Abstract
Sonic hedgehog (Shh), an axis-determining secreted protein, is expressed during early vertebrate embryogenesis in the notochord and ventral neural tube. In this site it plays a role in the phenotypic specification of ventral neurons along the length of the CNS. For example, Shh induces the differentiation of motor neurons in the spinal cord and dopaminergic neurons in the midbrain. Shh expression, however, persists beyond this induction period, and we have asked whether the protein shows novel activities beyond phenotype specification. Using cultures derived from embryonic day 14.5 (E14.5) rat ventral mesencephalon, we show that Shh is also trophic for dopaminergic neurons. Interestingly, Shh not only promotes dopaminergic neuron survival, but also promotes the survival of midbrain GABA-immunoreactive (GABA-ir) neurons. In cultures derived from the E15–16 striatum, Shh promotes the survival of GABA-ir interneurons to the exclusion of any other cell type. Cultures derived from E15–16 ventral spinal cord reveal that Shh is again trophic for interneurons, many of which are GABA-ir and some of which express the Lim-1/2 nuclear marker, but it does not appear to support motorneuron survival. Shh does not support the survival of sympathetic or dorsal root ganglion neurons. Finally, using the midbrain cultures, we show that in the presence of MPP+, a highly specific neurotoxin, Shh prevents dopaminergic neuron death that normally would have occurred. Thus Shh may have therapeutic value as a protective agent in neurodegenerative disease.
Keywords: Sonic hedgehog, patched, midbrain, striatum, spinal cord, Parkinson’s disease
In Drosophila, the hedgehoggene was first discovered for the role it plays in early embryo patterning (Nusslein-Volhard and Wieschaus, 1980). Further study showed that the product of this gene is secreted and as an intercellular signaling protein plays a critical role in body segmentation and patterning of imaginal disk derivatives such as eyes and wings (Lee et al., 1992; Mohler and Vani, 1992; Tabata et al., 1992). There are, at present, three mammalian homologs of Drosophila hedgehog protein that have been identified: Sonic hedgehog (Shh), Desert hedgehog, and Indian hedgehog (Fietz et al., 1994). During the course of vertebrate development, these secreted peptide molecules are involved in axial patterning and consequently regulate the phenotypic specification of precursor cells into functional differentiated cells.
The embryonic expression pattern of Shh has been shown to be closely linked to the development and differentiation of the entire ventral neuraxis (Marti et al., 1995a). Using naive neural tube explants derived from the appropriate levels of the rostrocaudal axis, it has been demonstrated that the induction of spinal motor neurons (Roelink et al., 1994; Tanabe et al., 1995), midbrain dopaminergic neurons (Hynes et al., 1995; Wang et al., 1995), and basal forebrain cholinergic neurons (Ericson et al., 1995) are dependent on exposure to Shh. This molecule appears to be crucial for such patterning and phenotype specification in vivo, because mouse embryos deficient in the expression of functional Shh gene product manifest a lack of normal ventral patterning in the CNS as well as gross atrophy of the entire cranium (Chiang et al., 1996) .
In this study we have explored the issue of whether Shh may have activities at stages in neural development later than those studied previously. Namely, we have asked whether Shh is trophic for particular neural populations, and under toxic conditions, whether Shh is neuroprotective. Using cultures derived from the embryonic day 14–16 (E14–16) rat, we find that Shh is trophic for midbrain, striatal, and spinal neurons. In the first case the factor is trophic for both dopaminergic and GABA-immunoreactive (GABA-ir) neurons. From the striatum, the surviving neurons are exclusively GABA-ir, whereas in the spinal cultures Shh promotes survival of a heterogeneous population of putative interneurons. Shh does not support survival of any peripheral nervous system neurons tested. Finally, we show that Shh protects cultures of midbrain dopaminergic neurons from the toxic effects of MPP+, a specific neurotoxin that induces Parkinsonism in vivo. Together, these observations indicate a novel role for Shh in nervous system development and its potential role as a therapeutic.
MATERIALS AND METHODS
Whole-mount in situ hybridization.Whole-mount in situ hybridization on bisected E14.5 Sprague Dawley rat embryos was performed with digoxigenin-labeled (Boehringer Mannheim, Indianapolis, IN) mouse RNA probes as described previously (Wilkinson, 1992). Bound probe was detected with alkaline phosphatase-conjugated anti-digoxigenin Fab fragments (Boehringer Mannheim). The 0.7 kb Shh probes were transcribed using T3 (antisense) or T7 (sense) RNA polymerase from HindIII (antisense) or BamHI (sense) linearized templates as described by Echelard et al. (1993). The 0.9 kb Ptc probes were transcribed using T3 (antisense) or T7 (sense) RNA polymerase fromBamHI (antisense) or HindIII (sense) linearized templates as described by Goodrich et al. (1996).
Shh protein and anti-Shh antibody. Rat Sonic hedgehog amino terminal signaling domain (amino acids 2–198) (Porter et al., 1995) was cloned into a baculovirus expression vector (Invitrogen, San Diego, CA) (virus encoding Shh insert was a gift of Dr. Henk Roelink, University of Washington). High Five insect cells (Invitrogen) were infected with the baculovirus according to manufacturer’s instructions. The culture supernatant was batch-adsorbed to heparin agarose type I (Sigma, St. Louis, MO), and Shh was eluted with PBS containing a total of 0.75 m NaCl and 0.1 mmβ-mercaptoethanol. Shh concentration was determined by the method ofEricson et al. (1996). Escherichia coli-derived Shh was obtained as described previously (Wang et al., 1995) and purified as described above. All samples were sterile-filtered, and aliquots were frozen in liquid nitrogen. Anti-Shh polyclonal antibody was a gift from Dr. Andy McMahon (Harvard University). Preparation of this reagent, directed against the amino peptide of Shh, is described by Bumcrot et al. (1995). Anti-Shh monoclonal antibody (5E1) was a gift of Dr. Thomas Jessell (Columbia University), and preparation of this reagent is described by Ericson et al. (1996).
Dissociation and culture of neural tissue. E14.5 rat ventral mesencephalon was dissected as described by Shimoda et al. (1992). Striatal cultures were established from E15–16 embryos from the regions identified by Altman and Bayer (1995) as the striatum and pallidum. Spinal cultures used the ventral one-third of the E15–16 spinal cord (Camu and Henderson, 1992). Tissues were dissociated for ∼30 min in 0.10–0.25% trypsin-EDTA (Life Technologies, Gaithersburg, MD), and the digestion was stopped using an equal volume of Ca2+/Mg2+-free HBSS (Life Technologies) containing 2.5 mg/ml soybean trypsin inhibitor (Sigma) and 0.04% DNase (Grade II, Boehringer Mannheim). Cells were than plated at 1 × 105 to 2 × 105 cells/well in the medium of Krieglstein et al. (1995) (a modified N2 medium) in 24-well tissue culture plates (Falcon) coated with poly-l-lysine or poly-l-ornithine (Sigma) after one wash in the same medium. Note that this procedure results in cultures in which the cells have never been exposed to serum and stands in contrast to cultures in which serum has been used to neutralize dissociation proteases or to initially “prime” the cells before serum withdrawal. The following peptide growth factors were added as indicated in the results: basic fibroblast growth factor, transforming growth factor β1(TGFβ1), TGFβ2, glial-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF) (all from PeproTech, Rocky Hill, NJ; additional lots of BDNF and GDNF were purchased from Promega, Madison, WI). Anti-TGFβ antibodies were purchased from R & D Systems (Minneapolis, MN). Antibody was added at the time of Shh addition to the cultures. Cultures were maintained for up to 2 weeks, and the medium was changed every 3 d.
Immunoctyochemistry and cell scoring. For all cell staining, cultures were fixed with 4% paraformaldehyde in PBS (plus 0.1% glutaraldehyde if staining for GABA) and blocked using 2% goat serum (Sigma), 0.5% Triton X-100 in PBS. Antibody incubations were performed in the blocking solutions. Antibodies used in this study were anti-tubulin βIII (Sigma), anti-tyrosine hydroxylase (TH) (Boehringer Mannheim and Sigma), anti-GABA (Sigma), and anti-glial fibrillary acidic protein (GFAP) (Sigma). Primary antibodies were detected using horseradish peroxidase-, alkaline phosphatase- (Vector, Burlingame, CA), or fluorochrome-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA). Peroxidase-linked secondaries were visualized using an Ni/DAB kit (Zymed, South San Francisco, CA), and phosphatase-linked secondaries were visualized using Vector Blue (Vector).
Cell counting was performed using an Olympus inverted microscope at a total magnification of 200×. Data presented are representative and have been confirmed by repeating the cultures at least 3–10 independent times for each neural population discussed. Cell numbers are reported as cells/field (the average of 20–40 fields from a total of 4 wells/condition; 3–10 independent experiments were assessed for each culture condition examined). Consistency of counting was verified by at least two observers. Errors are reported as SEM, and significance is calculated by Student’s t test.
Measurement of dopamine (DA) transport. To detect the presence of the DA transporter (Cerruti et al., 1993; Ciliax et al., 1995), cultures were incubated with a mixture consisting of 5 × 10−8m3H-DA (Amersham, Arlington Heights, IL; 48 Ci/mmol), 100 μm ascorbic acid (Sigma), 1 μm fluoxetine (Eli Lilly, Indianapolis, IN), 1 μm desmethylimipramine (Sigma), and 10 μmpargyline (Sigma) in DME-F12. Nonspecific labeling was measured by the addition of 5 × 10−5m unlabeled DA. Cells were incubated for 30 min at 37°C, rinsed three times with PBS, and processed for either scintillation counting or autoradiography. For scintillation counting, cells were first lysed with 150 μl of 0.1% SDS and then added to 500 μl of Microscint 20 (Packard, Meridian, CT) and counted in a Packard Instrument Topcount scintillation machine. For autoradiography, sister plates were coated with NTB-2 autoradiographic emulsion (Kodak, Rochester, NY) that had been diluted 1:3 with 10% glycerol. The plates were then air-dried, exposed for 1–2 weeks, and developed.
Quantitative competitive PCR (QC-PCR). RNA was isolated from cells and tissue using Trizol (Life Technologies) as prescribed by the manufacturer. Genomic DNA was removed from the RNA by incubation with 0.5 U of DNase (Life Technologies) at room temperature for 15 min. The solution was heated to 65°C for 10 min to inactivate the DNase. Reverse transcription was performed using random hexamer and MuLV reverse transcriptase (RT) (Life Technologies) as suggested by the manufacturer. All of the quantitative RT-PCR internal controls, or mimics, were synthetic single-stranded DNA oligonucleotides corresponding to the target sequence with an internal deletion from the central region (Oligos, Etc., Wilsonville, OR). For actin, target = 180 bp, mimic = 130 bp; for ptc, target = 254 bp, mimic = 100 bp. PCR was performed using the Clontech PCR kit; for actin: annealing temperature 54°C, oligos GGCTCCGGTATGTGC, GGGGTACTTC- AGGGT; for ptc: annealing temperature 62°C, oligos CATTGGCAGG-AGGAGTTGATTGTGG, AGCACCTTTTGAGTGGAGTTTGGGG. In each QC-PCR reaction, four reactions were set up with equal amounts of sample cDNA in each tube and fourfold serial dilution of mimic. Also, for each sample, an aliquot of cDNA was saved and amplified along with quantitative PCR as control for contamination. PCR reactions were performed in an MJ Research PTC-200 thermal cycler, and the following cycling profile was used: 95°C for 35 sec, 54° or 62°C for 25 sec, 72°C for 20 sec, for 30 cycles. The reaction mixtures were then fractionated by agarose electrophoresis, negative films were obtained, and the films were digitally scanned and quantified by area integration according to established procedures (Wang et al., 1995, and references therein). The quantity of target molecules was normalized to the competing mimic and expressed as a function of cDNA synthesized and used in each reaction.
N-methyl-4-phenylpyrridinium (MPP+) administration. Culture and MPP+ treatment of dopaminergic neurons were performed as described previously (Hyman et al., 1994; Krieglstein et al., 1995). MPP+ (Aldrich, St. Louis, MO) was added at day 2 of culture to a final concentration of 2 μm for 48 hr. Cultures were then washed extensively to remove MPP+, cultured for an additional 24–48 hr to allow clearance of dying TH+ neurons, and then processed for immunocytochemistry.
RESULTS
Shh and Ptc continue to be expressed in the rat CNS after the major period of dorsoventral patterning
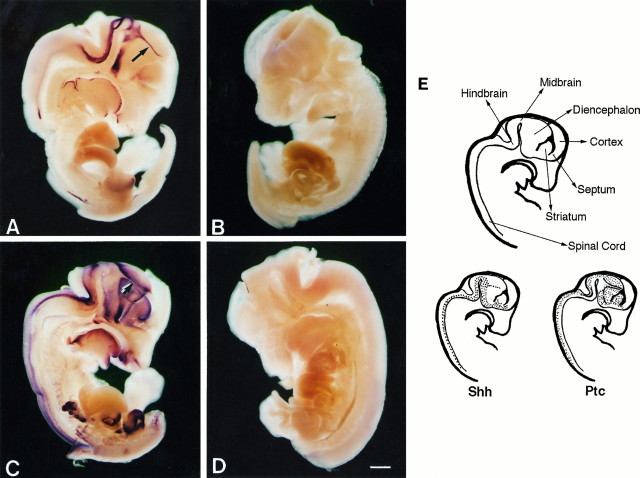
Previous studies have shown that shh is expressed in the vertebrate embryo in the period during which dorsoventral patterning manifests (approximately E9–10 in the rat). Within the CNS,shh expression persists beyond this period and can be detected at a very high level in the E14–16 rat embryo. For example,in situ hybridization studies of the E14.5 embryo (Fig.1A,E) reveal that shh is expressed in ventral regions of the spinal cord, hindbrain, midbrain, and diencephalon. Lower levels of expression are observed in the ventral striatum and septum, whereas no expression is observed in the cortex within the limits of detection of this method. Interestingly, a “streak” of shh expression (Fig. 1A,arrow) is observed to bisect the diencephalon into rostral and caudal halves. This is likely to be the zona limitans intrathalamica that separates prosomeres 2 and 3 and has been observed previously in the studies of shh expression in the developing chick embryo (Marti et al., 1995b).
Fig. 1.
Shh and Ptc in the E14.5 rat embryo. Shh (A, antisense;B, sense control), and ptc(C, antisense; D, sense control) expression as detected by in situ hybridization with digoxigenin-labeled riboprobes and alkaline phosphatase-conjugated anti-digoxigenin. The arrow in A and thedouble arrow in C designate the zona limitan intrathalamica. Major anatomical structures and summary diagrams of shh and ptc expression are shown in E. Scale bar, 1 mm.
Recent biochemical evidence supports the view that the ptcgene product can act as a high affinity Shh receptor (Marigo et al., 1996a; Stone et al., 1996). Ptc shows an expression pattern complementary to that of shh (Fig. 1C,E) and is observed primarily lateral and dorsal to the sites of shhexpression. The complementarity of expression is most dramatic in the diencephalon, where ptc mRNA is absent from the zona limitans (Fig. 1C, double arrow), but is expressed at a very high level on either side of this structure. Of further interest is the observation that rostral of the zonal limitans,ptc expression no longer seems as restricted to regions immediately dorsal of shh expression. Again, within the detection limits of this technique, ptc is not expressed in the cortex. Thus in regions where shh is expressed, adjacent tissue appears capable of responding to the gene product, as evidenced by expression of the putative receptor.
Shh promotes dopaminergic neuron survival
In the developing midbrain (approximately E9), Shh was first characterized for its ability to induce the production of dopaminergic neurons. Thus the trophic potential of Shh was tested on this neuronal population at a stage when these neurons have already been induced. Using cultures derived from the E14.5 mesencephalon, we found that Shh increases the survival of TH+ neurons in a dose-dependent manner (Fig. 2A). These cells exhibited a neuronal morphology (Fig. 2C,E), and >95% of the TH+ cells were also positive for the neuron-specific marker tubulin βIII (Banerjee et al., 1990); GFAP staining revealed no glial cells (data not shown). Differences in TH+ neuron survival between control and Shh-treated wells could be observed as early as day 4. Note that under these stringently serum-free conditions (i.e., at no time were the cells exposed to serum), baseline levels of survival are even lower than those conventionally reported for cultures that have been maintained in low serum or that have been briefly serum-“primed”. By 2 weeks in culture, <5% of the total TH+ cells plated were present in the control condition, whereas 25–30% survive in 50 ng/ml of Shh (from 4 to 14 d; p < 0.001 at 25 and 50 ng/ml).
Fig. 2.
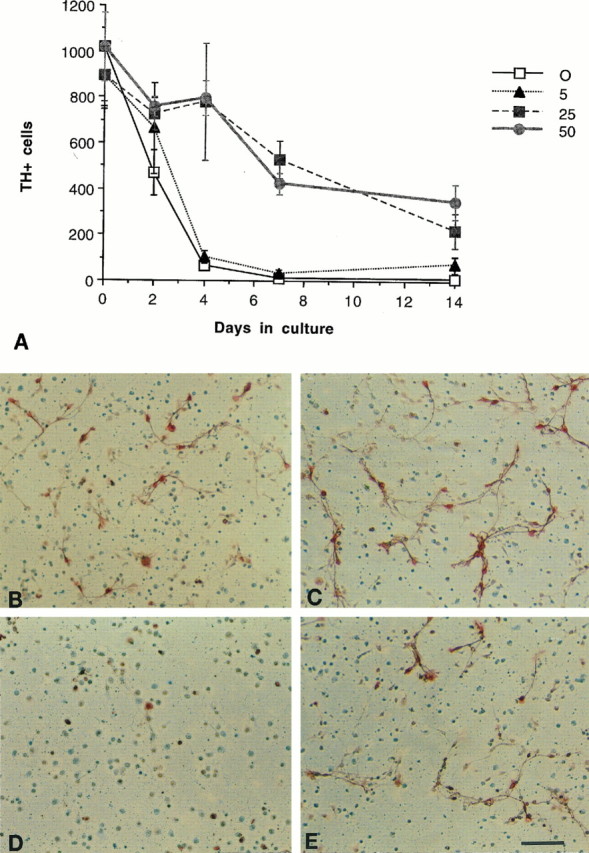
Shh promotes the survival of TH+ neurons of the ventral mesencephalon.A, Time course and dose–response of the Shh effect. The number of TH+ neurons in control cultures (0 ng/ml Shh) began to decline dramatically by 4 d in vitro. In cultures treated with Shh at 25 and 50 ng/ml, there were significantly greater numbers of TH+ neurons over control through 14 d in vitro (from 4 to 14 d,p < 0.001 at 25 and 50 ng/ml). The 50 ng/ml dose typically gave a 50–100% increase over controls at all time points (error bars represent SEM). Photomicrographs of TH+neurons in control (B, D) and 50 ng/ml Shh-treated (C, E) cultures, 2 d (B, C) and 7 d (D, E) after plating. Note that in addition to an increased number of TH+ cell bodies, the Shh-treated cells show extensive neuritic processes. Scale bar (shown in E): 200 μm.
All catecholaminergic neurons express TH, but the presence of a specific high-affinity DA uptake system is indicative of midbrain dopaminergic neurons (Di Porzio et al., 1980; Denis-Donini et al., 1984; Cerruti et al., 1993; Ciliax et al., 1995). As further evidence that the cells supported by Shh are bona fide dopaminergic neurons, specific, high-affinity DA uptake was also demonstrated (Fig.3). Midbrain cultures treated with Shh transported and retained 3H-DA with a dose-response profile paralleling that of the survival curves (Fig. 3A) (p < 0.005 at 25 and 50 ng/ml). Emulsion autoradiography also demonstrated that the cells taking up3H-DA were neuronal in morphology (Fig. 3B). In addition, immunohistochemistry for DA itself demonstrated high cellular content (data not shown).
Fig. 3.
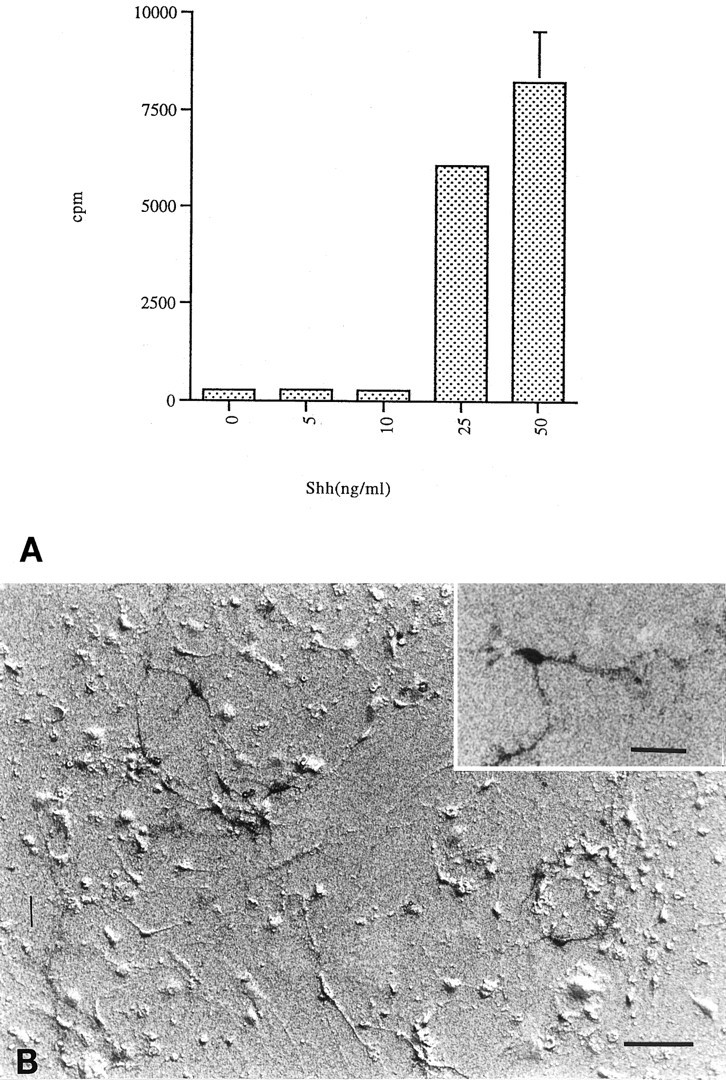
Transport of 3H-DA. The identity and functionality of the surviving midbrain neurons was assessed by their ability to specifically transport DA. A, Addition of 25 ng/ml Shh resulted in a 22-fold increase in 3H-DA cell uptake over controls and lower Shh concentrations; 50 ng/ml Shh gave a 30-fold increase in 3H-DA uptake (error bars represent SD) (p < 0.005 at 25 and 50 ng/ml).B, Autoradiography was performed on sister plates to visualize DA transport. Only cells with neuronal morphology transported3H-DA (inset). Scale bar, 50 μm; inset, 15 μm.
The observed effect of Shh on increased TH+ neuron number is unlikely to be attributable to differentiation of latent progenitor cells, because previous studies demonstrated that the ability of Shh to induce dopaminergic neurons in explanted tissue is lost at later stages of development (Hynes et al., 1995; Wang et al., 1995). Furthermore, the effects are unlikely to be attributable to a mitogenic response of committed neuroblasts, because pulse-labeling the cultures with 5-bromo-2′-deoxyuridine (BrdU) at 1, 2, or 4 din vitro revealed very low mitotic activity in the presence or absence of Shh (data not shown). Thus, in addition to inducing dopaminergic neurons in the naive mesencephalon, Shh is a trophic factor for these neurons.
Specificity of Shh action on midbrain neurons: regulated expression of ptc
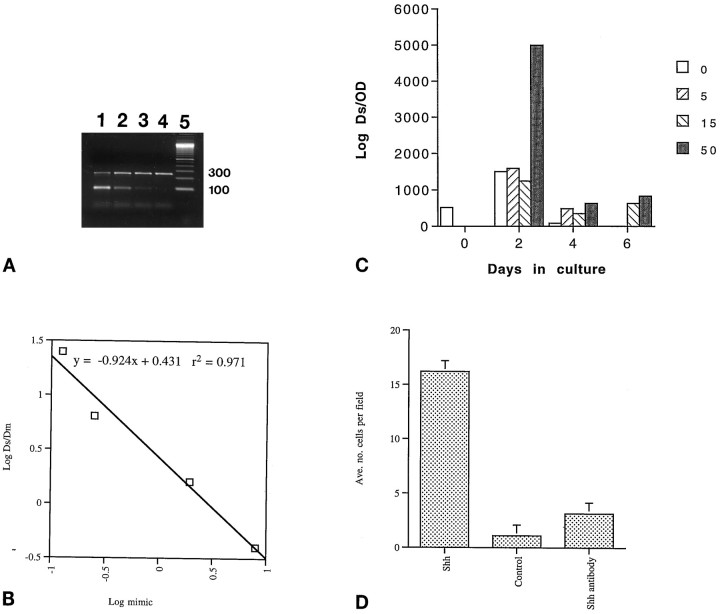
Expression of ptc has previously been shown to be regulated by Shh (Goodrich et al., 1996; Marigo et al., 1996b), and to date, Shh is the only factor known to transcriptionally upregulateptc expression. Therefore, the expression of ptcby mesencephalic explants would reinforce the view that these cells are capable of responding to Shh, and upregulation of ptc mRNA in response to Shh would strongly indicate the specificity of such a response. Therefore, QC-PCR was used to measure the level ofptc expression.
Ptc mRNA levels were measured at 0, 2, 4, and 6 d of culture by the method described by Wang et al. (1995). For each culture condition at each time point, four separate cDNA samples were coamplified with a different known amount of mimic substrate (DNA that can be amplified by the same primers but yielding a product of molecular weight lower than that being sought in the sample). Thus for each condition and time point, a gel like that shown in Figure4A was generated (upper bands correspond to amplified ptc transcripts; lower bands correspond to amplified mimic). Using scanning densitometry to quantify the observed bands, a graph was produced for each sample (Fig.4B corresponds to 4A). When the density of the target band and the mimic band are equal, the concentration of the unknown target can be taken to be equal to the known concentration of mimic. Based on a linear curve fit, the concentration of mimic at the point at which the density of the mimic and the target substrate are equal (LogDs/Dm = 0) was taken to be the concentration of the substrate in the sample; this value was then normalized to the total amount of cDNA added to the reaction. These values are plotted in Figure 4C; correlation coefficients (r2) of the curve fits always exceeded 0.95, and thus the margin of error for the values presented is <5%. This experiment was performed two independent times with independent cultures, and the results were nearly identical.
Fig. 4.
Specificity of Shh activity. A, QC-PCR gel. Lanes 1–4 are cDNA from midbrain cultures that have been coamplified with successive fourfold dilutions of mimic oligo. Lane 5 is DNA marker lane. Ptctarget is 254 bp and mimic is 100 bp. B, Representative plot (corresponding to A) of the log concentration of competitive mimic versus the log of the obtained band densities of target and mimic PCR substrates demonstrates the linearity of the amplification reaction. The extrapolated value of ptcmessage in the cDNA tested is determined to be equal to the value of mimic concentration where LogDs/Dm = 0. See main text for details of the procedure. Doses in ng/ml;Ds = density of test substrate;Dm = density of competitive mimic. Ther2 value shows that determinations made within this range vary within 3%. C, Administration of Shh induces ptc expression in a dose– response that parallels the survival curve. The values are expressed as number of target molecules (Log Ds) per total amount of cDNA used in each reaction as measured by optical density at 260 nm (OD) and were determined as demonstrated in A and B. At 4 din vitro Shh at 5 ng/ml increases ptcexpression over control, and 50 ng/ml increases expression ofptc over the level found in the ventral mesencephalon at the time of dissection. D, Affinity-purified anti-Shh antibody inhibited the Shh neurotrophic response (p < 0.001). Cultures were maintained for 5 d. Shh was added at a concentration of 50 ng/ml, and in the coadministration of Shh and anti-Shh (“Shh antibody”) Shh was added at 50 ng/ml and anti-Shh was added as a fivefold molar excess (error bars represent SEM).
As shown in Figure 4C, significant ptc expression was observed in the E14.5 ventral mesencephalon (time 0). After 2 d of culture, higher levels of ptc expression were observed than at the time of dissection; in control cultures this might reflect the loss of ptc nonexpressing cell types, because a constant amount of RNA was analyzed. There was no difference in ptcexpression between control cultures and those treated with either 5 or 15 ng/ml of Shh at this time; however, cultures treated with 50 ng/ml of Shh showed a 10-fold induction of ptc mRNA expression relative to time of dissection and at least fourfold over other culture conditions. By 4 d of culture, ptc message levels had declined significantly in comparison to the 2 d level of expression, but high levels of expression were still observed in 50 ng/ml Shh. By 6 d, no ptc expression was observed in either the control or 5 ng/ml Shh-treated cultures, although actin could still be detected (data not shown). It is important to note that in the 15 and 50 ng/ml Shh-treated cultures,ptc expression matched or exceeded the time 0 expression ofptc in the mesencephalon despite the overall decrease in cell number. These results indicate that (1) ptc is expressed in the E14.5 ventral mesencephalon (confirming the observation made by in situ hybridization), (2) Shh is necessary for the maintenance of ptc gene expression, and (3) the expression of ptc shows an Shh dose dependence that parallels the neurotrophic activity described above.
Specificity of Shh action on midbrain neurons: immunoneutralization
As further evidence that the trophic activity of the Shh preparation used for these studies, purified from a baculovirus expression system, was attributable to Shh and not to a contaminating factor, antibody neutralization experiments were performed. As shown in Figure 4D, a saturating dose of Shh (50 ng/ml) promotes midbrain neuron survival (p < 0.001), whereas the same dose of Shh in the presence of a fivefold molar excess of activity-neutralizing, anti-Shh, monoclonal antibody (5E1) [Ericson et al. (1996)] inhibits this trophic response (p < 0.001). In earlier studies (data not shown), an affinity-purified, polyclonal, anti-Shh antibody dramatically reduced the activity of Shh in the dopaminergic neuron survival assay (p < 0.005), whereas purified rabbit IgG antibody from preimmune sera had no significant effect. Anti-TGFβ antibodies used at a twofold molar excess to Shh did not inhibit the trophic activity, whereas they did inhibit the previously reported (Krieglstein et al., 1995) trophic effects of exogenously applied TGFβs (data not shown). Addition of β-galactosidase, expressed and purified in a manner identical to Shh, failed to show any trophic effect (data not shown), and thus renders unlikely the possibility that an undefined baculovirus protein is responsible for the observed trophic effects. Finally, Shh purified from an E. coli expression system (Wang et al., 1995) also had trophic activity for TH+ cells, whereas β-galactosidase purified identically to Shh from the E. coli expression system gave no such activity even at concentrations as high as 10 μg/ml (data not shown).
Shh supports the survival of other midbrain neurons
Because the original observations concerning the role of Shh in midbrain development were concerned with induction of dopaminergic neurons (Hynes et al., 1995; Wang et al., 1995), the current study initially focused on possible trophic effects on these neurons. Interestingly, the cultures in which the above described trophic effects were observed also demonstrated that the trophic effect of Shh extended to nondopaminergic neurons (i.e., TH−neurons). Within the dopaminergic nucleus of the midbrain, the substantia nigra, GABA is also a major neurotransmitter (Masuko et al., 1992). Staining for GABA in these cultures (Fig. 5) showed that GABA+ cells are supported by the presence of Shh, with a dose-response profile comparable to that of TH+ cells. Furthermore, GABA+cells outnumber TH+ cells by a ratio of ∼2:1. The two cell types together account for ∼95% of the total neurons as gauged by staining for tubulin βIII (data not shown), and thus it is clear that the trophic effect of Shh on midbrain neurons extends to multiple neuron subtypes (for TH, p < 0.001 at 25 and 50 ng/ml; for GABA, p < 0.001 at 25 and 50 ng/ml).
Fig. 5.
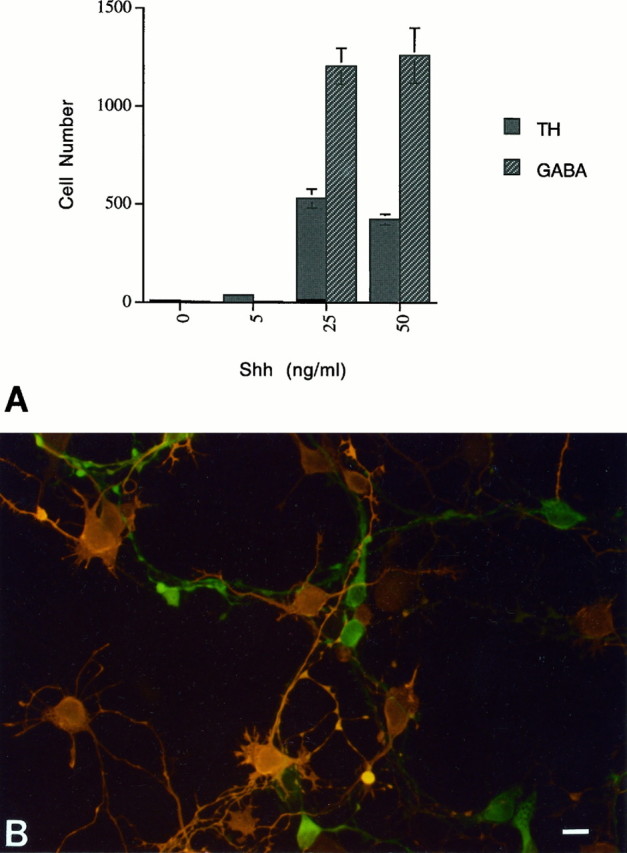
Shh supports the survival of midbrain GABA+ neurons. A, In addition to supporting the survival of TH+ cells in the midbrain cultures, Shh promotes the survival of GABA-ir neurons with a similar dose– response (error bars represent SEM) (for TH,p < 0.001 at 25 and 50 ng/ml; for GABA,p < 0.001 at 25 and 50 ng/ml). B, Double-label immunofluorescence of Shh-treated cultures shows that the majority of the GABA+ cells (orange) do not overlap with the TH+ cells (green). Scale bar, 15 μm.
Shh effects on striatal neurons
Because Shh is strongly expressed in the ventral and lateral forebrain (Echelard et al., 1993; Ericson et al., 1995) and the Shh knockout mouse exhibits striatal defects (Chiang et al., 1996), Shh neurotrophic activity was examined in striatum-derived cultures as well. As assessed after 4 d in vitro (Fig.6), Shh is a potent trophic factor for neurons cultured from the E15–16 striatum and shows a dose–response comparable to that of the midbrain. In comparing the number of total neurons (tubulin βIII+ cells) with that of GABA+neurons, it is clear that essentially all of the neurons supported by Shh are GABAergic (Fig. 6) (tubulin βIII, p < 0.001 at 25 and 50 ng/ml; GABA, p < 0.001 at 25 and 50 ng/ml). That this effect is strictly trophic was confirmed by the observation that BrdU labeling indices over the course of the culture period were low and did not vary with dose (data not shown). Closer inspection reveals that the intensity of GABA staining is variable, and it is thus possible that various subtypes of GABA+interneurons (reviewed by Kawaguchi et al., 1995) are all supported by Shh.
Fig. 6.
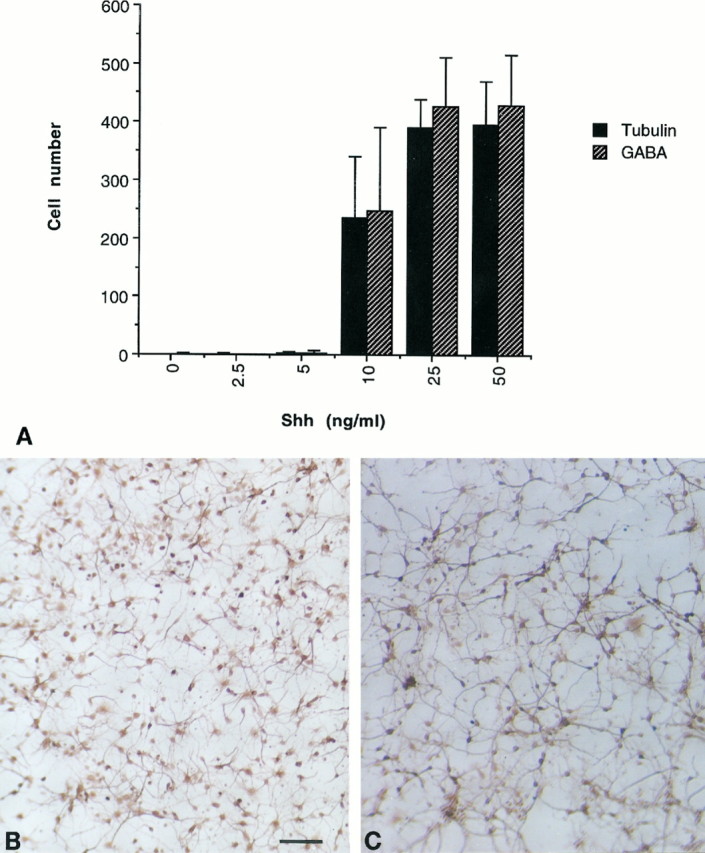
Shh effects on striatal cultures.A, At concentrations of 10 ng/ml and higher, Shh promotes neuronal survival as gauged by staining for tubulin βIII, and these cells are exclusively GABA+ (error bars represent SD) (tubulin βIII, p < 0.001 at 25 and 50 ng/ml; GABA, p < 0.001 at 25 and 50 ng/ml). Typical fields of neurons treated with 50 ng/ml Shh and stained for tubulin βIII (B) and GABA+(C) are shown. Scale bar, 100 μm.
Shh effects on spinal neurons
As a further examination of the postinductive effects of Shh on ventral neural tube derivatives, cultures of the E14–15 ventral neural tube were cultured with varying amounts of Shh. Again, with a dose–response identical to that observed in the mesencephalic and striatal cultures, Shh promotes the survival of tubulin βIII+ neurons as scored after 4 d in vitro (Fig. 7A). A majority but not all of these cells also stain for GABA, and a smaller subset stain for a nuclear marker of spinal interneurons, Lim-1/2 (Tsuchida et al., 1994) (Fig. 7A–C) (tubulin βIII, p < 0.001 at 25 and 50 ng/ml; Lim-1/2, p < 0.001 at 5, 10, 25, and 50 ng/ml; GABA, p < 0.001 at 25 and 50 ng/ml). It is important to note that although there is overlap between the GABA+ and Lim-1/2+ populations, the latter is not merely a subset of the former, because there are Lim-1/2+ cells that do not stain for GABA. Interestingly, immunoreactivity for the low-affinity nerve growth factor receptor (Camu and Henderson, 1992), Islet-1 (Ericson et al., 1992), or galectin-1 (Hynes et al., 1990), all markers of rat motorneurons, was not detectable in these cultures (although such staining could be demonstrated in acutely dissociated spinal preparations), and thus it appears that Shh is not trophic for spinal motorneurons.
Fig. 7.
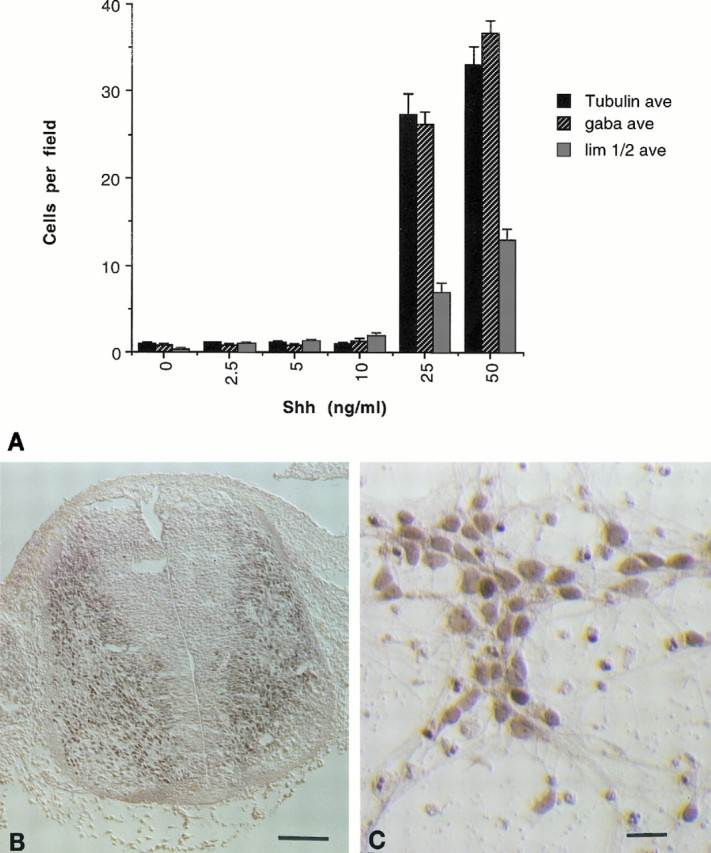
Shh effects on ventral spinal cultures.A, At concentrations of 25 ng/ml and higher, Shh promotes neuronal survival as gauged by staining for tubulin βIII. The majority of the cells stain positively for GABA, whereas a subset of cells stains for the nuclear marker of spinal interneurons Lim-1/2 (error bars represent SEM) (tubulin βIII, p < 0.001 at 25 and 50 ng/ml; lim 1/2, p < 0.001 at 5, 10, 25, and 50 ng/ml; GABA, p < 0.001 at 25 and 50 ng/ml). Typical staining for Lim-1/2 in the E14 rat spinal cord (B) (scale bar, 100 μm), and spinal neurons cultured in the presence of 50 ng/ml Shh (C). Scale bar, 20 μm.
Shh protects TH+ cells against MPP+ toxicity
The toxin 4-phenyl-1,2,3,6-tetrahydropterine (MPTP) and its active metabolite MPP+ are selectively toxic to mesencephalic dopaminergic neurons (Kopin and Markey, 1988; Forno et al., 1993). Because other agents that promote survival of TH+ cells also protect against chemical toxicity of MPP+ (Hyman et al., 1991; Krieglstein et al., 1995), we tested the ability of Shh to protect TH+ cells in E14 rat mesencephalon explants from the effects of MPP+. As shown in Figure 8, the presence of Shh in cultures treated for 48 hr with MPP+ significantly increased the numbers of TH+ cells that were observed in culture after removal of the MPP+. MPP+treatment caused a >90% reduction in the numbers of TH+ cells compared with non-MPP+-treated control cultures, whereas incubation with Shh protected the TH+ cells so that only a 65% reduction in TH+ cells occurred after MPP+ treatment. Thus, the addition of Shh resulted in a net 3.5-fold increase in TH+ cells after MPP+ treatment versus controls. Sister cultures tested for 3H-DA transport demonstrated a sevenfold increase in transport in Shh-treated cultures versus controls (data not shown).
Fig. 8.
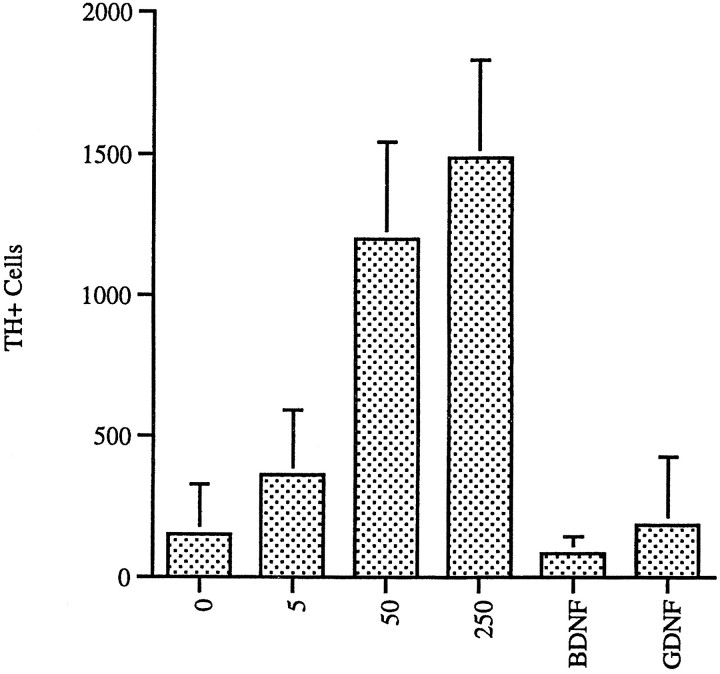
Shh protects midbrain TH+neurons from neurotoxic insult. Ventral mesencephalon neurons were cultured in the indicated concentrations of Shh (ng/ml). MPP+ was added at 4 d in vitrofor 48 hr. Cultures were then washed extensively and cultured for an additional 48 hr to allow clearance of dying neurons. Protection from MPP+ neurotoxicity could be seen at 5 ng/ml, with the effect saturating at 50 ng/ml. BDNF was used at 10 ng/ml and GDNF at 20 ng/ml (error bars represent SEM) (Shh, p < 0.001 at 50 and 250 ng/ml; BDNF, no significance; GDNF,p < 0.05). Note that the plating density used in this experiment was twice that used in Figure 2.
Shh was significantly more active in protecting TH+ cells from the effects of MPP+ than the other growth factors tested: GDNF (Lin et al., 1993) and BDNF (Hyman et al., 1991) (Shh, p < 0.001 at 50 and 250 ng/ml; BDNF, no significance; GDNF,p < 0.05). In the serum-free conditions used in these experiments, none of the other growth factors tested showed as significant a level of TH+ cell protection from MPP+ toxicity as Shh, even when tested at levels previously shown to be optimal for neuroprotection (Fig. 8).
DISCUSSION
Shh is neurotrophic for various ventral neurons
The hypothesis that Shh may play roles in the nervous system in addition to its initial function in neural tube ventralization was first suggested by the observation that Shh expression in ventral neural tissue along the entire neuraxis continues well past the period during which phenotypic specification has occurred (Echelard et al., 1993). Moreover, preliminary evidence generated in our laboratory indicates the presence of significant levels of Shh mRNA in specific regions of the adult human CNS (e.g., spinal cord and substantia nigra) (P. Jin, unpublished observations). We report here the first evidence that Shh can indeed exert effects independent of its induction and patterning activity.
Unlike its role at earlier stages of neural development, this novel neurotrophic activity acts on postmitotic neurons rather than on dividing progenitor cells. Although the general trophic effect is apparent in a number of CNS regions (Figs. 2, 5, 6, 7), there are both differences and similarities in the effects observed among the regions examined. Given the fact that Shh is necessary for the induction of both spinal motor neurons and midbrain dopaminergic neurons, one might predict that Shh would subsequently be trophic for these cells. Strikingly, Shh is a very potent trophic factor for the midbrain dopaminergic neurons (Fig. 2), but in the cultures of ventral spinal neurons no such effect on motor neurons was observed. Thus there is no direct correlation between the neuron phenotypes induced by Shh and those supported by Shh in a trophic manner. Interestingly, a common feature among the three CNS regions examined was the trophic effect for GABAergic neurons (Figs. 5, 6, 7). Although it is not obvious whether these specific GABA+ populations are directly or indirectly induced by Shh during early development (cf. Pfaff et al., 1996), it is plausible that the trophic actions on these neurons are direct.
It is important to note that the neurotrophic effects reported herein are not lacking in specificity. For example, neurons of the peripheral nervous system show no survival in response to Shh administration, and preliminary studies of cultures derived from E15–16 dorsal CNS regions (e.g., neocortex and dorsal spinal cord) show high baseline levels of neuron survival with no significant response to exogenous Shh application (J. Ott and N. Mahanthappa, unpublished observation). Thus there appears to be a general restriction of the trophic effects of Shh to regions of the CNS specified by Shh, but the actual targets of trophic activity need not encompass the phenotypes whose induction is Shh dependent. Nevertheless, the fact that Shh also protects neurons from toxic insult (Fig. 8) suggests previously unforeseen therapeutic roles for Shh as well.
Possible mechanisms of Shh action
As stated above, the neurotrophic effect of Shh observed in these cultures is not attributable to the stimulation of proliferation. One could argue, however, that the observed effects are indirect. In one scenario, Shh may act on a non-neuronal cell that in turn responds by secreting a neurotrophic factor. We observed no sign of astrocytes in any of our neural cultures, either by morphology or by staining for GFAP. Furthermore, in the purely neuronal cultures established from the midbrain, ptc is greatly upregulated in response to Shh, and thus the reported survival effects must be attributable to a response by neurons (Fig. 4C).
In another scenario, it is possible that Shh acts directly on some or all of the neurons, but the response is to secrete another factor(s) that actually possesses the survival activity. For example, Shh has been shown to induce the expression of TGFβ family members such as Bone morphogenetic proteins in vivo (Laufer et al., 1994;Levin et al., 1995), and these proteins are trophic for midbrain dopaminergic neurons (Krieglstein et al., 1995). That induced expression of TGFβs is the trophic mechanism seems unlikely, because exogenous TGFβs show only modest trophic activity in our culture system, and the presence of neutralizing, anti-pan-TGFβ antibodies failed to inhibit the neurotrophic effects of Shh. Thus, at a minimum, Shh supports the survival of a subset of ventral CNS neurons. The mechanism by which Shh supports neuron survival is yet to be determined. Although we favor the hypothesis that these trophic effects are direct, it remains possible that the survival response is attributable to Shh-induced expression of a secondary trophic factor.
As in the case of many secreted peptide factors, it now appears that Shh has activities that can vary greatly, depending on the spatiotemporal context in which the factor is expressed. Although it was initially thought that the primary role of Shh in the CNS is in early patterning events that are critical to phenotypic specification, it is now clear that Shh can also contribute to the survival and maturation of these CNS regions. Interestingly, the cell types acted on in these two distinct roles of Shh do not necessarily overlap. Thus a more thorough understanding of this multifaceted molecule will require a better understanding of its patterns of expression beyond early embryogenesis. Moreover, it will be critical to ascertain the significance of the trophic effects of Shh in vivo.
Footnotes
We thank Drs. D. Melton, T. Jessell, C. Tabin, and T. Ingolia for critical review of this manuscript; A. McMahon and M. Scott forin situ probe templates; A. McMahon and T. Jessell for antibodies; and H. Roelink for Shh-encoding baculovirus. We also thank P. Jin for advice on QC-PCR.
Correspondence should be addressed to Kevin Pang, Ontogeny, Inc., 45 Moulton Street, Cambridge, MA 02138.
REFERENCES
- 1.Altman J, Bayer SA. Atlas of prenatal rat brain development. CRC; Boca Raton, FL: 1995. [Google Scholar]
- 2.Banerjee A, Roach MC, Trcka P, Luduena RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of β-tubulin. J Biol Chem. 1990;1990:1794–1799. [PubMed] [Google Scholar]
- 3.Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of Sonic hedgehog. Mol Cell Biol. 1995;15:2294–2303. doi: 10.1128/mcb.15.4.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J Neurosci Methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 5.Cerruti C, Walther DM, Kuhar MJ, Uhl GR. Dopamine transporter mRNA expression is intense in rat midbrain neurons and modest outside midbrain. Mol Brain Res. 1993;18:181–186. doi: 10.1016/0169-328x(93)90187-t. [DOI] [PubMed] [Google Scholar]
- 6.Chiang C, Litingung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 7.Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM. The dopamine transporter: immunochemical characterization and localization in the brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis-Donini S, Glowinski J, Prochiantz A. Glial heterogeneity may define the three dimensional shape of mouse mesencephalic DA neurons. Nature. 1984;307:641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- 9.Di Porzio U, Daguet M-C, Glowinski J, Prochiantz A. Effect of striatal cells on in vitro maturation of mesencephalic dopaminergic neurons grown in serum-free conditions. Nature. 1980;288:370–373. doi: 10.1038/288370a0. [DOI] [PubMed] [Google Scholar]
- 10.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 11.Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Isl-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 12.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 13.Ericson J, Mortin S, Kawakami A, Roelink H, Jessell TM. Two critical periods of sonic hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 14.Fietz MJ, Concordet J-P, Barbosa R, Johnson R, Krauss S, McMahon AP, Tabin C, Ingham PW. The hedgehog gene family in Drosophila and vertebrate development. Development [Suppl] 1994;120:43–51. [PubMed] [Google Scholar]
- 15.Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonism and Parkinson’s disease: neuropathologic considerations. Adv Neurol. 1993;60:600–608. [PubMed] [Google Scholar]
- 16.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 17.Hyman C, Hofer M, Barde Y-A, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 18.Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes MA, Gitt M, Barondes SH, Jessell TM, Buck LB. Selective expression of an endogenous lactose-binding lectin gene in subsets of central and peripheral neurons. J Neurosci. 1990;10:1004–1013. doi: 10.1523/JNEUROSCI.10-03-01004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes MA, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 22.Kopin IJ, Markey SP. MPTP toxicity: implications for research in Parkinson’s disease. Annu Rev Neurosci. 1988;11:81–96. doi: 10.1146/annurev.ne.11.030188.000501. [DOI] [PubMed] [Google Scholar]
- 23.Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K. TGFβ superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995;14:736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and FGF-4 act along a signaling cascade with a feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ, Von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- 26.Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- 27.Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 28.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that Patched is the Hedgehog receptor. Nature. 1996a;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 29.Marigo V, Scott MP, Johnson RL, Goodrich LV, Tabin CJ. Conservation of hedgehog signaling: induction of a chicken patched homologue by sonic hedgehog in the developing limb. Development. 1996b;122:1225–1233. doi: 10.1242/dev.122.4.1225. [DOI] [PubMed] [Google Scholar]
- 30.Marti E, Bumcrot DA, Takada R, McMahon AP. Requirement of 19K sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995a;375:322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- 31.Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995b;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- 32.Masuko S, Nakajima S, Nakajima Y. Dissociated high-purity dopaminergic neuron cultures from the substantia nigra and the ventral tegmental area of the postnatal rat. Neuroscience. 1992;49:347–364. doi: 10.1016/0306-4522(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 33.Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–971. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- 34.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 36.Porter JA, Ekker SC, Young KE, Von Kessler DP, Lee JJ, Moses D, Beach PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signaling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- 37.Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by Vhh-1, a vertebrate homologue of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 38.Shimoda K, Sauve Y, Schwartz JP, Commissiong JW. A high percentage yield of tyrosine hydroxylase-positive cells from rat E14 mesencephalic cell culture. Brain Res. 1992;586:319–331. doi: 10.1016/0006-8993(92)91642-r. [DOI] [PubMed] [Google Scholar]
- 39.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Hooper JE, Sauvage FD, Rosenthal A. The tumor-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 40.Tabata T, Eaton S, Kornberg TB. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992;6:2635–2645. doi: 10.1101/gad.6.12b.2635. [DOI] [PubMed] [Google Scholar]
- 41.Tanabe Y, Roelink H, Jessel TM. Induction of motor neurons by sonic hedgehog is independent of floor plate. Curr Biol. 1995;5:651–658. doi: 10.1016/s0960-9822(95)00130-8. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang MZ, Jin P, Bumcrot DA, Marigo V, McMahon AP, Wang EA, Woolf T, Pang K. Induction of dopaminergic neuron phenotype in the midbrain by sonic hedgehog protein. Nature Med. 1995;1:1184–1188. doi: 10.1038/nm1195-1184. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In situ hybridization: a practical approach. IRL; Oxford: 1992. pp. 75–83. [Google Scholar]