Abstract
The actions of the endogenous ORL1-receptor ligand nociceptin on the membrane properties and synaptic currents in rat periaqueductal gray (PAG) neurons were examined by the use of whole-cell patch-clamp recording in brain slices. Nociceptin produced an outward current in all neurons tested, with an EC50 of 39 ± 7 nm. The outward current was unaffected by naloxone. Outward currents reversed polarity at −110 ± 3 mV in 2.5 mm extracellular potassium, and the reversal potential increased when the extracellular potassium concentration was raised (slope = 66.3 mV/log[K+]omm). Thus, the nociceptin-induced outward current was attributable to an increased K+ conductance. Nociceptin inhibited evoked fast GABAergic (IPSCs) and glutamatergic (EPSCs) postsynaptic currents and increased paired-pulse facilitation in a subpopulation of PAG neurons. Nociceptin inhibited evoked IPSCs and EPSCs in ∼50% of neurons throughout the PAG, except in the ventrolateral PAG, where nociceptin inhibited evoked IPSCs in most neurons. Nociceptin decreased the frequency of spontaneous miniature postsynaptic currents (mIPSCs and mEPSCs) in a subpopulation of PAG neurons but had no effect on their amplitude distributions. Thus, nociceptin had a presynaptic inhibitory effect on transmitter release. These findings suggest that nociceptin, via its pre- and postsynaptic actions, has the potential to modulate the analgesic, behavioral, and autonomic functions of the PAG.
Keywords: orphan receptor, ORL1 receptor, opioid receptor, nociceptin, potassium channel, presynaptic inhibition, periaqueductal gray, analgesia
An orphan opioid-like receptor, ORL1, recently has been identified that is highly homologous to the cloned μ-, δ-, and κ-receptors (Mollereau et al., 1994; Lachowicz et al., 1995). An endogenous heptadecapeptide nociceptin, or orphanin FQ, has been identified more recently that acts as a potent agonist at the ORL1 receptor (Meunier et al., 1995; Reinscheid et al., 1995). Nociceptin has been demonstrated to inhibit potently the forskolin-stimulated adenylyl cyclase activity in cells transfected with ORL1, whereas etorphine and dynorphin A were weak agonists, and other opioids were inactive (Mollereau et al., 1994;Lachowicz et al., 1995; Meunier et al., 1995; Reinscheid et al., 1995;Zhang and Yu, 1995). Recently, we have demonstrated that nociceptin increases a K+ conductance in dorsal raphe (Vaughan and Christie, 1996a) and locus coeruleus neurons (Connor et al., 1996a). The K+ conductance activated by nociceptin is the same as that activated by various receptors known to couple to inhibitory G-proteins in these neurons, including μ-receptors (North et al., 1987; Williams et al., 1988; Chieng et al., 1996; Vaughan and Christie, 1996a) .
The midbrain periaqueductal gray (PAG) plays a crucial role in the integration of an animal’s behavioral, somatic, and autonomic responses to threat, stress, and pain (Lovick, 1993; Bandler and Shipley, 1994). In particular, the PAG is a major site for modulation of nociception, forming part of a descending antinociceptive system that relays via the rostral ventromedial medulla to the spinal cord (Basbaum and Fields, 1984). In situ hybridization and immunohistochemical studies have demonstrated high densities of ORL1 receptors within the PAG (Lachowicz et al., 1995;Anton et al., 1996). Furthermore, concentrations of nociceptin precursor mRNA in the PAG are among the highest in the CNS, suggesting that nociceptin might participate in modulation of nociception in this region (Houtani et al., 1996). However, intracerebroventricular injections of nociceptin, which might be expected to act on the PAG, either produced hyperalgesia or had little or no antinociceptive action (Meunier et al., 1995; Reinscheid et al., 1995). We have used the thin-slice patch-clamp technique to characterize the pre- and postsynaptic actions of nociceptin on PAG neurons.
MATERIALS AND METHODS
Sprague Dawley rats, 9–16 d old, were anesthetized with halothane and decapitated; their brains were removed quickly and immersed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl 126, KCl 2.5, NaH2PO41.4, MgCl2 1.2, CaCl2 2.4, glucose 11, and NaHCO3 25 and equilibrated with 95%O2:5%CO2. A vibratome was used to prepare horizontal slices (200–250 μm thick) containing the PAG, which were placed in a holding chamber containing oxygenated ACSF maintained at 32°C.
Brain slices were placed in a chamber (1.5 ml vol) mounted on the stage of an upright microscope (Olympus BH-2 with a fixed-stage modification) and viewed with a water immersion objective (Zeiss, 40×). Slices were superfused continuously (2 ml/min) with ACSF (32°C). Neurons located in the PAG were viewed with Nomarski optics and cleaned with a micropipette containing ACSF; then whole-cell voltage-clamp recordings were made with patch electrodes (3–7 MΩ). In experiments examining postsynaptic K+ conductance effects, the electrodes contained (in mm): potassium gluconate 140, NaCl 15, MgCl2 1, HEPES 10, EGTA 11, MgATP 2, and NaGTP 0.25, adjusted to a pH of 7.3 with KOH. In experiments examining evoked and miniature synaptic currents, the electrodes contained (in mm): CsCl 140, EGTA 10, HEPES 5, CaCl2 2, MgATP 2, and NaGTP 0.25, adjusted to a pH of 7.3 with CsOH. The osmolarity was adjusted to 270–290 mOsm/l. Liquid junction potentials of −11 mV for the K-gluconate solution and −4 mV for CsCl solution were calculated by using JPCalc and corrected (Barry, 1994). At the completion of recording, the location of the neuron was mapped onto standard horizontal midbrain sections (Paxinos and Watson, 1986).
Evoked postsynaptic currents were elicited via bipolar tungsten stimulating electrodes (tip separation 100 μm), which were placed 200–600 μm from the recorded neuron. Single stimuli or paired stimuli (interpulse interval 20–80 msec) were applied at a frequency of 0.03 Hz (stimuli: 5–50 V, 20–400 μsec), and four to eight consecutive responses were averaged for subsequent analysis with Clampfit (Axon Instruments, Foster City, CA).
Spontaneous, miniature postsynaptic currents were recorded on video tape via PCM, low-pass-filtered at 2–5 kHz, and digitized at 5–10 kHz (in 10–20 sec segments) for later off-line analysis (Axograph 3.0, Axon Instruments). Events were detected automatically by selecting events in which the difference between a 0.5–1 msec baseline epoch compared with a similar epoch 0.5–1 msec later exceeded a preset threshold (set to 6–15 pA for a rejection rate of at least 10%). Then automatically selected events were examined visually, and erroneous events were rejected before their amplitude and time of occurrence were measured. Events were ranked by amplitude and inter-event interval for preparation of cumulative probability distributions. The cumulative distributions were compared by the Kolmogorov–Smirnov (K–S) test. All data are expressed as mean ± SEM.
Stock solutions of all drugs were made in distilled water, except CNQX (made in dimethyl sulfoxide, DMSO). These were diluted to working concentrations by using ACSF and applied by superfusion. Nociceptin (Meunier et al., 1995; Reinscheid et al., 1995) was synthesized (>95% pure by HPLC) by Chiron Mimotopes (Clayton, Victoria, Australia). Methionine enkephalin (met-enkephalin) and baclofen were obtained from Sigma (St Louis, MO); (−)-bicuculline methiodide, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and naloxone hydrochloride were obtained from Research Biochemicals (Natick, MA).
RESULTS
Nociceptin produces an outward current in all PAG neurons
When PAG neurons were voltage-clamped to a potential of −60 mV, superfusion of nociceptin (Noc, 300 nm; n = 34) produced an outward current in all PAG neurons that responded to the subsequent application of the GABAB agonist baclofen (30 μm). A number of PAG neurons did not respond to baclofen, nociceptin, or met-enkephalin (n = 9). We previously have demonstrated with intracellular electrodes that baclofen hyperpolarizes all PAG neurons (Chieng and Christie, 1995). These nonresponding neurons were not considered in the following analysis, because it was uncertain whether they were damaged or formed a distinct population of PAG neurons not previously observed.
Nociceptin produced an outward current, irrespective of whether the neuron did (n = 15) or did not (n = 10) respond to met-enkephalin (ME, 10 μm; Fig.1A,B). Although naloxone (1 μm) reversed the outward current produced by met-enkephalin (n = 3), it had no effect on the outward current produced by nociceptin (n = 3). The mean amplitudes of the agonist-induced currents were 45 ± 4 pA for nociceptin (n = 19; 300 nm), 44 ± 5 pA for met-enkephalin (n = 15; 10 μm), and 55 ± 6 pA for baclofen (n = 15; 30 μm). The outward current produced by nociceptin was dose-dependent, with an EC50 of 39 ± 7 nm(Fig. 1C).
Fig. 1.
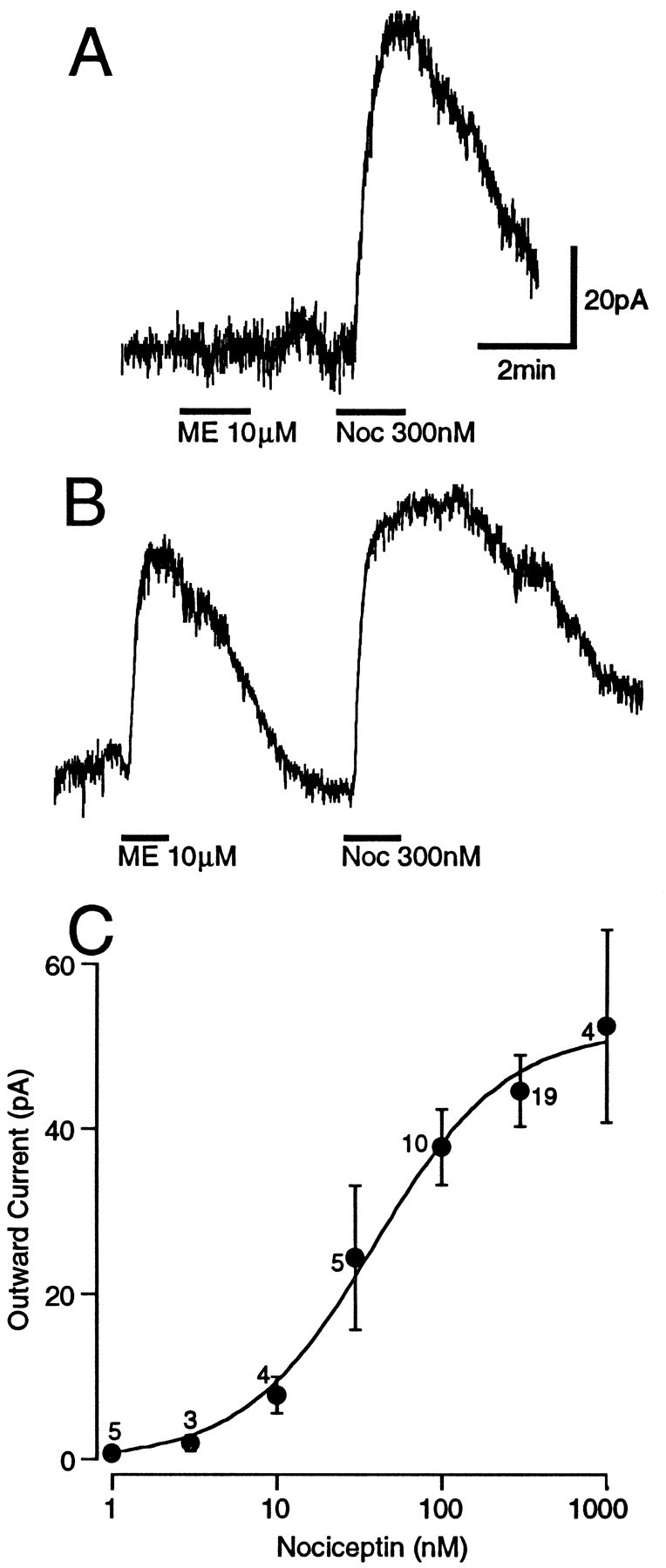
Nociceptin produces a dose-dependent outward current in all PAG neurons. Membrane current responses of PAG neurons in which A, met-enkephalin (ME 10 μm) had no effect, but nociceptin (Noc 300 nm) produced an outward current, andB, both met-enkephalin (ME 10 μm) and nociceptin (Noc 300 nm) produced an outward current. C, Concentration–response relationship for outward currents in PAG neurons produced by nociceptin. Each point shows the mean ± SEM of responses of several different neurons, with thenumber of cells indicated above thepoint. A logistic function was fit to determine the EC50 (39 ± 7 nm). Neurons were clamped to a potential of −60 mV.
Nociceptin opens an inwardly rectifying potassium current
The effect of nociceptin (300 nm) on the current–voltage relationship of PAG neurons was examined. The resting conductance showed inward rectification with slope conductances of 2.0 ± 0.4 nS and 3.1 ± 0.5 nS when measured between −60 to −90 mV and −110 to −130 mV, respectively (n = 6; Fig. 2A,B). Nociceptin increased the conductances to 2.7 ± 0.5 nS (p < 0.05, paired t test) and 5.0 ± 1.1 nS (p < 0.05, paired t test), when measured over the same potentials. The nociceptin-induced current reversal potential was −110 ± 3 mV, which is similar to the predicted value of −106 mV for the Nernst equation (Fig.2B). The reversal potential was shifted with increasing external potassium concentrations to −83 ± 4 mV in 6.5 mm [K+]o (n = 4) and to −68 ± 6 mV in 10.5 mm[K+]o (n = 3; slope = 66.3 mV/log[K+]o), which is similar to that predicted by the Nernst equation (slope = 60.9 mV/log[K+]o).
Fig. 2.
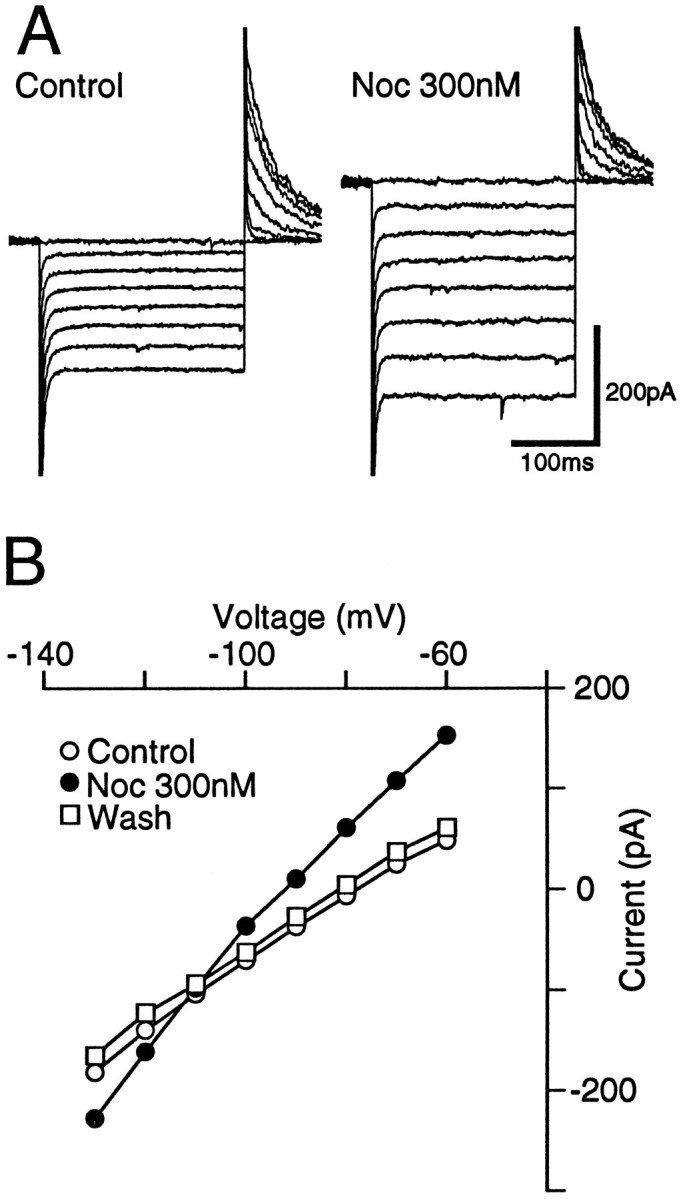
Nociceptin increases inwardly rectifying K+ conductance of PAG neurons. A, Voltage command steps of 250 msec duration were made in 10 mV increments from a holding potential of −60 to −140 mV. The resulting membrane currents in the absence (Control) and presence of nociceptin (Noc 300 nm) in a single neuron are shown. B, The current–voltage relationship for control (○), nociceptin (•), and wash (□) is plotted from the amplitudes of evoked currents shown in A.
In the presence of maximal concentrations of baclofen (30 μm; n = 3) and met-enkephalin (30 μm; n = 2), maximal concentrations of nociceptin (300 nm) produced no additional outward current. Thus, the outward current produced by nociceptin was mediated by the same K+ conductance produced by baclofen and met-enkephalin.
Effect of nociceptin on evoked synaptic currents
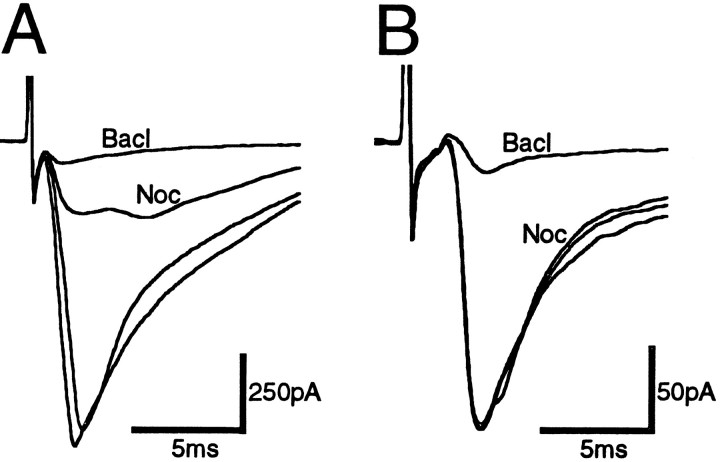
In the presence of the non-NMDA antagonist CNQX (10 μm), local stimulation evoked GABAergic IPSCs in PAG neurons, which had a mean amplitude of 656 ± 36 pA at −74 mV (n = 64) and were abolished by the GABAAantagonist bicuculline (30 μm; n = 3). Superfusion of nociceptin (300 nm) had a variable effect on the amplitude of evoked IPSCs (Fig. 3E). In 44 neurons, nociceptin reduced the amplitude of evoked IPSCs by 23–88% (mean = 49 ± 2%, Fig. 3A,C,E). In 20 neurons, nociceptin reduced the amplitude of evoked IPSCs by <15% (range = −13 to 11%; mean = 0 ± 2%; Fig.3B,D,E). In contrast, superfusion of the GABABagonist baclofen (10–30 μm) reduced the amplitude of evoked IPSCs in all PAG neurons tested by 63–98% (mean 83 ± 2%; n = 25), whether or not nociceptin inhibited evoked synaptic currents (Fig. 3A–D,F). The application of nociceptin or baclofen had no effect on the membrane current or the conductance of the neurons at −74 mV (Cs-filled electrodes used).
Fig. 3.
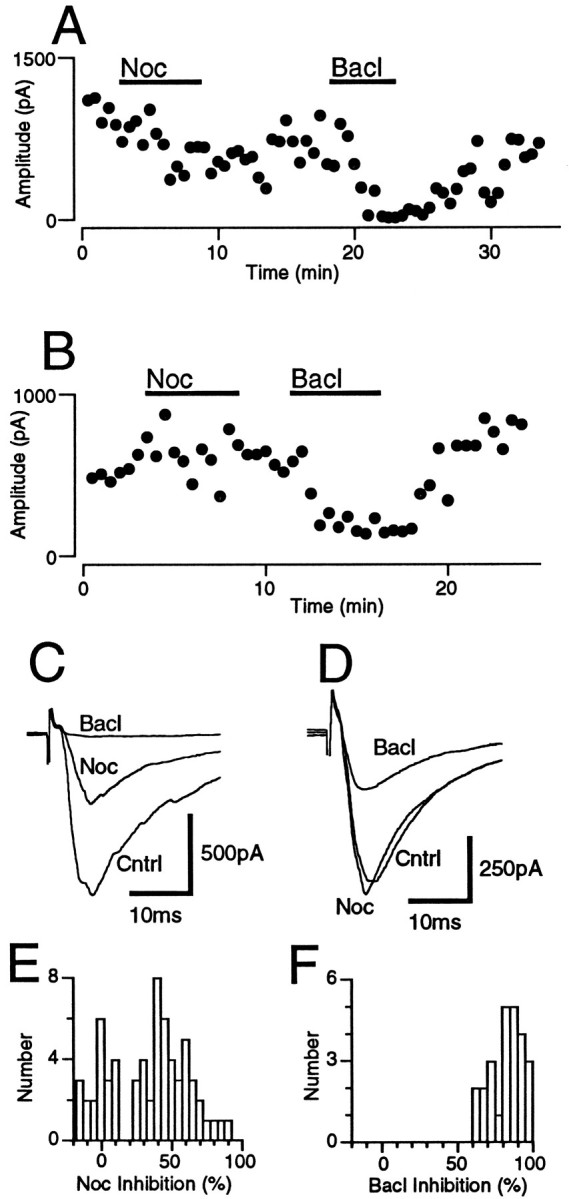
Nociceptin inhibited evoked GABAergic IPSCs in a subpopulation of PAG neurons. Evoked IPSCs were produced by local stimulation in the presence of CNQX (10 μm). Shown is time course of the amplitude of evoked IPSCs (eIPSC) during the application of nociceptin (Noc; 300 nm) and then baclofen (Bacl; 10 μm) for PAG neurons in which nociceptin did (A) and did not (B) inhibit synaptic transmission. IPSCs were evoked every 30 sec. C, D, Averaged traces (n = 4) of evoked IPSCs before and then during application of nociceptin (Noc; 300 nm) and baclofen (Bacl; 10 μm) for the time plots of A and B, respectively.E, F, Histograms of the inhibition of the evoked IPSC produced by (E) nociceptin and (F) baclofen in all neurons. Neurons were clamped to a potential of −74 mV.
In the presence of bicuculline (30 μm), local stimulation evoked non-NMDA EPSCs in PAG neurons, which had a mean amplitude of 338 ± 33 pA at −74 mV (n = 27) and were abolished by CNQX (10 μm; n = 2). Superfusion of nociceptin (300 nm) had a variable effect on the amplitude of evoked EPSCs. In 13 neurons, nociceptin reduced the amplitude of evoked EPSCs amplitude by 23–80% (mean = 46 ± 4%; Fig. 4A). In 14 neurons, nociceptin reduced the amplitude of evoked EPSCs by <15% (range = −15 to 14%; mean = 3 ± 2%; Fig. 4B). In contrast, superfusion of the GABAB agonist baclofen (10–30 μm) reduced the amplitude of evoked EPSCs in all PAG neurons tested by 62–92% (mean 82 ± 4%; n= 9; Fig. 4).
Fig. 4.
Nociceptin inhibited evoked glutamatergic EPSCs in a subpopulation of PAG neurons. Evoked EPSCs were produced by local stimulation in the presence of bicuculline (30 μm).A, Neuron in which nociceptin (300 nm) inhibited the evoked EPSC. B, Neuron in which nociceptin had no effect on the evoked EPSC. The subsequent application of baclofen (10 μm) inhibited the evoked EPSCs in bothA and B. Averaged traces (n = 4) are shown before, during nociceptin (Noc) and baclofen (Bacl), and after washout. Neurons were clamped to a potential of −74 mV.
Two stimuli of identical strength were applied in close succession (interstimulus interval 20–80 msec) to determine whether the nociceptin-induced inhibition of the first evoked response was associated with paired-pulse facilitation of the second evoked response (for neurons with inhibition > 20%). Under control conditions, the mean ratio of the amplitude of the paired evoked synaptic currents was 1.26 ± 0.16 for IPSCs (IPSC2/IPSC1;n = 11) and 1.58 ± 0.18 for the EPSCs (EPSC2/EPSC1; n = 8), with both paired-pulse facilitation and depression being observed. Superfusion of nociceptin produced an increase in the mean ratio of IPSC2/IPSC1 to 1.81 ± 0.19 (n = 11) and of EPSC2/EPSC1 to 2.04 ± 0.21 (n = 8). Thus, inhibition of the evoked synaptic currents was associated with an increase in paired-pulse facilitation of 54 ± 13% for the IPSCs (p < 0.005, paired t test;n = 11) and 61 ± 41% for the EPSCs (p < 0.05, paired t test;n = 8).
Effect of nociceptin on miniature synaptic currents
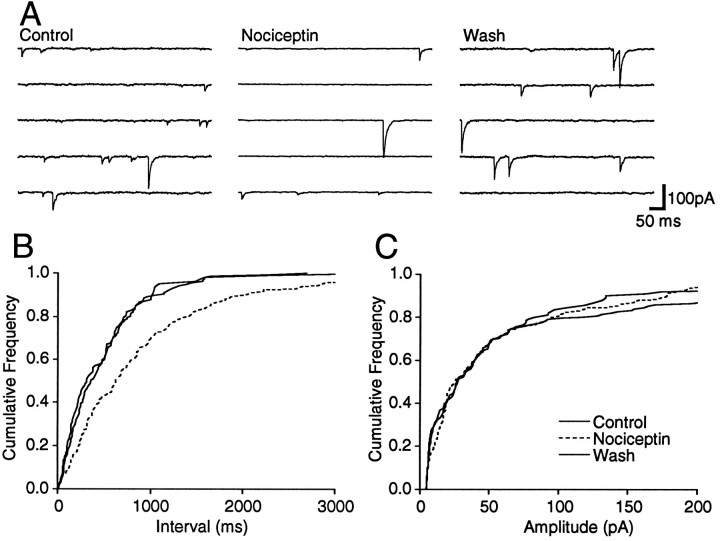
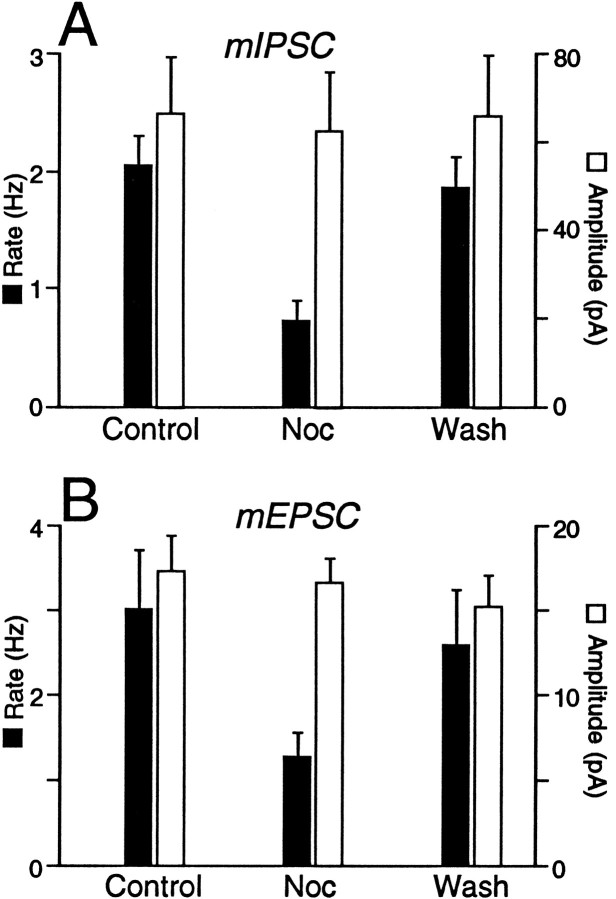
Spontaneous miniature IPSCs (mIPSCs) were readily observed during whole-cell voltage-clamp recordings in the presence of TTX (0.3 μm), a concentration that prevented Na-dependent action potentials and evoked postsynaptic currents. The mIPSCs were isolated by addition of CNQX (10 μm; Fig.5A) and were abolished by the addition of bicuculline (30 μm; n = 3). Superfusion of nociceptin (300 nm) had a variable effect on the frequency of mIPSCs. In six neurons, nociceptin produced a reversible reduction in the frequency of mIPSCs (Fig. 5A), as measured by a significant increase in the proportion of longer inter-event intervals between mIPSCs for each of these neurons (p < 0.05, K–S statistic for control/nociceptin; Fig. 5B). In contrast, nociceptin had no significant effect on the distribution of mIPSC amplitudes (p > 0.05, K–S statistic for control/nociceptin; Fig. 5C). On average, the mean mIPSC frequency was reduced by 63 ± 7% during superfusion of nociceptin, whereas the mean amplitude was reduced by 6 ± 12% in these neurons (Fig. 6A). In another five neurons, nociceptin had no significant effect on the distribution of mIPSC inter-event intervals or amplitudes (p> 0.05, K–S statistic for control/nociceptin).
Fig. 5.
Nociceptin decreases the frequency of spontaneous mIPSCs. Spontaneous mIPSCs were recorded in the presence of TTX (0.3 μm) and CNQX (10 μm). A, Five consecutive segments before, during, and after application of nociceptin (300 nm). B, C, Cumulative distribution plots of mIPSC inter-event interval and amplitude before, during (dashed line), and after application of nociceptin (number of events = 163, 177, and 112 for 80, 160, and 60 sec epochs of Control,Nociceptin, and Wash, respectively). Nociceptin had no effect on the amplitude distribution (p > 0.3, K–S statistic for control/nociceptin) but reversibly shifted the frequency distribution to longer inter-event intervals (p < 0.03, K–S statistic for control/nociceptin). All data are from the same neuron, which was clamped to a potential of −74 mV.
Fig. 6.
Nociceptin decreases the frequency, but has no effect on the amplitude, of both mIPSCs and mEPSCs in a subpopulation of PAG neurons. A, mIPSC andB, mEPSC frequency (filled bars) and mean amplitude (open bars) before, during, and after the application of nociceptin (300 nm) for those neurons in which there was a significant increase in inter-event interval (p < 0.05, K–S statistic for control/nociceptin). The pooled results of six neurons for mIPSCs and eight neurons for mEPSCs are shown. Error bars indicate ± SEM.
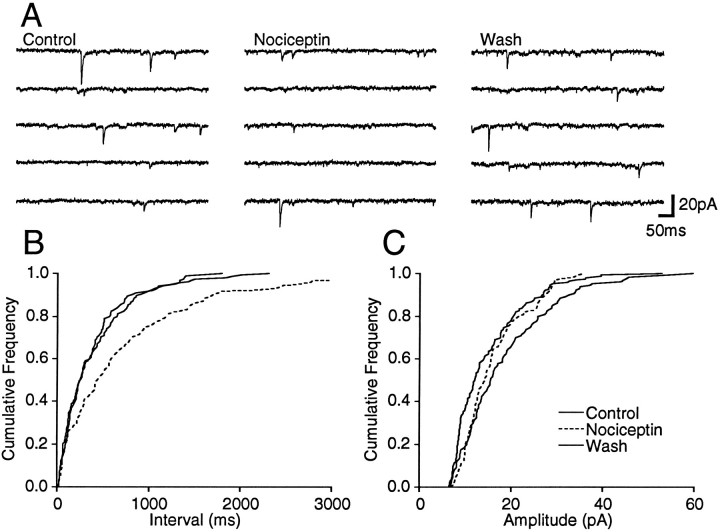
Spontaneous miniature excitatory postsynaptic currents (mEPSCs) were isolated by addition of TTX (0.3 μm) and bicuculline (30 μm) (Fig. 7A) and were abolished by the addition of CNQX (10 μm;n = 2). Superfusion of nociceptin (300 nm) had a variable effect on the frequency of spontaneous mEPSCs. In eight neurons, nociceptin produced a reversible reduction in the frequency of spontaneous mEPSCs (Fig. 7A), as measured by a significant increase in the proportion of longer inter-event intervals between mIPSCs for each of these neurons (p < 0.05, K–S statistic for control/nociceptin; Fig. 7B). In contrast, nociceptin had no significant effect on the distribution of mEPSC amplitudes (p > 0.1, K–S statistic for control/nociceptin; Fig. 7C). On average, the mean mIPSC frequency was reduced by 59 ± 3% during superfusion of nociceptin, whereas the mean amplitude was reduced by 4 ± 6% in these neurons (Fig. 6B). In another seven neurons, nociceptin had no significant effect on the distribution of mIPSC inter-event intervals or amplitudes (p > 0.1, K–S statistic for control/nociceptin).
Fig. 7.
Nociceptin decreases the frequency of spontaneous mEPSCs. Spontaneous mEPSCs were recorded in the presence of TTX (0.3 μm) and bicuculline (30 μm).A, Shown are five consecutive segments before, during, and after application of nociceptin (300 nm).B, C, Shown are cumulative distribution plots of mEPSC inter-event interval and amplitude before, during (dashed line), and after application of nociceptin (number of events = 131, 105, and 168 for 50, 80, and 70 sec epochs of Control, Nociceptin, andWash, respectively). Nociceptin had no effect on the amplitude distribution (p > 0.3, K–S statistic for control/nociceptin) but reversibly shifted the frequency distribution to longer inter-event intervals (p < 0.05, K–S statistic for control/nociceptin). All data are from the same neuron, which was clamped to a potential of −74 mV.
Distribution of nociceptin-responding PAG neurons
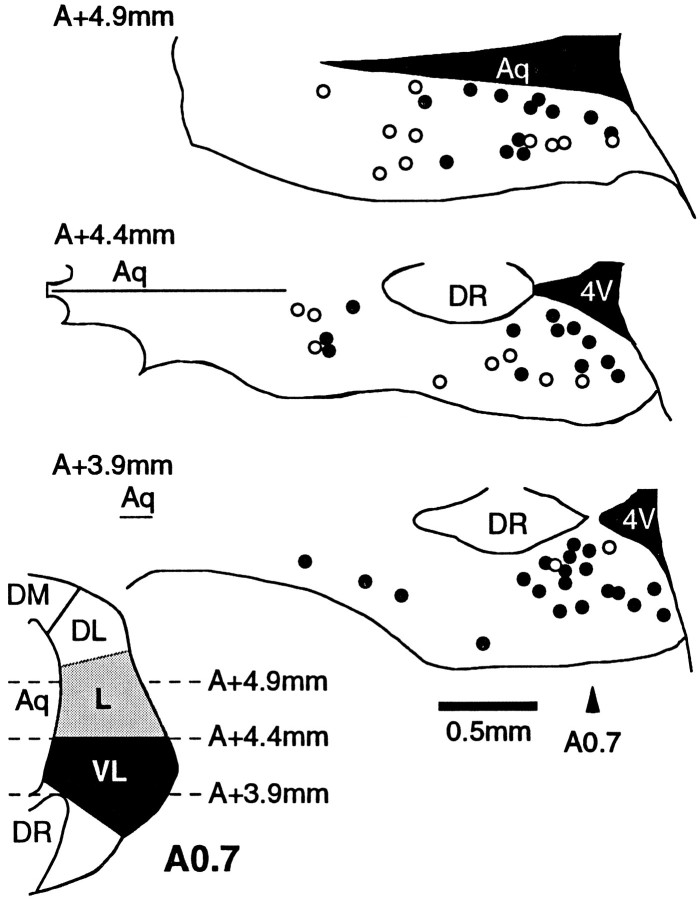
The location of recorded neurons was plotted onto standard horizontal PAG sections according to their location dorsal to the interaural plane. Presynaptic effects of nociceptin were observed in a subpopulation of PAG neurons (Fig. 8). The proportion of PAG neurons in which nociceptin inhibited evoked IPSCs varied with their dorsoventral location (p = 0.02; χ2 = 7.81; df = 2). Thus, 55% of neurons in the lateral PAG (n = 12/22; A +4.9 mm, 4.9 mm dorsal to the interaural plane), 60% of neurons in the intermediate PAG (n = 12/20; A +4.4 mm), and 91% of neurons in the ventrolateral PAG (n = 20/22; A +3.9 mm) displayed nociceptin-induced inhibition of GABAergic synaptic transmission of >15%. In contrast, the proportion of PAG neurons in which nociceptin inhibited evoked non-NMDA synaptic currents did not vary with their dorsoventral location (p > 0.5; χ2 = 0.06; df = 2). Thus, 45% of neurons in the lateral PAG (n = 5/11), 50% of neurons in the intermediate PAG (n = 3/6), and 50% of neurons in the ventrolateral PAG (n = 5/10) displayed nociceptin-induced inhibition of glutamatergic synaptic transmission of >15%.
Fig. 8.
Anatomical location of PAG neurons displaying nociceptin-sensitive and nociceptin-insensitive evoked IPSCs. Locations of PAG neurons in which evoked IPSCs were unaffected (○, inhibition <15%; n = 20) or inhibited (•;n = 44) by nociceptin (300 nm). Three dorsoventral levels of horizontal midbrain sections are shown (3.9, 4.4, and 4.9 mm dorsal to the interaural plane). Insetshows a coronal PAG section 0.7 mm rostral to the interaural plane, indicating the levels at which horizontal sections were taken.4V, Fourth ventricle; Aq, aqueduct;DL, dorsolateral PAG; DM, dorsomedial PAG; DR, dorsal raphe; L, lateral PAG;VL, ventrolateral PAG.
Unlike the presynaptic effects, all PAG neurons displayed nociceptin-induced outward currents with the use of K-gluconate-filled electrodes, whether they were located in the lateral (n= 22), intermediate (n = 7), or ventrolateral PAG (n = 5). Of the nociceptin-responding neurons, 69% in the lateral PAG (n = 9/13), 86% in the intermediate PAG (n = 6/7), and 0% in the ventrolateral PAG (n = 0/5) also responded to met-enkephalin.
DISCUSSION
In the present study, it has been demonstrated that the ORL1 receptor ligand nociceptin increases an inwardly rectifying K+ conductance in all tested neurons of the midbrain lateral and ventrolateral PAG, regions that mediate distinct autonomic and behavioral responses to threat, stress, and pain. It also was demonstrated that nociceptin had a presynaptic inhibitory action on excitatory glutamatergic and inhibitory GABAergic synaptic transmission in a subpopulation of PAG neurons. The proportion of neurons displaying nociceptin-induced inhibition of GABAergic synaptic transmission differed between the lateral and ventrolateral PAG.
Nociceptin was likely to have acted via ORL1 receptors and not other opioid receptors. Nociceptin produced an outward current in all PAG neurons, including those that did not display μ-opioid-mediated responses. The agonist action of nociceptin also was unaffected by naloxone, as has been demonstrated in the dorsal raphe (Vaughan and Christie, 1996a). It is unlikely that the actions of nociceptin were attributable to activation of δ- or κ-receptors, because PAG neurons do not respond to δ- and κ-specific agonists (Chieng and Christie, 1994). However, suitable antagonists are required to confirm that nociceptin does act via ORL1 receptors. Although in situ hybridization and immunohistochemical studies have demonstrated a relatively high density of ORL1receptors throughout the PAG (Lachowicz et al., 1995; Anton et al., 1996), the receptors seem to be localized in fiber processes, rather than in cell bodies (Anton et al., 1996). It is difficult to reconcile these findings to the observation of a postsynaptic action of nociceptin in the present study.
The potency of nociceptin observed in the present study (EC50 = 39 nm) was comparable to that previously found for K+ conductance increase in the dorsal raphe (EC50 = 22 nm; Vaughan and Christie, 1996a) and the locus coeruleus (EC50 = 90 nm;Connor et al., 1996a), for calcium current inhibition in SH-SY5Y cells (EC50 = 42 nm; Connor et al., 1996b), and for the inhibition of adenylate cyclase in CHO cells expressing cloned ORL1 receptors (EC50 = 1 nm;Meunier et al., 1995; Reinscheid et al., 1995). Differences among these EC50 values might be attributable to overexpression in cell lines and varying densities of ORL1 receptors.
The outward current produced by nociceptin was the result of an increase in an inwardly rectifying K+ conductance, as has been observed for the dorsal raphe (Vaughan and Christie, 1996a) and locus coeruleus (Connor et al., 1996a). Nociceptin produced an increase in conductance that was greater at more negative potentials. The reversal potential for the nociceptin-induced current varied with the external K+ concentration in a manner similar to that predicted by the Nernst equation for a K+ conductance. The mutual occlusion of each of the membrane currents produced by nociceptin, met-enkephalin, and baclofen indicates that activation of ORL1, μ-opioid, and GABAB receptors increases the same inwardly rectifying K+ conductance in PAG neurons as has been observed for the locus coeruleus (Chieng et al., 1996;Connor et al., 1996a).
Nociceptin also inhibited glutamatergic (non-NMDA) and GABAergic synaptic transmission in a subpopulation of PAG neurons. A number of observations demonstrated that nociceptin acted directly on presynaptic GABAergic and glutamatergic terminals. Nociceptin reduced the amplitude of evoked postsynaptic currents while increasing paired-pulse facilitation of these evoked postsynaptic currents. An increase in paired-pulse facilitation is associated with manipulations that decrease transmitter release and is indicative of a presynaptic locus of action (del Castillo and Katz, 1954). In addition, nociceptin had no effect on postsynaptic conductance at the potentials used to study evoked and spontaneous miniature postsynaptic currents when Cs+-filled electrodes were used. In another series of experiments, nociceptin reduced the frequency of spontaneous action potential-independent miniature postsynaptic currents without affecting their amplitude distributions. These observations indicate that the nociceptin-mediated inhibition of synaptic transmission was attributable to a reduced probability of presynaptic transmitter release and was not attributable to a reduction in postsynaptic receptor sensitivity. Thus, in addition to its postsynaptic actions, nociceptin has been demonstrated to have a presynaptic inhibitory action on transmitter release.
ORL1 receptors are expressed selectively by subpopulations of GABAergic and glutamatergic terminals innervating different PAG neurons. Nociceptin inhibited evoked glutamatergic synaptic currents in approximately one-half of the neurons throughout the lateral and ventrolateral PAG. In contrast, the proportion of neurons in which nociceptin inhibited evoked GABAergic synaptic currents varied with the dorsoventral location. Nociceptin inhibited evoked GABAergic synaptic currents in most ventrolateral PAG neurons tested but in only one-half of the lateral PAG neurons.
The widespread pre- and postsynaptic actions of nociceptin observed here suggest that this peptidergic system could play an important role in the modulation of nociception by the PAG. μ-Opioid induced antinociception is thought to arise via disinhibition of PAG output neurons, which project to the rostral ventromedial medulla (Basbaum and Fields, 1984; Reichling, 1991), by inhibiting transmitter release within the presynaptic GABAergic terminals (Vaughan and Christie, 1996b). The finding that nociceptin inhibited evoked IPSCs in most ventrolateral PAG neurons could indicate a similar disinhibitory action of nociceptin in facilitating analgesia. However, nociceptin inhibited evoked EPSCs in approximately one-half of the ventrolateral PAG neurons examined, and all PAG neurons were inhibited directly by nociceptin.
The pattern of pre- and postsynaptic effects of nociceptin more closely resembles that produced by GABAB-receptor agonists than by μ-opioid receptor agonists. GABAB-receptor agonists inhibit both GABAergic and glutamatergic transmission (present study) as well as directly hyperpolarizing all lateral and ventrolateral PAG neurons (Chieng and Christie, 1995). Like GABAB-agonists, μ-opioid agonists inhibit GABAergic and glutamatergic transmission in all PAG neurons (Vaughan and Christie, 1996b) but directly hyperpolarize few ventrolateral PAG neurons (Chieng and Christie, 1994;Osborne et al., 1996). However, it is difficult to predict the potential antinociceptive actions of nociceptin, because microinjections of both GABAB (Levy and Proudfit, 1979) and μ-opioid (Yaksh et al., 1988) agonists into the ventrolateral PAG produce antinociception.
These observations suggest a complex role for nociceptin in the modulation of nociception in the PAG. This might be expected to result from the balance of actions on PAG neurons that project to the rostral ventromedial medulla, including inhibition of excitatory and inhibitory transmission as well as direct postsynaptic inhibition. The role of nociceptin might be defined more clearly by determining the actions of nociceptin in PAG output neurons with identified projections to the rostral ventromedial medulla. In addition, recent studies indicate that quite different behavioral and autonomic response strategies are mediated by functionally distinct lateral (fight–flight/freezing, hypertension, and tachycardia) and ventrolateral (quiescence, hyporeactivity, hypotension, and bradycardia) PAG neuronal columns (Yaksh et al., 1988; Lovick, 1993; Bandler and Shipley, 1994). Thus, nociceptin also might be involved in the modulation of the other behavioral and autonomic functions of the PAG.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia. We thank Dr. M. Connor for his helpful comments.
Correspondence should be addressed to Dr. C. W. Vaughan, Department of Pharmacology, University of Sydney, NSW 2006, Australia.
REFERENCES
- 1.Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL1 in the central nervous system of the rat. J Comp Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Bandler R, Shipley MT. Columnar organisation in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 3.Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial, and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 5.Chieng B, Christie MJ. Hyperpolarisation by opioids acting on μ-receptors of a subpopulation of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chieng B, Christie MJ. Hyperpolarisation by GABAB receptor agonists in mid-brain periaqueductal gray neurones in vitro. Br J Pharmacol. 1995;116:1583–1588. doi: 10.1111/j.1476-5381.1995.tb16376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chieng B, Connor M, Christie MJ. The μ-receptor antagonist, CTOP (but not CTAP), produces a non-opioid receptor-mediated increase in K+ conductance of rat locus coeruleus neurons. Mol Pharmacol. 1996;50:650–655. [PubMed] [Google Scholar]
- 8.Connor M, Vaughan CW, Chieng B, Christie MJ (1996a) Receptors for nociceptin couple to an increase in potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol, in press. [DOI] [PMC free article] [PubMed]
- 9.Connor M, Yeo A, Henderson G. The effect of nociceptin on Ca+ channel current and intracellular Ca+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol. 1996b;118:205–207. doi: 10.1111/j.1476-5381.1996.tb15387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Castillo J, Katz B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol (Lond) 1954;124:574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houtani T, Nishi M, Takeshima H, Nukada T, Sugimoto T. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem Biophys Res Commun. 1996;219:714–719. doi: 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- 12.Lachowicz JE, Shen Y, Monsma FJ, Sibley DR. Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem. 1995;64:34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- 13.Levy RA, Proudfit HK. Analgesia produced by microinjection of baclofen and morphine at brain stem sites. Eur J Pharmacol. 1979;57:43–55. doi: 10.1016/0014-2999(79)90102-x. [DOI] [PubMed] [Google Scholar]
- 14.Lovick TA. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993;40:631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- 15.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 16.Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family—cloning, functional expression, and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- 17.North RA, Williams JT, Surprenant A, Christie MJ. μ and δ receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol (Lond) 1996;490:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 20.Reichling DB. GABAergic neuronal circuitry in the periaqueductal gray matter. In: Depaulis A, Bandler R, editors. The midbrain periaqueductal grey matter. Plenum; New York: 1991. pp. 139–150. [Google Scholar]
- 21.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol. 1996a;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughan CW, Christie MJ (1996b) Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal greyin vitro. J Physiol (Lond), in press. [DOI] [PMC free article] [PubMed]
- 24.Williams JT, North RA, Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988;8:4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaksh TL, Al-Rodhan NRF, Jensen TS. Sites of action of opiates in production of analgesia. Prog Brain Res. 1988;77:371–394. doi: 10.1016/s0079-6123(08)62803-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang SW, Yu L. Identification of dynorphins as endogenous ligands for an opioid receptor-like orphan receptor. J Biol Chem. 1995;270:22772–22776. doi: 10.1074/jbc.270.39.22772. [DOI] [PubMed] [Google Scholar]