Abstract
Rats treated with the AMPA receptor-facilitating drug 1-(quinoxolin-6-ylcarbonyl)piperidine (BDP-12) during training acquired fear conditioning to a tone faster than vehicle-treated controls. The effect on acquisition was dependent on the dose given. BDP-12-treated rats and vehicle-treated controls reached the same level of conditioned fear and extinguished at the same rate. The dissociation of learning rate from these other normally covariant measures suggests that the drug had a specific and isolated effect on acquisition. Controls for drug effects on stimulus sensitivity, locomotor activity, generalized fearfulness, and other performance factors support this interpretation. The known action of BDP-12 on receptor dynamics suggests that its effect on acquisition may be attributed to specific modulation of an AMPA and NMDA receptor-dependent plasticity mechanism. The finding that the drug accelerates acquisition but does not affect the level of conditioned fear acquired parallels the effect of the drug on long-term potentiation (LTP) (increasing the rate but not the ceiling of potentiation) and suggests that common mechanisms may underlie fear conditioning and LTP.
Keywords: glutamate, AMPA receptors, NMDA receptors, learning, fear, amygdala
The amygdala and its afferent and efferent connections constitute essential components of the neural circuit through which an innocuous auditory conditioned stimulus (CS) comes to elicit fear reactions after it is temporally paired with an aversive unconditioned stimulus (US) (Davis, 1992; Maren and Fanselow, 1996;Rogan and LeDoux, 1996). Anatomical (LeDoux and Farb, 1991; Farb et al., 1992, 1995) and physiological (Li et al., 1995, 1996; Maren and Fanselow, 1995) studies have implicated the excitatory amino acidl-glutamate and its AMPA and NMDA receptors in the functioning of this circuit. Importantly, NMDA receptor blockade in the amygdala disrupts fear conditioning (Miserendino et al., 1990; Kim et al., 1991; Campeau et al., 1992; Fanselow et al., 1994; Maren and Fanselow, 1995; Maren et al., 1996); however, interpretations regarding the role of NMDA receptors in fear conditioning have been complicated by the fact that NMDA receptors also play an important role in routine (nonplastic) synaptic transmission in the afferent pathways that transmit CS inputs to the amygdala (Li et al., 1995, 1996; Maren and Fanselow, 1995; Rogan and LeDoux, 1995b).
The present experiment attempts to examine plasticity mechanisms in the fear conditioning circuit by facilitating rather than blocking glutamatergic transmission. We used 1-(quinoxolin6-ylcarbonyl)piperidine (BDP-12), one of a family of closely related compounds known as ampakines (Stäubli, 1995). These drugs increase the mean open time of AMPA receptors (Arai et al., 1994, 1996), allowing a given level of presynaptic stimulation and glutamate release to produce a greater current flow through postsynaptic AMPA receptors. Because NMDA receptor activation is dependent on the degree of AMPA receptor-mediated depolarization of the postsynaptic cell (Collingridge et al., 1983), facilitation of AMPA-mediated synaptic responses effectively lowers the threshold level of presynaptic input required to activate NMDA receptors. One consequence of this is that the amount of afferent stimulation required to induce maximal levels of long-term potentiation (LTP), a form of NMDA and AMPA receptor-dependent synaptic plasticity (Bliss and Collingridge, 1993; Malenka and Nicoll, 1993), is reduced both in vitro and in vivo in the hippocampus (Arai and Lynch, 1992; Stäubli et al., 1994b). Because the synaptic changes occurring during LTP induction have been widely hypothesized to be similar to those occurring during memory formation (Lynch, 1986; Brown et al., 1988; Teyler, 1992; Bliss and Collingridge, 1993; Barnes et al., 1995; Stäubli, 1995), the facilitating effects of ampakines on LTP have stimulated researchers to ask whether these drugs enhance the acquisition and storage of memories. Ampakines have now been shown to enhance retention in several tasks, including the radial arm maze (Granger et al., 1993; Stäubli et al., 1994a,b, 1996), water maze (Stäubli et al., 1994a), and olfactory delayed match to sample (Stäubli et al., 1994b, 1996). Ampakines have also been found to render normally subthreshold stimulus parameters capable of supporting learning in odor discrimination (Stäubli et al., 1994a; Larson et al., 1995) and eyeblink conditioning (Shors et al., 1995) tasks.
The present study was designed to test whether facilitation of AMPA receptor transmission (and the consequent synergistic enhancement of NMDA receptor function) might enhance fear conditioning. The study was premised on four interrelated observations: (1) the involvement of NMDA receptors in hippocampal synaptic plasticity; (2) the putative role of NDMA receptors in fear conditioning (see above); (3) the presence of LTP-like mechanisms in the fear conditioning pathways (Chapman et al., 1990; Clugnet and LeDoux, 1990), and (4) the ability of LTP induction to modulate amygdala responses to auditory stimuli in the fear conditioning pathways (Rogan and LeDoux, 1995a).
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (300–325 gm) were given free access to rat chow and water and maintained on a 12 hr light/dark cycle. One week elapsed between delivery of the rats to the animal facility and the start of the experiment.
Experimental design
BDP-12 (Cortex Pharmaceuticals) was dissolved in vehicle solution [30% 2-hydroxypropyl-β-cyclodextrin (cyclodextrin 30%); Research Biochemicals, Natick, MA] and administered via interperitoneal injection. Doses were varied by adjusting the concentration of the drug solution: the ratio of injected volume to body weight (1.25 ml/kg) was held constant in all drug and vehicle conditions.
The effect of BDP-12 on acquisition of fear conditioning was tested by the administration of BDP-12 (50 mg/kg; n = 19) or vehicle (cyclodextrin 30%; n = 25) 30 min before each training day (days 1 and 2). On all subsequent days, all animals received injections of vehicle 30 min before testing.
To test the dose dependence of this effect, the experiment was repeated with the following additional doses of BDP-12: 4.00 mg/kg (n = 6), 6.25 mg/kg (n = 5), 8.00 mg/kg (n = 6), 10.00 mg/kg (n = 6), and 12.50 mg/kg (n = 5).
To distinguish between drug effects on acquisition and expression, and to test for the presence of a drug-induced performance effect, the experiment was repeated with a new drug group (n = 12) receiving BDP-12 (50 mg/kg) on day 1 only, and vehicle on days 2 and 3. Controls received vehicle on all days.
To test whether a drug-induced increase of CS sensitivity might contribute to observed effects on learning, we compared the effect of the drug on CS-elicited behaviors in the course of conditioning with the effect of increased CS intensity on the same behaviors. Specifically, we observed the effect of the drug on responding to the CS before training and performed a separate experiment with two groups of vehicle-treated animals: one group (CS standard, n = 7) was trained with the CS used in all other experiments described here; the other group (CS × 2, n = 7) was trained with a CS that was 6 dB more intense than the standard CS. This intensity was chosen for both theoretical (an increase of 6 dB quadruples the power and doubles the intensity of an acoustic stimulus) and practical (the animals had a dramatically different reaction to the more intense stimulus, as described below) reasons.
Behavioral procedures
Fear conditioning. Fear conditioning took place in a rodent conditioning chamber (model E10–10; Coulbourn Instruments, Lehigh Valley, PA) enclosed by a sound-attenuating cubicle (model E10–20, Coulbourn Instruments). Stimulus presentation was controlled by a microprocessor and a digital I/O board.
To habituate the animals to extraneous experimental procedures, they were handled, weighed, and injected with vehicle (1.25 ml/kg cyclodextrin 30%) once daily for the 5 d before training. On the day before training, rats were exposed to the conditioning room and chamber. They were then randomly assigned to drug or vehicle groups. The rats were then trained with two pairings a day for 2 d of an acoustic tone CS (10 kHz, 20 sec, 75 dB) and a footshock US (0.3 mA, 0.5 sec). The US occurred during the last 0.5 sec of the CS, and the interval between the two CSs on a day varied between 60 and 120 sec. This US intensity was chosen because it is known to yield reliable conditioned responding to the tone in normal animals, but at moderate levels that leave ample room for the measurement of drug-induced increases in conditioned responding through the course of training (Phillips and LeDoux, 1992).
Thirty minutes before training, the drug groups received an intraperitoneal injection of BDP-12, and the vehicle groups received the equivalent volume of vehicle. On all subsequent days, all rats were weighed and received injections of vehicle 30 min before two trials in which the tone was delivered with the same schedule as on the 2 training days, but without the US. Freezing, a species-specific response to threat, was used as an index of conditioned fear (Blanchard and Blanchard, 1969a,b; Bouton and Bolles, 1980; Fanselow, 1980; LeDoux et al., 1984, 1990).
Freezing is defined as the assumption of the characteristic crouching posture, and the cessation of all but respiration-related motion. Conditioning to the tone was assessed by measuring the time spent freezing during the 20 sec tone. Only data obtained in the first trial of each day were analyzed [measurements of behavior during the first trial reflect the learning that occurred as a result of the previous day’s experience; measurements of behavior in the second trial of the day are potentially confounded by persistent responses to the stimuli presented in first trial, especially the US (Phillips and LeDoux, 1992)]. Extinction of the conditioned response was quantified as the number of days from the start of training until the second of 2 consecutive days during which freezing to the first tone fell below 5 sec. Observations were conducted blind to drug condition.
Open field activity. Naive rats were handled, weighed, and injected with vehicle (cyclodextrin 30%) intraperitoneally once daily for the 5 d before testing and then were randomly assigned to drug (n = 8) or vehicle (n = 8) groups. Thirty minutes before testing, each rat received an intraperitoneal injection of either drug or vehicle. During testing each rat was permitted to range freely for 10 min in a novel, evenly illuminated 120 cm × 120 cm white Plexiglas chamber with 40-cm-high walls. The floor of the chamber was divided by black lines into 20 cm squares. The rat’s behavior during the test period was recorded by the observer on a paper map of the chamber, so that the number and locations of line crossings and rearings could be extracted later. Rearing was scored when both front paws left the floor and grooming did not occur. Defecation in open field was also measured. The chamber was thoroughly washed and dried before each test. Observations were conducted blind to drug condition.
RESULTS
BDP-12 accelerates acquisition
The effects of BDP-12 on fear conditioning were assessed by administration of BDP-12 30 min before training on both training days and administration of vehicle before testing on all subsequent days. A control group received an injection of vehicle before training and testing on all days.
The vehicle group (n = 25) displayed normal acquisition of conditioning to the tone, freezing ∼7 sec (mean 7.36 ± 2.78 sec) after 1 d of training, and 12 sec (mean 11.72 ± 4.37 sec) after the second (final) day of training (Fig. 1). This is in accord with data from previous experiments using these training parameters with normal rats (Phillips and LeDoux, 1992: reprinted as part of Fig. 7). In contrast, drug-treated animals required half as much training to reach the same level as controls, freezing 13 sec (mean 13.11 ± 3.11 sec) after 1 d of training, and maintaining this level after the second day of training (mean 11.63 ± 1.13 sec).
Fig. 1.
Acceleration of tone conditioning in BDP-12-treated rats. Vehicle- and drug-treated (50 mg/kg) rats were trained on a task that consisted of two pairings a day of an auditory CS coterminating with a footshock US for 2 consecutive d (day 1, day 2). On day 3, the CS was presented with the same schedule as on the 2 training days but without the US. Freezing was scored during the presentation of the first CS each day. Drug-treated rats acquired fear conditioning at an accelerated rate compared with vehicle-treated rats, but they expressed the same level of conditioned responding at the completion of training (day 3) as vehicle-treated rats.
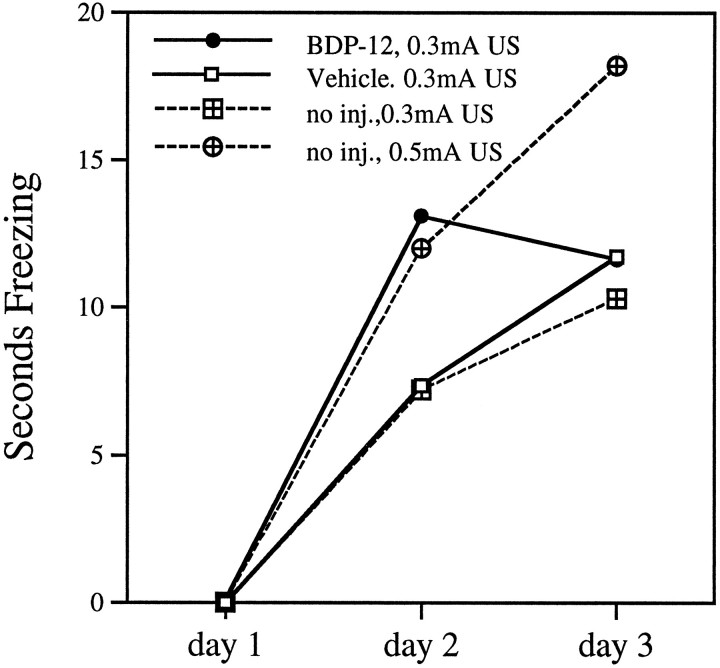
Fig. 7.
BDP-12 effect on acquisition cannot be attributed to an increase in US sensitivity. Training normal, untreated rats with a greater US intensity increases freezing on both day 2 and day 3 (no inj., 0.3 mA US and no inj. 0.5 mA US; data taken from Phillips and LeDoux, 1992); however, BDP-12 treatment increases freezing only on day 2, indicating that the drug effect is not ascribable to an increase in US sensitivity (vehicle and drug data are taken from Fig. 1).
A two-factor ANOVA was performed, with drug treatment as a between-subjects factor and days as within-subject factor. A significant effect of drug treatment was observed [F(1,42) = 9.07; p < 0.05], as well as a significant drug × day interaction [F(2,84) = 14.04; p < 0.001], reflecting the accelerated rate of learning in the drug-treated animals. Post hoc analysis performed on this data revealed a significant effect of drug on day 2 alone (Duncan, p < 0.05). Measurements obtained on day 2 reflect learning acquired during the training delivered on day 1. The slight decrease in freezing exhibited by drug-treated animals on day 3 was not statistically significant, compared with day 2 (Duncan, p > 0.05)
BDP-12 does not affect conditioned response ceiling (asymptotic level)
The first test after completion of training occurred on day 3. Controls are at asymptote for these training parameters on this day (Fig. 1). There was no difference in the level of freezing to the tone exhibited by drug- and vehicle-treated rats on this day (vehicle: mean 11.72 ± 4.37 sec; drug: mean 11.63 ± 1.13 sec; Duncan,p > 0.05). Moreover, the level of freezing in drug-treated animals on day 2 was not different from the level of controls on day 3 (Duncan, p > 0.05).
BDP-12 does not affect extinction of the conditioned response
To determine the effect of the drug treatment during acquisition on subsequent extinction, two groups of rats were tested until extinction of the conditioned response. There was no difference in rate of extinction of the conditioned response to the tone between the vehicle group (n = 7; mean 7.29 ± 0.78 d to extinction) and the drug group (n = 7; mean 6.29 ± 0.57 d to extinction) (t test; p > 0.3) (Fig. 2). Extinction rate provides a sensitive index of the amount of conditioning that has resulted from training (Mackintosh, 1975), and the absence of a drug effect on extinction supports the interpretation that both groups were conditioned to the same degree at completion of training.
Fig. 2.

Accelerated acquisition is not accompanied by a difference in extinction rate. A subset of the animals from the data set shown in Figure 1 were tested until extinction of the conditioned response. There was no effect of drug on the rate of extinction, strongly suggesting that the identical level of freezing expressed by both the drug and vehicle groups at completion of training (day 3) truly reflects an equal level of conditioned fear in both groups.
Drug effect on acquisition is dose dependent
The experiment was repeated with five additional doses of BDP-12 to determine how acquisition is modulated by drug dose. The overall pattern of conditioned response expression during acquisition was the same as in the first experiment: there was no drug effect on the amount of freezing after completion of training, but there was a dose-dependent increase in the level of conditioned responding expressed after 1 d of training (Fig. 3). With progressively higher doses, conditioned responding at the midpoint of the training schedule (day 2) came progressively closer to the level of conditioned response expressed at the completion of training in vehicle-treated animals.
Fig. 3.

The effect of BDP-12 on acquisition is dose dependent. Although there was no effect of drug on the level of conditioned response expressed at the completion of training, with increasing dose levels the conditioned response exhibited by drug-treated animals at the midpoint of training (testing on day 2) came progressively closer to the maximal level of conditioned response expressed at the completion of training (testing on day 3: mean response of the vehicle group represented by horizontal bar).
A two-factor ANOVA was performed, with drug treatment as a between-subjects factor and days as within-subject factor. A significant effect of drug was not observed; however, there was a significant drug × day interaction [F(12,130) = 4.09; p < 0.001), reflecting the effect of drug on acquisition. Post hocanalysis performed on this data revealed a significant effect of drug versus vehicle on day 2 alone for doses of 10, 12.5, and 50 mg/kg (Duncan, all p < 0.05). A dose of 12.5 mg/kg had the same effect as the larger dose tested (50 mg/kg) (Duncan,p > 0.05).
Accelerative effect of BDP-12 on acquisition is the same in the presence or absence of the drug during testing
In the experiments described thus far, the drug was administered on both training days, and the drug effect on acquisition was measured in the presence of the drug during the CS presentation on the second day of training. It is therefore possible that the measured effect was in some way caused by the presence of the drug during testing on day 2 rather than by the effect of the drug on learning mechanisms during the previous day’s training. To determine this, an additional experiment was performed in which the only change in procedure was that the drug group (n = 11) received BDP-12 on only the first day of training; vehicle was given on the second day of training and on test days.
The results of this study were indistinguishable from the first study using this dose (50 mg/kg), in which drug was administered on both days 1 and 2 (Fig. 4). Drug-treated animals froze to the tone 13 sec after the first day of training (mean 13.18 ± 4.12 sec) and maintained this level of conditioned response after the second day of training (mean 11.91 ± 1.34 sec). A two-factor ANOVA was performed, with drug treatment as a between-subjects factor and days as within-subject factor. A significant effect of drug treatment was not observed; however, there was a significant drug × day interaction [F(4,104) = 8.72; p < 0.001).Post hoc testing revealed a significant effect of both drug groups versus the vehicle group on day 2 only (Duncan,p < 0.05) and no significant difference between the two drug groups on any day. Measurement of enhanced freezing (accelerated acquisition) after 1 d of training with the drug was the same when tested in the presence or absence of the drug, indicating that the enhanced freezing measured on day 2 is ascribable entirely to a drug effect on acquisition during training on day 1, rather than to a drug-induced performance or state-dependent effect occurring on day 2.
Fig. 4.
Accelerated acquisition in BDP-12-treated animals is not attributable to the presence of the BDP-12 during testing. Rats given the drug only on day 1 exhibited the same pattern and magnitude of conditioned responding as rats that received the drug on day 1 and day 2 (redrawn here from Fig. 1), showing that the measured accelerative effect was independent of the drug’s presence during testing. These results show that the drug effect is on acquisition, rather than expression, of fear conditioning.
Accelerative effect of BDP-12 on acquisition is not ascribable to a drug effect on CS sensitivity
Because both AMPA and NMDA receptors are involved in routine transmission in some components of the fear conditioning circuit, it is necessary to control for the possibility that a drug effect on routine sensory processing of the CS could have contributed to the observed increase in acquisition rate. Because inhibitory as well as excitatory neural activity can be affected by BDP-12, the effect of the drug on sensory transmission in a particular circuit is hard to predict. Previous studies, however, have suggested that ampakines can enhance some behavioral responses to auditory stimuli (Shors et al., 1995), so we expected that if there were an effect of BDP-12 on CS processing, it would take the form of a heightened CS sensitivity (i.e., that drug-treated rats would respond to the CS as though it were more intense). We examined this issue by comparing the effects of BDP-12 on CS responses during the course of fear conditioning with the effect of increasing the intensity of the CS.
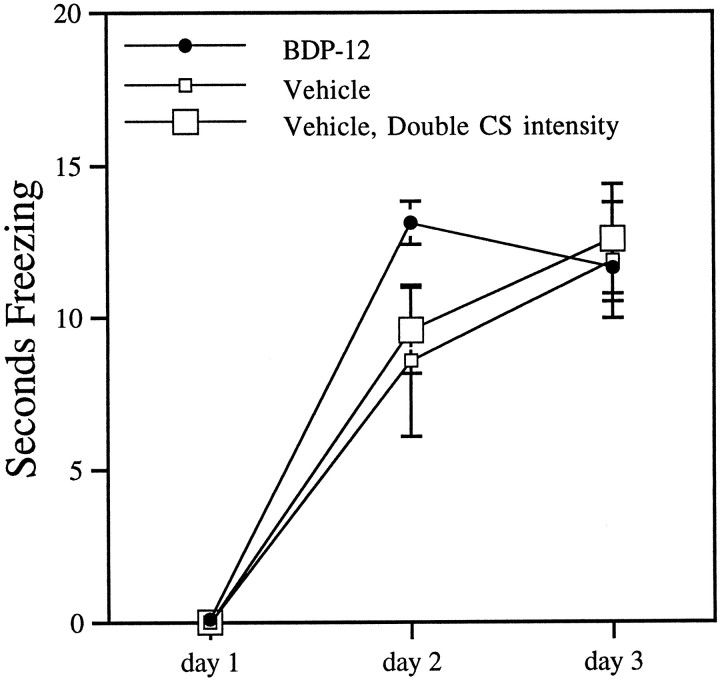
Examination of the response to the first CS presented on day 1 of training revealed that drug-treated animals (at all doses) showed no unusual responsiveness to the CS before conditioning, compared with vehicle-treated animals: responses ranged from a mild orienting response (turn of the head) to no discernible response. This is in accord with abundant observations of the response of normal, untreated animals to a CS of this intensity (which is our laboratory standard). On the other hand, pretraining exposure to a CS 6dB more intense than the standard CS (a 6dB increase doubles the intensity of the CS) invariably evoked a robust response at CS onset, ranging from a jerking movement of the head and shoulders and a momentary cessation of ongoing activity to a jump and a short run. Nevertheless, the amount of conditioned freezing was not different in rats trained with the greater intensity CS (n = 7) as compared with rats trained with standard CS in the other studies discussed (n = 7) (Fig. 5). A two-factor ANOVA was performed, with CS intensity as a between-subjects factor and days as within-subject factor. A significant effect of CS intensity was not observed [F(1,12) = 0.11; p > 0.5)], nor was there a significant CS intensity × day interaction [F(2,24) = 0.09; p > 0.5)].
Fig. 5.
Increasing CS intensity does not increase acquisition rate. Doubling CS intensity (an increase of 6 dB) failed to produce any increase in acquisition rate or final level of conditioning in vehicle-treated animals. Doubling CS intensity, however, does increase responding to pretraining exposure, an effect not observed with drug treatment (see Discussion). Also pictured are drug data taken from Figure 1.
The absence of drug effect on CS responsiveness before conditioning and the insensitivity of our fear conditioning protocol to behaviorally significant increases in CS intensity strongly suggest that BDP-12 does not have its effect on fear conditioning via an action on routine (nonassociative) transmission of the CS.
Open field activity
It is possible that the drug induces a generalized increase in fearfulness or anxiety, which contributes to a higher freezing score during conditioning. A measure of exploratory behavior that speaks to this question involves a rat’s tendency to move predominantly along the walls of an open field chamber and avoid the more exposed center area (thigmotaxis) (Gray, 1987). Our analysis of open field behavior included quantification of the animal’s movements into the center of the chamber versus its movement along the walls. Defecation in open field, a common indicator of fearfulness (Gray, 1987), was also measured.
Open field behavior also provides a simple control for drug-induced impairment of locomotor capacity. It is possible that the drug could induce alterations in locomotor behavior that could lead to an exaggerated freezing score, either by producing behavior that is misinterpreted as fear-related freezing or by interfering with the shift from freezing to nonfreezing behavior. Measurement of the total number of line crossings and instances of spontaneous rearing behavior were used to examine this question.
No significant difference between drug group (n = 8) and vehicle group (n = 8) was found in any measure of open field activity (Fig. 6) (t tests; allp > 0.05), indicating that our measurement of freezing behavior during fear conditioning was not confounded by drug effects on locomotor capacity or fearfulness per se.
Fig. 6.
No effect of BDP-12 on locomotor capacity or fear-related behaviors. There was no effect of drug treatment (50 mg/kg) on open field measures of locomotor capacity (left panel: rearing, total line crossings) or fear-modulated behaviors such as thigmotaxic behavior (right panel: line crossings in the center of the open field vs line crossings along the walls of the chamber) and defecation in open field (not shown).
DISCUSSION
When examining the possibility of a drug effect on learning, it is necessary to consider what aspect of neural function is contributing to the observed effect. It is of particular interest to distinguish between a drug effect that facilitates the processes that transmit sensory information to the sites of plasticity and a drug effect that facilitates plasticity mechanisms themselves. Furthermore, a drug that has a specific and isolated effect on plasticity mechanisms during acquisition can be expected to dissociate behavioral measures of acquisition from other normally covariant behavioral measures and from strict dependence on stimulus parameters that normally determine these measures.
How might drug-induced changes in neural response properties affect normal sensory processing?
Even though ampakines can be expected to enhance AMPA transmission, and in certain circumstances to increase NMDA-mediated transmission, the net effect on signal processing in vivo is not easy to predict, especially because inhibitory as well as excitatory interactions may be affected. Thus, it is important to consider the possible effects of BDP-12 on routine (nonplastic) sensory processing within the context of the circuit (system) under study.
Shors et al. (1995) showed that treatment with a related ampakine allows nictitating membrane conditioning to occur with stimulus intensities that do not lead to learning in untreated rats. They also found that α responding (baseline nictitating membrane responsiveness to the CS before training) is increased in ampakine-treated animals. These data suggest a drug effect that amplifies sensory processing (i.e., increases CS sensitivity) in the eyeblink conditioning system, although a drug action on the plasticity mechanisms underlying this kind of learning could be involved as well.
Our results are not consistent with a drug-induced amplification of CS or US sensory processing. Behavioral data from many sources have shown that more intense aversive unconditional stimuli lead to higher levels of conditioned responding (for review, see Klein, 1987). This has been demonstrated in parametric studies using the same conditioning protocol as the present experiment (Phillips and LeDoux, 1992). Specifically, increasing US intensity resulted in a particular behavioral pattern characterized by an increase in freezing on day 2 as well as an increased level of conditioned response expression at the completion of training and a decreased rate of extinction (Fig. 7). If BDP-12 induced a greater sensitivity to the US, one would expect training in the presence of the drug to lead to more freezing to the CS on days 2and 3, and slower extinction, than is exhibited with training without the drug. These indicators of increased US sensitivity did not occur in the present study. It is important to note that the level of freezing exhibited by drug-treated animals on days 2 and 3 do not represent a “performance ceiling”; for example, rats trained with a 1.0 mA US will freeze for the full 20 sec measurement period after just 1 d of training (Phillips and LeDoux, 1992).
An increase in the perceived intensity of the CS would be expected to enhance the rate of conditioning without altering the terminal level of conditioning (Rescorla and Wagner, 1972); however, our comparisons of the effect of BDP-12 on CS-elicited behaviors with the effect of increasing CS intensity on the same behaviors provide no evidence for a drug effect on nonplastic CS processing in the fear conditioning system (circuit). The absence of drug effect on CS responsiveness before conditioning, and the insensitivity of our fear conditioning protocol to behaviorally significant increases in CS intensity, suggests that whatever the effects of BDP-12 on routine CS transmission may be, they are unlikely to have contributed to our measures of conditioned fear. It is possible that increasing the CS intensity even more might reveal a significant effect on conditioning; however, the intensity used altered CS responses before conditioning (without affecting conditioning), and higher intensities tested in pilot studies elicited persistent agitation and hyperactivity over the duration of the tones, responses that suggest that the rats found the tones to be aversive, an effect that would tend to confound freezing measures.
Within this context, it is interesting to ask why the presence of the drug on day 2 did not increase performance on day 2 simply by increasing the degree to which the CS activated the amygdala. This is a reasonable expectation, but it is not borne out by the data. As we discuss above, because the drug is administered systemically, and inhibitory as well as excitatory transmission is likely to be affected, the effects of the BDP-12 on CS transmission, or on the degree of activation of the amygdala in response to any stimulus, are hard to predict. Although it has been reported that blocking AMPA transmission in the amygdala blocks expression of conditioned fear (Kim et al., 1993), it does not follow that an across-the-board increase in AMPA-mediated currents would necessarily increase amygdala activation or conditioned response expression. Indeed, the fact that the presence of the drug during testing on day 2 does not alter conditioned response expression suggests that an across-the-board increase of AMPA-mediated currents does not simply modulate information processing within the fear conditioning circuit in a way that increases the behavioral output of this system. On the contrary, these data suggest, in accord with our general conclusions, that in this system, increasing the gain of AMPA-mediated currents has a specific effect on acquisition mechanisms, an effect presumably attributable to a synergy of AMPA/NMDA receptors, and that other possible effects are not reflected in the behavioral indices of fear learning.
How might drug-induced changes in neural response properties affect experience-dependent plasticity?
Because the drug lowers the amount of afferent activity necessary for NMDA receptor activation, it can be predicted that processes dependent on NMDA receptor-mediated currents would be activated more efficiently in the presence of the drug. A range of findings have implicated NMDA receptor function in experience-dependent plasticity in the fear conditioning circuit, including the putative dependence of fear conditioning on NMDA receptor function, and the presence of LTP-like mechanisms in fear conditioning pathways capable of modulating the responsiveness of the amygdala to tones (see introductory remarks). The results of the present experiment are consistent with a drug effect that increases the efficiency of NMDA receptor-mediated plasticity mechanisms.
The dynamics of the effect of ampakine on NMDA currents is paralleled by the dynamics of ampakine’s effect on LTP induction in CA1 of hippocampus and on fear conditioning. In the presence of ampakine, afferent activity that would normally induce an intermediate level of potentiation at CA1 synapses instead induces a maximal level of potentiation (LTP ceiling); additional episodes of afferent activity, which in untreated slices are necessary to reach the LTP ceiling, do not increase the level of potentiation beyond the LTP ceiling (Arai and Lynch, 1992). Likewise, in the present experiment, the number of training trials needed to achieve an intermediate level of conditioned responding in untreated animals resulted in a maximal level of conditioned responding in ampakine-treated animals; once this conditioned response level is reached, additional CS/US pairings do not increase the level of conditioned responding in these animals. These effects add to the growing body of evidence for the involvement of LTP-like mechanisms in fear conditioning (for review, see Maren and Fanselow, 1996; Rogan and LeDoux, 1996).
Dissociation of acquisition rate from strength and content of association
The amount of conditioned responding is a function of the learned predictive value of the CS (strength of the CS/US association) and the aversiveness of the US (Rescorla and Wagner, 1972) (Fig.7). The fact that the drug-treated animals freeze the same amount after 1 and 2 d of training can be interpreted as evidence for a conditioned response asymptote for these training parameters. Such a conditioned response limit could reflect the behaviorally sensible proposition that a rat is going to be only so afraid of a tone that predicts a 0.3 mA shock (which is rather mildly aversive), no matter how well it learns the predictive value of the tone. Perhaps the asymptote corresponds to the level of CS-induced freezing that results from a “perfect” learning of both the predictive value of the CS and the “badness” of what it predicts (which within the context of this discussion we refer to as the “content” of the association), and this level of fear (or freezing) is determined by evolutionary processes that balance freezing behaviors with other adaptively valuable behaviors in the face of a relatively mild threat. The alternative is an animal that grows increasingly terrified (and immobile) with the day-by-day accumulation of repetitive experiences of certain predictable irritations. Because all training parameters were identical for drug and vehicle groups, the fact that both groups reached the same level of conditioned response and extinguished at the same rate strongly suggests that the level of conditioned response exhibited at the completion of training reflected identical association strengths, and that the memory of the “badness” of the US was likewise similarly encoded by both groups.
It is perhaps true that our ability to interpret our data in terms of a lack of drug effect on the “content” of the association may be to some extent a product of the poverty of our measure; freezing behavior, by its nature, may lack the subtlety to reflect small differences in information acquired during conditioning. It is important to point out, however, that our measure is neither arbitrary nor artificial but is a natural consequence of natural learning that has evolved under considerable selective pressure.
Conclusion
The pattern of conditioned responding exhibited by BDP-12-treated animals suggests that the drug has a specific and isolated effect on the mechanisms of acquisition and does not alter the strength or content of the acquired association. The specificity of the drug’s effect has provided a unique dissection of the processes underlying fear conditioning at both a behavioral and molecular level. It appears that the facilitation of AMPA receptors (and the presumed resulting effects on NMDA receptor function) during fear conditioning has the effect of dissociating the rate of acquisition from its usual dependence on stimulus parameters, suggesting that the mechanisms involved in the acquisition of contingency information can be biochemically isolated from the information being acquired, because in our protocol at least, these mechanisms can be modulated without altering what is learned. In combination with information about this drug’s effect on receptor dynamics, and other evidence for NMDA receptor involvement in fear conditioning, the present findings are consistent with the view that in fear conditioning, acquisition is mediated by an LTP-like synergy of AMPA and NMDA receptor activation.
Footnotes
This work was supported by Public Health Service Grants R01 MH46517, R37 MH38824, K02 MH0096, and F31 MH10919. We thank Professor T. J. Mathews of the Department of Psychology of New York University for his contribution to our behavioral analysis, and Jeff Muller, Dr. Katherine Melia, and Dr. Russell Phillips for expert assistance in data collection.
Correspondence should be addressed to Michael T. Rogan, Center for Neural Science, 6 Washington Place, Room 803, New York, NY 10003.
REFERENCES
- 1.Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- 2.Arai A, Kessler M, Xiao P, Ambros-Ingerson J, Rogers G, Lynch G. A centrally active drug that modulates AMPA receptor gated currents. Brain Res. 1994;638:343–346. doi: 10.1016/0006-8993(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 3.Arai A, Kessler M, Rogers G, Lynch G. Effects of a memory-enhancing drug on dl-α-amino-3-hydroxy-5-methyl-4-isoxazoleproionic acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther. 1996;278:627–638. [PubMed] [Google Scholar]
- 4.Barnes CA, Erickson CA, Davis S, McNaughton BL. Hippocampal synaptic enhancement as a basis for learning and memory: a selected review of current evidence from behaving animals. In: McGaugh JL, Weinberger NM, Lynch G, editors. Brain and memory: modulation and mediation of neuroplasticity. Oxford UP; New York: 1995. pp. 259–276. [Google Scholar]
- 5.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969a;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969b;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 7.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 8.Bouton ME, Bolles RC. Conditioned fear assessed by freezing and by the suppression of three different baselines. Anim Learn Behav. 1980;8:429–434. [Google Scholar]
- 9.Brown TH, Chapman PF, Kairiss EW, Keenan CL. Long-term synaptic potentiation. Science. 1988;242:724–728. doi: 10.1126/science.2903551. [DOI] [PubMed] [Google Scholar]
- 10.Campeau S, Miserendino MJD, Davis M. Intra-amygdala infusion of the N-methyl-d-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- 11.Chapman PF, Kairiss EW, Keenan CL, Brown TH. Long-term synaptic potentiation in the amygdala. Synapse. 1990;6:271–278. doi: 10.1002/syn.890060306. [DOI] [PubMed] [Google Scholar]
- 12.Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol (Lond) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis M. The role of the amygdala in conditioned fear. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 255–306. [Google Scholar]
- 15.Fanselow MS. Conditional and unconditional components of postshock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 16.Fanselow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-d-aspartate antagonist dl-2-amino-5-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behav Neurosci. 1994;108:235–240. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- 17.Farb C, Aoki C, Milner T, Kaneko T, LeDoux J. Glutamate immunoreactive terminals in the lateral amygdaloid nucleus: a possible substrate for emotional memory. Brain Res. 1992;593:145–158. doi: 10.1016/0006-8993(92)91303-v. [DOI] [PubMed] [Google Scholar]
- 18.Farb CR, Aoki C, LeDoux JE. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: a light and electron microscopic study. J Comp Neurol. 1995;362:86–108. doi: 10.1002/cne.903620106. [DOI] [PubMed] [Google Scholar]
- 19.Granger R, Stäubli U, Davis M, Perez Y, Nilsson L, Rogers GA, Lynch G. A drug that facilitates glutamaturgic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse. 1993;15:326–329. doi: 10.1002/syn.890150409. [DOI] [PubMed] [Google Scholar]
- 20.Gray JA. The psychology of fear and stress, Vol 2. Cambridge UP; New York: 1987. [Google Scholar]
- 21.Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-d-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav Neurosci. 1991;105:126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression fear-potentiated startle. Behav Neural Biol. 1993;59:5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- 23.Klein SB. Learning: Principles and applications. McGraw-Hill; New York: 1987. [Google Scholar]
- 24.Larson J, Lieu T, Petchpradub V, LeDuc B, Ngo H, Rogers GA, Lynch G. Facilitation of olfactory learning by a modulator of AMPA receptors. J Neurosci. 1995;15:8023–8030. doi: 10.1523/JNEUROSCI.15-12-08023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeDoux JE, Farb CR. Neurons of the acoustic thalamus that project to the amygdala contain glutamate. Neurosci Lett. 1991;134:145–149. doi: 10.1016/0304-3940(91)90527-z. [DOI] [PubMed] [Google Scholar]
- 26.LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned by acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Phillips RG, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp Brain Res. 1995;105:87–100. doi: 10.1007/BF00242185. [DOI] [PubMed] [Google Scholar]
- 29.Li XF, Stutzmann GE, LeDoux JL. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Memory. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- 30.Lynch G. Synapses, circuits, and the beginnings of memory. MIT; Cambridge, MA: 1986. [Google Scholar]
- 31.Mackintosh NJ. The psychology of animal learning. Academic; New York: 1975. [Google Scholar]
- 32.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 33.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 35.Maren S, Aharonov G, Stote DL, Fanselow MS. N-Methyl-d-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of the conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 36.Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 37.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 38.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AA, Prokasy WF, editors. Classical conditioning II: current research and theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- 39.Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995a;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 40.Rogan MT, LeDoux JE. Intra-amygdala infusion of APV blocks both auditory evoked potentials in the lateral amygdala and thalamo-amygdala transmission, but spares cortico-amygdala transmission. Soc Neurosci Abstr. 1995b;21:1930. [Google Scholar]
- 41.Rogan MT, LeDoux JE. Emotion: Systems, cells, synaptic plasticity. Cell. 1996;85:469–475. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- 42.Shors TJ, Servatius RJ, Thompson RF, Rogers G, Lynch G. Enhanced glutamatergic neurotransmission facilitates classical conditioning in the freely moving rat. Neurosci Lett. 1995;186:153–156. doi: 10.1016/0304-3940(95)11309-k. [DOI] [PubMed] [Google Scholar]
- 43.Stäubli UV. Parallel properties of long-term potentiation and memory. In: McGaugh JL, Weinberger NM, Lynch G, editors. Brain and memory: modulation and mediation of neuroplasticity. Oxford UP; New York: 1995. pp. 303–318. [Google Scholar]
- 44.Stäubli U, Izrael Z, Xu F. Remembrance of odors past: enhancement via central facilitation of glutamate receptors. Behav Neurosci. 1996;110:1067–1073. doi: 10.1037//0735-7044.110.5.1067. [DOI] [PubMed] [Google Scholar]
- 45.Stäubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994a;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stäubli U, Perez Y, Xu F, Rogers G, Ingvar M, Stone-Elander S, Lynch G. Centrally active modulators of glutamate receptors facilitate the induction of long-term potentiation in vivo. Proc Natl Acad Sci USA. 1994b;91:1158–1162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teyler JT. Long-term potentiation and memory. In: Smith B, Adelman G, editors. Neuroscience year: Suppl 2, encyclopedia of neuroscience. Brikhauser Boston; Cambridge, MA: 1992. pp. 91–93. [Google Scholar]