Abstract
To determine the subunit composition of high-affinity kainate receptors in native neurons is a challenging problem because of the expression of more than one GluR subunit. In the present study the question of whether GluR5 and/or GluR6 subunits combine with KA-1 or KA-2 subunits in vivo is addressed by performing detailed physiological, pharmacological, and molecular characterization of functional kainate receptor channels in acutely dissociated trigeminal ganglion (TG) neurons. The results show that (1) smaller diameter TG neurons (<30 μm) respond to l-glutamate and kainate, and the currents gated by kainate desensitize with prolonged agonist exposure; (2) all kainate receptor subunits are detected to some extent by reverse transcriptase-PCR, whereas glutamate receptor subunits GluR5 and KA-2 are expressed at high levels in the TG; (3) there is an obvious similarity between the features of native kainate receptor channels in TG neurons and of heteromeric recombinant GluR5(R)/KA-2 channels in pharmacological properties, desensitization, rectification, ion permeability, and mean channel conductance; and (4) the age-dependent increase in GluR5 and KA-2 RNA levels in the TG is correlated well with an increased number of kainate-sensitive cells during postnatal development. Our data suggest that the heteromeric GluR5/KA2 combination actually occurs in TG neurons and give a clue as to the subunit composition of native kainate receptor channels.
Keywords: kainate receptor channel, GluR5, KA-2, trigeminal ganglion neurons, Concanavalin A, RT-PCR, Q/R editing
The non-NMDA ionotropic glutamate receptors have been classified into two different groups according to their binding affinities for the agonists AMPA and kainate. AMPA receptors are formed by four subunits, GluR1 through GluR4 (GluR-A through GluR-D) (Hollmann et al., 1989; Keinänen et al., 1990), and are involved in fast glutamatergic transmission in the mammalian CNS. Five subunits, GluR5 through GluR7 and KA-1 and KA-2, have been demonstrated to generate high-affinity kainate receptors in expression systems (Bettler et al., 1990, 1992; Egebjerg et al., 1991; Werner et al., 1991; Herb et al., 1992), but much less is known about the function of the kainate receptors. Kainate receptor channels are characterized by rapid desensitization of responses on application of kainate, which is greatly reduced by Concanavalin A (Egebjerg et al., 1991; Herb et al., 1992; Sommer et al., 1992). Recombinant experiments have demonstrated that (1) GluR5 and GluR6 subunits form functional homomeric channels when expressed in vitro (Egebjerg et al., 1991; Herb et al., 1992; Sommer et al., 1992); (2) homomeric KA-2 expression does not generate agonist-sensitive channels, but currents are observed when KA-2 is coexpressed with GluR5 or GluR6 subunits (Herb et al., 1992; Howe, 1996; Swanson et al., 1996); and (3) RNA editing of the Q/R site, located in the second transmembrane domain (TMII) of GluR5 and GluR6 subunits, and the I/V and Y/C sites, located in the TMI of the GluR6 subunit, is a critical determinant of the Ca2+ permeability and rectification properties of kainate receptor channels (Sommer et al., 1991; Egebjerg and Heinemann, 1993; Köhler et al., 1993).
Although native kainate receptor channels have been characterized in the DRG neurons (Huettner, 1990), in cultured hippocampal neurons (Paternain et al., 1995; Wilding and Huettner, 1997), and in glia cells (Patneau et al., 1994), the subunit composition of native kainate receptor channels remains unknown. To address the question of the molecular composition of native glutamate receptors, researchers recently have used a combination of whole-cell patch-clamp recording and PCR in single identified neurons and have shown that responses of kainate receptors in cultured hippocampal neurons match GluR6(Q) homomeric responses (Ruano et al., 1995). On the other hand, in situ hybridization studies have shown that the mRNAs encoding the five kainate receptor subtypes have distinct but overlapping patterns of expression in the brain (Wisden and Seeburg, 1993; Bahn et al., 1994), and immunoprecipitation studies of native brain membranes suggest that some kainate receptors probably exist in vivoas heteromeric assemblies of different types of subunit (Puchalski et al., 1994; Wenthold et al., 1994).
In the present study the question of whether GluR5 and/or GluR6 subunits combine with KA-1 or KA-2 subunits in vivo is addressed by performing a detailed characterization of kainate receptor channels in trigeminal ganglion (TG) neurons with the whole-cell patch-clamp and reverse transcriptase-PCR (RT-PCR) methods. Our results show that the functional properties of kainate receptor channels in TG neurons match those reported for recombinant heteromeric GluR5(R)/KA2 receptor channels and provide evidence that the heteromeric GluR5/KA2 combination actually occurs in native neurons.
MATERIALS AND METHODS
Cell isolation and culture. Trigeminal ganglia were isolated from neonatal (2–14 d) or adult (2 month) Wistar rats. Dissected ganglia were incubated in Hank’s solution (Life Technologies, Gaithersburg, MD) with papain (20 U/ml) (Worthington Biochemical, Freehold, NJ) and/or collagenase (590 U/ml) (Sigma, St. Louis, MO) dispase (2.4 U/ml) (Boehringer Mannheim, Mannheim, Germany) at 37°C for 20–30 min. Cells were dissociated by trituration with a sterile Pasteur pipette and subsequently were plated onto poly-l-lysine pretreated 35 mm culture dishes at a density of 2 × 103 cells/plate. The plating medium contained Leibovitz’s L-15 solution (Life Technologies), 10% fetal calf serum, penicillin–streptomycin (20 U/ml), 26 mmNaHCO3, and 30 mm glucose. Cells were maintained in a humidified atmosphere of 95% air/5% CO2at 37°C. The cells were used for recording between 6 and 8 hr after plating.
Electrophysiological recordings. Whole-cell recording was performed with an Axopatch 1-D amplifier (Axon Instruments, Foster City, CA) at room temperature. Cells were visualized under phase contrast on an inverted microscope (Olympus IX-70, Tokyo, Japan). Pipettes for whole-cell recording contained (in mm) CsCl 150, CaCl2 1, MgCl2 2, EDTA 11, and HEPES 10, pH 7.3; osmolarity was adjusted to 320 mOsm. Spermine (100 μm) was added to prevent washout of intracellular polyamines (Bowie and Mayer, 1995; Kamboj et al., 1995). The extracellular solution contained (in mm) NaCl 160, KCl 2.5, CaCl2 2, MgCl2 1, HEPES 10, glucose 10, and tetraethylammonium (TEA) chloride 5, with 1 μmtetrodotoxin (TTX), pH 7.2 (osmolarity, 325 mOsm). An array of six quartz-glass tubes (200 μm in diameter, Polymicro Tech, Phoenix, AZ) was positioned within 200 μm of neuronal cell bodies by using a mechanical manipulator (Narishige, Tokyo, Japan). Each flow tube was connected to a gravity-fed reservoir and controlled by a three-way solenoid valve. Neurons were always bathed in a flowing stream of control solution, except during the application of drugs. Rapid solution exchange was attained by switching the flow between adjacent flow tubes by simultaneously closing one valve and opening another. The solution exchange time constant was ∼20 msec, estimated by using the 10–90% rise time of responses to a saturating concentration of kainate (200 μm) or l-glutamate (1 mm). In some experiments the array of flow tubes was moved with a piezoelectric bimorph (Vernitron, Bedford, OH), and the solution exchange time constant was <20 msec, as estimated, using a method described in Vyklicky et al. (1990). For noise analysis, solution flow was controlled manually, and the onset responses of kainate (200 μm) varied from 200 msec to 2–3 sec. Agonist applications were made at 70–120 sec intervals. Kainate,l-glutamate, quisqualate, NMDA, GABA, glycine, Concanavalin A (Con A), succinyl-Con A, and wheat germ agglutinin (WGA) were purchased from Sigma. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), cyclothiazide (CTZ), and AMPA were purchased from Tocris Cookson (Bristol, UK). TTX was from Sankyo (Tokyo, Japan). The junction potential between internal and external solutions and the series resistance was compensated.
Ramp current–voltage (I–V) relationships from −100 to +100 mV (0.2 V/sec) were generated with pClamp (Axon Instruments). The I–V relationships for kainate responses were leak-subtracted from a control response. Agonist-evoked currents were recorded on a PCM data recorder (TEAC RD-180T, band width DC to 20 kHz, −3 dB). For the analysis of kainate-induced current noise and spectral densities, records were replayed from tapes, high-pass-filtered at 0.2 Hz and low-pass-filtered at 2 kHz (Bessel eight-pole, 48 dB/oct), and digitized with pClamp (sampling rate 5 kHz) on a computer (Compaq Prolinear 4/33i) with a TL-1 DMA digital interface. Digitized records were edited to remove artifacts and analyzed with an Axograph (Axon Instruments). Spectral densities were measured by a custom program (Robinson et al., 1991). Data are presented as mean ± SEM. When statistical analysis was performed, ANOVA was used.
Reverse transcriptase-PCR. Total RNA was isolated from the TG and brains (cerebellum and hippocampus) of rats, aged P1, P3, P7, P14, and P56, using the guanidine thiocyanate method, followed by centrifugation in cesium chloride solution. First-strand cDNA was synthesized from 5 μg of rat RNA with reverse transcriptase in the presence of random hexamer (First-strand cDNA synthesis kit, Pharmacia, Piscataway, NJ). The GluR1–4, GluR5, GluR6, GluR7, KA-1, and KA-2 subunits were amplified by PCR with the following set primers (from 5′ to 3′): GluR1–4 sense, CCTTTGGCCTATGAGATCTGGATGTG (position 1600–1625; Hollmann et al., 1989); GluR1–4 antisense, TCGTACCACCATTTGTTTTTCA (position 2327–2348); GluR5 sense, GCCCCTCTCACCATCACATAC (position 1730–1750; Bettler et al., 1990); GluR5 antisense, ACCTCGCAATCACAAACAGTACA (position 1893–1915); GluR6 sense, TTCCTGAATCCTCTCTCCCCT (position 1963–1983; Egebjerg et al., 1991), GluR6 antisense, CACCAAATGCCTCCCACTATC (position 2182–2202); GluR7 sense, TGGAACCCTACCGCTACTCG (position 713–732; Bettler et al., 1992), GluR7 antisense, ACTCCACACCCCGACCTTCT (position 1096–1115); KA-1 sense, AGCGTTATGTCATGCCCAGACCAG (position 26–49; Werner et al., 1991), KA-1 antisense, AGGCATTCTGCTTTGGCACAGATGA (position 544–568); KA-2 sense, TGAGGAGGGGAGGAAGATGC (position 187–206; Herb et al., 1992); KA-2 antisense, TGCAGCTCAAAGATGTC (position 382–398). Rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Clontech, Palo Alto, CA) was used as a control for the PCR. PCR was performed for 20 cycles of amplification with denaturing at 95°C for 30 sec, annealing at 55°C for KA-1 and KA-2 and 58°C for others for 1 min, and elongating at 72°C for 1 min in the presence of 1× Vent buffer (New England Biolabs, Beverly, MA), 0.5 mm dNTP, 0.5 μm each primer, and 1.25 U of AmpliTaq polymerase (Perkin-Elmer, Norwalk, CT). PCR products were separated by electrophoresis on 2.5% Metaphor agarose gel (FMC Bioproducts, Rockland, ME) containing 0.5 μg/ml ethidium bromide.
RNA editing analysis. The regions across the Q/R edited sequence, located in the second transmembrane domain, of GluR5 and GluR6 were amplified by PCR, using the cDNA as template. The PCR primers for GluR5 were as follows: 5′ primer, GTTTGTGATTGCGAGGTTCACA (22 mers); 3′ primer, CAGGTTGGCCGTGTAGGATGA (21 mers). The amplified product was purified from the gel to remove residual nucleotides. The Q/R site of the GluR5 subunit was analyzed by digesting the PCR products with the restriction enzyme BbvI (New England Biolabs). BbvI recognizes the sequence GC AGC, which is identical with the sequence of unedited GluR5 mRNA, but not the edited sequence GC GGC. The digestion of the PCR-generated fragment (233 bp) was expected to be cut into two fragments (139 and 94 bp). The PCR primers for GluR6 were as follows: 5′ primer, TTCCTGAATCCTCTCTCCCCT (21 mers); 3′ primer, CACCAAATGCCTCCCACTATC (21 mers). The Q/R site of the GluR6 subunit was analyzed by digesting the PCR products with the restriction enzymeAciI (New England Biolabs). AciI recognizes the sequence CCGC of the edited version of GluR6 (coding for R), but not that of the unedited version. The digestion of the PCR-generated fragment (259 bp) predicts two fragments of 197 and 62 bp. After 1 hr of incubation with BbvI or AciI at 37°C, the digests were separated on a 2.5% Metaphor agarose gel, supplemented with ethidium bromide.
Northern blot analysis. Total RNA was isolated from TG of rats of various ages (P1, P3, P7, P14, P28, and P56), using the guanidine thiocyanate procedure. RNA samples (10 μg/lane) were electrophoresed through a 1.4% agarose-formaldehyde denaturing gel and transferred onto GeneScreen Plus membranes. The blot was hybridized with 32P-labeled cDNA probes specific for GluR5, KA-1, or KA-2 at 42°C for 1 hr and washed in high-stringency condition with 0.1× SSC/0.5% SDS at 65°C; autoradiography was performed on Kodak X-Omat AR5 film (Rochester, NY) with an intensifying screen at −80°C for 18 hr.
RESULTS
Expression of kainate receptor subunit RNAs in the TG
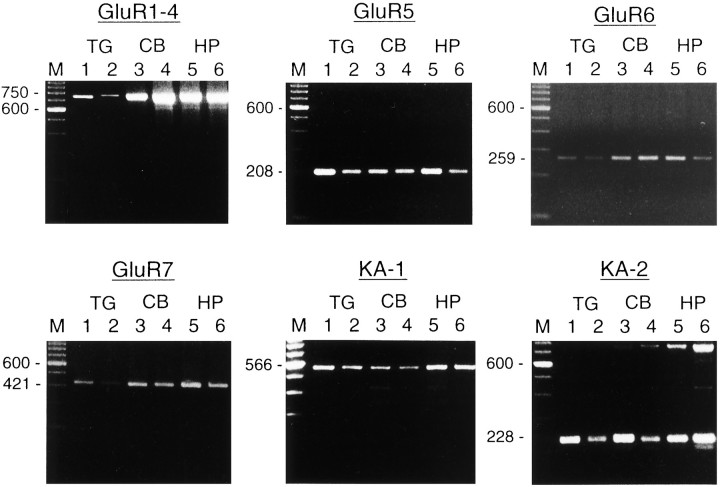
To determine which glutamate receptor subunits are expressed in the TG, we examined the expression of the GluR1–4, GluR5, GluR6, GluR7, KA-1, and KA-2 transcripts. Total cellular RNA was isolated and analyzed by RT-PCR. PCR primers for the GluR5, GluR6, GluR7, KA-1, and KA-2 subunits were derived from specific sites for each kainate receptor subunit, and a primer for AMPA receptor subunits (GluR1–4) was derived from a highly conserved region among AMPA receptors. The predicted sizes of the PCR-generated fragments were 750, 208, 259, 421, 566, and 228 bp for the GluR1–4, GluR5, GluR6, GluR7, KA-1, and KA-2 subunits, respectively. As shown in Figure1, PCR products for all subunits were detected in the TG, although a greater degree of variation in the expression of GluR subunits was apparent in the TG than in the cerebellum and hippocampus. In both P7 and adults the expression of GluR5, KA-1, and KA-2 subunits was high, but GluR6 and GluR7 were seen only at a barely detectable level in the TG.
Fig. 1.
RT-PCR to identify GluRs subunit expression in the TG. RNAs from tissues were reverse-transcribed and subjected to PCR, using primers specific for each kainate receptor subunit (GluR5, GluR6, GluR7, KA-1, and KA-2) and for a highly conserved region among AMPA receptor subunits (GluR1–4). Tissues analyzed included trigeminal ganglion (TG), cerebellum (CB), and hippocampus (HP) of 7-d-old (odd-numbered lanes) and 56-d-old rats (even-numbered lanes). Equal volumes of PCR product were analyzed in each lane.Lanes marked M contain a 100 bp ladder.
Small diameter TG neurons show desensitizing kainate responses
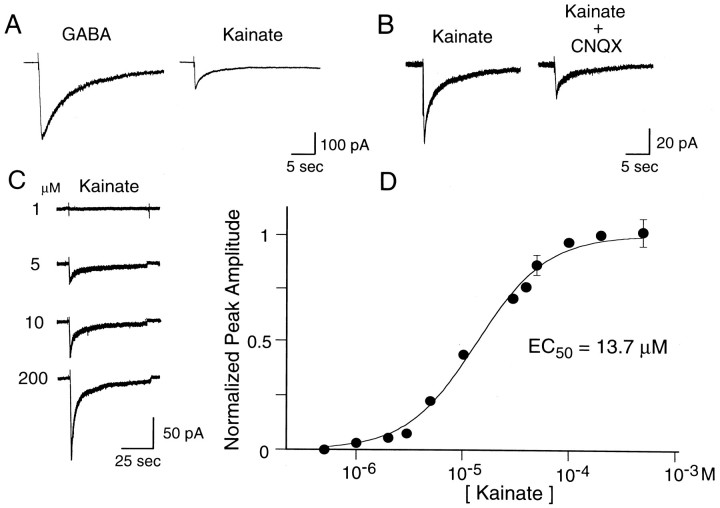
Acutely dissociated TG neurons from P4–P8 rats were tested with several excitatory and inhibitory amino acids, includingl-glutamate, kainate, quisqualate, AMPA, NMDA, GABA, and glycine. Of 70 cells tested, all cells responded to GABA (100 μm) (Fig. 2A). Approximately 50% of TG neurons for which the diameter was smaller than 30 μm responded tol-glutamate (1 mm) and kainate (200 μm). Two types of TG neurons (large light and small dark neurons) have been described on the basis of cytoplasmic appearance under the light microscope; however, none of the large diameter neurons (>30 μm) responded to kainate. Figure 2C shows currents evoked in a TG neuron by five doses of kainate, ranging from 1 to 200 μm. Responses increased in amplitude with increasing agonist dose, up to maximal levels at ∼200 μm. The EC50 was 13.7 ± 0.8 μm (Hill coefficient, 1.3 ± 0.1; six trials in four cells) (Fig.2D). The peak amplitude of kainate currents ranged from barely detectable to a maximum of 180 pA, with a mean of 63.4 ± 5.8 pA (n = 41) at −80 mV. Prolonged application of kainate produced an initial peak of current that decayed over several seconds to reach a steady-state level at ∼10% of the peak response. The extent of desensitization was quantified by theIss/Ipeak ratio, where Iss (steady-state current amplitude) was measured at 50 sec after agonist application, andIpeak represents the peak amplitude. In the TG neurons, theIss/Ipeak value was 0.14 ± 0.03. The kainate response was reversibly antagonized by CNQX (20 μm) (Fig.2B). We never encountered a TG neuron that responded to AMPA (up to 1 mm), quisqualate (up to 0.5–1 mm), NMDA (up to 1 mm), or glycine (up to 500 μm).
Fig. 2.
Whole-cell currents evoked by kainate in TG neurons. A, Kainate (200 μm) and GABA (100 μm) produced desensitizing responses in a small diameter TG neuron. B, CNQX (20 μm) inhibited kainate currents. C, Currents activated by 1–200 μm kainate in a TG neuron. D, Dose–response data for kainate from four TG neurons. Concentration–response curve was fit with the functionI =Imax · {1/(1+(K/[agonist])n)},whereK represents the half-maximal effective concentration (EC50) and ndenotes the Hill coefficient. EC50 was 13.7 ± 0.8 μm; the Hill coefficient was 1.3 ± 0.1. Holding potential was −80 mV.
Expression of functional kainate receptors in TG neurons
To assess which glutamate receptor subunits are functionally expressed in TG neurons, we performed detailed pharmacological, physiological, and molecular characterization of kainate receptor channels, using the whole-cell patch-clamp technique and RT-PCR.
Con A and WGA, but not CTZ, suppress desensitization
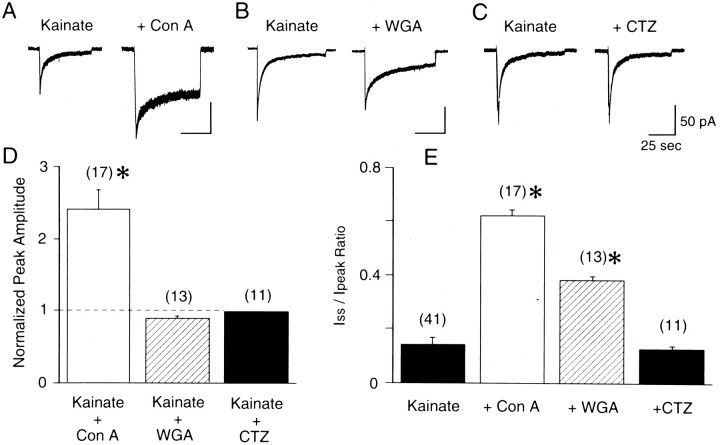
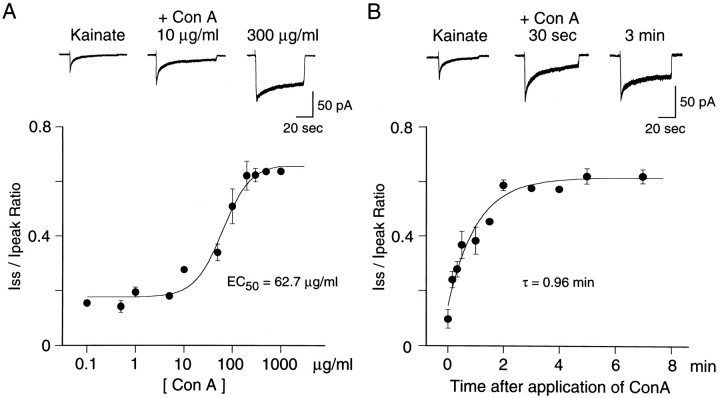
We examined the effect of lectins and CTZ on the desensitization of kainate responses. Exposure to Con A (300 μg/ml for 3 min) augmented the peak kainate currents and partially eliminated desensitization of kainate responses (Fig.3A). The effects of Con A persisted even after the neurons were washed with control solution. In 17 cells tested before and after Con A treatment, the peak current elicited by kainate increased significantly (2.4-fold ± 0.3;p < 0.05) (Fig. 3D), and the extent of desensitization (Iss/Ipeak ratio) was reduced significantly (0.62 ± 0.02; p < 0.05) (Fig. 3E). Con A did not change the EC50for kainate (14.4 ± 1.1 μm; Hill coefficient, 1.0 ± 0.1; five trials in three cells). The effects of Con A were both dose-dependent (EC50 = 62.7 μg/ml; n= 3) (Fig. 4A) and time-dependent. Figure 4B shows that the onset of action of Con A was fit reasonably well by a single exponential curve with a time constant of 0.96 min (n = 3). However, substantial desensitization persisted even after prolonged treatment with Con A.
Fig. 3.
Con A and WGA, but not CTZ, modulate desensitization of kainate currents. A, Con A (300 μg/ml for 3 min) irreversibly increased peak kainate currents and strongly attenuated desensitization. B, WGA (300 μg/ml for 3 min) irreversibly attenuated desensitization to kainate.C, CTZ (100 μm) did not change the kainate response. D, Peak amplitudes of kainate responses, normalized to the control response to kainate, after treatment with Con A, WGA, and CTZ. An asterisk indicates that Con A increased the peak current elicited by kainate significantly (p < 0.05). E,Iss/Ipeakfor kainate responses before and after treatment with Con A, WGA, and CTZ. Asterisks indicate that Con A and WGA reduced the extent of desensitization significantly (p< 0.05). Cells were treated with 0.3 mg/ml Con A or 0.3 mg/ml WGA for >3 min before application of kainate. Error bars show SEM.Numbers in parentheses show the number of recorded cells. Holding potential was −80 mV.
Fig. 4.
Dose- and time-dependent effects of Con A on desensitizing kainate responses. A, Dose–response curve for desensitization by Con A.Iss/Ipeak= Iss/Ipeakmax· {1/(1+(K/[Con A])n)} + Iss/Ipeakoffset, where K represents half-maximal effective concentration (EC50), n denotes the Hill coefficient, and Iss/Ipeakoffsetcorresponds to theIss/Ipeakvalues of kainate response in the absence of Con A. EC50 was 62.7 ± 11.7 μg/ml; the Hill coefficient was 1.5 ± 0.5 (n = 3). Top traces show responses to 200 μm kainate recorded in the absence and in the presence of Con A (10 and 300 μg/ml).B, Time course of the onset of Con A-evoked potentiation of kainate responses. The curve was fit with an exponential function.Top traces show responses to 200 μmkainate recorded before and after application of Con A (30 sec and 3 min). Con A (300 μg/ml for 3 min) did not completely eliminate desensitization.
In addition to Con A, WGA and succinyl-Con A reduced desensitization tol-glutamate and kainate. WGA applied at 300 μg/ml was much less effective than Con A in blocking desensitization of the response to kainate in TG neurons: normalized peak amplitude, 0.89 ± 0.03; Iss/Ipeakratio, 0.38 ± 0.01 (n = 13) (Fig.3B,D,E). The ability to modulate desensitization of kainate responses in TG neurons also was mimicked by succinyl-Con A (300 μg/ml), a dimeric form of Con A that does not cross-link receptors. This suggests that Con A works mainly in ways other than cross-linking. Considering that Con A interacts with α-mannose or α-glucose, that WGA specifically binds N-acetylglucosamine andN-acetylneuraminic acid, and that amino acid sequence analysis for glutamate receptors subunits predicts multiple N-linked glycosylation sites in GluR5–GluR7 and KA-1 and 2 subunits, it seems that glycosylation contributes to the desensitization of kainate currents.
Cyclothiazide (100 μm), which modulates responses to glutamate at non-NMDA receptors, also was tested for its effect on the desensitizing response to kainate in TG neurons (Fig. 3C). However, it produced neither potentiation of the peak kainate current nor reduction in the degree of desensitization of kainate responses in TG neurons (normalized peak amplitude, 0.98 ± 0.01;Iss/Ipeak ratio, 0.13 ± 0.01; n = 11) (Fig. 3D,E).
As summarized in Figure 3, D and E, desensitization of kainate responses in TG neurons was modulated strongly by Con A but it was insensitive to cyclothiazide. Considering that CTZ selectively modulates AMPA receptor subunits, but not kainate receptors (Partin et al., 1993), functional expression of AMPA receptors in the TG seems unlikely.
Time course of desensitization of kainate responses
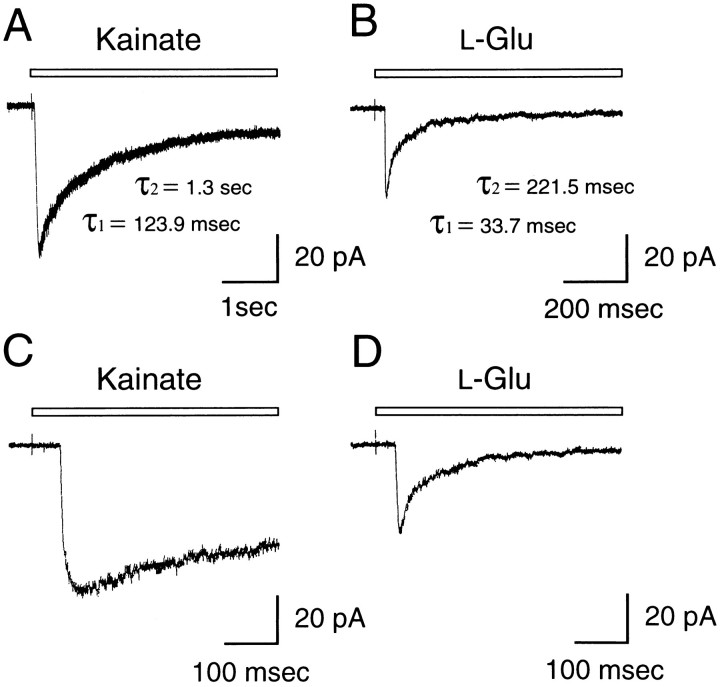
The time constants of desensitization have been shown to be affected by the subunit composition in recombinant kainate receptor channels (Herb et al., 1992). We analyzed the time course of desensitization of kainate responses in TG neurons, using a piezo-driven application system. Figure5, A and C, shows the current evoked by a saturating dose of kainate (200 μm). With prolonged agonist applications, lasting several minutes, the currents appeared to reach a steady-state level of ∼10% of the peak response to kainate. The onset of desensitization of kainate currents was well fit by the sum of two exponentials. In this case the faster time constant was 123.9 msec and the slower one was 1.3 sec. The mean values for nine TG neurons were τ1 = 128.1 ± 8.1 msec and τ2 = 1.3 ± 0.1 sec. Figure 5, B and D, shows responses to 1 mml-glutamate, which shows a fast current component (τ1 = 33.7 msec) and a slow component (τ2 = 221.5 msec). The mean values for three TG neurons were τ1 = 33.3 ± 2.5 msec and τ2 = 239.2 ± 30.6 msec.
Fig. 5.
Time course of desensitization of kainate (200 μm) and l-glutamate (1 mm) currents. Desensitization time constants were determined by fitting the decay phase with the sum of two exponentials. A, The decay time constants of kainate currents (200 μm) in TG neurons were τ1 = 123.9 msec and τ2 = 1.3 sec. B, A response to 1 mml-glutamate. A fast current component (τ1 = 33.7 msec) and a slow component (τ2 = 221.5 msec) were observed. The fitted range extended to 3 sec after the peak.C, D, Fast sweeps of the records inA and B, respectively. Holding potential was −80 mV.
Results from recombinant experiments with whole-cell recordings that used a rapid perfusion system (Herb et al., 1992) have shown that responses for homomeric GluR6(Q) or GluR6(R) and the combination GluR6(Q)/KA-2 are rapidly and completely desensitizing, unlike those in TG neurons. Homomeric recombinant receptors generated from GluR5(Q) showed slow desensitization (τ1 = 15.3 msec and τ2 = 281 msec), whereas GluR5(R) was inactive unless it was combined with other subunits (Herb et al., 1992; Sommer et al., 1992) or had an extremely low conductance (Swanson et al., 1996). For the combination GluR5(Q)/KA-2, the desensitization was more rapid (τ1 = 15.1 msec and τ2 = 688 msec) than that observed in TG neurons, but for GluR5(R)/KA-2, desensitization was like that seen in TG neurons. Together, these results suggest that the major glutamate receptor in TG neurons is likely to be composed of GluR5, not GluR6, in combination with KA-2.
Colquhoun et al. (1992) reported that the rise time of the AMPA receptor-mediated current in the hippocampal slice was 0.2–0.6 msec, the decay time constant of the current after short pulses of 1 mml-glutamate was ∼3.0 msec, and the time constant for desensitization was ∼10 msec for patches from hippocampal neurons. Thus, it is likely that the time constant of desensitization in the present experiments is determined mainly by the speed of agonist application. Kinetics of native kainate receptor channels still remain to be elucidated.
Current–voltage relation
It has been demonstrated that the presence of a glutamine (Q) or an arginine (R) residue in the Q/R site of GluR5 and GluR6 subunits and at the I/V and Y/C sites of the GluR6 subunit are critical determinants of Ca2+ permeability and rectification of kainate receptor channels (Sommer et al., 1991; Egebjerg and Heinemann, 1993;Köhler et al., 1993). In particular, editing of the Q/R site in the GluR6 subunit seems to govern the rectification properties of homomeric GluR6 channels (Egebjerg and Heinemann, 1993; Köhler et al., 1993).
Q/R editing. We analyzed the extent of GluR5 editing in RNAs extracted from both the TG and the cerebellum. After RT-PCR, theBbvI digestion of the PCR products was analyzed. In a typical experiment with rat TG (P7) and cerebellum (P14) PCR products, ethidium bromide staining of an agarose gel revealed amplification products of 233 bp (Fig. 6A, left). BbvI digested the PCR product from unedited GluR5 subunit and completely cut it into two smaller fragments (139 and 94 bp), whereas the fraction from edited GluR5 subunit was not cut byBbv1. As shown in Figure 6A(right), gel analysis revealed three fragments (233, 139, and 94 bp) in both the TG and cerebellum, suggesting that both the edited and unedited forms are expressed. Because it has been reported that 40% of GluR5 mRNA is edited in the cerebellum at P4–P25, with the degree of editing increasing to 80% in the adult (Paschen et al., 1995), we analyzed the extent of GluR5 editing during the course of postnatal development (P3, P14, and P56). In the TG, both the edited and unedited forms of the GluR5 subunit were detected at all ages (Fig.6A, right).
Fig. 6.
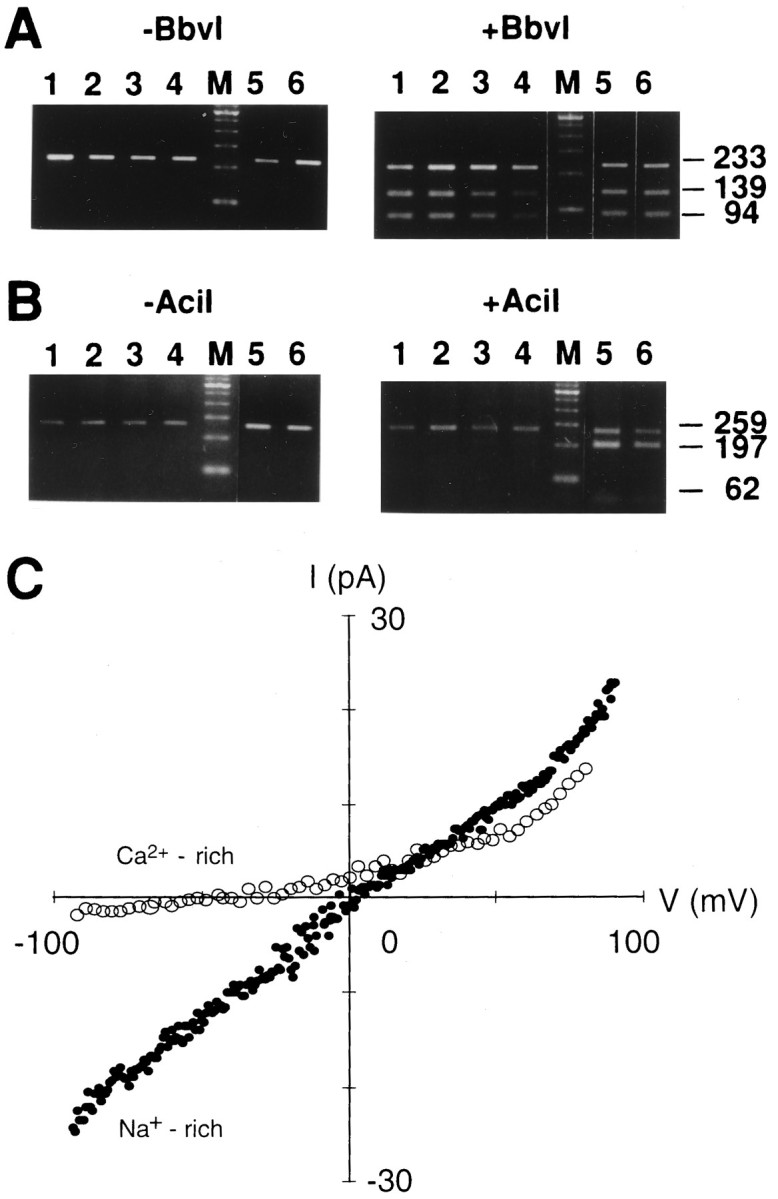
RNA editing analysis of the Q/R site of the GluR5 and GluR6 subunit in the TG and I–V relationships of the kainate-induced responses in a TG neuron. A, RNA editing analysis of the Q/R site of the GluR5 fragment. RNA editing of the Q/R site was analyzed by isolating total RNA from the TG, reverse transcription into cDNA, PCR across the edited region with GluR5 specific primers, and restriction analysis of the PCR products withBbvI. Shown are ethidium bromide-stained agarose gels of the PCR products (left, expected size 233 bp) and the PCR products incubated with BbvI (right, expected fragment sizes 139 and 94 bp). Lanes 1–4, TG samples taken from P3, P7, P14, and P56; lane 5, cerebellum sample from P14; lane 6, hippocampal sample from P7. Lanes marked M contain a 100 bp ladder.B, RNA editing analysis of the Q/R site of the GluR6 fragment. Shown are ethidium bromide-stained agarose gels of the GluR6 PCR products (left, expected size 259 bp) and the PCR products incubated with AciI (right, expected fragment sizes 197 and 62 bp). Lanes 1–4, GluR6 TG samples taken from P3, P7, P14, and P56; lane 5, hippocampal sample from P7; lane 6, cerebellum sample from P14. Lanes marked M contain a marker. C, Current–voltage (I–V) relations of kainate-activated (200 μm) currents in a TG neuron with Na+-rich extracellular solution (filled circles) and Ca2+-rich solution (open circles). Recordings were performed after 3 min of Con A (300 μg/ml) treatment. The I–Vrelation was recorded by using a voltage ramp from −100 to +100 mV (0.2 V/sec) and by subtracting the I–V curve obtained before and after 200 μm kainate application.
We also determined the extent of GluR6 editing in the TG, although PCR products of GluR6 were barely detectable. AciI restriction enzyme cuts the edited version of GluR6 (coding for R) but cuts neither the unedited version (coding for Q) nor GluR5. The AciI digestion of the GluR6 PCR-generated fragment (259 bp) predicts two fragments of 197 and 62 bp. In agreement with the report that GluR6 mRNA is edited by 50–60% at the Q/R site in the hippocampus (Bernard and Khrestchatisky, 1994), gel analysis revealed three fragments in the hippocampus (P7) (Fig. 6B, right). However, the GluR6 subunit in the TG was nearly exclusively in the unedited form at all ages (Fig. 6B, right).
I–V relation and Ca2+ permeability.The I–V relationship of the steady-state kainate currents in TG neurons was measured by applying voltage ramps. To prevent washout of intracellular polyamine, which produces linear instead of inwardly rectifying I–V relationships of the kainate response (Bowie and Mayer, 1995; Kamboj et al., 1995), we included spermine (100 μm) in the patch pipettes. In all TG neurons tested, the I–V relation for kainate was approximately linear or slightly outwardly rectifying. Figure6C shows the I–V relation for kainate from −100 mV to +90 mV. In Na+-rich (160 mm) extracellular solution, the reversal potential of the kainate-activated current was close to 0 mV (−2.4 ± 0.4 mV; n = 44). The ratio of conductances (G+40/G−60) was taken as the rectification index, which had a mean value of 0.81 ± 0.05 (n = 44) in TG neurons. Inward current at negative membrane potentials was recorded in high extracellular Na+ but was barely detectable in high extracellular Ca2+ solution (10 mmCa2+ + 150 mm NMG). In the high extracellular Ca2+ solution the reversal potential of the kainate-activated current shifted to −37.5 ± 7.7 mV (n = 4). Variations of the extracellular Ca2+ concentration (0.1–10 mm) had no measurable effect on the I–V relation. These indicate a low Ca2+ permeability of kainate receptor channels in the TG neurons.
The slightly outward rectification of the steady-state I–Vrelationship and the low Ca2+ permeability of kainate receptor channels in TG neurons match the properties of the edited form of the GluR5 subunit. We never encountered a doubly rectifying shaped I–V curve, which is characteristic of unedited channels, although the present RT-PCR data revealed the unedited form of GluR5 and GluR6 subunits in the TG. This discrepancy could be explained if the GluR5(Q) subunit coexists in the same cell with GluR5(R) or GluR5(R)/KA-2 channels. Coexpression of the edited and unedited form of the GluR5 or GluR6 subunits has been reported to make the I–V relationship more linear and the divalent permeability low (Sommer et al., 1992; Köhler et al., 1993).
Whole-cell current noise analysis
To estimate the mean channel conductance, we plotted the variance of whole-cell kainate current noise against mean inward current. Figure7A shows the onset of the steady-state current response to 200 μm kainate after treatment with Con A (300 μg/ml). All of the current–variance relationships obtained were linear rather than parabolic, indicating that the probability of channel opening was low during the steady-state response to kainate. Mean conductance was derived according to γ = δ2/[μ(Vc −Vrev)], where γ is the mean conductance, δ2 is the current variance, μ is the mean current, Vrev is the reversal potential, andVc is the holding potential.Vrev was near 0 mV for all recordings. The mean channel conductance estimated from the slope of the variance versus mean current relationship in Figure 7B was 1.5 pS. The mean value for 10 TG neurons was 1.6 ± 0.2 pS. As illustrated in Figure 7C, the power spectrum was well fit by the sum of two Lorentzian components. Cutoff frequencies were 13.1 ± 2.4 and 127.3 ± 12.7 Hz, yielding mean time constants of 14.9 ± 3.2 and 1.1 ± 0.1 msec (n = 10). Swanson et al. (1996) reported that (1) Q/R site editing dramatically reduces single-channel conductance [GluR6(Q), 5.4 pS; GluR6(R), 225 fS; GluR5(Q), 2.9 pS; GluR5(R), <200 fS] and (2) heteromeric GluR5(R)/KA-2 and GluR6(R)/KA-2 receptors have higher conductances (950 and 700 fS) than the respective homomeric channels. The value from TG neurons corresponds to that obtained from recombinant GluR5(R)/KA-2.
Fig. 7.
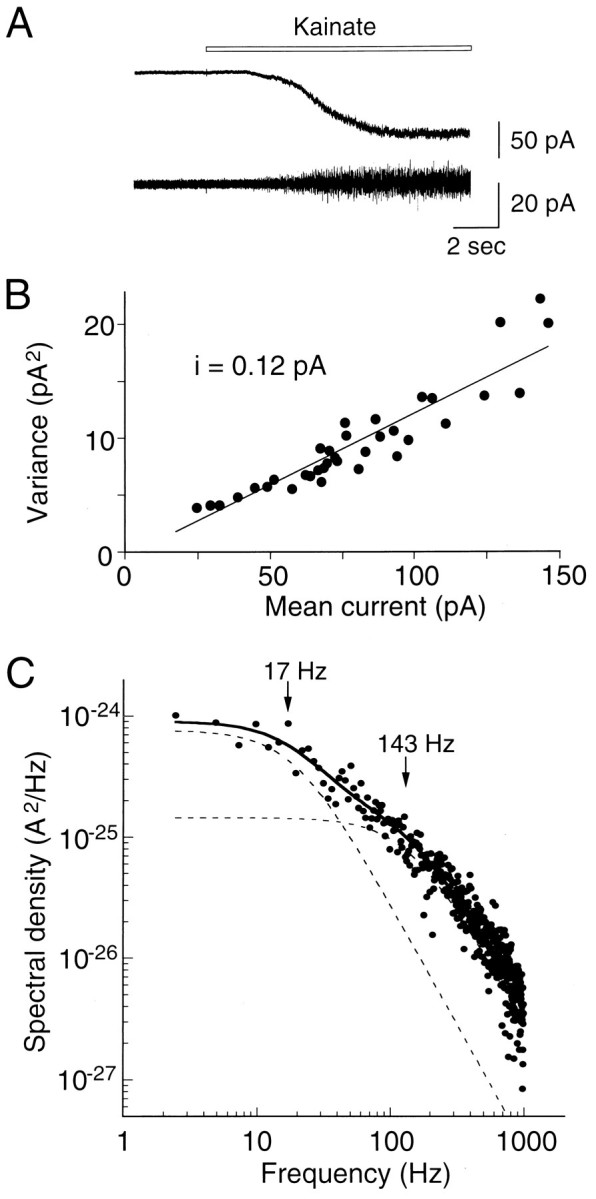
Whole-cell current noise analysis of kainate currents in TG neurons. A, Onset of the whole-cell current evoked by 200 μm kainate at −80 mV after treatment with Con A (300 μg/ml for 3 min). The lower trace shows the high-pass-filtered record at 0.2 Hz.B, Variance to mean current plots calculated from fluctuation analysis of a kainate response. Background variance was subtracted from the test variance. The straight line is the least-squares linear fit to the results. The slope of the relationship (i) has a value of 0.12 pA at −80 mV, corresponding to a mean conductance of 1.5 pS.C, Power spectrum obtained from analysis of the current noise shown in A. The spectral density of base line noise was subtracted from that of the kainate-induced noise. The spectrum was fit with the sum of two Lorentzian components according to the equation: G(f =G(0)1/(1 + (f/fc1)2) + G(0)2/(1 + (f/fc2)2). The individual components are shown as dashed lines, and their respective cutoff frequencies (fc) were 17 and 143 Hz.
In summary, the data obtained from the Con A and CTZ sensitivities, the time course of desensitization of kainate currents, the I–Vrelationships, and the mean channel conductance from noise analysis all point to the possibility of coassembly of GluR5(R) and KA2 subunits in TG neurons.
Postnatal changes of kainate receptor subunits
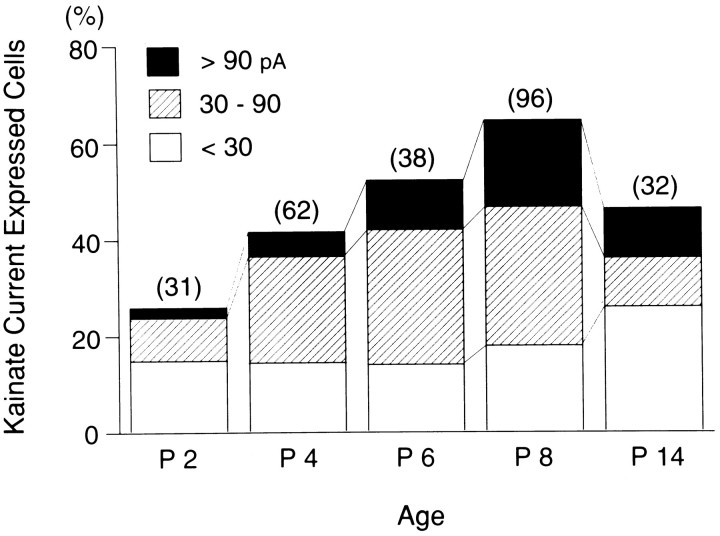
It has been reported that the GluR5 gene, in contrast to other GluRs subunits, undergoes clear qualitative changes in its expression over time and that the time course of the GluR5 expression peak is consistent with that of the period of greatest developmental plasticity (Bettler et al., 1990; Bahn et al., 1994). We examined kainate responses at various postnatal developmental ages (P2–P14). The number of kainate-sensitive TG neurons increased until P8, although only small diameter cells (<30 μm) responded to kainate. The peak amplitude of kainate current was small at birth (>10 pA) and gradually increased during the first postnatal week. As shown in Figure8, an increasing number of neurons had large kainate responses (peak amplitude >90 pA at −80 mV) until P8. After P8, the numbers of neurons showing small kainate responses increased.
Fig. 8.
Postnatal developmental changes of kainate responses in small- diameter TG neurons. Percentage of kainate current expressing cells plotted as a function of age. Numbersin parentheses show the number of cells for which kainate (200 μm) was applied. Until postnatal day 8 (P8), kainate-sensitive TG neurons increased with development, and an increasing number of neurons had large kainate responses (peak amplitude >90 pA). At later stages of development the number of neurons showing small kainate responses (peak amplitude <30 pA) increased. Holding potential was −80 mV.
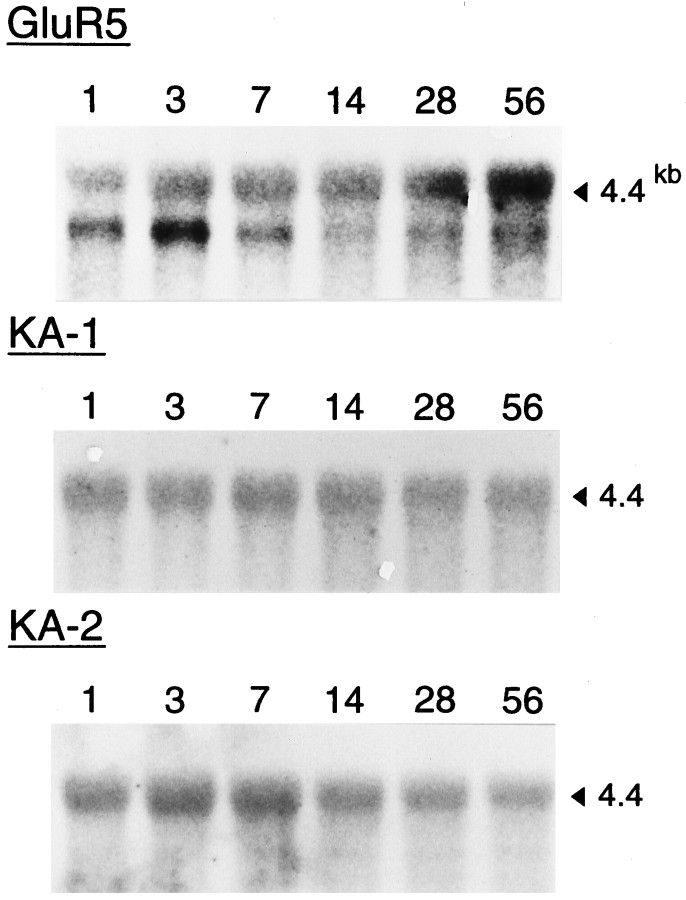
Figure 9 shows Northern blot analysis of GluR5, KA-1, and KA-2 messages at various postnatal ages (P1, P3, P7, P14, P28, and P56). The relative levels of RNA for GluR5 in the TG changed age-dependently and were changed only slightly for KA-2, whereas the RNA levels for KA-1 remained relatively constant (Fig. 9). The GluR5 3.6 kb message showed a high level at birth and reached its peak by 3 d, subsequently dropping to a barely detectable level in the adult. In contrast, 4.4 kb GluR5 message was detectable at birth and increased steadily until the adulthood. By P7 the relative abundance of the two isoforms appeared to be approximately equal. By the adulthood it was clear that the 4.4 kb isoform comprised the majority of the products, although there was some 3.6 kb product still visible. KA-2 messages also reached a peak by 3–7 d and subsequently dropped to a barely detectable level in the adult. The increase in GluR5 and KA-2 RNA correlates well with the increased number of kainate-sensitive cells and increased peak amplitude of kainate-evoked currents. The changes in GluR5 and KA-2 RNA levels we have observed could reflect the appearance and disappearance of particular types of GluRs during postnatal development.
Fig. 9.
Northern blot analysis of GluR5,KA-1, and KA-2 message in TG at various postnatal ages. Total RNAs (10 μg per lane) isolated from TGs of P1, P3, P7, P14, P28, and P56 rats were hybridized with cDNA probes specific for GluR5, KA-1, or KA-2 under stringent conditions. Numberson the right represent molecular size as derived from an RNA standard.
DISCUSSION
We have demonstrated that (1) all five known kainate receptor subunits are expressed to some extent, although particularly high levels of GluR5 and KA-2 RNA are observed in the TG; (2) there is an obvious similarity between the features of native kainate receptor channels in TG neurons and of recombinant channels assembled from the heteromeric GluR5(R)/KA2 receptors with respect to the specific physiological and pharmacological properties of currents gated by kainate; and (3) the age-dependent increase in GluR5 and KA-2 RNA levels in the TG is correlated with an increased number of kainate-sensitive cells during postnatal development. Our results suggest that the heteromeric GluR5/KA2 combination actually occurs in native neurons.
AMPA receptors in TG neurons
Low levels of GluR1–4 RNA were detected in the TG, consistent with other studies on cranial and spinal ganglia (Bettler et al., 1990;Sato et al., 1993; Niedzielski and Wenthold, 1995). In contrast, the present physiological and pharmacological experiments showed that AMPA (up to 1 mm) did not induce any detectable responses in TG neurons. One concern might be that AMPA responses may not be detected in the present experiments because of the speed of solution exchange. Very rapid desensitization of AMPA receptor channels (∼10 msec,Colquhoun et al., 1992; Jonas and Sakmann, 1992) has been reported with a fast perfusion technique with outside-out patches. However, significant functional expression of AMPA receptors in the TG seems unlikely, based on the following facts: (1) AMPA is a potent agonist at AMPA receptors that produces rapidly and strongly desensitizing responses, whereas kainate is a low-affinity agonist at AMPA receptors that produces nondesensitizing responses with whole-cell recording (Keinänen et al., 1990; Sommer et al., 1991); (2) all kainate responses in TG neurons have desensitization, which was not modulated by CTZ but was modulated by Con A; and (3) inhibition of kainate responses after prolonged application of AMPA or quisqualate (cross-desensitization; Patneau and Mayer, 1991) was not observed in TG neurons. Possibilities that might account for the presence of GluR1–4 would be receptors on glia in the TG and/or expressed AMPA receptor subunits that are not correctly assembled, transported to, or inserted into the plasma membrane of TG neurons.
Composition of glutamate receptor subunits in TG neurons
The heteromeric recombinant GluR5(R)/KA-2 channels matched the native kainate receptor channels in all functional properties, and high levels of expression of GluR5 and KA-2 RNAs were detected in the TG. This is consistent with the fact that the functional properties of ion channels are highly dependent on the level of expression (Geiger et al., 1995). On the other hand, single-cell PCR analysis suggests that native AMPA responses are likely to result from the assembly of different combinations of subunits (Lambolez et al., 1992; Bochet et al., 1994; Geiger et al., 1995). It remains possible that the KA-1 or GluR7 subunit combines with GluR5(R)/KA-2 subunits to produce kainate receptors. The effects of KA-1 on desensitization kinetics and the behavior of receptors containing GluR5(R)/KA-2 have not been assessed in transfected mammalian cell lines. GluR7 was expected to have physiological properties similar to those observed for homomeric GluR6 subunits, based on amino acid sequence homology. However, GluR7 was found not to assemble into either functional homomeric channels or heteromeric channels with KA-1 or KA-2 in Xenopus oocytes and HEK 293 cells (Bettler et al., 1992; Lomeli et al., 1992). It would be interesting to examine the subunit composition in TG neurons by single-cell PCR/patch-clamp technique.
The replacement of a glutamine by an arginine in the Q/R site of GluR2, GluR5, and GluR6 results in (1) a reduction in Ca2+permeability (Hume et al., 1991; Burnashev et al., 1992), (2) a change of inward rectification because of a loss of sensitivity to intracellular polyamines (Bowie and Mayer, 1995; Kamboj et al., 1995), and (3) reduction of the single channel conductance of AMPA and kainate receptors (Swanson et al., 1996; Swanson et al., 1997). Coexpression of the edited and nonedited forms of the GluR(B), GluR5, or GluR6 subunits has been reported to change the shape of the I–Vrelationship to become more linear (Sommer et al., 1992; Köhler et al., 1993), and heteromeric GluRB(Q)/B(R) channels generate cells with low divalent permeability (Burnashev et al., 1992). Thus, some of the GluR5(Q) could coexist with GluR5(R) or GluR5(R)/KA-2 in the same cell. However, inward rectifying responses to GluR5(Q) were reported to be unaltered on cotransfection with GluR-B, and the inward rectification of GluR-D responses also was unchanged on cotransfection with GluR5(R) (Partin et al., 1993). Thus, any cross-assembly between AMPA and kainate receptor subunits seems unlikely.
KA-2 does not form functional homomeric channels, whereas coexpression of KA-2 with GluR5 or GluR6 results in channels with novel properties (Herb et al., 1992). It has been demonstrated that the KA-2 subunit causes striking effects on single-channel conductance when coexpressed with GluR5(R) or GluR6(R) (Howe, 1996; Swanson et al., 1996). In addition, the involvement of the KA-2 subunit was found to influence the kinetics of low-affinity subunits: the channel produced by GluR5(Q)/KA-2 has shorter channel burst lengths than that of homomeric GluR5(Q) (Swanson et al., 1996) and results in more rapid desensitization than that seen in channels formed from GluR5(Q) subunits (Herb et al., 1992). Our present data suggest that the involvement of KA-2 could change sensitivity to Con A. Desensitization of glutamate receptors expressed by invertebrate muscle, vertebrate CNS, and mammalian DRG neurons as well as of recombinant homomeric GluR5(Q), GluR5(R), and GluR6(Q) is eliminated almost completely by Con A (Huettner, 1990; Wong and Mayer, 1993; Howe, 1996; Swanson et al., 1996). In TG neurons, however, treatment with Con A did not eliminate desensitization completely. Interestingly, Con A-insensitive desensitizations were visible in domoate responses of both heteromeric GluR5(R)/KA-2 and GluR6(R)/KA-2 channels (Swanson et al., 1996). The abundantly and widely expressed KA-2 subunit could lead to functional diversity in kainate receptor channels.
DRG neurons are known to respond to AMPA (Huettner, 1990), which has been explained by homomeric expression of GluR5(Q). This raises the question of whether the lack of AMPA response in TG depends on the receptor isoform. It has been reported that GluR5(Q) forms functional homomeric AMPA-activated receptors (Sommer et al., 1992) and that kainate receptors become sensitive to AMPA when GluR5 coassembles with KA-1, or GluR6 assembles with KA-1 or KA-2 (Herb et al., 1992). The involvement of the KA-2 subunit was found to influence agonist affinity for the low-affinity subunits [e.g., the EC50 for kainate activation of homomeric GluR6(R) channel is 0.47 μm and GluR6(R)/KA-2 channels is 1.62 μm (Howe, 1996)]. Thus, it is likely that the KA-2 subunit influences AMPA affinity, depending on the receptor isoform coexpressed.
Functional significance of kainate receptors on TG neurons
There is no direct electrophysiological evidence for an involvement of kainate receptors in synaptic transmission. However, there is a general belief that kainate receptors play a role in nociception, based on the facts that (1) kainate responses have been identified by using the whole-cell patch-clamp technique in a subpopulation of smaller diameter DRG neurons; (2) C-fibers arise from small to intermediate diameter sensory neurons (Harper and Lawson, 1985), and most axons in the C-fiber range convey nociceptive or thermoreceptive information; and (3) the kainate receptor seems to be expressed preferentially on the afferent axons rather than on the DRG cell body, because local administration of kainate is less effective when applied at the DRG (Agrawl and Evans, 1986; Tölle et al., 1993). However, it is not known whether functional kainate receptors reside on presynaptic terminals, where kainate receptors may function as presynaptic autoreceptors.
An involvement of kainate receptors in development has been suggested.Bettler et al. (1990) first described the regulated expression of GluR5 in the developing mouse brain, where a peak in expression of high-affinity kainate binding sites occurred in the first 2 weeks after birth, coincident with periods of synaptogenesis. We observed a marked transient increase of GluR5 RNAs in the TG, and the increase of GluR5 expression correlated well with both an increased number of kainate-sensitive cells and an increased peak amplitude of kainate-evoked currents. Trigeminal axons, however, already have reached their peripheral and central targets by day E11 (Stainier and Gilbert, 1990; Li et al., 1994); thus, the changes in GluR5 RNA levels we have observed seem not to reflect the formation of synapses or the generation of new neuronal networks during synaptogenesis. The GluR5 subunit may play a role in synapse refinement. In the developing spinal cord (at E18–E21) of the rat, Seebach and Ziskind-Conhaim (1994)showed that a significant percentage of motoneurons initially was innervated by inappropriate primary afferents of antagonistic muscles, but the percentage of functionally inappropriate synapses was reduced within 3–5 d after birth, which was correlated with an increase in the frequency of spontaneous activity and the onset of myelination. Endogenous lectins expressed in both CNS and PNS neurons, which bind to sugar residues of cellular surface glycoproteins and carbohydrate moieties, are believed to play important roles in neuron–neuron or neuron–glia interactions and are good candidate modulators of receptor desensitization in vivo.
Footnotes
This work was supported by the Japanese Ministry of Education, Science, and Culture and the Uehara Memorial Foundation. We thank Drs. Craig Jahr, Hugh Robinson, and Gary Bennett for reading this manuscript.
Correspondence should be addressed to Dr. Yoshinori Sahara, Department of Physiology, Faculty of Dentistry, Tokyo Medical and Dental University, 1-5-45, Yushima, Bunkyo-ku, Tokyo 113, Japan.
Dr. Noro’s present address: Yoshizato Morphomatrix Project, Exploratory Research for Advanced Technology, Japan Science and Technology Corporation, Tokyo, Japan.
REFERENCES
- 1.Agrawl SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard A, Khrestchatisky M. Assessing the extent of RNA editing in the TMII regions of GluR5 and GluR6 kainate receptors during rat brain development. J Neurochem. 1994;62:2057–2060. doi: 10.1046/j.1471-4159.1994.62052057.x. [DOI] [PubMed] [Google Scholar]
- 4.Bettler B, Boulter J, Hermans-Borgmeyer I, O’Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- 5.Bettler B, Egebjerg J, Sharma G, Pecht G, Hermans-Borgmeyer I, Moll C, Stevens CF, Heinemann S. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron. 1992;8:257–265. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- 6.Bochet P, Audinat E, Lambolez B, Crépel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 7.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 8.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 9.Colquhoun D, Jonas P, Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J Physiol (Lond) 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci USA. 1993;90:755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- 12.Geiger JRP, Melcher T, Koh D-S, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 13.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol (Lond) 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- 15.Hollmann M, O’Shea-Greenfield A, Rogers SW, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989;342:643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- 16.Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- 17.Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 18.Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- 19.Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol (Lond) 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol (Lond) 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- 22.Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- 23.Lambolez B, Audinat E, Bochet P, Crépel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 25.Lomeli H, Wisden W, Köhler M, Keinänen K, Sommer B, Seeburg PH. High-affinity kainate and domoate receptors in rat brain. FEBS Lett. 1992;307:139–143. doi: 10.1016/0014-5793(92)80753-4. [DOI] [PubMed] [Google Scholar]
- 26.Niedzielski AS, Wenthold RJ. Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. J Neurosci. 1995;15:2338–2353. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and Concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- 28.Paschen W, Schmitt J, Dux E, Djuricic B. Temporal analysis of the upregulation of GluR5 mRNA editing with age: regional evaluation. Dev Brain Res. 1995;86:359–363. doi: 10.1016/0165-3806(95)00042-c. [DOI] [PubMed] [Google Scholar]
- 29.Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 30.Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- 31.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glia cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 32.Puchalski RB, Louis J-C, Brose N, Traynelis SF, Egebjerg J, Kukekov V, Wenthold RJ, Rogers SW, Lin F, Moran T, Morrison JH, Heinemann SF. Selective RNA editing and subunit assembly of native glutamate receptors. Neuron. 1994;13:131–147. doi: 10.1016/0896-6273(94)90464-2. [DOI] [PubMed] [Google Scholar]
- 33.Robinson HPC, Sahara Y, Kawai N. Nonstationary fluctuation analysis and direct resolution of single channel currents at postsynaptic sites. Biophys J. 1991;59:295–304. doi: 10.1016/S0006-3495(91)82223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruano D, Lambolez B, Rossier J, Paternain AV, Lerma J. Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron. 1995;14:1009–1017. doi: 10.1016/0896-6273(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA, and NMDA receptors are expressed in the rat DRG neurones. NeuroReport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Seebach BS, Ziskind-Conhaim L. Formation of transient inappropriate sensorimotor synapses in developing rat spinal cords. J Neurosci. 1994;14:4520–4528. doi: 10.1523/JNEUROSCI.14-07-04520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer B, Köhler M, Sprengel R, Seeburg SH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 38.Sommer B, Burnashev N, Verdoorn TA, Keinänen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. J Physiol (Lond) 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tölle TR, Berthele A, Zieglgänsberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vyklicky L, Jr, Benveniste M, Mayer ML. Modulation of N-methyl-d-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol (Lond) 1990;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenthold RJ, Trumpy VA, Zhu W-S, Petralia RS. Biochemical and assembly properties of GluR6 and KA2, two members of the kainate receptor family, determined with subunit-specific antibodies. J Biol Chem. 1994;269:1332–1339. [PubMed] [Google Scholar]
- 45.Werner P, Voigt M, Keinänen K, Wisden W, Seeburg PH. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- 46.Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong LA, Mayer ML. Differential modulation by cyclothiazide and Concanavalin A of desensitization at native α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol. 1993;44:504–510. [PubMed] [Google Scholar]