Abstract
The influence of 5-HT receptor agonists on the expression of BDNF in brain was determined. Administration of a hallucinogenic 5-HT2A /2C receptor agonist, but not a 5-HT1A receptor agonist, resulted in a significant but differential regulation of BDNF mRNA levels in hippocampus and neocortex. In the hippocampus, the 5-HT2A /2C receptor agonist significantly decreased BDNF mRNA expression in the dentate gyrus granule cell layer but did not influence expression of the neurotrophin in the CA subfields. In parietal cortex and other neocortical areas, but not piriform cortex, the 5-HT2A /2C receptor agonist dramatically increased the expression of BDNF mRNA. The effect of the 5-HT2A /2Creceptor agonist on BDNF mRNA in both the hippocampus and the neocortex was blocked by pretreatment with a selective 5-HT2A, but not 5-HT2C, receptor antagonist. The expression of BDNF mRNA in the hippocampus is reported to be decreased by stress, raising the possibility that the 5-HT2A receptor mediates this effect. Pretreatment with ketanserin, a 5-HT2A /2Creceptor antagonist, significantly blocked the stress-induced downregulation of BDNF mRNA in hippocampus, in support of this hypothesis. The results of this study raise the possibility that regulation of BDNF expression by hallucinogenic 5-HT2Areceptor agonists leads to adaptations of synaptic strength in the hippocampus and the neocortex that may mediate some of the acute and long-term behavioral effects of these agents.
Keywords: BDNF, 5-HT2A, 5-HT2C, hippocampus, cortex, DOI, stress
The neurotrophin family, which consists of NGF (Levi-Montalcini and Angeletti, 1968), BDNF (Leibrock et al., 1989), neurotrophin-3 (Ernfors et al., 1990; Hohn et al., 1990; Maisonpierre et al., 1990; Rosenthal et al., 1990), and neurotrophin-4/5 (Hallbook et al., 1991; Ip et al., 1992), plays an important role in the development, differentiation, maintenance, and survival of distinct and overlapping neuronal populations within the central and peripheral nervous system (Levi-Montalcini, 1987; Barde, 1989; Barde, 1994;Davies, 1994). BDNF has the widest distribution of the neurotrophins in the CNS, and is expressed at highest levels in the hippocampus and the cerebral cortex (Ernfors et al., 1990; Hofer et al., 1990; Wetmore et al., 1990, Phillips et al., 1990). In the hippocampus and/or cerebral cortex, BDNF regulates survival, differentiation, synaptic strength, and neuronal morphology (Ghosh et al., 1994; Korte et al., 1995;McCallister et al., 1995; Thoenen, 1995). BDNF also influences the expression of phenotypic markers such as neurotransmitter synthesizing enzymes, neuropeptides, and calcium-binding proteins (Alderson et al., 1990; Ip et al., 1993; Croll et al., 1994; Jones et al., 1994; Nawa et al., 1994).
Expression of BDNF mRNA is activity dependent, undergoes regulation during development, and also shows marked and transient changes in response to a number of neuronal insults, including ischemia, hypoglycemia, epileptic activity, immobilization stress, and trauma (Maisonpierre et al., 1990; Ernfors et al., 1991; Lindvall et al., 1994; Smith et al., 1995). Regulation of BDNF mRNA levels under basal conditions, as well as in response to insult, involves a complex interplay between different neurotransmitter systems (Thoenen et al., 1991). For example, glutamate plays a role in upregulation of BDNF mRNA, whereas GABAergic pathways are involved in downregulation of BDNF expression in the hippocampus (Zafra et al., 1991; Berninger et al., 1995). Both NMDA and non-NMDA glutamate receptors mediate the effects of glutamate on BDNF mRNA expression (Zafra et al., 1990; Gwag et al., 1993; Wetmore et al., 1994). In addition, cholinergic and noradrenergic pathways also modulate levels of BDNF mRNA in vivo (Zafra et al., 1992; da Penha Berzaghi et al., 1993; Lapchak et al., 1993;Knipper et al., 1994; Hutter et al., 1996). Basal expression of BDNF mRNA in the hippocampus and cerebral cortex is stimulated by glucocorticoids, which also influence the regulation of BDNF in response to a neuronal insult (Barbany and Persson, 1993).
The role of 5-HT in the in vivo regulation of BDNF mRNA has not been examined in detail. Recent work has shown that chronic administration of 5-HT-selective reuptake inhibitors, used clinically as antidepressants, leads to an upregulation of BDNF mRNA in the hippocampus (Nibuya et al., 1995, Nibuya et al., 1996). 5-HT exerts its actions through a large family of receptors expressed in the periphery and throughout the CNS (Martin and Humphrey, 1994; Boess and Martin, 1994). This family of receptors serves as targets for the treatment of a number of disorders, including anxiety, depression, schizophrenia, obsessive–compulsive disorder, migraine, eating disorders, and panic disorders (Roth, 1994). Two of the best characterized 5-HT receptor subtypes are the 5-HT1A and 5-HT2A receptors. Both the 5-HT1A and 5-HT2A receptors have been of interest in the etiology and treatment of depression and other affective disorders (Schreiber and De Vry, 1993; Berendsen, 1995). In addition, 5-HT2A receptors are also involved in the actions of hallucinogens and atypical antipsychotics and have been implicated in the pathogenesis of schizophrenia (Titeler et al., 1988; Levy and Van de Kar, 1992; Sorensen et al., 1993; Gellman and Aghajanian, 1994;Marek and Aghajanian, 1994; Fiorella et al., 1995). The closely related 5-HT2C receptor may also play a role in some of these actions (Sanders-Bush and Breeding, 1991; Baxter et al., 1995).
The present study examines the influence of 5-HT1A and 5-HT2A/2C receptor stimulation on the expression of BDNF mRNA in the brain. The results demonstrate that 5-HT2A, but not 5-HT1A or 5-HT2C, receptors mediate a dramatic, differential regulation of BDNF mRNA expression in neocortex and hippocampus. The results also demonstrate that 5-HT2A/2C receptors mediate, at least in part, the stress-induced downregulation of BDNF mRNA expression in hippocampus. The potential cellular mechanisms mediating the differential regulation of BDNF expression in these brain regions are discussed.
MATERIALS AND METHODS
Animal treatment paradigms. Male Sprague Dawley rats (170–210 gm; CAMM, Wayne, NJ) were group housed and maintained on a 12 hr light/dark cycle with access to food and water ad libitum. For treatment with 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), animals were administered 1 mg/kg of 8-OH-DPAT or vehicle (0.9% saline) via an i.p. injection and sacrificed 2 hr after treatment (n = 4). This dose of 8-OH-DPAT was selected so as to activate postsynaptic 5-HT1A receptors (Tricklebank et al., 1984). For treatment with 4-iodo-2,5-dimethoxyphenylisopropylamine (DOI), animals were administered 8 mg/kg of DOI or vehicle (0.9% saline) via an i.p. injection and sacrificed 3 hr after treatment (n = 6). The dose–response experiment had the following treatment groups: vehicle (n = 4), 0.1 mg/kg DOI (n = 3), 0.5 mg/kg DOI (n = 3), and 2 mg/kg of DOI (n = 3). All groups were administered drug or vehicle via an i.p. injection, and animals were sacrificed 2 hr later. For the time course experiment, each time point had a separate vehicle group (n = 3–4) that was sacrificed along with the DOI (2 mg/kg) group. The groups were as follows: 30 min DOI (n= 4), 60 min DOI (n = 4), 120 min DOI (n = 3), and 180 min DOI (n = 4). To examine the effects of chronic treatment with DOI, the vehicle group (n = 6) received 0.9% saline, and the DOI group (n = 6) was administered 2 mg/kg DOI for 7 d and sacrificed 2 hr after the last treatment.
For the receptor antagonists experiments, the drugs were administered (i.p.) 30 min before a 2 mg/kg injection of DOI, and the vehicle group received injections of saline at both time points. The 5-HT2C receptor antagonist 5-methyl-1-(3-pyridylcarbamoyl)−1,2,3,5-tetrahydropyrrolo[2,3-f]indole (SB 206553) (Kennett et al., 1996) was administered (15 mg/kg, s.c.) as a suspension in 0.1% methyl cellulose; the 5-HT2A receptor antagonist R-(+)-α-(2,3-dimethoxyphenyl)−1-[2(4-flurophenylethyl)]−4-piperidine-methanol (MDL 100,907) (Schreiber et al., 1994) was administered (1 mg/kg, i.p.) in saline that was titrated into solution with citric acid. The treatment groups were as follows: experiment 1: vehicle/vehicle (n = 4), vehicle/DOI (n= 4), 5 mg/kg ketanserin/DOI (n = 4); experiment 2: vehicle/vehicle (n = 4), vehicle/DOI (n= 4), 1 mg/kg MDL 100,907/DOI (n = 4), 15 mg/kg SB 206553/DOI (n = 4). All groups were decapitated 2 hr after the last injection. The effects of the antagonists alone were examined by treating animals with either drug or vehicle and decapitation 2 hr later. The treatment groups were as follows: vehicle (0.9% saline, n = 4); 5 mg/kg of ketanserin (n = 4), 1 mg/kg of MDL 100,907 (n = 4), and 15 mg/kg of SB 206553 (n = 4). To study the effect of DOI in the absence of circulating glucocorticoids, animals were subjected to bilateral adrenalectomy (ADX) under sodium pentobarbital anesthesia (50 mg/kg). Sham animals were exposed to the same anesthesia and surgical procedure but without removal of adrenal glands. All animals (sham = 5, ADX = 5, ADX + DOI = 5) were returned to home cages for 1 week. They were then administered vehicle or DOI (8 mg/kg) and decapitated 2 hr later. For the stress experiments, the treatment groups were as follows: vehicle/sham (n = 12), vehicle/stress (n = 12), ketanserin/stress (n = 12). Sprague Dawley rats (250–280 gm) were administered vehicle or ketanserin 30 min before administration of stress; rats were then subjected to immobilization stress in plastic restraint cone bags (Harvard Apparatus) for 2 hr.
After decapitation, the brains were removed and frozen on dry ice and stored at −80° C for in situ hybridization analysis. All animal use procedures were in strict accordance with the guidelines of the National Institutes for the Care and Use of Laboratory Animals and were approved by the Yale Animal Care and Use Committee. The drugs utilized in this study were obtained as follows: DOI and ketanserin tartarate were purchased from Research Biochemicals, Inc. (Natick, MA); MDL 100,907 was a gift from Hoechst Marion Roussel, Inc. (Cincinnati, OH); and SB 206553 was a gift from SmithKline Beecham (Essex, England).
In situ hybridization. In situ hybridization for BDNF and trkB mRNA was carried out as described previously (see Nibuya et al., 1995). In brief, coronal sections of 14 μm thickness were cut on the cryostat and thaw mounted onto RNase free Probe-on (+) slides (Fisher). Tissue sections were fixed in 4% formaldehyde, acetylated, and dried. Levels of BDNF and trkB mRNA were examined by probing with35S-labeled riboprobes (see Nibuya et al., 1995). Rat BDNF and mouse trkB cDNA clones were obtained from Regeneron (Tarrytown, NY) and the National Cancer Institute (Frederick, MD). The sections were hybridized with 2 × 106 cpm/section for 18 hr at 55°C in hybridization buffer (50% formamide, 0.6 m NaCl, 10 mm Tris, 1× Denhardt’s solution, 2 mmEDTA, 10 mm DTT, 10% dextran sulfate, 50 μg/ml salmon sperm DNA, 250 mg/ml tRNA). After hybridization, sections were washed in 2× SSC (0.15 m NaCl, 0.015 m sodium citrate, pH 7.0) at 25°C and then treated with 20 μg/ml RNase A for 30 min in RNase buffer (0.5 m NaCl, 10 mm Tris, 1 mm EDTA). The sections were then washed for 10 min in 2× SSC at room temperature and twice for 20 min in 0.2× SSC at 55°C. The sections were then rinsed in 0.2× SSC, dried, and exposed to Hyperfilm (Amersham) for 7–14 d. 35S-labeled sense riboprobes for BDNF and trkB did not yield any significant hybridization (not shown), indicating that the signal observed with BDNF and trkB antisense riboprobes are specific.
Quantitation and data analysis. Levels of BDNF and trkB mRNA were analyzed using the Macintosh-based National Institutes of Health Image program, version 1.57. The regions that were analyzed forin situ hybridization were parietal cortex, piriform cortex, dentate gyrus granule cell layer, and CA1 and CA3 pyramidal cell layers. These regions were analyzed by outlining the area of interest; an equivalent area was outlined for each sample. For each animal, the optical density measurements from both sides of 3–4 individual sections were analyzed, yielding 6–8 determinations, from which the mean was calculated. To correct for nonlinearity, 14C step standards were used for calibration. To determine cellular localization, some sections were dipped in emulsion (Kodak, NTB-2) and developed with D-19 developer after 3–4 weeks. These sections were stained with cresyl violet to visualize location of the silver grains.
Results were then subjected to statistical analysis. Experiments with two groups were analyzed for differences using the unpaired Student’st test, with significance determined at p < 0.05. Experiments with three or more groups were subjected to ANOVA, followed by the post hoc Newmann–Keuls test with a significance level of p < 0.05.
RESULTS
Influence of 5-HT1A and 5-HT2A/2C receptor agonists on BDNF mRNA expression in the hippocampus and neocortex
The effect of the 5-HT1A receptor agonist 8-OH-DPAT on the expression of BDNF mRNA was examined by in situ hybridization analysis. Treatment with 8-OH-DPAT did not significantly regulate BDNF mRNA levels in the brain regions examined, including the hippocampus and the neocortex (Table 1). The hallucinogenic phenylalkylamine DOI, a 5-HT2A/2Creceptor agonist (Shannon et al., 1984), was utilized to characterize the role of 5-HT2 receptors in the regulation of BDNF expression. Administration of DOI led to a dramatic, differential regulation of BDNF mRNA in hippocampus and neocortex. DOI significantly decreased BDNF mRNA levels within the dentate gyrus region of the hippocampus (Fig. 1). Levels of BDNF mRNA expression within the CA1 and CA3 regions were not influenced by DOI. In contrast, in parietal cortex DOI significantly increased levels of BDNF mRNA (Fig. 1). The upregulation in BDNF mRNA was also seen within other regions of cerebral cortex, including frontal and temporal cortex, and in a subcortical area, the claustrum (Fig. 2). The induction of BDNF mRNA by DOI in parietal cortex was observed in layers II/III and layers V/VI (Fig. 3). Levels of BDNF mRNA in piriform cortex were not influenced significantly. The effect of DOI on trkB, the receptor for BDNF, was also examined. There was no regulation of trkB mRNA in either neocortex or hippocampus after acute administration of DOI (data not shown).
Table 1.
Influence of the 5-HT1A agonist 8-OH-DPAT on BDNF mRNA levels in hippocampus and neocortex
| Region | BDNF mRNA (% of vehicle ± SEM) | |
|---|---|---|
| Sham | 8-OH-DPAT | |
| Parietal cortex | 100 ± 7 | 101 ± 4 |
| CA1 | 100 ± 2 | 97 ± 5 |
| CA3 | 100 ± 3 | 98 ± 4 |
| Dentate gyrus | 100 ± 3 | 97 ± 3 |
Rats were administered vehicle or 8-OH-DPAT (1 mg/kg), and levels of BDNF mRNA were determined 2 hr later by in situhybridization analysis. Levels of BDNF mRNA were quantified by densitometry. The results are expressed as percent of vehicle and are the mean ± SEM (n = 4).
Fig. 1.
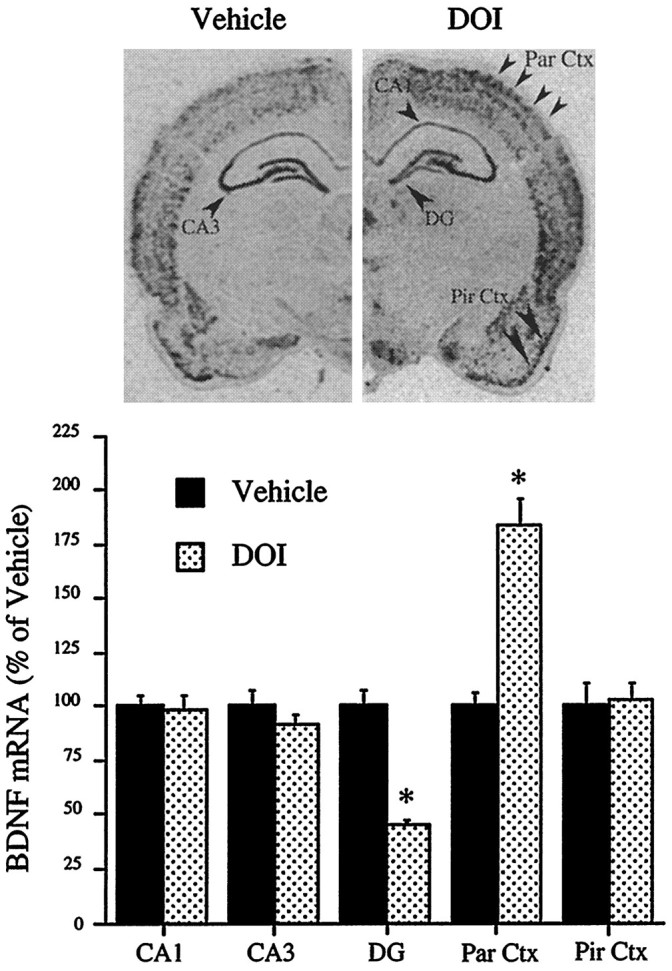
Regulation of BDNF mRNA in hippocampus and neocortex by DOI. The influence of an acute injection of vehicle or DOI (8 mg/kg) on levels of BDNF mRNA was determined by in situ hybridization as described in Materials and Methods. Representative autoradiographs from the vehicle- and DOI-treated groups are shown. Levels of BDNF mRNA in the CA1, CA3, dentate gyrus (DG), parietal cortex (Par Ctx) and piriform cortex (Pir Ctx) were determined by quantitative densitometry. Results are expressed as percent of vehicle and are the mean ± SEM (n = 6). *p < 0.05 compared with vehicle (Student’st test).
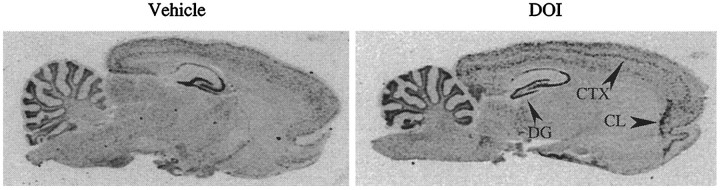
Fig. 2.
Regulation of BDNF mRNA by DOI. Rats were administered vehicle or DOI (8 mg/kg), and levels of BDNF mRNA were determined by in situ hybridization. Representative autoradiographs of sagittal brain sections from vehicle- and DOI-treated groups are shown. Arrowheads indicate the decrease in BDNF expression in the dentate gyrus (DG) and the induction in neocortex (CTX) and claustrum (CL). The induction in BDNF mRNA levels in cortex is widespread and seems to be in specific layers.
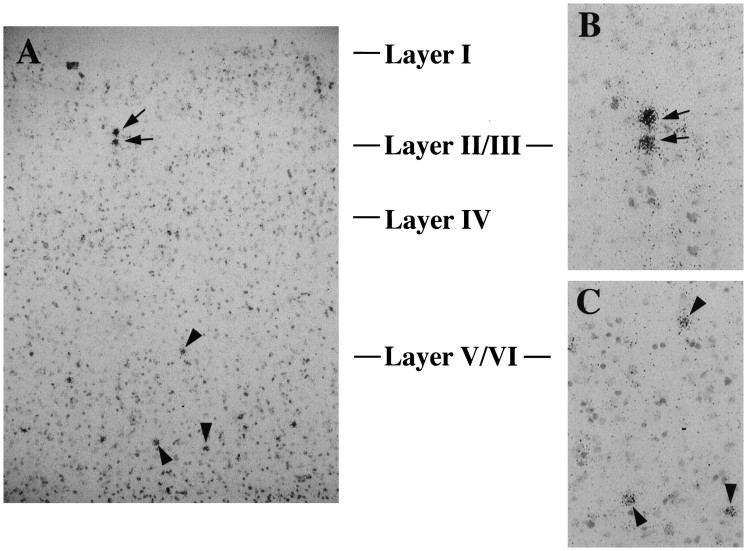
Fig. 3.
Cellular localization of DOI induction of BDNF mRNA in neocortex. Brain sections from DOI-treated (8 mg/kg) rats were subjected to emulsion autoradiography and then counterstained with cresyl violet. A, Note cells within layers II/III and layers V/VI with black silver grains indicating hybridization to BDNF mRNA. Cells specified by arrows in layers II/III (B) and arrowheads in layers V/VI (C) are shown at higher magnification.
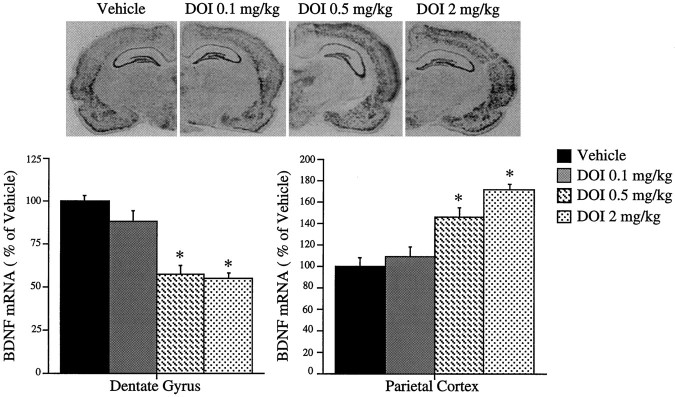
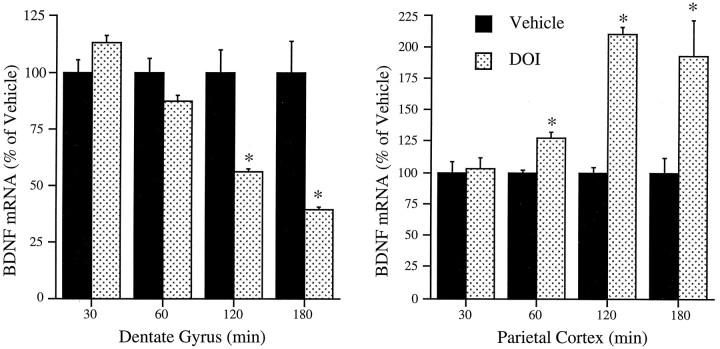
The dose- and time-dependent effects of DOI on the expression of BDNF were examined next. The downregulation of BDNF mRNA within the dentate gyrus of the hippocampus reached a near maximal response with a DOI dose of 0.5 mg/kg, whereas the same dose resulted in a half maximal induction of BDNF mRNA in neocortex (Fig. 4). The regulation of BDNF mRNA by DOI was time dependent and reached a maximal or near maximal response at 2 hr in both the parietal cortex and dentate gyrus granule cell layer (Fig. 5). The influence of repeated DOI (seven daily treatments) on the expression of BDNF mRNA was also examined. Although there was a tendency for levels of BDNF mRNA to be decreased in hippocampus and increased in parietal cortex, these effects were not significant after repeated treatment [86 ± 4, 112 ± 4% of control hippocampus and neocortex, respectively (mean ± SEM); n = 6]. This could result from the downregulation of 5-HT2 receptors in response to chronic agonist treatment.
Fig. 4.
Regulation of BDNF mRNA by different doses of DOI. Rats were administered vehicle or different doses of DOI from 0.1 to 2.0 mg/kg, and levels of BDNF mRNA were determined by in situ hybridization. Representative autoradiographs from each treatment group are shown. The results are expressed as percent of vehicle and are the mean ± SEM (n = 3–4). *p < 0.05 compared with vehicle (ANOVA; Newmann–Keuls post hoc test).
Fig. 5.
Time-dependent regulation of BDNF mRNA by DOI. Rats were administered vehicle or DOI (2 mg/kg), and levels of BDNF mRNA were determined at different times as indicated by in situ hybridization. Each time point had a separate vehicle treatment group. The results are expressed as percent of vehicle and are the mean ± SEM (n = 4). *p < 0.05 compared with vehicle (Student’st test).
Regulation of BDNF mRNA by DOI is mediated via the 5-HT2A receptor subtype
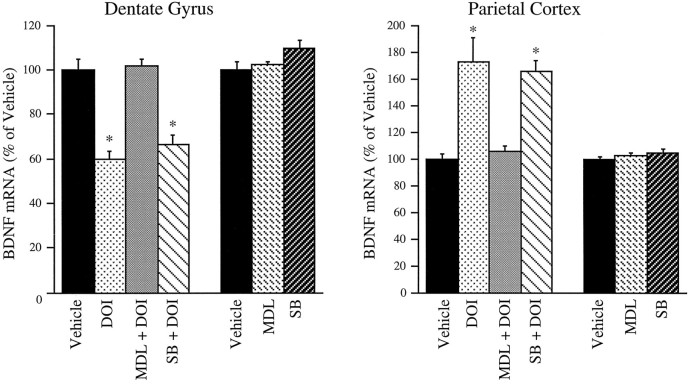
To study the pharmacological specificity of DOI regulation of BDNF mRNA in hippocampus and neocortex, selective 5-HT receptor antagonists were examined. Pretreatment with the 5-HT2A/2C antagonist ketanserin, which has a 30-fold selectivity for the 5-HT2Areceptor over the 5-HT2C receptor (Sanders-Bush and Breeding, 1991), before administration of DOI completely blocked DOI regulation of BDNF mRNA in both the hippocampus and parietal cortex (Fig. 6). Administration of ketanserin alone did not influence basal expression of BDNF mRNA in either region (Fig. 6). Selective 5-HT2A and 5-HT2C receptor antagonists were also examined. MDL 100,907 has a 100-fold greater affinity for the 5-HT2A than the 5-HT2Creceptor subtype (Kehne et al., 1996). Pretreatment with MDL 100,907 completely blocked DOI regulation of BDNF mRNA in the dentate gyrus and the parietal cortex (Fig. 7). SB 206553 has a >100-fold selectivity for the 5-HT2C than the 5-HT2Areceptor (Forbes et al., 1995, Kennett et al., 1996). Pretreatment with SB 206553 did not block DOI regulation of BDNF mRNA expression in either brain region (Fig. 7). BDNF mRNA levels were not regulated by administration of MDL 100,907 or SB 206553 alone (Fig. 7). These findings indicate that DOI regulation of BDNF mRNA in the hippocampus and the neocortex is mediated via activation of the 5-HT2Areceptor subtype.
Fig. 6.
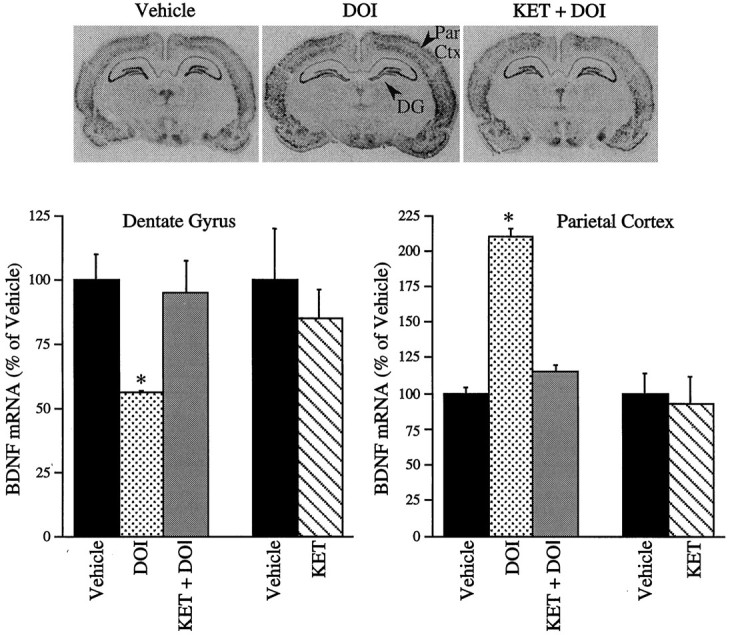
Influence of ketanserin (KET) pretreatment on the DOI regulation of BDNF mRNA. Ketanserin (5 mg/kg) was administered 30 min before DOI (2 mg/kg), and levels of BDNF mRNA were determined 2 hr later by in situ hybridization. In a separate experiment, the influence of ketanserin (5 mg/kg) alone was examined. Representative autoradiographs from the vehicle-, DOI-, and KET + DOI-treated groups are shown. Results are expressed as percent of vehicle and are the mean ± SEM (n = 4). *p < 0.05 compared with vehicle (ANOVA; Newmann–Keuls post hoctest).
Fig. 7.
Influence of MDL 100,907 (MDL) or SB 206553 (SB) pretreatment on DOI regulation of BDNF mRNA. Rats were pretreated (30 min) with vehicle, MDL 100,907 (1 mg/kg), or SB 206553 (15 mg/kg) before administration of DOI (2 mg/kg), and levels of BDNF mRNA were determined 2 hr later by in situ hybridization. In a separate experiment, rats were administered vehicle, MDL 100,907, and SB 206553, and levels of BDNF mRNA were determined 2 hr later. Results are expressed as percent of vehicle and are the mean ± SEM (n = 4). *p < 0.05 compared with vehicle (ANOVA; Newmann–Keuls post hoc test).
Regulation of BDNF mRNA expression by DOI does not involve the hypothalamic-pituitary-adrenocortical (HPA) axis
DOI is reported to cause a dose-dependent activation of the HPA axis, including elevated levels of circulating glucocorticoids (Rittenhouse et al., 1994; Welch and Saphier, 1994). To examine the possibility that DOI regulation of BDNF mRNA is mediated via activation of the HPA axis, the influence of adrenalectomy was examined. Adrenalectomy did not influence DOI regulation of BDNF mRNA in either the dentate gyrus or the parietal cortex (Fig. 8). In this experiment, DOI regulation of BDNF mRNA in the dentate gyrus, and parietal cortex was similar to that observed in intact animals. ADX alone did not significantly regulate the expression of BDNF mRNA in either brain region.
Fig. 8.
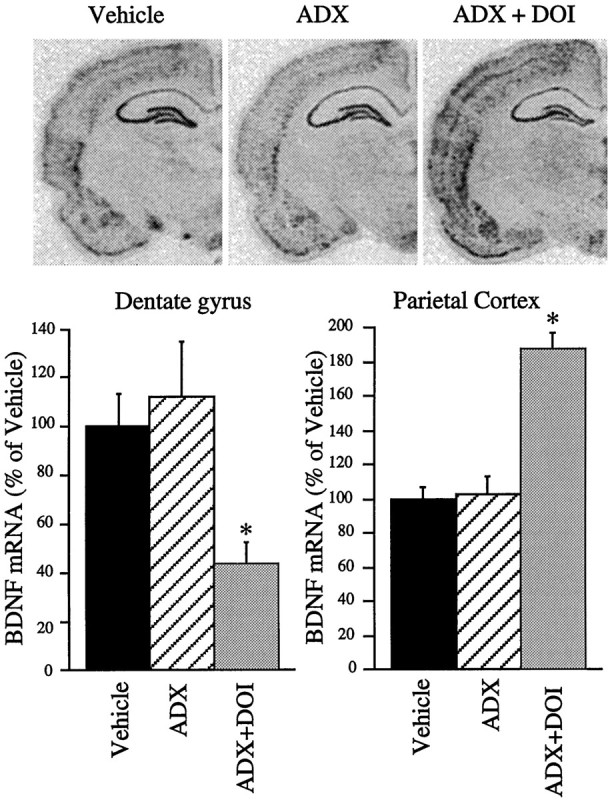
Influence of adrenalectomy on DOI regulation of BDNF mRNA. Rats underwent sham surgery or bilateral adrenalectomy (ADX). Seven days later, animals received vehicle or DOI (8 mg/kg), and levels of BDNF mRNA were determined by in situ hybridization. Representative autoradiographs for the different treatment groups are shown. Results are expressed as percent of vehicle and are the mean ± SEM (n = 5). *p < 0.05 compared with vehicle (ANOVA; Newmann–Keuls post hoc test).
5-HT2A/2C receptors are involved in the downregulation of BDNF mRNA induced by immobilization stress
Immobilization stress has been shown to downregulate BDNF mRNA expression in the hippocampus (Smith et al., 1995), an effect similar to the DOI regulation of BDNF mRNA in hippocampus observed in the present study. In addition, previous studies have demonstrated that immobilization stress causes an increase in 5-HT release and turnover in the hippocampus (Joseph and Kennett, 1983; Tanaka et al., 1983;Richardson, 1984; Chauloff, 1993; Vahabzadeh and Fillenz, 1994). To examine the possibility that 5-HT2A/2C receptors mediate the stress-induced downregulation of BDNF mRNA levels, the influence of ketanserin on this effect was examined. Pretreatment with ketanserin lead to a partial but highly significant blockade of the stress-induced downregulation of BDNF mRNA in the dentate gyrus granule cell layer (Fig. 9). In the CA3 pyramidal cell layer, stress resulted in a small, nonsignificant downregulation of BDNF mRNA, although the level of BDNF mRNA in the stress group was significantly different from that in the ketanserin plus stress group (Fig. 9). The results indicate that the 5-HT2A/2C receptor mediates, at least in part, the effects of stress on BDNF mRNA levels in the hippocampus. Although the combined results indicate a significant blockade of the stress-induced downregulation of BDNF mRNA, interexperimental variability was observed. Ketanserin was found to completely or partially block the stress-induced decrease in BDNF mRNA levels in the different experiments conducted. The reason for this variability is not known but may be related to the previous unknown exposure of animals to stress, or other factors that influence the stress response. Because of this variability, a further characterization of the 5-HT2 receptor subtypes that mediate the stress effect will require an extended series of experiments.
Fig. 9.
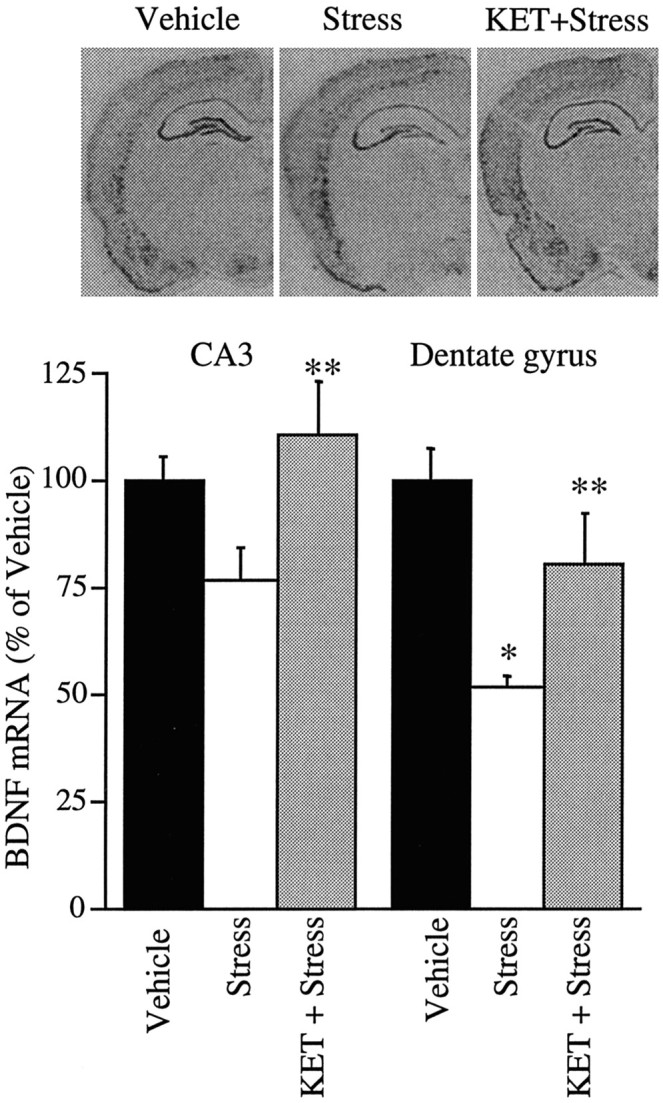
Influence of ketanserin (KET) on the stress-induced downregulation of BDNF mRNA. Rats were pretreated with vehicle or ketanserin (5 mg/kg) 30 min before being subjected to immobilization stress (2 hr). Levels of BDNF mRNA were determined immediately after stress by in situ hybridization. Representative autoradiographs from the different treatment groups are shown. Results are expressed as percent of vehicle and are the mean ± SEM (n = 12). *p < 0.05 compared with vehicle; **p < 0.05 compared with stress (ANOVA; Newmann–Keuls post hoc test).
DISCUSSION
The results of this study demonstrate that administration of 8-OH-DPAT, a 5-HT1A receptor agonist, did not influence the expression of BDNF in any of the brain regions examined. However, 8-OH-DPAT is also an agonist of the 5-HT7 receptor (Lovenberg et al., 1993) and could thereby oppose the actions of the 5-HT1A receptor via its opposing effects on the cAMP system. Additional studies with more selective 5-HT1A and 5-HT7 receptor drugs are needed to further characterize the role of these receptors in regulation of BDNF expression. In contrast, administration of DOI, a 5-HT2A/2C receptor agonist, differentially regulated the expression of BDNF mRNA in cerebral cortex and hippocampus. The induction of BDNF mRNA by DOI was observed in different regions of neocortex, including the frontal, parietal, and temporal cortex, but was absent in the piriform cortex, a part of paleocortex. Within the hippocampus, the DOI-induced downregulation of BDNF mRNA was observed in the granule cell layer of the dentate gyrus, but not in the CA1 or CA3 pyramidal cell layers. The effects of DOI in both regions were dose and time dependent, and repeated administration of the agonist resulted in desensitization of the BDNF mRNA response in both hippocampus and neocortex. This probably results from downregulation of 5-HT2 receptors after chronic agonist treatment (Buckholtz et al., 1988; Eison et al., 1989; Leysen et al., 1989).
The neocortical localization of DOI-induced upregulation of BDNF mRNA, which is observed in layers II/III and layers V/VI, is similar to the distribution of the 5-HT2A receptor subtype (Mengod et al., 1990; Pompeiano et al., 1994; Wright et al., 1995). In addition, the 5-HT2A receptor subtype is the most abundant 5-HT receptor in neocortex, whereas expression of the 5-HT2C receptor subtype is relatively low in neocortical areas (Wright et al., 1995). The possibility that the 5-HT2A receptor subtype mediates the DOI induction of BDNF mRNA is supported by the results of the receptor antagonist studies. Pretreatment with ketanserin, a 5-HT2A/2C receptor antagonist, or MDL 100,907, a selective 5-HT2A receptor antagonist, completely blocked DOI regulation of BDNF mRNA in both the neocortex and hippocampus. In contrast, pretreatment with SB 206553, a selective 5-HT2Creceptor antagonist, did not significantly influence DOI regulation of BDNF mRNA in either brain region.
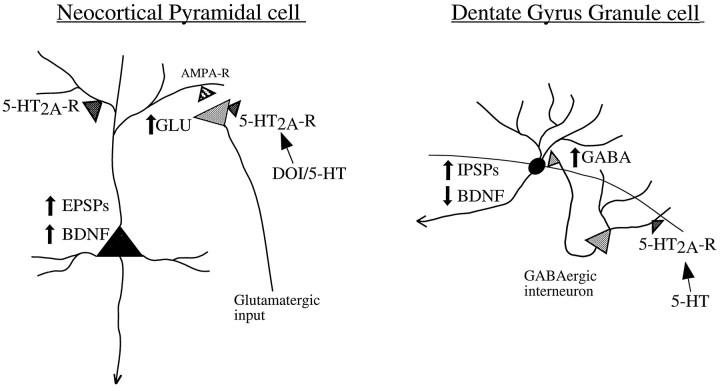
The mechanism by which activation of the 5-HT2A receptors increases the expression of BDNF mRNA in neocortex may be explained by electrophysiological studies. Bath application of 5-HT to cerebral cortical slices has been shown to increase EPSPs in pyramidal neurons of the neocortex (Marek and Aghajanian, 1996), and this effect is mimicked by application of DOI (G. J. Marek and G. K. Aghajanian, personal communication). In addition, the EPSPs induced by 5-HT are blocked by MDL 100,907 and are observed in the same population of pyramidal neurons (layer V) as the elevation of BDNF mRNA in response to DOI. The increase in EPSPs has been shown to be a result of the release of glutamate and can be blocked by an AMPA receptor antagonist. This is consistent with previous reports that the activity-dependent induction of BDNF mRNA in cerebral cortex is mediated by glutamate (Zafra et al., 1990; Ernfors et al., 1991; Lindholm et al., 1994). Taken together, the results suggest that the upregulation of BDNF mRNA by DOI is mediated via the presynaptic release of glutamate and the activation of AMPA receptors (Fig. 10). This hypothesis is currently being tested by examining the influence of an AMPA receptor antagonist on DOI induction of BDNF mRNA.
Fig. 10.
Cellular models for 5-HT2A receptor regulation of BDNF mRNA in neocortex and hippocampus. Within the neocortex, application of 5-HT/DOI has been shown to induce EPSPs in layer V pyramidal neurons via activation of 5-HT2Areceptors (5-HT2A-R). The increase in EPSPs is a result of increased release of glutamate (GLU) and activation of AMPA receptors (AMPA-R) and can be blocked by the selective AMPA receptor antagonist LY 293558. This suggests that the upregulation of BDNF mRNA by DOI may be mediated via a release of glutamate and an increase in neuronal activity. In contrast, DOI treatment leads to a decrease in levels of BDNF mRNA in the dentate gyrus granule cell layer of the hippocampus. This could be mediated by activation of 5-HT2A receptors located on GABAergic interneurons. Activation of 5-HT2A receptors increases the firing rate of GABAergic interneurons and thereby increases IPSPs in the granule cells. The increased inhibitory control of dentate gyrus granule cells could lead to downregulation of BDNF mRNA.
DOI also upregulates BDNF mRNA in other areas of neocortex in addition to parietal cortex, such as frontal and temporal cortex. In contrast, levels of BDNF mRNA in piriform cortex, a part of paleocortex, are not influenced by DOI. In piriform cortex, DOI activation of 5-HT2A receptors has been shown to induce IPSCs in the pyramidal neurons through the excitation of GABAergic interneurons (Sheldon et al., 1990, 1991; Marek et al., 1994). This inhibitory effect on GABAergic interneurons seems to balance any stimulatory effect of DOI on 5-HT2A/2C receptors located postsynaptically on the pyramidal cells and may explain the absence of regulation of BDNF mRNA by DOI in this brain region.
The mechanism responsible for the downregulation of BDNF mRNA in the dentate gyrus granule cell layer by DOI may also be explained by electrophysiological and receptor distribution studies. Serotonergic inputs from the dorsal raphe are thought to exert a global control over the hippocampus via modulation of local inhibitory interneurons (Freund et al., 1990). The 5-HT2A receptors are expressed at relatively high levels on the GABAergic interneurons and hilar cells, but are present at lower levels in the granule cell layer of hippocampus (Pompeiano et al., 1994; Wright et al., 1995). The 5-HT2C receptor is distributed lightly through the principal layers, with dense distribution in the temporal hippocampus, CA1, and subiculum (Pompeiano et al., 1994; Wright et al., 1995). Activation of the 5-HT2A receptors on GABAergic interneurons is thought to increase spontaneous GABA release and lead to an increased inhibitory control of dentate granule cells (Piguet and Galvan, 1994). Because stimulation of the GABAergic system leads to a decrease in levels of BDNF mRNA, the effects of DOI might be mediated via its activation of 5-HT2A receptors on GABAergic interneurons (Fig. 10).
Levels of 5-HT in the hippocampus are increased in response to several different types of stress, including immobilization (Joseph and Kennett, 1983; Richardson, 1984; Chauloff, 1993). Recent studies have demonstrated that stress decreases the expression of BDNF in hippocampus, particularly in the dentate gyrus granule cell layer (Smith et al., 1995). The downregulation of BDNF by stress is not blocked by adrenalectomy, indicating that factors other than adrenal glucocorticoids mediate this effect. Considering our findings with DOI, we hypothesized that the influence of stress on BDNF expression is mediated by 5-HT2A receptor activation of GABAergic interneurons. This possibility is supported by the results of the receptor antagonist studies, which demonstrate that pretreatment with ketanserin significantly blocks the downregulation of BDNF mRNA in response to stress. However, ketanserin did not completely reverse the stress effect, and the response to ketanserin varied between experiments. Therefore, additional studies are required to further characterize the 5-HT2 receptor subtype that mediates this effect, and to examine the potential role of other neurotransmitter receptors in the stress response.
BDNF is known to influence the survival and function of neurons in the brain, including 5-HT neurons (Mamounas et al., 1995; Celada et al., 1996). In addition, BDNF has recently been demonstrated to acutely influence the synaptic efficacy of neurons. Several electrophysiological studies have demonstrated that application of BDNF to hippocampal slices results in increased synaptic strength, and endogenous BDNF has been implicated in formation of long-term potentiation, a cellular model of learning and memory (Kang and Schuman, 1995; Korte et al., 1995, Levine et al., 1995; Figurov et al., 1996; Patterson et al., 1996). Thus, it is conceivable that the effects of BDNF on neuronal survival and function, as well as synaptic efficacy, could contribute to the actions of drugs known to influence 5-HT2A receptors. Upregulation of BDNF in neocortex by certain antidepressant treatments could be mediated, at least in part, by 5-HT2A receptors (Nibuya et al., 1995), although the induction observed in hippocampus seems to involve other 5-HT and monoamine receptor subtype(s) (Nibuya et al., 1995, 1996). Induction of BDNF, through its positive effects on target neurons in neocortex, as well as on serotonergic neurons, could play a role in the therapeutic actions of antidepressants. In cerebral cortex, the enhanced sensory perception that is observed in response to hallucinogenic compounds could also be mediated, in part, by induction of BDNF (Abraham et al., 1996). Further studies will be required to determine the acute and long-term cellular and behavioral effects of antidepressants and hallucinogenic drugs that are related to the regulation of BDNF in cerebral cortex, as well as hippocampus.
Footnotes
This work was supported by U.S. Public Health Service Grants MH45481, MH 51399, and 2 PO1 MH25642, and a Veterans Administration National Center Grant for PTSD (VA hospital in West Haven, CT). We thank Rosemarie Terwilliger for technical assistance.
Correspondence should be addressed to Dr. Ronald S. Duman, Division of Molecular Psychiatry, Yale University School of Medicine, CMHC, 34 Park Street, New Haven, CT 06508.
REFERENCES
- 1.Abraham HD, Aldridge AM, Gogia P. The psychopharmacology of hallucinogens. Neuropsychopharmacology. 1996;14:285–298. doi: 10.1016/0893-133X(95)00136-2. [DOI] [PubMed] [Google Scholar]
- 2.Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- 3.Barbany G, Persson H. Adrenalectomy attenuates kainic acid-elicited increases of messenger RNAs for neurotrophins and their receptors in the rat brain. Neuroscience. 1993;54:909–922. doi: 10.1016/0306-4522(93)90584-3. [DOI] [PubMed] [Google Scholar]
- 4.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 5.Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 6.Baxter G, Kennett G, Blaney F, Blackburn T. 5-HT2 receptor subtypes: a family reunited? Trends Pharmacol Sci. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- 7.Berendsen HH. Interactions between 5-hydroytryptamine receptor subtypes: is a disturbed receptor balance contributing to the symptomatology of depression in humans? Pharmacol Ther. 1995;66:17–37. doi: 10.1016/0163-7258(94)00075-e. [DOI] [PubMed] [Google Scholar]
- 8.Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- 9.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 10.Buckholtz NS, Zhou D, Freedman DX. Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 1988;42:2439–2445. doi: 10.1016/0024-3205(88)90342-6. [DOI] [PubMed] [Google Scholar]
- 11.Celada P, Siuciak JA, Tran TM, Altar CA, Tepper JM. Local infusion of brain-derived neurotrophic factor modifies the firing pattern of dorsal raphe serotonergic neurons. Brain Res. 1996;712:293–298. doi: 10.1016/0006-8993(95)01469-1. [DOI] [PubMed] [Google Scholar]
- 12.Chaouloff F. Physiopharmacolgical interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 13.Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci. 1994;6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 14.da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, Lindholm D. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci. 1993;13:3818–3826. doi: 10.1523/JNEUROSCI.13-09-03818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies AM. Neurotrophic factors. Switching neurotrophin dependence. Curr Biol. 1994;4:273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 16.Eison AS, Eison MS, Yocca FD, Gianutsos G. Effects of imipramine and serotonin-2 agonists and antagonists on serotonin-2 and β-adrenergic receptors following noradrenergic or serotonergic denervation. Life Sci. 1989;44:1419–1427. doi: 10.1016/0024-3205(89)90400-1. [DOI] [PubMed] [Google Scholar]
- 17.Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 18.Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor; developmental and topographical expression in the brain. Proc Natl Acad Sci USA. 1990a;87:5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990b;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 20.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1990;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 21.Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I. Antagonist correlation analysis. Psychopharmacology. 1995;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- 22.Forbes IT, Ham P, Booth DH, Martin RT, Thompson M, Baxter GS, Blackburn TP, Glen A, Kennett GA, Wood MD. 5-Methyl-1(3-pyridylcarbamoyl)-1,2,3,5-tetrahyrdropyrrolo[2,3-f]indole: a novel 5HT2C/5-HT2B receptor antagonist with improved affinity, selectivity, and oral activity. J Med Chem. 1995;38:2524–2530. doi: 10.1021/jm00014a004. [DOI] [PubMed] [Google Scholar]
- 23.Freund TF, Gulyas AI, Acsady L, Gorcs T, Toth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci USA. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gellman RL, Aghajanian GK. Serotonin2 receptor-mediated excitation of interneurons in piriform cortex: antagonism by atypical antipsychotic drugs. Neuroscience. 1994;58:515–525. doi: 10.1016/0306-4522(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 26.Gwag BJ, Springer JE. Activation of NMDA receptors increases brain-derived neurotrophic factor (BDNF) mRNA expression in the hippocampal formation. NeuroReport. 1993;5:125–128. doi: 10.1097/00001756-199311180-00007. [DOI] [PubMed] [Google Scholar]
- 27.Hallbook F, Ibanez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- 28.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 30.Hutter P, Johansson M, Saria A, Humpel C. Acute and chronic noradrenergic regulation of neurotrophin messenger RNA expression in rat hippocampus: evidence from lesions and organotypic cultures. Neuroscience. 1996;70:15–29. doi: 10.1016/0306-4522(95)00346-k. [DOI] [PubMed] [Google Scholar]
- 31.Ip NY, Ibanez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, Le Beau MM, Espinosa R, III, Squinto SP. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ip NY, Li Y, Yancopoulos GD, Lindsay RM. Cultured hippocampal neurons show responses to BDNF, NT-3 and NT-4, but not NGF. J Neurosci. 1993;13:3394–3405. doi: 10.1523/JNEUROSCI.13-08-03394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph MH, Kennett GA. Stress-induced release of 5-HT in the hippocampus and its dependence on increased tryptophan availability: an in vivo electrochemical study. Brain Res. 1983;270:251–257. doi: 10.1016/0006-8993(83)90598-x. [DOI] [PubMed] [Google Scholar]
- 35.Kang HJ, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 36.Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, Van Giersbergen PLM, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- 37.Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, Blackburn TP. In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 39.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8865–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapchak PA, Araujo DM, Hefti F. Cholinergic regulation of hippocampal brain-derived neurotrophic factor mRNA expression: evidence from lesion and chronic cholinergic drug treatment studies. Neuroscience. 1993;52:575–585. doi: 10.1016/0306-4522(93)90407-7. [DOI] [PubMed] [Google Scholar]
- 41.Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y-A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 42.Leysen JE, Janssen PFM, Niemegeers CJE. Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol. 1989;163:145–149. doi: 10.1016/0014-2999(89)90409-3. [DOI] [PubMed] [Google Scholar]
- 43.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 44.Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- 45.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy AD, Van de Kar LD. Endocrine and receptor pharmacology of serotonergic anxiolytics, antipsychotics and antidepressants. Life Sci. 1992;51:83–94. doi: 10.1016/0024-3205(92)90001-6. [DOI] [PubMed] [Google Scholar]
- 47.Lindholm D, Castren E, Berzaghi M, Blochl A, Thoenen H. Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain: implications for neuronal plasticity. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- 48.Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 49.Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 50.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990a;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 51.Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990b;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- 52.Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marek GJ, Aghajanian GK. Excitation of interneurons in piriform cortex by 5-hydroxytryptamine: blockade by MDL 100,907, a highly selective 5-HT2A receptor antagonist. Eur J Pharmacol. 1994;259:137–141. doi: 10.1016/0014-2999(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 54.Marek GJ, Aghajanian GK. Serotonergic amplification of apically-derived EPSPs in neocortical pyramidal cells via a persistent sodium current. Soc Neurosci Abstr. 1996;22:1323. [Google Scholar]
- 55.Martin GR, Humphrey PP. Receptors for 5-hydroxytryptamine: current perspectives on classification and nomenclature. Neuropharmacology. 1994;33:261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 56.McCallister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 57.Mengod G, Pompeiano M, Martinez-Mir MI, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- 58.Nawa H, Pelleymounter MA, Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electoconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 62.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- 63.Piguet P, Galvan M. Transient and long-lasting actions of 5-HT on rat dentate gyrus neurones in vitro. J Physiol. 1994;481:629–639. doi: 10.1113/jphysiol.1994.sp020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparision between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 65.Richardson JS. Brain part monoamines in the neuroendocrine mechanisms activated by immobilization stress in the rat. Int J Neurosci. 1984;23:57–68. doi: 10.3109/00207458408985345. [DOI] [PubMed] [Google Scholar]
- 66.Rittenhouse PA, Bakkum EA, Levy AD, Li Q, Carnes M, Van de Kar LD. Evidence that ACTH secretion is regulated by serotonin2A/2C (5-HT2A/2C) receptors. J Pharmacol Exp Ther. 1994;271:1647–1655. [PubMed] [Google Scholar]
- 67.Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, Nikolics K, Winslow JW. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990;4:767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- 68.Roth BL. Multiple serotonin receptors: clinical and experimental aspects. Ann Clin Psychiatry. 1994;6:67–78. doi: 10.3109/10401239409148985. [DOI] [PubMed] [Google Scholar]
- 69.Sanders-Bush E, Breeding M. Choroid plexus epithelial cells in primary culture: a model of 5HT1C receptor activation by hallucinogenic drugs. Psychopharmacol. 1991;105:340–346. doi: 10.1007/BF02244428. [DOI] [PubMed] [Google Scholar]
- 70.Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effects of DOI by MDL 100,907 and the “atypical” antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- 71.Schreiber R, De Vry J. 5-HT1A receptor ligands in animal models of anxiety, impulsivity and depression: multiple mechanisms of action? Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:87–104. doi: 10.1016/0278-5846(93)90034-p. [DOI] [PubMed] [Google Scholar]
- 72.Shannon M, Battaglia G, Glennon RA, Titeler M. 5HT1 and 5HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA). Eur J Pharmacol. 1984;102:23–29. doi: 10.1016/0014-2999(84)90333-9. [DOI] [PubMed] [Google Scholar]
- 73.Sheldon PW, Aghajanian GK. Serotonin (5-HT) induces IPSPs in pyramidal layer cells of rat piriform cortex: evidence for the involvement of a 5-HT2-activated interneuron. Brain Res. 1990;506:62–69. doi: 10.1016/0006-8993(90)91199-q. [DOI] [PubMed] [Google Scholar]
- 74.Sheldon PW, Aghajanian GK. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse. 1991;9:208–218. doi: 10.1002/syn.890090307. [DOI] [PubMed] [Google Scholar]
- 75.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- 77.Tanaka M, Kohno Y, Nakagawa R, Nishikawa T, Tsuda A, Nagasaki N. Immobilization stress increases serotonin turnover in the extended brain regions in the rat. Kurume Med J. 1983;30:35–43. doi: 10.2739/kurumemedj.30.35. [DOI] [PubMed] [Google Scholar]
- 78.Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- 79.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 80.Thoenen H, Zafra F, Hengerer B, Lindholm D. The synthesis of nerve growth factor and brain-derived neurotrophic factor in hippocampal and cortical neurons is regulated by specific neurotransmitter systems. Ann NY Acad Sci. 1991;640:86–90. doi: 10.1111/j.1749-6632.1991.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 81.Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioral response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- 82.Vahabzadeh A, Fillenz M. Comparision of stress-induced changes in noradrenergic and serotonergic neurons in the rat hippocampus using microdialysis. Eur J Neurosci. 1994;6:1205–1212. doi: 10.1111/j.1460-9568.1994.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 83.Welch JE, Saphier D. Central and peripheral mechanisms in the stimulation of adrenocortical secretion by the 5-hydroxytryptamine2 agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. J Pharmacol Exp Ther. 1994;270:918–928. [PubMed] [Google Scholar]
- 84.Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 85.Wetmore C, Olson L, Bean AJ. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J Neurosci. 1994;14:1688–1700. doi: 10.1523/JNEUROSCI.14-03-01688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin 1A, 1C and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 87.Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and γ-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]