traumatic causes (e.g. war injuries and work accidents) (Varma 2014; Ziegler‐Graham 2008). Amongst these causes, dysvascular disease is most common, and includes diseases such as diabetes and peripheral vascular disease (Varma 2014; Ziegler‐Graham 2008). The incidence of lower limb amputation is estimated to be 24 per 100,000 in the USA (Moxey 2011), and 26 per 100,000 in the UK (Ahmad 2014). These estimates increase in people with diabetes, and estimates range from 410 to 3100 per 100,000 in the USA and from 147 to 248 per 100,000 in the UK (Moxey 2011). Trauma is the second most common cause of limb loss (Varma 2014; Ziegler‐Graham 2008), and accounts for 16% of amputations in the USA (Tintle 2010), and 7% to 9% of amputations in the UK (Perkins 2012). Approximately half of all lower limb amputations are transtibial (below the knee) amputations (Curran 2014; Fortington 2013; Kayssi 2015; Moxey 2010; Zayed 2014).
Poor outcomes are commonly reported post‐lower limb amputation. High mortality rates have been reported in patients with non‐traumatic amputations, with almost 50% dying within one year and 70% dying within three years, mostly due to underlying co morbidities (e.g. heart failure, renal failure, cancer and chronic obstructive pulmonary disease) (Jones 2013). The rate of hospital readmission within 30 days ranges from 10% to 30%, with a large proportion readmitted due to wound complications and stump revisions (Curran 2014; Kayssi 2015; Ries 2015). In patients with traumatic amputations, half have been reported to have substantial disability at two‐year and seven‐year follow‐up (MacKenzie 2004; MacKenzie 2005). Rehospitalisation rates are similar at less than 30%, with 34% developing wound infections and 15% requiring revision (Harris 2009). Consequently, the cost of acute and post‐acute care of an initial episode of amputation is high, costing more than USD 8.3 billion yearly in the USA (Ma 2014). In the UK, up to GBP 985 million is spent on care related to foot ulcers and amputations (Hex 2012).
Description of the intervention
Two main types of dressings can be applied after a transtibial amputation, namely soft and rigid dressings. The dressings applied to amputation sites differ from local wound dressings (e.g. hydrogel dressings, negative wound therapy, honey, aloe Vera) in that they deliver a degree of compression in order to reduce stump swelling and help to prepare and shape the stump for prosthetic fitting (Choudhury 2001; Smith 2003). Soft dressings (e.g. elastic or crepe bandages) are the conventional choice of dressing due to their low cost and easy applicability (Choudhury 2001). However, rigid dressings have grown in popularity due to the belief that a hard exterior provides greater compression, greater reduction in swelling and hence faster wound healing and shorter time to prosthetic fitting (Churilov 2014; Nawijn 2005). Rigid dressings are the intervention of interest in this systematic review and the various types are outlined below (Smith 2003).
Non‐removable rigid dressings
These are multi‐layered dressings made out of gauze pads and bandages, cotton/woollen/synthetic fibre stump socks and a plaster of Paris cast. Dressings are moulded up to the thigh level of the stump with the knee immobilised in full extension. The earliest report of their use is in 1961 (Baker 1977; Golbranson 1968). These dressings are sometimes combined with an immediate postoperative prosthesis (Johannesson 2010). Plaster of Paris casts are also sometimes replaced with a prefabricated plastic dressing held by neoprene and Velcro straps (Sumpio 2013).
Removable rigid dressings
These are similar to non‐removable rigid dressings except they do not contain the knee which can be flexed. Use of a removable rigid dressing was first reported in 1979 (Wu 1979). The main advantages of a removable rigid dressing over a non‐removable rigid dressing are that it allows frequent observation of the wound and does not require another cast to be made. If stump volume decreases, socks can be added to the cast and the cast placed back on the stump (Wu 1979). Removable rigid dressings may increase susceptibility to knee flexion contractures because the knee is not held in extension. In order to keep the knee extended and minimise the chances of knee flexion contractures, the use of pouches on patients' wheelchairs (Hughes 1998), or custom‐made removable bivalved rigid shells have also been suggested (Duwayri 2012). Plaster of Paris casts are also sometimes replaced with a fibreglass/synthetic cast for a lighter cast (Duwayri 2012; Taylor 2008).
Immediate postoperative prostheses
These allow for early weight‐bearing on the stump and can vary in terms of their top or bottom parts. The top part surrounding the stump can come as a custom‐made plaster of Paris cast (Burgess 1968; Condon 1969; Folsom 1992), a prefabricated pneumatic air bladder/air splint (Pinzur 1989; Schon 2002) or a prefabricated plastic dressing held by neoprene and Velcro straps (Ali 2013). The bottom part that is in contact with the ground can be either a metal cylinder (Pinzur 1989), or an adjustable aluminium pylon attached to an artificial foot (Ali 2013; Burgess 1968; Condon 1969; Folsom 1992; Schon 2002).
Other treatments
These include combinations of the above (e.g. non‐removable rigid dressings and immediate postoperative prostheses) or dressings and prostheses that are not yet described. These include the Sterishield Controlled Environment Unit (CEU) and semi‐rigid dressings. The CEU consists of a sterile transparent pneumatic plastic cylinder, which allows the flow of warm filtered air through the system but does not allow weight‐bearing (Ruckley 1986). Semi‐rigid dressings consist of a bandage imbedded with Unna paste (zinc oxide, calamine, gelatin and glycerine) which forms a semi‐rigid inextensible dressing (MacLean 1994; Wong 2000).
How the intervention might work
The main postulated benefits of rigid dressings over soft dressings are:
greater reduction in swelling via application of more consistent pressure around the stump (Duwayri 2012; Golbranson 1968); and
greater protection of the stump from trauma due to the hard surface of a rigid dressing (Duwayri 2012; Wu 1979).
These factors are believed to lead to faster wound healing, reduced risk of wound infection/breakdown, reduced pain, shorter time to prosthetic fitting and reduced length of stay in the hospital (Churilov 2014; Schon 2002).
Why it is important to do this review
There is uncertainty about the most appropriate and effective type of dressings following transtibial amputations. Several reviews have been conducted to investigate the efficacy of rigid dressings in improving outcomes in transtibial amputations, though only two were systematic reviews (Churilov 2014; Nawijn 2005). Of these two systematic reviews, one review was published more than a decade ago (Nawijn 2005), and one only investigated the efficacy of rigid dressings on one outcome (i.e. time from amputation to prosthetic fitting) (Churilov 2014). Despite being the first meta‐analysis to be conducted on the literature, Churilov 2014 drew conclusions in support of rigid dressings without consideration of the inconsistency and imprecision of the results from the studies included in the systematic review. Several amputee care guidelines have also recommended the use of rigid dressings for transtibial amputations (BACPAR 2012; US Department of Veterans Affairs 2017), though these recommendations are largely based on poorly‐conducted randomised controlled trials (RCTs), observational studies, case‐control studies and retrospective audits. Due to the scepticism surrounding the certainty of evidence on rigid dressings and the belief that rigid dressings can lead to wound breakdowns in some patients with poor skin integrity, there remains wide variation in practice concerning dressings in transtibial amputations (Barnes 2014; Choudhury 2001). It is therefore important to conduct a comprehensive and rigorous systematic review to summarise recent evidence on the benefits and harms of rigid dressings in transtibial amputations.
Objectives
To assess the benefits and harms of rigid dressings versus soft dressings for treating people after transtibial amputation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs. The latter included studies with quasi‐randomised allocation procedures, such as alternation, hospital record number or date of birth (Lefebvre 2011).
Types of participants
We included people of all ages with transtibial amputation irrespective of the reason for amputation. The reasons for amputation were expected to include dysvascular disease (diabetes, peripheral vascular disease), trauma and cancer. If a study had mixed populations (e.g. transtibial and transfemoral amputations), we included the study if more than 75% of participants had transtibial amputations.
Types of interventions
Rigid dressings, including non‐removable rigid dressings, removable rigid dressings, immediate postoperative prostheses and others;
Soft dressings, including crepe bandaging and elastic/compression bandaging.
Types of outcome measures
Timing of outcome measures
Outcomes could be obtained at any time point following amputation. We grouped outcomes according to the time since amputation:
short‐term outcomes: outcomes obtained less than one month since amputation;
medium‐term outcomes: outcomes obtained between one to three months of amputation;
long‐term outcomes: outcomes obtained after three months of amputation.
Where data (i.e. time point of outcome measurements) were available, we presented dichotomous and continuous outcomes as short‐term, medium‐term and long‐term outcomes. We presented time‐to‐event outcomes at the median or mean follow‐up reported by the authors. We used our judgement as to whether statistical pooling within these outcomes was appropriate.
Primary outcomes
Wound healing measured as time from amputation to wound healing and proportion of wounds healed.
Complications/adverse events measured as proportion of skin‐related complications/adverse events (e.g. wound infections/breakdowns/stump revisions/further amputations/pressure areas), proportion of non skin‐related complications/adverse events (e.g. deaths, chest infections, falls, pain) and severity of pain on the visual analogue scale.
Secondary outcomes
Prescription of prosthetics measured as time from amputation to first prosthetic fit/cast.
Physical function measured as time to independent ambulation, proportion of participants mobilising independently and functional assessment scales (e.g. Functional Independence Measure scale).
Length of hospital stay measured as time from hospital admission to discharge.
Patient comfort measured with a validated scale used to measure patient’s ease, comfort or satisfaction with the dressing.
Quality of life data measured with generic or wound‐specific questionnaires.
Cost measured as any cost relating to dressings or other resources (e.g. personnel costs).
Swelling measured as girth measurements or any other measures of stump volume reported by study authors. (We note that swelling is a potential surrogate outcome for other outcomes such as wound healing, physical function and length of hospital stay. Conclusions regarding efficacy of rigid dressings were therefore not based on swelling).
We anticipated that study authors would define wound healing in different ways (Gethin 2015). We did not try to enforce a single definition of wound healing across all trials but instead extracted data according to each authors’ definition of wound healing. We also aligned our methods of data extraction and data analysis/synthesis of wound outcomes with previous Cochrane systematic reviews on wound healing for consistency (Dumville 2015a; Dumville 2015b). We covered these methods further in the sections on Data extraction and management, Measures of treatment effect, Unit of analysis issues and Data synthesis.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
Cochrane Wounds Specialised Register (searched 5 December 2018);
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (searched 5 December 2018);
Ovid MEDLINE (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE Daily) (1946 to 5 December 2018);
Ovid Embase (1974 to 5 December 2018);
EBSCO CINAHL Plus (1937 to 5 December 2018);
Ovid AMED (1985 to 18 November 2018);
PEDro (www.pedro.org.au) (to 14 December 2018).
The search strategies used for these databases can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008) revision (Lefebvre 2011). We combined the Embase search with the Ovid Embase trial filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus search with the trial filter terms developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018).There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 5 December 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 5 December 2018).
Searching other resources
In order to identify further published, unpublished and ongoing studies, we also:
searched the reference lists of relevant studies and reviews identified in prior searches;
used the Cited Reference Search facility on ThomsonReuters Web of Science;
contacted relevant individuals and organisations for unpublished and ongoing studies;
searched the grey literature using Open Grey and Google Scholar.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Kwah 2016), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors (LKK and LG) independently screened titles and abstracts to determine eligibility of potential studies. We resolved any disagreements through discussion and the third review author (LH) arbitrated if there was still disagreement. We obtained full‐text publications of the potentially eligible studies and two review authors (LKK and LG) independently screened these publications for inclusion. We excluded studies that did not meet the inclusion criteria at this point. We recorded the excluded studies and their reasons for exclusion in the Characteristics of excluded studies table. If we required more information to determine the eligibility of studies, we contacted the investigators of relevant studies for more information. If there were disagreements regarding the eligibility of the full‐text publications, we consulted a third review author (LH) to resolve these disagreements.
We completed a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow‐chart to summarise this process (Liberati 2009). We used the reference management software EndNote (EndNote 2014) to manage the records retrieved in the selection process.
Data extraction and management
Two review authors (LKK and MW) independently extracted data on study characteristics and outcomes from the included studies using a data extraction form. The categories of data extracted included:
methods: study design, method of randomisation, country of study, type of incision (skew flap or long posterior flap), care setting (acute/surgical or rehabilitation);
participants: sample size (by group), number of dropouts (by group), inclusion criteria, exclusion criteria, baseline characteristics of participants (age, gender, traumatic or non‐traumatic amputation, co‐morbidities and skin integrity (e.g. measured with the NPUAP Pressure Ulcer Stages/Categories), by group if provided);
interventions: type of dressing, time to first application of dressing, person applying dressing, duration of dressing (hours per day, days/weeks), duration of dressing removal, frequency of dressing change and the same information for comparator therapy;
outcomes: primary outcomes (units and definitions), secondary outcomes (units and definitions), other outcomes included in review (units and definitions), other outcomes not included in review (units and definitions), timing of outcomes included in review (short term, medium term or long term with specific time frames) and timing of outcomes not included in review;
notes: publication status, funding of trials and conflicts of interest.
We used a piloted data extraction form. We resolved all disagreements by discussion or arbitration with the third review author (LH). One review author (LKK) entered the extracted data into Review Manager (RevMan) and a second author (MW) cross‐checked the data to ensure accuracy (Review Manager 2014). We screened for potential duplicate publications by cross‐checking authors’ names, year of publication and journal titles. We downloaded and assessed full‐text copies of the studies if we were uncertain whether or not the publication was a duplicate. Where important information was missing or unclear we attempted to contact study authors.
If several measures of a similar outcome (e.g. wound healing) were present in a study, we extracted all data and listed them in a summary of outcomes table (Table 2), but we only entered the preferred data type into the meta‐analyses. The preferred data type were time‐to‐event outcomes, followed by dichotomous outcomes and, lastly, continuous outcomes. Time‐to‐event outcomes (e.g. time from amputation to wound healing) and dichotomous outcomes (e.g. proportion of wounds healed) were preferred as these are likely to have more clinical relevance than continuous outcomes (e.g. wound size). Time‐to‐event outcomes were preferred over dichotomous outcomes as they allow more comparisons between studies with different follow‐up time points and are less prone to selective outcome reporting bias, which can occur in studies with dichotomous outcomes since investigators can intentionally select time points that show the least or greatest difference between groups (Tierney 2007).
1. Summary of primary and secondary outcomes.
| Authors (Year) |
Primary outcome: wound healing |
Primary outcome: adverse events |
Secondary outcome: prescription of prosthetics | Secondary outcome: physical function | Secondary outcome: length of hospital stay | Secondary outcome: swelling |
| Baker 1977 |
Proportion of primary wound healinga Rigid: 18/27e Soft: 14/24e (short term) Proportion of secondary wound healinga Rigid: 5/27 Soft: 6/24 |
Proportion of skin‐related adverse events: revisions Rigid: 4/27e Soft: 4/24e Proportion of non skin‐related adverse events: deaths Rigid: 0/27e Soft: 0/24e (medium term) |
NR |
Averageb time to gait training Rigid: 29.6 days; n = 13 Soft: 36.5 days; n = 11 |
NR by group | NR |
| Deutsch 2005 |
Mean (SD) time to wound healing Rigid: 51.2 (19.4) days; n = 17 Soft: 64.7 (29.5) days; n = 14 |
Proportion of skin‐related adverse events: revisions Rigid: 1/26e Soft: 2/24e Proportion of skin‐related adverse events: surgical debridements Rigid: 1/26 Soft: 2/24 Proportion of skin‐related adverse events: stump damage post‐fall Rigid: 0/4 Soft: 3/6 Proportion of non skin‐related adverse events: deaths Rigid: 3/26e Soft: 3/24e Proportion of non skin‐related adverse events: falls Rigid: 4/26 Soft: 6/24 Proportion of non skin‐related adverse events: not for prosthetic rehab Rigid: 1/26 Soft: 1/24 Proportion of non skin‐related adverse events: medical complications Rigid: 2/26 Soft: 0/24 |
Mean (SD) time to prosthetic fitting Rigid: 23.3 (19.5) days; n = 22 Soft: 22.6 (15.7) days; n = 19 |
NR |
Mean (SD) time to discharge from acute and rehabilitation settings Rigid: 46.1 (32.7) days; n = 17 Soft: 44.6 (28.0) days; n = 17 Mean (SD) time to discharge from acute setting Rigid: 15.5 (9.2) days; n = 22 Soft: 17.4 (14.3) days; n = 19 |
Proportion of prosthetic sockets required within 6 months Rigid: 1.33/20 Soft: 1.47/17 |
| Hidayati 2013 | NR |
Average (SD) time to resolution of non skin‐related adverse event: stump pain Rigid: 4.83 (1.946) weeks; n = 12e Soft: 5.18 (2.31) weeks; n = 11e Proportion of non skin‐related adverse events: deaths NR by group Average (SD) of pain VAS score Rigid: 2.5 (1.24); n = 12 Soft: 1.73 (1.00); n = 11 (short term)c Rigid: 3.66 (1.56); n = 12 Soft: 3.36 (1.28); n = 11 (medium term)c |
NR | NR | NR |
Time to "decrease in stump oedema volume" Relative Risk = 3.088 (95% CI 1.128 to 4.916) Mean (SD) time to stump being "free of oedema" Rigid: 5.08 (1.17) weeks; n = 12 Soft: 6.82 (1.31) weeks; n = 11 Average (SD) decrease in stump volume Rigid: 133.33 (62.24); n = 12e Soft: 94.55 (33.57); n = 11e (short term)c Rigid: 87.92 (70.6); n = 12e Soft: 106.45 (76.17); n = 11e (medium term)c |
| Janchai 2008 | NR |
Proportion of skin‐related adverse events: wound trauma from falls Rigid: 1 / 12e Soft: 1 / 14e Proportion of non skin‐related adverse events: falls Rigid: 1 / 12e Soft: 1 / 14e |
NR | NR | NR |
Mean (SD) decrease in stump volume Rigid: 42.73 (62.70)cm3; n = 12e Soft: 21.89 (118.49)cm3; n = 14e (short term) Rigid: 79.90 (103.33)cm3; n = 11e Soft: 83.03 (113.05)cm3; n = 11e (medium term) |
| MacLean 1994 | NR |
Proportion of skin‐related adverse events: revisions Rigid: 3/19e Soft: 7/21e Proportion of non skin‐related adverse events: deaths Rigid: 1/19e Soft: 2/21e Proportion of non skin‐related adverse events: medical complications Rigid: 0/19e Soft: 1/21e Proportion of non skin‐related adverse events: stump pain Rigid: 5/19 Soft: 8/21 Proportion of non skin‐related adverse events: phantom pain Rigid: 8/19 Soft: 9/21 |
Time to prosthetic fitting Survival curves presented but data not used as outcome was lack of readiness of prosthetic fitting, instead of readiness of prosthetic fitting. |
NR | NR | NR |
| Mueller 1982 | NR |
Proportion of skin‐related adverse events: pressure areas Rigid: 0/7e Soft: 0/8e (short term) |
NR | NR | NR |
Mean (SD) decrease in stump volume Rigid: 70.7 (21.3) cm3; n = 8 (limb)e Soft: 31.2 (49.0) cm3; n = 8 (limb)e (short term) |
| Vigier 1999 |
Mean (SD) time to wound healing Rigid: 71.2 (31.7) days; n = 28e Soft: 96.8 (54.9) days; n = 28e |
NR | NR |
Mean (SD) time to walking with offload prosthesis Rigid: 30.3 (16.2) days; n = 28e Soft: 33.3 (9.5) days; n = 28e Mean (SD) time to walking with prosthesis and contact socket Rigid: 63.5 (20.8) days; n = 28 Soft: 73.3 (31.2) days; n = 28 |
Mean (SD) time to discharge from acute and rehabilitation settings Rigid: 99.8 (22.4) days; n = 28e Soft: 129.9 (48.3) days; n = 28e Mean (SD) time to discharge from acute setting Rigid: 22.4 (13.0) days; n = 28 Soft: 20.5 (9.1) days; n = 28 |
Time‐to‐event: nil |
| Wong 2000 | NR |
Proportion of non skin‐related adverse events: deaths Rigid: 1/12e Soft: 2/9e (long term) |
Time to prosthetic fitting HR calculated: 0.27 (95% CI 0.09 to 0.84)ed |
Proportion of fit and ambulatory Estimates presented but data not used as functional outcome scale was not validated "Average"score for FIM – transfer item Rigid: 5.9 (range 3 to 7) Soft: 5.6 (range 2.5 to 7) (long term) "Average"score for FIM – gait with or without prosthesis item Rigid: 5.0 (range 2 to 6) Soft: 2.6 (range 0 to 6) (long term) |
NR by group | NR |
| Woodburn 2004 | NR |
Proportion of skin‐related adverse events: revisions Rigid: 2/78e Soft: 3/76e Proportion of skin‐related adverse events: wound infections Rigid: 12/78 Soft: 10/76 Proportion of non skin‐related adverse events: deaths Rigid: 14/78e Soft: 10/76e |
Median time to prosthetic casting Rigid: 36 days (95% CI 30 to 47); n = 78 Soft: 42 days (95% CI 36 to 45); n = 76 |
NR | NR | NR |
Data for dichotomous outcomes are presented as n / N (number of participants with outcomes/total number of participants in group)
Data for continuous outcomes are presented as mean (SD), median (SD), or average (SD)
Data for time‐to‐event outcomes are presented as HR
aPrimary wound healing defined as healing that occurred when sutures removed at 14 to 21 days; Secondary wound healing defined as healing that occurred after 21 days
b Term "average" used when it is unclear if estimate refers to mean or median
cAs outcomes were measured weekly, data for short‐term outcomes were taken from week 0 to 3, and long‐term outcomes were taken from week 0 to 8.
dHR calculated using Method 9 from Tierney 2007. (Data input into calculations included P value, total events and numbers randomised to each arm)
eData entered into meta‐analyses
CI = Confidence interval; FIM = Functional Independence Measure; HR = Hazard ratio; NR = Not reported; VAS = Visual Analogue Scale
Assessment of risk of bias in included studies
Two review authors (LKK and MW) independently rated the risk of bias in each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We assessed the risk of bias using the following domains (see Appendix 2):
random sequence generation;
treatment allocation;
blinding of participants, care providers and outcome assessors;
incomplete outcome data;
selective outcome reporting;
other potential sources of bias (e.g. industry funding).
We rated each potential source of bias as either high, low or unclear in each included study and provided justification for our rating in the 'Risk of bias' table. If there was ambiguity, we contacted the study investigators for clarification. We also summarised the overall risk of bias of all studies for each domain and for each outcome so that the final results for outcome measures were deemed as either at high, low or unclear risk of bias.
Measures of treatment effect
For time‐to‐event data (e.g. time from amputation to wound healing), we calculated results as hazard ratios using the ‘O‐E’ (observed minus expected events) and ‘V’ (logrank variance) statistics derived from number of events and times to events in control and interventions groups (Tierney 2007). If these statistics were not readily available, we referred to further guidance (Tierney 2007), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If study authors provided a mean or median time to outcomes and clearly stated that all outcomes (e.g. wound healing) were achieved, we pooled these data in a meta‐analyses as continuous data. If it was unclear whether all participants achieved the outcome, we documented, but did not pool the data. We used the generic inverse variance method in RevMan (Review Manager 2014) for time‐to‐event data, and for dichotomous and continuous data when data could not be entered in the usual form (e.g. if study only reported odds ratio or relative risk and its standard error for dichotomous outcomes, or if a study only reported difference between the means for two groups and the standard error of this difference for continuous outcomes) (Higgins 2011a). If dichotomous and continuous data were presented in the usual form, we used the Mantel‐Haenszel method for dichotomous data and the inverse variance method for continuous data in Revman (Review Manager 2014).
For dichotomous data (e.g. proportion of wounds healed), we presented results as risk ratios (RRs) with 95% confidence intervals (CIs). We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) from the risk difference for easier interpretability of results (see Effects of interventions).
For continuous data (e.g. wound sizes, girth measurements, pain scores), we calculated results as means or changes in mean scores with 95% CIs. If studies used different scales to measure the same outcome, we planned to report standardised mean differences with 95% CIs. If ordinal data were present, we analysed these as continuous data.
Unit of analysis issues
If studies had more than one intervention group (e.g. non‐removable rigid dressings and removable rigid dressings) or more than one control group (e.g. crepe bandaging and elastic bandaging), we combined the groups such that we made only a single pair‐wise comparison, i.e. we compared data from both non‐removable rigid and removable rigid dressing groups against data from crepe bandaging and elastic bandaging groups. The unit of analysis was the participant. In the event that studies had participants with double amputations and treatment was carried out on both legs, we adjusted for intra‐patient correlation (intra‐cluster correlation) in the effect estimates of relevant outcome measures. However, if this number was very small (e.g. less than 10% of participants had double amputations) and studies had analysed data on limbs instead of participants, we included these studies in our meta‐analyses of outcome measures but conducted sensitivity analyses to determine if there were changes in effect estimates if such studies are omitted.
Dealing with missing data
If information was missing on the methods or results (e.g. data from dropouts, data reported at baseline but not at follow‐up, statistics such as standard deviations (SDs)), we contacted study investigators to request missing information. We contacted study investigators via email addresses provided in the publication or by searching the staff directory of authors’ affiliated organisations as stated in the publication. If we were unable to obtain the missing information, we estimated the missing SD values according to methods described in the Cochrane Handbook for Systematic Reviews of Interventions, Section 16.1.3 (Higgins 2011b). We performed sensitivity analyses to determine the influence of missing data on the results. We discussed findings of the review based on the results of our sensitivity analyses. To deal with missing data in analyses, we did the following: where studies measured dichotomous outcomes (e.g. proportion of participants experiencing adverse events) and had dropouts, we included the number of dropouts in the denominator but not in the numerator. This means that we assumed dropouts did not develop the outcomes of interest. Where studies measured continuous outcomes (e.g. wound size), or time‐to‐event outcomes (e.g. time from amputation to wound healing) and had dropouts, only complete‐case data were used. This means that we reported continuous outcomes and time‐to‐event outcomes of participants remaining in studies.
Assessment of heterogeneity
Before combining studies in meta‐analyses, we checked for clinical and statistical heterogeneity. We based judgements about clinical heterogeneity on clinical reasoning after reviewing participant, intervention and outcome characteristics of studies. We based judgements about statistical heterogeneity on the Chi² test and the I² statistic values (Higgins 2011a).
Assessment of reporting biases
We minimised reporting biases by searching several databases and clinical trial registries. We ensured that we did not enter data in duplicate publications twice into the meta‐analysis. If there were more than 10 studies for each outcome, we planned to create funnel plots and looked for signs of asymmetry. In our protocol, we stated that if there were fewer than 10 studies for each outcome, we would summarise the findings of the review based on the results of our sensitivity analyses.
Data synthesis
We combined details of included studies according to the type of comparator and outcomes. We considered clinical and methodological heterogeneity and undertook pooling when studies appeared appropriately similar in terms of intervention type, duration of follow‐up and outcome type.
We were unable to pre‐specify the amount of clinical, methodological and statistical heterogeneity in the included studies. Thus, we used a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. We only used a fixed‐effect approach when clinical and methodological heterogeneity was assessed to be minimal, and the assumption that a single underlying treatment effect was being estimated held. We used Chi2 and I2 to quantify heterogeneity but these were not used to guide choice of model for meta‐analysis (Kontopantelis 2013). We would have exercised caution when meta‐analysed data were at risk of small‐study effects because use of a random‐effects model may be unsuitable here. In this case, or where there were other reasons to question the selection of a fixed‐effect or random‐effects model, we planned to assess the impact of the approach using sensitivity analyses to compare results from alternate models, but this was not implemented (Thompson 1999).
We presented data using forest plots where possible. For dichotomous outcomes we presented the summary estimate as a risk ratio (RR) with 95% CI. Where continuous outcomes were measured, we presented a mean difference (MD) with 95% CI; we planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and if appropriate pool) estimates of hazard ratios (HR) and 95% CIs as presented in the study reports using the generic inverse variance method in RevMan 5. Where time to healing was analysed as a continuous measure, but it was not clear if all wounds had healed, we documented use of the outcome in the study, but did not summarise or use these data in any meta‐analysis.
We obtained pooled estimates of the treatment effect using Cochrane Review Manager 5 software (RevMan 2014).
'Summary of findings' table and GRADE assessment of the certainty of evidence
We presented the main results of the review in a 'Summary of findings' table using GRADEpro GDT (GRADEpro GDT 2015). This table presents key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). Two review authors (LKK and MW) independently applied the GRADE approach to the body of evidence for all outcomes.
We presented the following outcomes in the 'Summary of findings' table; wound healing, complications/adverse events, physical function and length of hospital stay. As there were no data reported for patient comfort, quality of life and cost in any of the published studies, these outcomes appear in the 'Summary of findings' table as empty rows. For relevant outcomes reported for comparisons not listed above (i.e. prescription of prosthetics and swelling), we presented GRADE assessments narratively within the Results section without inclusion in a 'Summary of findings' table. In terms of the GRADE assessment, when making decisions for the risk of bias domain, we downgraded only when studies were classed at high risk of bias for one or more domains. We did not downgrade for unclear 'Risk of bias' assessments. In assessing the precision of effect estimates, we also followed GRADE guidance (GRADE 2013); we assessed the size of confidence intervals, downgrading twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity using the methods described in Section 9.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We planned to perform subgroup analyses to determine whether the size of treatment effects were influenced by the following:
type of rigid or soft dressings (e.g. non‐removable rigid dressings vs crepe bandaging, removable rigid dressings versus crepe bandaging, non‐removable rigid dressings versus elastic bandaging, removable rigid dressings versus elastic bandaging).
We were unable to perform subgroup analyses as fewer than 10 studies were included in the meta‐analysis.
Sensitivity analysis
We performed sensitivity analyses to determine if the results were robust to arbitrary decisions that we made during the review process. Specifically, we performed sensitivity analyses to determine the influence of studies with transfemoral amputations and studies that had analysed data on limbs instead of participants (provided less than 10% of participants had double amputations). We also assessed whether these results differed when we only considered studies at low risk of bias versus studies of high and unclear risk of bias in specific methodological aspects of the study. These methodological aspects include:
randomisation (true random versus quasi‐random);
concealed allocation (concealed versus non‐concealed);
blinding of assessors (blinding versus no blinding); and
dropout rate (greater than 15% versus less than 15%).
Results
Description of studies
Results of the search
We present the results of our search in Figure 1. After duplicates were removed, we screened the titles and abstracts of 1483 records and obtained 22 articles for eligibility assessment. Of the 22 articles, 20 were full‐text articles and two were trial protocols of ongoing studies.
1.
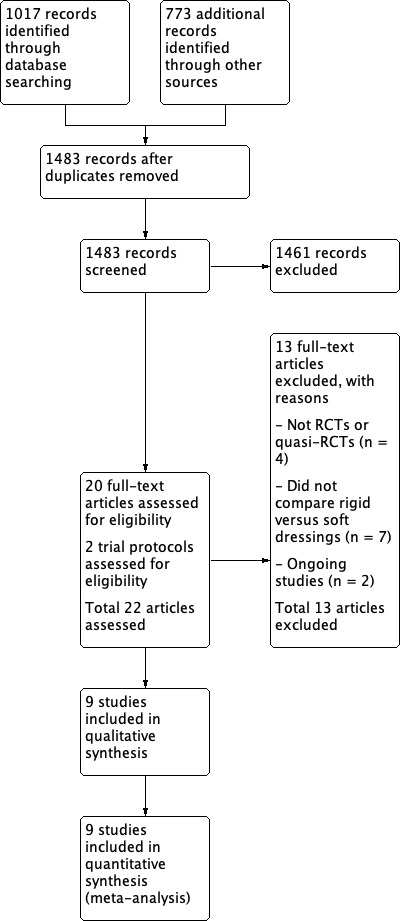
Study flow diagram.
Included studies
Of the 20 full‐text articles, we included nine randomised controlled trials (RCTs) and quasi‐RCTs involving 436 participants (441 limbs) (Baker 1977; Deutsch 2005, Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000; Woodburn 2004). Details of the nine included studies are provided in the Characteristics of included studies. We summarise the characteristics of included studies as follows.
Methods
Of the nine studies, seven were RCTs (Baker 1977; Deutsch 2005; Hidayati 2013; Janchai 2008; Vigier 1999; Wong 2000; Woodburn 2004) and two were quasi‐RCTs (MacLean 1994; Mueller 1982). Studies were conducted in seven countries. These included the USA (Baker 1977; Mueller 1982; Wong 2000), Australia (Deutsch 2005), Indonesia (Hidayati 2013), Thailand (Janchai 2008), Canada (MacLean 1994), France (Vigier 1999) and the UK (Woodburn 2004). Of the nine studies, five studies recruited participants from a single site (i.e. one hospital) (Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000) and four studies recruited participants from multiple sites (i.e. two hospitals (Baker 1977; Deutsch 2005), three hospitals (Hidayati 2013) and seven hospitals (Woodburn 2004)). The care setting of hospitals were a mix of acute and rehabilitation settings (Baker 1977; Deutsch 2005; MacLean 1994) or rehabilitation settings (Janchai 2008; Mueller 1982; Vigier 1999; Wong 2000), with two studies not reporting specific care settings (Hidayati 2013; Woodburn 2004). The types of incision used during surgery were generally not reported although a long posterior flap was used in two studies (Baker 1977; Deutsch 2005), a posterior or non‐posterior flap was used in one study (Hidayati 2013), and a skew or long posterior flap was used in one study (Woodburn 2004).
Participants
The number of participants in studies ranged from 15 to 154 (Baker 1977; Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000; Woodburn 2004). The mean age of participants ranged from 54 to 75 (Baker 1977; Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000) although age was not always reported. The number of male participants ranged from 10 to 114, and the number of female participants ranged from five to 40 (Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000; Woodburn 2004), although these numbers were not always reported. In studies that reported information about co‐morbidities of participants and causes of amputation, more than half of all participants in studies had diabetes (Baker 1977; Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Wong 2000) and other co‐morbidities (e.g. anaemia, smoking history, hypertension, cardiac disease) (Baker 1977; Hidayati 2013; MacLean 1994; Mueller 1982; Wong 2000). It was specified in eight of the nine studies that the amputations were secondary to vascular conditions (e.g. arterial occlusive disease, peripheral vascular disease) (Baker 1977; Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000). Skin integrity of participants was not reported in any of the studies.
Intervention ‐ rigid dressings
Several rigid dressing types were used in the studies. Most studies used removable rigid dressings (Deutsch 2005; Hidayati 2013; Janchai 2008; Mueller 1982; Vigier 1999), followed by non‐removable rigid dressings (Baker 1977; Woodburn 2004) and semi‐rigid dressings with Unna paste (MacLean 1994; Wong 2000). Dressings were applied by prosthetists (Baker 1977; Deutsch 2005), physiotherapists (MacLean 1994; Mueller 1982; Wong 2000), surgeons (Woodburn 2004), participants, relatives or caregivers (Janchai 2008; Mueller 1982). In studies that clearly reported time of dressing application, dressings were applied immediately post‐operation (Deutsch 2005; MacLean 1994; Woodburn 2004), within the first month post‐operation (Hidayati 2013) and on rehabilitation admission (Mueller 1982). Dressings remained in‐situ for varying amounts of time (e.g. "continuously", 30 minutes, 23.5 hours and five hours daily) over a period of three days to eight weeks (Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000; Woodburn 2004). Most studies did not report how long dressings were removed for. Only one study reported that the removable rigid dressing was not removed for more than 15 minutes (Deutsch 2005).
Comparator therapy ‐ soft dressings
Soft dressing types included elastic bandage (Baker 1977; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Vigier 1999; Wong 2000), crepe bandage (Deutsch 2005) and soft bandage (Woodburn 2004). Dressings were applied by ward physicians (Baker 1977), surgeons (MacLean 1994; Woodburn 2004), physiotherapists (MacLean 1994; Mueller 1982), nurses (MacLean 1994; Wong 2000), participants, relatives or caregivers (Hidayati 2013; Janchai 2008; Mueller 1982; Wong 2000). In studies that clearly reported time of dressing application, dressings were applied immediately post‐operation (Deutsch 2005; MacLean 1994; Woodburn 2004), within the first month post‐operation (Hidayati 2013) and on rehabilitation admission (Mueller 1982). Dressings remained in‐situ for varying amounts of time (e.g. "continuously", "at all times", "permanently" and 23.5 hours daily) (Deutsch 2005; Janchai 2008; Mueller 1982; Vigier 1999), with only one study reporting dressing remained in‐situ over a period of eight weeks (Hidayati 2013). Most studies did not report how long dressings were removed for. Only one study reported that the soft dressing was not removed for more than 15 minutes (Deutsch 2005).
Comparisons of intervention (rigid dressings) and comparator therapy (soft dressings)
Most studies compared the effects of removable rigid dressings with either crepe bandaging (Deutsch 2005) or elastic bandaging (Hidayati 2013; Janchai 2008; Mueller 1982; Vigier 1999). The other comparisons included semi‐rigid dressings with elastic bandaging (MacLean 1994; Wong 2000), non‐removable rigid dressings with either elastic bandaging (Baker 1977) or soft bandaging (Woodburn 2004).
Outcomes
Few studies clearly stated the time point of outcome measurements (Baker 1977; Hidayati 2013; Janchai 2008; Mueller 1982). Hence, most outcomes could not be grouped according to the time since amputation (i.e. outcomes could not be categorised into short‐term, medium‐term or long‐term outcomes). Based on the few studies that clearly stated time point of outcome measurements, only two outcomes could be grouped according to the time since amputation. These two outcomes were wound healing (categorised as short‐term outcome) (Baker 1977), and swelling (categorised as short‐term and medium‐term outcomes) (Hidayati 2013; Janchai 2008; Mueller 1982).
Several studies measured time‐to‐event outcomes (Baker 1977; Deutsch 2005; Hidayati 2013; MacLean 1994; Vigier 1999; Wong 2000; Woodburn 2004), but reported the data as continuous data (e.g. mean time from amputation to wound healing/adverse events/prescription of prosthetics/physical function/discharge/swelling) rather than time‐to‐event data (e.g. survival curves, hazard ratios). As per protocol, we only pooled such data in meta‐analyses if studies clearly stated that all outcomes were achieved in participants. We considered these data as usable data; data that were not included in meta‐analyses were documented in the summary of outcomes table (Table 2). We made attempts to contact authors for time point of outcome measurements, and additional information that might render their data usable in meta‐analyses (e.g. standard deviation (SD) for time to walking independently (Baker 1977), SD for time to prescription of prosthetics (Woodburn 2004), and HR for "decreasing stump volume" (Hidayati 2013)); however we either received no responses from the authors (Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Woodburn 2004), or could not locate the email addresses of authors (Baker 1977).
In total, two studies provided usable data on wound healing (Baker 1977; Vigier 1999), eight studies provided usable data on adverse events (Baker 1977; Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Mueller 1982; Wong 2000; Woodburn 2004), one study provided usable data on prescription of prosthetics (Wong 2000), one study provided usable data on physical function (Vigier 1999), one study provided usable data on length of hospital stay (Vigier 1999), and three studies provided usable data on swelling (Hidayati 2013; Janchai 2008; Mueller 1982). Further details on outcomes are provided in Effects of interventions. In addition, three studies analysed limbs instead of participants (MacLean 1994; Mueller 1982; Vigier 1999) and one study included a small number of participants with transfemoral amputations (Wong 2000). Thus, sensitivity analyses were conducted to examine the effects of analyses conducted by limbs rather than participants on adverse events and swelling, and the effects of transfemoral amputations on adverse events and prescription of prosthetics.
Ongoing studies
Our search of trial registries identified two ongoing studies (TCTR20170928004; NCT03593174) which met inclusion criteria. No study results or publications are available yet (see details of trials in Characteristics of ongoing studies).
Excluded studies
We excluded 11 out of the 20 articles we screened in full text; four were not RCTs or quasi‐RCTs (Jones 1970; Kane 1980; Ladenheim 2007; Mooney 1971), and seven did not investigate the efficacy of rigid dressings compared with soft dressings in transtibial amputations (Choksy 2006; Graf 2003; Johannesson 2008; Louie 2010; Manella 1981; Ruckley 1986; Topuz 2012). Instead, these studies compared rigid dressings with a different type of rigid dressing (Graf 2003; Johannesson 2008; Ruckley 1986), soft dressings with a different type of soft dressings (Louie 2010; Manella 1981), soft dressings with complex decongestive physiotherapy (which included manual lymphatic drainage, skin care, short‐stretch bandages and exercise) (Topuz 2012), or compared using a tourniquet with no tourniquet (Choksy 2006) (see further details in Characteristics of excluded studies).
Risk of bias in included studies
The nine included studies were assessed for risk of bias. Results are summarised in Figure 2 and Figure 3, with further details provided below and in the Characteristics of included studies.
2.
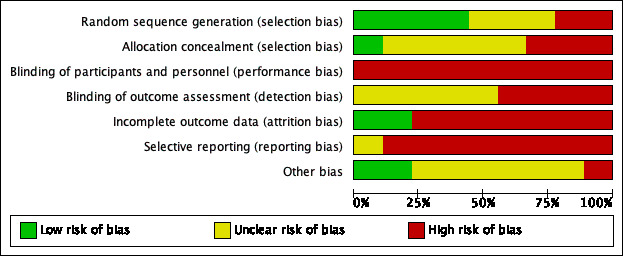
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
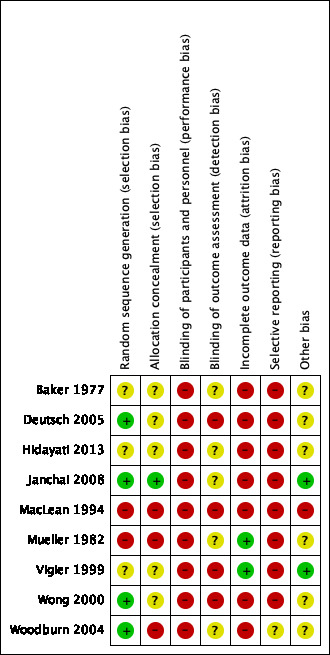
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Four studies (44%) were classified as being at low risk of bias for random sequence generation, and used various randomisation methods including ballot, table of random numbers and a central telephone randomisation service (Deutsch 2005; Janchai 2008; Wong 2000; Woodburn 2004). The remaining studies were rated either unclear for risk of bias due to insufficient details (Baker 1977; Hidayati 2013; Vigier 1999), or at high risk of bias due to alternate assignment of participants to groups (MacLean 1994; Mueller 1982).
Allocation concealment
One study (11%) was rated low risk of bias for allocation concealment. Allocation was concealed by having a secretary open a series of opaque envelopes which had group allocation numbers in them (Janchai 2008). The remaining studies were rated either unclear for risk of bias due to insufficient details (Baker 1977; Deutsch 2005; Hidayati 2013; Vigier 1999; Wong 2000), or high risk of bias due to alternate assignment of participants to groups (MacLean 1994; Mueller 1982) and surgeons knowing the randomisation schedule while assessing eligibility of participants (Woodburn 2004).
Blinding
Blinding of participants and personnel (performance bias)
Due to the nature of the intervention, it is not possible to blind participants and personnel. Therefore, although all studies were rated at high risk of bias of performance bias, we did not downgrade the evidence for performance bias when using the GRADE assessment.
Blinding of outcome assessor (detection bias)
No study was rated as low risk of bias for blinding of outcome assessor. Studies were rated as either unclear for risk of bias due to insufficient details (Baker 1977; Hidayati 2013; Janchai 2008; Mueller 1982; Woodburn 2004) or high risk of bias due to a clear statement that blinded assessors were not used (Vigier 1999), or evidence that outcome assessors were staff on the ward, and were likely to be aware of the type of dressings participants had during their hospital admission (Deutsch 2005; MacLean 1994; Wong 2000). We determined that blinding of outcome assessors was less important for hard outcomes (e.g. adverse events); hence, if studies did not blind outcome assessors, we did not downgrade the evidence for detection bias when using the GRADE assessment on adverse events. For other outcomes that were not adverse events (i.e. wound healing, physical function, length of hospital stay and prescription of prosthetics), evidence was downgraded for detection bias if outcome assessors were not blinded.
Incomplete outcome data
Two studies (22%) were rated as low risk of bias for incomplete outcome data. These studies were considered to be of low risk as there were either no loss to follow up (Mueller 1982), or the numbers lost to follow up were small (≤ 15%) and balanced in both groups (Vigier 1999). The remaining studies were rated as being at high risk of bias as the numbers lost to follow up were large (>15%) and / or unbalanced between groups (Baker 1977; Deutsch 2005; Janchai 2008; MacLean 1994; Wong 2000; Woodburn 2004). In some studies, participants’ data were also excluded from analyses by researchers (Baker 1977; Hidayati 2013) and in one case the treating physician made the decision to exclude data (MacLean 1994).
Selective reporting
None of the studies was rated low risk for selective reporting bias because we did not have study protocols to enable us to check whether all data for pre‐specified outcomes were reported. To determine whether studies scored an unclear or high risk of bias, we compared outcomes reported in Methods with outcomes reported in Results/Discussion. One study was rated as unclear for risk of reporting bias because all outcomes reported in the Methods were reported in the Results/Discussion (Woodburn 2004). The remaining studies were rated as being at high risk of reporting bias due to clear evidence that outcomes specified in Methods were not reported in Results/Discussion (Baker 1977; Wong 2000), outcomes not specified in Methods were reported in Results/Discussion (Deutsch 2005; Hidayati 2013; Janchai 2008; Mueller 1982; Vigier 1999), or outcomes specified in Methods were changed (MacLean 1994). Three studies were also rated as being at high risk of reporting bias because outcomes were inadequately reported. For example, they did not provide measures of variability (e.g. SD) (Baker 1977; Hidayati 2013; Wong 2000).
Other potential sources of bias
Two studies (22%) were rated as low risk of bias for other potential sources of bias (Janchai 2008; Vigier 1999). The remaining studies were either rated unclear for risk of other biases due to discrepancies in data or insufficient/unclear reporting of data in publications (Baker 1977; Deutsch 2005; Hidayati 2013; Woodburn 2004), potential opportunistic stopping of the trial based on results (Deutsch 2005) or unit of analysis issues with limbs rather than participants included in analyses (MacLean 1994; Mueller 1982; Vigier 1999). One study was rated as at high risk of other bias because there was a notable imbalance in baseline characteristics of participants (MacLean 1994).
Effects of interventions
See: Table 1
Comparison: effects of rigid dressings compared with soft dressings for transtibial amputations
See Table 1 for the main comparison of outcomes between rigid and soft dressing groups. (We did not report the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes because the point estimates and 95% confidence intervals indicated no differences between groups, and the certainty of evidence was very low; presenting NNTB and NNTH would not have helped with interpretation of the results). As readers might be interested in the comparison of individual types of complications/adverse events between rigid and soft dressing groups, we present the data in an additional table (Table 3) in a similar format to a 'Summary of findings' table.
2. Rigid dressings compared with soft dressings for transtibial amputations.
| Additional summary of findings for rigid dressings compared with soft dressings for transtibial amputations | ||||||
| Patient or population: people who had undergone transtibial amputations Setting: acute and rehabilitation hospitals Intervention: rigid dressings Comparison: soft dressings | ||||||
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with soft dressings | Risk with rigid dressings | |||||
| Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Revisions to above knee amputations) | Study population | RR 0.62 (0.29 to 1.30) | 295 (4 RCTs) | ⊕⊝⊝⊝ Very low 1 2 | We are uncertain if rigid dressings increase or decrease the proportion of revisions to above knee amputations. The true effect is likely to be substantially different from the estimate of effect. | |
| 11 per 100 | 7 per 100 (3 to 14) | |||||
| Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Wound breakdown/trauma/infections) | Study population | RR 1.07 (0.53 to 2.18) | 230 (3 RCTs) | ⊕⊝⊝⊝ Very low 2 3 | We are uncertain if rigid dressings increase or decrease the proportion of wound breakdown/trauma/infections. The true effect is likely to be substantially different from the estimate of effect. | |
| 11 per 100 | 12 per 100 (6 to 25) | |||||
| Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Pressure areas) | Study population | not estimable | 15 (1 RCT) | ⊕⊝⊝⊝ Very low 4 5 | We are uncertain if rigid dressings increase or decrease the proportion of pressure areas. The true effect is likely to be substantially different from the estimate of effect. | |
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Deaths) | Study population | RR 1.09 (0.59 to 2.01) | 316 (5 RCTs) | ⊕⊝⊝⊝ Very low 2 6 | We are uncertain if rigid dressings increase or decrease the proportion of deaths. The true effect is likely to be substantially different. | |
| 11 per 100 | 12 per 100 (7 to 22) | |||||
| Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Medical complications) | Study population | RR 1.37 (0.11 to 16.47) | 90 (2 RCTs) | ⊕⊝⊝⊝ Very low 7 8 | We are uncertain if rigid dressings increase or decrease the proportion of medical complications. The true effect is likely to be substantially different from the estimate of effect. | |
| 2 per 100 | 3 per 100 (1 to 20) | |||||
| Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Falls) | Study population | RR 0.68 (0.24 to 1.93) | 76 (2 RCTs) | ⊕⊝⊝⊝ Very low 8 9 | We are uncertain if rigid dressings increase or decrease the proportion of falls. The true effect is likely to be substantially different from the estimate of effect. | |
| 18 per 100 | 13 per 100 (4 to 36) | |||||
| Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Stump pain) | Study population | RR 0.69 (0.27 to 1.75) | 40 (1 RCT) | ⊕⊝⊝⊝ Very low 8 10 | We are uncertain if rigid dressings increase or decrease the proportion of participants experiencing stump pain. The true effect is likely to be substantially different. | |
| 38 per 100 | 26 per 100 (10 to 67) | |||||
| Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Phantom pain) | Study population | RR 0.98 (0.48 to 2.02) | 40 (1 RCT) | ⊕⊝⊝⊝ Very low 8 10 | We are uncertain if rigid dressings increase or decrease the proportion of participants experiencing phantom pain. The true effect is likely to be substantially different from the estimate of effect. | |
| 43 per 100 | 42 per 100 (21 to 87) | |||||
| aThe risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by two levels due to all 4 studies scoring high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting.
2 Downgraded by one level due to 95% CI crossing no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect).
3 Downgraded by two levels due to all 3 studies scoring high risk of bias for at least two of the following items: concealed allocation, incomplete outcome data and selective reporting.
4 Downgraded by two levels as no data (no events) available to assess imprecision.
5 Downgraded by two levels due to high risk of bias for random allocation, concealed allocation and selective reporting.
6 Downgraded by two levels due to all 5 studies scoring high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting.
7 Downgraded by two levels due to all 2 studies scoring high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting.
8 Downgraded by two levels due to optimal information size criterion not met for dichotomous outcome (i.e. at least 190 patients from a sample size power calculation based on an absolute target difference of 20%, two‐sided alpha of 0.05 and power of 80%), and 95% CI crossing no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect).
9 Downgraded by two levels due to both studies scoring high risk of bias for incomplete outcome data and selective reporting.
10 Downgraded by two levels due to high risk of bias for random sequence generation, concealed allocation, incomplete outcome data and selective reporting.
Primary outcome 1: wound healing
Two studies (107 participants) provided usable data in the form of continuous data (i.e. mean time from amputation to wound healing) (Vigier 1999) or dichotomous data (i.e. proportion of wounds healed) (Baker 1977).
Wound healing – time from amputation to wound healing
Based on one study of 56 participants, it is not clear whether rigid dressings decrease the time to wound healing compared with soft dressings (mean difference (MD) ‐25.60 days; 95% confidence interval (CI) ‐49.08 to ‐2.12; Analysis 1.1) (Vigier 1999), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels due to very high risk of bias for blinding of outcome assessor and selective reporting, and unclear risk of bias for random sequence generation and concealed allocation, and further downgraded by one level due to serious imprecision as the optimal information size criterion was not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004) (see Table 1).
1.1. Analysis.
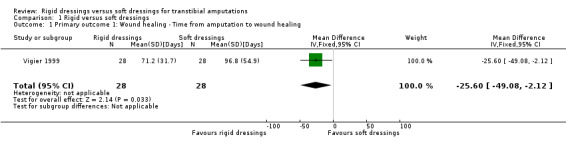
Comparison 1 Rigid versus soft dressings, Outcome 1 Primary outcome 1: Wound healing ‐ Time from amputation to wound healing.
Wound healing – proportion of wounds healed
Based on one study of 51 participants, it is not clear whether rigid dressings increase the proportion of wounds healed at 14 to 21 days compared with soft dressings (risk ratio (RR) 1.14; 95% CI 0.74 to 1.76; Analysis 1.2) (Baker 1977), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels due to very high risk of bias for incomplete outcome data and selective reporting, and unclear risk of bias for random sequence generation, concealed allocation and blinding of outcome assessor, and further downgraded by two levels due to very serious imprecision as the optimal information size criterion was not met for dichotomous outcome (i.e. at least 190 patients from a sample size power calculation based on an absolute target difference of 20%, two‐sided alpha of 0.05 and power of 80%), and the 95% CI of point estimate crossed no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect) (see Table 1).
1.2. Analysis.
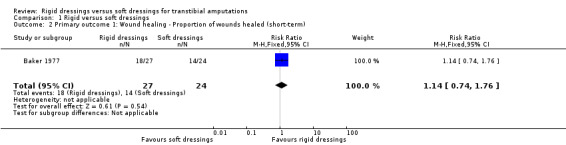
Comparison 1 Rigid versus soft dressings, Outcome 2 Primary outcome 1: Wound healing ‐ Proportion of wounds healed (short‐term).
Primary outcome 2: complications/adverse events
Adverse events were categorised as skin‐related adverse events (e.g. wound infections/breakdowns/stump revisions/further amputations/pressure areas) and non skin‐related adverse events (e.g. deaths/chest infections/medical complications/falls/pain). When studies had multiple skin‐related or non skin‐related adverse events, we used the data for the most severe adverse event in each category. For example, two studies had multiple skin‐related adverse events (e.g. revisions to above knee amputations, surgical debridement, wound infections) (Deutsch 2005; Woodburn 2004); however we only used data for revisions to above knee amputations for the meta‐analysis for skin‐related adverse events. Two studies had multiple non skin‐related adverse events (e.g. deaths, medical complications, falls, stump pain, phantom pain) (Deutsch 2005; MacLean 1994). For these two studies, we used the death data in the meta‐analysis for non skin‐related adverse events.
Six studies (336 participants) provided usable data in the form of dichotomous data for adverse events (i.e. proportion of adverse events) (Baker 1977; Deutsch 2005; Janchai 2008; MacLean 1994; Mueller 1982; Woodburn 2004), six studies (342 participants) provided usable data in the form of dichotomous data for non skin‐related adverse events (i.e. proportion of adverse events) (Baker 1977; Deutsch 2005; Janchai 2008; MacLean 1994; Wong 2000; Woodburn 2004) and one study (23 participants) provided usable data in the form of continuous data for non skin‐related adverse event (i.e. time from amputation to no pain) (Hidayati 2013).
In addition, we provided a further breakdown of the proportion of adverse events. For individual skin‐related adverse events, four studies reported proportion of revisions to above knee amputations (Baker 1977; Deutsch 2005; MacLean 1994; Woodburn 2004), three studies reported proportion of wound breakdowns/trauma/infections (Deutsch 2005; Janchai 2008; Woodburn 2004) and one study reported proportion of pressure areas (Mueller 1982). For individual non skin‐related adverse events, five studies reported proportion of deaths (Baker 1977; Deutsch 2005; MacLean 1994; Wong 2000; Woodburn 2004), two studies reported proportion of medical complications (Deutsch 2005; MacLean 1994), two studies reported proportion of falls (Deutsch 2005; Janchai 2008), and one study reported proportion of stump pain and phantom pain (MacLean 1994).
Complications/adverse events – proportion of skin‐related adverse events
Based on six studies of 336 participants (340 limbs), it is not clear whether rigid dressings increase the proportion of skin‐related adverse events in the rigid dressings group compared with the soft dressings group (RR 0.65; 95% CI 0.32 to 1.32; I2 = 0%; Analysis 1.3) (Baker 1977; Deutsch 2005; Janchai 2008; MacLean 1994; Mueller 1982; Woodburn 2004), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels as all six studies scored very high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting; evidence was further downgraded by one level due to serious imprecision as the 95% CI of point estimate crossed no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect) (see Table 1).
1.3. Analysis.
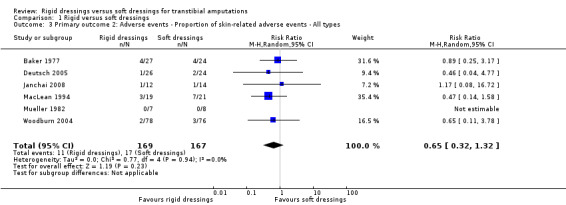
Comparison 1 Rigid versus soft dressings, Outcome 3 Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ All types.
Results were similar for individual skin‐related adverse events. It is not clear whether rigid dressings increase the proportion of proportion of revisions to above knee amputations, wound breakdown / trauma / infections, and pressure areas in the rigid dressings group compared with the soft dressings group (see Analysis 1.4; Analysis 1.5; Analysis 1.6). The evidence was downgraded by one and two levels due to very high risk of bias and serious/very serious imprecision (see Table 3).
1.4. Analysis.
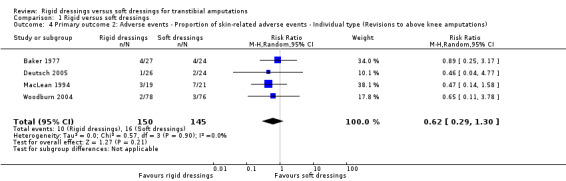
Comparison 1 Rigid versus soft dressings, Outcome 4 Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Revisions to above knee amputations).
1.5. Analysis.
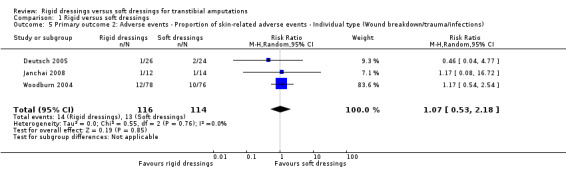
Comparison 1 Rigid versus soft dressings, Outcome 5 Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Wound breakdown/trauma/infections).
1.6. Analysis.
Comparison 1 Rigid versus soft dressings, Outcome 6 Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Pressure areas).
Complications/adverse events – proportion of non skin‐related adverse events
Based on six studies of 342 participants (346 limbs), it is not clear whether rigid dressings increase the proportion of non skin‐related adverse events in the rigid dressings group compared with the soft dressings group (RR 1.09; 95% CI 0.60 to 1.99; I2 = 0%; Analysis 1.7) (Baker 1977; Deutsch 2005; Janchai 2008; MacLean 1994; Wong 2000; Woodburn 2004), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels as all six studies scored very high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting; evidence was further downgraded by one level due to serious imprecision as the 95% CI of point estimate crossed no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect) (see Table 1).
1.7. Analysis.
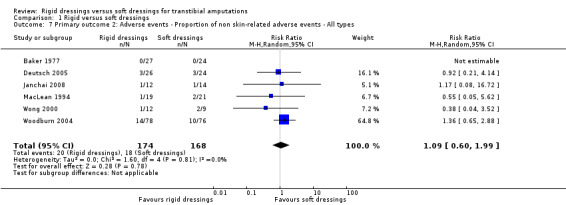
Comparison 1 Rigid versus soft dressings, Outcome 7 Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ All types.
Results were similar for individual non skin‐related adverse events. It is not clear whether rigid dressings increase the proportion of deaths, falls, medical complications, stump pain and phantom pain in the rigid dressings group compared with the soft dressings group (see Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12). The evidence was downgraded by one and two levels due to very high risk of bias and serious/very serious imprecision (see Table 3).
1.8. Analysis.

Comparison 1 Rigid versus soft dressings, Outcome 8 Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Deaths).
1.9. Analysis.

Comparison 1 Rigid versus soft dressings, Outcome 9 Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Medical complications).
1.10. Analysis.

Comparison 1 Rigid versus soft dressings, Outcome 10 Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Falls).
1.11. Analysis.

Comparison 1 Rigid versus soft dressings, Outcome 11 Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Stump pain).
1.12. Analysis.

Comparison 1 Rigid versus soft dressings, Outcome 12 Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Phantom pain).
Complications/adverse events – time from amputation to no pain
Based on one study of 23 participants, it is not clear whether rigid dressings decrease the time to no pain in the rigid dressings group compared with the soft dressings group (MD ‐0.35 weeks; 95% CI ‐2.11 to 1.41; Analysis 1.13) (Hidayati 2013), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels due to very high risk of bias for incomplete outcome data and selective reporting, and unclear risk of bias for random sequence generation, concealed allocation and blinding of outcome assessor; evidence was further downgraded by two levels due to very serious imprecision as the optimal information size criterion was not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004), and the 95% CI of point estimate crossed no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect) (see Table 1).
1.13. Analysis.
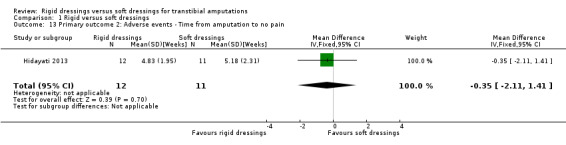
Comparison 1 Rigid versus soft dressings, Outcome 13 Primary outcome 2: Adverse events ‐ Time from amputation to no pain.
Secondary outcome 1: prescription of prosthetics
One study (21 participants; 22 limbs) provided data that allowed us to calculate time‐to‐event data (i.e. hazard ratio (HR) for readiness of prosthetic prescription) (Wong 2000), using methods recommended by Tierney 2007. Based on the study, it is not clear whether rigid dressings decrease the time to readiness of prosthetic prescription in the rigid dressings group compared with the soft dressings group (HR of 0.27; 95% CI 0.09 to 0.84; Analysis 1.14) (Wong 2000), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels due to very high risk of bias for blinding of outcome assessor, incomplete outcome data and selective reporting; evidence was further downgraded by one level due to serious imprecision as the optimal information size criterion was not met for dichotomous outcome (i.e. at least 190 patients from a sample size power calculation based on an absolute target difference of 20%, two‐sided alpha of 0.05 and power of 80%). (Even though this outcome was time‐to‐event, we had data to calculate events (i.e. participants who achieved a first prosthetic fit/cast and thus calculated optimal information size for a dichotomous outcome). Prescription of prosthetics was not included as an outcome for the 'Summary of findings' table as it was considered a surrogate outcome for more clinically important outcomes (e.g. wound healing, physical function), as per protocol.
1.14. Analysis.
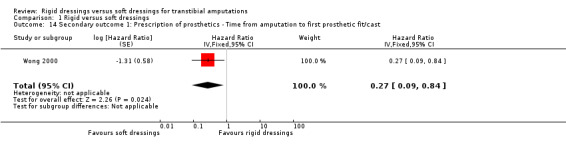
Comparison 1 Rigid versus soft dressings, Outcome 14 Secondary outcome 1: Prescription of prosthetics ‐ Time from amputation to first prosthetic fit/cast.
Secondary outcome 2: physical function
One study (56 participants) provided usable data in the form of continuous data (i.e. mean time from amputation to walking) (Vigier 1999). Based on the study, it is not clear whether rigid dressings decrease the time to walking in the rigid dressings group compared with the soft dressings group (MD ‐3 days; 95% CI ‐9.96 to 3.96 days; Analysis 1.15) (Vigier 1999), as the certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels due to very high risk of bias for blinding of outcome assessor and selective reporting, and unclear risk of bias for random sequence generation and concealed allocation; evidence was further downgraded by two levels due to very serious imprecision as the optimal information size criterion was not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004), and the 95% CI of point estimate crossed no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect) (see Table 1).
1.15. Analysis.

Comparison 1 Rigid versus soft dressings, Outcome 15 Secondary outcome 2: Physical function ‐ Time from amputation to walking.
Secondary outcome 3: length of hospital stay
One study (56 participants) provided usable data in the form of continuous data (i.e. mean time from amputation to discharge from hospital) (Vigier 1999). Based on the study, it is not clear whether rigid dressings decrease the length of hospital stay in the rigid dressings group compared with the soft dressings group (MD ‐30.10 days; 95% CI ‐49.82 to ‐10.38 days; Analysis 1.16) (Vigier 1999), as the certainty of evidence has been assessed as very low. The evidence was downgraded by two levels due to very high risk of bias for blinding of outcome assessor and selective reporting, and unclear risk of bias for random sequence generation and concealed allocation; evidence was further downgraded by one level due to serious imprecision as the optimal information size criterion was not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004) (see Table 1).
1.16. Analysis.
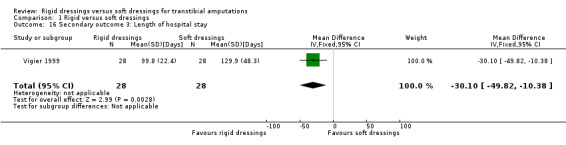
Comparison 1 Rigid versus soft dressings, Outcome 16 Secondary outcome 3: Length of hospital stay.
Secondary outcome 4: patient comfort
None of the studies measured patient comfort.
Secondary outcome 5: quality of life
None of the studies measured quality of life.
Secondary outcome 6: cost
None of the studies measured cost.
Secondary outcome 7: swelling
Three studies (65 participants) provided usable data in the form of continuous data (i.e. change scores/reduction in stump volume from baseline) (Hidayati 2013; Janchai 2008; Mueller 1982). We report short‐term and medium‐term effects of swelling as follows.
Swelling – change in swelling (short term)
Based on three studies (65 participants; 66 limbs), it is not clear whether rigid dressings decrease stump volume in the rigid dressings group compared with the soft dressings group when swelling was measured less than one month after amputation (MD 36.84 cm3; 95% CI 11.33 to 62.34; I2 = 0%; Analysis 1.17) (Hidayati 2013; Janchai 2008; Mueller 1982). The certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels as all three studies scored very high risk of bias for at least two of the following items: random sequence generation, concealed allocation, incomplete outcome data and selective reporting; evidence was further downgraded by one level due to serious imprecision as the optimal information size criterion was not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004).
1.17. Analysis.
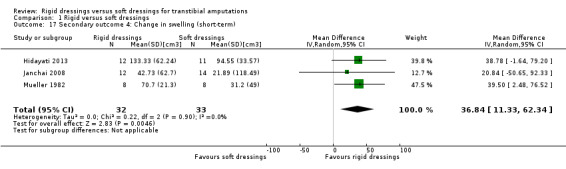
Comparison 1 Rigid versus soft dressings, Outcome 17 Secondary outcome 4: Change in swelling (short‐term).
Swelling – change in swelling (medium term)
Based on two studies (45 participants), it is not clear whether rigid dressings decrease stump volume in the rigid dressings group compared with the soft dressings group when swelling was measured within one to three months after amputation (MD ‐13.81 cm3; 95% CI ‐63.92 to 36.31; I2 = 0%; Analysis 1.18) (Hidayati 2013; Janchai 2008). The certainty of the evidence has been assessed as very low. The evidence was downgraded by two levels as both studies scored very high risk of bias for incomplete outcome data and selective reporting; evidence was further downgraded by two levels due to very serious imprecision as the optimal information size criterion was not met for continuous outcome (i.e. at least 300 patients from a sample size power calculation by Woodburn 2004), and the 95% CI of point estimate crossed no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect). Swelling was not included as an outcome for the 'Summary of findings' table as it was considered a surrogate outcome for more clinically important outcomes (e.g. wound healing, physical function), as per protocol.
1.18. Analysis.
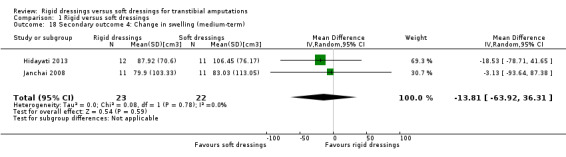
Comparison 1 Rigid versus soft dressings, Outcome 18 Secondary outcome 4: Change in swelling (medium‐term).
Subgroup analyses
We did not create funnels plots to look for publication bias, and we did not perform any subgroup analyses as there were less than 10 studies included in the meta‐analyses.
Sensitivity analyses
We conducted three types of sensitivity analyses. The first type of sensitivity analysis examined the effects of random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data on outcomes. The second type of sensitivity analysis examined the effects of limb data on outcomes. The third type of sensitivity analysis examined the effects of transfemoral amputations on outcomes. We present the data in Additional Tables (Table 4; Table 5; Table 6) and report the results in Appendix 3. Briefly, excluding the three types of studies (i.e. studies at high or unclear risk of bias for random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data, studies that analysed limbs and studies that included transfemoral amputations) made no difference to the interpretation of outcome estimates as the certainty of remaining evidence remained very low.
3. Sensitivity analysis – Bias (omitting studies at high or unclear risk of bias for random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data).
| Outcomes | Pooled results from all studies | Pooled results from studies with random sequence generation | Pooled results from studies with concealed allocation | Pooled results from studies with blinded outcome assessors | Pooled results from studies with complete outcome data |
| Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ All types | RR 0.65 (0.32 to 1.32) (from 6 studies of 336 participants) | RR 0.67 (0.19 to 2.32) (from 3 studies of 230 participants) | RR 1.17 (0.08 to 16.72) (from 1 study of 26 participants) | Not estimable as no studies | Not estimable as no events from Mueller 1982 |
| Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Revisions to above knee amputations) | RR 0.62 (0.29 to 1.30) (from 4 studies of 295 participants) | RR 0.57 (0.14, 2.34) (from 2 studies of 204 participants) | Not estimable as no studies | Not estimable as no studies | Not estimable as no studies |
| Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Wound breakdown/trauma/infections) | RR 1.07 (0.53 to 2.18) (from 3 studies of 230 participants) | RR 1.07 (0.53 to 2.18) (from 3 studies of 230 participants) | RR 1.17 (0.08, 16.72) (from 1 study of 26 participants) | Not estimable as no studies | Not estimable as no studies |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ All types | RR 1.09 (0.60 to 1.99) (from 6 studies of 342 participants) | RR 1.14 (0.61 to 2.13) (from 4 studies of 251 participants) | RR 1.17 (0.08 to 16.72) (from 1 study of 26 participants) | Not estimable as no studies | Not estimable as no studies |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Deaths) | RR 1.09 (0.59 to 2.01) (from 5 studies of 316 participants) | RR 1.14 (0.60 to 2.17) (from 3 studies of 225 participants) | Not estimable as no studies | Not estimable as no studies | Not estimable as no studies |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Medical complications) | RR 1.37 (0.11 to 16.47) (from 2 studies of 90 participants) | RR 4.63 (0.23 to 91.81) (from 1 study of 50 participants) | Not estimable as no studies | Not estimable as no studies | Not estimable as no studies |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Falls) | RR 0.68 (0.24 to 1.93) (from 2 studies of 76 participants) | RR 0.68 (0.24 to 1.93) (from 2 studies of 76 participants) | RR 1.17 (0.08 to 16.72) (from 1 study of 26 participants) | Not estimable as no studies | Not estimable as no studies |
| Secondary outcome 4: Change in swelling (short term) (cm3) | MD 36.84 (11.33 to 62.34) (from 3 studies of 65 participants) | MD 20.84 (‐50.65 to 92.33) (from 1 study of 26 participants) | MD 20.84 (‐50.65 to 92.33) (from 1 study of 26 participants) | Not estimable as no studies | MD 39.50 (2.48 to 76.52) (from 1 study of 16 participants) |
| Secondary outcome 4: Change in swelling (medium term) (cm3) | MD ‐13.81 (‐63.92 to 36.31) (from 2 studies of 45 participants) | MD ‐3.13 (‐93.64 to 87.38) (from 1 study of 22 participants) | MD ‐3.13 (‐93.64 to 87.38) (from 1 study of 22 participants) | Not estimable as no studies | Not estimable as no studies |
4. Sensitivity analysis – Unit of analysis issue (omitting studies that analysed limbs instead of participants, i.e. Maclean 1994, Mueller 1982, Wong 2000).
| Outcomes | Pooled results from all studies | Pooled results from studies that analysed participant data (no limb data) |
| Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ All types | RR 0.65 (0.32 to 1.32) (from 6 studies of 336 participants) | RR 0.77 (0.32 to 1.87) (from 4 studies of 281 participants) |
| Primary outcome 2: Adverse events ‐ Proportion of skin‐related adverse events ‐ Individual type (Revisions to above knee amputations) | RR 0.62 (0.29 to 1.30) (from 4 studies of 295 participants) | RR 0.73 (0.28 to 1.88) (from 3 studies of 255 participants) |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ All types | RR 1.09 (0.60 to 1.99) (from 6 studies of 342 participants) | RR 1.26 (0.66 to 2.40) (from 4 studies of 281 participants) |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Deaths) | RR 1.09 (0.59 to 2.01) (from 5 studies of 316 participants) | RR 1.26 (0.65 to 2.46) (from 3 studies of 255 participants) |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Medical complications) | RR 1.37 (0.11 to 16.47) (from 2 studies of 90 participants) | RR 4.63 (0.23 to 91.81) (from 1 study of 50 participants) |
| Secondary outcome 4: Change in swelling (short term) (cm3) | MD 36.84 (11.33 to 62.34) (from 3 studies of 65 participants) | MD 34.43 (‐0.75 to 69.62) (from 2 studies of 49 participants) |
5. Sensitivity analysis – Study population (omitting study with transfemoral amputations, i.e. Wong 2000).
| Outcomes | Pooled results from all studies | Pooled results from studies with only transtibial amputations |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ All types | RR 1.09 (0.60 to 1.99) (from 6 studies of 342 participants) | RR 1.18 (0.63 to 2.21) (from 5 studies of 321 participants) |
| Primary outcome 2: Adverse events ‐ Proportion of non skin‐related adverse events ‐ Individual type (Deaths) | RR 1.09 (0.59 to 2.01) (from 5 studies of 316 participants) | RR 1.18 (0.62 to 2.25) (from 4 studies of 295 participants) |
| Secondary outcome 1: Prescription of prosthetics ‐ Time from amputation to first prosthetic fit / cast | HR 0.27 (0.09 to 0.84) (from 1 study of 21 participants) | Not estimable as no studies |
Discussion
Summary of main results
The aim of this systematic review was to assess the benefits and harms of rigid dressings for postoperative management of transtibial amputations. Despite the inclusion of nine randomised controlled trials (RCTs) and quasi‐RCTs involving 436 participants (441 limbs), we remain uncertain of the benefits and harms of rigid dressings due to limited and very low‐certainty evidence for outcomes including wound healing, adverse events, physical function, length of hospital stay, prescription of prosthetics and swelling/stump volume.
We considered evidence to be limited, because most of the outcomes in our systematic review included data from only one study with a small sample size ranging from 15 to 56 participants. We considered the certainty of evidence to be very low due to very high risk of bias (limitations in study design and execution) and serious/very serious imprecision of study results for all outcomes.
Overall completeness and applicability of evidence
The evidence in our review was complete and generalisable in some aspects. For example, participants were recruited from acute and rehabilitation hospitals, and were representative of the population seen after a transtibial amputation. The type of rigid dressings were also similar to those commonly reported in clinical practice (Barnes 2014) and practice guidelines (ACI 2017; Randolph 2017; Smith 2017).
However, very few studies provided details about the acuity of amputation or timing of first application of dressings, or both. Information such as the duration of dressing, duration of dressing removal and frequency of dressing change were also missing in most studies (see Interventions in Included studies). This lack of information about the interventions makes it difficult for clinicians to replicate interventions, or to know when to first apply dressings, how long to apply dressings for, how long to remove dressings for and how often to change dressings. The choice of outcomes reported in studies was also problematic. Outcomes that we deemed important and most relevant to patients were either not measured, or not reported adequately. For example, no studies measured patient comfort, quality of life and cost related to dressings or personnel. Only two out of nine studies provided usable data for wound healing, and only one out of nine studies provided usable data for outcomes such as prescription of prosthetics, physical function and length of hospital stay (see Potential biases in the review process and Table 2). If outcomes in future trials can be standardised in terms of measurement, analysis and reporting, it will help with the synthesis of evidence in future systematic reviews.
Quality of the evidence
The evidence was downgraded to very low‐certainty because of limitations in study design (risk of bias) and imprecision. For example, most of the included studies had high or unclear risk of selection bias (56% and 89%), detection bias (100%), attrition bias (78%), reporting bias (100%) or other biases (78%) (see Risk of bias in included studies). In other words, the evidence was often downgraded due to the following limitations in study design: no random allocation, no concealed allocation, no blinding of outcome assessor, large losses to follow‐up and selective reporting of outcomes in studies. Although performance bias was present in all studies, we did not downgrade the evidence in the 'Summary of findings' table on the basis of performance bias, as it was not possible to blind participants and therapists due to the nature of the intervention. If outcomes were adverse events, we also did not downgrade the evidence for the blinding of outcome assessors as we considered adverse events less prone to subjective interpretation by assessors. We often also downgraded evidence for imprecision. The optimal information size criterion was often not met for continuous outcomes (i.e. at least 300 participants based on a sample size power calculation by Woodburn 2004) and dichotomous outcomes (i.e. at least 190 participants based on an absolute target difference of 20%, two‐sided alpha of 0.05 and power of 80%), and the 95% CI of results often crossed the line of no effect (i.e. the 95% CI fails to rule in or rule out a treatment effect). This is not surprising, considering most meta‐analyses only included one study consisting of less than 60 participants.
Potential biases in the review process
There was limited opportunity for bias in our review. We conducted a comprehensive search strategy on electronic databases, clinical trial registries, reference lists, citation references of studies and the grey literature. Two review authors independently screened eligibility of studies, extracted data on characteristics and effect estimates, and rated risk of bias. Where there was uncertainty regarding inclusion of studies or analysis of data that were not considered in the protocol, we consulted with the Cochrane editorial board in order to seek independent advice. These changes are specified in the Differences between protocol and review section. Specifically, they related to the inclusion of studies with mixed populations (e.g. transtibial and transfemoral amputations), the inclusion of data on limbs and transfemoral amputations and the conduct of subsequent sensitivity analyses. These ensured transparency in the report and conduct of our review.
However, there is one potential limitation of the review process. In some instances where there were missing data for meta‐analyses (e.g. SD for time to walking independently (Baker 1977), SD for time to prescription of prosthetics (Woodburn 2004), or unclear reporting (e.g. RR provided instead of HR for "decreasing stump volume" (Hidayati 2013)), we emailed study authors to request the data or provide clarification, but either received no response (Deutsch 2005; Hidayati 2013; Janchai 2008; MacLean 1994; Woodburn 2004) or could not locate the email addresses of authors (Baker 1977). Data from these studies could therefore not be pooled in meta‐analyses for the specific outcomes. It is possible that the missing data could change the pooled effect estimate. Nonetheless, we are only able to make conclusions based on the available information and it is unlikely that the small amount of missing data would substantially change the conclusions of the review.
Agreements and disagreements with other studies or reviews
To date, only two systematic reviews have been published on the efficacy of dressings on outcomes in people with transtibial amputations (Churilov 2014; Nawijn 2005). Compared with these reviews, our review is more comprehensive due to the inclusion of studies that were either published recently or measured other outcomes besides time from amputation to prosthetic fitting. Our results are also less susceptible to bias as we did not include case‐control and cross‐sectional studies in our review.
Our conclusions are broadly similar to Nawijn 2005 in that no firm conclusions can be drawn about the benefits and harms of rigid dressings due to limited and very low‐certainty evidence, despite the inclusion of four new/additional trials in our review. However, our conclusions differ from Churilov 2014 who concluded that rigid dressings led to a significantly shorter time from amputation to prosthetic fitting compared with soft dressings. The differences in our conclusions may be explained by differences in our analyses and interpretation of the data. First, Churilov 2014 included data from retrospective cross‐sectional studies, while such studies were excluded from our review. Second, Churilov 2014 included data from studies that reported the mean time from amputation to prescription of prosthetics. In our review, such studies were only included if studies clearly stated that all outcomes were achieved in participants (since reporting the mean time to event in studies with censored participants is not recommended as the mean time to event would only reflect that of a subset of participants remaining in the study) (Deeks 2011). Third, despite acknowledging the poor quality of studies in their review (Churilov 2014), conclusions were made based on all studies (including studies at high risk of bias). In contrast, our conclusions were made after using GRADE to downgrade evidence accordingly if studies were at high risk of bias. Fourth, we are uncertain whether rigid dressings decrease the time from amputation to prosthetic fitting in the rigid dressings group compared with the soft dressings group (calculated HR 0.27; 95% CI 0.09 to 0.84; one study of 21 participants) (Wong 2000), as results were based on very low‐certainty evidence, downgraded twice for very high risk of bias and once for serious imprecision (see Effects of interventions; Characteristics of included studies).
Authors' conclusions
Implications for practice.
We are uncertain if rigid dressings are superior to soft dressings for improving outcomes related to wound healing, adverse events, prosthetic prescription, walking function, length of hospital stay and swelling. Our uncertainty stems from the very low‐certainty evidence. Clinicians should exercise clinical judgement as to which type of dressing they use, and consider the pros and cons of each. For example, a rigid dressing might protect the stump of a patient who is at high risk of falling, and a soft dressing may decrease the chance of skin breakdown in a patient with poor skin integrity.
Implications for research.
High‐quality trials are now required to determine the benefits and harms of rigid dressings. To improve the reporting and conduct of future trials, we suggest that researchers do the following:
a) publish a protocol and register the trial prior to commencement,
b) refer to CONSORT to guide reporting of study (Schulz 2010);
c) refer to TIDieR to guide reporting of interventions (Hoffman 2014);
d) develop a core set of outcome measures to be collected as part of the COMET initiative (Williamson 2017);
e) collect important and meaningful outcome measures (e.g. wound healing, adverse events, patient comfort, quality of life and costs) rather than surrogate outcome measures (e.g. prescription of prosthetics and swelling);
f) increase sample sizes, or perform sample size power calculations to increase the precision of study results; and
g) minimise risk of bias by using random allocation, concealing allocation, blinding outcome assessor, following up all participants, and reporting all outcomes as per protocol.
Acknowledgements
The review authors are grateful to Jacqueline Fan for providing feedback on the initial protocol draft. The authors would like to thank peer reviewers for their comments on the protocol; Joan Webster, Joern Klein, Abitha Senthinathan, Helen Castledine and Camila Pino, and Gill Norman, Victoria Clemmett and Ann Fonfa for their feedback on the review. They would also like to thank Deidre Walshe for copy editing the protocol and Heather Maxwell for copy editing the review.
Appendices
Appendix 1. Search strategies
The Cochrane Wounds Specialised Register
1 MeSH DESCRIPTOR Amputation Explode All AND INREGISTER
2 MeSH DESCRIPTOR Amputees Explode All AND INREGISTER
3 MeSH DESCRIPTOR Amputation Stumps Explode All AND INREGISTER
4 ((transtibia* or trans‐tibia*) near3 amput*) AND INREGISTER
5 (below knee or below‐knee) near3 amput* AND INREGISTER
6 (lower limb* near3 amput*) AND INREGISTER
7 (lower extremit* near3 amput*) AND INREGISTER
8 (amput* next stump*) AND INREGISTER
9 (residua* next limb*) AND INREGISTER
10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 AND INREGISTER
11 MeSH DESCRIPTOR Bandages Explode All AND INREGISTER
12 MeSH DESCRIPTOR Artificial Limbs Explode All AND INREGISTER
13 MeSH DESCRIPTOR Casts, Surgical Explode All AND INREGISTER
14 MeSH DESCRIPTOR Splints Explode All AND INREGISTER
15 ((rigid or plastic* or compress* or unna) near3 (dressing* or bandage*)) AND INREGISTER
16 gauze AND INREGISTER
17 (sock* near5 (amput* or stump*)) AND INREGISTER
18 (prosth* near3 (amput* or stump* or transtibia* or trans‐tibia* or "below knee" or below‐knee or low* next limb*)) AND INREGISTER
19 ((plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) near3 cast*) AND INREGISTER
20 splint* AND INREGISTER
21 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 AND INREGISTER 1662
22 #10 and #21 AND INREGISTER
The Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Amputation] explode all trees
#2 MeSH descriptor: [Amputation Stumps] explode all trees
#3 MeSH descriptor: [Amputees] explode all trees
#4 ((transtibia* or trans‐tibia*) near/3 amput*):ti,ab,kw
#5 (("below knee" or below‐knee) near/3 amput*):ti,ab,kw
#6 ((low* next limb*) near/3 amput*):ti,ab,kw
#7 ((low* next extremit*) near/3 amput*):ti,ab,kw
#8 BKA:ti,ab,kw
#9 amput* next stump*:ti,ab,kw
#10 residua* next limb*:ti,ab,kw
#11 {or #1‐#10}
#12 MeSH descriptor: [Bandages] explode all trees
#13 MeSH descriptor: [Artificial Limbs] explode all trees
#14 MeSH descriptor: [Casts, Surgical] explode all trees
#15 MeSH descriptor: [Splints] explode all trees
#16 ((rigid or plastic* or compress* or unna) near/3 (dressing* or bandage*)):ti,ab,kw
#17 gauze:ti,ab,kw
#18 (sock* near/5 (amput* or stump*)):ti,ab,kw
#19 (prosth* near/3 (amput* or stump* or transtibia* or trans‐tibia* or "below knee" or below‐knee or low* next limb* or low* next extremit* or residua* next limb*)):ti,ab,kw
#20 ((plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) near/3 cast*):ti,ab,kw
#21 splint*:ti,ab,kw
#22 {or #12‐#21}
#23 (#11 and #22) in Trials
Ovid MEDLINE
1 exp Amputation/
2 exp Amputation Stumps/
3 exp Amputees/
4 ((transtibia* or trans‐tibia*) adj3 amput*).ti,ab.
5 ((below knee or below‐knee) adj3 amput*).ti,ab.
6 (low* limb* adj3 amput*).ti,ab.
7 (low* extremit* adj3 amput*).ti,ab.
8 BKA.ti,ab.
9 amput* stump*.ti,ab.
10 residua* limb*.ti,ab.
11 or/1‐10
12 exp Bandages/
13 exp Artificial Limbs/
14 exp Casts, Surgical/
15 exp Splints/
16 ((rigid or plastic* or compress* or unna) adj3 (dressing* or bandage*)).ti,ab.
17 gauze.ti,ab.
18 (sock* adj5 (amput* or stump*)).ti,ab.
19 (prosth* adj3 (amput* or stump* or transtibia* or trans‐tibia* or below knee or below‐knee or low* limb* or low* extremit* or residua* limb*)).ti,ab.
20 ((plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) adj3 cast*).ti,ab.
21 splint*.ti,ab.
22 or/12‐21
23 11 and 22
24 randomized controlled trial.pt.
25 controlled clinical trial.pt.
26 randomi?ed.ab.
27 placebo.ab.
28 clinical trials as topic.sh.
29 randomly.ab.
30 trial.ti.
31 or/24‐30
32 exp animals/ not humans.sh.
33 31 not 32
34 23 and 33
Ovid Embase
1 limb amputation/ or leg amputation/ or below knee amputation/
2 amputation/
3 amputation stump/
4 ((transtibia* or trans‐tibia*) adj3 amput*).ti,ab.
5 ((below knee or below‐knee) adj3 amput*).ti,ab.
6 (low* limb* adj3 amput*).ti,ab.
7 (low* extremit* adj3 amput*).ti,ab.
8 BKA.ti,ab.
9 amput* stump*.ti,ab.
10 residua* limb*.ti,ab.
11 or/1‐10
12 bandage/ or exp "bandages and dressings"/
13 exp limb prosthesis/
14 orthopedic cast/ or plaster cast/
15 exp splint/
16 ((rigid or plastic* or compress* or unna) adj3 (dressing* or bandage*)).ti,ab.
17 gauze.ti,ab.
18 (sock* adj5 (amput* or stump*)).ti,ab.
19 (prosth* adj3 (amput* or stump* or transtibia* or trans‐tibia* or below knee or below‐knee or low* limb* or low* extremit* or residua* limb*)).ti,ab.
20 ((plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) adj3 cast*).ti,ab.
21 splint*.ti,ab.
22 or/12‐21
23 11 and 22
24 Randomized controlled trials/
25 Single‐Blind Method/
26 Double‐Blind Method/
27 Crossover Procedure/
28 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
29 (doubl* adj blind*).ti,ab.
30 (singl* adj blind*).ti,ab.
31 or/24‐30
32 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
33 human/ or human cell/
34 and/32‐33
35 32 not 34
36 31 not 35
37 23 and 36
EBSCO CINAHL Plus
S36 S22 AND S35
S35 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34
S34 TI allocat* random* or AB allocat* random*
S33 MH "Quantitative Studies"
S32 TI placebo* or AB placebo*
S31 MH "Placebos"
S30 TI random* allocat* or AB random* allocat*
S29 MH "Random Assignment"
S28 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S27 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S26 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S25 TI clinic* N1 trial* or AB clinic* N1 trial*
S24 PT Clinical trial
S23 MH "Clinical Trials+"
S22 S10 AND S21
S21 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20
S20 TI splint* OR AB splint*
S19 TI ( (plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) n3 cast*) ) OR AB ( (plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) n3 cast*) )
S18 TI ( prosth* n3 (amput* or stump* or transtibia* or trans‐tibia* or below knee or below‐knee or low* limb* or low* extremit* or residua* limb*) ) OR AB ( prosth* n3 (amput* or stump* or transtibia* or trans‐tibia* or below knee or below‐knee or low* limb* or low* extremit* or residua* limb*) )
S17 TI ( sock* n5 (amput* or stump*) ) OR AB ( sock* n5 (amput* or stump*) )
S16 TI gauze OR AB gauze
S15 TI ( (rigid or plastic* or compress* or unna) n3 (dressing* or bandage*) ) OR AB ( (rigid or plastic* or compress* or unna) n3 (dressing* or bandage*) )
S14 (MH "Splints")
S13 (MH "Casts")
S12 (MH "Limb Prosthesis")
S11 (MH "Bandages and Dressings+")
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 TI residua* limb* OR AB residua* limb*
S8 TI ( amput* and stump* ) OR AB ( amput* and stump* )
S7 TI BKA OR AB BKA
S6 TI low* extremit* n3 amput* OR AB low* extremit* n3 amput*
S5 TI low* limb* n3 amput* OR AB low* limb* n3 amput*
S4 TI ( ((below knee or below‐knee) n3 amput*) ) OR AB ( ((below knee or below‐knee) n3 amput*) )
S3 TI ( ((transtibia* or trans‐tibia*) n3 amput*) ) OR AB ( ((transtibia* or trans‐tibia*) n3 amput*) )
S2 (MH "Amputees")
S1 (MH "Amputation") OR (MH "Amputation Stumps") OR (MH "Below‐Knee Amputation")
Ovid AMED
1 amputation/
2 ((transtibia* or trans‐tibia*) adj3 amput*).ti,ab.
3 ((below knee or below‐knee) adj3 amput*).ti,ab.
4 (low* limb* adj3 amput*).ti,ab.
5 (low* extremit* adj3 amput*).ti,ab.
6 BKA.ti,ab.
7 amput* stump*.ti,ab.
8 residua* limb*.ti,ab.
9 or/1‐8
10 bandages/
11 prosthesis/
12 casting/ or splinting/
13 splints/
14 cast.ti,ab.
15 ((rigid or plastic* or compress* or unna) adj3 (dressing* or bandage*)).ti,ab.
16 gauze.ti,ab.
17 (sock* adj5 (amput* or stump*)).ti,ab.
18 (prosth* adj3 (amput* or stump* or transtibia* or trans‐tibia* or below knee or below‐knee or low* limb* or low* extremit* or residua* limb*)).ti,ab.
19 ((plaster or fibreglass or fiberglass or plastic* or surgical or synthetic*) adj3 cast*).ti,ab. 20
20 splint*.ti,ab.
21 or/10‐20
22 9 and 21
23 exp randomized controlled trials/
24 exp double blind method/
25 exp random allocation/
26 (random$ or control$ or placebo$ or factorial).mp.
27 (double adj blind).mp.
28 (single adj blind).mp.
29 exp comparative study/
30 or/23‐29
31 22 and 30
PEDro
Under Advanced Search, combine the following terms using "AND":
[Abstract & Title field] amput*
[Body part] lower leg or knee
[Method] clinical trial
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid I Amputation
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | Amputation Stump
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | Amputation, Traumatic
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | Amputation; Traumatic, Leg, Lower
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | Amputation Stump Complication
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | amputee
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | transtibial
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | trans‐tibial
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | below knee
dressing OR bandage OR sock OR gauze OR cast OR splint OR prosthesis OR prosthetic OR rigid | below‐knee
World Health Organization International Clinical Trials Registry Platform
amputation [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
amputation [Condition] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
amputee [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
amputee [Condition] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
transtibial [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
transtibial [Condition] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
trans‐tibial [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
trans‐tibial [Condition] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
below knee [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
below knee [Condition] [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
below‐knee [Title] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
below‐knee [Condition] AND dressing or bandage or sock or gauze or cast or splint or prosthesis or prosthetic or rigid [Intervention]
Appendix 2. Cochrane 'Risk of bias' assessment tool
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
There is insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
no blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding;
blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken;
either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
no blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding;
blinding of key study participants and personnel attempted, but likely that the blinding could have been broken;
either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following:
insufficient information to permit judgement of low or high risk of bias;
the study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
no missing outcome data;
reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size;
missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
reason for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate;
for continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size;
'as‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation;
potentially inappropriate application of simple imputation.
Unclear
Either of the following:
insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided);
the study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
the study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way;
the study protocol is unavailable but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
not all of the study’s prespecified primary outcomes have been reported;
one or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified;
one or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
one or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis;
the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that most studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study either:
had a potential source of bias related to the specific study design used;
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Sensitivity analyses
We conducted three types of sensitivity analyses. The first type of sensitivity analysis examined the effects of random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data on outcomes. The second type of sensitivity analysis examined the effects of limb data on outcomes. The third type of sensitivity analysis examined the effects of transfemoral amputations on outcomes. We describe the results of sensitivity analyses as follows:
Complications/adverse events – proportion of skin‐related adverse events
Excluding studies at high or unclear risk of bias for the following criteria: random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data had no effect on the proportion of skin‐related adverse events. It remains unclear whether rigid dressings increase the proportion of skin‐related adverse events in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with random sequence generation (RR 0.67; 95% CI 0.19 to 2.32; 3 studies, 230 participants) (Deutsch 2005; Janchai 2008; Woodburn 2004), and only studies with concealed allocation (RR 1.17; 95% CI 0.08 to 16.72; 1 study, 26 participants) (Janchai 2008). As none of the relevant studies blinded outcome assessor, the pooled results was not estimable. As the remaining study had no skin‐related adverse events (Mueller 1982), the pooled result was also not estimable when the analysis included only studies with complete outcome data.
Excluding studies that analysed limbs also made no difference to the proportion of skin‐related adverse events. It remains unclear whether rigid dressings increase the proportion of skin‐related adverse events in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with participant data (i.e. no limb data) (RR 0.77; 95% CI 0.32 to 1.87; 4 studies, 281 participants) (Baker 1977; Deutsch 2005; Janchai 2008; Woodburn 2004). In total, we excluded two to six studies across analyses. Results were similar for the proportion of individual type of skin‐related adverse events (see Additional tables on sensitivity analyses Table 4; Table 5).
Complications/adverse events – proportion of non skin‐related adverse events
Excluding studies at high or unclear risk of bias for the following criteria: random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data had no effect on the proportion of non skin‐related adverse events. It remains unclear whether rigid dressings increase the proportion of non skin‐related adverse events in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with random sequence generation (RR 1.14; 95% CI 0.61 to 2.13; 4 studies, 251 participants) (Deutsch 2005; Janchai 2008; Wong 2000; Woodburn 2004), and only studies with concealed allocation (RR 1.17; 95% CI 0.08 to 16.72; 1 study, 26 participants) (Janchai 2008). As none of the relevant studies blinded outcome assessors, or had complete outcome data, the pooled results were not estimable.
Excluding studies that analysed limbs, or studies that included transfemoral amputations also made no difference to the proportion of non skin‐related adverse events. It remains unclear whether rigid dressings increase the proportion of skin‐related adverse events in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with participant data (i.e. no limb data) (RR 1.26; 95% CI 0.66 to 2.40; 4 studies,281 participants) (Baker 1977; Deutsch 2005; Janchai 2008; Woodburn 2004), and only studies with transtibial amputations (RR 1.18; 95% CI 0.63 to 2.21; 5 studies, 321 participants) (Baker 1977; Deutsch 2005; Janchai 2008; MacLean 1994; Woodburn 2004). In total, we excluded one to six studies across analyses. Results were similar for the proportion of individual type of non skin‐related adverse events (see Additional tables on sensitivity analyses Table 4; Table 5; Table 6).
Swelling – change in swelling (short term)
Excluding studies at high or unclear risk of bias for the following criteria: random sequence generation, concealed allocation and incomplete outcome data had no effect on the change in swelling less than one month after amputation. It remains unclear whether rigid dressings decrease stump volume in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with random sequence generation (MD 20.84; 95% CI ‐50.65 to 92.33; 1 study, 26 participants) (Janchai 2008), only studies with concealed allocation (MD 20.84; 95% CI ‐50.65 to 92.33; 1 study, 26 participants) (Janchai 2008) and only studies with complete outcome data (MD 39.50; 95% CI 2.48 to 76.52; 1 study, 16 participants) (Mueller 1982).
Excluding studies that analysed limbs also made no difference to the change in swelling. It remains unclear whether rigid dressings decrease stump volume in the rigid dressings group in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with participant data (i.e. no limb data) (MD 34.43; 95% CI ‐0.75 to 69.62; 2 studies, 49 participants) (Hidayati 2013; Janchai 2008).
Swelling – change in swelling (medium term)
Excluding studies at high or unclear risk of bias for the following criteria: random sequence generation, concealed allocation, blinding of outcome assessor and incomplete outcome data had no effect on the change in swelling between one to three months of amputation. It remains unclear whether rigid dressings decrease stump volume in the rigid dressings group compared with the soft dressings group based on the sensitivity analysis which included only studies with random sequence generation (MD ‐3.13; 95% CI ‐93.64 to 87.38; 1 study, 22 participants) (Janchai 2008), and only studies with concealed allocation (MD ‐3.13; 95% CI ‐93.64 to 87.38; 1 study, 22 participants) (Janchai 2008). As none of the relevant studies blinded outcome assessor, or had complete outcome data, the pooled results were not estimable. In total, we excluded one to three studies across analyses (see Additional tables on sensitivity analyses Table 4; Table 5).
Data and analyses
Comparison 1. Rigid versus soft dressings.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baker 1977.
| Methods | Study design: randomised controlled trial Method of randomisation: not reported Country of study: United States of America Type of incision: long posterior flap Care setting: two hospitals (acute and rehabilitation settings) |
|
| Participants | Sample size: 51 participants (group 1 rigid dressing: 27; group 2 soft dressing: 24) Number of dropouts: possibly one dropout for wound healing ‐ unclear if dropout is from group 1 or 2 Inclusion criteria: participants who were candidates for below‐knee amputation for Ischaemic indications, and had warm, non‐infected, neurologically intact skin at proposed incision site and a functional knee joint Exclusion criteria: not reported Gender: not reported Mean age: (group 1: 65.3 years, range 43 to 86 years; group 2: 63.2 years, range 41 to 76 years) Participants with diabetes: 33 with diabetes (16 in group 1; 17 in group 2) Participants with other co‐morbidities: 11 with previous lumbar sympathectomy, all with absent pedal pulses, 34 with no popliteal pulse (18 in group 1; 16 in group 2), 3 with no femoral pulse (all in group 1) Cause of amputation: 39 due to skin necrosis as indication for amputation (21 in group 1; 18 in group 2), 12 due to incapacitating Ischaemic rest pain (6 in group 1; 6 in group 2), 6 due to failure of minor amputation, 13 due to unsuccessful vascular reconstruction Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: non‐removable rigid dressing Materials: fine mesh silk and fluffed lamb's wool held in place by Lycra spandex stocking, relief pads of orthopaedic felt glued to either side of tibia and patella, 4‐inch rolls of elastic plaster and regular plaster, strap incorporated into cast and attached to waist belt. (Plaster around patellar removed to allow for patellar motion.) Time to first dressing application: not reported Person applying dressing: certified prosthetist Duration of dressing: not reported Duration of dressing removal: not reported Frequency of dressing change: changed every 10 to 14 days (earlier if clinical indication of infection) Group 2 soft dressing – type: elastic bandaging Materials: self‐adherent gauze bandage and elastic bandage. (A posterior splint was used to maintain knee extension in some participants.) Time to first application: not reported Person applying dressing: ward physicians Duration of dressing: not reported Duration of dressing removal: not reported Frequency of dressing change: changed as needed |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) Primary wound healing: wounds healed when sutures removed at 14 to 21 days (count and percentage) aAdverse events – skin‐related: revisions to transfemoral/above‐knee amputations (count and percentage) aAdverse events – non skin‐related: deaths (count) Secondary outcomes (units and definitions, if provided) bPhysical function: time from amputation to rehabilitation/start of gait training (days) Other outcomes not included in this review Secondary wound healing: wounds not healed at suture removal but healed eventually (count and percentage) bLength of hospital stay: time from amputation to discharge from hospital (days) Time points included in this review Wound healing – observed at 14 to 21 days (short term) Adverse events – non skin‐related: deaths – observed at 30 days (medium term) Other time points not included in this review None reported |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported aOutcome reported in Results/Discussion but not specified as outcome in Methods bOutcome not pooled in meta‐analysis due to insufficient data; either because data not reported by group, missing measure of precision (e.g. SD, 95% CI), or both. Due to dropouts, the data were only presented as continuous data (i.e. mean/median times to event) rather than time‐to‐event data (i.e. hazard ratios, or Kaplan‐Meier curves). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random allocation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: "the twenty‐seven patients from the Veterans Administration Hospital were excluded from both this analysis as well as the rehabilitation time analysis because of different patient motivations, discharge and disposition problems, and physical therapy philosophies ... one patient with one arm required hospitalization until she was independent with a special walker (35 days) and is excluded from statistical analysis" Comment: large number of dropouts ( > 15%) for outcomes length of hospital stay and physical function (e.g. 53% (27/51)), and intentional exclusion of data by researchers |
| Selective reporting (reporting bias) | High risk | Comment 1: type of outcome prespecified in Methods but not reported in Results/Discussion (e.g. physical function as dichotomous outcome) Comment 2: outcomes missing data for inclusion in a meta‐analysis (e.g. length of hospital stay data not reported by group and missing SD, physical function data missing SD) |
| Other bias | Unclear risk | Comment: discrepancy between number of wounds healed (n = 51) and missing outcome data for wounds healed (n = 1). Although discrepancy in data reported was small and only affect 1 estimate, uncertain of other sources of bias due to unclear reporting |
Deutsch 2005.
| Methods | Study design: randomised controlled trial Method of randomisation: ballot Country of study: Australia Type of incision: long posterior flap Care setting: two hospitals (acute and rehabilitation settings) |
|
| Participants | Sample size: 50 participants (group 1 rigid dressing: 26; group 2 soft dressing: 24) Number of dropouts: differs depending on outcomes:
Inclusion criteria: consecutive dysvascular trans‐tibial amputees from vascular units of hospitals who consented to participate Exclusion criteria: not reported aGender: (group 1: 9 female 13 male; group 2: 6 female 13 male) aMean age: (group 1: 74.5 years; group 2: 72 years) aParticipants with diabetes: 21 (10 in group 1; 11 in group 2) Participants with other co‐morbidities: not reported Cause of amputation: all due to peripheral vascular disease Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: removable rigid dressing Materials: non‐adherent dressing (Tegaderm), terry‐towelling sock (Coolmax), fibreglass and resin sock Time to first application: in theatre post‐operation or recovery room within 20 minutes of wound closure Person applying dressing: treating prosthetist and assistant Duration of dressing: worn for 23.5 hours/day until casting for prosthetic fitting, then worn interchangeably with prosthesis until wound healed Duration of dressing removal: removed for no longer than 15 minutes during wound dressing or inspection Frequency of dressing change: not reported Group 2 soft dressing – type: crepe bandaging Materials: gauze, wool pad, crepe bandage Time to first application: in theatre post‐operation Person applying dressing: not reported Duration of dressing: worn for 23.5 hours/day until casting for prosthetic fitting, then replaced with blue‐line elastic bandage and worn interchangeably with prosthesis until wound healed Duration of dressing removal: removed for no longer than 15 minutes during wound dressing or inspection Frequency of dressing change: not reported |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) aWound healing: time from amputation to wound healing (days; wound healing defined as "complete, pink epithelial bridging of the suture line") Adverse events – skin‐related: revisions to transfemoral amputations (count) Adverse events – skin‐related: surgical debridements (count) Adverse events – skin‐related: stump damage post‐fall (count) Adverse events – non skin‐related: deaths (count) Adverse events – non skin‐related: falls (count) Adverse events – non skin‐related: medical complications (count; defined as organ failure or illness not directly related to residual wound healing) Secondary outcomes (units and definitions, if provided) aPrescription of prosthetics: time from amputation to first prosthetic fitting (days) aLength of hospital stay (acute and rehabilitation): time from amputation to discharge from hospital (days) Other outcomes not included in this review Length of acute stay: time from amputation to discharge from acute/admission to rehabilitation (days) Swelling/Stump volume: prosthetic sockets required within 6 months (count) Time points included in this review None reported Other time points not included in this review Swelling/stump volume – observed within 6 months |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported aThese data were from 41 participants. 9 participants were excluded due to deaths, transfemoral amputations and ineligibility for prosthetic rehabilitation ‡Outcome not pooled in meta‐analysis due to dropouts, and the data were only presented as continuous data (i.e. mean/median times to event) rather than time‐to‐event data (i.e. hazard ratios, or Kaplan‐Meier curves) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocated by ballot" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the physiotherapist treating amputees at the acute site reported that she was much more confident to exercise new amputees with the stump more protected" Comment: reported for personnel (i.e. physiotherapist), not reported for other personnel or participants but unlikely for other personnel and not possible for participants to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the nursing staff performed an assessment and documentation of date of wound healing" Comment: not clear if nursing staff blinded but unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: large number of dropouts ( > 15%) for all four outcomes mentioned in results (e.g. 38% (19/50) missing for time to wound healing, 18% (9/50) missing for time to prosthetic fitting, 18% (9/50) missing for length of acute stay, and 32% (16/50) missing for length of hospital stay), and no reasons stated for some missing outcomes (see table 2) |
| Selective reporting (reporting bias) | High risk | Quote: "the physiotherapist treating amputees at the acute site reported that she was much more confident to exercise new amputees with the stump more protected. Nursing staff report a decreased amount of time spent dressing and rebandaging stump wounds" Comment: outcomes not prespecified in Methods but reported in Results/Discussion (e.g. paragraph on "other benefits") |
| Other bias | Unclear risk | Quote: "there were no significant differences between the two groups of amputees in terms of age, sex, side amputated, or incidence of diabetes (Table I)"; numbers in Table 2 only add up to 41 when 50 participants were included in study Comment 1: despite text to state no imbalance in baseline characteristics between rigid dressing and soft dressing groups, and a reference to Table 2, it appears that baseline characteristics of all participants were not reported Quote: "a larger sample group would have been necessary to achieve statistical significance at (p ≤ 0.05) with regard to wound healing in the present study. Because of the apparent success of the RRD, it was deemed unethical to persuade surgeons and other staff that it was appropriate to continue to withhold the RRD from half of the new amputee population. BHS now fits RRDs to all new trans‐tibial amputees" Comment 2: trial appears to have been stopped early by researchers due to trend in faster wound healing results in rigid dressings group. Unclear whether this decision was made by an Independent Data Monitoring Board or by repeated looks at the data by the authors. If latter, potential for opportunistic stopping of the trial |
Hidayati 2013.
| Methods | Study design: randomised controlled trial Method of randomisation: unclear – "randomization through a block of two to divide subjects into two groups" Country of study: Indonesia Type of incision: posterior flap, and non‐posterior flap Care setting: three hospitals |
|
| Participants | Sample size: 23 participants (group 1 rigid dressing: 12; group 2 soft dressing: 11) Number of dropouts: two participants died "during the study period" but it is unclear if they had been randomised Inclusion criteria: participants with diabetes mellitus (DM) that have transtibial amputation within 1 month, visual analogue scale (VAS) > 3, controlled blood glucose, ankle brachial index (ABI) from 0.8 to 1.2 Exclusion criteria: participants with impaired immunity (leucocytes < 1500/μL), other causes of amputation aside from DM, presence of cognitive impairment and handicap in hand (unless participant had a care assistant who can help with the application of the elastic bandage), and damage to sutures skin flap in the removable rigid dressing group Gender: (group 1: 7 female 5 male; group 2: 3 female 8 male) Mean age: (group 1: 54.3 ± 8.1 years; group 2: 59.9 ± 9.0years) Participants with diabetes: all Participants with other co‐morbidities: 5 with uncontrolled blood glucose (3 in group 1; 2 in group 2), 15 with infection (8 in group 1; 7 in group 2) Cause of amputation: all due to peripheral vascular disease Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: removable rigid dressing Materials: plaster of Paris, extra layer of socks (maximum 4 layers) Time to first application: within first month of amputation Person applying dressing: not reported Duration of dressing: 8 weeks Duration of dressing removal: not reported Frequency of dressing change: applied at first week, reapplied every 7 days Group 2 soft dressing – type: elastic bandaging Materials: elastic bandage (figure of 8 technique) Time to first application: within first month of amputation Person applying dressing: participant and caregiver Duration of dressing: 8 weeks Duration of dressing removal: not reported Frequency of dressing change: reapplied every 4 hours each day |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) aAdverse events – non skin‐related: time from amputation to resolution of stump pain (weeks) Adverse events – non skin‐related: stump pain – measured via visual analogue scale (number between 0 to 10; 0 indicates no pain and 10 indicates unbearable pain) Secondary outcomes (units and definitions, if provided) Swelling/Stump volume: change in stump volume between weeks – measured via amount of water spilled out from volume glass (cm³) cSwelling/Stump volume: time from amputation to "decrease in stump edema volume" ("Relative Risk") Other outcomes not included in this review bAdverse events – non skin‐related: deaths (count) Time points included in this review Adverse events – non skin‐related: stump pain – observed at 3 weeks (short term) and 8 weeks (medium term) Swelling/Stump volume – observed at 3 weeks (short term) and 8 weeks (medium term) Other time points not included in this review Adverse events – non skin‐related: stump pain and swelling/stump volume – observed every week for 8 weeks. Hence, other time points that are not included in this review for these two outcomes include 1, 2, 4, 5, 6 and 7 week(s) |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported aOutcome reported in Results/Discussion but not specified as outcome in Methods bOutcome not pooled in meta‐analysis due to insufficient data; either because data not reported by group, missing measure of precision (e.g. SD, 95% CI), or both. cOutcome not pooled in meta‐analysis due to insufficient data; no hazard ratios or information to calculate hazard ratios provided. (Authors provided RR (Relative Risk) instead of HR (Hazard Ratio), and it was unclear how "decrease in stump edema volume" was dichotomised.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "sampling was conducted by using consecutive sampling and randomization through a block of two to divide subjects into two groups" Comment: randomisation mentioned, but unclear how participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "two subjects died during the study period and one person refused to participate in the study" Comment 1: unclear if the 2 participants that died had been randomised prior, and now excluded by researchers. Although data removed were small (2 participants), uncertain of other sources of bias due to unclear reporting Quote: "stumps were not considered if edema obtained was accompanied by the change in volume of stump edema in three consecutive measurements" Comment 2: data from some participants were intentionally excluded by researchers |
| Selective reporting (reporting bias) | High risk | Comment 1: type of outcome not prespecified in Methods but reported in Results/Discussion, (e.g. stump pain as time‐to‐event outcome) Comment 2: outcomes missing data for inclusion in a meta‐analysis, (e.g. stump pain and stump volume missing hazard ratios (HR), or Kaplan Meier curves) |
| Other bias | Unclear risk | Quotes: "then performed survival analysis using Cox regression to assess the RR (relative risk) between the relationships of time it takes for the stump become not edema ... Multivariate analysis was performed by using backward technique in Cox regression test to evaluate the influences of each variables in the acceleration of decreasing stump edema volume and stump pain. Based on these tests, it was found that the intervention was statistically significant for RRD and elastic bandages to decrease stump edema volume. A value of RR obtained 3.088 (CI 95%: 1.128–4.916). It means that the RRD decreased stump edema volume three times faster than the elastic bandage" Comment: reporting of RR instead of HR for Cox regression analyses. Uncertain of other sources of bias due to unclear reporting |
Janchai 2008.
| Methods | Study design: randomised controlled trial Method of randomisation: random number table Country of study: Thailand Type of incision: not reported Care setting: one hospital (rehabilitation setting) |
|
| Participants | Sample size: 26 participants (group 1 rigid dressing: 12; group 2 soft dressing: 14) Number of dropouts: differs depending on time point 0‐2 weeks: 0 dropouts 2‐4 weeks: 4 dropouts (1 in group 1; 3 in group 2) 0‐4 weeks: 4 dropouts (1 in group 1; 3 in group 2) Inclusion criteria: participants with below‐knee amputations (no longer than 3 months) Exclusion criteria: participants who could not follow protocol, had severe infected stump wound, or were from other provinces and could not return for follow‐up Gender: 15 female 11 male (group 1: 7 female 5 male; group 2: 8 female 6 male) Mean age: 68.2 ± 10.8 years (group 1: 67.6 ± 13.6 years; group 2: 68.7 ± 8.3 years) Participants with diabetes: 21 (10 in group 1; 11 in group 2) Participants with other co‐morbidities: not reported Cause of amputation: 9 due to infection (3 in group 1; 6 in group 2), 15 due to vascular (8 in group 1; 7 in group 2), 2 due to tumour (1 in group 1; 1 in group 2) Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: removable rigid dressing Materials: stump sock = thin and thick cotton stockinette, below‐knee cast = plaster of Paris, suspension stockinette = polyester stockinette, supracondylar suspension cuff = plaster of Paris Time to first application: not reported Person applying dressing: participants initially (participants taught to put on dressing), then participants, relatives or caregivers after. (Prosthetist prepared cast.) Duration of dressing: worn continuously Duration of dressing removal: removed every 4‐6 hours for massage, wound inspection or bathing as prescribed in practice guideline sheet Frequency of dressing change: stump socks added according to stump shrinkage and new cast replaced as needed during follow‐up. Group 2 soft dressing – Type: elastic bandaging Materials: Standard 4 inches elastic bandage Time to first application: not reported Person applying dressing: participants initially (participants taught to put on dressing), then participants, relatives or caregivers after Duration of dressing: worn continuously Duration of dressing removal: removed every 4‐6 hours for massage, wound inspection or bathing as prescribed in practice guideline sheet Frequency of dressing change: not reported |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) aAdverse events – skin‐related: wound trauma from falls (count) aAdverse events – non skin‐related: falls (count) Secondary outcomes (units and definitions) Swelling stump volume: change in stump volume between weeks – measured via circumferential measurements entered into Katch and Katch mathematical formula (cm³) Other outcomes not included in this review None reported Time points included in this review Swelling/stump volume – observed at 2 weeks (short term) and 4 weeks (medium term) Other time points not included in this review None reported |
|
| Notes | Publication status: published Funding source: Ratchadaphiseksompotch Fund of the Faculty of Medicine, Chulalongkorn University Conflicts of interest: none reported aOutcome reported in Results/Discussion but not specified as outcome in Methods. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization was generated by using a table of random numbers and blocks of four techniques" |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation was concealed and in separate opaque envelopes, which was sequentially numbered. The secretary opened the next in a series of envelopes when a new patient was enrolled" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:"all circumference measurements for an individual patient were taken by the same therapist" Comment: not clear if therapist blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: although number of dropouts (e.g. 15% (4/26)) was small, imbalance in number of dropouts between groups (e.g. 4% (1/26) in rigid dressing group and 12% (3/26) in soft dressing group) and no reasons mentioned (see table 2) |
| Selective reporting (reporting bias) | High risk | Quote: "the patients in RRD group were ready for prosthetic fitting faster than the EB group and learned to use the prosthetic socket faster, too" Comment: outcome not prespecified in Methods but reported in Results/Discussion, (e.g. prescription of prosthetics) |
| Other bias | Low risk | Comment: study appears free of other biases |
MacLean 1994.
| Methods | Study design: quasi‐randomised controlled trial Method of randomisation: alternate assignment ‐ "alternate assignment of subjects to one of two groups as they became available" Country of study: Canada Type of incision: not reported Care setting: one hospital (acute and rehabilitation settings) |
|
| Participants | Sample size: 40 participants (group 1 rigid dressing: 19; group 2 soft dressing: 21); 43 limbs (group 1 rigid dressing: 22; group 2 soft dressing: 21) Number of dropouts: 16 dropouts (6 in group 1; 10 in group 2) Inclusion criteria: persons with below‐knee amputations as consequence of peripheral vascular disease Exclusion criteria: none reported Gender: 15 female 25 male (group 1: 6 female 13 male; group 2: 9 female 12 male) Mean age: (group 1: 61.3 ± 9.4 years; group 2: 61.8 ± 11.2 years) Participants with diabetes: 24 (13 in group 1; 11 in group 2) Participants with other co‐morbidities: 29 with anaemia (12 in group 1; 17 in group 2), 27 smokers (12 in group 1; 15 in group 2), 27 with hypertension (12 in group 1; 15 in group 2), 21 with cardiac disease (13 in group 1; 8 in group 2) – note 2 incomplete observations for anaemia in group 1, 1 incomplete observation for smoker and 1 incomplete observation for hypertension in group 2 Cause of amputation: all due to peripheral vascular disease Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: semi‐rigid dressing/"Unna boot" Materials: Jelonet, Dome‐Paste (gauze with zinc oxide, calamine and gelatin), Kling gauze Time to first application: immediately in operating room or half hour post‐op in recovery room Person applying dressing: physical therapist Duration of dressing: up to 7 days Duration of dressing removal: not reported Frequency of dressing change: removed once when drains removed 48 hours post‐operation, changed thereafter as needed (i.e. if it became loose, or if physician wanted to inspect wound) and reapplied every 7 days, until incision healed Group 2 soft dressing ‐ type: elastic bandaging Materials: tensor bandage Time to first application: immediately in operating room Person applying dressing: surgeon (in operating room), nurse or physical therapist (after 48 hour dressing change), or unidentified person who is not "skilled personnel" Duration of dressing: not reported Duration of dressing removal: not reported Frequency of dressing change: daily, or several times daily in early post‐surgical period |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) aAdverse events – skin‐related: above‐knee amputations (count) aAdverse events – non skin‐related: deaths (count) aAdverse events – non skin‐related: medical complications (count) aAdverse events – non skin‐related: stump pain (count) aAdverse events – non skin‐related: phantom pain (count) Secondary outcomes (units and definitions, if provided) bPrescription of prosthetics: time from amputation to lack of readiness for first prosthetic fitting (days; readiness for first prosthetic fitting defined as "the absence of edema and no further changes in girth of the stump on three separate measurements taken every 2 days") Other outcomes not included in this review None reported Time points included in this review None reported Other time points not included in this review None reported |
|
| Notes | Publication status: published Funding source: Research and Development Committee of Foothills Hospital Conflicts of interest: none reported aOutcome reported in Results/Discussion but not specified as outcome in Methods bOutcome not included in meta‐analysis as differs from other studies (i.e. event was lack of readiness for first prosthetic fitting instead of readiness for first prosthetic fitting/first prosthetic fitting) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotes: "we did not randomize treatment to subjects; rather, we alternately assigned the subjects to one of two groups as they became available ... we believe our results are generalizable; however, random assignment to treatment group may have been a more rigorous approach" Comment: sequence generation was not random |
| Allocation concealment (selection bias) | High risk | Comment: probably not done, considering the lack of random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "two experienced therapists (having practiced physical therapy more than 3 years) examined each patient separately, and each therapist determined separately when the patient was ready for prosthetic fitting. The first therapist who measured the patients also treated the patients throughout their rehabilitation, and subsequently knew to which group each patient belonged. The second therapist who measured the patients, however, was not informed as to which group the patients belonged, nor as to the results of the measurements taken by the first therapist" Comment: two physical therapists assessed – one was blinded, one was not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "six subjects in the SRD group did not complete the study. Three subjects underwent above‐knee amputations, 1 subject was transferred out of town, 1 subject died, and 1 subject was removed at the request of the physician. In the soft dressing group, 10 subjects did not complete the study. Seven subjects underwent above‐knee amputations, 2 subjects died, and we were unable to determine the time to fitting of 1 subject due to other medical problems. (The patient was very ill in the intensive care unit at the time his residuum was healed)" Comment 1: large number of dropouts ( > 15%) for outcome time to prosthetic fitting (e.g. 40% (16/40) missing), and numbers were not balanced with a higher number of dropouts in soft dressings group (25% (10/40)) compared with rigid dressings group (15% (6/40)) Quote: "1 subject was removed at the request of the physician" Comment 2: although data removed were small and only affected 1 estimate, uncertain of other sources of bias due to intentional exclusion of data by physician who treated participants in study |
| Selective reporting (reporting bias) | High risk | Comment: outcome was prespecified in Methods as "readiness for prosthetic fitting". However, this was changed to "lack of readiness for prosthetic fitting" in Data Analysis and subsequent results |
| Other bias | High risk | Quote: "All but two of the factors were distributed fairly evenly between the two groups. There were nearly twice as many patients with cardiac disease in the SRD group than in the soft dressing group. There was also an uneven distribution of drain types between the two groups" Comment 1: imbalance in two potentially important baseline characteristic (i.e. cardiac disease and drain types). Likely due to the lack of random sequence generation Quote: "Three patients were included in the study twice because they were later readmitted for amputation of the other leg. One patient received SRDs on both legs, and the other two patients received the soft dressing on one leg and the SRD on the other leg" Comment 2: unit of analysis issue. Data from 6 limbs (instead of 3 participants) used in outcome time from amputation to readiness for first prosthetic fitting |
Mueller 1982.
| Methods | Study design: quasi‐randomised controlled trial Method of randomisation: order of admission ‐ "by order of admission to use either the conventional elastic bandage or the plaster RRD" Country of study: USA Type of incision: not reported Care setting: one hospital (rehabilitation setting) |
|
| Participants | Sample size: 15 participants (group 1 rigid dressing: 7; group 2 soft dressing: 8); 16 limbs (group 1 rigid dressing: 8; group 2 soft dressing: 8) Number of dropouts: no dropouts Inclusion criteria: participants who a) had undergone below‐knee amputation no longer than two months before the study and b) were at least 55 years old Exclusion criteria: none reported Gender: 5 female 10 male (group 1: 4 female 3 male; group 2: 1 female 7 male) Mean age: (group 1: 72 ± 14 years; group 2: 74 ± 6 years) Participants with diabetes: 12 (6 in group 1; 6 in group 2) Participants with other co‐morbidities: 3 with thromboemboli (2 in group 1; 1 in group 2), 1 with peripheral vascular insufficiency (0 in group 1; 1 in group 2) Cause of amputation: all due to peripheral vascular disease Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: removable rigid dressing Materials: plaster rolls, hexalite (or polyform), velcro, cast stockinette and cotton cast padding, athletic tube socks (two‐ply) Time to first application: at admission to rehabilitation Person applying dressing: physical therapist initially, then participant after (participant taught how to put on dressing independently) Duration of dressing: worn at all times except during hygiene, wound care, or excessive pain; worn for 3 weeks (period of study) Duration of dressing removal: not reported Frequency of dressing change: not reported Group 2 soft dressing – type: elastic bandaging Materials: conventional elastic bandage Time to first application: at admission to rehabilitation Person applying dressing: physical therapist initially, then participant after (participant taught how to put on dressing independently) Duration of dressing: worn at all times except during hygiene, wound care, or excessive pain; worn for 3 weeks (period of study) Duration of dressing removal: not reported Frequency of dressing change: not reported |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) Adverse events – skin‐related: pressure areas (count) Secondary outcomes (units and definitions, if provided) Swelling/stump volume: change in stump volume between weeks – measured via circumferential measurements entered into Katch and Katch mathematical formula (cm³) Other outcomes not included in this review Ability of participant to apply dressing independently (count; defined as "able to achieve total contact of the dressing on the residual limb with no assistance") Tendency of dressing to remain secure (count; defined as "if it remained in total contact with the amputated limb about 75 percent of the time") Time points included in this review All outcomes – observed at 3 weeks (short term) Other time points not included in this review None reported |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "all patients were randomly assigned by order of admission to use either the conventional elastic bandage or the plaster RRD" Comment: sequence generation was not random |
| Allocation concealment (selection bias) | High risk | Comment: probably not done, considering the lack of random sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "those assigned to use the RRDs were fitted at admission by the author following the procedure outlined by Wu" Comment: reported for personnel (i.e. physiotherapist), not reported for other personnel or participants but unlikely for other personnel and not possible for participants to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "To further ensure reliability and to eliminate investigator bias, all circumference measurements for an individual patient were taken by the same therapist using a blank ribbon, which was then measured on a flat surface" Comment: unclear if the therapist is the same therapist who had applied the dressings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts ‐ same numbers in Table 2 and 2 |
| Selective reporting (reporting bias) | High risk | Quote: "although not objectively measured, pain seemed to be minimized by the RRD in the patients fitted at our institution. A reason for this may be that the dressing provided a rigid protection for the tender stump against bumping. The patients with bilateral below‐knee amputations also noted that bed mobility and transfers were much easier because they were able to push down on the ends of their stumps with no pain" Comment: outcomes not prespecified in Methods but reported in Results/Discussion, (e.g. pain, physical function) |
| Other bias | Unclear risk | Quote: "eleven of the patients had unilateral amputations, and four of the patients had bilateral amputations; however, three of the four patients with bilateral amputations had undergone one amputation more than two months before the study was initiated, and measurements of these residual limbs were not included. Therefore, the treatment results for 16 limbs, in all, were evaluated in the study" Comment: unit of analysis issue. Data from 2 limbs (instead of 1 participant) used in outcome swelling/stump volume |
Vigier 1999.
| Methods | Study design: randomised controlled trial Method of randomisation: not reported Country of study: France Type of incision: not reported Care setting: one hospital (rehabilitation setting) |
|
| Participants | Sample size: 56 participants (group 1 rigid dressing: 28; group 2 soft dressing: 28) Number of dropouts: 4 dropouts (2 in group 1; 2 in group 2) Inclusion criteria: participants with recent ( < 3 months) unilateral below‐knee amputation at the junction of the upper and middle third of the leg, amputation resulting from arterial occlusive disease, stump initially open by design, with a wound surface between 8 cm2 and 24 cm2 measured by a tracing of the wound on a sterile transparent graph sheet; and transcutaneous oxygen tension (TcPoz) of ≥ 35 mmHg. Exclusion criteria: participants with contraindications to use of artificial limbs because of general health problems (e.g. serious heart failure, arrhythmia, neurologic disorders), with stump problems other than healing that hindered socket contact (e.g. algodystrophy, infections, secondary necrosis, neuroma, intense contact pressure pain), or if there was ischaemia of the non‐amputated limb (e.g. ankle systolic index, measured by Doppler, of < 0.6). Gender: 11 female 45 male (group 1: 5 female 23 male; group 2: 6 female 22 male) Mean age: (group 1: 65.2 ± 12.4 years; group 2: 66.8 ± 10.8 years) Participants with diabetes: not reported Participants with other co‐morbidities: not reported Cause of amputation: all due to peripheral vascular disease/"arterial occlusive disease" Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: removable rigid dressing Materials: silicone sleeve with tubular piece of jersey sewn up at stump end, plaster contact cast Time to first application: not reported Person applying dressing: not reported Duration of dressing: 30 minutes for first 2 days, then 5 hours daily Duration of dressing removal: not reported Frequency of dressing change: not reported Group 2 soft dressing – type: elastic bandaging Materials: elastic bands Time to first application: not reported Person applying dressing: not reported Duration of dressing: worn "permanently" and only removed for dressing changes Duration of dressing removal: not reported Frequency of dressing change: not reported |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) Wound healing: time from amputation to complete healing of stump (days; wound healing defined as "achieved epithelialization") Secondary outcomes (units and definitions, if provided) Physical function: time from amputation to ability to walk with an off‐load prosthesis (days) Length of hospital stay: time from amputation to discharge from hospital (days) Other outcomes not included in this review Physical function: time from amputation to ability to walk more than 20 m with a prosthesis with contact socket (days) Length of acute stay: time from amputation to discharge from acute/admission to rehabilitation (days) Time points included in this review None reported Other time points not included in this review None reported |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random allocation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "it would have been useful to take blinded measures of the wound surface at regular intervals to observe healing progress. We were unable to put this method into practice reliably enough for statistical evaluation" Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "from the total number of patients used for this study, two for each group had to be left out because of medical problems unrelated to the stump, but nonetheless making use of a prosthesis impossible" Comment: small number of dropouts ( < 15%) for all outcomes (e.g. 7% (4/56) missing) and numbers balanced in both groups. |
| Selective reporting (reporting bias) | High risk | Comment: outcome not prespecified in Methods but reported in Results/Discussion, (e.g. physical function ‐ "walking with an offload prosthesis") |
| Other bias | Low risk | Comment: study appears free of other biases. |
Wong 2000.
| Methods | Study design: randomised controlled trial Method of randomisation: random number table Country of study: USA Type of incision: not reported Care setting: one hospital (rehabilitation setting) |
|
| Participants | Sample size: 21 participants (group 1 rigid dressing: 12; group 2 soft dressing: 9); 22 limbs (group 1 rigid dressing: 12; group 2 soft dressing: 10) Number of dropouts: 8 dropouts (3 in group 1; 5 in group 2) Inclusion criteria: participants who had undergone transtibial (TT) or transfemoral (TF) amputation within 30 days of surgery, deemed appropriate by attending physician for inpatient rehabilitation, had amputations secondary to peripheral vascular disease and can comply with instructions presented in rehabilitation unit Exclusion criteria: participants who had an infectious cellular culture or fever or nonviable amputation limb Gender: 11 female 10 male (group 1: 5 female 7 male; group 2: 6 female 3 male) Mean age: (group 1: 70.8 ± 10.1 years; group 2: 64.1 ± 9.3 years) Participants with diabetes: (58% in group 1; 80% in group 2) Participants with other co‐morbidities: diabetic neuropathy (17% in group 1; 0% in group 2), hypertension (42% in group 1; 60% in group 2), coronary artery disease (17% in group 1; 10% in group 2), previous amputation (8% in group 1; 20% in group 2), others such as myocardial infarction, cerebrovascular accident, pulmonary disease and cancer (42% in group 1; 0% in group 2) Cause of amputation: all due to peripheral vascular disease Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: semi‐rigid dressing/"Unna boot" Materials: sterile gauze, op‐site transparent adhesive film, dome‐Paste (Unna) bandages ± felt pads Time to first application: not reported (time appeared to vary with the mention of felt pads, e.g. "immediately after surgery", or "several weeks after surgery") Person applying dressing: physical therapists Duration of dressing: 3 to 5 days Duration of dressing removal: not reported Frequency of dressing change: reapplied if significant atrophy has occurred or on wound inspection (usually every 3‐5 consecutive days) Group 2 soft dressing – type: elastic bandaging Materials: conform stretch bandage, micropore tape, 4‐inch wide elastic bandages (for transtibial limbs) or 4‐inch and 6‐inch wide elastic bandages (for transfemoral limbs) Time to first application: not reported Person applying dressing: nurses, family members and participants Duration of dressing: not reported Duration of dressing removal: not reported Frequency of dressing change: reapplied as needed throughout the day (usually every 4 hours) depending on wound inspection, loosening or soiling of bandages |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) aAdverse events – non skin‐related: deaths (count) Secondary outcomes (units and definitions, if provided bPrescription of prosthetics: time from amputation to readiness for first prosthetic fitting (days; readiness for first prosthetic fitting defined as "(1) no opening in the incision larger than 1cm, (2) cylindrical limb shape as confirmed by girth measurements, (3) stable girth for at least 1 week and (4) the subject’s ability to transfer from bed to wheelchair with minimal assistance" cPhysical function: – measured via items of the Functional Independence Measure (FIM). Items include transfers, non‐prosthetic gait, gait with or without prosthesis (scores on items) Other outcomes not included in this review Prescription of prosthetics: time from rehabilitation admission to readiness for prosthetic fitting (days; readiness criteria for prosthetic fitting defined as above) Functional outcome: – measured via a 3‐point scale classified as (1) never fitted with a prosthesis, (2) fitted but unable to ambulate independently with the prosthesis and (3) fitted and able to ambulate independently with the prosthesis and a cane or walker – scale not validated. (count and percentage) cLength of hospital stay (rehabilitation): time from rehabilitation admission to discharge from hospital (days) Time points included in this review Adverse events – non skin‐related: deaths – observed at follow‐up which ranged from 6‐20 months (long term) Other time points not included in this review Physical function: FIM and 3‐point scale – observed at discharge but no specific time point mentioned |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported aOutcome reported in Results/Discussion but not specified as outcome in Methods bHR (Hazard ratio) calculated and included in meta‐analysis as authors presented P value of log‐rank tests, total events and numbers randomised to each arm cOutcome not pooled in meta‐analysis due to insufficient data; either because data not reported by group, missing measure of precision (e.g. SD, 95% CI), or both. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "once informed consent was obtained, each was assigned with the use of a random number table to the ED or SRD group" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "twice a week, two therapists took girth measurements with a blank tape that was subsequently laid on a ruler. Although not blinded to the type of dressing, the therapists always included a clinician who was not treating a given subject. Measurements were averaged, and the mean value was recorded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "of those in the ED group, 2 died, including the one person who had been discharged home ambulatory with a prosthesis. Three others could not be reached ... in contrast, at the time of follow‐up, only 1 of the 12 subjects in the SRD group remained unsuitable for a permanent prosthesis. One died and 2 could not be located" (see Table 6) Comment: large number of dropouts ( > 15%) for all outcomes (e.g. 38% (8/21) missing) |
| Selective reporting (reporting bias) | High risk | Comment 1: outcome prespecified in Methods but not reported in Results/Discussion (e.g. length of hospital stay) Comment 2: outcome missing data for inclusion in a meta‐analysis, (e.g. physical function missing SD) |
| Other bias | Unclear risk | Quote: "the ED group consisted of 9 subjects (6 women, 3 men) with 10 recent amputations (table 1). One woman had both legs amputated at the TT level during the same hospitalization period; data were collected for each leg" Comment: unit of analysis issue. Data from 2 limbs (instead of 1 participant) used in outcome time from amputation to readiness for first prosthetic fitting and physical function measures |
Woodburn 2004.
| Methods | Study design: randomised controlled trial Method of randomisation: central telephone randomisation service Country of study: UK Type of incision: skew flap or long posterior flap Care setting: 7 hospitals |
|
| Participants | Sample size: 154 participants (group 1 rigid dressing: 78; group 2 soft dressing: 76) Number of dropouts: 42 dropouts (22 in group 1; 20 in group 2) Inclusion criteria: new transtibial amputation Exclusion criteria: not reported Gender: 40 female; 114 male Mean age: not reported Participants with diabetes: not reported Participants with other co‐morbidities: not reported Cause of amputation: not reported Skin integrity: not reported |
|
| Interventions |
Group 1 rigid dressing – type: non‐removable rigid dressing Materials: gauze swabs, tubinette stocking, moulded polyurethane distal end cap, elasticated stump sock, orthopedic felt, elasticated or conventional Plaster of Paris. Suspension strap after 21 days Time to first application: in theatre post‐operation Person applying dressing: surgeon Duration of dressing: 21 days (worn for 7 days up to first review, then worn for further 14 days if satisfactory) Duration of dressing removal: not reported Frequency of dressing change: dressing removed and wound inspected at 7 days. If wound satisfactory, dressing reapplied for further 14 days before final removal Group 2 soft dressing – type: soft bandaging Materials: customary soft dressings and bandaging Time to first application: in theatre post‐operation Person applying dressing: surgeon Duration of dressing: not reported Duration of dressing removal: not reported Frequency of dressing change: not reported |
|
| Outcomes |
Outcomes included in this review Primary outcomes (units and definitions, if provided) Adverse events – skin‐related: wound infections (count and percentage) aAdverse events – skin‐related: revisions to transfemoral amputations (count and percentage) aAdverse events – non skin‐related: deaths (count) Secondary outcomes (units and definitions, if provided) bPrescription of prosthetics: time from amputation to first prosthetic fitting/casting (days) Other outcomes not included in this review Clinicians' beliefs and preferences for dressings – measured via a questionnaire with 8 statements on rigid dressings (number of responders who agree with each of the 8 statements) Time points included in this review None reported Other time points not included in this review None reported |
|
| Notes | Publication status: published Funding source: none reported Conflicts of interest: none reported aOutcome reported in Results/Discussion but not specified as outcome in Methods. bOutcome not pooled in meta‐analysis due to dropouts, and the data were only presented as continuous data (i.e. mean/median times to event) rather than time‐to‐event data (i.e. hazard ratios, or Kaplan‐Meier curves). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participating surgeons were then expected to randomise all new trans‐tibial amputations using a central telephone randomisation service" |
| Allocation concealment (selection bias) | High risk | Quote: "participating surgeons were then expected to randomise all new trans‐tibial amputations using a central telephone randomisation service" Comment: highly unlikely if surgeons (who included participants in study) knew the randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but not possible for participants and unlikely for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "although patients were referred for limb fitting after removal of the rigid dressing, at a time when the nursing and physiotherapy staff felt that this was appropriate, the final decision as to the suitability of a limb for casting was made by the local prosthetic team who were blinded to the post‐operative dressing regime employed" Comment: blinding for one outcome ‐ prescription of prosthetics but unclear if blinding was carried out for other outcome (e.g. wound infections) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "between March 1997 and September 1999, 14 surgeons from 7 centres enrolled 154 patients in the trial ... of the 112 patients who completed the trial, wound infection was documented ..."; reasons not stated for "incomplete data" (see Table 2) Comment: large number of dropouts ( > 15%) for outcome time to prosthetic fitting (e.g. 27% (42/154) missing). Reasons for "incomplete data" not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: although outcomes prespecified in Methods match outcomes reported in Results/Discussion, there was no published protocol. Unable to check if outcomes prespecified in protocol match outcomes reported in publication |
| Other bias | Unclear risk | Comment: no baseline data of participants provided. Uncertain if participants with certain prognostic factors were equal in both groups |
CI ‐ confidence interval EB ‐ elastic bandage FIM ‐ functional independence measure m ‐ metres RRD ‐ removable rigid dressing SD ‐ standard deviation SRD ‐ semi‐rigid dressing TT ‐ trans‐tibial TF ‐ trans‐femoral
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Choksy 2006 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between tourniquet and no tourniquet) |
| Graf 2003 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between removable rigid dressing and removable rigid dressing with polymer gel sock) |
| Johannesson 2008 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between vacuum‐formed removable rigid dressing and conventional rigid dressing) |
| Jones 1970 | Design of study: not randomised controlled trial (RCT) or quasi‐RCT |
| Kane 1980 | Design of study: not randomised controlled trial (RCT) or quasi‐RCT |
| Ladenheim 2007 | Design of study: not randomised controlled trial (RCT) or quasi‐RCT |
| Louie 2010 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between residual limb bandages and elastic residual limb socks) |
| Manella 1981 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between elastic bandages and elastic shrinker socks) |
| Mooney 1971 | Design of study: not randomised controlled trial (RCT) or quasi‐RCT |
| Ruckley 1986 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between controlled environment unit and plaster dressings) |
| Topuz 2012 | Interventions: comparison of interventions was not between rigid and soft dressings (comparison was between elastic bandages and complex decongestive physiotherapy which included manual lymphatic drainage, skin care, short‐stretch bandages and exercise) |
Characteristics of ongoing studies [ordered by study ID]
NCT03593174.
| Trial name or title | Étude de l'efficacité du Pansement Rigide Amovible Sous Vide à Titre de modalité de Pansement Post‐amputation Tibiale |
| Methods | Parallel‐group (2‐arm) randomised controlled trial |
| Participants | Adults (40 to 75 years old) with transtibial amputations of atraumatic cause |
| Interventions | Intervention: Ossur rigid dressing (type of removable rigid dressing) Comparator therapy: elastic bandage |
| Outcomes | Stump size (leg circumference measure) |
| Starting date | June 19 2018 |
| Contact information | Michel Tousignant Position: Professor, PhD Telephone: Email: Postal address: Universite de Sherbrooke, Sherbrooke, Quebec, Canada, J1K 2R1 |
| Notes | Currently recruiting (Estimated study completion date June 2019) |
TCTR20170928004.
| Trial name or title | Comparison of removable rigid dressing and elastic bandage for preprosthetic training in transtibial amputees: a randomised controlled trial |
| Methods | Parallel‐group (2‐arm) randomised controlled trial |
| Participants | Adults (at least 18 years old) with transtibial amputations |
| Interventions | Intervention: removable rigid dressing Comparator therapy: elastic bandage |
| Outcomes | Duration of stump maturation (days) Complications ‐ wound infection, pressure ulcer, joint contracture, shape abnormality, others Patient satisfaction (Likert scale) |
| Starting date | 24 June 2017 |
| Contact information | Name: Siriporn Janchia Address: Faculty of Medicine, Rehabilitation Chulalongkorn University Pathumwan, Bangkok 10330, Thailand Telephone: 0‐2256‐4433 Email: jju09@yahoo.com Affiliation: Faculty of Medicine, Chulalongkorn University |
| Notes | Currently recruiting |
Differences between protocol and review
"Types of participants", as per advice from the Cochrane editorial board, we added new text that states "If a study had mixed populations (e.g. transtibial and transfemoral amputations), we included the study if more than 75% of participants had transtibial amputations.".
"Types of outcome measures ‐ timing of outcome measures", we further clarified a sentence by adding the words "Where data (i.e. time point of outcome measurements) were available" to previous sentence in protocol. New text states "Where data (i.e. time point of outcome measurements) were available, we presented dichotomous and continuous outcomes as short‐term, medium‐term and long‐term outcomes."
"Data extraction and management", LKK and MW independently extracted data instead of LKK and LG. In the 'Characteristics of studies' tables, additional details were also extracted for participants, interventions and outcomes. For participants, we added details on co‐morbidities if listed by authors. For interventions, we added details on person applying dressing, duration of dressing removal and frequency of dressing change. For outcomes, we added details on units of outcomes, and divided outcomes into outcomes included in review (i.e. data which were pooled in meta‐analysis or summarised as narrative review) or outcomes not included in review (i.e. data which were neither pooled in meta‐analysis nor summarised as narrative review). Time points were also divided into time points included in review and time points not included in review.
"Measures of treatment effect", we further clarified when generic inverse variance method will be used with new text that states "We used the generic inverse variance method in RevMan (Review Manager 2014) for time‐to‐event data, and for dichotomous and continuous data when data cannot be entered in the usual form (e.g. if study only reports odds ratio or relative risk and its standard error for dichotomous outcome, or if study only reports difference between the means for two groups and the standard error of this difference) (Higgins 2011a). If dichotomous and continuous data were presented in the usual form, we used the Mantel‐Haenszel method for dichotomous data and the inverse variance method for continuous data in Revman (Review Manager 2014)."
"Unit of analysis issues", as per advice from the Cochrane editorial board, we added new text that states "However, if this number was very small (e.g. less than 10% of participants had double amputations) and studies had analysed data on limbs instead of participants, we included these studies in our meta‐analyses of outcome measures but conducted sensitivity analyses to determine if there are changes in effect estimates if such studies are omitted."
"Dealing with missing data", as per advice from the Cochrane editorial board, we added new text that states "To deal with missing data in analyses, we did the following: where studies measured dichotomous outcomes (e.g., proportion of adverse events) and had dropouts, we included the number of dropouts in the denominator but not in the numerator. This means that we assumed dropouts did not develop the outcomes of interest. Where studies measured continuous outcomes (e.g., wound size), or time‐to‐event outcomes (e.g. time from amputation to wound healing) and had dropouts, only complete‐case data were used. This means that we reported continuous outcomes and time‐to‐event outcomes of participants remaining in studies."
"Sensitivity analyses", as per advice from the Cochrane editorial board, we added new text that states "Specifically, we performed sensitivity analyses to determine the influence of studies with transfemoral amputations and studies that have analysed data on limbs instead of participants (provided less than 10% of participants had double amputations)."
As readers might be interested in the comparison of individual types of complications/adverse events between rigid and soft dressing groups, we present the data in an additional table (Table 3) in a similar format to a 'Summary of findings' table.
Contributions of authors
Li Khim Kwah: conceived, designed and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study author/experts/companies; approved the final review prior to submission; and is a guarantor of the review.
Matthew Webb: extracted data; analysed or interpreted data; undertook quality assessment; performed statistical analysis; contributed to writing or editing the review; and approved the final review prior to submission.
Lina Goh: conceived and designed the review; extracted data; contributed to writing or editing the review; and approved the final review prior to submission.
Lisa Harvey: conceived and designed the review; checked the quality of data extraction; analysed or interpreted data; checked quality assessment; checked the quality of the statistical analysis; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Contributions of the editorial base
Kurinchi Gurusamy: (Editor): edited the protocol, advised on methodology, interpretation and content; approved the final protocol prior to submission.
Nicky Cullum (Coordinating Editor): edited the review, advised on methodology, interpretation and content; approved the final review prior to submission.
Gill Rizzello (Managing Editor) : coordinated the editorial process; advised on interpretation and content and edited the review.
Naomi Shaw and Sophie Bishop (Information Specialists): designed the search strategy, ran the searches and edited the search methods sections.
Ursula Gonthier (Editorial Assistant): edited the Plain language summary and reference sections of the review.
Sources of support
Internal sources
Health and Social Sciences Cluster, Singapore Institute of Technology, Singapore.
Department of Physiotherapy, St George Hospital & Community Health Services, South Eastern Sydney Local Health District, Kogarah, Sydney, Australia.
Department of Physiotherapy, Bankstown‐Lidcombe Hospital, Bankstown, Australia.
John Walsh Centre for Rehabilitation Research, Kolling Institute, Northern Sydney Local Health District, St Leonards, Sydney, Australia.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and not necessarily those of the NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
Li Khim Kwah: none known.
Matthew Webb: none known.
Lina Goh: none known.
Lisa Harvey: two government organisations, Lifetime Care and Support Authority of NSW, Australia, fund my academic unit and position at the University of Sydney, Australia. I am Editor‐in‐Chief of Spinal Cord.
New
References
References to studies included in this review
Baker 1977 {published data only}
- Baker WH, Barnes RW, Shurr DG. The healing of below‐knee amputations: a comparison of soft and plaster dressing. American Journal of Surgery 1977;133(6):716‐8. [DOI] [PubMed] [Google Scholar]
Deutsch 2005 {published data only}
- Deutsch A, English RD, Vermeer TC, Murray PS, Condous M. Removable rigid dressings versus soft dressings: a randomized, controlled study with dysvascular, trans‐tibial amputees. Prosthetics and Orthotics International 2005;29(2):193‐200. [DOI] [PubMed] [Google Scholar]
Hidayati 2013 {published data only}
- Hidayati ER, Ilyas E, Murdana IN, Tarigan TJ, Werdhani RA. Efficacy of removable rigid dressing after transtibial amputation in diabetes mellitus patients. Medical Journal of Indonesia 2013;22:16‐21. [Google Scholar]
Janchai 2008 {published data only}
- Janchai S, Boonhong J, Tiamprasit J. Comparison of removable rigid dressing and elastic bandage in reducing the residual limb volume of below knee amputees. Journal of the Medical Association of Thailand 2008;91(9):1441‐6. [PubMed] [Google Scholar]
MacLean 1994 {published data only}
- MacLean N, Fick GH. The effect of semirigid dressings on below‐knee amputations. Physical Therapy 1994;74(7):668‐73. [DOI] [PubMed] [Google Scholar]
Mueller 1982 {published data only}
- Mueller MJ. Comparison of removable rigid dressings and elastic bandages in preprosthetic management of patients with below‐knee amputations. Physical Therapy 1982;62(10):1438‐41. [DOI] [PubMed] [Google Scholar]
Vigier 1999 {published data only}
- Vigier S, Casillas JM, Dulieu V, Rouhier‐Marcer I, D'Athis P, Didier JP. Healing of open stump wounds after vascular below‐knee amputation: plaster cast socket with silicone sleeve versus elastic compression. Archives of Physical Medicine and Rehabilitation 1999;80(10):1327‐30. [DOI] [PubMed] [Google Scholar]
Wong 2000 {published data only}
- Wong CK, Edelstein JE. Unna and elastic postoperative dressings: comparison of their effects on function of adults with amputation and vascular disease. Archives of Physical Medicine and Rehabilitation 2000;81(9):1191‐8. [DOI] [PubMed] [Google Scholar]
Woodburn 2004 {published data only}
- Woodburn KR, Sockalingham S, Gilmore H, Condie ME, Ruckley CV, Scottish Vascular Audit Group, Scottish Physiotherapy Amputee Research Group. A randomised trial of rigid stump dressing following trans‐tibial amputation for peripheral arterial insufficiency. Prosthetics and Orthotics International 2004;28(1):22‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Choksy 2006 {published data only}
- Choksy SA, Lee Chong P, Smith C, Ireland M, Beard J. A randomised controlled trial of the use of a tourniquet to reduce blood loss during transtibial amputation for peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2006;31(6):646‐50. [DOI] [PubMed] [Google Scholar]
Graf 2003 {published data only}
- Graf M, Freijah N. Early trans‐tibial oedema control using polymer gel socks. Prosthetics and Orthotics International 2003;27(3):221‐6. [DOI] [PubMed] [Google Scholar]
Johannesson 2008 {published data only}
- Johannesson A, Larsson GU, Oberg T, Atroshi I. Comparison of vacuum‐formed removable rigid dressing with conventional rigid dressing after transtibial amputation: similar outcome in a randomized controlled trial involving 27 patients. Acta Orthopaedica 2008;79(3):361‐9. [DOI] [PubMed] [Google Scholar]
Jones 1970 {published data only}
- Jones RF, Burniston GG. A conservative approach to lower‐limb amputations. Review of 240 amputees with a trial of the rigid dressing. Medical Journal of Australia 1970;2(16):711‐8. [DOI] [PubMed] [Google Scholar]
Kane 1980 {published data only}
- Kane TJ, Pollak EW. The rigid versus soft postoperative dressing controversy: a controlled study in vascular below‐knee amputees. American Surgeon 1980;46(4):244‐7. [PubMed] [Google Scholar]
Ladenheim 2007 {published data only}
- Ladenheim E, Oberti‐Smith K, Tablada G. Results of managing transtibial amputations with a prefabricated polyethylene rigid removable dressing. Journal of Prosthetics and Orthotics 2007;19(1):2‐4. [Google Scholar]
Louie 2010 {published data only}
- Louie S, Lai F, Poon C, Leung S, Wan I, Wong S. Residual limb management for persons with transtibial amputation: Comparison of bandaging technique and residual limb sock. Journal of Prosthetics and Orthotics 2010;22(3):194‐201. [Google Scholar]
Manella 1981 {published data only}
- Manella KJ. Comparing the effectiveness of elastic bandages and shrinker socks for lower extremity amputees. Physical Therapy 1981;61(3):334‐7. [DOI] [PubMed] [Google Scholar]
Mooney 1971 {published data only}
- Mooney V, Harvey JP Jr, McBride E, Snelson R. Comparison of postoperative stump management: plaster vs. soft dressings. Journal of Bone and Joint Surgery 1971;53(2):241‐9. [PubMed] [Google Scholar]
Ruckley 1986 {published data only}
- Ruckley CV, Rae A, Prescott RJ. Controlled environment unit in the care of the below‐knee amputation stump. British Journal of Surgery 1986;73(1):11‐3. [DOI] [PubMed] [Google Scholar]
Topuz 2012 {published data only}
- Topuz S, Ülger Ö, Bakar Y, Sener G. Comparison of the effects of complex decongestive physiotherapy and conventional bandaging on edema of geriatric amputees: a pilot study. Topics in Geriatric Rehabilitation 2012;28(4):275‐80. [Google Scholar]
References to ongoing studies
NCT03593174 {published data only}
- NCT03593174. Étude de l'efficacité du Pansement Rigide Amovible Sous Vide à Titre de modalité de Pansement Post‐amputation Tibiale. https://clinicaltrials.gov/ct2/show/NCT03593174 (first registered on 19 June 2018).
TCTR20170928004 {published data only}
- TCTR20170928004. Comparison of removable rigid dressing and elastic bandage for preprosthetic training in transtibial amputees: a randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20170928004 (first registered 24 June 2017).
Additional references
ACI 2017
- NSW Agency for Clinical Innovation (ACI). Care of the person following amputation: minimum standards of care. www.aci.health.nsw.gov.au/__data/assets/pdf_file/0019/360532/The‐care‐of‐the‐person‐following‐amputation‐minimum‐standards‐of‐care.pdf (accessed 23 March 2018).
Ahmad 2014
- Ahmad N, Thomas GN, Gill P, Chan C, Torella F. Lower limb amputation in England: prevalence, regional variation and relationship with revascularisation, deprivation and risk factors. A retrospective review of hospital data. Journal of the Royal Society of Medicine 2014;107(12):483‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2013
- Ali MM, Loretz L, Shea A, Poorvu E, Robinson WP, Schanzer A, et al. A contemporary comparative analysis of immediate postoperative prosthesis placement following below‐knee amputation. Annals of Vascular Surgery 2013;27(8):1146‐53. [DOI] [PubMed] [Google Scholar]
BACPAR 2012
- British Association of Chartered Physiotherapists in Amputee Rehabilitation (BACPAR). Guidance for the multidisciplinary team on the management of post‐operative residuum oedema in lower limb amputees. bacpar.csp.org.uk/publications/guidance‐multi‐disciplinary‐team‐management‐post‐operative‐residuum‐oedema‐lowe (accessed 23 March 2018).
Barnes 2014
- Barnes R, Souroullas P, Chetter IC. A survey of perioperative management of major lower limb amputations: current UK practice. Annals of Vascular Surgery 2014;28(7):1737‐43. [DOI] [PubMed] [Google Scholar]
Burgess 1968
- Burgess EM, Romano RL. The management of lower extremity amputees using immediate postsurgical prostheses. Clinical Orthopaedics and Related Research 1968;57:137‐46. [PubMed] [Google Scholar]
Choudhury 2001
- Choudhury SR, Reiber GE, Pecoraro JA, Czerniecki JM, Smith DG, Sangeorzan BJ. Postoperative management of transtibial amputations in VA hospitals. Journal of Rehabilitation Research and Development 2001;38(3):293‐8. [PubMed] [Google Scholar]
Churilov 2014
- Churilov I, Churilov L, Murphy D. Do rigid dressings reduce the time from amputation to prosthetic fitting? A systematic review and meta‐analysis. Annals of Vascular Surgery 2014;28(7):1801‐8. [DOI] [PubMed] [Google Scholar]
Condon 1969
- Condon RE, Jordan PH Jr. Immediate postoperative prostheses in vascular amputations. Annals of Surgery 1969;170(3):435‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curran 2014
- Curran T, Zhang JQ, Lo RC, Fokkema M, McCallum JC, Buck DB, et al. Risk factors and indications for readmission after lower extremity amputation in the American College of Surgeons National Surgical Quality Improvement Program. Journal of Vascular Surgery 2014;60(5):1315‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dumville 2015a
- Dumville JC, Keogh SJ, Liu Z, Stubbs N, Walker RM, Fortnam M. Alginate dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2015b
- Dumville JC, Stubbs N, Keogh SJ, Walker RM, Liu Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011226.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duwayri 2012
- Duwayri Y, Vallabhaneni R, Kirby JP, Mueller MJ, Volshteyn O, Geraghty PJ, et al. Early protection and compression of residual limbs may improve and accelerate prosthetic fit: a preliminary study. Annals of Vascular Surgery 2012;26(2):242‐9. [DOI] [PubMed] [Google Scholar]
EndNote 2014 [Computer program]
- Thomson Reuters. EndNote. Version X7.2.1. New York: Thomson Reuters, 2014.
Folsom 1992
- Folsom D, King T, Rubin JR. Lower‐extremity amputation with immediate postoperative prosthetic placement. American Journal of Surgery 1992;164(4):320‐2. [DOI] [PubMed] [Google Scholar]
Fortington 2013
- Fortington LV, Geertzen JH, Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. European Journal of Vascular and Endovascular Surgery 2013;46(1):124‐31. [DOI] [PubMed] [Google Scholar]
Gethin 2015
- Gethin G, Killeen F, Devane D. Heterogeneity of wound outcome measures in RCTs of treatments for VLUs: a systematic review. Journal of Wound Care 2015;24(5):211‐26. [DOI] [PubMed] [Google Scholar]
Golbranson 1968
- Golbranson FL, Asbelle C, Strand D. Immediate postsurgical fitting and early ambulation. A new concept in amputee rehabilitation. Clinical Orthopaedics and Related Research 1968;56:119‐31. [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro Guideline Development Tool. Version last updated: December 04, 2015. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Harris 2009
- Harris AM, Althausen PL, Kellam J, Bosse MJ, Castillo R, Lower Extremity Assessment Project (LEAP) Study Group. Complications following limb‐threatening lower extremity trauma. Journal of Orthopaedic Trauma 2009;23(1):1‐6. [DOI] [PubMed] [Google Scholar]
Hex 2012
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabetic Medicine 2012;29(7):855‐62. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hughes 1998
- Hughes S, Ni S, Wilson S. Use of removable rigid dressing for transtibial amputees rehabilitation: a Greenwich Hospital experience. Australian Journal of Physiotherapy 1998;44(2):135‐7. [DOI] [PubMed] [Google Scholar]
Johannesson 2010
- Johannesson A, Larsson GU, Ramstrand N, Lauge‐Pedersen H, Wagner P, Atroshi I. Outcomes of a standardized surgical and rehabilitation program in transtibial amputation for peripheral vascular disease: a prospective cohort study. American Journal of Physical Medicine & Rehabilitation 2010;89(4):293‐303. [DOI] [PubMed] [Google Scholar]
Jones 2013
- Jones WS, Patel MR, Dai D, Vemulapalli S, Subherwal S, Stafford J, et al. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. American Heart Journal 2013;165(5):809‐15. [DOI] [PubMed] [Google Scholar]
Kayssi 2015
- Kayssi A, Mestral C, Forbes TL, Roche‐Nagle G. Predictors of hospital readmissions after lower extremity amputations in Canada. Journal of Vascular Surgery 2015;63(3):688‐95. [DOI] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PLOS One 2013;8(7):e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2014
- Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Archives of Physical Medicine and Rehabilitation 2014;95(5):986‐95.e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 2004
- MacKenzie EJ, Bosse MJ, Castillo RC, Smith DG, Webb LX, Kellam JF, et al. Functional outcomes following trauma‐related lower‐extremity amputation. Journal of Bone and Joint Surgery 2004;86(8):1636‐45. [DOI] [PubMed] [Google Scholar]
MacKenzie 2005
- MacKenzie EJ, Bosse MJ, Pollak AN, Webb LX, Swiontkowski MF, Kellam JF, et al. Long‐term persistence of disability following severe lower‐limb trauma. Results of a seven‐year follow‐up. Journal of Bone and Joint Surgery 2005;87(8):1801‐9. [DOI] [PubMed] [Google Scholar]
Moxey 2010
- Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. British Journal of Surgery 2010;97(9):1348‐53. [DOI] [PubMed] [Google Scholar]
Moxey 2011
- Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, et al. Lower extremity amputations ‐ a review of global variability in incidence. Diabetic Medicine 2011;28(10):1144‐53. [DOI] [PubMed] [Google Scholar]
Nawijn 2005
- Nawijn SE, Linde H, Emmelot CH, Hofstad CJ. Stump management after trans‐tibial amputation: a systematic review. Prosthetics and Orthotics International 2005;29(1):13‐26. [DOI] [PubMed] [Google Scholar]
Perkins 2012
- Perkins ZB, De'Ath HD, Sharp G, Tai NR. Factors affecting outcome after traumatic limb amputation. British Journal of Surgery 2012;99(Suppl 1):75‐86. [DOI] [PubMed] [Google Scholar]
Pinzur 1989
- Pinzur MS, Littooy F, Osterman H, Schwartz D. A safe, pre‐fabricated, immediate postoperative prosthetic limb system for rehabilitation of below‐knee amputations. Orthopedics 1989;12(10):1343‐5. [DOI] [PubMed] [Google Scholar]
Randolph 2017
- Randolph B, Webster J, Highsmith MJ. VA/DoD Clinical practice guideline for rehabilitation of individuals with lower limb amputation. www.healthquality.va.gov/guidelines/Rehab/amp/ (accessed 23 March 2018).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ries 2015
- Ries Z, Rungprai C, Harpole B, Phruetthiphat OA, Gao Y, Pugely A, et al. Incidence, risk factors, and causes for thirty‐day unplanned readmissions following primary lower‐extremity amputation in patients with diabetes. Journal of Bone and Joint Surgery 2015;97(21):1774‐80. [DOI] [PubMed] [Google Scholar]
Schon 2002
- Schon LC, Short KW, Soupiou O, Noll K, Rheinstein J. Benefits of early prosthetic management of transtibial amputees: a prospective clinical study of a prefabricated prosthesis. Foot and Ankle International 2002;23(6):509‐14. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search‐filters.html (accessed 22 November 2018).
Smith 2003
- Smith DG, McFarland LV, Sangeorzan BJ, Reiber GE, Czerniecki JM. Postoperative dressing and management strategies for transtibial amputations: a critical review. Journal of Rehabilitation Research and Development 2003;40(3):213‐24. [PubMed] [Google Scholar]
Smith 2017
- Smith S, Pursey H, Jones A. Clinical guidelines for the pre and post‐operative physiotherapy management of adults with lower limb amputations. 2nd edition March 2017. bacpar.csp.org.uk/publications/2nd‐edition‐clinical‐guidelines‐pre‐post‐operative‐physiotherapy‐management‐adu (accessed 23 March 2018).
Sumpio 2013
- Sumpio B, Shine SR, Mahler D, Sumpio BE. A comparison of immediate postoperative rigid and soft dressings for below‐knee amputations. Annals of Vascular Surgery 2013;27(6):774‐80. [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor L, Cavenett S, Stepien JM, Crotty M. Removable rigid dressings: a retrospective case‐note audit to determine the validity of post‐amputation application. Prosthetics and Orthotics International 2008;32(2):223‐30. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; Vol. 8, issue 16:available from trialsjournal.biomedcentral.com/articles/10.1186/1745‐6215‐8‐16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed]
Tintle 2010
- Tintle SM, Keeling JJ, Shawen SB, Forsberg JA, Potter BK. Traumatic and trauma‐related amputations: part I: general principles and lower‐extremity amputations. Journal of Bone and Joint Surgery 2010;92(17):2852‐68. [DOI] [PubMed] [Google Scholar]
US Department of Veterans Affairs 2017
- US Department of Veterans Affairs (VA) and Department of Defense (DoD). VA/DoD Clinical practice guideline for rehabilitation of individuals with lower limb amputation. Version 2.0. September 2017. www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf (accessed 21 November 2018).
Varma 2014
- Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Physical Medicine and Rehabilitation Clinics of North America 2014;25(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2017
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18(Suppl 3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 1979
- Wu Y, Keagy RD, Krick HJ, Stratigos JS, Betts HB. An innovative removable rigid dressing technique for below‐the‐knee amputation. Journal of Bone and Joint Surgery 1979;61(5):724‐9. [PubMed] [Google Scholar]
Zayed 2014
- Zayed M, Bech F, Hernandez‐Boussard T. National review of factors influencing disparities and types of major lower extremity amputations. Annals of Vascular Surgery 2014;28(5):1157‐65. [DOI] [PubMed] [Google Scholar]
Ziegler‐Graham 2008
- Ziegler‐Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Archives of Physical Medicine and Rehabilitation 2008;89(3):422‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kwah 2016
- Kwah LK, Goh L, Harvey LA. Rigid dressings versus soft dressings for transtibial amputations. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012427] [DOI] [PMC free article] [PubMed] [Google Scholar]


