Abstract
Cocaine exposure in utero is known to cause a variety of behavioral and motor deficits that may be attributable to alterations in the dopamine neurocircuitry. To ascertain cocaine effects in the fetus, we developed a nonhuman primate model in which pregnant monkeys were administered cocaine from day 20 through day 60 or 70 of gestation. Fetuses from these pregnancies develop a repertoire of neural deficiencies, including decreased mRNA expression of tyrosine hydroxylase in the midbrain and increased mRNA expression of dopamine receptor subtypes in the rostral forebrain. Presently, we studied the effects of maternal cocaine treatment on the mRNA expression of the endogenous opioids preprodynorphin (PPD) and preproenkephalin (PPE) in fetal monkey brains. Fetuses exposed to saline (0.9%) or cocaine (3 mg/kg) were delivered by Caesarean section, the fetal brains were dissected, and tissue RNA was extracted and quantified using ribonuclease protection assay analysis. The opioid peptides PPD and PPE were expressed in the fetal monkey brain by day 60, and even higher levels were found in day 70 fetuses. Maternal exposure to cocaine increased gene expression of PPD and PPE in the fetus at both day 60 and day 70 of gestation. Dynorphin mRNA levels were significantly elevated in the striatum, whereas enkephalin mRNA was elevated in both the frontal cortex and the striatal area of fetuses whose mothers received cocaine. Changes in the expression of these opioid peptides in presumed dopamine target neurons, which mediate motivation and reward, as well as motor control, provide further evidence for profound consequences of in utero cocaine exposure on the developing dopamine neurocircuitry.
Keywords: fetal monkey, development, cocaine, enkephalin mRNA, dynorphin mRNA, frontal cortex, striatal area
Cocaine is a CNS stimulant that affects many aminergic neurons in the brain (Williams and Lacey, 1988; Woolverton and Johnson, 1992). Its addictive properties are attributable to alterations in dopamine neural transmission (Ritz et al., 1987; Kuhar et al., 1991). The free base form of the drug crack is relatively pure, and the smoking of crack causes subjective and physiological effects similar to those produced by intravenous cocaine injections (Cregler and Mark, 1986). Cocaine crosses the placenta, and infants and children who have been exposed to cocaine in utero often exhibit aberrant behavior and learning difficulties, which may be due to abnormal organization of the nervous system (Cregler and Mark, 1986;Hume et al., 1989; Struthers and Hansen, 1992; Azuma and Chasnoff, 1993; Fries et al., 1993; Mayes et al., 1995; Regalado et al., 1995). However, the specific effects of cocaine in infants and children are difficult to determine because of the limitations that accompany research with human subjects. Therefore, we are using the rhesus monkey as a model because its fetal development is similar to the human (Gribnau and Geijsberts, 1981). Using this model, we have found that the midbrain of monkeys exposed to cocaine early in gestation contains reduced expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis (Rönnekleiv and Naylor, 1995). In the same animal model, dopamine D1, D2, and D5 receptor subtype mRNAs are elevated in the rostral forebrain regions, and dopamine D1 and D2 receptor-binding capacity is increased in cocaine-exposed fetuses (Choi and Rönnekleiv, 1996; Fang et al., 1996). These findings suggest that dopamine receptor sensitivity has been affected by chronic treatment with cocaine during pregnancy (Kostrzewa, 1995).
Dynorphin- and enkephalin-containing neurons in the rostral forebrain express dopamine D1 and D2 receptors, which are regulated by dopaminergic neurons (Li et al., 1988; Sivam, 1989; Gerfen et al., 1990; Le Moine et al., 1990, 1991; Surmeier et al., 1992; Hyman et al., 1994; Cole et al., 1995). For example, in Parkinson’s and Huntington’s diseases, in which the nigrostriatal dopaminergic system is disrupted, there are alterations in the mRNA expression of enkephalin (Nisbet et al., 1995; Richfield et al., 1995). Thus, experimentally induced Parkinson’s disease in various animal models, including nonhuman primates, results in increased expression of enkephalin mRNA (Young et al., 1986; Li et al., 1988; Augood et al., 1989; Gerfen et al., 1990; Asselin et al., 1994). Additionally, the mRNA expression of dynorphin is elevated in rostral forebrain regions of cocaine-addicted animals (Sivam, 1989; Hurd et al., 1992; Daunais et al., 1993; Spangler et al., 1993). In the present study, we examined the effects of chronic treatment of pregnant rhesus monkeys with cocaine on the development of enkephalin and dynorphin mRNAs in their fetuses. The study focuses on fetal days 60 and 70 because these are the ages when cocaine-induced alterations of the dopamine neurocircuitry can first be detected in the fetal monkey (Rönnekleiv and Naylor, 1995; Choi and Rönnekleiv, 1996).
MATERIALS AND METHODS
Animals
These studies were conducted in accordance with the principle and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult female rhesus monkeys (Macaca mulatta) were paired with fertile males for 3 d beginning on day 9 through day 18 of their menstrual cycle, based on an analysis of their previous menstrual cycle lengths. Pregnancy was determined by radioimmunoassay analysis of estrogen (>100 pg/ml) and progesterone (>2.5 ng/ml) in blood samples obtained at days 13–17 after pairing (Hess et al., 1981). The second day of pairing was chosen as the day of conception; gestation times were calculated from that point.
Cocaine treatment
Synthesized cocaine hydrochloride was obtained from the Research Triangle Institute (Research Triangle Park, NC) through the National Institute on Drug Abuse (Bethesda, MD). In these experiments, we used the cocaine treatment paradigm that we have shown previously to decrease TH mRNA expression in the fetal midbrain and to increase dopamine receptor expression in the rostral forebrain (Rönnekleiv and Naylor, 1995; Choi and Rönnekleiv, 1996). Therefore, as soon as pregnancy was confirmed, at approximately day 20 after mating, cocaine (3 mg dissolved in 50 μl of 0.9% saline/kg) was injected intramuscularly four times daily at 8 A.M., noon, 4 P.M., and 8 P.M. As determined in detail previously, this cocaine regimen gave peak plasma levels of 800–1000 ng/ml in the mother at 10–15 min after the injection, and 150–460 ng/ml in the fetus at ∼45 min after the cocaine injection (Rönnekleiv and Naylor, 1995). Animals receiving saline injections were used as controls. In both groups, the injection site was rotated between hips and shoulders.
The fetuses were delivered by Cesarean (C) section at days 60 and 70 of gestation. On the day of C section, each animal received the final saline or cocaine injection at 8:40 A.M. Shortly thereafter, the animal was sedated with ketamine, transported to the surgical area, and anesthetized with halothane. Each fetus was delivered ∼50 min after the final cocaine injection.
Tissue preparation
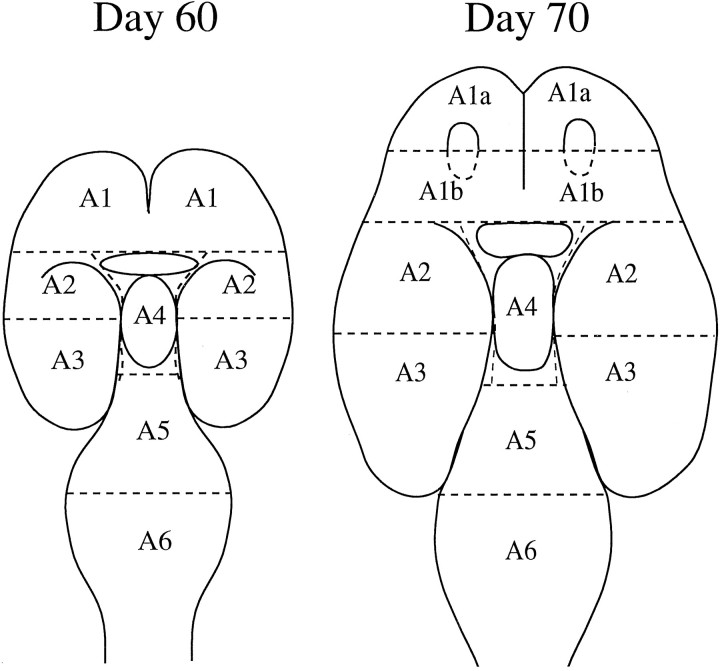
Before dissection, three parameters were recorded: body weight, crown-rump length, and head circumference. Then, each brain was dissected under a microscope into six regions, as illustrated in Figure1.
Fig. 1.
Diagrammatic representation of fetal monkey brains (horizontal view) illustrating the dissections of day 60 and day 70 brains. The day 60 fetal brains were dissected into six regions from rostral to caudal: A1 contains the FC and the rostral part of the ST; A2 contains the caudal part of the ST, rostral part of the temporal lobe, and adjacent cortical regions;A3 contains the caudal part of the temporal lobe and adjacent cortical regions; A4 contains the preoptic area, the thalamus, and the hypothalamus; A5 contains the MB; and A6 contains the developing pons, cerebellum, and the BS. The day 70 fetal brains were dissected similar to the day 60 brains except that A1 was divided into area A1a andA1b, which contain primarily the prefrontal cortex and the striatal area, respectively. Also, A6 (the BS) was not analyzed in the day 70 fetal groups.
Day 60 fetus. Area (A) 1, rostral forebrain, which includes the frontal cortex (FC) and striatum (ST). A2, rostral temporal (RT), which includes the rostral part of the temporal lobe, the caudal part of the ST, and dorsal cortical areas. A3, caudal temporal (CT), which includes the caudal part of the temporal lobe and the corresponding dorsal cortical areas. A4, diencephalon (DI), which includes the preoptic area, hypothalamus, and thalamus. A5, midbrain (MB), which includes the ventral tegmental area, substantia nigra, and collicular regions. A6, brainstem (BS), which includes the cerebellum, pons, and medulla.
Day 70 fetus. The day 70 fetal brain was dissected similarly to the day 60 fetal brain, except that A1 of the rostral forebrain was separated into A1a (FC) and A1b (ST and the surrounding cortex). The separation between the FC and the ST was made immediately rostral to the base of the olfactory bulbs. Thus, the olfactory bulbs were included with the striatal area tissue block. Day 70 fetal brains, which were subjected to similar dissections, have been analyzed histologically to verify that A1a contains primarily the FC and A1b contains the ST and the surrounding cortex. The freshly dissected brain blocks were frozen in isopentane at −55°C and stored in liquid nitrogen. The BS block was not analyzed in the day 70 fetal monkey.
RNA isolation
Total RNA was isolated according to a modification of the procedure described by Chirgwin et al. (1979). Briefly, the frozen brain blocks were homogenized in 4 m guanidium isothiocyanate, 10 mm EDTA, 2% sodium N-lauryl sarcosine, 1% (v/v) β-mercaptoethanol, and 50 mm Tris, pH 7.6, in the presence of 10 mm vanadyl ribonucleoside complexes. The homogenized extracts were ultracentrifuged through a 5.7m cesium chloride gradient overnight at 35,000 rpm in a Beckman SW55TI rotor. The pellet was resuspended in Tris-EDTA buffer containing 0.1% SDS extracted with phenol chloroform and precipitated with ice-cold 100% ethanol. Total RNA pellets were dissolved in RNase-free (diethyl pyrocarbonate-treated) water, and a 1 μl aliquot was removed to measure the RNA concentration by spectrophotometry. The remaining RNA was aliquoted and stored at −80°C.
Preproenkephalin and preprodynorphin subcloning
The cDNAs from human preproenkephalin (PPE) and preprodynorphin (PPD) were generous gifts from Dr. James Douglas (Amgen, Inc., Thousand Oaks, CA). Template DNAs for the in vitro transcription of probe and reference (sense) RNAs were cDNA fragments that were inserted into the polylinker region of pGEM3fz(+) (Promega, Madison, WI). For enkephalin, a 411 bp PstI PPE fragment (bases 334–745) (Comb et al., 1982) was subcloned into the PstI restriction site of pGEM3Zf(+) (Promega). For dynorphin, a 305 bp NcoI and EcoRI DNA fragment (bases 1535–1840) containing the entire PPD coding region (Horikawa et al., 1983) was subcloned into theSmaI restriction site of pGEM3Zf(+) (Promega). The DNA sequences of both the PstI fragment of PPE andNcoI/EcoRI fragment of PPD were analyzed by sequencing (Sequenase 2.0; United States Biochemical).
Synthesis of cRNA probes and sense RNAs
Both PPE and PPD cRNA probes were synthesized from the pGEM3Z(+) recombinants. The antisense cRNA was transcribed with T7 RNA polymerase from both PPD and PPE linearized with HindIII. The [32P]UTP (DuPont NEN)-labeled probes were prepared using the MAXIscript in vitro Transcription kit (Ambion, Austin, TX) to a specific activity of 1.0 × 109 cpm/μg. The antisense cRNA probes were purified by electrophoresis in a denaturing gel (7.2 m urea, 5% polyacrylamide), eluted in 2m ammonium acetate, 1% SDS, and 25 μg/ml tRNA at 37°C, and then ethanol-precipitated. Sense RNAs for PPD and PPE were transcribed by SP6 RNA polymerase from the recombinants that had been linearized with EcoRI. The protected sense RNA and tissue mRNA for PPE was 356 and 305 bp, respectively. For PPD, the protected sense RNA and tissue mRNA were 462 and 411 bp, respectively.
Cyclophilin mRNA was measured using a 185 bp [32P]cRNA probe that was transcribed from a rhesus monkey p1B15 cyclophilin cDNA clone (pGEM-5Zf vector; courtesy of Dr. Sergio Ojeda, Oregon Regional Primate Research Center, Beaverton, OR). The protected cyclophilin mRNA fragment in the ribonuclease protection assay (RPA) was 158 bp.
The [32P]rUTP-labeled PPE, PPD, and cyclophilin probes were initially purified in 7.1 m urea acrylamide gel and eluted with elution buffer as described previously (Choi et al., 1995). Recently, the labeled probes have been purified by electrophoresis through an 8.0 m urea/5% polyacrylamide gel using the Fullengther Preparative Gel Apparatus (Dwarf Scientific, Aloha, OR) and run at 60 V for 60 min. Fractions were collected at 3 min intervals, and the peak fraction (determined by liquid scintillation counting) was ethanol-precipitated and used in the RPA.
RPA
All reagents used in the RPA are from the Ambion RPA II kit (Ambion) unless otherwise specified. The gel-purified probes were reconstituted in hybridization buffer to a concentration of 500,000 dpm/20 μl and hybridized to 5 μg (PPE) or 20 μg (PPD) of total RNA, and reference RNA (62.5–4000 fg) were used as standards. The tissue RNA samples were hybridized simultaneously to 5000 dpm of the monkey radiolabeled [32P]rUTP cyclophilin probe to correct for differences in amount of sample RNA (Danielson et al., 1988). The reaction was incubated overnight at 45°C. The hybridization mixture was then digested with ribonuclease T1 (700 U/200 μl; Life Technologies, Grand Island, NY) for 1 hr at 37°C. The ribonuclease digestion was terminated by the addition of 300 μl of RNase inactivation precipitation solution (Ambion kit; solution D), and the protected fragment was precipitated at −20°C for 30 min. The pellets were dissolved in loading buffer (Ambion kit; solution E) and subjected to electrophoresis in denaturing gel (7.1 m urea, 6% polyacrylamide) at 250 V for 2 hr. The gel was dried and exposed to autoradiographic film (Reflection; Dupont NEN Research Products, Boston, MA) for 4–16 hr at −80°C using intensifying screens.
Data and statistical analysis
RPA autoradiograms were analyzed by densitometry using a computer-based video imaging system (Imaging Research, St. Catharines, Ontario, Canada). The relative amount of Enk and Dyn mRNA in each sample was determined from the RPA standard curve derived from a linear regression analysis using GraphPad Inplot Computer program (GraphPad Software, San Diego, CA). Results were expressed as mean ± SEM and analyzed by a paired Student’s t test (two-tailed). The distribution data were analyzed with ANOVA followed by a Tukey–Kramer multiple comparisons test.
RESULTS
Maternal effects of cocaine
In this study, we treated pregnant rhesus monkeys with cocaine from day 20 to day 60 or day 70 of gestation, according to a previously described procedure (Rönnekleiv and Naylor, 1995). We did not observe overt signs of cocaine intolerance, such as anorexia or seizures, in our experimental subjects.
Morphology of the placenta
In each case, the placenta was inspected and analyzed histologically. We found no obvious signs of cocaine-induced placental or decidual abnormalities.
Fetal growth
On day 60 of gestation, data from four male and two female fetuses were obtained, whereas on day 70 six males were studied. Maternal cocaine exposure did not significantly affect body weight, crown-rump length, or head circumference of fetuses on day 60 or day 70 of gestation. Body weight of control and cocaine-treated fetuses (n = 2 males and 1 female in each group) were 12.50 ± 1.85 gm and 12.34 ± 2.10 gm, respectively, on day 60, and 25.28 ± 1.63 gm (n = 3 males) and 27.54 ± 2.35 gm (n = 3 males), respectively, on day 70. The crown-rump lengths of control and cocaine-treated fetuses were 5.80 ± 0.39 and 5.81 ± 0.38 cm, respectively, on day 60, and 7.27 ± 0.25 and 7.57 ± 0.26 cm, respectively, on day 70. The head circumference was 6.34 ± 0.31 and 6.43 ± 0.48 cm, respectively, on day 60 control and cocaine-treated subjects, and 7.80 ± 0.29 and 8.10 ± 0.08 cm, respectively, on day 70 control and cocaine-treated subjects.
PPD and PPE mRNA in fetal monkey brain
We used a sensitive RPA to quantify the levels of PPD and PPE mRNA in the fetal monkey brain. Figures2A, 3, 4, 5A illustrate the levels of PPE and PPD mRNA found in RNA extracts from individual control and cocaine-exposed fetal brains. Figures2A, 3, 4, 5A also give the standard curves of different concentrations of sense RNA hybridized with32P-labeled human PPE and PPD antisense RNA probes. These figures illustrate that single bands of protected tissue mRNA were obtained. Figures 2B, 3, 4, 5B depict the regression lines for the respective standard curves shown in panelA.
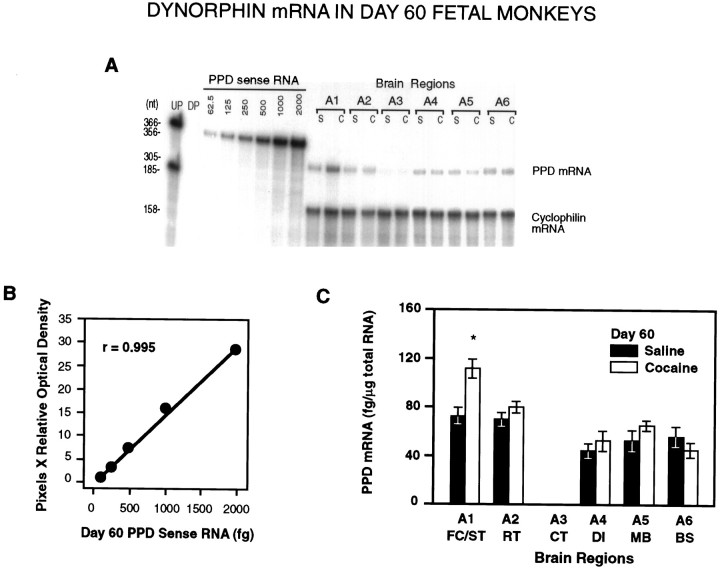
Fig. 2.
A, A representative RPA of total RNA (20 μg/lane) from saline- and cocaine-treated day 60 fetal monkey brain tissues illustrating the levels of PPD mRNA detected in the different brain regions of individual animals. UP, undigested probe; DP, digested probe; S, saline-treated; C, cocaine-treated.A1–A6 represent defined brain regions (see Materials and Methods). B, Linear regression analysis of the optical densities of the PPD mRNA sense standard curve revealedr = 0.995. C, Distribution and quantitative analysis of PPD mRNA in brain tissue obtained from saline-treated and cocaine-treated fetal macaques (n = 3 each). Densitometric scannings were normalized to cyclophilin mRNA and quantified from each sense mRNA standard curve. PPD mRNA was significantly increased in A1 (FC/ST) in cocaine-treated animals (*p < 0.05; paired two-tailed Student’st test).
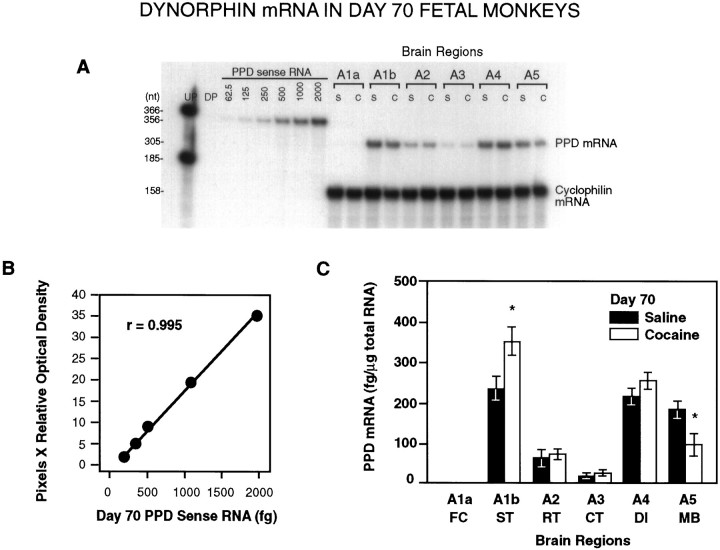
Fig. 3.
A, A representative RPA of total RNA (10 μg/lane) from saline- and cocaine-treated day 70 fetal monkey brain tissues illustrating the levels of PPD mRNA detected in the different brain regions of individual animals. UP, Undigested probe; DP, digested probe; S, saline-treated; C, cocaine-treated.A1–A5 represent defined brain regions (see Materials and Methods). B, Linear regression analysis of the optical densities of the PPD mRNA sense standard curve revealedr = 0.995. C, Distribution and quantitative analysis of PPD mRNA in brain tissue obtained from saline-treated and cocaine-treated fetal macaques (n = 3 each). Densitometric scannings were normalized to cyclophilin mRNA and quantified from each sense mRNA standard curve. PPD mRNA was significantly increased in A1b (ST and surrounding cortical regions) and significantly decreased in A5 (MB) in cocaine-treated animals (*p < 0.05; paired two-tailed Student’st test).
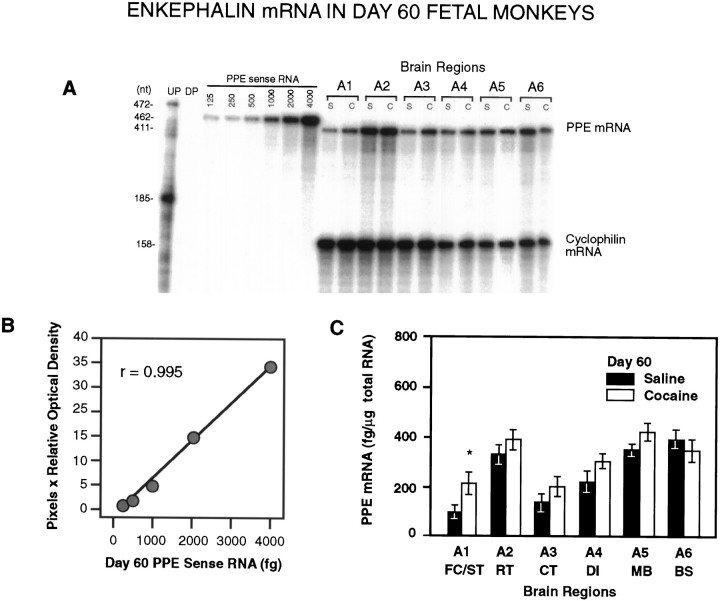
Fig. 4.
A, A representative RPA of total RNA (5 μg/lane) from saline- and cocaine-treated day 60 fetal monkey brain tissues illustrating the levels of PPE mRNA detected in the different brain regions of individual animals. UP, undigested probe; DP, digested probe; S, saline-treated; C, cocaine-treated.A1–A6 represent defined brain regions (see Materials and Methods). B, Linear regression analysis of the optical densities of the PPE mRNA sense standard curve revealedr = 0.995. C, Distribution and quantitative analysis of PPE mRNA in brain tissue obtained from saline-treated and cocaine-treated fetal macaques (n = 3 each). Densitometric scannings were normalized to cyclophilin mRNA and quantified from each sense mRNA standard curve. PPE mRNA was significantly increased in A1 (FC/ST) in cocaine-treated animals (*p < 0.05; paired two-tailed Student’st test).
Fig. 5.
A, A representative RPA of total RNA (3 μg/lane) from saline- and cocaine-treated day 70 fetal monkey brain tissues illustrating the levels of PPE mRNA detected in the different brain regions of individual animals. UP, Undigested probe; DP, digested probe; S, saline-treated; C, cocaine-treated.A1–A5 represent defined brain regions (see Materials and Methods). B, Linear regression analysis of the optical densities of the PPE mRNA sense standard curve revealedr = 0.995. C, Distribution and quantitative analysis of PPE mRNA in brain tissue obtained from saline-treated and cocaine-treated fetal macaques (n = 3 each). Densitometric scannings were normalized to cyclophilin mRNA and quantified from each sense mRNA standard curve. PPE mRNA was significantly increased in A1a (FC) and A1b (ST and surrounding cortical regions), and significantly decreased in A5 (MB) in cocaine-treated animals (*p< 0.05, **p < 0.005; paired two-tailed Student’st test).
In day 60 control fetuses (n = 3), all brain regions showed approximately equal amounts of PPD mRNA, with the exception of the caudal part of the temporal lobe (A3), where PPD mRNA was not found (Table 1, Fig. 2). In the areas showing PPD mRNA expression on day 60, the levels ranged from 73.7 ± 9.9 fg/μg in the rostral forebrain (A1) to 59.3 ± 4.58 fg/μg in the diencephalon (A4) (Table 1, Fig. 2). On day 70 (n = 3), the highest concentrations of PPD mRNA were found in the ST (A1b), the diencephalon (A4), and the MB (A5) (Table 2, Fig.3). PPD mRNA levels differed significantly between day 60 and day 70 in these regions (p < 0.05, 0.001, and 0.001, respectively; Tables 1, 2). PPD mRNA was not expressed in the FC (A1a) on day 70 of gestation, but a low level of expression was found in the caudal temporal lobe (A3) (Table 2).
Table 1.
PPE and PPD mRNA levels in day 60 fetal rhesus macaques
| Brain area | PPE (fg/μg) | PPD (fg/μg) | ||
|---|---|---|---|---|
| Saline | Cocaine | Saline | Cocaine | |
| A1 | 97.17 ± 9.51 | 225.23 ± 26.49* | 73.7 ± 9.9 | 114.13 ± 8.47* |
| A2 | 337.63 ± 21.72 | 395.9 ± 21.53 | 65.86 ± 7.6 | 82.1 ± 9.23 |
| A3 | 144.02 ± 22.95 | 198.9 ± 20.69 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| A4 | 252.8 ± 24.51 | 315.0 ± 13.65 | 43.16 ± 6.03 | 52.86 ± 12.26 |
| A5 | 353.9 ± 24.48 | 430.77 ± 27.63 | 54.26 ± 6.98 | 68.43 ± 6.63 |
| A6 | 400.1 ± 28.12 | 334.2 ± 28.67 | 59.3 ± 4.58 | 44.03 ± 4.81 |
The quantities of PPE and PPD mRNA were determined in total RNA extracts from dissected brain areas A1–A6 in saline- and cocaine-treated day 60 fetal monkeys. A1–A6, Areas through the brain from rostral A1 to caudal A6. The numbers are expressed as mean ± SEM.
p < 0.05 versus saline controls.
Table 2.
PPE and PPD mRNA levels in day 70 fetal rhesus macaques
| Brain area | PPE (fg/μg) | PPD (fg/μg) | ||
|---|---|---|---|---|
| Saline | Cocaine | Saline | Cocaine | |
| Ala | 155.42 ± 24.89 | 385.86 ± 28.142-160 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Alb | 352.0 ± 41.35 | 538.1 ± 33.53* | 238.7 ± 21.2 | 352.4 ± 23.53* |
| A2 | 480.87 ± 43.75 | 593.6 ± 34.75 | 62.9 ± 9.6 | 76.6 ± 4.76 |
| A3 | 129.2 ± 13.85 | 163.53 ± 15.92 | 17.1 ± 3.66 | 24.1 ± 4.54 |
| A4 | 648.2 ± 21.22 | 578.01 ± 45.39 | 213.3 ± 14.4 | 255.0 ± 12.46 |
| A5 | 603.03 ± 16.17 | 502.77 ± 14.96* | 181.9 ± 9.8 | 121.0 ± 19.14* |
The quantities of PPE and PPD mRNA were determined in total RNA extracts from dissected brain areas A1–A5 in saline- and cocaine-treated day 70 fetal monkeys. A1–A5, Areas through the brain from rostral A1 to caudal A5. The numbers are expressed as mean ± SEM.
p < 0.05,
F2-160: p < 0.005 versus saline controls.
Gene expression for PPE was higher than PPD in all brain regions on days 60 and 70 of gestation (Tables 1, 2). On day 60 (n= 3), PPE mRNA was highly expressed in the rostral part of the temporal lobe (A2), the MB (A5), and the BS (A6) (Table 1, Fig.4). PPE mRNA was moderately expressed in the diencephalon (A4) and was found in lower concentrations in the rostral forebrain (A1) and the caudal part of the temporal lobe (A3) (Table 1, Fig. 4). On day 70 (n = 3), PPE mRNA was found in high concentrations in the rostral temporal lobe (A2), the diencephalon (A4), and the midbrain (A5) (Table 2, Fig. 5). PPE mRNA expression was significantly elevated in the same areas compared with amounts measured on day 60 (p < 0.05, 0.001, and 0.001, respectively; Tables 1, 2). On day 70, PPE mRNA was found in relatively high concentrations in the FC (A1a) and the ST (A1b) (Table2). The lowest level of PPE mRNA was detected in the caudal part of the temporal lobe (A3) (Table 2).
Effect of cocaine on PPD and PPE gene expression in fetal monkey brain
On day 60 of gestation (n = 3), chronic cocaine treatment of the mother caused a significant increase in the mRNA expression of both PPD (p < 0.05) and PPE (p < 0.05) in the rostral forebrain of the fetus (Table 1, Figs. 2, 4). On day 70 (n = 3), fetal PPD mRNA was significantly increased in the ST (A1b; p< 0.05) and significantly decreased in the MB (A5; p< 0.05) of cocaine-treated animals (Table 2, Fig. 3). In day 70 fetuses, cocaine treatment significantly affected PPE gene expression in the greatest number of tissues (Table 2, Fig. 5) (n= 3). At this time in gestation, PPE mRNA levels were significantly elevated both in the most rostral tissue block, the developing FC (A1a;p < 0.005), and in the striatal area (A1b;p < 0.05) of cocaine-treated fetuses (Table 2, Fig.5). Similar to PPD mRNA, PPE mRNA levels declined significantly in the MB region after cocaine (A5; p < 0.05) (Table 2, Fig.5).
DISCUSSION
The present study demonstrates that the mRNA of the endogenous opioid peptides PPD and PPE were expressed in the fetal monkey brain by day 60 of gestation, and even higher levels were found in day 70 fetuses. In utero cocaine exposure from day 20 to day 60 of gestation significantly increased the mRNA concentrations of these opioid peptides in the rostral forebrain region. By day 70 of gestation, PPD mRNA expression increased in the striatal area and decreased in the MB of cocaine-exposed subjects. In comparison, PPE mRNA expression on day 70 increased in both the FC and the ST, and declined in the MB in fetuses from cocaine-treated mothers.
These data indicate that PPD and PPE mRNA expression increases in most brain areas as the fetus grows between day 60 and day 70 of a 165 d gestation period. Therefore, PPD and PPE mRNAs appear to be actively transcribed in the fetal monkey brain already in the beginning of the second trimester. In rat brains, PPE and PPD mRNAs are detectable on fetal days 14 and 16, respectively, of a 21 d gestation period (Tecott et al., 1989). In the rat striatum, PPE mRNA expression develops gradually during fetal life and continues postnatally, until it reaches the adult levels on postnatal day 14 (Tecott et al., 1989). Total PPE mRNA levels increase sharply just before birth (Zagon et al., 1994). In the adult rat brain, PPE mRNA-containing neurons are found in high concentrations in the striatal area, but are also present in many other brain regions, including the cortex, hypothalamus, MB, and BS (Harlan, 1987). In the adult monkey, PPE mRNA is concentrated in the striatal area and surrounding cortical regions (Haber and Lu, 1995). In the present study, PPE mRNA was expressed in the FC in day 70 fetal monkeys, but we did not detect PPD mRNA. These findings have been confirmed in preliminary experiments using in situhybridization (O. K. Rönnekleiv, unpublished observations). In the rat brain, PPD mRNA is found in the ST and hypothalamus as early as day 16 of gestation; however, the expression of this peptide is first detected in the cerebral cortex on postnatal day 7 (Sato et al., 1991;Laurent-Huck et al., 1993). The approximate time at which PPD mRNA is first expressed in the monkey cerebral cortex is currently not known. A more comprehensive study of the cellular distribution of PPE and PPD mRNAs in the fetal monkey is needed.
We have found that chronic prenatal cocaine treatment increased PPD gene expression in the rostral forebrain region of day 60 and day 70 fetal monkeys. These results agree with earlier findings that chronic cocaine treatment increases PPD mRNA expression and peptide levels in the striatal region in adult mammals (Sivam, 1989; Smiley et al., 1990;Hurd et al., 1992; Spangler et al., 1993; Daunais and McGinty, 1994,1995). The elevation in PPD mRNA and peptide expression after chronic cocaine treatment is thought to be mediated by dopamine D1 receptors, because PPD gene expression in the ST is regulated primarily by D1 receptors through activation of GS and adenylyl cyclase (Sivam, 1989; Gerfen et al., 1990; Le Moine et al., 1990; Gerfen, 1992;Cole et al., 1995). The D1 receptor activation of adenylyl cyclase causes phosphorylation of cAMP response element (CRE) binding proteins (CREB), which bind to CREs in the PPD promotor and stimulate PPD synthesis (Cole et al., 1995).
Previously, we have found that dopamine D1 receptor mRNA and binding capacity in the fetal monkey increase significantly in the striatal area after chronic cocaine-treatment (Choi and Rönnekleiv, 1996;Fang et al., 1996). These data indicate that cocaine exposure upregulates dopamine receptors in the fetus. Collectively, these observations suggest that PPD gene expression in the fetal monkey is increased because of cocaine-induced activation of the D1-receptor/adenylyl cyclase/cAMP-dependent protein kinase A (PKA) cascade. However, the specific mechanism by which PPD gene expression is regulated in cocaine-exposed fetal monkeys remains to be elucidated. It is interesting that rabbits exposed to cocaine in uteroexhibit uncoupling of the dopamine D1 receptor from its G-protein, as measured by dopamine stimulation of [35S]GTPγS binding to Gαs in striatal membranes at postnatal days 10, 50, and 100 (Wang et al., 1995). In contrast to our findings, these data suggest that prenatal cocaine has long-term inhibitory effects on dopamine D1-like functions. A possible explanation is that fetal exposure followed by a period of withdrawal from cocaine may induce compensatory alterations of dopamine neural transmission, which have been shown in adult animals after cocaine withdrawal (Bonci and Williams, 1996).
Our observation that PPE mRNA levels increase significantly in the rostral forebrain of day 60 and day 70 cocaine-treated fetuses contradicts findings in adult animals. Most reports suggest that cocaine-treatment increases striatal PPD mRNA levels, but not immunoreactive enkephalin or PPE mRNA in adult rats (Sivam, 1989;Branch et al., 1994). However, adult rats that self-administer cocaine for 24 hr exhibit increased levels of both PPD and PPE mRNA in the nucleus accumbens (Hurd et al., 1992), suggesting that these peptides covary in response to certain cocaine treatment paradigms. The mechanism through which PPE gene expression is effected by cocainein utero is not known. We do know that enkephalin-containing neurons express dopamine D2 receptors and that PPE biosynthesis is inhibited by dopamine acting at the level of the D2 receptor (Gerfen et al., 1990; Pollack and Wooten, 1992). Thus, lesions of the dopamine input to the striatal area in rodents and primates are associated with elevated PPE mRNA levels (Li et al., 1988; Augood et al., 1989; Gerfen et al., 1990; Soghomonian, 1993; Asselin et al., 1994). Similarly, lesions of MB dopamine neurons or depletion of dopamine by reserpine result in increases in D2 receptor binding and mRNA levels in the striatal area (Jaber et al., 1992; Radja et al., 1993; Soghomonian, 1993). Thus, our current results showing increased PPE mRNA, coupled with our previous findings that D2 receptor mRNA and binding increase in the striatal area (Choi and Rönnekleiv, 1996; Fang et al., 1996), suggest that the dopamine input to the striatal area is reduced in cocaine-exposed fetuses. Our observation that TH mRNA levels are significantly reduced in the substantia nigra/ventral tegmental area of cocaine-exposed fetal monkeys, which may be related to reduced dopamine synthesis and release, also supports this conclusion (Rönnekleiv and Naylor, 1995). A possible explanation for the increased expression of dopamine D2 receptors, which inhibits adenylyl cyclase concurrent with increased PPE gene expression, is that the D2 receptor is uncoupled from its G-protein/cAMP effector system in the rostral forebrain of the fetal monkey (Civelli et al., 1993; Choi and Rönnekleiv, 1996). In this respect, chronic cocaine treatment decreases Gi and Go in the nucleus accumbens of the adult rat (Self and Nestler, 1995).
Enkephalin neurons are also regulated by dopamine D1 receptors, and electrophysiological studies have demonstrated that dopamine D1 and D2 receptors are colocalized in striatal neurons (Surmeier et al., 1992;Surmeier and Kitai, 1994). Dopamine D1- and D2-receptor agonist treatment increases and decreases the levels of PPE mRNA, respectively, presumably through activation (D1) and inhibition (D2) of adenylyl cyclase and PKA. These data suggest that D1 and D2 receptor activation differentially regulates striatal PPE mRNA levels (Angulo, 1992;Pollack and Wooten, 1992; Weisinger, 1995). Similar to the PPD gene, promoter regions of the PPE gene contain CREs, and activation of the adenylyl cyclase/PKA pathway leads to phosphorylation of CREB, which then induces PPE transcription (Konradi et al., 1993). Chronic cocaine treatment increases levels of adenylyl cyclase and PKA in the rat nucleus accumbens, and it is postulated that such adaptations may be partly responsible for drug reinforcement and addiction (Self and Nestler, 1995). Therefore, it is possible that in the fetal monkey, chronic, intermittent exposure to cocaine upregulates adenylyl cyclase/PKA in the FC and striatal neurons, which in turn stimulate PPE (and PPD) synthesis. This hypothesis, however, remains to be elucidated.
An interesting finding in the present study is that the PPD and PPE mRNA levels were reduced in the MB block of cocaine-treated fetal monkeys at day 70 of gestation. Experiments are in progress to elucidate the cellular distribution of the opioid peptides and mRNAs in control and cocaine-treated fetuses. Preliminary observations usingin situ hybridization have found that PPE mRNA is widely distributed in the fetal MB in areas such as the central gray, raphé, mesencephalic reticular nucleus, and the geniculate complex. In contrast, PPD mRNA exhibits a more limited distribution in the lateral central gray area, ventrolateral MB, and the geniculate complex (unpublished observations). At present, we have limited information on the regulation of enkephalin and dynorphin neurons in the MB region of the fetal monkey. Also, in other species the specific regulation of PPD and PPE gene expression in the MB and its modulation by cocaine has not been well explored. One could speculate that the reduction in PPD and PPE mRNAs in the MB is attributable to cocaine’s actions at the serotonergic or noradrenergic neuronal systems (Felten and Sladek, 1983; Hökfelt et al., 1984; Costa et al., 1994;Battaglia et al., 1995). Clearly, further studies are needed to elucidate the mechanism by which cocaine decreases the mRNA levels of PPD and PPE in the fetal MB.
We have reported previously that cocaine exposure up to day 60 of gestation does not affect fetal growth, findings confirmed in the present study (Rönnekleiv and Naylor, 1995; Choi and Rönnekleiv, 1996). In addition, we observed that cocaine exposure for an additional 10 days did not affect the body weight, crown-rump length, or head circumference in day 70 fetuses. Therefore, it appears that cocaine exposure from day 20 to day 70 of gestation does not compromise fetal growth. To our knowledge, studies of blood flow and oxygen delivery to the fetus have not been done in cocaine-treated monkeys. However, in the pregnant ewe, cocaine administration at day 105 of gestation (term 145 days) does not produce fetal hypoxemia or impede blood flow and oxygen delivery to the fetus (Peña et al., 1996). Collectively, these observations suggest that, at least early in gestation, fetal growth and general brain development is maintained after cocaine exposure because the flow of nutrients and oxygen to the fetus is maintained. Therefore, we believe that our observations of changes within the dopamine neurocircuitry in cocaine-exposed fetuses are attributable to specific binding of cocaine to the dopamine transporter and not to an alteration in dopaminergic functions as a result of reduced blood flow (Madras and Kaufman, 1994;Rönnekleiv and Naylor, 1995; Choi and Rönnekleiv, 1996).
In summary, we have found that cocaine exposure during early gestation in the primate increases enkephalin and dynorphin gene expression in the dopamine terminal field region of the rostral forebrain. The mechanism through which these changes occur in the fetal brain is currently unknown. However, alterations in the expression of these opioid peptides in presumed dopamine target neurons that mediate motivation and reward, as well as motor control, provide further evidence for profound consequences of in utero cocaine exposure on the developing dopamine neurocircuitry.
Footnotes
This work was supported by the Medical Research Foundation of Oregon and U.S. Department of Health and Services Grant DA-07165, Population P30 Program Project Grant HD-18185, and Animal Resources Branch Grant RR-00163 for operation of the Oregon Regional Primate Research Center. L.C. was supported in part by Public Health Service Grant T32 HD-01733. Cocaine hydro chloride was obtained from the Research Triangle Institute (Research Triangle Park, NC) through the National Institute of Drug Abuse (Bethesda, MD) distribution program. We thank Drs. Martin J. Kelly, Charles E. Roselli, and John A. Resko for helpful suggestions in the preparation of this manuscript. The technical assistance of Martha A. Bosch, Barry R. Naylor, and Brett Hall is gratefully acknowledged. This is Publication No. 2021 of the Oregon Regional Primate Research Center.
Correspondence should be addressed to Dr. Oline K. Rönnekleiv, Department of Physiology and Pharmacology, L334, Oregon Health Sciences University, 3181 SW Sam Jackson Park Road, Portland, OR 97201-3098.
Dr. Choi’s present address: Department of Anatomy School of Medicine, Gyeongsang National University, Chinju, Korea.
REFERENCES
- 1.Angulo JA. Involvement of dopamine D1 and D2 receptors in the regulation of proenkephalin mRNA abundance in the striatum and accumbens of the rat brain. J Neurochem. 1992;58:1104–1109. doi: 10.1111/j.1471-4159.1992.tb09368.x. [DOI] [PubMed] [Google Scholar]
- 2.Asselin M-C, Soghomonian J, Côté P, Parent A. Striatal changes in preproenkephalin mRNA levels in parkinsonian monkeys. NeuroReport. 1994;5:2137–2140. doi: 10.1097/00001756-199410270-00037. [DOI] [PubMed] [Google Scholar]
- 3.Augood SJ, Emson PC, Mitchell IJ, Boyce S, Clarke CE, Crossman AR. Cellular localization of enkephalin gene expression in MPTP-treated cynomolgus monkeys. Mol Brain Res. 1989;6:8592. doi: 10.1016/0169-328x(89)90032-6. [DOI] [PubMed] [Google Scholar]
- 4.Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three-year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]
- 5.Battaglia G, Cabrera TM, Van de Kar LD. Prenatal cocaine produces biochemical and functional changes in brain serotonin systems in rat progeny. In: Thadani PV, editor. Biological mechanisms and perinatal exposure to abused drugs. National Institute on Drug Abuse; Rockville, MD: 1995. pp. 115–148. [PubMed] [Google Scholar]
- 6.Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- 7.Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Mol Brain Res. 1994;14:231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- 8.Chirgwin JM, Pryzbyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 9.Choi WS, Rönnekleiv OK. Effects of in utero cocaine exposure on the expression of mRNAs encoding the dopamine transporter and the D1, D2 and D5 dopamine receptor subtypes in fetal rhesus monkey. Dev Brain Res. 1996;96:249–260. doi: 10.1016/0165-3806(96)00123-x. [DOI] [PubMed] [Google Scholar]
- 10.Choi WS, Machida CA, Rönnekleiv OK. Distribution of dopamine D1, D2, and D5 receptor mRNAs in the monkey brain: ribonuclease protection assay analysis. Mol Brain Res. 1995;31:86–94. doi: 10.1016/0169-328x(95)00038-t. [DOI] [PubMed] [Google Scholar]
- 11.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 12.Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comb M, Seeberg PH, Adelman JP, Eiden L, Herbert E. Primary structure of the human met- and leu-enkephalin precursor and its mRNA. Nature. 1982;295:663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- 14.Costa JJL, Averill S, Saaverdra JP, Priestley JV. Serotonin innervation of enkephalin containing neurones in the rat spinal trigeminal nucleus. Neurosci Lett. 1994;168:167–171. doi: 10.1016/0304-3940(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 15.Cregler LL, Mark H. Medical complications of cocaine abuse. N Engl J Med. 1986;315:1495–1500. doi: 10.1056/NEJM198612043152327. [DOI] [PubMed] [Google Scholar]
- 16.Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, Sutcliffe JG. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- 17.Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- 18.Daunais JB, McGinty JF. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Mol Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]
- 19.Daunais JB, Roberts DCS, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. NeuroReport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Fang Y, Janowsky A, Rönnekleiv OK. Effect of chronic cocaine exposure on the densities of dopamine D1- and D2-like receptor binding in fetal rhesus monkey brain. Soc Neurosci Abstr. 1996;22:1881. [Google Scholar]
- 21.Felten DL, Sladek JR. Monoamine distribution in primate brain. V. Monoaminergic nuclei: anatomy, pathways and local organization. Brain Res Bull. 1983;10:171–284. doi: 10.1016/0361-9230(83)90045-x. [DOI] [PubMed] [Google Scholar]
- 22.Fries MH, Kuller JA, Norton ME, Yankowitz J, Kobori J, Good WV, Ferriero D, Cox V, Donlin SS, Golabi M. Facial features of infants exposed prenatally to cocaine. Teratology. 1993;48:413–420. doi: 10.1002/tera.1420480505. [DOI] [PubMed] [Google Scholar]
- 23.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 24.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 25.Gribnau AAM, Geijsberts LGM. Developmental stages in the rhesus monkey (Macaca mulatta). In: Brodal A, Hild W, Limborgh JV, Ortmann R, Pauly JE, Schiebler TH, Wolff E, editors. Advances in anatomy, embryology and cell biology. Springer; New York: 1981. pp. 1–84. [DOI] [PubMed] [Google Scholar]
- 26.Haber SN, Lu W. Distribution of preproenkephalin messenger RNA in the basal ganglia and limbic-associated regions of the monkey telencephalon. Neuroscience. 1995;65:417–429. doi: 10.1016/0306-4522(94)00490-v. [DOI] [PubMed] [Google Scholar]
- 27.Harlan RE. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987;258:159–184. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- 28.Hess DL, Spies HG, Hendrickx AG. Diurnal steroid patterns during gestation in the rhesus macaque: onset, daily variation, and the effects of dexamethasone treatment. Biol Reprod. 1981;24:609–616. doi: 10.1095/biolreprod24.3.609. [DOI] [PubMed] [Google Scholar]
- 29.Horikawa S, Talkai T, Toyosato M, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H, Miyata T, Numa S. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306:611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- 30.Hökfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy. Elsevier; Amsterdam: 1984. pp. 277–379. [Google Scholar]
- 31.Hume RF, O’Donnell KJ, Stanger CL, Killiam AP, Gingras JL. In utero cocaine exposure: observations of fetal behavioral state may predict neonatal outcome. Am J Obstet Gynecol. 1989;161:685–690. doi: 10.1016/0002-9378(89)90380-3. [DOI] [PubMed] [Google Scholar]
- 32.Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- 33.Hyman SE, Konradi C, Robierski L, Cole R, Senatus P, Green D (1994) Pharmacologic regulation of striatal proenkephalin gene expression via transcription factor CREB. Mol Neurobiol 155–171. [PubMed]
- 34.Jaber M, Fournier MC, Bloch B. Reserpine treatment stimulates enkephalin and D2 dopamine receptor gene expression in the rat striatum. Mol Brain Res. 1992;15:189–194. doi: 10.1016/0169-328x(92)90108-n. [DOI] [PubMed] [Google Scholar]
- 35.Konradi C, Kobierski LA, Nguyen TV, Heckers S, Hyman SE. The cAMP-response-element-binding protein interacts, but Fos protein does not interact, with the proenkephalin enhancer in rat striatum. Proc Natl Acad Sci USA. 1993;90:7005–7009. doi: 10.1073/pnas.90.15.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostrzewa RM. Dopamine receptor supersensitivity. Neurosci Biobehav Rev. 1995;19:1–17. doi: 10.1016/0149-7634(94)00019-w. [DOI] [PubMed] [Google Scholar]
- 37.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 38.Laurent-Huck FM, Anguelova E, Rene F, Stoeckel ME, Felix JM. Ontogeny of prodynorphin gene expression in the rat hypothalamus. Dev Brain Res. 1993;75:45–53. doi: 10.1016/0165-3806(93)90064-h. [DOI] [PubMed] [Google Scholar]
- 39.Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci USA. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li SJ, Sivam SP, McGinty JF, Jiang HK, Douglass J, Calavetta L, Hong JS. Regulation of the metabolism of striatal dynorphin by the dopaminergic system. J Pharmacol Exp Ther. 1988;246:403–408. [PubMed] [Google Scholar]
- 42.Madras BK, Kaufman MJ. Cocaine accumulates in dopamine-rich regions of primate brain after i.v. administration: comparison with mazindol distribution. Synapse. 1994;18:261–275. doi: 10.1002/syn.890180311. [DOI] [PubMed] [Google Scholar]
- 43.Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessment in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995;95:539–545. [PubMed] [Google Scholar]
- 44.Nisbet AP, Foster OJF, Kingsbury A, Eve DJ, Daniel SE, Marsden CD, Lees AJ. Preproenkephalin and preprotachykinin messenger RNA expression in normal human basal ganglia and in Parkinson’s disease. Neuroscience. 1995;66:361–376. doi: 10.1016/0306-4522(94)00606-6. [DOI] [PubMed] [Google Scholar]
- 45.Peña AE, Burchfield DJ, Abrams RM. Myocardial and cerebral oxygen delivery are not adversely affected by cocaine administration to early-gestation fetal sheep. Am J Obstet Gynecol. 1996;174:1028–1032. doi: 10.1016/s0002-9378(96)70345-9. [DOI] [PubMed] [Google Scholar]
- 46.Pollack AE, Wooten GF. Differential regulation of striatal preproenkephalin mRNA by D1 and D2 dopamine receptors. Mol Brain Res. 1992;12:111–119. doi: 10.1016/0169-328x(92)90074-l. [DOI] [PubMed] [Google Scholar]
- 47.Radja F, El Mansari M, Soghomonian J-J, Dewar KM, Ferron A, Reader TA, Descarries L. Changes of D1 and D2 receptors in adult rat neostriatum after neonatal dopamine denervation: quantitative data from ligand binding, in situ hybridization and iontophoresis. Neuroscience. 1993;57:635–648. doi: 10.1016/0306-4522(93)90011-4. [DOI] [PubMed] [Google Scholar]
- 48.Regalado MG, Schechtman VL, Del Angel AP, Bean XD. Sleep disorganization in cocaine-exposed neonates. Infant Behav Dev. 1995;18:319–327. [Google Scholar]
- 49.Richfield EK, Maguire-Zeiss KA, Cox C, Gilmore J, Voorn P. Reduced expression of preproenkephalin in striatal neurons from Huntington’s disease patients. Ann Neurol. 1995;37:335–343. doi: 10.1002/ana.410370309. [DOI] [PubMed] [Google Scholar]
- 50.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 51.Rönnekleiv OK, Naylor BR. Chronic cocaine exposure in the fetal rhesus monkey: consequences for early development of dopamine neurons. J Neurosci. 1995;15:7330–7343. doi: 10.1523/JNEUROSCI.15-11-07330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato M, Morita Y, Saika T, Fujita M, Ohhata K, Tohyama M. Localization and ontogeny of cells expressing preprodynorphin mRNA in the rat cerebral cortex. Brain Res. 1991;541:41–49. doi: 10.1016/0006-8993(91)91071-8. [DOI] [PubMed] [Google Scholar]
- 53.Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 54.Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- 55.Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramidal and limbic dynorphin systems. J Pharmacol Exp Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- 56.Soghomonian J. Effects of neonatal 6-hydroxydopamine injections on glutamate decarboxylase, preproenkephalin and dopamine D2 receptor mRNAs in the adult rat striatum. Brain Res. 1993;621:249–259. doi: 10.1016/0006-8993(93)90113-2. [DOI] [PubMed] [Google Scholar]
- 57.Spangler R, Unterwald EM, Kreek MJ. “Binge” cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 58.Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. Dev Behav Pediat. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Surmeier DJ, Kitai ST. Dopaminergic regulation of striatal efferent pathways. Curr Opin Neurobiol. 1994;4:915–919. doi: 10.1016/0959-4388(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 60.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tecott LH, Rubenstein LR, Paxinos G, Evans CJ, Eberwine JH, Valentino KL. Developmental expression of proenkephalin mRNA and peptides in rat striatum. Dev Brain Res. 1989;49:75–86. doi: 10.1016/0165-3806(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 62.Wang H-Y, Runyan S, Yadin E, Friedman E. Prenatal exposure to cocaine selectively reduces D1 dopamine receptor-mediated activation of striatal Gs proteins. J Pharmacol Exp Ther. 1995;273:492–498. [PubMed] [Google Scholar]
- 63.Weisinger G. The transcriptional regulation of the preproenkephalin gene. Biochem J. 1995;307:617–629. doi: 10.1042/bj3070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams JT, Lacey MG. Actions of cocaine on central monoamine neurons: intracellular recordings in vitro. In: Harris LS, editor. Problems of drug dependence. National Institute on Drug Abuse; Rockville, MD: 1988. pp. 234–242. [PubMed] [Google Scholar]
- 65.Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- 66.Young WS, III, Bonner TI, Brann MR. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci USA. 1986;83:9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zagon IS, Isayama T, McLaughlin PJ. Preproenkephalin mRNA expression in the developing and adult rat brain. Mol Brain Res. 1994;21:85–98. doi: 10.1016/0169-328x(94)90381-6. [DOI] [PubMed] [Google Scholar]