Abstract
Potent neurotoxicity is associated with both apolipoprotein E (apoE)-related synthetic peptides and the 22 kDa N-terminal thrombin-cleavage fragment of apoE. Furthermore, the E4 isoform of the 22 kDa fragment is significantly more toxic than the same fragment derived from the E3 isoform, suggesting the possibility of a direct role of apoE-associated neurotoxicity in the pathophysiology of Alzheimer’s disease. In the present study, the potential role of cell surface receptors in mediating neurotoxicity was assessed by using a variety of agents that should block the heparin-binding and receptor-binding activity of apoE. Effective inhibitors of neurotoxicity of both the apoE peptides and the apoE fragment include heparin, heparan sulfate, sodium chlorate and heparinase, the low-density lipoprotein (LDL) receptor-related protein receptor-associated protein, and a polyclonal anti-LDL receptor-related protein antibody. These results suggest that the neurotoxicity of the 22 kDa thrombin cleavage fragment of apoE and related peptides is receptor-mediated, and that the most likely candidate receptor is a heparan sulfate proteoglycan–LDL receptor-related protein complex.
Keywords: apolipoprotein E, synthetic peptides, neurotoxicity, LRP, HSPG, Alzheimer’s disease
Apolipoprotein E (apoE) has been implicated in the pathogenesis of late-onset Alzheimer’s disease (AD) by several lines of evidence. Perhaps the most compelling finding is the association between inheritance of the allele for the E4 isoform and the increased risk (4.5-fold greater), and earlier age of onset (16 years earlier on average), of the disease (Corder et al., 1993; Saunders et al., 1993a,b; Strittmatter et al., 1993a). Although several hypotheses have been proposed to account for the isoform-specific association of apoE with AD, none has yet gained sufficient support to provide a clear explanation of how apoE contributes to the pathology.
Based on the demonstration that apoE synthetic peptides exhibit cytotoxicity (Clay et al., 1995) and cause neurite degeneration (Crutcher et al., 1994), apoE has been suggested to play a direct role in AD pathogenesis by contributing directly to neurodegenerative events. This hypothesis has gained additional support from the recent finding that a 22 kDa thrombin cleavage fragment of apoE, which may be analogous to a similar fragment found in brain and CSF, exhibits neurotoxicity and that the E4-derived fragment is significantly more toxic than the E3-derived fragment (Marques et al., 1996). Furthermore, apoE4 has been shown to exhibit greater neurotoxicity than apoE3 (Marques et al., 1997). However, the mechanism underlying these toxic effects, including the question of whether the effects are mediated by specific receptors, is unknown. The cytotoxic apoE peptides and the 22 kDa thrombin fragment include residues 141–155, which reside within the receptor-binding region of apoE (residues 140–160) (Innerarity et al., 1983; Weisgraber et al., 1983; Lalazar et al., 1988), as well as the overlapping high-affinity heparin-binding region (residues 142–147) (Weisgraber et al., 1986), suggesting that these regions are important for cytotoxicity (Clay et al., 1995). The present investigation was performed to determine whether any of the cell surface molecules that have been shown previously to mediate the uptake of apoE lipoproteins are implicated in the neurotoxicity of the synthetic peptides or N-terminal thrombin-cleavage fragment of apoE. The results demonstrate that these neurotoxic effects are specific and likely involve interaction with heparan sulfate (HS) proteoglycans (HSPGs) and a receptor that is the same as, or very similar to, the low-density lipoprotein (LDL) receptor-related protein (LRP).
MATERIALS AND METHODS
Cell death assay. Embryonic day 9 (E9) chick lumbar sympathetic ganglia, E7 chick cortical tissue, or E19 rat hippocampal tissue were procured and dissected in Ham’s F12 medium (Sigma, St. Louis, MO). The tissue was dissociated by initial incubation with 0.25% trypsin (Sigma) for 15 min at 37°C. After incubation with 100% fetal calf serum for 5 min to end trypsinization, the tissue was washed two times in F12 medium, transferred to Neurobasal medium (Life Technologies, Gaithersburg, MD), and dissociated by trituration with a flamed Pasteur pipette. Dissociated cells were then plated onto poly-dl-ornithine-coated 96-well plates and incubated in a humidified environment with 5% CO2/95% O2 in Neurobasal medium overnight. This procedure yielded ∼95%-pure cultures of chick sympathetic neurons and 75%-pure cultures of chick cortical neurons as determined by neurofilament immunocytochemistry (our unpublished observations).
On the following day, dissociated chick sympathetic cultures were transferred to F12 medium supplemented with 20 nmprogesterone, 100 μm putrescine, 30 nmselenium, 100 μg/ml human transferrin, 1% penicillin/streptomycin, and 5 μg/ml bovine insulin. Sympathetic neurons were exposed to the apoE peptides or 22 kDa fragment and potential inhibitors diluted in the supplemented F12 medium. Dissociated chick cortical neurons and dissociated rat hippocampal neurons were maintained in Neurobasal medium for all treatments and also cultured for 20 hr before being used for toxicity experiments. Experiments in which the ability of various agents to block neurotoxicity of apoE peptides or the 22 kDa apoE fragment were performed by treating the cultures with one of the following before addition of the peptides or fragments: heparin (catalog #H3393, Sigma), HS (catalog #H5393, Sigma), a combination of sodium chlorate (Sigma) and heparinase I (catalog #H2519, Sigma), LRP receptor-associated protein (RAP), 30 μg/ml each of affinity-purified rabbit polyclonal anti-LRP antibody (Strickland et al., 1991) or monoclonal anti-apoA1 antibodies (de Silva et al., 1990).
The proportion of living cells remaining after overnight incubation with the experimental treatments was determined by incubating the cultures with a vital dye (5-carboxyfluorescein diacetate, acetoxymethyl ester, Molecular Probes, Eugene, OR) for 30–45 min at 37°C. The vital dye was removed, and fresh unsupplemented F12 medium was added to the cultures. The center of each well was visualized under fluorescent illumination (using a fluorescein filter) using the 4× objective of a Nikon Diaphot fluorescence microscope. A field covering ∼15% (2.7 × 2.1 mm) of the total area of the well was captured with a video camera linked to a Macintosh IIfx computer equipped with a Data Translation framegrabber card and running Image 1.57 software (National Institutes of Health). Images were captured from four to eight wells per treatment, thresholded, and converted into a binary file, and the number of stained cells was counted by the computer.
Propidium iodide (Sigma), a fluorescent stain for nucleic acids used previously for quantification of cell death (Juurlink et al., 1993;Keilhoff et al., 1993), was used to independently confirm the sensitivity of the vital dye method. Cultures were stained with 50 μg/ml propidium iodide for 5 min, and the images were captured under fluorescent illumination using a rhodamine filter. Treatment of cultures with synthetic apoE peptides resulted in extensive cell death as determined by propidium iodide staining. However, the vital dye staining method appeared to be more sensitive than the propidium iodide method and was used for all of the experiments reported here. Each data point in the figures represents the average number of cells from four to eight wells per treatment. Experimental results were replicated in at least two subsequent experiments. Statistical comparisons between groups of measurements were made using ANOVA and, where appropriate, a two-tailed, unpaired Student’s t test adjusted appropriately for multiple comparisons.
ApoE peptides. Studies were performed with a series of peptides that have been shown previously to have cytotoxic effects as well as several control peptides. Toxic peptides used for these studies included the two tandem peptides E(141–149)2 (consisting of a duplicated sequence of apoE amino acids 141 through 149) and E(141–155)2 (duplicated sequence of apoE amino acids 141 through 155). Additional studies were performed with E130–169 and E263–286, the former exhibiting neurotoxic activity but the latter, from the C-terminal region of apoE, showing no toxicity. The monomeric sequence of peptide E141–149, previously shown to lack the appropriate secondary structure of the receptor-binding domain (Clay et al., 1995), was also found not to be toxic. Bovine serum albumin (BSA) was used in some experiments to control for nonspecific protein effects.
Binding of ApoE peptides to the heparin agarose beads. The affinity of various apoE peptides for heparin was determined by incubating 20 μg of each peptide, diluted in 1 ml of 0.137m NaCl-0.05 m Tris buffer, pH 7.4 (TBS), with 20 μl of heparin-agarose beads (Sigma) that were prewashed with TBS. After incubation overnight at 4°C, the following washing operation was repeated three times: the beads in all solutions were allowed to sediment by gravitational force, the supernatant was discarded, and the pellets were resuspended with 1 ml of TBS. The peptides were recovered by SDS-Laemmli sample buffer and loaded onto a Tris–tricine SDS-PAGE under reducing conditions (Schagger et al., 1987). Proteins were subsequently visualized by Coomassie blue staining.
Preparation of recombinant RAP. Recombinant RAP was prepared based on human placental RAP cDNA (Strickland et al., 1991) as described previously (Williams et al., 1992). Briefly, RAP cDNA was cloned into pGEX2T vector (Pharmacia, Piscataway, NJ) designed to produce a protein fusion of the insert-encoded protein and glutathione S-transferase from Schistosoma japonicum. The construct also contains a thrombin cleavage site, which permits the release of RAP. The expression vector was subsequently transformed into the DH5αF′ strain of Escherichia coli. The fusion protein was purified from other proteins contained in the bacterial lysate on a glutathioneS- transferase (GST) affinity column (Herz et al., 1991). After purification, the GST was removed by digestion with thrombin (Calbiochem, La Jolla, CA), and GST was removed by passing the digestion mix once again over the GST affinity column.
Purification of the 22 kDa apoE fragment. Transfected HEK cells were cultured as described previously (LaDu et al., 1994). The apoE was concentrated from conditioned medium by ultrafiltration (10 kDa cut-off membrane, Amicon, Beverly, MA) followed by heparin column chromatography (heparin-coupled agarose beads, Sigma) and subsequent elution with a linear salt concentration. ApoE was then purified using HPLC gel filtration chromatography (BIO SEC, Sigma) and dialyzed against 0.1 m NH4HCO3 before being concentrated with Centricon 10 (Amicon). The purified apoE was then digested with thrombin (1% wt/wt) in 0.1 mNH4HCO3 for 24 hr and loaded onto a DEAE-5PW HPLC column, 7.5 cm × 7.5 mm (Supelco, Bellefonte, PA). The running buffer was 20 mm Tris, pH 7.5, and the elution buffer was 20 mm Tris with 0.5 m NaCl, pH 7.5. The protein solution was loaded using a 60 min gradient program with a 1 ml/min flow rate beginning with the running buffer. The elution buffer concentration was increased at 1%/min for the first 40 min, then up to 100% from 40 to 47 min and held for an additional 1 min. Finally, the elution buffer concentration was brought down to 0% from 48 to 50 min where it was maintained for an additional 10 min. The 22 kDa fragment, which eluted at 26 min, was concentrated in 0.1m NH4HCO3 using Centricon 10. Protein concentration was measured by a Bradford protein assay (Bio-Rad, Hercules, CA) that was found previously to match the protein concentration as determined by amino acid analysis (Marques et al., 1996). After lyophilization, the fragment was stored at −20°C.
RESULTS
Synthetic apoE peptides cause dose-dependent neuronal cell death
Cytotoxicity of apoE peptides has been demonstrated previously with lymphocyte cultures (Clay et al., 1995). Neurite-degenerative effects of these peptides were subsequently demonstrated with explant cultures of chick sympathetic neurons (Crutcher et al., 1994). Although the explant assay is sensitive to changes in neurite morphology, it is not suitable for quantifying cell death. Therefore, initial experiments were performed to determine whether the apoE peptides would also result in the death of dissociated neurons in culture. Synthetic peptides, prepared as described previously (Clay et al., 1995), were added to dissociated embryonic chick sympathetic neurons that had been in culture for 24 hr. The cells were exposed for 20 hr to different concentrations of each of three toxic apoE peptides, E(141–149)2, E(141–155)2, or E130–169, or to peptides that have been found previously to be inactive in other assays. The extent of cell death was then determined by staining with the carboxyfluorescein vital dye, which stains only cells with an intact membrane (Fig. 1).
Fig. 1.
Blockade of the toxicity of peptide E(141–149)2 by anti-LRP antibody. Dissociated embryonic chick sympathetic neurons in tissue culture as revealed by phase-contrast (A, C,E) or fluorescence microscopy (B,D, F) after staining with the vital dye. Cultures were photographed 20 hr after being exposed to vehicle solution (A, B), 4 μm E(141–149)2(C, D) or 8 μm peptide in the presence of 30 μg/ml polyclonal anti-LRP antibody (E, F). Vehicle treatment did not result in cell death. However, exposure to the peptide led to swelling of cell bodies (C, arrowheads), beading of neurites (C, arrow), and extensive cell death (D). The few cells that survived at this time point appeared to be swollen (D,arrowhead). The anti-LRP antibody protected the cells against the toxic effects of the peptide (E,F). Scale bar, 100 μm.
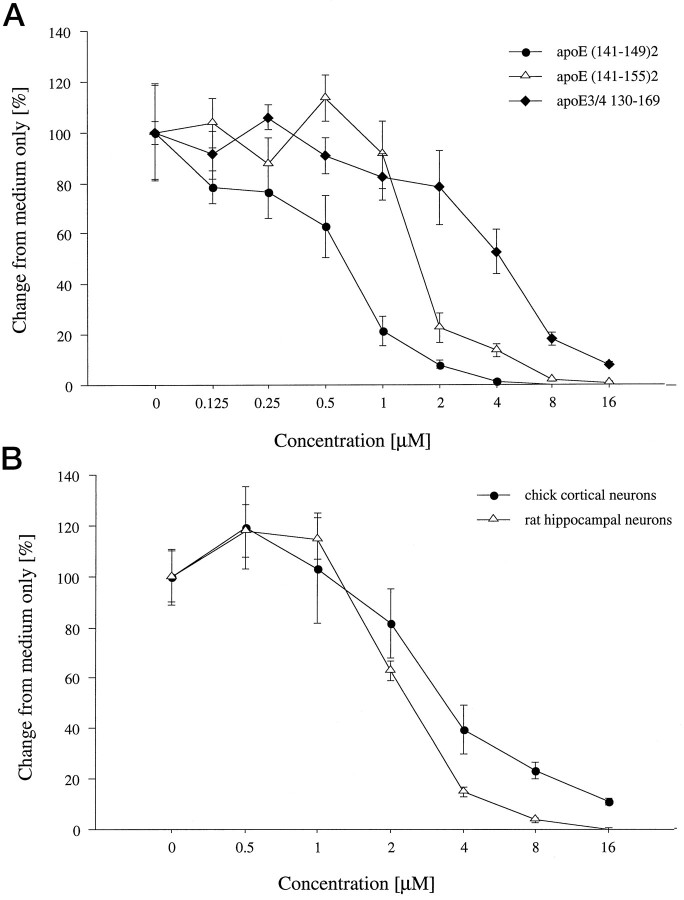
All three peptides incorporating the receptor-binding domain were found to cause dose-dependent neuronal cell death (Fig.2A). The control peptides and BSA did not show any toxicity when applied to chick sympathetic neurons (data not shown). The toxic peptides resulted in neurite degeneration (Fig.1C), similar to that observed with explant cultures of sympathetic neurons (Crutcher et al., 1994), in addition to swelling of the cell body. The half-maximal effective concentrations for causing neuronal death, ranging from 1–4 μm, were similar to the concentrations found to be effective previously in causing degeneration of sympathetic neurites (Crutcher et al., 1994). Therefore, this range of peptide concentrations was used in subsequent studies aimed at blocking the neurotoxic effects.
Fig. 2.
Dose-dependent toxic effects of apoE peptides in chick and rat neuronal cultures. A, Cultures of chick sympathetic neurons were exposed to increasing concentrations of peptides E(141–149)2, E(141–155)2, or E130–169for 20 hr. The graph shows the number of surviving cells as revealed by vital dye staining. Dose–response curves of peptides showed half-maximal toxic concentrations ranging from 1 to 4 μm. B, Similar dose–response curves were obtained with cultures of dissociated chick cortical neurons and rat hippocampal neurons after exposure to peptide E(141–149)2. Error bars indicate mean ± SEM.
To determine whether other neuronal populations are susceptible to the neurotoxic effects of the apoE peptides, additional experiments were performed with dissociated cultures of embryonic chick cortical neurons or fetal rat hippocampal neurons exposed to peptide E(141–149)2. As shown in Figure2B, this peptide caused dose-dependent death of both neuronal cell types with a potency similar to that observed with cultures of sympathetic neurons.
The time course of the apoE peptide-induced cell death was examined in chick sympathetic neurons. Cultures were exposed to 4 μmpeptide E(141–149)2 for up to 24 hr, and the number of surviving cells was determined by vital dye staining. Exposure of the cells to the apoE peptide for 20 hr resulted in death of ∼75% of the cells (Fig. 3).
Fig. 3.
Time course of the apoE peptide-induced cell death in chick sympathetic neuronal cultures. Cultures of chick sympathetic neurons were exposed to 4 μm peptide E(141–149)2 for up to 24 hr. Theline graph shows the number of surviving cells as revealed by vital dye staining. Error bars indicate mean ± SEM.
Neurotoxicity involves a heparin-binding site
Only peptides that include the receptor-binding domain of apoE and the overlapping N-terminal heparin-binding site are toxic (Crutcher et al., 1994; Clay et al., 1995). In previous work, heparin was found to block the toxicity of apoE peptides when tested with lymphocytes (Clay et al., 1995). To determine whether functional heparin binding may also be critical in mediating neurotoxicity, E(141–149)2, E(141–155)2, E130–169, the control peptide E263–286, or BSA was tested for heparin-binding activity. All tested peptides were incubated with heparin-agarose beads, washed, recovered from the beads by SDS-Laemmli sample buffer, and then loaded onto a Tris–tricine gel under reducing conditions. Coomassie blue staining of the gel, shown in Figure4, revealed that only those apoE peptides that include the heparin-binding domain and exhibit neurotoxicity were recovered from the buffer. The E(141–149)2peptide showed a high tendency to aggregate with prolonged incubation in the TBS buffer and gave rise to a smear above the expected molecular weight.
Fig. 4.
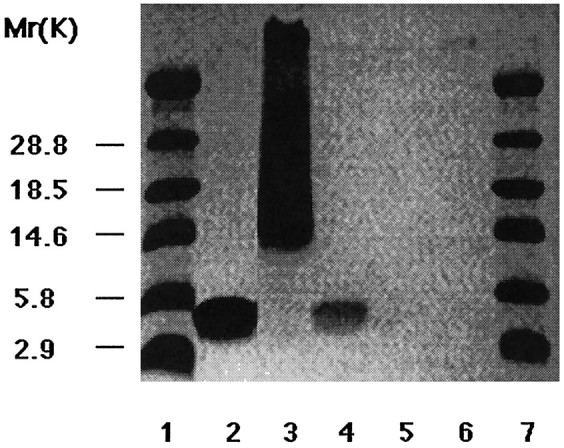
Heparin binding of apoE peptides. Peptides were incubated with heparin-agarose beads, washed, and loaded on the SDS-PAGE gel in the following order: E(141–155)2 (lane 2); E(141–149)2 (lane 3); E130–169 (lane 4); a control peptide from the C-terminal region of apoE, E268–284 (lane 5); and BSA (lane 6). Coomassie blue staining reveals that only neurotoxic apoE peptides are recovered under these conditions. Bands are present at the expected molecular weights for the peptides in addition to a smear in lane 3 attributable to aggregation of this peptide. Molecular weight markers are inlanes 1 and 7.
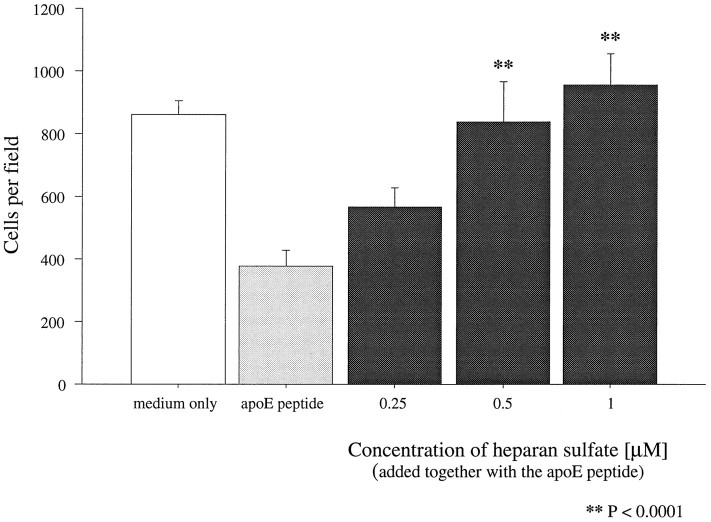
To determine whether blockade of the heparin-binding region would also block the neurotoxic effects of the peptides, saturation of the heparin-binding region was attempted by preincubating the peptides for 10 min with either heparin or HS. Both agents completely abolished the neurotoxic effects of the apoE peptides in a dose-dependent manner when tested with either embryonic chick sympathetic or cortical neurons. Figure 5 shows the dose-dependent blockade of the neurotoxicity of peptide E(141–149)2 by HS. Heparin showed similar effectiveness (data not shown). Complete abolition of toxicity of all peptides was achieved with an HS concentration of 1 μm.
Fig. 5.
Blockade of peptide toxicity with HS. Preincubation (10 min) of 2.5 μm peptide E(141–149)2 with increasing concentrations of HS before addition to cultures of chick sympathetic neurons led to complete blockade of the toxicity. Statistically significant protection was achieved with 1 μm HS. Error bars indicate mean ± SEM.
Pretreatment with sodium chlorate and heparinase blocks neurotoxicity
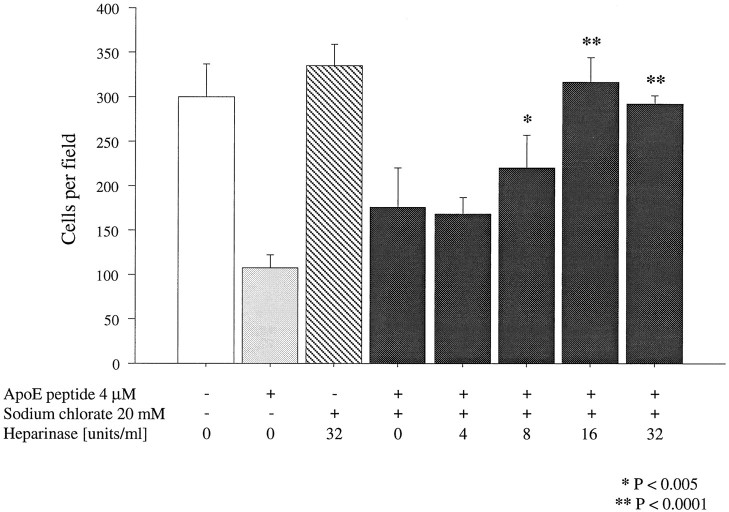
To determine whether the neurotoxicity of apoE peptides requires interaction with HSPGs, cultures of dissociated sympathetic neurons were pretreated simultaneously with heparinase I, which degrades the glycosaminoglycan moiety of HSPGs, and sodium chlorate, which prevents sulfation of newly synthesized glycosaminoglycans (Ji et al., 1993;Nathan et al., 1995). These agents have been shown previously to inhibit internalization of apoE-containing lipoproteins by interfering with the synthesis of, or by degrading, HSPGs. Cells were preincubated overnight with 20 mm sodium chlorate and with increasing concentrations of heparinase I. Cell viability was assessed after 20 hr of exposure to 2 μm peptide E(141–149)2. Significant protection was achieved with 16 U/ml heparinase I (Fig. 6), suggesting that PGs play some role in mediating the neurotoxic effects of the peptides.
Fig. 6.
Blockade of peptide toxicity with sodium chlorate and heparinase. All cells in this experiment, with the exception of the medium-only group, were pretreated with 20 mm sodium chlorate. The preincubation did not have any effect on cell viability in the absence of peptide. Overnight preincubation with sodium chlorate together with increasing concentrations of heparinase protected chick sympathetic neurons from 2 μm peptide E(141–149)2. Statistically significant protection was achieved with 16 U/ml heparinase. Error bars indicate mean ± SEM.
RAP, which blocks ligand interaction with the LDL family of receptors, prevents neurotoxicity
The RAP is a 39 kDa protein co-purified with LRP that prevents binding of all known LRP ligands (Herz et al., 1991; Willnow et al., 1995) and also of several ligands that bind to the other members of the LDL receptor family, such as gp330 and very low density lipoprotein (VLDL) receptors (Kounnas et al., 1992b; Medh et al., 1995). To determine whether the apoE peptide-mediated neurotoxicity might be mediated by receptors within the LDL family, the toxicity of the peptides was tested in the presence of recombinant human RAP. Sympathetic neurons were preincubated with RAP for 1 hr followed by exposure to peptide E(141–149)2 or peptide E130–169. This RAP preparation protected the neurons against toxicity of both peptides (Fig. 7) in a dose-dependent manner with a half-maximal effective concentration of 100 nm, indicating that one of the members of the LDL receptor family is involved in the neurotoxic effects.
Fig. 7.
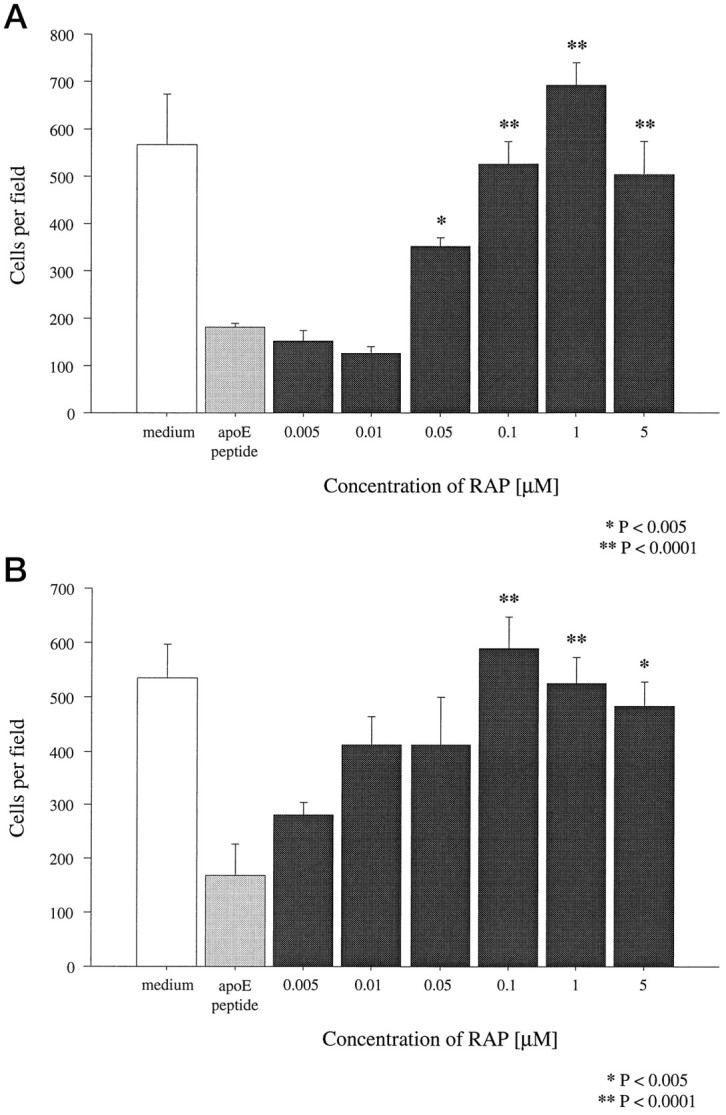
Blockade of peptide toxicity with RAP. After 1 hr preincubation with increasing concentrations of RAP, peptides E(141–149)2 (A) and E130–169 (B) were applied to chick sympathetic neurons at concentrations of 2 and 4 μm, respectively. Vital dye staining 20 hr later revealed that 100 nm RAP completely blocked the toxicity of the peptides. Error bars indicate mean ± SEM.
Neurotoxicity is blocked by anti-LRP antibodies
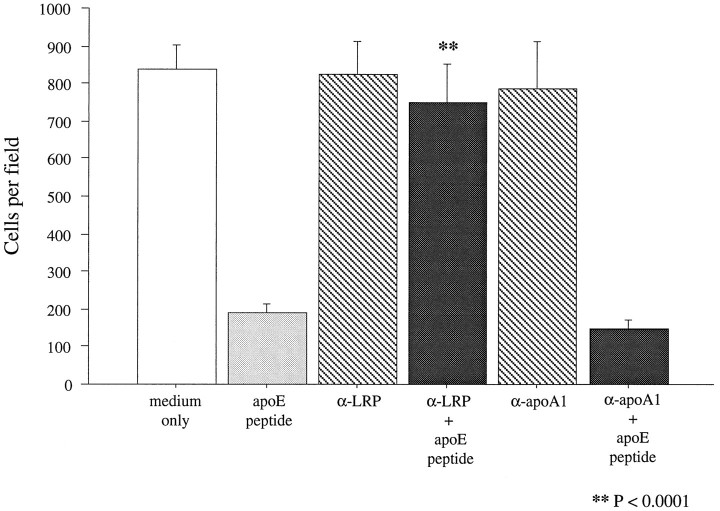
To obtain additional information on which member of the LDL receptor family might mediate apoE peptide toxicity, a polyclonal antibody raised against LRP was tested for its ability to block neurotoxicity. Cultures were incubated for 2 hr with 30 μg/ml either polyclonal anti-LRP or a control monoclonal antibody raised against apolipoprotein A1. After incubation with the antibodies, neurons were exposed to 2 μm peptide E(141–149)2 for 20 hr. Cultures preincubated with the control anti-apoA1 antibodies showed the same extent of neuronal cell death as cultures exposed to the peptide in the absence of antibody treatment. However, cultures treated with the anti-LRP antibody were completely protected from the toxic effects of the peptide (Figs. 1, 8). This result suggests that LRP is the most likely member of the LDL receptor family mediating the neurotoxic effects of the peptides.
Fig. 8.
Blockade of peptide toxicity with anti-LRP antibody. Chick sympathetic neurons were preincubated for 2 hr with 30 μg/ml either polyclonal anti-LRP or monoclonal anti-apoA1 antibodies and then treated with 2 μg/ml peptide E(141–149)2. Vital dye staining of neuronal cultures revealed that only cultures pretreated with the anti-LRP antibody were protected from peptide toxicity. Error bars indicate mean ± SEM.
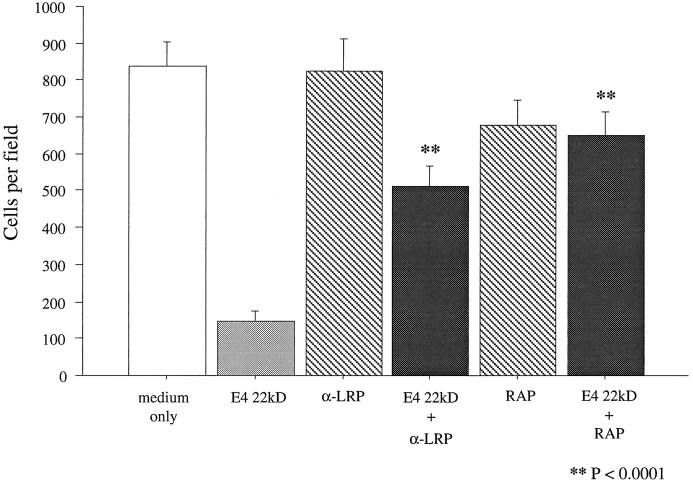
RAP and anti-LRP also block the toxicity of the 22 kDa fragment of apoE
Recent evidence suggests that the N-terminal 22 kDa thrombin-cleavage fragment of apoE is present in the human brain and exhibits neurotoxicity. In addition, the E4 fragment is significantly more toxic than the E3 fragment (Marques et al., 1996). To determine whether the mechanism of toxicity of this naturally occurring fragment is similar to that of the synthetic apoE peptides, the E4-derived 22 kDa fragment was applied to sympathetic neuronal cultures at a previously determined half-maximal toxic concentration of 250 nm (Marques et al., 1996) in the presence or absence of RAP or anti-LRP antibodies. Significant neuronal death occurred in cultures exposed to the 22 kDa fragment. However, both 1 μm RAP and 30 μg/ml anti-LRP polyclonal antibody provided significant protection (Fig. 9), suggesting that neurotoxicity of the 22 kDa fragment is likely to be mediated by the same receptor complex as that caused by the synthetic peptides.
Fig. 9.
Blockade of toxicity of the 22 kDa fragment of apoE with RAP and anti-LRP. Dissociated cultures of chick sympathetic neurons were preincubated with either 1 μmRAP or 30 μg/ml polyclonal anti-LRP antibody for 1 and 2 hr, respectively. After preincubation, cultures were exposed to 250 nm 22 kDa thrombin cleavage fragment of apoE4 for 20 hr and then stained with vital dye. Both agents significantly protected cells from the toxicity of the 22 kDa fragment. Error bars indicate mean ± SEM.
DISCUSSION
Although apoE has been shown to play an important role in lipid transport and uptake, there is evidence that it has other functions. Studies of the immunosuppressive effects of apoE led to the discovery that peptides derived from the receptor-binding domain that show strong LDL receptor-binding activity (Dyer and Curtiss, 1991; Dyer et al., 1991) also have potent anti-proliferative and cytotoxic effects on T lymphocytes (Clay et al., 1995). Neuritotoxic effects of the same peptides were discovered subsequently (Crutcher et al., 1994), followed by the demonstration that a naturally occurring fragment of apoE exhibits neurotoxicity with the E4-derived fragment being more toxic than the E3-derived fragment (Marques et al., 1996). However, previous studies have not addressed whether the neurotoxicity involves specific interactions with the cell. The present results suggest that synthetic apoE peptides and the 22 kDa fragment exert neurotoxic effects via receptors of the LDL family, most probably LRP.
Synthetic apoE-related peptides were found to be toxic when tested in various neuronal cultures, including primary cultures of chick sympathetic and cortical neurons, and fetal rat hippocampal neurons. These results suggest that several neuronal populations are susceptible to such toxicity, at least under the in vitro conditions used here. ApoE peptides were also found to be toxic when tested with both undifferentiated and NGF-differentiated PC12 cells (data not shown). However, these peptides are not toxic to all cells. For example, high concentrations of peptide E(141–155)2 failed to cause lysis of erythrocytes (Clay et al., 1995), and non-neuronal cells within cultures of sympathetic explants appear to be relatively resistant (Crutcher et al., 1994), suggesting that toxicity involves specific interaction of the apoE peptides with the cell membrane.
The search for likely receptors mediating apoE peptide effects on neurons began with candidate receptors identified in other cell types. Several structurally related cell surface receptors of the LDL receptor family mediate the internalization of apoE-containing lipoproteins. In addition to the LDL receptor (Yamamoto et al., 1984), this family includes LRP, also known as the α2-macroglobulin receptor (Herz et al., 1988; Beisiegel et al., 1989), epithelial glycoprotein gp330 (Raychowdhury et al., 1989; Willnow et al., 1992), the VLDL receptor (Takahasi et al., 1992), and two recently described receptors, apoE receptor 2 (apoER2) (Kim et al., 1996) and LR8B (Novak et al., 1996).
Each of the known apoE receptors is found on distinct CNS cell populations. The LDL receptor, for example, is localized primarily to astrocytes and the neuropil (Pitas et al., 1987; Swanson et al., 1988;Rebeck et al., 1993) but does not appear to be expressed by neurons. LRP, which was originally cloned from human cells (Herz et al., 1988), but includes highly homologous receptors in the chicken (Nimpf et al., 1994) and Caenorhabditis elegans (Yochem et al., 1993), is widely expressed by neurons (Moestrup et al., 1992; Wolf et al., 1992;Rebeck et al., 1993; Bu et al., 1994; Ishiguro et al., 1995). The VLDL receptor is expressed by microglia and some cortical pyramidal neurons (Okuizumi et al., 1995; Christie et al., 1996), but gp330 is only expressed by ependymal cells (Zheng et al., 1994). ApoER2 appears to be expressed highly in the brain, as shown by in situhybridization (Kim et al., 1996). LR8B receptor expression is highly restricted to the brain but expression in specific cell types has not yet been determined (Novak et al., 1996).
The 39 kDa LDL RAP co-purifies with LRP and is thought to play a role as a chaperone protein for newly synthesized receptors (Willnow et al., 1996). It has been found to regulate ligand binding to most members of the LDL receptor family (Strickland et al., 1990; Herz et al., 1991;Williams et al., 1992), a property that has made it a useful tool for examining ligand interactions with this receptor family. The mechanism of blockade is not completely known but may involve conformational changes in the receptor, thereby reducing its affinity for other ligands (Strickland et al., 1995). The fact that RAP blocks the neurotoxic effects of the peptides and the 22 kDa fragment strongly suggests that a member of the LDL receptor family participates in the neurotoxic effects. The LDL receptor itself can be excluded based on the fact that the E130–169 peptide, which has low LDL receptor-binding activity (Dyer et al., 1995), shows significant neurotoxicity.
Perhaps the strongest piece of evidence for an LRP-like receptor mediating the neurotoxicity is the ability of antibodies raised against LRP to block such effects. This antibody does not block ligand interaction with other known members of the LDL receptor family (Chappell et al., 1992; Kounnas et al., 1992a). Although the polyclonal anti-LRP antibodies used in this study were raised against the human receptor, and their interaction with the chicken receptor has not been determined, chicken LRP has been reported to cross-react with polyclonal antibodies against human LRP (Nimpf and Schneider, 1994). In addition, the sequence homology between human and chicken LRP is high (83% identity, where ∼50% of the differences are conservative substitutions), and the features of the protein are conserved, e.g., the cysteine residues within the ligand-binding domain align perfectly (Nimpf et al., 1994). This structural similarity leads to similar affinity for ligands (Stifani et al., 1989) and suggests that the chicken LRP is comparable to the human LRP.
Other evidence consistent with a role for LRP includes the fact that LRP is highly expressed in neurons, as noted above. For example, immunohistochemical studies have demonstrated LRP expression in hippocampal pyramidal neurons, granule cells of the dentate gyrus, and throughout the neuropil (Moestrup et al., 1992; Wolf et al., 1992;Rebeck et al., 1993; Bu et al., 1994; Ishiguro et al., 1995; Tooyama et al., 1995). Furthermore, previous studies have implicated LRP in mediating the effects of apoE on neurite outgrowth (Bellosta et al., 1995; Holtzman et al., 1995). However, in contrast to the present results, apoE effects on neurite outgrowth were reported to require the presence of exogenous β-VLDL lipoproteins (Nathan et al., 1994;Holtzman et al., 1995).
Although the present results implicate LRP’s involvement in the toxicity of apoE peptides, the fact that heparin, HS, and the combination of heparinase and sodium chlorate all protect against apoE peptide neurotoxicity also implicates cell surface PGs in this phenomenon. These results, together with the heparin-binding activity of the apoE peptides, are consistent with previous studies demonstrating that many ligands interact with LRP through an HSPG–receptor complex (Kounnas et al., 1995; Mikhailenko et al., 1995) and the fact that the toxic peptides and 22 kDa peptide include one of the two heparin-binding domains of apoE. They also indicate that the heparin-binding site is functional, a conclusion that is consistent with earlier results in which heparin was found to block toxic effects of apoE peptides on lymphocytes (Clay et al., 1995).
ApoE4 is a risk factor for late-onset AD. Several hypotheses regarding the role of apoE in the pathogenesis of AD have been proposed, but none has been established with certainty (Handelmann et al., 1992; Rebeck et al., 1993; Strittmatter et al., 1993a,b, 1994; Huang et al., 1994;Nathan et al., 1994; Wisniewski et al., 1994; Genis et al., 1995;Guillaume et al., 1995; Masliah et al., 1995; Nathan et al., 1995). These hypotheses rest on the assumption that apoE plays an indirect role in the pathology. An alternative possibility is that apoE plays a direct neurotoxic role in the disease. In fact, some studies indicate that the E4 isoform of apoE has an inhibitory effect on neurite outgrowth, an effect that is mediated by the HSPG–LRP receptor complex (Nathan et al., 1994; Bellosta et al., 1995) and that may involve depolymerization of microtubules (Nathan et al., 1995). It is not clear whether the inhibitory effect on neurite outgrowth reported for E4 in the presence of β-VLDL is related to neurotoxicity. However, recent work has shown that apoE can also exhibit neurotoxic effects (Marques et al., 1997) in the absence of exogenous lipoproteins. Furthermore, the fact that apoE peptides and the N-terminal proteolytic fragment of apoE, which does not contain the lipid-binding domain, exhibit neurotoxic effects suggests that toxicity does not depend on lipoprotein interactions.
The presence of a 22 kDa apoE fragment in brain and CSF samples, as well as the strikingly higher toxicity of the 22 kDa thrombin cleavage fragment derived from apoE4 as compared with apoE3 (Marques et al., 1996), has led to the suggestion that apoE plays a direct role in the pathogenesis of AD (Crutcher et al., 1994, 1997; Marques et al., 1996). Although speculative, this hypothesis provides a novel approach to investigating the pathogenesis of the disease. Of particular interest is the fact that both LRP (Van Gool et al., 1993; Rebeck et al., 1995) and HSPGs (Snow et al., 1988, 1990) are localized to neuritic plaques. Thus, the critical players in this hypothetical scenario of AD pathogenesis, i.e., apoE, LRP, and HSPGs, are present in sites where extensive neurite degeneration occurs. Future studies will be needed to determine to what extent the neurotoxic effects of apoE-related molecules contribute to neurological disorders such as AD or other diseases in which apoE has been implicated.
Note added in proof. After submission of this paper an error was discovered in the calculation of the concentrations of the peptide E(144–149)2 used in this study. The actual concentrations of this peptide are 56% of the reported values. This correction does not in any way affect the results or conclusions of the study.
Footnotes
This work was supported by National Institutes of Health Grants NS31410 and HL27333. We thank William Stuart and Esly Caldwell for technical assistance and Meena Mistry for helpful discussions. The transfected HEK 293 cell lines were kindly provided by Dr. M. J. LaDu (Department of Pathology, University of Chicago). The polyclonal anti-LRP antibody was a generous gift from Dr. D. Strickland (Department of Biochemistry, American Red Cross, Rockville, MD).
Correspondence should be addressed to Prof. Keith A. Crutcher, Department of Neurosurgery, University of Cincinnati, ML 515, Cincinnati, OH 45267-0515.
REFERENCES
- 1.Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 2.Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produce differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 3.Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J Biol Chem. 1994;269:18521–18528. [PubMed] [Google Scholar]
- 4.Chappell DA, Fry GL, Waknitz MA, Iverius P-H, Williams SE, Strickland DK. The low density lipoprotein receptor-related protein/alpha2 macroglobulin receptor binds and mediates catabolism of bovine milk lipoprotein lipase. J Biol Chem. 1992;267:25764–25767. [PubMed] [Google Scholar]
- 5.Christie RH, Freeman M, Hyman BT. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am J Pathol. 1996;148:399–403. [PMC free article] [PubMed] [Google Scholar]
- 6.Clay MA, Anantharamaiah GM, Mistry MJ, Balasubramaniam A, Harmony JAK. Localization of a domain in apolipoprotein E with both cytostatic and cytotoxic activity. Biochemistry. 1995;34:11142–11151. doi: 10.1021/bi00035a020. [DOI] [PubMed] [Google Scholar]
- 7.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 8.Crutcher KA, Clay MA, Scott SA, Tian X, Tolar M, Harmony JAK. Neurite degeneration elicited by apolipoprotein E peptides. Exp Neurol. 1994;130:120–126. doi: 10.1006/exnr.1994.1191. [DOI] [PubMed] [Google Scholar]
- 9.Crutcher KA, Tolar M, Harmony JAK, Marques MA. A new hypothesis for the role of apolipoprotein E in Alzheimer’s disease pathology. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM, editors. Alzheimer’s disease: biology, diagnosis and therapeutics. Wiley; Chichester, UK: 1997. pp. 545–554. [Google Scholar]
- 10.de Silva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, Albers HW, Smith WR, Harmony JA. A 70 kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990;265:13240–13247. [PubMed] [Google Scholar]
- 11.Dyer CA, Curtiss LK. A synthetic peptide mimic of plasma apolipoprotein E that binds the LDL receptor. J Biol Chem. 1991;266:22803–22806. [PubMed] [Google Scholar]
- 12.Dyer CA, Smith RS, Curtiss LK. Only multimers of a synthetic peptide of human apolipoprotein E are biologically active. J Biol Chem. 1991;266:15009–15015. [PubMed] [Google Scholar]
- 13.Dyer CA, Cistola DP, Parry GC, Curtiss LK. Structural features of synthetic peptides of apolipoprotein E that bind the LDL receptor. J Lipid Res. 1995;36:80–88. [PubMed] [Google Scholar]
- 14.Genis I, Gordon I, Sehayek E, Michaelson DM. Phosphorylation of tau in apolipoprotein E-deficient mice. Neurosci Lett. 1995;199:5–8. doi: 10.1016/0304-3940(95)12007-q. [DOI] [PubMed] [Google Scholar]
- 15.Guillaume D, Dea D, Davignon J, Poirier J. Low density lipoprotein pathways in the central nervous system and apolipoprotein E isoform-specific differences. In: Iqbal K, Mortimer JA, Winblad B, Wisniewski HM, editors. Research advances in Alzheimer’s disease and related disorders. Wiley; Chichester, UK: 1995. pp. 385–395. [Google Scholar]
- 16.Handelmann GE, Boyles JK, Weisgraber KH, Mahley RW, Pitas RE. Effects of apolipoprotein E, β-very low density lipoproteins, and cholesterol on the extension of neurites by rabbit dorsal root ganglion neurons in vitro. J Lipid Res. 1992;33:1677–1688. [PubMed] [Google Scholar]
- 17.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39 kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/α2-macroglobulin receptor. J Biol Chem. 1991;266:1–7. [PubMed] [Google Scholar]
- 19.Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang DY, Goedert M, Jakes R, Weisgraber KH, Garner CC, Saunders AM, Pericak-Vance MA, Schmechel DE, Roses AD, Strittmatter WJ. Isoform-specific interactions of apolipoprotein E with the microtubule-associated protein MAP2c: implications for Alzheimer’s disease. Neurosci Lett. 1994;182:55–58. doi: 10.1016/0304-3940(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 21.Innerarity TL, Friedlander EJ, Rall SJ, Weisgraber KH, Mahley RW. The receptor-binding domain of human apolipoprotein E. Binding of apolipoprotein E fragments. J Biol Chem. 1983;258:12341–12347. [PubMed] [Google Scholar]
- 22.Ishiguro M, Imai Y, Kohsaka S. Expression and distribution of low density lipoprotein receptor-related protein mRNA in the rat central nervous system. Brain Res. 1995;33:37–46. doi: 10.1016/0169-328x(95)00104-z. [DOI] [PubMed] [Google Scholar]
- 23.Ji ZS, Brecht WJ, Miranda RD, Hussain MM, Innerarity TL, Mahley RW. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–10167. [PubMed] [Google Scholar]
- 24.Juurlink BH, Herz L. Ischemia-induced death and neurons in primary culture: pitfalls in quantifying neuronal cell death. Brain Res. 1993;71:239–246. doi: 10.1016/0165-3806(93)90175-a. [DOI] [PubMed] [Google Scholar]
- 25.Keilhoff G, Wolf G. Comparison of double fluorescence staining and LDH-test for monitoring cell viability in vitro. NeuroReport. 1993;5:129–132. doi: 10.1097/00001756-199311180-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kim D-H, Iijima H, Goto K, Sakai J, Ishii H, Kim H-J, Suzuki H, Kondo H, Saeki S, Yamamoto T. Human apolipoprotein E receptor 2. J Biol Chem. 1996;271:8373–8380. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- 27.Kounnas MZ, Morris RE, Thompson MR, FitzGerald DJ, Strickland DK, Saelinger CB. The α2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas Exotoxin A. J Biol Chem. 1992a;267:12420–12423. [PubMed] [Google Scholar]
- 28.Kounnas MZ, Argraves WS, Strickland DK. The 39 kDa receptor-associated protein interacts with two members of the low density lipoprotein receptor family, alpha2-macroglobulin receptor and glycoprotein 330. J Biol Chem. 1992b;267:21162–21166. [PubMed] [Google Scholar]
- 29.Kounnas MZ, Chappell DA, Wong H, Argraves WS, Strickland D. The cellular internalization and degradation of hepatic lipase is mediated by low density lipoprotein receptor-related protein and requires cell surface proteoglycans. J Biol Chem. 1995;270:9307–9312. doi: 10.1074/jbc.270.16.9307. [DOI] [PubMed] [Google Scholar]
- 30.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 31.Lalazar A, Weisgraber KH, Rall SC, Jr, Giladi H, Innerarity TL, Levanon AZ, Boyles JK, Amit B, Gorecki M, Mahley RW, Vogel T. Site-specific mutagenesis of human apolipoprotein E: receptor binding activity of variants with single amino acid substitutions. J Biol Chem. 1988;263:3542–3545. [PubMed] [Google Scholar]
- 32.Marques MA, Tolar M, Harmony JAK, Crutcher KA. A thrombin cleavage fragment of apolipoprotein E exhibits isoform-specific neurotoxicity. NeuroReport. 1996;7:2529–2532. doi: 10.1097/00001756-199611040-00025. [DOI] [PubMed] [Google Scholar]
- 33.Marques MA, Tolar M, Crutcher KA. Apolipoprotein E exhibits isoform-specific neurotoxicity. Alzheimer Res. 1997;3:1–6. doi: 10.1097/00001756-199611040-00025. [DOI] [PubMed] [Google Scholar]
- 34.Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- 35.Medh JD, Fry GL, Bowen SL, Pladet MW, Strickland DK, Chappell DA. The 39 kDa receptor-associated protein modulates lipoprotein catabolism by binding to LDL receptors. J Biol Chem. 1995;270:536–540. doi: 10.1074/jbc.270.2.536. [DOI] [PubMed] [Google Scholar]
- 36.Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptor-related protein/α2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J Biol Chem. 1995;270:9543–9549. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- 37.Moestrup SK, Gliemann J, Pallesen G. Distribution of the α2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- 38.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 39.Nathan BP, Chang KC, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- 40.Nimpf J, Schneider WJ. The chicken LDL receptor-related protein/α2-macroglobulin receptor family. Ann NY Acad Sci. 1994;737:145–153. doi: 10.1111/j.1749-6632.1994.tb44308.x. [DOI] [PubMed] [Google Scholar]
- 41.Nimpf J, Stifani S, Bilous PT, Schneider WJ. The somatic cell-specific low density lipoprotein receptor-related protein of the chicken. J Biol Chem. 1994;269:212–219. [PubMed] [Google Scholar]
- 42.Novak S, Hiesberger T, Schneider WJ, Nimpf J. A new low density lipoprotein receptor homologue with 8 ligand binding repeats in brain of chicken and mouse. J Biol Chem. 1996;271:11732–11736. doi: 10.1074/jbc.271.20.11732. [DOI] [PubMed] [Google Scholar]
- 43.Okuizumi K, Onodera O, Namba Y, Ikeda K, Yamamoto T, Seki K, Ueki A, Nanko S, Tanaka H, Takahashi H, Oyanagi K, Mizusawa H, Kanazawa I, Tsuji S. Genetic association of the very low density lipoprotein receptor (VLDL) with sporadic Alzheimer’s disease. Nat Genet. 1995;11:207–209. doi: 10.1038/ng1095-207. [DOI] [PubMed] [Google Scholar]
- 44.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 45.Raychowdhury RJ, Niles L, McCluskey RT, Smith JA. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989;244:1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- 46.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 47.Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the α2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 48.Saunders AM, Schmader K, Breitner JCS, Benson MD, Brown WT, Goldfarb L, Goldgaber D, Manwaring MG, Szymanski MH, McCown N, Dole KC, Schmechel DE, Strittmatter WJ, Pericak-Vance MA, Roses AD. Apolipoprotein E ε4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet. 1993a;342:710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- 49.Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-McLachlin DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993b;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 50.Schagger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 51.Snow AD, Mar H, Nochlin D, Kimata K, Kato M, Suzuki S, Hassell J, Wight TN. The presence of heparan sulfate proteoglycan in the neuritic plaques and congophilic angiopathy in Alzheimer’s disease. Am J Pathol. 1988;133:456–463. [PMC free article] [PubMed] [Google Scholar]
- 52.Snow AD, Mar H, Nochlin D, Sekiguchi RT, Kimata K, Koike Y, Wight TN. Early accumulation of heparan sulfate in neurons and in the β-amyloid protein-containing lesions of Alzheimer’s disease and Down’s syndrome. Am J Pathol. 1990;137:1253–1270. [PMC free article] [PubMed] [Google Scholar]
- 53.Stifani S, Barber DL, Aebersold R, Steyrer E, Shen X, Nimpf J, Schneider WJ. The laying-hen expresses two different low density lipoprotein receptor-related proteins. J Biol Chem. 1989;266:19079–19087. [PubMed] [Google Scholar]
- 54.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the α2-macroglobulin receptor and low density lipoprotein receptor-related protein suggest that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- 55.Strickland DK, Ashcom JD, Williams S, Battey F, Behre E, McTigue K, Battey JF, Argraves WS. Primary structure of α2-macroglobulin receptor-associated protein. J Biol Chem. 1991;266:13364–13369. [PubMed] [Google Scholar]
- 56.Strickland DK, Kounnas MZ, Argraves WS. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- 57.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high avidity binding to β-amyloid and increased frequency of type-4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993a;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strittmatter WJ, Weisgraber KH, Huang DY, Dong L-M, Salvesen GS, Pericak-Vance M, Schmechel D, Sanders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid β-peptide: isoform specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993b;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong L-M, Jakes R, Alberts MJ, Gilbert JR, Han S-H, Hulette C, Einstein G, Schmechel DE, Pericak-Vance MA, Roses AD. Hypothesis: microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- 60.Swanson LW, Simmons DM, Hoffmann SL, Goldstein JL, Brown MS. Localization of mRNA for low density lipoprotein receptor and a cholesterol synthetic enzyme in rabbit nervous system by in situ hybridization. Proc Natl Acad Sci USA. 1988;85:9821–9825. doi: 10.1073/pnas.85.24.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahasi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci USA. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tooyama I, Kawamata T, Akiyama H, Kimura H, Moestrup SK, Gliemann J, Matsuo A, McGeer PL. Subcellular localization of the low density lipoprotein receptor-related protein (alpha2-macroglobulin receptor) in human brain. Brain Res. 1995;691:235–238. doi: 10.1016/0006-8993(95)00735-9. [DOI] [PubMed] [Google Scholar]
- 63.Van Gool D, De Strooper B, Van Leuven F, Triau E, Dom R. α2-macroglobulin expression in neuritic-type plaques in patients with Alzheimer’s disease. Neurobiol Aging. 1993;14:233–237. doi: 10.1016/0197-4580(93)90006-w. [DOI] [PubMed] [Google Scholar]
- 64.Weisgraber KH, Innerarity TL, Harder KJ, Mahley RW, Milne RW, Marcel YL, Sparrow JT. The receptor-binding domain of human apolipoprotein E: monoclonal antibody inhibition of binding. J Biol Chem. 1983;258:12348–12354. [PubMed] [Google Scholar]
- 65.Weisgraber KH, Rall SC, Mahley RW, Milne RW, Marcel YL, Sparrow JT. Human apolipoprotein E: determination of the heparin binding sites of apolipoprotein E. J Biol Chem. 1986;261:2068–2076. [PubMed] [Google Scholar]
- 66.Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of α2-macroglobulin receptor/low density lipoprotein receptor-related protein. J Biol Chem. 1992;267:92035–92040. [PubMed] [Google Scholar]
- 67.Willnow TE, Goldstein JL, Orth K, Brown MS, Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem. 1992;267:26172–26180. [PubMed] [Google Scholar]
- 68.Willnow TE, Armstrong SA, Hammer RE, Herz J. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci USA. 1995;92:4537–4541. doi: 10.1073/pnas.92.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willnow TE, Rohlmann A, Horton J, Otani H, Braun JR, Hammer RE, Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996;15:2632–2639. [PMC free article] [PubMed] [Google Scholar]
- 70.Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 71.Wolf BB, Lopes MBS, Vandenberg SR, Gonias SL. Characterization and immunohistochemical localization of α2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto T, Davis GC, Brown MS, Schneider WJ, Casey ML, Goldstein JL, Russel DW. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984;39:27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- 73.Yochem J, Greenwald I. A gene for a low density lipoprotein receptor-related protein in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1993;90:4572–4576. doi: 10.1073/pnas.90.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng G, Bachinsky DR, Stamenkovic I, Strickland DK, Brown D, Andres G, McCluskey RT. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/α2-MR, and the receptor-associated protein (RAP). J Histochem Cytochem. 1994;42:531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]