Abstract
Amyloid β-protein (Aβ), the principal constituent of the senile plaques seen in Alzheimer’s disease (AD), is derived by proteolysis from the β-amyloid precursor protein (βPP). The distribution and trafficking of cell surface βPP are of particular interest because some of these molecules are direct precursors of secreted Aβ and because the localization of βPP at the cell surface may be related directly to its physiological functions. Recently, we reported that, in cultured hippocampal neurons, cell surface βPP is preferentially expressed on axons in a striking discontinuous pattern. In this study, we describe the colocalization of cell surface βPP and integrins in primary cultured cells. In rat hippocampal neurons, cell surface βPP was colocalized selectively with α1β1 and α5β1 integrin heterodimers at these characteristic segmental locations. In rat cortical astrocytes, both cell surface βPP and β1 integrin were located at the cell periphery in the “spreading” stage shortly after plating. In “flattened” astrocytes cultured for several days, βPP was found in punctate deposits called point contacts. In these sites, βPP was colocalized with α1β1, but not with α5β1 integrin heterodimers, the latter of which were situated at focal contact sites. In both neurons and astrocytes examined after shearing, clathrin and α-adaptin were colocalized with βPP on the surface that directly contacts the substratum. These results are consistent with the putative role of βPP in cell adhesion and suggests that βPP either interacts with selected integrins or shares similar cellular machinery to promote cell adhesion.
Keywords: amyloid β-protein precursor, amyloid β-protein, integrins, cell adhesion, clathrin, substrate attachment, point contacts
Alzheimer’s disease (AD) is a progressive, neurodegenerative disorder characterized by the extracellular deposition of the 39–43 amino acid amyloid β-protein (Aβ) in the brain parenchyma (Selkoe, 1994). Aβ is derived by proteolytic cleavages from the β-amyloid precursor protein (βPP) (Kang et al., 1987). Constitutive cleavage of βPP by “α-secretase” occurringin vivo and in vitro results in the secretion of the N-terminal ectodomain βPPS. The ubiquitous α-secretase cleavage occurs within the Aβ region and therefore precludes the generation of an intact Aβ peptide (Esch, 1990). In contrast to most non-neural cells, neurons and glia secrete relatively small amounts of βPPS for unclear reasons (Haass et al., 1991). Some βPP molecules not cleaved on the cell surface are internalized and targeted to endosomes and lysosomes. A smaller population of βPP molecules seems to be recycled back to the surface for secretion or internalization (Koo et al., 1996). Both the secretory and endocytic pathways have been shown to contribute to Aβ released into medium by cultured cells, although endocytic processing seems to be the predominant source (Koo and Squazzo, 1994).
The physiological role of βPP remains to be defined. Proposed functions for βPPS include neurite-promoting properties, wound healing, cell adhesion, cell growth and differentiation, and inhibition of proteases and coagulation factor XIa (for review, seeSaitoh and Mook-Jung, 1996). Full-length membrane-bound βPP may function as a cell surface receptor capable of interacting with G-proteins (Nishimoto et al., 1993) and in cell adhesion. The latter function is consistent with the binding of βPP with laminin and proteoglycans (Small et al., 1996). Which of these diverse functions predominates in brain is unclear.
In cultured neurons, cell surface βPP is located predominantly in axons, where it subsequently can undergo retrograde and trans-cytotic transport. Surprisingly, on the axonal surface, βPP displayed a characteristic patchy pattern. In particular, βPP is distributed in discontinuous and irregularly spaced segments along the entire length of the axon (Yamazaki et al., 1995). This pattern of βPP distribution on the axonal surface is in sharp contrast to the diffuse localization seen intracellularly. We hypothesize, therefore, that this unique βPP localization on the axonal surface is related to its putative role of βPP in cell adhesion.
To investigate further the basis for this intriguing distribution of βPP on the surface of neurons and to gain insights into the function of surface molecules, we conducted a detailed immunocytochemical analysis of the distribution of βPP, integrins, clathrin, and α-adaptin in different neural cells. Our results demonstrate that βPP is colocalized with integrins on the surface of both neurons and astrocytes. In the latter cells, βPP accumulates at sites of point contact, but not at focal contact sites. In both cell types, βPP shows a tight association with the α1β1 integrin heterodimer. These observations suggest that βPP either interacts directly with selected integrins or, more likely, shares similar cellular machinery to promote attachment of cells to the substratum.
MATERIALS AND METHODS
Cultures
Rat hippocampal neurons. Hippocampal cultures were prepared from embryonic day 18 rats as previously described (Yamazaki et al., 1995). In brief, cells from the dissected hippocampi were dissociated by trypsin (0.25% for 15 min at 37°C), followed by trituration with fire-polished Pasteur pipettes. The cells were plated at a density of 100,000 cells/60 mm culture dish on glass coverslips coated with poly-l-lysine (1 mg/ml) in MEM with 10% horse serum. After 2–4 hr, the medium was changed to 1 ml of MEM with N2 supplements, ovalbumin (0.1%), and pyruvate (0.01 mg/ml) that had been conditioned in cultures of astroglial cells for 24 hr. Coverslips plated with neurons were cocultured with astroglia.
Rat sympathetic neurons. Methods of dissecting rat cervical sympathetic neurons from P1 newborn rats were described previously (Yamazaki et al., 1995). Dissociated neurons were plated on glass coverslips coated with either rat type 1 collagen (50 ng/cm2) or laminin (10 μg/ml). Cultures were maintained in serum-free medium for 7 d without astroglial coculture.
Rat type 1 astrocytes. Cultures highly enriched in type 1 astrocytes (>95%) were prepared according to methods described previously (Tawil et al., 1993). Postnatal day 1 rat cerebral cortices were treated with trypsin (0.25% in MEM) for 30 min at 37°C, and cells were seeded into poly-l-lysine-coated flasks after trituration. After 10 d in culture, flasks were shaken overnight. Then the cells were seeded on glass coverslips coated with poly-l-lysine (5 μg/ml), laminin (0.5–5.0 μg/cm2), collagen (50 ng/cm2), and fibronectin (2.0–10.0 μg/cm2). Cells were cultured for 3 hr (“spreading cells”) or 3 d (“flattened cells”) after plating before being prepared for immunocytochemistry.
Antibodies
The monoclonal antibodies 5A3 and 1G7 (Koo and Squazzo, 1994) and the goat polyclonal antibody 207 (gift of Dr. B. Greenberg, Cephalon, West Chester, PA) (Shoji et al., 1992) made against human βPPS from transfected Chinese hamster ovary cells (CHO) or baculovirus-infected Sf9 cells, respectively, were used in the studies. 5A3 and 1G7 recognize nonoverlapping epitopes in the midregion of the βPP ectodomain, and these two monoclonal antibodies were used together to obtain higher signal (Yamazaki et al., 1995). The polyclonal antibody C7 (Podlisny et al., 1991) was raised against the C-terminal 20 amino acids of βPP. At the immunocytochemical level, these anti-βPP antibodies do not recognize amyloid precursors like protein 2 (APLP2) expressed in transfected CHO cells (Yamazaki et al., 1995). The monoclonal antibody 3A3 (gift of Dr. S. Carbonetto, McGill University, Montreal, Canada) was directed against the extracellular domain of the rat α1 integrin subunit (Turner et al., 1989). Rabbit polyclonal anti-β1 antiserum (gift of Dr. Carbonetto) was made to a purified extracellular domain of the rat β1 integrin subunit (Tawil et al., 1990). Monoclonal antibodies X22 (Brodsky, 1985) and AP.6 (Chin et al., 1989) were raised against clathrin heavy chain and 100 kDa α-adaptin, respectively (gift of Dr. F. Brodsky, University of California, San Francisco, CA). Additional antibodies included those directed at the intracellular domain of the β1 integrin subunit (Chemicon, Temecula, CA), the α5 subunit of integrins (Chemicon), transferrin receptor (gift of Dr. I. Trowbridge, Salk Institute, La Jolla, CA), synaptophysin (Boehringer Mannheim, Indianapolis, IN), MAP2 (Sigma, St. Louis, MO), focal adhesion kinase (FAK; Transduction Laboratories, Lexington, KY), L1 (Developmental Studies of Hybridoma Bank, Iowa City, IA), and neural cell adhesion molecule (NCAM; Chemicon).
Immunocytochemistry
Cultured cells were fixed for 20 min with warm 4% formaldehyde in PBS containing 0.12 m sucrose. If necessary, cells were permeabilized in 0.3% Triton X-100 for 5 min at room temperature after fixation. After being blocked in 10% BSA for 1 hr at 37°C, the fixed cultures were exposed to primary antibodies overnight at 4°C. After several PBS washes, the cells were incubated for 1 hr with rhodamine or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). To label intracellular molecules together with cell surface βPP, we labeled cell surface βPP first, followed by permeabilization and incubation with a second primary antibody. In some experiments, double-labeling immunocytochemistry was performed with two mouse monoclonal antibodies as described previously (Yamazaki et al., 1995). The staining patterns of the antibodies used in double-labeling studies are identical to immunostaining with each of the respective antibodies alone. So that bleedthrough of the fluorescence images in double-labeling experiments could be further excluded, no signal could be detected from single-labeled cultures using FITC-conjugated secondary antibody when they were visualized with the rhodamine filter set, and vice versa. Experiments in which cells were sheared were performed essentially as described (Avnur and Geiger, 1981). Briefly, cells on coverslips were rinsed in buffer A (50 mm 4-morpholine-ethanesulfonic acid (MES), 5 mm MgCl2, and 3 mm EGTA, pH 6.0) and then incubated in buffer B (buffer A plus 1 mmZnCl2) for 2 min at room temperature. The cells were sheared from the coverslips with several brisk streams of PBS, pH 7.2, from a Pasteur pipette, thereby leaving behind only those cellular regions on the ventral surface in contact with the substratum. Then these preparations were fixed and labeled as described.
RESULTS
Cell surface βPP is colocalized with integrins in cultured neurons
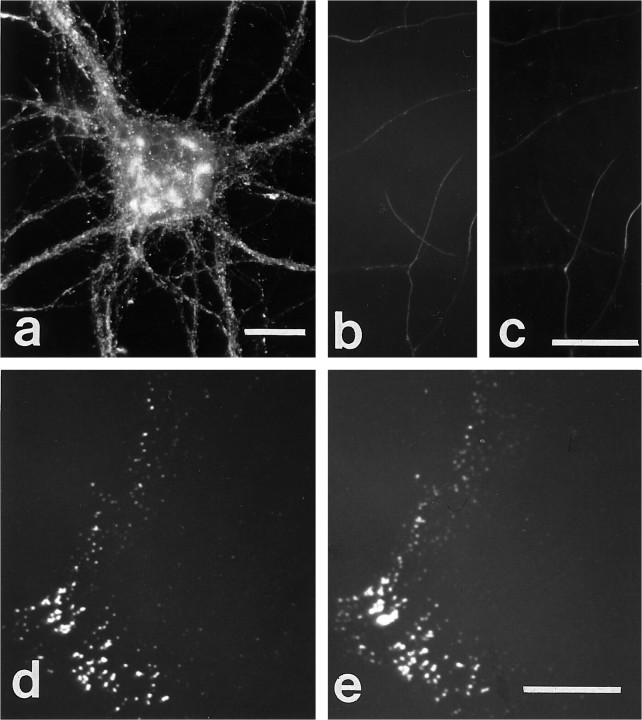
We recently showed that full-length βPP on neurites has a characteristic discontinuous patchy pattern of fine granularity on the axonal surface in cultured hippocampal neurons (Yamazaki et al., 1995). To investigate the basis for this characteristic distribution of neuronal surface βPP, we initially examined neurons for colocalization of integrins and βPP, because earlier reports suggested that βPP might play a role in cell adhesion. Hippocampal neurons cultured for 14 d were fixed and incubated with anti-βPP monoclonal antibodies (1G7/5A3) without permeabilization and well characterized anti-β1 integrin antibodies. The β1 integrin antibody produced a patchy staining pattern consisting of fine granularity on the neurites of hippocampal neurons (Fig. 1a) that completely colocalized with cell surface βPP staining (Fig.1b). This association between β1 integrin and cell surface βPP also was observed for the α1 and α5 integrin subunits. Immunostaining by antibodies to these two α subunits demonstrated the same colocalization of patchy cell surface staining with cell surface βPP (Fig. 1c–f), implying that βPP is colocalized with the α1β1 and α5β1 integrin heterodimers. The distribution of βPP on the cell surface has been shown to be predominantly on axons. Accordingly, the integrins also localized preferentially on axons (data not shown). In addition, we have observed previously that βPP is only variably present on the surface of growth cones (Yamazaki et al., 1995). This observation extends to the association with integrins as well, such that β1 integrin and βPP seemed to be present on growth cones coordinately (Fig.2a–d).
Fig. 1.
Immunocytochemical colocalization of cell surface βPP and integrins in cultured hippocampal neurons. A hippocampal neuron cultured for 14 d was double-labeled for β1 integrin (a) and cell surface βPP (5A3/1G7)(b). The axonal pattern from both immunostaining reactions was patchy and overlapped entirely. β1 integrin and βPP on the perikaryal surface cannot be compared clearly, because that region of the cell body is not within the plain of focus of the photomicrographs. α1 (c) and α5 (e) subunits of integrins also were colocalized with cell surface βPP (d, f) along neurites. Scale bars, 5 μm.
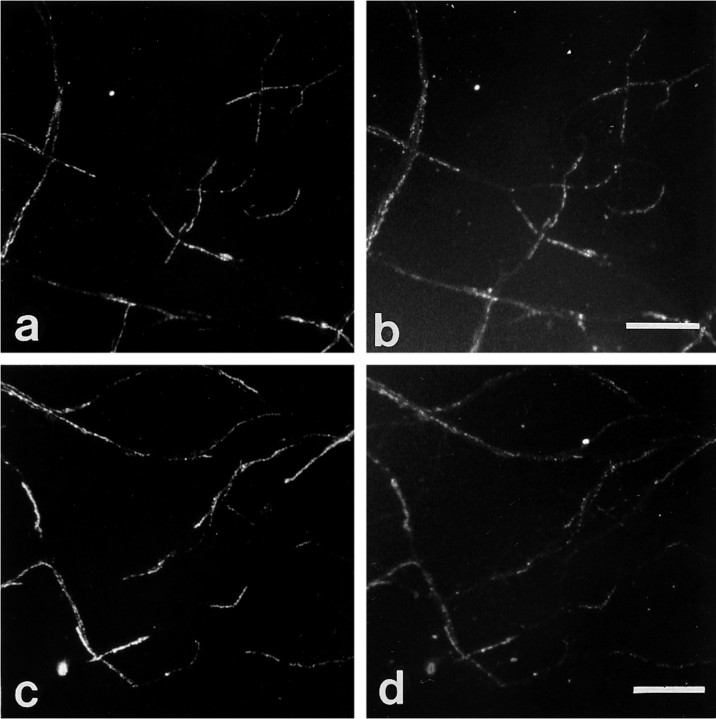
Fig. 2.
Immunocytochemical colocalization of cell surface βPP and integrins at growth cones. Shown are two examples of colocalization of cell surface βPP (a, c) at growth cones with β1 (b) and α5 (d) integrins in mature hippocampal neurons in culture, as seen by double-labeling. The patchy surface distribution of βPP is highlighted in a, where the arrowheadstrace out the segment of axon devoid of surface βPP immunoreactivity. To examine cell substrate contact sites, we sheared neurons (see Materials and Methods) and stained them with anti-βPP antibodies (5A3/1G7)(e). βPP was localized on neurites (arrows) and on the cell body in a granular pattern. Neuronal cell adhesion molecule (NCAM) staining (f) showed a diffuse, rather than a patchy, pattern on neurites. Scale bars, 5 μm.
At the cell body, βPP showed a fine punctate surface staining pattern. Immunoreactivity for the integrins was distributed similarly, although the resolution of the microscopy was such that it could not be ascertained whether the reactivity was located on the cell body itself or on axons that had traversed the soma. To examine more closely the ventral surfaces of the neuronal cell body and processes, we used a technique that shears the apical surface of the neuron, leaving behind only portions of the ventral surface that are adherent to the substratum (Avnur and Geiger, 1981). Labeling these sheared cells with anti-βPP antibodies demonstrated granular staining in both cell bodies and neurites (Fig. 2e; see also Fig.5b), and this staining colocalized with that for β1 integrin (data not shown, see below). These observations suggest that at least a portion of βPP molecules is located on both cell bodies and axons that are tightly attached to the substratum.
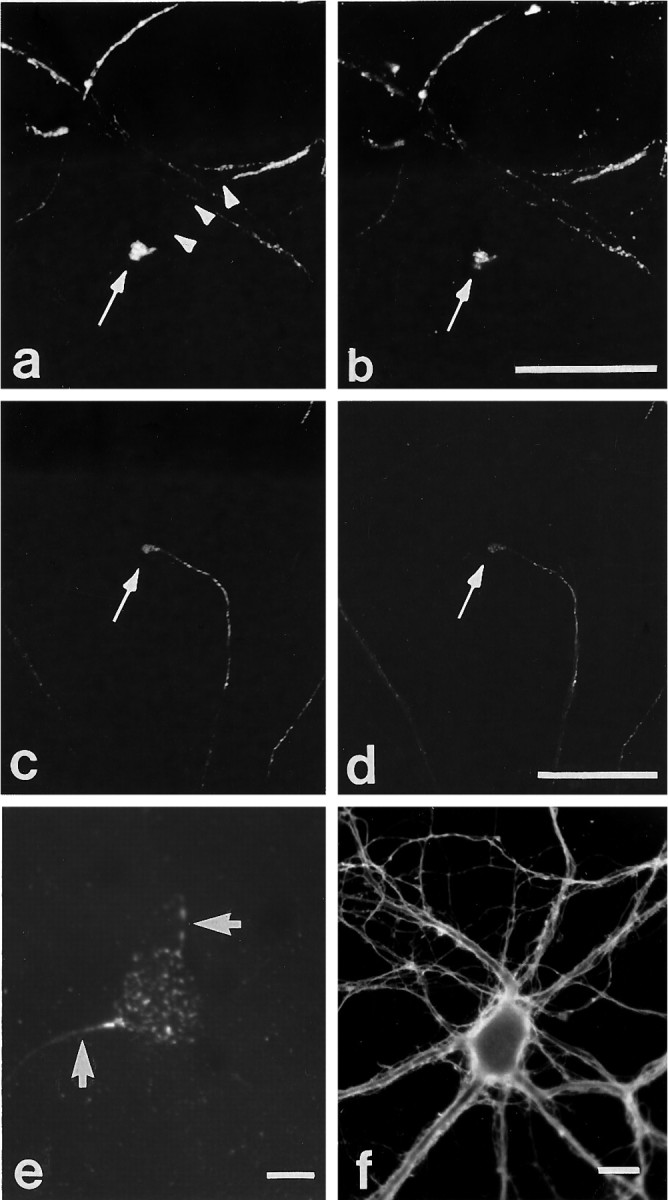
Fig. 5.
Immunocytochemical colocalization of cell surface βPP with clathrin and α-adaptin. a, A hippocampal neuron cultured for 10 d labeled with an anti-clathrin antibody (X22) showed a fine punctate staining pattern on neurites, but its distribution was not patchy and occurred predominantly in axons (compare with βPP shown in Fig. 1b). On the other hand, at substrate contact sites visualized in sheared neurons, βPP (antibody 207) (b) and clathrin (c) were specifically colocalized. Within the immunoreactive patches, there is a suggestion of fine granular staining. In sheared astrocytes, βPP (207) (d) also was tightly colocalized with α-adaptin, as demonstrated by antibody AP.6 (e). Scale bars, 5 μm.
To investigate whether this association between βPP and integrins is substrate-dependent, we cultured neurons in defined medium without serum and without cocultured astrocytes. Extracellular matrix components are present in serum and also are released by astrocytes, and these could affect our analyses. Therefore, peripheral sympathetic neurons were cultured in serum-free medium on either type 1 collagen- or laminin-coated coverslips. Regardless of the substrate, the tight colocalization of βPP and β1 integrin remained and showed the characteristic discontinuous distribution (Fig. 3).
Fig. 3.
Immunocytochemical colocalization of cell surface βPP and β1 integrin in sympathetic ganglion neurons cultured for 7 d on type 1 collagen- or laminin-coated glass coverslips in serum-free medium. On both type 1 collagen (a, b) and laminin (c, d), cell surface βPP (a, c) and β1 integrin (b, d) showed the characteristic segmental pattern and tight colocalization by double labeling. Scale bars, 10 μm.
It should be noted that antibodies raised against either the extracellular or the intracellular domains of β1 integrin (the latter examined in permeabilized cells) showed identical colocalization with cell surface βPP (examined without permeabilization) in all of the experiments described above. Moreover, double staining of neurons for synaptophysin or for transferrin receptor demonstrated no specific colocalization with cell surface βPP (data not shown). Finally, in all experiments described above, no staining was observed when the cells were incubated with secondary antibodies alone or with nonimmune mouse IgG. Antibodies to NCAM demonstrated a diffuse distribution along the axonal and dendritic processes, with no specific colocalization with cell surface βPP (Fig. 2f), and did not show an axonal predominance. Furthermore, another neuronal adhesion molecule, L1, also showed a continuous staining pattern similar to NCAM on both axons and dendrites, with no colocalization with βPP (data not shown). Therefore, the association between βPP and integrins is not generalized to other classes of adhesion molecules.
Cell surface βPP is specifically expressed at point contact sites in rat type 1 astrocytes
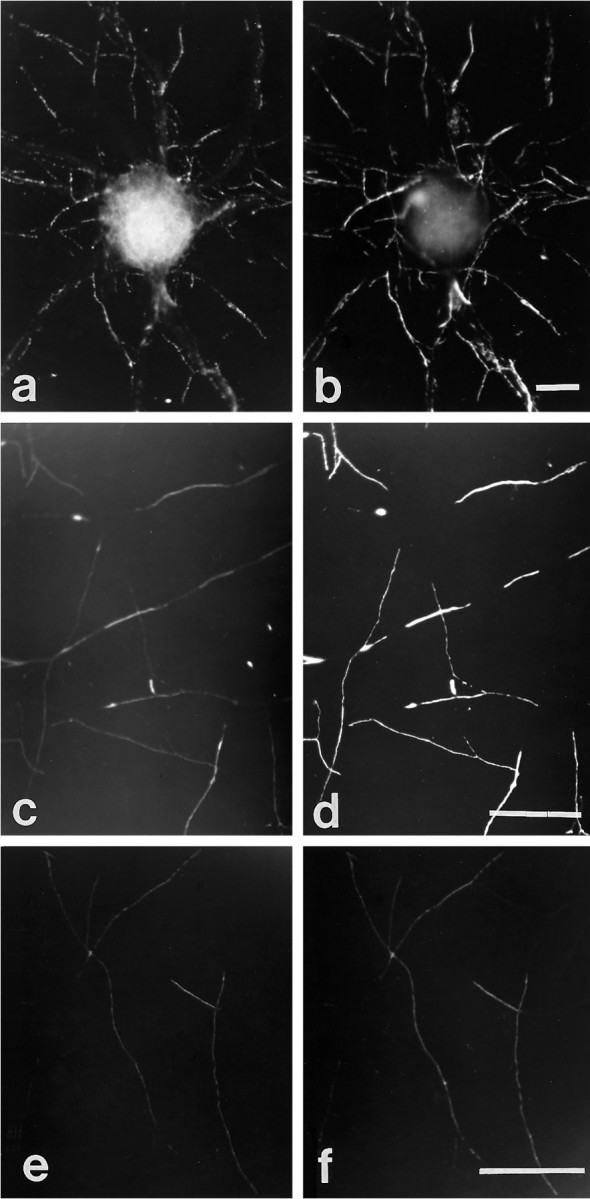
To investigate further the relationship between cell surface βPP and integrins, we analyzed rat type 1 astrocytes. The rationale is based on the observations that the heterodimers of integrin subunits are differentially expressed during astrocyte attachment and spreading in culture: α1β1 integrin accumulates at point contacts, whereas α5β1 integrin is associated with focal contacts, the latter seen in flattened astrocytes after long-term culture (Tawil et al., 1993). Using this culture paradigm, we located cell surface βPP at the periphery of the spreading astrocytes shortly after plating, where the staining colocalized tightly with β1 integrin (Fig. 4a,b). In contrast, cell surface βPP in fully flattened astrocytes that had been cultured for 3 d appeared in a fine punctate pattern diffusely distributed on the plasma membrane (Fig. 4c). In these flattened cells it has been shown that the anti-β1 integrin antibody we used showed two distinct patterns: linear (corresponding to focal contacts) and small punctate staining (corresponding to point contacts) (Tawil et al., 1993). We found that only the latter pattern of β1 integrin reactivity colocalized with cell surface βPP (Fig.4c,d). More specifically, the labeling of sheared, flattened astrocytes with anti-βPP showed punctate staining that colocalized with the α1 integrin subunit (Fig.4e,f), which has been found only at the point contact sites on the plasma membrane that are in contact with the substratum (Tawil et al., 1993). In contrast, the α5 integrin subunit, located at linear focal contact sites (Fig. 4h), colocalized with vinculin and FAK (data not shown), but not with βPP (Fig. 4g). Moreover, antibodies raised against both extracellular (1G7/5A3) and intracellular (C7) domains of βPP labeled these punctate sites (Fig. 4i,j) in sheared preparations, indicating that the cell surface βPP located at point contacts on the ventral surface represents full-length molecules. Taken together, these observations suggest that, in cultured type 1 astrocytes, cell surface full-length βPP is tightly colocalized with α1β1 integrins at point contact sites, but not with α5β1 integrins at focal contact sites. Essentially identical results were obtained when astrocytes were cultured on laminin, fibronectin, collagen, or poly-l-lysine substrates (data not shown).
Fig. 4.
Immunocytochemical colocalization of cell surface βPP and integrins in type 1 astrocytes. Type 1 astrocytes were allowed to attach and spread on laminin or fibronectin for 3 hr (spreading stage) and then were labeled with βPP (5A3/1G7) (a) and β1 integrin (b) antibodies. Both molecules were located mainly at the periphery and in the middle of the spreading cells. In cells cultured for 3 d before fixation (“flattened” astrocytes), cell surface βPP (c) and β1 integrin (d) were now colocalized at point contact sites. More specifically, surface βPP (antibody 207)(e) was tightly colocalized with the α1 subunit of integrins (f) when examined in sheared astrocytes. In contrast, the α5 subunit of integrins was localized in focal contact sites, appearing as linear streaks in the sheared cells (h, arrows), and it did not colocalize with βPP (5A3/1G7) (g). At point contact sites, staining with βPP midregion (1G7/5A3) (i) and C-terminal (C7) (j) antibodies in sheared cells showed complete colocalization, suggesting that βPP at the substrate surface represents full-length molecules. Scale bars:a–h, 10 μm; i, j, 5 μm.
βPP at contact sites is colocalized with clathrin and α-adaptin
βPP is internalized via the receptor-mediated pathway, an observation consistent with the presence of a signal (GNENPTY) for internalization in the cytoplasmic domain at residues 756–762 (βPP770 numbering) (Lai et al., 1995). A previous immunocytochemical study showing partial colocalization of the βPP C terminus with clathrin could not ascertain whether the molecules are associated with sites of substrate attachment (Ferreira at al., 1993). Recent evidence suggests that integrin, α-adaptin, and clathrin are colocalized with each other at point contacts in astrocytes and fibroblasts (Nermut et al., 1991; Tawil et al., 1993). On the basis of these findings, we examined hippocampal cultures with antibodies recognizing clathrin heavy chain and α-adaptin. Unlike βPP on the axonal surface (Fig.1b,d), the staining of clathrin after permeabilization of neurons was distributed diffusely throughout all neurites (Fig.5a). When these neurons were examined after shearing, however, βPP and clathrin, as well as α-adaptin (data not shown), showed specific colocalization (Fig. 5b,c) at these substrate contact sites. Similarly, in sheared astrocytes, βPP at point contact sites (Fig. 5d) was strongly colocalized with α-adaptin (Fig. 5e) and clathrin (data not shown).
DISCUSSION
The aim of this study was to investigate a potential function of βPP in cell adhesion by characterizing the distribution of cell surface βPP with respect to a number of adhesion molecules in different neural cell types, namely, cultured rat neurons and astrocytes. Our earlier observation that βPP on the axonal surface shows an unusual and distinctive patchy appearance prompted us to postulate that this discontinuous distribution is related to the putative role of βPP in adhesion (Yamazaki et al., 1995). Our novel results demonstrate that βPP surface molecules do, indeed, colocalize with specific integrin heterodimers. In particular, the α1β1 integrin heterodimer at point contacts in neurons and in spreading astrocytes showed essentially complete colocalization with surface βPP. This association is selective for the integrins, because other adhesion molecules examined, NCAM and L1 (Schachner, 1989), did not exhibit this colocalization. Thus, the results suggest that surface βPP molecules accumulate preferentially at contact sites where they may interact with membrane-bound integrin receptors or function in an integrin-like manner in cell adhesion.
Integrins serve as cell adhesion molecules and receptors for the extracellular matrix, and they consist of two nonidentical subunits, α and β (Hynes, 1992). The precise combination of integrin subunits determines their cellular distribution, functions, and types of ligands. In cultured rat type 1 astrocytes, for example, the α5β1 integrin mediates adhesion to fibronectin, whereas the α1β1 heterodimer mediates adhesion to laminin and collagen (Tawil et al., 1993). In addition, α5β1 accumulates preferentially at focal contact sites and α1β1 at point contacts. By microscopy, point contacts are regions of the cell surface closely apposed to the substratum (Streeter and Rees, 1987; Nermut et al., 1991). The exact functional role of point contacts is not known, although they seem to mediate cell adhesion during the stage of cell spreading. The strong colocalization between βPP and integrins shown here, particularly in sheared cell preparations, indicates that full-length βPP inserted in the plasma membrane contributes to the adhesion of cells to the substrate. These contact sites on neurites occur as patches and not punctate dot-like structures, as seen in astrocytes at point contacts. Whether these segments represent the coalescence of multiple fine granules or point contacts cannot be ascertained from our preparations. Furthermore, the absence of colocalization of βPP with integrins at focal contact sites argues against βPP functioning in an integrin-like manner in signal transduction involving focal adhesion kinase, as occurs in these latter sites (Clark and Brugge, 1995). Finally, our studies show that, in astrocytes, cell surface βPP was colocalized only with α1β1, but not with α5β1 integrin, whereas in hippocampal neurons it localized with both α1β1 and α5β1 integrins. Thus, these distinct patterns of βPP distribution and integrin association may reflect different functions of cell surface βPP molecules in different cell types.
In spreading type 1 astrocytes, cell surface βPP at the periphery colocalized not only with the α1β1 integrin heterodimer but also with clathrin and α-adaptin. Clathrin and α-adaptin are components of coated pits that participate in the endocytosis of cell surface receptors. The consensus sequence, NPXY, known to mediate such internalization via clathrin-coated pits, is present not only in β1 integrin but also in the βPP cytoplasmic tail (Chen et al., 1990). It has been suggested that endocytosis of integrins is linked intimately with cell motility via cycling of surface receptors at the cell periphery in the clathrin-mediated pathway (Bretscher, 1989; Tawil et al., 1993). Interestingly, surface βPP also enters clathrin-coated vesicles during trafficking in the receptor-mediated endocytic pathway (Nordstedt et al., 1993; Yamazaki et al., 1996). In addition, we have shown that a population of surface βPP is recycled rapidly after its internalization (Koo et al., 1996; Yamazaki et al., 1996). In this context, the association of selected integrins and βPP at the cell surface, followed by processing in the the endocytic pathway, may be important in the transient cell–substratum contacts that participate in the motility of cells and neurites. This proposed mechanism would be consistent with the observations that both βPP and integrins are associated with the cytoskeleton (Refolo et al., 1991; Allinquant et al., 1994; Arregui et al., 1994). Therefore, the similarities of these two molecules potentially extend to providing a mechanical link between the internal cytoskeletal network and extracellular matrix proteins.
Several studies have provided direct or indirect evidence that the βPPS and other βPP secretory products can mediate cell–cell or cell–substrate adhesion in culture (Schubert et al., 1989; Chen and Yankner, 1991; Milward et al., 1992; Koo et al., 1993). One active domain that mediates this effect seems to be within the Aβ domain itself, specifically at the RHDS tetrapeptide motif (at residues 5–8 of Aβ) (Ghiso et al., 1992; Saporito-Irwin and Van Nostrand, 1995). Similarity of the Aβ RHDS sequence to the RGDS binding motif recognized by many integrin receptors has led to the demonstration that secreted Aβ modulates adhesion via interaction with integrin-like receptors (Ghiso et al., 1992; Sabo et al., 1995). This interaction at the RHDS tetrapeptide motif has not been demonstrated with membrane-bound full-length βPP, although cell surface βPP molecules have been shown to promote neuronal adhesion and neurite outgrowth (Breen et al., 1991; Qiu et al., 1995). The latter effects of cell surface βPP are consistent with the impairment of neurite outgrowth and adhesion from neurons incubated with antisense oligonucleotides (Allinquant et al., 1995) or transfected with antisense βPP construct to lower βPP synthesis (Leblanc et al., 1992), respectively. Interaction of βPP at the cell surface with extracellular matrix (ECM) molecules, such as laminin and heparin (Kibbey et al., 1993;Small et al., 1996), may be one mechanism by which βPP contributes to neurite outgrowth. However, it is not clear which βPP substrate, surface-bound full-length βPP or βPPS or both, actually interacts with the ECM in vivo (Klier et al., 1990). Nonetheless, taken together, we hypothesize that βPP at the cell surface functions in an integrin-like manner by interacting with similar ECM and intracellular cytoskeletal constituents at sites of point contacts to facilitate neuronal adhesion and outgrowth. Although our study did not address the nature of this βPP/integrin association, it is tempting to postulate that a direct βPP and integrin interaction occurs at the cell surface, possibly via the RHDS sequence, to produce a synergistic effect on cell adhesion.
Finally, it is not clear whether the association of βPP and integrins plays a role in Alzheimer’s disease. Aβ is, of course, the principal constituent of senile plaques, and, in “classical” plaques, βPP-containing dystrophic neurites are found within and around these deposits. Interestingly, various integrin receptors and their ligands also have been detected within senile plaques by immunocytochemistry (Eikelenboom et al., 1994). It is possible that these molecules participate in the cascade of events that result in amyloidogenesis in brain and in the subsequent formation of the senile plaque. Whether the association between βPP and integrins we have described in this study impacts on AD pathology remains an interesting speculation that awaits further investigation.
Footnotes
This work was supported by National Institutes of Health Grants AG06173 and HL49552 (D.J.S.) and AG12376 (E.H.K.) and the Paul Beeson Physician Faculty Scholar in Aging Research from the American Federation for Aging Research (E.H.K.). The monoclonal antibody ASCS4 (L1), developed by Dr. Paul Patterson, was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, and the Department of Biological Sciences, University of Iowa, Iowa City, IA, under contract N01-HD-2-3144 from the National Institute of Child Health and Human Development. We thank Drs. Elizabeth Hay, Martin Hemmler, Tomas Kirchausen, and Carl Lagenaur for helpful discussions; Dr. Lisa Flanagan for reviewing this manuscript; and Drs. Frances Brodsky, Salvatore Carbonetto, Barry Greenberg, and Ian Trowbridge for their generous gift of antibodies.
Correspondence should be addressed to Dr. Edward H. Koo, Department of Neurosciences 0691, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0691.
Dr. Yamazaki’s present address: Department of Neuropathology, Institute for Brain Research, University of Tokyo School of Medicine, Tokyo 113, Japan.
REFERENCES
- 1.Allinquant B, Moya KL, Bouillot C, Prochiantz A. Amyloid precursor protein in cortical neurons: coexistence of two pools differentially distributed in axons and dendrites and associated with cytoskeleton. J Neurosci. 1994;14:6842–6854. doi: 10.1523/JNEUROSCI.14-11-06842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allinquant B, Hantraye P, Mailleux P, Moya J, Bouillot C, Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol. 1995;128:919–927. doi: 10.1083/jcb.128.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arregui CO, Carbonetto S, McKerracher L. Characterization of neural cell adhesion sites: point contacts are the sites of interaction between integrins and the cytoskeleton in PC12 cells. J Neurosci. 1994;14:6967–6977. doi: 10.1523/JNEUROSCI.14-11-06967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avnur Z, Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol. 1981;153:361–379. doi: 10.1016/0022-2836(81)90283-7. [DOI] [PubMed] [Google Scholar]
- 5.Breen KC, Bruce M, Anderton BH. Beta amyloid precursor protein mediates neuronal cell-cell and cell-surface adhesion. J Neurosci Res. 1991;28:90–100. doi: 10.1002/jnr.490280109. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher MS. Endocytosis and recycling of fibronectin receptor in CHO cells. EMBO J. 1989;8:1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodsky FM. Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J Cell Biol. 1985;101:2047–2054. doi: 10.1083/jcb.101.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Yankner BA. An antibody to β amyloid and the amyloid precursor protein inhibits cell-substratum adhesion in many mammalian cell types. Neurosci Lett. 1991;125:223–226. doi: 10.1016/0304-3940(91)90034-q. [DOI] [PubMed] [Google Scholar]
- 9.Chen W-J, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated-pit mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 10.Chin DJ, Straubinger RM, Acton S, Nathke I, Brodsky FM. 100 kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Eikelenboom P, Zhan SS, Kamphorst W, van der Valk P, Rozemuller JM. Cellular and substrate adhesion molecules (integrins) and their ligands in cerebral amyloid plaques in Alzheimer’s disease. Virchows Arch. 1994;424:421–427. doi: 10.1007/BF00190565. [DOI] [PubMed] [Google Scholar]
- 13.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993;13:3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiso J, Rostagno A, Gardella JE, Liem L, Gorevic PD, Frangione B. A 109-amino-acid C-terminal fragment of Alzheimer’s-disease amyloid precursor protein contains a sequence, -RHDS-, that promotes cell adhesion. Biochem J. 1992;288:1053–1059. doi: 10.1042/bj2881053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haass C, Hung AY, Selkoe DJ. Processing of β-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991;11:3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 18.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 19.Kibbey MC, Jucker M, Weeks BS, Neve RL, Van Nostrand WE, Kleinman HK. β-Amyloid precursor protein binds to the neurite-promoting IKVAV site of laminin. Proc Natl Acad Sci USA. 1993;90:10150–10153. doi: 10.1073/pnas.90.21.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klier FG, Cole G, Stallcup W, Schubert D. Amyloid β-protein precursor is associated with extracellular matrix. Brain Res. 1990;515:336–342. doi: 10.1016/0006-8993(90)90619-m. [DOI] [PubMed] [Google Scholar]
- 21.Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 22.Koo EH, Park L, Selkoe DJ. Amyloid β-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci USA. 1993;90:4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell surface amyloid β-protein precursor. I. Secretion, endocytosis, and recycling detected by labeled monoclonal antibody. J Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- 24.Lai A, Sisodia SS, Trowbridge IS. Characterization of sorting signals in the β-amyloid precursor protein cytoplasmic domain. J Biol Chem. 1995;270:3565–3573. [PubMed] [Google Scholar]
- 25.LeBlanc AC, Kovacs DM, Chen HY, Villare F, Tykocinski M, Autilio-Gambetti L, Gambetti P. Role of amyloid precursor protein (APP): study with antisense transfection of human neuroblastoma cells. J Neurosci Res. 1992;31:635–645. doi: 10.1002/jnr.490310407. [DOI] [PubMed] [Google Scholar]
- 26.Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 27.Nermut MV, Eason P, Hirst EMA, Kellie S. Cell substratum adhesions in RSV-transformed rat fibroblasts. Exp Cell Res. 1991;193:382–397. doi: 10.1016/0014-4827(91)90111-7. [DOI] [PubMed] [Google Scholar]
- 28.Nishimoto I, Okamoto T, Matsuura Y, Takahashi S, Okamoto T, Murayama Y, Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G0. Nature. 1993;362:75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- 29.Nordstedt C, Caporaso GL, Thyberg J, Gandy SE, Greengard P. Identification of the Alzheimer BAP amyloid precursor protein in clathrin-coated vesicles purified from PC12 cells. J Biol Chem. 1993;268:608–612. [PubMed] [Google Scholar]
- 30.Podlisny MB, Tolan DR, Selkoe DJ. Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer’s disease. Am J Pathol. 1991;138:1423–1435. [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ. Cell-surface β-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. J Neurosci. 1995;15:2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Refolo LM, Wittenberg IS, Friedrich VL, Jr, Robakis NK. The Alzheimer amyloid precursor is associated with the detergent-insoluble cytoskeleton. J Neurosci. 1991;11:3888–3897. doi: 10.1523/JNEUROSCI.11-12-03888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabo S, Lambert MP, Kessey K, Wade W, Krafft G, Klein WL. Interaction of beta-amyloid peptides with integrins in a human nerve cell line. Neurosci Lett. 1995;184:25–28. doi: 10.1016/0304-3940(94)11159-g. [DOI] [PubMed] [Google Scholar]
- 34.Saitoh T, Mook-Jung I. Commentary. Is understanding the biological function of APP important in understanding Alzheimer’s disease? Alzheimer Dis Rev. 1996;1:30–36. doi: 10.3233/jad-1999-14-509. [DOI] [PubMed] [Google Scholar]
- 35.Saporito-Irwin SM, Van Nostrand WE. Coagulation factor XIa cleaves the RHDS sequence and abolishes the cell adhesive properties of the amyloid β-protein. J Biol Chem. 1995;270:26265–26269. doi: 10.1074/jbc.270.44.26265. [DOI] [PubMed] [Google Scholar]
- 36.Schachner M. Families of neural adhesion molecules. Ciba Found Symp. 1989;145:156–172. doi: 10.1002/9780470513828.ch10. [DOI] [PubMed] [Google Scholar]
- 37.Schubert D, Jin L-W, Saitoh T, Cole G. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 38.Selkoe DJ. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- 39.Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai X-D, McKay DM, Tintner R, Frangione B, Younkin SG. Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 40.Small DH, Clarris HL, Williamson TG, Reed G, Key B, Mok SS, Beyreuther K, Masters CL, Nurcombe V. Neurite outgrowth-regulating functions of the amyloid protein precursor of Alzheimer’s disease. Alzheimer Dis Rev. 1996;1:21–29. doi: 10.3233/jad-1999-14-508. [DOI] [PubMed] [Google Scholar]
- 41.Streeter HB, Rees DA. Fibroblast adhesion to RGDS shows novel features compared with fibronectin. J Cell Biol. 1987;105:507–515. doi: 10.1083/jcb.105.1.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tawil NJ, Houde M, Blacher R, Esch F, Reichert LF, Turner DC, Carbonetto S. α1β1 integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry. 1990;29:6540–6544. doi: 10.1021/bi00479a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tawil N, Wilson P, Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs focal contacts. J Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner DC, Flier LA, Carbonetto S. Identification of a cell surface protein involved in PC12 cell–substratum adhesion and neurite outgrowth on laminin and collagen. J Neurosci. 1989;9:3287–3296. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki T, Selkoe DJ, Koo EH. Trafficking of cell surface β-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J Cell Biol. 1995;129:431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki T, Koo EH, Selkoe DJ. Trafficking of cell surface amyloid β-protein precursor. II. Endocytosis, recycling, and lysosomal targeting detected by immunolocalization. J Cell Sci. 1996;109:999–1008. doi: 10.1242/jcs.109.5.999. [DOI] [PubMed] [Google Scholar]