Abstract
Whole-cell patch-clamp recordings were performed together with time-resolved measurements of membrane capacitance (Cm) in nerve terminals acutely dissociated from neurohypophysis of adult rats to investigate modulation of Ca2+ currents and secretion by activation of opioid receptors. Bath superfusion of the κ-opioid agonists U69,593 (0.3–1 μm), dynorphin A (1 μm), or U50,488H (1–3 μm) reversibly suppressed the peak amplitude of Ca2+ currents 32.7 ± 2.7% (in 41 of 56 terminals), 37.4 ± 5.3% (in 5 of 8 terminals), and 33.5 ± 8.1% (in 5 of 10 terminals), respectively. In contrast, tests in 11 terminals revealed no effect of the μ-opioid agonist [d-Pen2,5]-enkephalin (1–3 μm; n = 7) or of the δ-agonist Tyr-d-Ala-Gly-N-Me-Phe-Gly-ol (1 μm; n = 4) on Ca2+currents. Three components of high-threshold current were distinguished on the basis of their sensitivity to blockade by ω-conotoxin GVIA, nicardipine, and ω-conotoxin MVIIC: N-, L-, and P/Q-type current, respectively. Administration of U69,593 inhibited N-type current in these nerve terminals on average 32%, whereas L-type current was reduced 64%, and P/Q-type current was inhibited 28%. Monitoring of changes in Cm in response to brief depolarizing steps revealed that the κ-opioid-induced reductions in N-, L-, or P/Q-type currents were accompanied by attenuations in two kinetically distinct components of Ca2+-dependent exocytotic release. These data provide strong evidence of a functional linkage between blockade of Ca2+ influx through voltage-dependent Ca2+ channels and inhibitory modulation of release by presynaptic opioid receptors in mammalian central nerve endings.
Keywords: κ-opioid receptor, Ca2+ currents, membrane capacitance, secretion, neuroendocrine nerve terminals, patch- clamp
Opiates and endogenous opioid peptides (opioids) produce their analgesic effects primarily by reducing Ca2+-dependent release of neurotransmitters from nerve terminals, thereby disrupting the communication between neurons involved in sensory signaling (Jessell and Iversen, 1977;Macdonald and Nelson, 1978; Grudt and Williams, 1994). Opioids also depress neuropeptide release from the posterior pituitary, acting directly on vasopressin- (AVP) and oxytocin (OT)-containing neurons in the hypothalamus (Wuarin and Dudek, 1990; Renaud et al., 1992; Russell et al., 1995) and through presynaptic receptors located on their neurosecretory endings in the neurohypophysis (Zhao et al., 1988b; Kato et al., 1992). Although it remains unclear how the presynaptic inhibitory effects of opioids are manifested, measurements from neuronal somata suggest that the depression of release may result from reductions in Ca2+ influx through voltage-dependent Ca2+ channels (North, 1993). Activation of μ-, δ-, or κ-opioid receptors has been shown to inhibit somatic N- and P/Q-type Ca2+ currents in several types of neurons (Schroeder et al., 1991; Moises et al., 1994; Rhim and Miller, 1994;Rusin and Moises, 1995) and clonal cells (Seward et al., 1991; Taussig et al., 1992). These same channel types have been implicated to play a pivotal role in mediating Ca2+ influx that triggers depolarization-evoked neurotransmitter release from nerve endings in the periphery (Kongsamut et al., 1989; Toth et al., 1993) and CNS (Takahashi and Momiyama, 1993; Turner et al., 1993; Wheeler et al., 1994). Such data support the idea that presynaptic inhibition by opioids might result, at least in part, from suppression in the activity of exocytotic Ca2+ channels (Illes, 1989;North, 1993). However, this hypothesis awaits direct experimental confirmation in a mammalian neuronal system.
Elucidation of the role of Ca2+ channel modulation in opioid-induced presynaptic inhibition would be accomplished best through direct and simultaneous measurements of the ionic currents and release events in the terminal. However, technical limitations imposed by the size and fragility of mammalian nerve endings have primarily precluded such studies in CNS tissues. Instead, previous studies have relied on indirect measurements to probe the nature of this relationship, comparing opioid effects on intracellular free Ca2+([Ca2+]i), monitored fluorometrically, with changes in K+depolarization-evoked release of neurotransmitters in synaptosome preparations (Xiang et al., 1990) or of neurohormones from dissociated neurosecretomes and isolated neurohypophysis (Dayanithi et al., 1992;Kato et al., 1992). Although some studies report a good correspondence between opioid-induced reductions in evoked release and changes in [Ca2+]i (Dayanithi et al., 1992), these particular approaches lack the temporal resolution to characterize the rapid electrical and exocytotic events involved in excitation–secretion coupling at the terminal and hence afford only limited insight regarding a functional linkage between opioid-induced modulation of Ca2+ influx and release. Moreover, a requirement for precise temporal resolution within the millisecond domain is dictated by the need to ascertain whether an effect of opioids on release results directly from modulation of Ca2+ influx or might reflect alterations in downstream events that influence the exocytotic machinery in a Ca2+-dependent manner (Gillis et al., 1996), subsequent to any imposed changes in [Ca2+]i.
The use of patch-clamp techniques to record Ca2+currents in single neurosecretory endings of the rat neurohypophysis, simultaneously with time-resolved membrane capacitance (Cm) measurements as a monitor of exocytotic activity, enabled us to directly examine the involvement of Ca2+-dependent processes in the mechanism of opioid-induced presynaptic inhibition. The present experiments were designed to identify the specific types of Ca2+channels that serve as targets of inhibitory modulation by opioid receptors in neurohypophysial nerve endings and to decipher the role of this channel regulation in opioid depression of release.
MATERIALS AND METHODS
Preparation of isolated nerve endings. Male rats (150–250 gm) were rendered unconscious with CO2 and decapitated rapidly by guillotine. After isolation of the pituitary, the anterior and posterior lobes were separated by dissection, the isolated neural lobe was collected, and isolated neurohypophysial nerve endings were prepared as described previously (Cazalis et al., 1987;Stuenkel, 1994) by brief homogenization of the neural lobe in 100 μl of buffer containing (in mm): sucrose 270, EGTA 2, and HEPES 10, with pH adjusted to 7.0 with Tris. The resulting homogenate was directly aliquoted onto a glass coverslip forming the bottom of a specially designed superfusion and recording chamber of elliptical shape and 100 μl solution volume. After allowing time for adherence to the chamber bottom, we superfused the nerve endings under laminar flow with a physiological saline (PS) solution containing (in mm): NaCl 140, KHCO3 5, CaCl2 2.2, MgCl2 1, glucose 10, and NaOH-HEPES 10, with pH adjusted to 7.2. For recording of Ca2+ currents andCm responses, the superfusion was changed from PS to one normally consisting of (in mm): TEA 137, CaCl2 10, MgCl2 2, glucose 19, and HEPES 10, with pH adjusted to 7.2 with Tris. All studies were performed on spherical nerve endings having diameters of 5–12 μm to facilitate patch-clamp recording.
Recording of Ca2+ currents. Whole-cell patch-clamp recording techniques (Hamill et al., 1981; Lindau and Neher, 1988) were used to evoke and record Ca2+currents and measure changes in Cm (see below) under voltage clamp from single neurohypophysial nerve endings. Whole-terminal recordings (as appropriately termed) were made at room temperature (23–25°C) using patch pipettes fashioned from 1.5 mm outer diameter capillary glass (WP Instruments), coated to within 100 μm of the tip with Sylgard elastomer, and fire-polished to resistances of 3–8 MΩ. The standard pipette recording solution contained (in mm):N-methyl-d-glucamine 140, HEPES 40, MgATP 2, GTP 0.2, and Tris-EGTA 0.25, adjusted to pH 7.1 and ∼300 mOsm.
Current recordings and Cm measurements were obtained using an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA) that provides compensation circuitry to correct for pipette and whole-cell Cm and series resistance. Electrode capacitance was compensated electronically before transition to whole-terminal recording. Whole-terminal capacitance (0.8–3.5 pF) and 65–80% of the series resistance (3–35 MΩ) were compensated to eliminate membrane-charging transients and voltage and temporal errors, respectively. The evoked Ca2+currents were low-pass filtered (5–10 kHz) before digitization at 5 kHz and corrected for linear leak currents and capacitative transients by digital subtraction of an appropriately scaled current elicited by a series of small hyperpolarizing voltage commands, using a P/N routine. Voltage-clamp protocols, data acquisition, and analyses of current andCm recordings were performed using Pulse Control V4.5 Apple computer-based software routines generously distributed by Dr. Richard Bookman (Herrington et al., 1995).
Capacitance measurements. High-resolution membrane capacitance measurements under whole-terminal patch-clamp recording were used to monitor ΔCm, which directly reflects secretory activity. The fusion of synaptic vesicles or granules to the plasma membrane during exocytosis results in an increase in the total plasma membrane surface area (with corresponding increases in Cm) provided that the rate of exocytosis is much greater than the rate of endocytosis. Time-resolvable changes in Cm were monitored using an adaptation of the phase-tracking method of Fidler and Fernandez (1989). Briefly, changes in whole-terminalCm were measured by adding a 30 mV r.m.s., 1.2 kHz sine wave to the holding potential (usually −90 mV), and the resulting current output signal of the voltage clamp was analyzed at two orthogonal phase angles using a software-based, phase-sensitive detector with high time resolution. Current signals were sampled 16 times per sinusoidal period (19.1 kHz sampling rate), and aCm value was computed every 13.3 msec. TheCm signal is shifted 90° from changes in access resistance (Ra) and membrane conductance (Gm), eliminating interference with the Cm measurement. Imposing defined changes in Ra, by periodically inserting a 500 kΩ resistor between the bath electrode and electrical ground, allowed for immediate calculation by computer of the correct phase angle for the phase-sensitive detector. Lack of projection of the resistor-induced Ra changes in theCm trace ensured correct alignment of the phase-sensitive detector. Calibration of the Cmtrace was obtained by computer-controlled unbalancing of the whole-cell capacitance circuitry of the amplifier to provide a series of 100 fF signals. Data acquisition and the software-based phase-sensitive detector were controlled by software developed for the Apple Macintosh computer (Pulse Control 4.5) and generously distributed by Dr. Richard Bookman (Herrington et al., 1995).
Delivery of test substances. External solution was continuously passed at a rate of 1–1.5 ml/min through the recording chamber by means of a gravity-fed delivery system. For combined measurements of Ca2+ current andCm, rapid change of solution in the recording chamber (100 μl total volume) was accomplished through use of a manually controlled, eight port distribution valve connected at the chamber inflow to a series of solution reservoirs. The perfusion system maintains a constant bath volume, which is essential for accurate Cm measurements, and the presence of baffles on the chamber influx and efflux ports results in the generation of a laminar flow across individual nerve endings. Tyr-d-Ala-Gly-N-Me-Phe-Gly-ol (DAMGO), [d-Pen2,5]-enkephalin (DPDPE), and dynorphin A (all obtained from Peninsula Laboratories) were prepared as 1 mm stock solutions in sterile water, partitioned into 10 μl aliquots, lyophilized, and stored at −20°C. ω-Conotoxin GVIA (GVIA) (Peninsula Laboratories) and ω-conotoxin MVIIC (MVIIC) (Bachem California) were prepared as 500 μm and 1 mmstock solutions, respectively, and stored in the same manner. On the day of the experiment, aliquots of the above compounds and freshly weighed amounts of nicardipine (Sigma, St. Louis, MO), U69,593, U50,488H, naloxone, and nor-binaltorphimine (nor-BNI) (all obtained from Research Biochemicals, Natick, MA) were dissolved in the extracellular bathing solution to the desired concentrations. Nicardipine was held in a light-proof container and routinely applied to the recording chamber under restricted light conditions to minimize degradation caused by exposure to light. For all experiments involving use of Ca2+ channel blockers, only one nerve ending was examined from each dish in which recordings were obtained.
Statistical comparisons. Statistical comparisons were performed using either a paired or unpaired Student’s ttest. Values are given as mean ± SEM, unless otherwise indicated.
RESULTS
Opioid regulation of Ca2+ currents in neurohypophysial nerve terminals
Whole-terminal recordings (n = 67) were obtained using bath and pipette solutions of composition that suppressed K+ and Na+ conductances, with Ca2+ (10 mm) as the charge carrier. Step depolarizations of 5–200 msec were made to various test potentials from a holding potential (Vh) of −90 mV to evoke Ca2+ currents and associated increases inCm (see below). The Ca2+currents that we recorded consisted of inactivating and sustained components of high-voltage activated current, with a threshold for activation of −40 mV and peak amplitudes obtained at +10 mV, but contained no rapidly inactivating low-threshold T-type component. The amplitude of the Ca2+ currents, measured at the peak of the current–voltage curve, averaged 128.9 ± 10.9 pA (n = 67), yielding a mean current density of 71.8 ± 7.2 A/F for these nerve endings. Overall, the Ca2+ currents we measured corresponded closely to those described previously in acutely isolated rat neurohypophysial nerve endings (Lemos et al., 1994; Stuenkel, 1994) with regard to their amplitudes, voltage-dependent activation, and kinetic properties.
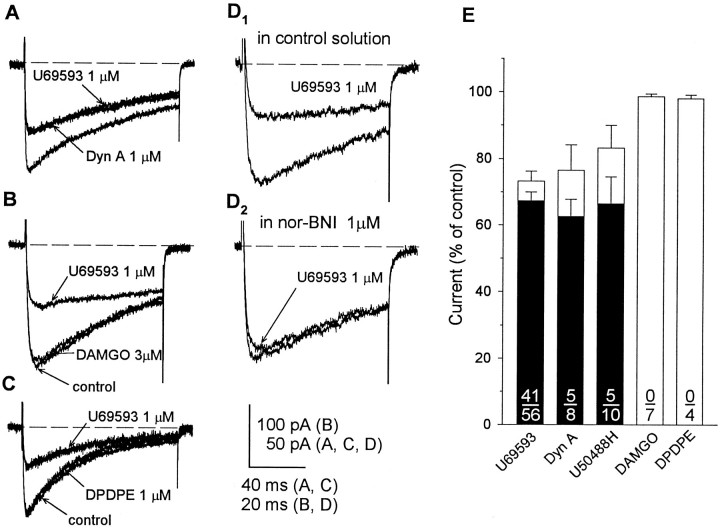
Binding studies and immunohistochemical localization of mRNA coding for the various opioid receptor subtypes indicate that opioid receptors on the nerve endings of rat neurohypophysial magnocellular secretory neurons are predominantly, if not exclusively, of the κ-type (Herkenham et al., 1986; Sumner et al., 1990; Mansour et al., 1995). Guided by these considerations, we first looked for regulation of the evoked Ca2+ current by agonists acting at κ-opioid receptors. Bath superfusion of the κ-opioid agonists dynorphin A (1 μm), U50,488H (1–3 μm), or U69,593 (0.3–1 μm) reversibly suppressed the peak amplitude of Ca2+ currents in 45 of 67 terminals examined. The inhibitory response to a particular agonist varied considerably among the group of nerve endings that showed κ-opioid sensitivity. For example, the maximal response to U69,593 ranged from 9 to 66% inhibition of the control current, while exceeding 50% suppression in Ca2+ current in approximately one-third (12) of 41 responsive nerve endings. However, comparisons of the effects of U69,593, dynorphin A (n = 5), and U50,488H (n = 2) in the same nerve ending revealed equivalent inhibitory responses to these agonists (Fig.1A). Moreover, when the data were pooled for a particular agonist, no significant differences were observed in the maximal inhibition of Ca2+current produced by U69,593 (32.7 ± 2.7%; 41 of 56 terminals), dynorphin A (37.4 ± 5.3%; 5 of 8 terminals), and U50,488H (33.5 ± 8.1%; 5 of 10 terminals). Although the predominant effect of the κ-opioid agonists was to decrease a rapidly inactivating current (τ, 142 ± 6 msec; n = 10), more-sustained components were also reduced in many of the terminals. We simplified the interpretation of experimental outcomes by using U69,593 for assessing κ-opioid regulation of Ca2+currents and Cm responses (see below), avoiding potential problems associated with the susceptibility of dynorphin peptides to breakdown and cross-reactivity with multiple opioid receptor subtypes.
Fig. 1.
Opioid-induced inhibition of Ca2+ currents mediated via κ-opioid receptors.A–C, Recordings from three different terminals of whole-terminal Ca2+ currents evoked by a 100 msec (A, C) or 50 msec (B) step to +10 mV from −90 mV before the application and in the presence of selective κ-opioid (dynorphin A, 1 μm; U69,593, 1 μm), δ-opioid (DPDPE, 1 μm), or μ-opioid (DAMGO, 3 μm) agonists. Only the κ-opioid agonists reversibly inhibited Ca2+ currents. D1,D2, Recordings from another terminal showing that this effect of U69,593 was blocked by the κ-selective antagonist nor-BNI (1 μm). E, Comparison of effects of κ-, μ-, and δ-opioid-selective drugs on Ca2+ current. Open bars represent the mean ± SEM of the values obtained in the presence of a particular agonist (number of terminals shown in denominator); values are expressed as a percentage of the predrug control current. Current amplitudes were reduced significantly (p < 0.05) by U69,593, dynorphin A, and U50,488H but not by DAMGO and DPDPE. Filled barsrepresent only the data obtained from opioid-responsive terminals (number shown in numerator) and demonstrate equivalent inhibitory efficacy of the three κ-opioid agonists examined.
In a separate set of experiments on 11 nerve terminals, the effects of administration of agonists selective for either μ- or δ-opioid receptors on Ca2+ currents were compared with those produced by a subsequent application of U69,593 (0.3–1 μm) to determine whether opioid-induced suppression of these currents resulted specifically from the activation of κ-opioid receptors. These tests revealed no effect of the μ-opioid-selective agonist DAMGO (1–3 μm; n = 7; Fig.1B) or of the δ-agonist DPDPE (1 μm;n = 4; Fig. 1C) on Ca2+currents, although reductions in current by the κ-opioid were registered in more than half (n = 8) of the same preparations. The graph depicted in Figure 1Esummarizes the results of all experiments in which we tested for regulation of nerve terminal Ca2+ currents by agonists having selectivity for different subtypes of opioid receptors. The inhibitory effects of the five κ-, μ-, or δ-opioid-selective agonists tested were compared by plotting the averaged amount of Ca2+ current evoked in the presence of a particular agonist (represented by open bars), expressed as a percentage of the predrug control current. Current amplitudes were reduced significantly (p < 0.05) by U69,593, dynorphin A, and U50,488H but not by DAMGO and DPDPE. Furthermore, recomputation of the effects of each κ-opioid agonist using only those data obtained from opioid-responsive terminals (filled bars) demonstrated equivalent inhibitory efficacy of the three κ-opioids examined. In additional experiments, inhibitory responses to U69,593 were tested for sensitivity to blockade by naloxone or nor-BNI, a κ-opioid receptor-selective antagonist (Portoghese et al., 1987), to confirm their mediation via activation of the corresponding subtype of opioid receptor. In all eight nerve endings examined, the ability of U69,593 to inhibit the evoked Ca2+ current was blocked by coadministration of naloxone (1 μm; n = 4) or nor-BNI (1 μm; n = 4; Fig.1D1,D2). These data, when taken together, indicate that only the κ-subtype of opioid receptors is functionally coupled to Ca2+channels in rat neurohypophysial nerve terminals.
Identification of high-threshold currents sensitive to κ-opioids
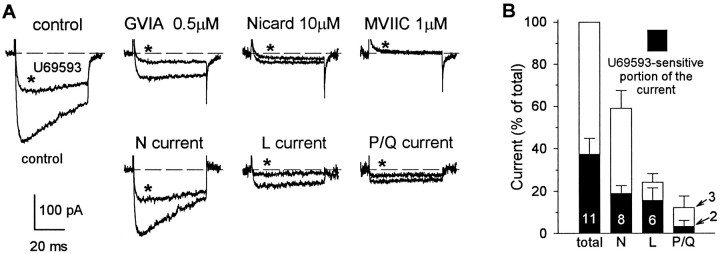
To identify the types of high-threshold Ca2+channels that are modulated by presynaptic κ-opioid receptors, we examined the ability of U69,593 to reduce Ca2+current before and after selective blockade of N-, L-, and P/Q-type current components (n = 11). The traces in Figure 2A illustrate the inhibitory effect of U69,593 (1 μm) on Ca2+ currents evoked by 50 msec steps to +10 mV from a Vh of −90 mV. Application of U69,593 (for 1 min) reduced the peak amplitude of the Ca2+ current from 260 to 110 pA (150 pA), and this effect was reversed after washout of the opioid. After the current recovered from the inhibition by U69,593, GVIA (0.5 μm) was administered for 40 sec. GVIA irreversibly suppressed the peak amplitude of the control current from 260 to 75 pA (185 pA, representing 71.2% of the control current), and after GVIA the fraction of current that was inhibited by U69,593 was reduced from 150 to 48 pA (representing a 68% blockade). The application of nicardipine (10 μm) subsequent to the establishment of the irreversible blockade by GVIA reduced peak current amplitude further from 75 to 29 pA (equivalent to 17.7% of the control current), and this was associated with a further reduction in the inhibitory response to U69,593 (from 48 to 17 pA). Administration of MVIIC (1 μm) in the presence of nicardipine abolished whole-cell current and any effect of U69,593 that remained after nicardipine. The pairs of traces depicted in Figure2A (lower) were obtained by subtraction of corresponding currents evoked before and after application of antagonists and illustrate sensitivity to U69,593 of pharmacologically distinguished N-, L-, and P/Q-type current components. Overall, in 3 of 11 terminals (28.9%) tested, Ca2+ currents showed sensitivity to blockade by selective Ca2+ channel blockers similar to that illustrated for the nerve terminal in Figure2. The majority of terminals that were studied (5 of 11, 45.5%) possessed only N- and L-type Ca2+ channels, whereas the remaining 3 terminals expressed L- and P/Q-type but not N-type channels. It should be noted, however, that all three pharmacologically distinct current components demonstrated sensitivity to κ-opioids. These data suggest that κ-opioid receptors are negatively coupled to all types of Ca2+ channels in rat neurohypophysial nerve terminals, including a GVIA-sensitive (N-type) channel, a nicardipine-sensitive (L-type) channel, and a MVIIC-sensitive (P/Q-type) channel.
Fig. 2.
κ-Opioids inhibited GVIA-sensitive, nicardipine-sensitive, and MVIIC-sensitive Ca2+current components. A, upper, Recordings from a single nerve terminal show the effects of U69,593 (traces marked with asterisks) on Ca2+ currents evoked by 50 msec steps to +10 mV from a holding potential of −90 mV before the application and in the presence of Ca2+ channel-type selective blockers. Sequential administration of GVIA (0.5 μm), nicardipine (10 μm), and MVIIC (1 μm) completely abolished whole-terminal current. The ability of U69,593 to suppress Ca2+ current was greatly reduced after application of each of these blockers. A, lower, Subtraction of the corresponding currents evoked before and after application of antagonists yielded pharmacologically distinguished N-, L-, and P/Q-type current components in control conditions and in the presence of U69,593. B, Relative contribution of pharmacologically distinguished N-, L-, and P/Q-type current components to the total whole-terminal current and their sensitivity to the inhibitory effect of U69,593. Bars in the graph represent the mean ± SEM of the normalized current amplitudes obtained from the number of terminals shown, examined using the experimental protocol depicted in A.
To quantify the relative contribution of individual current components to the total whole-terminal current and its κ-opioid-sensitive portion, we averaged measurements of current amplitude blocked by each antagonist that were obtained from the terminals examined using the same protocol as described for the experiment in Figure2A. This analysis revealed that 59 ± 8% (n = 8) of high-threshold Ca2+current was contributed through GVIA-sensitive N-type channels, 25 ± 4% (n = 6) through dihydropyridine-sensitive L-type channels, and 13 ± 5% (n = 3) through pharmacologically defined P/Q-type channels. Administration of U69,593 inhibited N-type current on average ∼32%, whereas nicardipine-sensitive current (L-type) was reduced ∼64%, and MVIIC-sensitive (P/Q-type) current was inhibited ∼28% (Fig.2B).
Opioid regulation of Ca2+-dependent exocytosis in nerve terminals
The changes in Cm that are measured in response to brief depolarizations reflect increases in surface membrane area caused by the fusion of vesicles (provided that the rate of exocytosis exceeds the rate of endocytosis), and these depend not only on Ca2+ entry (Fig. 3) and buffering but also on the pattern and duration of stimulation (Lindau and Neher, 1988; Lim et al., 1990; Horrigan and Bookman, 1994;Giovannucci and Stuenkel, 1997). Single-step depolarizations to potentials that yielded maximal inward Ca2+ currents were associated with Cm responses in virtually all nerve endings examined, and the evoked increases inCm measured in 43 of these were sufficiently stable to allow systematic examination of their modulation by κ-opioids (see below). Despite variation in the depolarization-evokedCm increases that were measured among individual nerve endings, each Cm response was resolvable into several kinetically and functionally distinct components, as has been described in detail in an earlier report (Giovannucci and Stuenkel, 1997). Briefly, depolarizations of single nerve endings achieved with 5 msec steps produced a transientCm increase that was independent of Ca2+ entry. It was shown for these peptidergic nerve endings that this transient Cm signal does not represent Ca2+-dependent exocytotic activity and is most likely related to a charge redistribution within the membrane (Giovannucci and Stuenkel, 1997). Depolarizations of longer duration (50–200 msec) to between 0 and +30 mV produced more-prolonged Ca2+-dependent increases inCm that could be resolved into two kinetically distinct phases (Fig. 3A): an immediate step-like jump (seen in all 43 terminals) reflecting fusion of an immediately releasable pool of “predocked vesicles” followed by a larger, slowCm increase of several seconds duration indicative of fusion and release from a distinct, larger readily releasable pool of vesicles (Lindau et al., 1992; Horrigan and Bookman, 1994; Hsu and Jackson, 1996; Giovannucci and Stuenkel, 1997). This latter component of Ca2+-dependent exocytosis was observed in 19 of the 43 nerve terminals studied. The presence of the transient Cm change together with slower Ca2+-dependent Cm components obfuscates the correct estimation of the immediate step-like exocytoticCm jump. Therefore, the transient ΔCm evoked by a 5 msec step was digitally subtracted from the Cm changes evoked with longer depolarizations to yield a measure of Ca2+-dependent exocytosis. All data described subsequently were obtained from transient ΔCm-subtracted records.
Fig. 3.
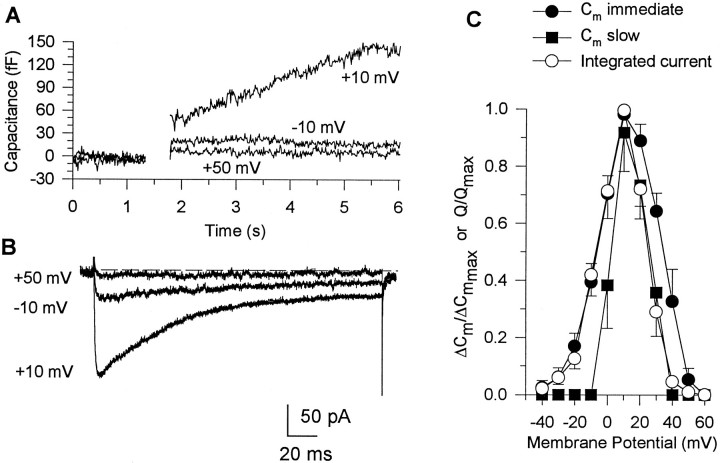
Comparison of current–voltage and ΔCm–voltage relationships determined for single nerve endings. Cm responses (A) and corresponding Ca2+currents (B) evoked in a single terminal by 200 msec step depolarizations to different command potentials (shown near corresponding traces) from a holding potential of −90 mV. The depolarizations to −10 and +50 mV produced small currents that were accompanied by an immediate, step-likeCm increase, whereas steps to +10 mV evoked a larger current and prolonged Cm increase lasting for several seconds. Ca2+-dependentCm responses were corrected for transient ΔCm artifacts (as explained in text) by subtracting the response to a 5 msec depolarization aligned with the pulse. C. Integrated Ca2+ currents (open circles; n = 8) and corresponding immediate (filled circles;n = 8) and slow (filled squares; n = 6)Cm responses plotted against command potential at which they were evoked.
The properties of the immediate and slow Cmresponses measured here, as illustrated in Figure 3A, for example, were similar to those described for these peptidergic nerve endings in previous studies (Lim et al., 1990; Seward et al., 1995; Hsu and Jackson, 1996; Giovannucci and Stuenkel, 1997). In contrast to the transient ΔCm, both the immediate jump and slowly increasing Cm responses were markedly altered by manipulations that resulted in attenuation of depolarization-evoked Ca2+ entry. Administration of Cd2+ (100 μm) to the external medium was found to abolish the inward Ca2+ currents and any corresponding immediate or slow Cmresponses, without appreciable change in the transientCm components [n = 2; see alsoGiovannucci and Stuenkel (1997), their Fig. 3A]. Conversely, depolarization-evoked inward currents were greatly enhanced, whereas both immediate and slow Cmresponses were reduced when Ba2+ (10 mm) was substituted for external Ca2+ (n= 2). These results support the notion that the immediate step and slowly increasing Cm changes reflect exocytotic activity that is dependent on Ca2+ influx. In additional experiments (n = 8), the dependence of theseCm responses on Ca2+ entry was examined further by comparing the relationships of the magnitude of the evoked Ca2+ current and associated changes inCm (measured in the same nerve ending) as a function of the step depolarization (Fig. 3). Depolarizing pulses of 200 msec duration were applied in a random order to membrane potentials between −40 and +60 mV from a Vh of −90 mV, and all changes in Cm were allowed to return to baseline (typically requiring 60–90 sec) before we initiated the next depolarization. To facilitate comparisons between current–voltage andCm–voltage relationships, we normalized the magnitude of the time-integrated Ca2+ currents and the amplitudes of immediate and slow Cmresponses measured in a given nerve ending to the corresponding maximal responses obtained, and the results from all experiments were pooled, yielding the plots shown in Figure 3C. The inward Ca2+ current began to activate at −30 mV and reached a maximum at +10 mV, at which point the time-integrated Ca2+ current averaged 12.0 ± 2.3 pC (n = 8). The corresponding immediate step increases inCm (n = 8) closely followed the current–voltage profile and also reached a maximal value (18.4 ± 5.0 fF) at +10 mV (Fig. 3C). When steps were made to potentials ≥ 0 mV (Figs.3A,C), a slowly increasing ΔCm was observed after the immediateCm response in six of the terminals. With a higher threshold, the plot of the slow Cmincrease as a function of test potential showed a rightward shift compared with the voltage relationships of the evoked Ca2+ and immediate Cmresponses but also peaked at +10 mV (57.6 ± 17.8 fF). On the other hand, voltage steps to test potentials positive to +50 mV evoked no inward currents and failed to induce immediate or slowCm responses in any of the nerve endings (Fig.3C). These results support the conclusion that both the immediate and slow Cm responses measured here are tightly coupled to elevations in [Ca2+]i produced by influx through voltage-sensitive Ca2+ channels. Nevertheless, the normalized amplitude of the immediate Cmincrease exceeded that of the slow Cm response at all test potentials, suggesting that the initial component of exocytotic release may have a different requirement for triggering by elevations in [Ca2+]i than the slow phase of release (Lim et al., 1990; Lindau et al., 1992; Seward et al., 1995; Hsu and Jackson, 1996; Giovannucci and Stuenkel, 1997).
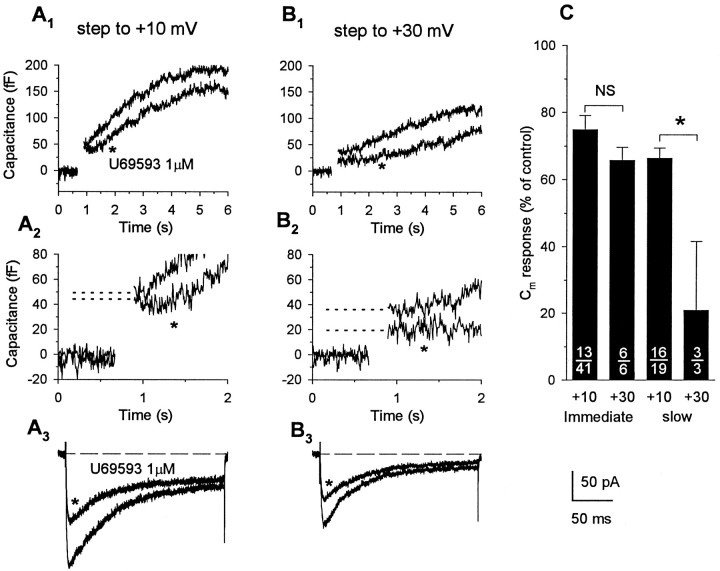
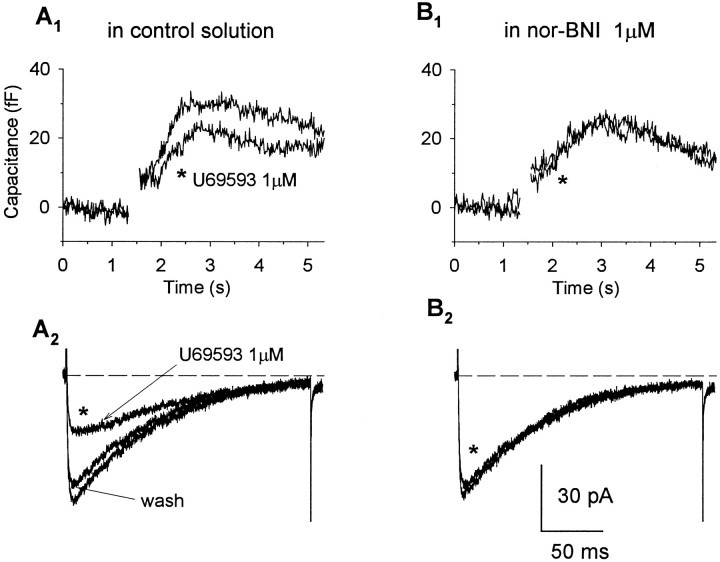
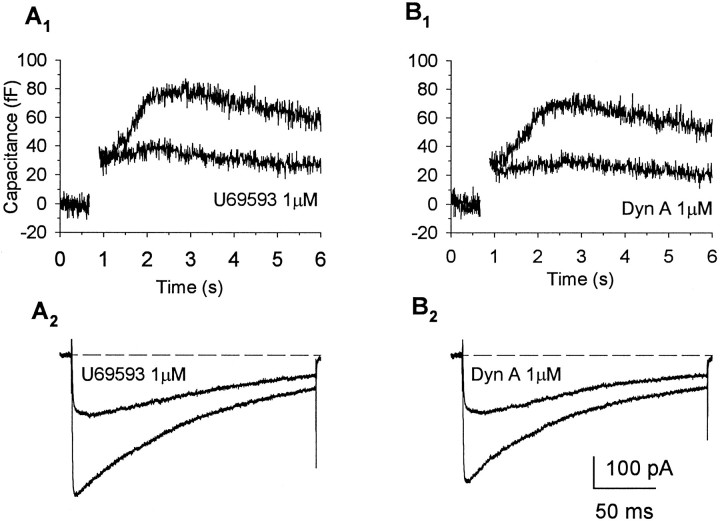
To determine whether the activation of κ-opioid receptors had an effect on Ca2+-dependent exocytosis, we tested the ability of U69,593 (1 μm) and dynorphin A (1 μm), administered at concentrations near-maximal for Ca2+ current inhibition, to modulate depolarization-evoked immediate (n = 43) and slow (n = 19) increases in Cm. The effects of U69,593 were examined in 41 individual nerve endings, of which 19 exhibited a slowly increasing phase ofCm in addition to the immediate stepCm response. The traces in Figure4 show Ca2+ currents (A3) and correspondingCm responses (A1,A2) elicited by a 200 msec step to +10 mV from a Vh of −90 mV. Bath application of U69,593 (1 μm; for 30 sec) decreased the peak amplitude of the Ca2+ current from 193 to 121 pA (Fig.4A3), resulting in a 32% reduction in the depolarization-evoked Ca2+ influx (from 18.5 to 12.6 pC). The immediate step-like increase inCm was only slightly attenuated (9%, from 49 to 44 fF) by κ-opioid administration in this terminal (Fig.4A2); however, the magnitude of the subsequent slowly increasing phase of Cm was appreciably reduced (19%, from 193 to 157 fF; Fig.4A1). In three additional terminals in which we used the same protocol to examine for mediation of agonist effects via κ-opioid receptors, both the inhibition in evoked Ca2+ current and corresponding attenuation in immediate or slow Cm responses produced by U69,593 (1 μm) were blocked reversibly by administration of nor-BNI (1 μm; Fig. 5). Overall, the administration of U69,593 reduced immediate step increases in Cm in 13 of 41 nerve endings examined (25.2 ± 4.2%; range, 8–40%), when measurements were obtained using step depolarizations to test potentials yielding maximal Ca2+ currents (Fig. 4C). Under these condition, an inhibition in the evoked Ca2+ current was observed in 31 of the nerve terminals (30.1 ± 3.0%; range, 9–67%), including 11 of those in which reductions in the immediateCm response were registered (26.2 ± 5.0%; range, 8–62%). In contrast, administration of the κ-opioid agonist reduced the slowly increasing Cm responses evoked by depolarizing steps to the peak of the current–voltage curve in 16 of the 19 terminals that showed this additional exocytotic component. In 14 of these 16 terminals, U69,593 reduced the evoked Ca2+ current by an average of 33.7 ± 5.1% (range, 9–66%), a value close to the mean reduction observed in the peak amplitude of the slow Cm response (33.0 ± 3.5%; range, 6–47%). Interestingly, in the other two terminals, opioid-induced reductions in the slowCm response (and in the immediate step increases that preceded them) were observed without appreciable changes in the Ca2+ current. Application of U69,593 had no effect on either the evoked Ca2+ currents or the associated slow (and immediate) Cm responses in the remaining three terminals. Tests with dynorphin A (1 μm) in seven terminals, including five that were screened initially with U69,593, revealed a similar profile of inhibitory effects on depolarization-evoked Ca2+ currents and the corresponding immediate and slow Cm responses. Administration of the κ-opioid peptide routinely suppressed inward currents (five of seven terminals) and the associated slowly increasing phase of Cm (three of three terminals), whereas significant inhibitory effects on immediateCm responses were observed in only a single terminal. Direct comparisons of the effects of U69,593 and dynorphin A in the same nerve ending (n = 5) demonstrated nearly equivalent inhibitory responses to these agonists (Fig.6). Moreover, when the data were pooled for all terminals that responded to a particular agonist, no significant differences were observed in the maximal inhibition of Ca2+ current produced by U69,593 (30.1 ± 3.0%; n = 31) and dynorphin (37.4 ± 5.3%;n = 5) and in agonist-induced attenuation of the slowCm response produced by U69,593 (33.7 ± 3.1%; n = 16) and dynorphin (37.9 ± 4.1%;n = 3).
Fig. 4.
Activation of κ-opioid receptors attenuates immediate and slow Cm responses evoked by steps inducing smaller Ca2+ influx.Cm increases (A1,A2, B1,B2) and corresponding Ca2+currents (A3, B3) recorded in response to 200 msec depolarizations to +10 mV (A) and +30 mV (B) from a holding potential of −90 mV. Administration of U69,593 appreciably reduced Ca2+ influx (32.1%, from 18.53 to 12.58 pC) evoked by depolarization to +10 mV but had little effect on the corresponding immediate Cm response (9% reduction, from 49 to 44 fF, indicated by dotted lines). Depolarization to +30 mV induced much smaller Ca2+influx (9.1 pC) that was attenuated further by activation of κ-opioid receptors (to 6.23 pC, representing 31.5% blockade). Under this condition of reduced driving force for Ca2+ influx, inhibition of the immediate Cm response by the κ-opioid agonist was markedly increased (47%, from 36 to 19 fF). Opioid administration also reduced the slowly increasingCm component recorded in this nerve ending, and this effect of U69,593 was greater on the response measured at +30 mV (33.9%, from 118 to 78 fF) compared with that recorded at +10 mV (17.7%, from 192 to 158 fF). C, Bar graph summarizes the effects of U69,593 on the immediate step and slowly increasing components of Cm responses. Bars represent the mean ± SEM of the normalized Cmresponses pooled from κ-opioid-responsive terminals (number shown in numerator, out of the total number of terminals examined, shown in denominator) for immediate and slowCm responses evoked at command potentials of +10 or +30 mV, expressed as a percent of the corresponding predrug control values. Reduction of depolarization-evoked Ca2+ influx to submaximal levels resulted in an increased probability for modulation of the immediateCm response by κ-opioid receptor activation from 32% (13 of 41) at +10 mV to 100% (6 of 6) at +30 mV, without significantly (p = 0.19,NS indicates not significant) altering the magnitude of the inhibitory effect of U69,593 on these initial exocytotic events. In contrast, the inhibitory effect of U69,593 on the slowCm responses was significantly increased under conditions in which the driving force for Ca2+influx and the net amount of depolarization-evoked Ca2+ influx was reduced (asteriskindicates p < 0.001).
Fig. 5.
Activation of κ-opioid receptors attenuates depolarization-evoked exocytotic Cmresponses in neurohypophysial nerve endings in nor-BNI-sensitive manner. Cm increases (A1, B1) and corresponding Ca2+ currents (A2, B2) recorded in response to 200 msec depolarizations to +10 mV from a holding potential of −90 mV in control solution (A) and in the presence of the selective κ-opioid antagonist nor-BNI (B). Administration of U69,593 reduced the evoked Ca2+ current and accompanying slowly increasingCm response, and these effects were prevented after blockade of κ-opioid receptors by nor-BNI (1 μm).
Fig. 6.
Comparison of effects of U69,593 and dynorphin A demonstrates the similar efficacy of the two κ-opioid agonists in inhibiting Ca2+ currents and slowCm responses. Cmincreases (A1, B1) and corresponding Ca2+ currents (A2, B2) recorded in the same nerve terminal in response to 200 msec depolarizations to +10 mV from a holding potential of −90 mV in control solution and in the presence of U69,593 (A) and dynorphin A (B). Administration of U69,593 reduced the evoked Ca2+ current and accompanying slowly increasingCm response to a degree similar to that seen with dynorphin A.
Despite a routine suppression of the slow phase of exocytosis, activation of κ-opioid receptors had variable and inconsistent effects on the immediate step-like increases inCm measured in response to depolarizations that yielded maximal Ca2+ currents. Given the close correlation observed between the opioid-induced inhibition in evoked Ca2+ currents and in slow Cmresponses, what might account for the lack of correspondence between changes in Ca2+ currents and the immediateCm increases that constitute the initial secretory event? One possibility is that such an outcome might derive from fundamental differences in the dependency of the initiation and maintenance of vesicular exocytosis on increases in Ca2+ concentration within functionally distinct intraterminal domains (Lindau et al., 1992; Horrigan and Bookman, 1994;Hsu and Jackson, 1996; but see Seward et al., 1995). We hypothesized that if the immediate Cm response reflects exocytosis of predocked vesicles (Lindau et al., 1992; Horrigan and Bookman, 1994), then activation of Ca2+ channels by depolarizations to the peak of the current–voltage curve may increase submembrane Ca2+ concentration within spatially restricted domains close to the release sites beyond the level of saturation for vesicle fusion. Under these conditions, a modest reduction in the evoked Ca2+ current by κ-opioids might not decrease submembrane concentrations of Ca2+ near the release sites below the level for saturation and, therefore, would have little if any effect on the immediate Cm response. To test this hypothesis, we examined the effects of U69,593 on Ca2+ currents and Cm responses that were evoked by stepping to more positive command potentials (+30 mV) closer toECa, thereby reducing the driving force for Ca2+ influx (Fig. 4B). We relied on this approach to manipulate the magnitude of Ca2+ entry induced by depolarization rather than to modify influx by altering [Ca2+]obecause the latter manipulations induce changes in basal levels of [Ca2+]i in these nerve endings with dramatic effects on the functional readiness of different vesicular pools (Stuenkel, 1994), thereby precluding valid comparisons between values obtained under control and experimental conditions. With delivery of single depolarizing steps to +30 mV, the amplitudes of the evoked currents and associated immediate and slowCm responses were reduced on average 45.8 ± 10.5% (n = 6), 20.7 ± 5.7% (n = 6), and 45.8 ± 6.4% (n = 3), respectively, compared with the corresponding values measured in the same terminals after depolarizations to +10 mV. Under this test condition, all response parameters recorded in each nerve terminal were inhibited by the κ-opioid receptor agonist, including the immediate step Cm increases in three terminals that did not show modulation by the κ-opioid when evoked by steps to +10 mV. For this entire subgroup of nerve terminals (n = 6), no significant difference was observed between the inhibitory effect of U69,593 on the peak Ca2+ currents evoked at +10 and +30 mV (34.2 ± 4.0% and 29.5 ± 5.2%, respectively), nor was there any difference in the extent of inhibition of the noninactivating current component measured at the end of the 200 msec pulse (32.2 ± 4.7% and 34.3 ± 4.4% at +10 and +30 mV, respectively). However, the U69,593-induced inhibition of the immediateCm increase tended to be greater for responses evoked at +30 mV (34.3 ± 3.9%) compared with responses evoked at +10 mV (14.1 ± 9.9%) and was comparable in magnitude with the κ-opioid inhibition of slow Cm increases (33.7 ± 3.1%; n = 16) measured in response to depolarizations (+10 mV) that yielded maximal Ca2+currents. Similarly, under the condition of reduced Ca2+ driving force, the inhibitory effect of U69,593 on the slow Cm response was markedly increased in two of three nerve terminals that exhibited this additional component of Ca2+-dependent exocytosis. Figure4C provides a graphical comparison of the inhibitory effects of U69,593 on the immediate step and slowly increasingCm responses evoked by depolarizations to +10 or +30 mV, expressed as a percent of the corresponding predrug control responses. The height of each bar represents the opioid effect computed by pooling results obtained only from nerve terminals that showed modulation of the particular Cm response. The principal effect of reducing depolarization-evoked Ca2+ influx to submaximal levels was to increase the probability for modulation of the immediate Cmresponse by activation of κ-opioid receptors (from 32 to 100%, Fig.4C), without significantly altering the magnitude of the inhibitory effect of U69,593 on the initial exocytotic events evoked by depolarizing steps yielding peak inward currents compared with submaximal Ca2+ influx (25.2 ± 4.2% at +10 mV compared with 34.3 ± 3.9% at +30 mV; p = 0.19). An additional effect of this manipulation was to significantly increase the extent to which the slowly increasing Cmcomponent can be inhibited by κ-opioid receptor activation (79.2 ± 20.8% at +30 mV compared with 33.7 ± 3.1% at +10 mV;p < 0.001). Taken together, the results of these experiments suggest that activation of κ-opioid receptors on the endings of rat neurohypophysial magnocellular neurons reduces the secretion of the neurohormones AVP and OT by modulating several kinetically distinct components of exocytotic vesicular release.
DISCUSSION
This work provides the first direct examination of the relationship between opioid-induced suppression of Ca2+ influx through voltage-gated Ca2+ channels and inhibitory modulation of neurotransmitter or neuropeptide release in mammalian nerve terminals. This became possible because of the development of an anatomically unique preparation of neurosecretory terminals from the rat neurohypophysis. These nerve endings maintain cytoplasmic constituents, release neuropeptides in a Ca2+-dependent manner, and respond to receptor-mediated modulatory influences (Cazalis et al., 1987; Nordmann et al., 1987).
Opioids have been shown to act at κ- and, possibly, μ-opioid receptors (Zhao et al., 1988a) to inhibit depolarization-evoked release of AVP and OT from isolated rat neural lobes and single neurohypophysial nerve terminals (Zhao et al., 1988b; Dayanithi et al., 1992; Kato et al., 1992). In some reports, κ-opioid agonists and nonselective opioids (etorphine) also reduced the associated rise in [Ca2+]i, measured with Fura-2, but the relationship between the changes in intraterminal [Ca2+]i and inhibition of K+-stimulated release of neuropeptides from isolated endings or intact neural lobes showed only a weak correlation (Dayanithi et al., 1992; Kato et al., 1992). To clarify the role of Ca2+-dependent processes in opioid-induced inhibition of release, we examined the effects of μ-, δ-, and κ-opioid-selective agonists on Ca2+ currents in single neurohypophysial nerve terminals using the whole-terminal recording configuration, while simultaneously monitoring changes in whole-terminal Cm as an assay of Ca2+-triggered exocytotic release (Lindau and Neher, 1988; Lim et al., 1990).
Inhibition of Ca2+ current by the κ-opioid agonists dynorphin A, U69,593, or U50,488H was observed in 67% of neurohypophysial nerve terminals, and these responses were blocked by naloxone and the κ-selective antagonist nor-BNI. Ca2+ currents were unaffected by either μ- or δ-opioid agonists, although reductions in current by κ-opioids were registered in most (8 of 11) of the same preparations. Thus, only κ-opioid receptors are negatively coupled to Ca2+channels in these neurosecretory endings.
The Ca2+ currents we recorded were high-threshold and consisted of both inactivating and sustained components, but these currents contained no rapidly inactivating low-threshold T-type current. Our findings that GVIA irreversibly blocked ∼59%, nicardipine suppressed 25%, and MVIIC (applied subsequent to GVIA and in the presence of nicardipine) blocked 13% of the total current are consistent with earlier reports that such terminals only express N-, L-, and Q-type channels (Wang et al., 1993; Lemos et al., 1994). However, only one-fourth of the terminals examined demonstrated this profile of sensitivity to Ca2+ channel blockers, whereas approximately one-half seemed to express only N- and L-type Ca2+ channels, and the remainder possessed only L- and P/Q-type channels.
Somatic recordings obtained from rat peripheral sensory (Schroeder et al., 1991; Moises et al., 1994; Rusin and Moises, 1995) and nucleus tractus solitarius neurons (Rhim and Miller, 1994) have identified several high-threshold Ca2+ channels that are modulated by opioid receptors. In these neurons, μ-opioids, and κ-selective agonists to a lesser extent, inhibit Ca2+ current contributed by GVIA-sensitive N-type and pharmacologically distinct P- and Q-type channels but spare L- and T-type currents, thereby regulating the principal Ca2+ channel types involved in exocytosis at central synapses and peripheral sites of release (Luebke et al., 1993;Takahashi and Momiyama, 1993; Wheeler et al., 1994; Dunlap et al., 1995). Studies in cortical synaptosomes suggest a similar pattern of coupling of presynaptic opioid receptors to Ca2+channels, in that here both N- and L-type channels contribute to depolarization-evoked Ca2+ entry, whereas the inhibitory effects of κ-opioid agonists on [Ca2+]i and exocytosis are blocked by GVIA but not by dihydropyridines (Adamson et al., 1989; Xiang et al., 1990). However, L-type channels have been shown in chromaffin cells to be more efficiently coupled to exocytosis than N- and P-type channels (Artalejo et al., 1994) and similarly play an important role in neurohormone release in rat neurosecretory endings (Stuenkel and Nordmann, 1993). It is not surprising, therefore, that L channels are the target for G-protein modulation in melanotrophs from rat pituitary gland (Ciranna et al., 1996) and that they are most sensitive to modulation by κ-opioids in isolated neurohypophysial nerve terminals as reported here. Administration of U69,593 in a saturating concentration inhibited L-type current on average by ∼64%, whereas N- and P/Q-type currents were reduced by ∼32 and ∼28%, respectively. The present results also serve to emphasize the necessity for direct examination of isolated terminal Ca2+currents to establish the involvement of particular Ca2+ channel subtypes in opioid regulation of exocytosis. Thus, recordings from magnocellular supraoptic neurons reveal that activation of μ-opioid receptors inhibits N- and P-type but not L-type Ca2+ channels, yet these recordings fail to demonstrate functional coupling between κ-opioid receptors and Ca2+ channels (Soldo and Moises, 1996).
We tested for a functional linkage between opioid suppression of voltage-dependent Ca2+ channels and inhibition of release by comparing the effects of opioid-induced alterations in Ca2+ influx on kinetically distinct components of the neurosecretory response. The slow increases inCm that we measured are thought to reflect the trafficking and membrane fusion of secretory vesicles from a readily releasable vesicular pool (Lindau et al., 1992; Horrigan and Bookman, 1994). Administration of U69,593 consistently attenuated the amplitude of these slow Cm responses evoked by depolarization to +10 mV as well as potentials (+30 mV) closer toECa. On the other hand, the immediate step-likeCm increases in response to depolarizations that induced maximal Ca2+ influx were not affected by the κ-opioids in more than two-thirds of the terminals examined, this despite the fact that Ca2+ currents were substantially reduced in three-quarters of these preparations. Interestingly, the effects produced by κ-opioid receptor activation mirror the relationship between changes in Ca2+influx and release described in chromaffin cells, wherein manipulations that reduce Ca2+ currents (reducing [Ca2+]o or stepping closer toECa) decreased the delayed rise inCm but had little effect on the immediateCm jump after 100 msec depolarizations (Horrigan and Bookman, 1994). It was proposed that in those cells high levels of submembrane Ca2+ might saturate the Ca2+ receptors, resulting in a constant probability of release of the vesicles from the immediately releasable pool (IRP). Similarly, saturation of Ca2+ receptors might account for the variable effects of opioids on immediate responses observed here. In fact, when we evoked smaller Ca2+currents by stepping closer to ECa, the amplitudes of the immediate Cm responses were correspondingly reduced, indicating that Ca2+ levels at the release sites were decreased to a level below saturation. Under these conditions, the immediate Cmresponses were reliably decreased by U69,593. The ability of κ-opioids to modulate any associated slowly increasingCm component was also related to the size of the depolarizing step used to evoke these responses and the corresponding Ca2+ currents. Thus, this slowly increasing phase ofCm was inhibited to a much greater extent (being abolished in two-thirds of the terminals) when U69,593 was applied under conditions in which the Ca2+ driving force was reduced (+30 mV) compared with the effects measured on responses evoked by stepping to test potentials that yielded maximal Ca2+ currents. This experimental outcome is in keeping with recent findings that suggest that maintenance of exocytotic activity in these nerve endings (presumably reflected in the slowly increasing Cm response) may involve a replenishment of vesicles into the IRP that is directly dependent on the level of intraterminal [Ca2+]i(Giovannucci and Stuenkel, 1997).
Overall, our results provide strong evidence that activation of κ-opioid receptors modulates several components of exocytotic release in rat neurohypophysial nerve endings, including both vesicle fusion and the recruitment of vesicles into an IRP. However, the molecular mechanism(s) whereby κ-opioid receptors produce these presynaptic inhibitory actions remains to be elucidated. Our findings support the hypothesis that modulation of Ca2+-dependent processes plays a role in κ-opioid-induced inhibition of exocytosis in neurohypophysial nerve endings. However, they do not preclude the possibility that the relationship between opioid-induced reduction of voltage-dependent Ca2+ influx and presynaptic modulation of release could be more complex and that the inhibition of Ca2+ channels may be selectively involved in regulatory mechanisms that govern distinct aspects of the exocytotic process. In this regard, recent findings obtained in rat hippocampal slice preparations that μ-opioid agonists reduced the occurrence of spontaneous, Ca2+-independent synaptic events raise the possibility of a direct opioid effect on the intracellular machinery that regulates vesicle exocytosis (Cohen et al., 1992;Capogna et al., 1993; Rekling, 1993). The possibility of a direct depressant action of opioids on the secretory apparatus is also supported by recent findings obtained in the GLC8 small-cell lung carcinoma cell line, wherein it was found that activation of endogenous δ-opioid receptors not only inhibited the depolarization-induced release of [3H]serotonin but also the Ca2+-dependent secretion of the labeled transmitter induced by thapsigargin and ionomycin (Sher et al., 1997). Our finding in a few terminals that administration of κ-opioids attenuated depolarization-evoked Cm responses despite little or no decrease in the associated Ca2+currents raises the additional possibility that opioids might regulate vesicle fusion and/or trafficking independently of Ca2+ current modulation. In any event, the present results suggest a potential mechanism whereby endogenous release of prodynorphin-derived opioid peptides, known to be colocalized in AVP- and OT-containing terminals (Zhao et al., 1988b; Russell et al., 1995), can exert inhibitory modulation of neuropeptide release from the neurohypophysis.
Footnotes
This work was supported by National Science Foundation Grant 9410834 to E.L.S., American Heart Association of Michigan Postdoctoral Fellowship 09F956 to D.R.G., and National Institute on Drug Abuse Grant DA03365 to H.C.M.
Correspondence should be addressed to Dr. Hylan C. Moises, Department of Physiology, University of Michigan Medical School, Ann Arbor, MI 48109-0622.
REFERENCES
- 1.Adamson P, Xiang JZ, Mantzourides T, Brammer MJ, Campbell IC. Presynaptic α2-adrenoceptor and κ-opiate receptor occupancy promotes closure of neuronal (N-type) calcium channels. Eur J Pharmacol. 1989;174:63–70. doi: 10.1016/0014-2999(89)90874-1. [DOI] [PubMed] [Google Scholar]
- 2.Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- 3.Capogna M, Gahwiler BH, Thompson SM. Mechanism of μ-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol (Lond) 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazalis M, Dayanithi G, Nordmann JJ. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol (Lond) 1987;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciranna L, Feltz P, Schlichter R. Selective inhibition of high voltage-activated L-type and Q-type Ca2+ currents by serotonin in rat melanotrophs. J Physiol (Lond) 1996;490.3:595–609. doi: 10.1113/jphysiol.1996.sp021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 7.Dayanithi G, Stuenkel EL, Nordmann JJ. Intracellular calcium and hormone release from nerve endings of the neurohypophysis in the presence of opioid agonists and antagonists. Exp Brain Res. 1992;90:539–545. doi: 10.1007/BF00230936. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–97. [PubMed] [Google Scholar]
- 9.Fidler NH, Fernandez J. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989;56:1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillis KD, Mobner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci DR, Stuenkel EL. Regulation of secretory granule recruitment and exocytosis at rat neurohypophysial nerve endings. J Physiol (Lond) 1997;498.3:735–751. doi: 10.1113/jphysiol.1997.sp021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grudt TJ, Williams JT. μ-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. J Neurosci. 1994;14:1646–1654. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth SJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Herkenham M, Rice KC, Jacobson AE, Rothman RB. Opiate receptors in rat pituitary are confined to the neural lobe and are exclusively kappa. Brain Res. 1986;382:365–371. doi: 10.1016/0006-8993(86)91346-6. [DOI] [PubMed] [Google Scholar]
- 15.Herrington J, Newton KR, Bookman RJ. Pulse control V4.5:IGOR XOPs for patch clamp data acquisition and capacitance measurements. Miami: University of Miami; 1995. [Google Scholar]
- 16.Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 17.Hsu SF, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. J Physiol (Lond) 1996;494.2:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illes P. Modulation of transmitter and hormone release by multiple neuronal opioid receptors. Rev Physiol Biochem Pharmacol. 1989;112:141–233. doi: 10.1007/BFb0027497. [DOI] [PubMed] [Google Scholar]
- 19.Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;268:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Chapman C, Bicknell RJ. Activation of κ-opioid receptors inhibits depolarisation-evoked exocytosis but not the rise in intracellular Ca2+ in secretory nerve terminals of the neurohypophysis. Brain Res. 1992;574:138–146. doi: 10.1016/0006-8993(92)90810-v. [DOI] [PubMed] [Google Scholar]
- 21.Kongsamut S, Lipscombe D, Tsien RW. The N-type Ca2+ channel in frog sympathetic neurons and its role in α-adrenergic modulation of transmitter release. Ann NY Acad Sci. 1989;560:312–333. doi: 10.1111/j.1749-6632.1989.tb24112.x. [DOI] [PubMed] [Google Scholar]
- 22.Lemos JR, Wang G, Wang X, Stuenkel EL, Nordmann JJ, Treistman SN. Effects of toxins on Ca2+ currents and peptide release from nerve terminals. Ann NY Acad Sci. 1994;710:11–29. doi: 10.1111/j.1749-6632.1994.tb26610.x. [DOI] [PubMed] [Google Scholar]
- 23.Lim NF, Nowycky MC, Bookman RJ. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990;344:449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- 24.Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- 25.Lindau M, Stuenkel EL, Nordmann JJ. Depolarization, intracellular calcium and exocytosis in single vertebrate nerve endings. Biophys J. 1992;61:19–30. doi: 10.1016/S0006-3495(92)81812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald RL, Nelson PG. Specific opiate-induced depression of transmitter release from dorsal root ganglion cells in culture. Science. 1978;199:1449–1551. doi: 10.1126/science.204015. [DOI] [PubMed] [Google Scholar]
- 28.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 29.Moises HC, Rusin KI, Macdonald RL. μ- and κ-Opioid receptors selectively reduce the same transient components of high-threshold calcium current in rat dorsal root ganglion sensory neurons. J Neurosci. 1994;14:5903–5916. doi: 10.1523/JNEUROSCI.14-10-05903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordmann JJ, Dayanithi G, Lemos JR. Isolated neurosecretory nerve endings as a tool for studying the mechanism of stimulus-secretion coupling. Biosci Rep. 1987;7:411–426. doi: 10.1007/BF01362504. [DOI] [PubMed] [Google Scholar]
- 31.North RA. Presynaptic actions of opioids. In: Dunwiddie TV, Lovinger DM, editors. Presynaptic receptors in the mammalian brain. Birkenhauser; Boston: 1993. pp. 71–76. [Google Scholar]
- 32.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective κ-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 33.Rekling JC. Effects of met-enkephalin on GABAergic spontaneous miniature IPSPs in organotypic slice cultures of the rat hippocampus. J Neurosci. 1993;13:1954–1964. doi: 10.1523/JNEUROSCI.13-05-01954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renaud LP, Allen AM, Cunningham JT, Jarvis CR, Johnston SA, Nissen R, Sullivan MJ, Van Vulpen E, Yang CR. Synaptic and neurotransmitter regulation of activity in mammalian hypothalamic magnocellular neurosecretory cells. In: Josse I, Buijis RM, Tilders FJH, editors. The peptidergic neuron. Elsevier; Amsterdam: 1992. pp. 277–288. [DOI] [PubMed] [Google Scholar]
- 35.Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J Neurosci. 1994;14:7608–7615. doi: 10.1523/JNEUROSCI.14-12-07608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusin KI, Moises HC. μ-Opioid receptor activation reduces multiple components of high-threshold calcium current in rat sensory neurons. J Neurosci. 1995;15:4315–4327. doi: 10.1523/JNEUROSCI.15-06-04315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell JA, Leng G, Bicknell RJ. Opioid tolerance and dependence in the magnocellular oxytocin system: a physiological mechanism? Exp Physiol. 1995;80:307–340. doi: 10.1113/expphysiol.1995.sp003850. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder JE, Fischbach PS, Zheng D, McCleskey EW. Activation of μ-opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991;6:13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- 39.Seward EP, Hammond C, Henderson G. μ-Opioid receptor-mediated inhibition of the N-type calcium channel current. Proc R Soc Lond [Biol] 1991;244:129–135. doi: 10.1098/rspb.1991.0061. [DOI] [PubMed] [Google Scholar]
- 40.Seward EP, Chernevskaya NI, Nowycky MC. Exocytosis in peptidergic nerve terminals exhibits two calcium-sensitive phases during pulsatile calcium entry. J Neurosci. 1995;15:3390–3399. doi: 10.1523/JNEUROSCI.15-05-03390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sher E, Cesare P, Codignola A, Clementi F, Tarroni P, Pollo A, Magnelli V, Carbone E. Activation of delta-opioid receptors inhibits neuronal-like calcium channels and distal steps of Ca2+-dependent secretion in human small-cell lung carcinoma cells. J Neurosci. 1997;16:3672–3684. doi: 10.1523/JNEUROSCI.16-11-03672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soldo BL, Moises HC. Ca2+ channels in rat supraoptic neurons are modulated by μ- but not δ- or κ-opioid receptors. Soc Neurosci Abstr. 1996;22:1752. [Google Scholar]
- 43.Stuenkel EL. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. J Physiol (Lond) 1994;481.2:251–271. doi: 10.1113/jphysiol.1994.sp020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stuenkel EL, Nordmann JJ. Intracellular calcium and vasopressin release of rat isolated neurohypophysial nerve endings. J Physiol (Lond) 1993;468:335–355. doi: 10.1113/jphysiol.1993.sp019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumner BEH, Coombes JE, Pumford KM, Russell JA. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37:635–645. doi: 10.1016/0306-4522(90)90095-l. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 47.Taussig R, Sanchez S, Rifo M, Gilman AG, Belardetti F. Inhibition of the omega-conotoxin-sensitive calcium current by distinct G proteins. Neuron. 1992;8:799–809. doi: 10.1016/0896-6273(92)90100-r. [DOI] [PubMed] [Google Scholar]
- 48.Toth PT, Bindokas VP, Bleakman D, Colmers WF, Miller RJ. Mechanism of presynaptic inhibition by neuropeptide Y at sympathetic nerve terminals. Nature. 1993;364:635–639. doi: 10.1038/364635a0. [DOI] [PubMed] [Google Scholar]
- 49.Turner TJ, Adams ME, Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Treistman SN, Lemos JR. Single channel recordings of Nt- and L-type Ca2+ currents in rat neurohypophysial terminals. J Neurophysiol. 1993;70:1617–1628. doi: 10.1152/jn.1993.70.4.1617. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler DB, Randall AD, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 52.Wuarin J-P, Dudek FE. Direct effects of an opioid peptide selective for μ-receptors: intracellular recordings in the paraventricular and supraoptic nuclei of the guinea-pig. Neuroscience. 1990;36:291–298. doi: 10.1016/0306-4522(90)90426-5. [DOI] [PubMed] [Google Scholar]
- 53.Xiang JZ, Adamson P, Brammer MJ, Campbell IC. The κ-opiate agonist U50488H decreases the entry of 45Ca into rat cortical synaptosomes by inhibiting N- but not L-type calcium channels. Neuropharmacology. 1990;29:439–444. doi: 10.1016/0028-3908(90)90165-n. [DOI] [PubMed] [Google Scholar]
- 54.Zhao BG, Chapman C, Bicknell RJ. Opioid-noradrenergic interactions in the neurohypophysis. I. Differential opioid receptor regulation of oxytocin, vasopressin, and noradrenaline release. Neuroendocrinology. 1988a;48:16–24. doi: 10.1159/000124984. [DOI] [PubMed] [Google Scholar]
- 55.Zhao BG, Chapman C, Bicknell RJ. Functional κ-opioid receptors on oxytocin and vasopressin nerve terminals isolated from the rat neurohypophysis. Brain Res. 1988b;462:62–66. doi: 10.1016/0006-8993(88)90585-9. [DOI] [PubMed] [Google Scholar]