Abstract
The electrogenesis of synaptically activated dendritic Ca2+-mediated potentials, which may contribute to synaptic signal integration in pyramidal cells, was examined in rat layers V–VI prefrontal cortical (PFC) neurons in vitro. Intrasomatically recorded suprathreshold synaptic responses evoked by stimulation of the distal dendrites were attenuated by focal Cd2+ application to the proximal apical dendritic stem (100–200 μm from soma), but not to the apical dendritic tuft (>500 μm from soma). With use of intracellular QX-314 and Cs+ to block Na+ and K+ currents, intrasomatic recordings revealed that the Cd2+-induced attenuation of synaptic responses was attributable to the blockade of a dendritic Ca2+-mediated “hump” potential and high-threshold Ca2+ spike activated by NMDA EPSPs. The hump potential was not blocked by bath application of Ni2+ (100 μm) but was blocked by focal application of Cd2+ to the proximal but not distal apical dendrites, suggesting that it was generated by Ca2+ channels located in the proximal dendrites. Direct patch-clamp recordings made from the distal apical tuft of layers V–VI PFC neurons revealed that layers I–II synaptic stimulation or intradendritic depolarizing current pulses evoked tetrodotoxin- and QX-314-sensitive Na+ spikes. Unlike in the stem of the apical dendrite, Ca2+spikes were not easily evoked in the distal apical tuft when Na+ channels were blocked. When triggered, the Cd2+-sensitive Ca2+ spikes in the dendritic tuft were nonregenerative and had very high activation thresholds (approximately +10 mV). These results suggested that the high voltage-activated Ca2+ potentials that amplify distal EPSPs are primarily generated in the proximal stem of the apical dendrite and not within the fine dendritic branches of the apical tuft of layers V–VI PFC neurons.
Keywords: dendrites, PFC, amplification, calcium, NMDA, EPSP, apical tuft, electrophysiology
Pyramidal neurons within layers V–VI of the neocortex possess a long ascending apical dendrite that extends to layers II–III where it bifurcates into the fine branches of the apical tuft (see Fig. 1A). The apical tuft in layers I–II is a major receptive zone for synaptic inputs (Ramón y Cajal, 1889; Peters, 1987). Signal integration by the soma of cortical neurons is a considerable task, because distal EPSPs arriving in the apical tuft are strongly filtered by the passive cable properties of the dendrite before reaching the soma (Cauller and Connors, 1992; Mel, 1994; Spruston et al., 1994; Johnston et al., 1996; Yuste and Tank, 1996). In addition, ongoing synaptic inputs provide a background activity that can serve to increase the electrotonic length of the neuron and thereby attenuate the distally generated synaptic signal even further (Holmes and Woody, 1989; Bernander et al., 1991). Furthermore, strategically placed inhibitory inputs within the dendrites may prevent a considerable portion of the synaptic signal from reaching the soma (Koch et al., 1983; Kim et al., 1995; Miles et al., 1996).
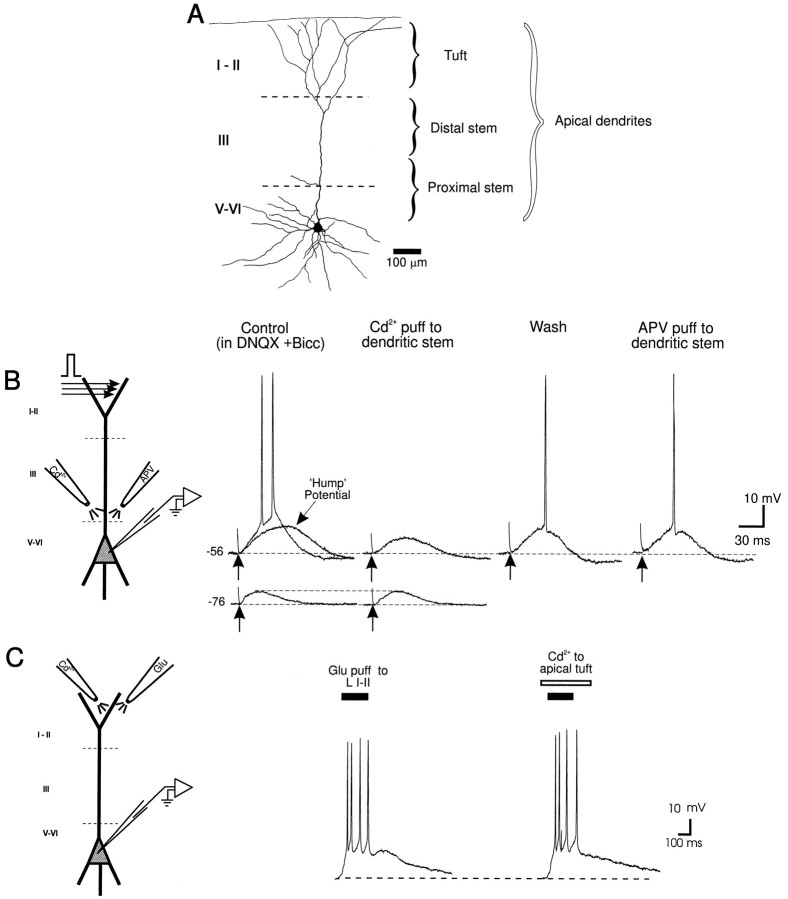
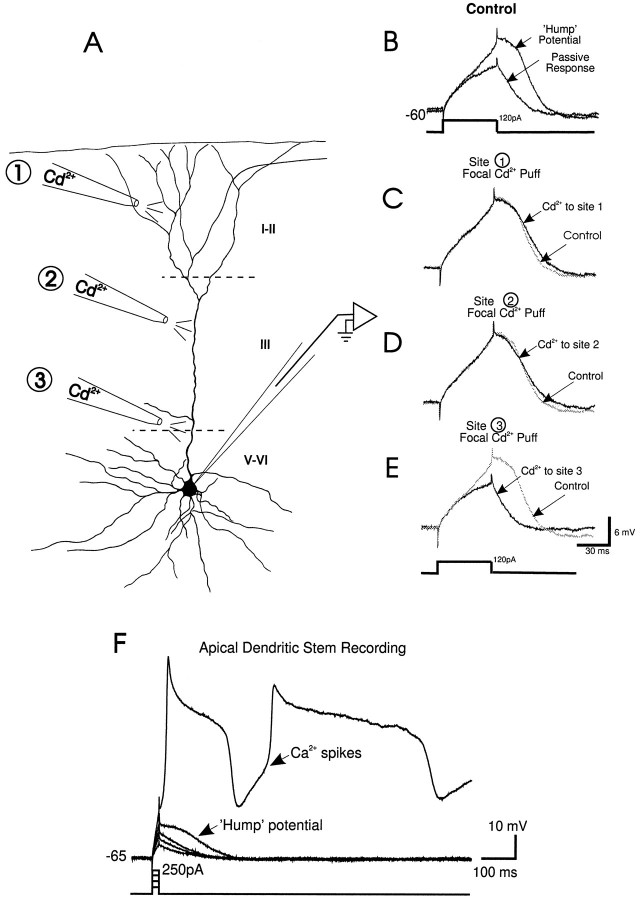
Fig. 1.
Cd2+-sensitive currents proximal to the soma of layer V PFC neurons enhance responses evoked by stimulation of layers I–II. A, Camera lucida tracing of a layer V PFC neuron, with the apical dendritic tuft and distal and proximal apical dendritic stem regions demarcated. B, (left to right) Schematic diagram of a layer V PFC neuron and the location of synaptic stimulation and Cd2+ (5 mm in puff pipette) and APV (250 μm in puff pipette) pressure ejection pipettes. Synaptic stimulation of layers I–II (in the presence of bath-applied DNQX, 10 μm, and bicuculline, 4 μm) evoked either a large subthreshold NMDA EPSP or a suprathreshold response at aVm of −56 mV. Note the hump-like potential during the late portion of the subthreshold EPSP. At aVm of −76 mV a smaller subthreshold NMDA EPSP was evoked. Cd2+ application to the proximal apical dendritic stem region (100–200 μm from the soma) reduced the large subthreshold EPSP and abolished synaptically evoked action potentials but had no effect on the EPSP evoked at −76 mV. After partial recovery from the effects of Cd2+application, focal application of APV to the same site had no effect on the evoked response. In all figures, an arrow denotes the time of synaptic stimulation. C, (left to right) Schematic diagram of a layer V PFC neuron and the location of the glutamate and Cd2+ pressure ejection pipettes. Focal pressure ejection of glutamate to the apical tuft evoked a suprathreshold response recorded at the soma. Cd2+ application just below the glutamate pipette in the apical tuft did not block the glutamate-evoked response.
One solution to overcome such problems has been to suggest that voltage-gated ionic conductances in the dendrites of pyramidal neurons boost the effects of local synaptic inputs by acting as either voltage or current amplifiers (Spencer and Kandel, 1961; Shepherd et al., 1985;Bernander et al., 1994; Yuste et al., 1994). Slowly inactivating Na+ currents have been shown to enhance distal EPSPs and currents evoked by iontophoresis of glutamate onto the apical dendrites of layers V–VI cells (Schwindt and Crill, 1995; Stuart and Sakmann, 1995). In addition, both low- and high-threshold Ca2+ currents have been recorded in the dendrites of pyramidal neurons and might also serve to amplify synaptic signals (Deisz et al., 1991; Amitai et al., 1993; Kim and Connors, 1993;Markram and Sakmann, 1994; Magee and Johnston, 1995a,b; Magee et al., 1995).
An understanding of signal integration by pyramidal neurons requires insight into the spatial locations of the voltage-gated channels that amplify distal EPSPs. One might expect that EPSPs generated within the apical tuft would be amplified by voltage-gated currents locally. In a recent study, Stuart and Sakmann (1995) demonstrated that this is not always the case. They showed that slowly inactivating Na+ channels located primarily in the axosomatic region, rather than along the apical dendrite of layer V cortical neurons, amplify distal EPSPs. It is unclear whether a similar arrangement exists with respect to Ca2+ channels. Although evidence from immunocytochemical and electrophysiological studies indicates that Ca2+ channels are located throughout the dendrites of pyramidal neurons, there is a clustering of large conductance (L-type) high-threshold Ca2+channels at the base of the major dendrites (Westenbroek et al., 1990;Magee and Johnston, 1995b). In addition, Ca2+imaging studies have shown that synaptic activation of the apical dendrites or back-propagating action potentials evoke significant Ca2+ influx in the proximal apical and basal dendrites of both cortical and hippocampal pyramidal neurons, and Ca2+ influx tends to decrease with distance from the soma (Jaffe et al., 1992; Miyakawa et al., 1992; Müller and Connor, 1992; Regehr and Tank, 1992; Schiller et al., 1995; Johnston et al., 1996; Svoboda et al., 1997). Furthermore, although there is a considerable rise in Ca2+ influx in the proximal dendrites of cortical neurons after whisker stimulation in vivo, no detectable rise in Ca2+ influx is observed within the distal apical tufts (Svoboda et al., 1997). However, a band of Ca2+ influx has been observed in the mid-distal portion of the apical dendritic stem of layer V cortical neurons after layer I stimulation (Yuste et al., 1994). Thus, Ca2+ influx can be greatest in different regions of pyramidal neurons under different conditions. It is presently unclear whether activation of Ca2+ channels located along the apical dendritic stem or the apical tufts of pyramidal neurons are responsible for electrical amplification of distal EPSPs.
The present study examined the functional roles of the dendritic Ca2+ channels distributed at different locations along layers V–VI prefrontal cortical (PFC) neurons. Intracellular and patch-clamp somatic recordings, used in combination with focal pressure application of the voltage-gated Ca2+ channel blocker Cd2+, revealed that layers I–II EPSPs triggered a late Ca2+-mediated potential and high-threshold Ca2+ spikes in the proximal dendrites. On the other hand, direct patch-clamp recordings from the apical tuft dendrites of deep layer neurons revealed that both intradendritic current injection and synaptic stimulation were largely ineffective in evoking Ca2+ spikes. The present results suggested that the Ca2+ currents that enhance distally generated EPSPs are located primarily along the apical dendritic stem of layer V PFC neurons and not within the apical tuft.
Preliminary results have been published previously in abstract form (Seamans et al., 1996).
MATERIALS AND METHODS
Brain slice preparation. Experimental procedures forin vitro brain slice preparation and intracellular recordings were modified from those described in detail by Yang et al. (1996a). Briefly, coronal brain slices (450 μm) containing the prelimbic portion of the medial PFC (Uylings and van Eden, 1990;Condé et al., 1995) were prepared from young adult (≥5 weeks) male Sprague Dawley rats (80–200 gm; University of British Columbia colony). The prelimbic cortex is that part of the medial PFC that is flanked by the corpus callosum. Slices were transferred to a submerged-style recording chamber (transilluminated so that the cortical layers could be identified) and continuously perfused with an oxygenated (95% O2, 5% CO2) solution containing (in mm): NaCl 126, KCl 3, NaHCO3 26, MgCl2 1.3, CaCl2 2.3, and glucose 10. Experiments were performed at 23–33°C.
Recording and stimulation. Standard sharp electrode intracellular recordings of layers V–VI PFC neurons were made in current-clamp mode, and whole-cell patch-clamp recordings were made in current-clamp or voltage-clamp mode. Intracellular microelectrodes were made from borosilicate tubing [1.2 mm outer diameter (o.d.), 0.69 mm inner diameter (i.d.); Sutter Instruments] and filled with 2–3m potassium acetate, 1 m cesium acetate, and 80–100 mm QX-314. Electrodes had a resistance between 110 and 170 MΩ. Patch pipettes (1.5 mm o.d., 1.1 mm i.d.) were filled with (in mm): potassium gluconate 130, KCl 10, EGTA 1, MgCl2 2, NaATP 2, and HEPES 10 and 0.3% biocytin, and had a resistance between 8 and 12 MΩ for dendritic recordings and <5 MΩ for somatic recordings. In some experiments QX-314 (1 mm) or CsCl (10 mm) was also added to the internal patch solution. Seal resistance before break-in was >2 GΩ, and after break-in, access resistance was routinely 60–90 MΩ for dendritic recordings and <20 MΩ for somatic recordings.
Microelectrodes were connected to the head stage of an Axoclamp-2B (Axon Instruments, Foster City, CA) amplifier by an Ag/AgCl wire. Bridge balance was monitored continually, and capacitive transients were compensated optimally. The recorded voltage or current signals were amplified, digitized by a Digidata 2000 analog-to-digital board (Axon), and sampled by a PC running pClamp software (version 5.7; Axon). Voltage-clamp recordings were obtained using the Axoclamp-2B in continuous single-electrode voltage-clamp mode. Series resistance was 80% compensated, and the gain was increased up to 25 μA/mV. Data were filtered at 10 kHz.
For most experiments a concentric bipolar stimulating electrode (SNE-100, David Kopf Company) was placed in layers I–II. Electrical stimulation (0.2 msec, 50–500 μA) was delivered to layers I–II at low frequencies (≤0.1 Hz) and consisted of monophasic square pulses delivered via an optically isolated stimulation unit (ISO-FLEX, A.M.P.I.). Stimulation frequencies were programmed by a Master-8 pulse generator (A.M.P.I.). In some experiments, to isolate layers I–II inputs, a vertical cut from the corpus callosum to layer II was made using a 30.5 ga syringe needle mounted on a micromanipulator. The stimulating electrode was then placed at least 0.3 mm to one side of the cut (Cauller and Connors, 1994). In other experiments, 300 μml-glutamic acid monosodium salt (Sigma, St. Louis, MO) plus fast green (Sigma) was applied to layers I–II via a glass pipette (tip diameter, 1–2 μm) using a Picospritzer II (General Valve, Fairfield, NJ) pressure ejection device (using 10–500 msec pressure pulses, 20–50 psi), and diffusion was monitored visually under a microscope.
Pharmacological treatments. In some cells a large EPSP could be evoked in the absence of externally applied drugs (nonisolated EPSP); however, in many cells a strong IPSP occluded the EPSP and prevented the initiation of Na+ or Ca2+ spikes. The concentrations of bicuculline (>1.5 μm) necessary to block this strong IPSP resulted in the emergence of a large plateau potential that showed highly variable onset latencies and reversed at ∼0 mV. This large potential was likely a giant polysynaptic EPSP (Johnston and Brown, 1981; Tasker and Dudek, 1991), which completely occluded synaptically evoked Ca2+ potentials and spikes. Application of APV (50 μm) only served to decrease the duration of the large potential, whereas DNQX (10 μm) (in the absence of APV) blocked it. In the presence of bicuculline and DNQX, an isolated monosynaptic NMDA EPSP was evoked. The layers I–II-evoked isolated NMDA EPSP was not contaminated by collateral inputs synapsing in deeper layers, because application of APV (250 μm) to the apical dendrite (100–200 μm from soma) had no effect on the magnitude of the evoked response. All glutamatergic and GABAergic antagonists were obtained from Precision Biochemicals (Vancouver, British Columbia, Canada).
In experiments in which Ca2+ spikes were evoked by intracellular current pulses, tetraethylammonium hydrochloride (TEA) (20 mm) and tetrodotoxin (TTX) (0.5–1 μm) were bath-applied to block K+ and Na+ channels, respectively. In other experiments NiCl2 (100 μm) was bath-applied to block low-threshold Ca2+ currents. The above agents were obtained from Research Biochemicals (Natick, MA) or Sigma.
Focal Cd2+ applications. To determine the site of electrogenesis of Ca2+-mediated potentials, CdCl2 (2–50 mm in puff pipette) plus fast green was pressure-ejected focally (using 100–500 msec pressure pulses, 20–50 psi) to the apical dendritic stem at the region between the dorsal borders of layers III and V (100–200 μm from the soma), the border between layers II–III (300–400 μm from the soma), or to layers I–II (>500 μm from the soma, <200 μm from pia) via a glass pipette (tip diameter, 1–2 μm) using a Picospritzer II pressure ejection device (General Valve). To minimize spreading, small diameter pipettes were used, and the speed of perfusion was increased to 5 ml/min. Diffusion was monitored visually through a microscope.
Apical dendritic tuft patch-clamp recordings and staining.Dendritic tuft recordings were made by patch pipettes in layers I–II, and biocytin was diffused passively throughout the neuron during the course of the experiments. At the end of each experiment, brain slices were fixed and stained for biocytin (Yang et al., 1996a). Dimethylsulfoxide was used as the mounting medium for cover-slipping. When viewed under a microscope, the soma of stained neurons were located in layers III–VI, and the corresponding recording was in the apical tuft. In some cells, the recording site could be observed clearly on the stained dendrite as a small notch (see Fig. 5).
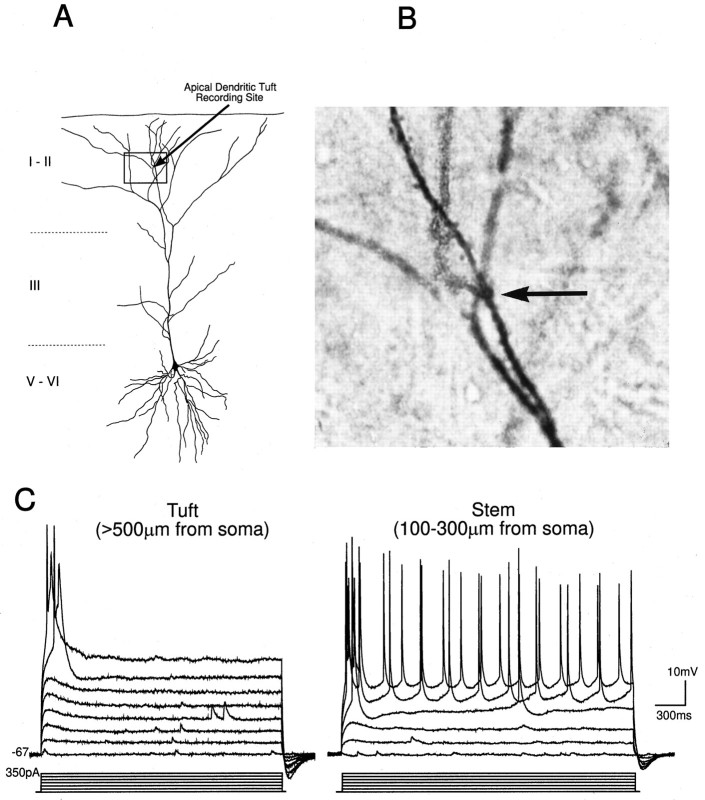
Fig. 5.
Typical recording sites and electrophysiological properties of the apical tuft of layer V PFC neurons. A, Camera lucida tracing of a biocytin-stained layer V PFC neuron in which a patch-clamp recording was made from the apical tuft.B, A photomicrograph of the region shown in thebox in A. The recording site within the tuft is indicated by the arrow. C,Left, A distal apical tuft dendrite recorded beyond the main bifurcation (>500 μm from the soma) responded to intradendritic current pulses with fast spikes; right, a recording from the main stem of the apical dendrite (∼200 μm from soma) showing the response to intradendritic depolarizing current pulses for comparison.
RESULTS
The database was derived from 127 neurons recorded from the prelimbic region of the PFC using either intracellular or patch pipettes. Only cells with a Vm more negative than −60 mV and action potentials (observed before QX-314 took effect) that overshot 0 mV were analyzed.
Contribution of Cd2+-sensitive dendritic potentials to layers I–II-evoked responses: intrasomatic recordings from layers V–VI PFC pyramidal neurons
To examine the spatial locations of Ca2+channels that contributed to distally generated suprathreshold responses, the Ca2+ channel blocker Cd2+ (2–50 mm in puff pipette) was pressure-ejected focally to the apical dendritic stem or apical dendritic tuft (Fig. 1A). A number of preliminary experiments were conducted to determine the possible contributions of polysynaptic collateral inputs (synapsing in layers III–V) to the synaptic response recorded at the soma, because these collaterals could potentially be affected by Cd2+applications. Although a vertical cut from the corpus collosum to layers I–II was made in the brain slices, it was not always possible to rule out completely the contribution of collateral inputs to layers III–V. Thus, APV (250 μm in puff pipette) or DNQX (50 μm in puff pipette) was applied focally to the apical dendritic stem in layers III–V either before or after recovery from Cd2+ application. Although nonisolated EPSPs evoked by layers I–II stimulation were often reduced by focal application of APV and DNQX to the proximal apical dendrite (not shown), isolated NMDA EPSPs (in the presence of bath-applied DNQX and bicuculline) were unaffected by focal application of APV to the same location (n = 5 of 5) (Fig. 1B). This indicated that the polysynaptic collateral inputs to layers III–V were primarily AMPA receptor-mediated and blocked by DNQX. Thus experiments were performed using vertically cut brain slices or in bicuculline and DNQX to reduce IPSPs and the effects of polysynaptic collateral inputs.
Layers I–II NMDA EPSPs that evoked action potentials (suprathreshold responses) were reduced significantly by Cd2+application to the proximal apical dendrite and soma (n= 8 of 9) (Fig. 1B). After Cd2+application, action potentials were not triggered synaptically unless the Vm was depolarized by at least 2–4 mV more positive than the control Vm. In contrast, focal Cd2+ application to the proximal apical dendrite had no effect on subthreshold NMDA EPSPs evoked >5 mV more negative than the threshold for triggering action potentials (Fig.1B). Thus, Cd2+ application to the apical dendritic stem reduced the suprathreshold synaptic responses evoked by synaptic stimulation of layers I–II.
To test whether Cd2+-sensitive Ca2+ channels in the distal apical tuft also contributed to the suprathreshold response recorded at the soma, responses were evoked nonsynaptically by focal glutamate application to the apical tuft (>500 μm from soma). The glutamate-evoked suprathreshold response recorded from the soma was unaffected by focal Cd2+ application to the apical tuft (>500 μm from the soma) just below the site of glutamate application (n = 5 of 5) (Fig. 1C). In contrast, Cd2+ application to the proximal apical dendrite (100–200 μm from soma; n = 8 of 8; not shown) reduced the glutamate-evoked response; however, with use of this preparation it was not possible to rule out that glutamate-activated collateral inputs were also blocked by Cd2+. Collectively, the results illustrated in Figure 1 suggested that dendritic voltage-gated Ca2+ channels proximal to the soma and not within the apical tuft functionally amplified distally evoked suprathreshold synaptic responses.
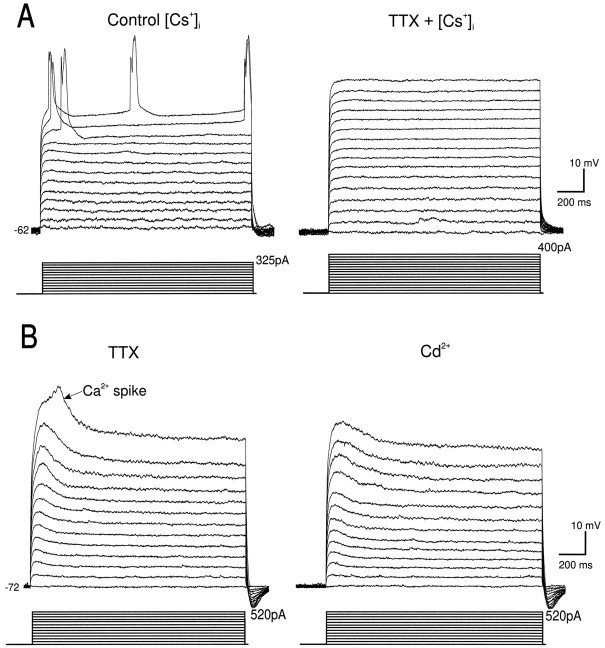
To examine the properties of the Cd2+-sensitive dendritic Ca2+-mediated potentials that amplified distally generated responses, the Na+ channel blocker QX-314 alone or in combination with the K+channel blocker Cs+ was included in the recording pipette. Under these conditions, if the intensity of layers I–II stimulation was increased gradually or the membrane potential was clamped more positive than −55 mV, a late hump potential (Fig.2A,C, asterisk) and Ca2+ spike (Fig. 2A,C, gray traces) were evoked on top of the nonisolated EPSP or the isolated NMDA EPSP. Ca2+ spikes evoked synaptically in the presence of QX-314 and Cs+ (n = 15) (Fig. 2A,C) or by intracellular current pulses in the presence of TTX and TEA (not shown; n = 12) were followed by a single long duration repolarizing potential that reversed in polarity at approximately −55 mV. The repolarizing potential was often accompanied by a short duration depolarizing afterpotential (Fig.2A, DAP). Unlike somatosensory cortical neurons (Reuveni et al., 1993), stepwise repolarization from the Ca2+ spike (in Cs+ and QX-314 or TTX and TEA) was absent in PFC neurons. Because this stepwise repolarization of Ca2+ spikes has been attributed to distal Ca2+ electrogenesis, this finding suggested that in PFC neurons there was a lack of such distal electrogenesis.
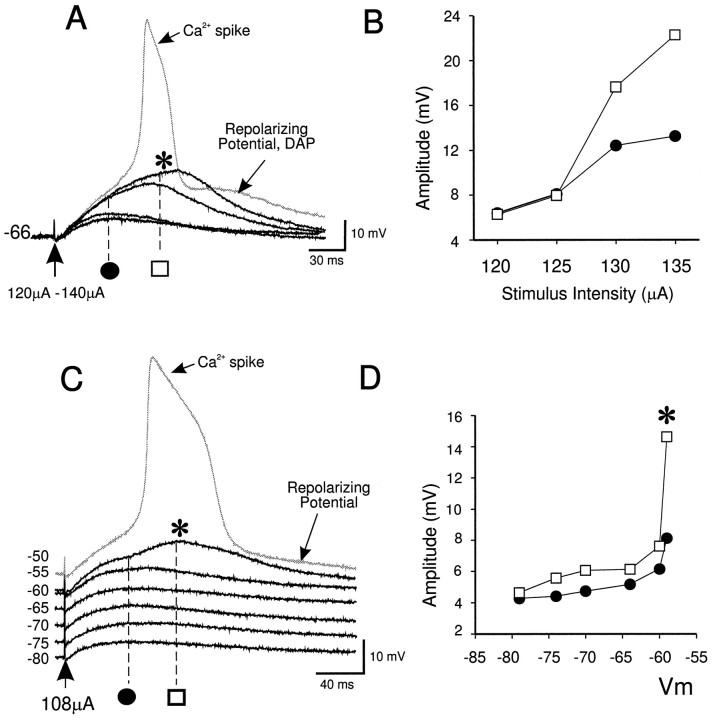
Fig. 2.
Layers I–II EPSPs and synaptically evoked depolarizing potentials in QX-314. A, A synaptically evoked hump potential (asterisk) and Ca2+ spike (gray trace) was evoked by progressive increases in stimulation current intensity, with the Vm held at a constant value (−66 mV) in cells recorded with QX-314 and Cs+-filled electrodes. B, Graph of subthreshold response amplitude with increased stimulation current intensity. Black circles, Amplitudes of the early component of the EPSPs illustrated in A; open squares, amplitudes of the late component of the EPSPs illustrated inA. C, The initiation of a synaptically evoked hump potential (asterisk) and Ca2+ spike (gray trace) by progressive membrane depolarization using a constant stimulation current (108 μA, 0.1 Hz). D, Graph of subthreshold response amplitude evoked at different membrane potentials.Black circles, Amplitudes of the early component of the EPSPs shown in C; open squares, amplitudes of the late component of the EPSPs shown inC. These particular responses were recorded in bicuculline and DNQX.
Ionic mechanisms underlying the dendritic hump potential
A number of experiments were conducted to examine the mechanisms responsible for the activation of the hump potential. Although cortical neurons posses a TTX- and intracellular QX-314-sensitive slowly inactivating Na+ current that is activated in the subthreshold voltage range (Connors et al., 1982; Stafstrom et al., 1982, 1985; Hirsch and Gilbert, 1991; Hwa and Avoli, 1992; Yang et al., 1996a), the synaptically evoked hump potential shown in this study was not mediated by this Na+ current, because the potential was evoked synaptically or by intrasomatic current injection in the presence of QX-314 (n > 100) or TTX (n = 4).
A low-threshold Ni2+-sensitive T-type Ca2+-current is also activated synaptically in pyramidal neurons (Markram and Sakmann, 1994; Magee et al., 1995b). The hump potential, however, was not mediated by this current because (1) it was evoked at membrane potentials near −50 mV where T-currents are inactivated (Tsien et al., 1988), and (2) it was not blocked by Ni2+ (100 μm) (n = 4 of 4) (Fig. 3A). Voltage-clamp analysis (Fig.3B) confirmed that this concentration of Ni2+ greatly reduced the low-threshold T-current in these neurons.
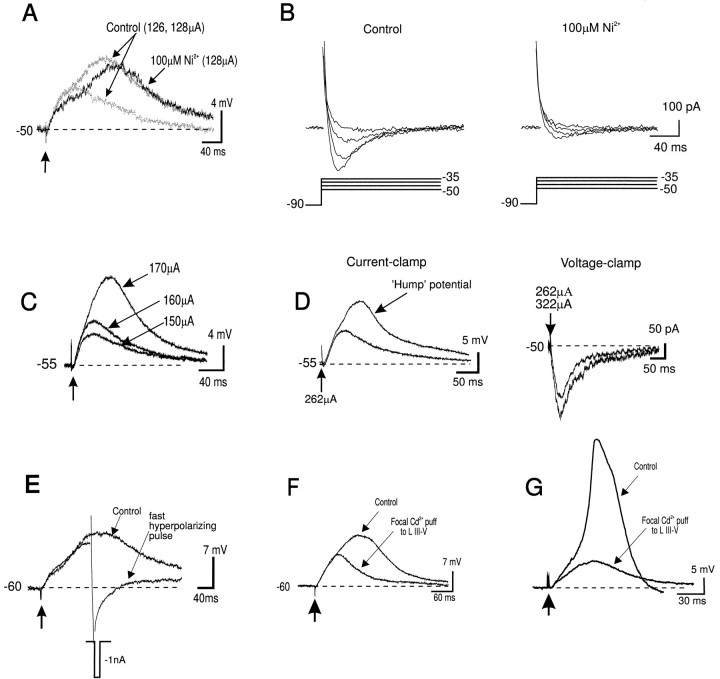
Fig. 3.
Properties of the synaptically evoked hump potential. A, The hump potential was not mediated by a low-threshold Ca2+ (T-type) current. The synaptically evoked hump potential was evoked at a steady stateVm of −50 mV and was not blocked by bath application of Ni2+ (100 μm).B, In the same neuron, however, the low-threshold T-current evoked under voltage clamp (with leak currents subtracted) was greatly attenuated by bath application of 100 μmNi2+. C, The hump potential was evoked in the presence of the NMDA antagonist APV (50 μm) (without DNQX), 1 mm[Ca2+]o and 4 mm[Mg2+]o, suggesting that it was not mediated by an NMDA-dependent polysynaptic EPSP. D, The ‘Hump’ potential was evoked synaptically in a voltage-dependent manner in current clamp mode (left). In the same neuron, the hump potential was absent in voltage-clamp mode even when the stimulation intensity was increased from 262 to 322 μA (right), indicating that it was mediated by a voltage-gated current. E, The synaptically evoked hump potential was repolarized by a fast hyperpolarizing somatic current pulse (−1000 pA/10 msec). F, G, The synaptically evoked hump potential and Ca2+ spike were blocked by focal Cd2+ puff to the proximal apical dendritic stem (100–200 μm from soma). All responses shown were recorded using QX-314 and Cs+-filled electrodes.
The hump potential appeared similar to a late polysynaptic EPSP recorded in layer V neocortical neurons (Sutor and Hablitz, 1989); however, the late hump potential could be evoked synaptically in high (10 mm) [Ca2+]o or APV and low (1 mm) [Ca2+]o + high (4 mm) [Mg2+]o(n = 6) (Fig. 3C). Because APV blocked NMDA receptors and these alterations in external [Ca2+] and [Mg2+] strongly attenuated polysynaptic responses (Berry and Pentreath, 1976), it is not possible that the late hump potential was mediated by a polysynaptic NMDA-dependent EPSP.
Additional evidence that the hump potential was not mediated by a polysynaptic EPSP came from comparisons of current-clamp and voltage-clamp responses during synaptic stimulation of layers I–II. In current-clamp mode, the hump potential was evoked synaptically in a voltage-dependent manner, e.g., at −50 to −55mV (Fig. 3D); however, in the same neuron when the soma was voltage-clamped just below the activation threshold for the hump potential, only an EPSC was evoked synaptically (Fig. 3D). Because the voltage clamp should not have prevented the activation of a polysynaptic EPSC, the present result suggested that the hump potential was mediated by a voltage-activated current and not a polysynaptic EPSP.
As a result of the considerable space-clamp limitations associated with voltage-clamping large pyramidal neurons (Spruston et al., 1993), the data shown in Figure 3D also suggested that the hump potential was generated electrotonically close to the soma in a region adequately voltage-clamped by the somatic electrode. In accordance with this hypothesis, the hump potential was repolarized by a fast hyperpolarizing (−1 to −3 nA/10–20 msec) somatic current pulse (Fig. 3E) (n = 5 of 5). Such fast intrasomatic hyperpolarizing pulses do not propagate far from the soma because they are strongly filtered by the passive cable properties of the dendrites (i.e., estimated to be ∼50% attenuation at 333 μm from the soma) (Jack et al., 1975; Johnston and Brown, 1983; Spruston et al., 1993, 1994; Spruston and Stuart, 1996). Thus the hump potential was generated by a voltage-activated current located electrotonically close to the soma. Accordingly, the synaptically evoked hump potential (Fig. 3F) was blocked by focal pressure ejection of Cd2+ to the proximal apical dendritic stem (100–200 μm from the soma) (n = 11 of 12). Likewise, the synaptically evoked Ca2+ spike was also blocked by focal Cd2+ application to this region (Fig. 3G) (n = 6).
The hump potential was also evoked by intrasomatic depolarizing current pulses (>50 msec) in the absence of synaptic stimulation using electrodes containing QX-314 and Cs+ or in the presence of external TTX and TEA (total n = 15). The hump potential generated by intrasomatic current pulses (Fig.4B) was blocked by Cd2+ application to the proximal apical dendritic stem and soma (Fig. 4A,E,Site 3) but unaffected by focal Cd2+application to the apical tuft (>500 μm from the soma) (Fig.4A,C, Site 1), or the distal apical dendritic stem (300–400 μm from the soma) (Fig.4A,D, Site 2). This suggested that the hump potential was not a Ca2+spike generated distally and attenuated en route to the soma.
Fig. 4.
Sites of electrogenesis of the hump potential.A, Camera lucida tracing of a typical layer V PFC neuron and three sites where Cd2+ was applied focally: the apical tuft (site 1), the distal apical dendritic stem (site 2), and the proximal apical dendritic stem (site 3) during somatic recordings with pipettes filled with QX-314 and Cs+.B, A passive membrane response was evoked by an intrasomatic depolarizing current pulse (50 msec, 120 pA) at aVm of −60 mV, whereas the hump potential was evoked by the same current pulse at a Vmof −58 mV. C, Focal Cd2+ application to the apical tuft (Site 1) or (D) distal apical dendritic stem (Site 2) had no effect on the hump potential recorded from the soma. E, The hump potential was blocked by focal Cd2+ application to the proximal apical dendritic stem (Site 3).F, Direct patch-clamp recording from the proximal apical dendritic stem (in TTX and TEA) revealed that a hump potential was evoked by intradendritic current pulses (20 msec, 50 pA) before the initiation of regenerative Ca2+ spikes.
Additional evidence that the hump potential was not Ca2+ spike-generated in the apical dendritic stem and degraded en route to the soma came from direct patch-clamp recordings from the apical dendritic stem (100–300 μm from soma;n = 3). These recordings revealed that a hump potential was evoked before prominent and distinctive regenerative Ca2+ spikes (Fig. 4F). Finally, the hump potential was not mediated indirectly by a [Ca2+]i-activated current, because it was recorded with electrodes filled with the intracellular Ca2+ chelator BAPTA (100 mm) (n = 5 of 5; not shown). Collectively, the present results suggest that the hump potential was generated by dendritic slowly activating Ca2+ channels in the proximal dendrites. It is likely that the proximally generated hump potential and/or Ca2+ spikes contributed to the Cd2+-sensitive Ca2+-mediated amplification of distally generated synaptic signals in PFC neurons, as shown in Figure 1.
Patch-clamp recordings from the apical dendritic stem and tuft of deep layer PFC cells
The results described above suggested that dendritic Ca2+ channels located in the proximal dendrites and not within the apical tuft were activated by layers I–II stimulation and shaped distal EPSPs. To determine directly whether dendritic Ca2+ channels at the site of synaptic input modulated the EPSP evoked by layers I–II stimulation, recordings were made from the dendrites of the apical tuft. Cells were stained for biocytin to confirm that recordings were made from the apical tuft dendrites of pyramidal cells whose soma could be traced to layers III–VI (Fig. 5A,B). The yield for such experiments was low because the mean diameter of the tuft branches was small, and patch recordings were made using the “blind” approach. Nevertheless, recordings were made from 17 apical tuft dendrites (above the main bifurcation). Eleven recordings were made from the apical tuft of histologically confirmed layer V neurons, whereas two recordings were made from histologically confirmed layer III neurons (no obvious differences were observed between these two groups of cells in the electrophysiological properties tested). Four additional presumed apical tuft dendrites were not stained but had electrophysiological properties similar to the apical tuft recordings from layers III–V cells. Four more stained dendrites were recorded from the main stem of the apical dendrite below the main bifurcation.
Electrophysiological responses to intracellular current pulse injection
Tuft dendrites had a mean resting Vmof −64 ± 2.89 mV and a mean Rin of 212 ± 29 MΩ (n = 9). The time course of the membrane voltage response to a hyperpolarizing current pulse (−10 pA) could be fitted by two exponentials with mean time constants of 1.65 ± 0.2 and 17.35 ± 3.83 msec. Tuft dendrites possessed voltage-dependent rectification and were capable of firing a mixture of fast and slow spikes with variable durations. Like recordings obtained from the main stem of the apical dendrite (Kim and Connors, 1993;Spruston et al., 1995b), intradendritic hyperpolarizing pulses injected into the dendritic tufts evoked a characteristic hyperpolarizing “sag” typically mediated by the time-dependent hyperpolarization-activated mixed cationic conductanceIh (n = 7 of 8; not shown). Also like the main stem of the apical dendrite (Fig. 5D), intradendritic depolarizing current pulses evoked spikes within the tufts (n = 11 of 14) (Fig. 5C). The spike threshold within the apical tufts was relatively high (−17.8 ± 5 mV; n = 11); however, spikes initiated in the main stem of the apical dendrite also have thresholds near 0 mV (Stuart and Sakmann, 1994). Such high spike thresholds may be caused by a low local Na+ channel density (Mainen et al., 1995).
Spikes recorded from the tuft usually consisted of fast and slow components that slowly disappeared during recordings with QX-314-filled pipettes or were blocked by bath application of TTX (Fig.6A), indicating that they were initiated by a Na+ current. Unlike in the apical dendritic stem, however, where Ca2+ spikes in isolation from Na+ spikes could be evoked readily (Fig. 4F) (Wong et al., 1979; Kim and Connors, 1993;Stuart and Sakmann, 1994), Ca2+ spikes evoked in the absence of Na+ spikes were rare in the apical tuft dendrites. No Ca2+-mediated potentials were evoked by intradendritic current injection in 9 of 13 tuft dendrites when internal QX-314 or external TTX were used in either the presence or absence of internal Cs+ (Fig. 6A). In the remaining four tuft dendrites, a broad low-amplitude spike could be evoked at a mean threshold of +10 ± 5.8 mV (Fig.5B). This spike was blocked by bath application of 200 μm Cd2+ (Fig. 5B), indicating that it was Ca2+-mediated.
Fig. 6.
Electrophysiological responses of apical tuft dendrites after blockade of Na+ and Ca2+ channels. A, In a dendrite recorded using a Cs+-filled electrode, spikes evoked by injecting depolarizing intradendritic current pulses (right) were blocked by bath application of TTX (500 nm) (left). B, In a different tuft dendrite it was possible to evoke Ca2+ spikes by injecting large depolarizing intradendritic current pulses in TTX (500 nm) (left). Ca2+spikes were blocked by bath application of Cd2+ (200 μm) (right).
Electrophysiological responses to layers I–II synaptic stimulation
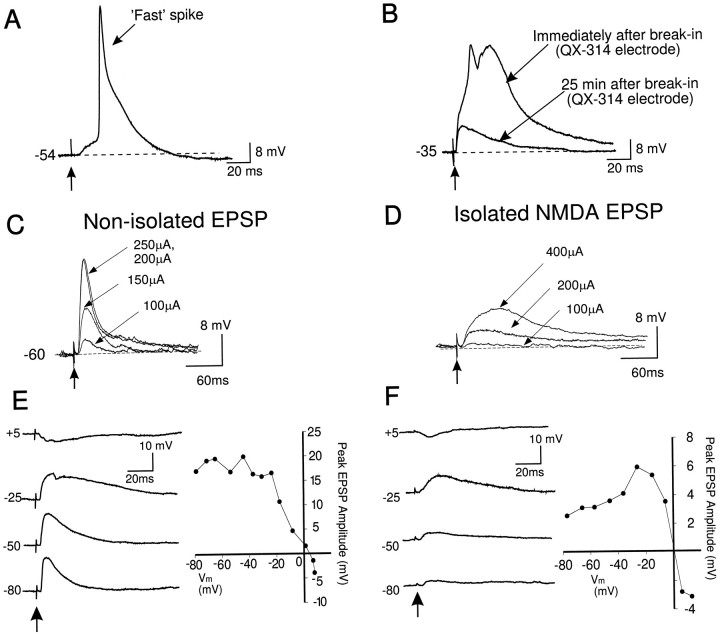
Fast, presumably Na+-mediated spikes (mean half-width = 5.3 ± 0.6 msec) were evoked synaptically in 50% of tuft dendrites tested (Fig. 7A). In 2 of 16 dendrites, synaptic stimulation of layers I–II evoked an initial fast spike that was followed by an additional long duration spike (mean half-width = 50.5 ± 17.8 msec) (Fig. 7B). In one of these two tuft dendrites, recorded close (∼50 μm) to the main bifurcation, long duration regenerative spikes were evoked (not shown) and appeared similar to dendritic putative Ca2+ spikes reported previously (Amitai et al., 1993). In the other tuft dendrite, synaptically activated mixed spikes were present immediately after break-in using a QX-314 electrode (Fig.7B); however, spikes disappeared later during the recording session when QX-314 produced a sufficient block of the Na+ channels (Fig. 7B). These observations suggested that in these two apical tuft dendrites, Ca2+ spikes may have been triggered by QX-314-sensitive Na+ spikes.
Fig. 7.
Synaptic responses of apical tuft dendrites.A, Synaptic stimulation of layers I–II often evoked an EPSP with an overriding fast spike. B, A dendrite recorded using a QX-314-filled electrode. Fast and slow spikes were evoked by synaptic stimulation of layers I–II immediately after break-in of the dendritic patch. These spikes disappeared during the recording period as QX-314 blocked Na+ channels, leaving an EPSP. C, After QX-314 sufficiently blocked Na+ channels, synaptic stimulation of layers I–II evoked a subthreshold EPSP that increased in amplitude uniformly with increases in stimulation intensity. No Ca2+ spikes were evoked synaptically. D, Left, Synaptic stimulation of the same dendrite as shown in Cafter application of bicuculline and DNQX. Current intensities were increased from 100 to 200 μA and then 400 μA, but Ca2+ spikes were not evoked. E,Left, In a different dendrite recorded in the presence of 0.5 μm bicuculline, EPSPs decreased in amplitude as the dendritic membrane was current-clamped from −80 mV and reversed at approximately +5 mV. Note that EPSP duration was enhanced with membrane depolarization from −80 to −25 mV because of activation of NMDA receptors. Right, A graph showing the changes in the amplitude of the layers I–II EPSP with changes in dendritic membrane voltage. F, Left, In the presence of bicuculline and DNQX, the NMDA EPSP increased in amplitude as the steady-state dendritic membrane potential was depolarized from −80 to −30 mV. With further membrane depolarization, the NMDA EPSP decreased in amplitude and reversed in polarity at potentials more positive than 0 mV. Again, no Ca2+ spikes were observed.Right, A graph showing the changes in the amplitude of the layers I–II NMDA EPSP, with changes in dendritic membrane voltage.
In the presence of QX-314 (>25 min after break-in), it was not possible to evoke spikes synaptically within the tuft under control conditions (n = 7) (Fig. 7C,D) or in the presence of DNQX and bicuculline (Fig. 7E,F) (n = 3), even when delivering up to 400 μA of stimulation current or during strong membrane depolarization. In contrast, during intrasomatic recordings in the presence of DNQX and bicuculline, a hump potential or Ca2+ spike could be evoked synaptically using ∼150 μA of stimulation current at rest or using ∼100 μA of stimulation during membrane depolarization (Fig.2). Thus, unlike the soma or apical dendritic stem, it was not possible to trigger Ca2+ potentials synaptically in the absence of Na+ spikes in the apical tuft.
The amplitude of NMDA EPSPs recorded from the apical tufts was influenced by dendritic membrane voltage and reversed in polarity at 0 mV (Fig. 7D,E). This finding indicated that (1) the NMDA-mediated response had properties consistent with NMDA responses recorded from the soma (Nowak et al., 1984; Hestrin et al., 1990) and main stem of the apical dendrite (Spruston et al., 1995a), and (2) the high access resistance (60–80 MΩ) of the dendritic patch pipette did not seriously alter the recorded membrane voltage.
DISCUSSION
The present results suggest that dendritic voltage-gated Ca2+ channels located proximal to the soma generated Cd2+-sensitive potentials that amplified distal EPSPs, whereas Ca2+ channels in the apical tuft contributed little to the shaping of distal EPSPs in layers V–VI PFC neurons.
Contribution of NMDA receptors to layers I–II EPSPs
There has been considerable controversy regarding the spatial locations of NMDA receptors that mediate distally generated EPSPs. Although NMDA R1 subunit immunoreactivity is found in virtually all lamina of the cortex (and PFC), including the superficial layers (Bröckers et al., 1994; Huntley et al., 1994; Rudolf et al., 1996), the electrical response of deep layer cortical neurons to iontophoresis of NMDA has been reported to decrease with distance from the soma (Currie et al., 1994; Dodt et al., 1995). During tuft recordings, the initial synaptic response to layers I–II stimulation was greatly reduced by DNQX, but an NMDA EPSP could be evoked if the stimulation intensity was increased. This is consistent with the notion that the peak synaptic current is largely dependent on activation of dendritic non-NMDA receptors (Spruston et al., 1995a). Furthermore, in the absence of non-NMDA receptors, synaptic inputs will produce less membrane depolarization and therefore less relief of the voltage-dependent Mg2+ block of NMDA receptors. Thus, although NMDA EPSPs could be recorded within the tuft after layers I–II stimulation, NMDA receptor activation is likely dependent on AMPA receptor stimulation normally.
Electrogenesis of dendritic Ca2+-mediated potentials
The late hump potential that rode atop layers I–II nonisolated or isolated NMDA EPSPs was clearly different from a late polysynaptic NMDA-mediated EPSP described previously (Sutor and Hablitz, 1989), because (1) the hump potential was evoked abruptly during membrane depolarization; (2) the hump potential was evoked in media containing APV and low [Ca2+]o, high [Mg2+]o or high [Ca2+]o, which block NMDA receptors and polysynaptic EPSPs, respectively (Berry and Pentreath, 1976); (3) the hump potential was repolarized by a fast hyperpolarizing pulse; (4) the hump potential was evoked by intrasomatic current pulses; and (5) a monosynaptic EPSC but no hump current was evoked synaptically when the soma was voltage-clamped below the activation threshold of the hump potential. The present results also suggested that the hump potential was not mediated by a QX-314-sensitive slowly inactivating Na+ current or a Ni2+-sensitive low-threshold Ca2+current (Figs. 2, 3A). Rather, the hump potential may have been mediated by a slowly activating Ca2+ current located along the proximal apical dendritic stem, similar to that recorded intradendritically in hippocampal pyramidal and Purkinje neurons (Llinas and Sugimori, 1980; Benardo et al., 1982; Masukawa and Prince, 1984). However, although Cd2+ application specifically to the proximal apical dendrite blocked the hump potential and Ca2+ spikes, the spread of Cd2+ could also have affected Ca2+ channels in the basal dendrites and soma, which may also contribute to Ca2+ potential electrogenesis. It is likely that L-type Ca2+channels contributed to the hump potential, because in other types of neurons, L-type Ca2+ channels are clustered along the proximal apical dendrite (Westenbroek et al., 1992; Magee and Johnston, 1995a,b) and mediate subthreshold membrane depolarization (Hernández-López et al., 1997).
During somatic or apical dendritic stem recordings, when a hump potential was evoked, slight membrane depolarization or further increases in stimulation intensity could trigger a Ca2+ spike. The relative contributions of the hump potential and Ca2+ spikes to the amplification of distal EPSPs is presently unclear; however, given the high input resistance, low capacitance, and relatively high density of Ca2+ channels along the proximal apical dendrite of pyramidal neurons (Westenbroek et al., 1992; Magee and Johnston, 1995a,b), it is possible that an orthodromically traveling EPSP could trigger both the hump potential and Ca2+ spikes en route to the soma. These Ca2+ potentials could then amplify EPSPs to ensure that spike firing is reached near the soma.
A key feature of Ca2+ spikes in layers V–VI PFC neurons evoked synaptically (in QX-314 and Cs+) or by intrasomatic depolarizing current pulses (in TTX and TEA) was that such spikes were followed by a single repolarizing potential and/or depolarizing after potential but no stepwise repolarization. Stepwise repolarization in layer V somatosensory cortical neurons has been attributed to Ca2+ electrogenesis in electrotonically distant sites of the neuron (Reuveni et al., 1993;Yuste et al., 1994), which suggests that PFC neurons lacked an electrotonically distal site of Ca2+ electrogenesis. Accordingly, Cd2+ application to the distal apical tufts had no effect on glutamate-evoked layers I–II suprathreshold responses or the Ca2+-mediated hump potential evoked by intrasomatic current pulses in layers V–VI PFC neurons. Furthermore, during direct apical tuft recordings, injection of depolarizing current pulses or synaptic stimulation was usually ineffective in evoking Ca2+ spikes locally. The low probability of evoking Ca2+ spikes and their high activation threshold may be attributable to a low density of Ca2+ channels for a given length of dendrite in PFC neurons. As a result, high-threshold Ca2+ channels within the apical tuft of PFC neurons may be activated only by very strong and fast membrane depolarization caused by back-propagating or intradendritic Na+-mediated action potentials.
In both somatosensory (Amitai et al., 1993; Kim and Connors, 1993;Stuart and Sakmann, 1994) and PFC neurons (Figs. 4F,5D) (Yang et al., 1996b), large and regenerative mixed Na+/Ca2+ spikes are evoked in the apical dendritic stem. Unlike PFC neurons, Ca2+spikes can also be evoked directly from the apical tuft of layer V somatosensory neurons (Schiller et al., 1996). In somatosensory neuronsin vivo, however, Ca2+ influx attributable to back-propagating action potentials or to whisker stimulation is large in the proximal dendrites but decreases dramatically with distance from the soma (Svoboda et al., 1997). Thus, although the contribution of Ca2+ channels within the apical tuft may be different in somatosensory and PFC neurons, it appears that in both types of cortical neurons the proximal dendrites are a prominent site for Ca2+ electrogenesis.
Signal integration by cortical pyramidal neurons: role of dendritic Ca2+ potentials
The results from the present study suggest that in layers V–VI PFC neurons, dendritic Ca2+ channels contribute to the amplification of layers I–II suprathreshold or large subthreshold responses but not small subthreshold EPSPs evoked well below action potential threshold (Fig. 1). Likewise, previous studies have also suggested that Ca2+ channels in the apical dendritic stem of layer V somatosensory neurons make only a minor contribution to the functional amplification of subthreshold EPSPs (Stuart and Sakmann, 1995). This differential contribution of high-threshold Ca2+ channels to subthreshold and suprathreshold EPSPs may be attributable to the relatively high activation threshold of such dendritic channels, which is not reached by small subthreshold EPSPs (Magee and Johnston, 1995a,b).
There is a functional advantage to having Ca2+potentials evoked synaptically within the apical dendritic stem but not the apical tuft of PFC neurons. Specific information encoded by individual EPSPs arriving at different intervals within the apical tuft would not be completely obscured by the large prolonged change in membrane voltage caused by a Ca2+ spike. In the more proximal apical dendritic stem, synaptic activation of Ca2+ channels might overcome certain problems associated with effective synaptic signal processing in layers V–VI pyramidal neurons. The axial resistance of the main stem of the apical dendritic stem strongly limits the axial current that enters the soma (Bernander et al., 1994). In contrast, there is minimal loss of synaptic current to membrane capacitance along the dendrite because the apical dendritic stem is very thin; however, a considerable portion of synaptic current is lost at the soma because of charging the large somatic membrane (Rall and Rinzell, 1973). Hence, synaptic activation of voltage-gated Ca2+ channels in the proximal apical dendritic stem would dramatically enhance the synaptic current before entering the signal-attenuating capacitive sink at the soma. In this way Ca2+ channels located in the proximal apical dendritic stem may serve as effective current amplifiers for distally generated EPSCs.
If Ca2+ channels proximal to the soma were to make a contribution to voltage amplification of distal EPSPs, they would have to be activated before an axosomatic action potential. This is because if action potentials were triggered, the soma would be so strongly depolarized that any subsequent depolarization by a Ca2+ spike would be minimal. The hump potential was observed before action potential initiation, and Cd2+ application to the proximal apical dendritic stem and soma inhibited EPSPs from reaching action potential threshold (Fig. 1). These data suggested that the Ca2+channels that boosted distal EPSPs were indeed activated before axosomatic action potentials. Thus, it appears that Ca2+ channels proximal to the soma can act as voltage amplifiers of synaptic signals. It should be emphasized, however, that such a hypothesis does not preclude a role for Ca2+ channels in the distal apical dendritic stem from also acting as amplifiers of distally generated synaptic inputs (Yuste et al., 1994). Ca2+ channels in the proximal and distal apical dendritic stem, in addition to Na+ channels in the axosomatic region or dendrites (Stuart and Sakmann, 1995; Lipowsky et al., 1996), may act in concert to effectively boost distally generated EPSPs.
The proximal apical dendritic region of layer V neurons, which corresponds roughly to the border between layers III and V, is a likely site for neuromodulation of signal transmission and integration. These cortical layers in the primate and rodent PFC receive dense monoaminergic innervation, including the well characterized mesocortical dopamine (DA) input (Berger et al., 1991). We have shown recently that DA, via D1 receptors, attenuates dendritic Ca2+ potentials evoked synaptically or by local intradendritic depolarizing pulses (Yang and Seamans, 1996; Yang et al., 1996b). If these Ca2+ potentials serve as current or voltage amplifiers of distally generated synaptic signals, then DA may reduce the gain of such amplifiers, functionally uncoupling the distal input zone from the soma of layer V PFC neurons (Yang et al., 1996a,b). Thus, the neuromodulation of dendritic function may represent a crucial event in signal processing by pyramidal neurons, and the voltage-gated Ca2+ channels in the apical dendrites of PFC cells have proven to be some of the most important targets.
Footnotes
This research was funded by grants from the British Columbia Health Research Foundation (BCHRF), the Medical Research Council of Canada, and the EJLB Foundation to C.R.Y. C.R.Y is a BCHRF Scholar, and J.K.S. is a recipient of the University of British Columbia Graduate Fellowship Award. We thank Drs. Paul Rhodes, Nelson Spruston, Paul MacKenzie, Charles Blaha, and Anthony Phillips for their helpful discussions and comments.
Correspondence should be addressed to Dr. Charles R. Yang, Departments of Psychology and Psychiatry, University of British Columbia, 2136 West Mall, Vancouver, British Columbia V6T 1Z4, Canada.
REFERENCES
- 1.Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993;3:26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- 2.Benardo LS, Masukawa LM, Prince DA. Electrophysiology of isolated hippocampal pyramidal dendrites. J Neurosci. 1982;2:1614–1622. doi: 10.1523/JNEUROSCI.02-11-01614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger B, Gasper P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- 4.Bernander Ö, Douglas RJ, Martin KAC, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci USA. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernander Ö, Koch C, Douglas RJ. Amplification and linearization of distal synaptic input to cortical pyramidal neurons. J Neurophysiol. 1994;72:2743–2753. doi: 10.1152/jn.1994.72.6.2743. [DOI] [PubMed] [Google Scholar]
- 6.Berry MS, Pentreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- 7.Bröckers TM, Zimmer M, Müller A, Bergman M, Brose N, Kreutz MR. Expression of the NMDA R1 receptor in selected human brain regions. NeuroReport. 1994;5:965–969. doi: 10.1097/00001756-199404000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Cauller LJ, Connors BW. Functions of very distal dendrites: experimental and computational studies of layer I synapses on neocortical pyramidal cells. In: McKenna T, Davis J, Zornetzer SF, editors. Single neuron computation. Academic; New York: 1992. pp. 199–230. [Google Scholar]
- 9.Cauller LJ, Connors BW. Synaptic physiology of horizontal afferents to layer I in slices of rat SI neocortex. J Neurosci. 1994;14:751–762. doi: 10.1523/JNEUROSCI.14-02-00751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condé F, Marie-Levoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 11.Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982;48:1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- 12.Currie SN, Wang XF, Daw NW. NMDA receptors in layers II and III of rat cerebral cortex. Brain Res. 1994;662:103–108. doi: 10.1016/0006-8993(94)90801-x. [DOI] [PubMed] [Google Scholar]
- 13.Dodt HU, Kampe K, Frink A, Rüther T, Zeiglgänsberger W. Differential distribution of excitatory amino acid receptors on rat layer V pyramidal neurons in vitro. Soc Neurosci Abstr. 1995;21:144.3. [Google Scholar]
- 14.Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Glalarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hestrin S, Sah P, Nicoll RA. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990;5:247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat’s visual cortex. J Neurosci. 1991;11:1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes WR, Woody CD. Effects of uniform and non-uniform synaptic “activation-distributions” on the cable properties of modeled cortical pyramidal neurons. Brain Res. 1989;505:12–22. doi: 10.1016/0006-8993(89)90110-8. [DOI] [PubMed] [Google Scholar]
- 18.Huntley GW, Vickers JC, Janssen W, Brose N, Heinemann SF, Morrison JH. Distribution and synaptic localization of immunocytochemically identified NMDA receptor subunit proteins in sensory-motor and visual cortices of monkey and human. J Neurosci. 1994;14:3603–3619. doi: 10.1523/JNEUROSCI.14-06-03603.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwa GGC, Avoli M. Excitatory postsynaptic potentials recorded from regular-spiking cells in layers II/III of rat sensorimotor cortex. J Neurophysiol. 1992;67:728–737. doi: 10.1152/jn.1992.67.3.728. [DOI] [PubMed] [Google Scholar]
- 20.Jack JJB, Noble D, Tsien RW. Electric current flow in excitable cells. Oxford UP; London: 1975. [Google Scholar]
- 21.Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN. The spread of Na+ spikes determines the pattern of Ca2+ entry into hippocampal neurons. Nature. 1992;357:244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- 22.Johnston D, Brown TH. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981;211:294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- 23.Johnston D, Brown TH. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983;50:464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- 24.Johnston D, Magee JC, Colbert CM, Christie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- 25.Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13:5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HG, Beierlein M, Connors BW. Inhibitory control of excitable dendrites in neocortex. J Neurophysiol. 1995;74:1810–1814. doi: 10.1152/jn.1995.74.4.1810. [DOI] [PubMed] [Google Scholar]
- 27.Koch C, Poggio T, Torre V. Nonlinear interactions in a dendritic tree: localization, timing, and role of information processing. Proc Natl Acad Sci USA. 1983;80:2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipowsky R, Gillessen T, Alzheimer C. Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. J Neurophysiol. 1996;76:2181–2191. doi: 10.1152/jn.1996.76.4.2181. [DOI] [PubMed] [Google Scholar]
- 29.Llinás R, Sugimori M. Electrophysiological properties of in vitro purkinje cell dendrites in mammalian cerebellar slices. J Physiol (Lond) 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995a;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- 31.Magee JC, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol (Lond) 1995b;481:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magee JC, Christofi G, Miyakawa H, Christie B, Lasser-Ross N, Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 1995;74:1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- 33.Mainen ZF, Joerges J, Huguenard JR, Sejnowski TJ. A model of spike initiation in neocortical pyramidal neurons. Neuron. 1995;15:1427–1439. doi: 10.1016/0896-6273(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 34.Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated Ca2+ channels. Proc Natl Acad Sci USA. 1994;91:5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masukawa LM, Prince DA. Synaptic control of excitability in isolated dendrites of hippocampal neurons. J Neurosci. 1984;4:217–227. doi: 10.1523/JNEUROSCI.04-01-00217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mel B. Information processing in dendritic trees. Neural Computat. 1994;6:1031–1085. [Google Scholar]
- 37.Miles R, Tóth K, Gulyás AI, Hájos N, Freund T. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 38.Miyakawa H, Ross WN, Jaffe D, Callaway JC, Lasser-Ross N, Lisman JE, Johnston D. Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron. 1992;9:1163–1173. doi: 10.1016/0896-6273(92)90074-n. [DOI] [PubMed] [Google Scholar]
- 39.Müller W, Connor JA. Ca2+ signalling in postsynaptic dendrites and spines of mammalian neurons in brain slice. J Physiol (Paris) 1992;86:57–66. doi: 10.1016/s0928-4257(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 40.Nowak L, Bregestovski P, Ascher P, Herbert A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature (Lond) 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 41.Peters A. Number of neurons and synapses in primary visual cortex. In: Jones E, Peters A, editors. Cerebral cortex: further aspects of cortical function, including hippocampus. Plenum; New York: 1987. pp. 267–294. [Google Scholar]
- 42.Rall W, Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys J. 1973;13:648–688. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramón y Cajal S (1889) Conexión general de los elementos nerviosos. Medicina Práctica.
- 44.Regehr WG, Tank DW. Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J Neurosci. 1992;12:4202–4223. doi: 10.1523/JNEUROSCI.12-11-04202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuveni I, Friedman A, Amitai Y, Gutnick MJ. Stepwise repolarization from Ca2+ plateaus in neocortical pyramidal cells: evidence for nonhomogeneous distribution of HVA Ca2+ channels in dendrites. J Neurosci. 1993;13:4609–4621. doi: 10.1523/JNEUROSCI.13-11-04609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudolf GD, Cronin CA, Landwehrmeyer GB, Standaert DG, Penney JB, Young AB. Expression of N-methyl-d-aspartate glutamate receptor subunits in the prefrontal cortex of the rat. Neuroscience. 1996;73:417–427. doi: 10.1016/0306-4522(96)00048-6. [DOI] [PubMed] [Google Scholar]
- 47.Schiller J, Helmchen F, Sakmann B. Spatial profile of dendritic calcium transients evoked by action potentials in rat neocortical pyramidal neurones. J Physiol (Lond) 1995;487.3:583–600. doi: 10.1113/jphysiol.1995.sp020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiller J, Schiller Y, Sakmann B. Calcium action potentials in apical dendrites of neocortical pyramidal neurons in rat brain slices. Soc Neurosci Abstr. 1996;315.22:794. [Google Scholar]
- 49.Schwindt PC, Crill WE. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol. 1995;74:2220–2224. doi: 10.1152/jn.1995.74.5.2220. [DOI] [PubMed] [Google Scholar]
- 50.Seamans JK, Gorelova N, Yang CR. The pattern of afferent input to the apical dendrites of layer V–VI prefrontal cortical neurons influences the immediate and prolonged amplification of synaptic signals by high threshold Ca2+ spikes. Soc Neurosci Abstr. 1996;22:1743. [Google Scholar]
- 51.Shepherd GM, Brayton RK, Miller JP, Segev I, Rinzel J, Rall W. Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proc Natl Acad Sci USA. 1985;82:2192–2195. doi: 10.1073/pnas.82.7.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer WA, Kandel ER. Electrophysiology of hippocampal neurons. IV. Fast prepotentials. J Neurophysiol. 1961;24:272–285. doi: 10.1152/jn.1961.24.3.272. [DOI] [PubMed] [Google Scholar]
- 53.Spruston N, Stuart G. Voltage attenuation and intracellular resistivity in neocortical pyramidal neurons. Soc Neurosci Abstr. 1996;22:792. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spruston N, Jaffe DB, Williams SH, Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol. 1993;70:781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- 55.Spruston N, Jaffe DB, Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994;17:161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 56.Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol (Lond) 1995a;482.2:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spruston N, Stuart G, Sakmann B. How do voltage-activated channels shape EPSPs in hippocampal CA1 neurons? Soc Neurosci Abstr. 1995b;21:584. [Google Scholar]
- 58.Stafstrom CE, Schwindt PC, Crill WE. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982;236:221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- 59.Stafstrom CE, Schwindt PC, Crill WE. Properties of persistent sodium and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985;53:153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- 60.Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- 61.Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 62.Sutor B, Hablitz JJ. EPSPs in rat neocortical neurons in vitro II. Involvement of N-methyl-d-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989;61:621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- 63.Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- 64.Tasker JG, Dudek FE. Electrophysiology of GABA-mediated synaptic transmission and possible roles in epilepsy. Neurochem Res. 1991;16:251–262. doi: 10.1007/BF00966088. [DOI] [PubMed] [Google Scholar]
- 65.Tsien RW, Lipscome D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 66.Uylings HBM, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 67.Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- 68.Westenbroek RE, Hell JN, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 69.Wong RKS, Prince DA, Basbaum AI. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci USA. 1979;76:986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang CR, Seamans JK. Dopamine D1 receptor actions in layer V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layer V–VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci. 1996a;16:1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang CR, Seamans JK, Gorelova N. Focal dendritic D1 receptor stimulation differentially modulates layer I–II and V–VI glutamate inputs to deep layer prefrontal cortical pyramidal neurons in vitro. Soc Neurosci Abstr. 1996b;22:1770. [Google Scholar]
- 73.Yuste R, Tank DW. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 74.Yuste R, Gutnick MJ, Saar D, Delaney KR, Tank DW. Ca2+ accumulations in dendrites of neocortical pyramidal neurons: an apical band and evidence for two functional compartments. Neuron. 1994;13:23–43. doi: 10.1016/0896-6273(94)90457-x. [DOI] [PubMed] [Google Scholar]