Abstract
Tumor necrosis factor (TNF) is a well characterized sleep-regulatory substance. To study receptor mechanisms for the sleep-promoting effects of TNF, sleep patterns were determined in control and TNF 55 kDa receptor knock-out (TNFR-KO) mice with a B6 × 129 background after intraperitoneal injections of saline or murine TNFα. The TNFR-KO mice had significantly less baseline sleep than the controls. TNFα dose-dependently increased non-rapid eye movement sleep (NREMS) in the controls but did not influence sleep in TNFR-KO mice. Although TNFR-KO mice failed to respond to TNFα, they had an increase in NREMS and a decrease in rapid eye movement sleep after interleukin-1β treatment. These results indicate that TNFα affects sleep via the 55 kDa receptor and provide further evidence that TNFα is involved in physiological sleep regulation. Current results also extend the list of species to mice in which TNFα and interleukin-1β are somnogenic.
Keywords: knock-out mice, REM sleep, slow-wave sleep, TNF receptor, EEG slow-wave activity, interleukin-1
Administration of exogenous tumor necrosis factor α (TNFα) (Shoham et al., 1987a; Kapás et al., 1992; Nistico et al., 1992) induces increases in non-rapid eye movement sleep (NREMS) in rabbits and rats. Conversely, inhibition of endogenous TNF using either anti-TNFα antibodies (Takahashi et al., 1995a), a TNF-soluble receptor, or a synthetic fragment of the TNF-soluble receptor (Takahashi et al., 1995b) inhibits spontaneous sleep. Inhibition of TNF also attenuates sleep rebound after sleep deprivation (Takahashi et al., 1996c), excess sleep induced by acute exposure to mild increases in ambient temperature (Takahashi et al., 1997), or sleep responses induced by bacterial cell wall products such as muramyl dipeptide (Takahashi et al., 1996b). TNFα mRNA (Hunt et al., 1992;Tchelingerian et al., 1994) and TNFα immunoreactivity (Breder et al., 1993) are present in neurons in several areas of the normal rat brain including the hypothalamus, an area involved in NREMS regulation. TNFα is also a product of astrocytes (Lieberman et al., 1989). Furthermore, hypothalamic levels of TNFα mRNA (Bredow et al., 1996) and TNFα (Floyd et al., 1996) in rats are greater during daylight hours, when rats sleep the most, than during the nighttime. TNF receptor mRNA is also expressed in normal brain (Hunt et al., 1992), and the soluble TNF receptor seems to be a normal constituent of CSF (Puccioni-Sohler et al., 1995). Circulating TNF may also be linked to sleep regulation. In humans, TNF plasma levels vary in phase with electroencephalographic (EEG) slow-wave activity (Darko et al., 1995). The ability of circulating monocytes to produce TNF is dependent on the sleep–wake cycle and increases during sleep deprivation (Yamasu et al., 1992; Hohagen et al., 1993; Uthgenannt et al., 1994). Collectively, these data support the hypothesis that TNFα is an important regulatory component in sleep regulation.
Two cell surface receptors for TNF [a 55 kDa receptor (TNF55kDR) and a 75 kDa receptor (TNF75kDR)] have been characterized (Hohmann et al., 1989; 1990; Schall et al., 1990). The extracellular domains of these receptors share significant amino acid homologies, although their intracellular domains do not. The actions mediated by these two receptors are distinct (Vilcek and Lee, 1991; for review, see Schutze et al., 1994). Which TNF receptor is involved in sleep regulation was heretofore unknown. Currently it is not possible to differentiate the two types of TNF receptors using pharmacological methods. However, the presence of mutant mice that lack TNF55kDR (Rothe et al., 1993) provided an opportunity to study the receptor mechanisms of TNF. These mutant mice still express TNF75kDR and develop normally with no apparent anomalies. It was, therefore, of interest to investigate sleep in these TNF55kDR knock-out (TNFR-KO) mice. We now report that the TNFR-KO mice sleep less than their strain controls and do not exhibit NREMS responses after the administration of exogenous TNFα, although they do retain the ability to express excess NREMS if given interleukin-1β (IL-1β), another well characterized NREMS-promoting cytokine.
MATERIALS AND METHODS
Adult TNFR-KO mice (n = 14), which have a B6 × 129 background (Rothe et al., 1993), and B6 × 129-F2 control mice (n = 13) were used in the experiments. The TNFR-KO mice were provided by Dr. W. Lesslauer (F. Hoffmann-LaRoche, Ltd., Basel, Switzerland). Mice used in these experiments were 79.36 ± 8.21 d old, weighing 28.12 ± 1.52 gm. Control mice of the same age and weight were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with ketamine (25 mg/kg) and xylazine (25 mg/kg) and implanted with three EEG electrodes (Plastics One, Inc., Roanoke, VA) in the skull over the parietal cortex and three electromyogram (EMG) electrodes in the muscle of the dorsal neck, respectively. The electrodes with attached wires were fixed to the skull with dental cement. Ten days were allowed for recovery from surgery. Mice were housed at 30 ± 1°C in separate recording cages in sound-attenuated environmental chambers with a 12 hr light/dark cycle (lights on at 5 A.M. and off at 5 P.M.). Each mouse received one (1.0 μg/mouse; n = 7 for control mice and n = 7 for TNFR-KO mice) or three doses (0.3, 1.0, or 3.0 μg/mouse; n = 6 for control mice andn = 7 for TNFR-KO mice) of the full-length recombinant murine TNFα (R & D Systems, Inc., Minneapolis, MN) by intraperitoneal injections at dusk (5 P.M.). Each TNFα injection day was preceded by a saline injection day. The effects of TNFα on sleep were tested in an increasing dose order. There was a separation of 5 d between the previous TNFα injection and the next saline injection. The control and TNFR-KO mice received the same treatment according to the same schedule. In a separate experiment, the same TNFR-KO mice that received only 1 μg TNFα (n = 6) were also injected intraperitoneally with saline on the control day (3 d after the TNFα test) and IL-1β (R & D Systems, Inc.) (0.4 μg/mouse) on the experimental day.
In all experiments, EEG and EMG were recorded for 24 hr after each injection. Sleep data were collected with a Grass Instruments (Quincy, MA) polygraph. The EEG signals were amplified with a 7P5 wide-band EEG preamplifier and a 7P-DA-G DC driver amplifier. The one-half cutoff for low and high frequencies was set at 0.5 and 35.0 Hz, respectively. The EMG signals were amplified with a 7P511J amplifier with one-half cutoff for low and high frequencies set at 100 and 10,000 Hz, respectively. The data collection was controlled by a 386 microcomputer. The J6 output from the DC drivers or 7P511J amplifiers was fed into a 12 bit PC30D analog-to-digital (AD) converter (Omega Engineering, Inc., Stamford, CT). The AD converter digitized the EEG and EMG signals at 128 Hz. The digitized data were transferred to the computer and displayed graphically on the computer monitor. An on-line fast Fourier transformation (FFT) was performed on EEG data in every 2 sec of data. The FFT analyses generated the power density values from 0.0 to 63.5 Hz at a 0.5 Hz resolution. The results of the FFT were averaged for every 10 sec. The sleep data and FFT results were saved to the hard disk for off-line analyses.
Data were scored to determine sleep parameters as described previously (Fang et al., 1996a). After data collection the EEG and EMG patterns and FFT data were displayed graphically on the screen of the computer monitor for sleep scoring. The behavioral states were categorized visually according to the following criteria: wakefulness was identified by low-voltage fast EEG and high-amplitude EMG; rapid eye movement sleep (REMS) by low-voltage EEG with clear (6–10 Hz) theta activity and dramatic suppression of EMG with occasional muscle twitches; and NREMS by high-voltage and low-frequency EEG and low-amplitude EMG. Sleep was scored in epochs of 10 sec. The behavioral state for each epoch was determined by the predominant state during the epoch. The number of episodes for each behavioral state was calculated by a computer program based on the criterion that the minimal episode length for each state should last at least 30 sec.
The FFT data were sorted by a computer program according to the scoring results. The total power in each 5 Hz frequency band was summed for each 10 sec epoch and then averaged for every 6 hr. Results from the 0.5–5 Hz frequency band are presented; these results are referred to as EEG slow-wave activity. Because the EEG amplitude was subject to the influences of subtle variations of EEG electrode placement, the average total power during NREMS on each saline injection day was normalized to 100. The relative changes of EEG power from the baseline were calculated for data collected after TNFα or IL-1β treatment.
Sleep and EEG spectrum data were analyzed with two-way ANOVA for repeated measures and followed by a Student–Newman–Keuls (SNK) multiple-comparison test.
RESULTS
TNFR-KO mice sleep less than strain controls
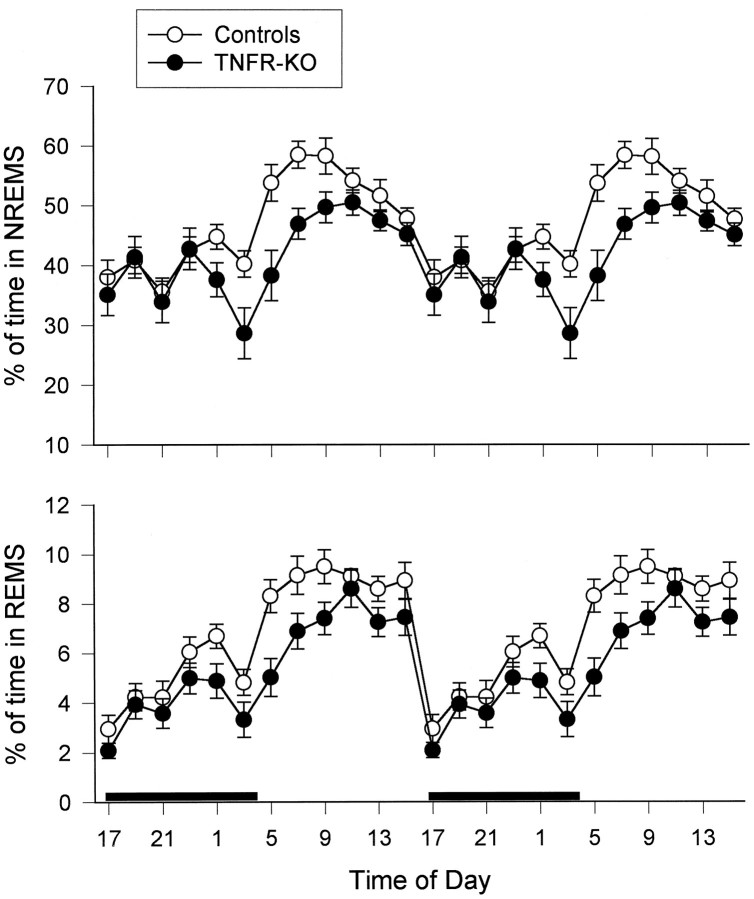
Compared with control mice, the TNFR-KO mice had significantly less NREMS [F(1,25) = 5.86; p< 0.05] and REMS [F(1,25) = 10.66;p < 0.004] (Fig. 1). These decreases in sleep occurred primarily during the light period and in the last few hours during the dark period. There was a significant treatment and time interaction for NREMS [F(3,75) = 4.10;p < 0.01]; SNK multiple-comparison tests indicated that NREMS was decreased significantly in TNFR-KO mice compared with the controls during the first 6 hr of the light period [q(4,75) = 5.299; p < 0.01]. The TNFR-KO mice also had fewer NREMS episodes of shorter duration than the control mice during the period in which they slept less than strain controls, although neither difference was statistically significant. The TNFR-KO mice also had longer REMS to REMS cycles than the controls, although this difference also did not reach statistical significance. The TNFR-KO mice recovered normally from surgery without any signs of infection around the wound. Although not quantified, waking behavior (eating, drinking, and motor activity) and sleep postures of the TNFR-KO mice seemed normal. Similarly, no atypical features in the EEG, EMG, or EEG-FFT transformations were apparent in recordings from either controls or TNFR-KO mice.
Fig. 1.
Double plot of 24 hr sleep patterns in control (n = 13) and TNFR-KO (n = 14) mice. The amounts of NREMS (top) and REMS (bottom) are expressed as percent of time in each state. Each data point is a 2 hr average. The vertical barsindicate SE. The black bar in the bottom panel indicates the dark period.
TNF55-kDR is involved in TNFα-induced sleep
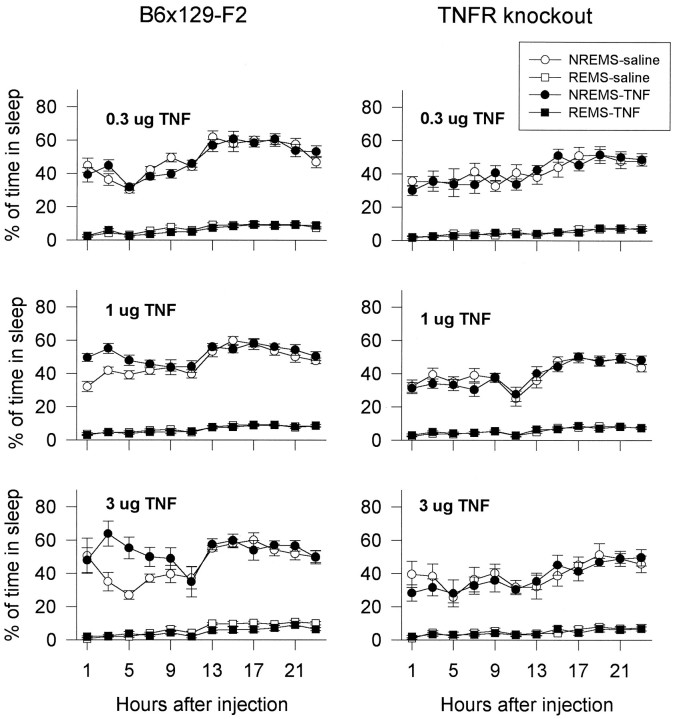
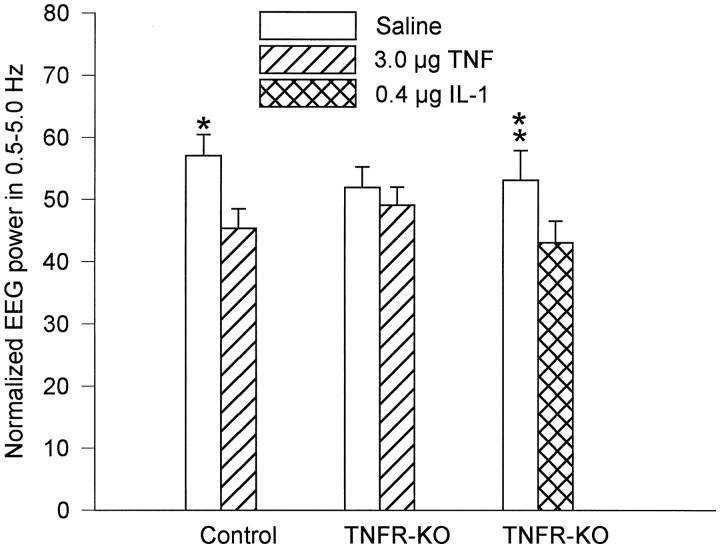
The effects of TNFα on sleep were examined in the control and TNFR-KO mice. Dose-dependent increases in NREMS were observed in control mice after TNFα treatment, whereas TNFR-KO mice did not respond to TNFα treatment (Fig. 2; see statistical details in figure legend). The increases of NREMS occurred during the first 9 hr after 1.0 and 3.0 μg of TNFα injection; they result from an increase in the number, but not the duration, of NREMS episodes. The number of NREMS episodes occurring in the first 6 hr (postinjection) was almost doubled after 3.0 μg of TNFα (62.50 ± 6.69 vs 121.17 ± 13.87; F(3,15) = 5.26;p < 0.02 for treatment and time interaction;q(8,15) = 6.7630; p < 0.01). The high dose (3.0 μg) of TNF also decreased the amount of REMS in control mice during the light period; this REMS inhibitory activity of TNFα was absent in the TNFR-KO mice (Fig. 2). The effects of TNFα on EEG slow-wave activity (0.5–5.0 Hz) were also determined. The EEG slow-wave activity was not influenced by the low doses of TNFα (0.3 and 1.0 μg), but it was decreased during the first 6 hr after the injection of 3.0 μg of TNFα (Fig. 3; see statistical details in figure legend). TNFα did not have any effects on EEG slow wave activity in TNFR-KO mice (Fig. 3).
Fig. 2.
Effects of various doses of mouse recombinant TNFα in control and TNFR-KO mice. Control mice (left) had a significant increase in NREMS after 1.0 μg [F(1,12) = 12.01; p < 0.005] and 3.0 μg [F(1,5) = 7.34;p < 0.05 for interaction;q(1,5) = 4.932 for hours 1–12] of TNFα and a significant decrease in REMS after 3.0 μg of TNFα [F(1,5) = 26.37; p < 0.004 for interaction; q(2,5) = 4.357;p < 0.05 for hours 13–24 ]. TNFR-KO mice (right) did not respond to TNFα treatments.
Fig. 3.
Effects of TNFα and IL-1β on EEG slow-wave activity (SWA). The average of total power (0.5–35.0 Hz) during NREMS on saline injection day was normalized to 100. *TNFα significantly decreased EEG SWA in control mice but not in TNFR-KO mice during the first 6 hr after injection [F(3,15) = 10.559; p = 0.0006; q(8,15) = 7.574;p < 0.01]. **IL-1β significantly decreased EEG SWA [F(3,15) = 6.68; p< 0.005 for treatment and time interaction;q(4,15) = 6.417; p < 0.01].
IL-1β induces NREMS in TNFR-KO mice
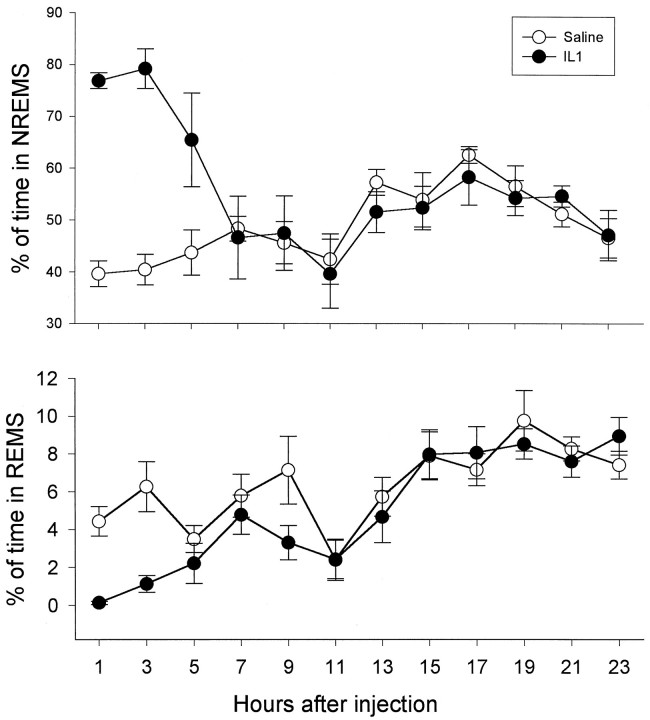
Although TNFR-KO mice failed to respond to TNFα, they displayed a robust increase in NREMS and a decrease in REMS after IL-1β treatment (Fig. 4). IL-1β significantly increased the number [F(3,15) = 6.02; p < 0.01 for treatment and time interaction; q(6,15)= 6.2303; p < 0.01] and the duration [F(3,15) = 5.489; p < 0.01 for treatment and time interaction; q(8,15) = 5.658;p < 0.05] of NREMS episodes in TNFR-KO mice during the first 6 hr after injection. IL-1β also significantly decreased the number [F(3,15) = 4.47; p< 0.02 for treatment and time interaction;q(3,15) = 4.00; p < 0.05] and duration [F(3,15) = 6.47; p < 0.005 for treatment and time interaction;q(4,15) = 6.5368; p < 0.01] of REMS during the same period (data not shown). The EEG slow wave activity was decreased during the first 6 hr after the injection of IL-1 (Fig. 3).
Fig. 4.
Effects of 0.4 μg IL-1 on sleep in TNFR-KO mice. IL-1 significantly increased NREMS [F(1,5)= 11.13; p < 0.025] and decreased REMS [F(1,5) = 8.31; p < 0.05 for treatment and time interaction;q(2,5) = 4.188; p < 0.05 for nighttime].
DISCUSSION
Results presented here extend the list of species, to include mice, in which TNFα and IL-1β are somnogenic. Although abundant evidence suggests that TNF is an important humoral agent involved in sleep regulation, the receptor mechanism for its sleep-promoting effects was heretofore unknown. The TNFR-KO mice fail to respond to TNFα. This was probably not attributable to alterations in physiological sleep in TNFR-KO mice, because NREMS was increased by TNFα in control mice during the first few hours of the dark period, the time during which the control and TNFR-KO mice have similar amounts of sleep. Furthermore, such failure strongly suggests that the sleep-promoting actions of TNFα are mediated by 55, but not 75 kDa, receptors. The importance of this finding is twofold. First, localization of TNF55 kDa receptors in the brain may provide information about the sleep-promoting sites of TNFα. Second, the 55 kDa and 75 kDa receptors mediate distinct actions, probably because of the differences in their intracellular domains. Knowledge of the TNF55 kDa receptor-mediated actions may provide important clues for the understanding of the intracellular mechanisms of sleep regulation.
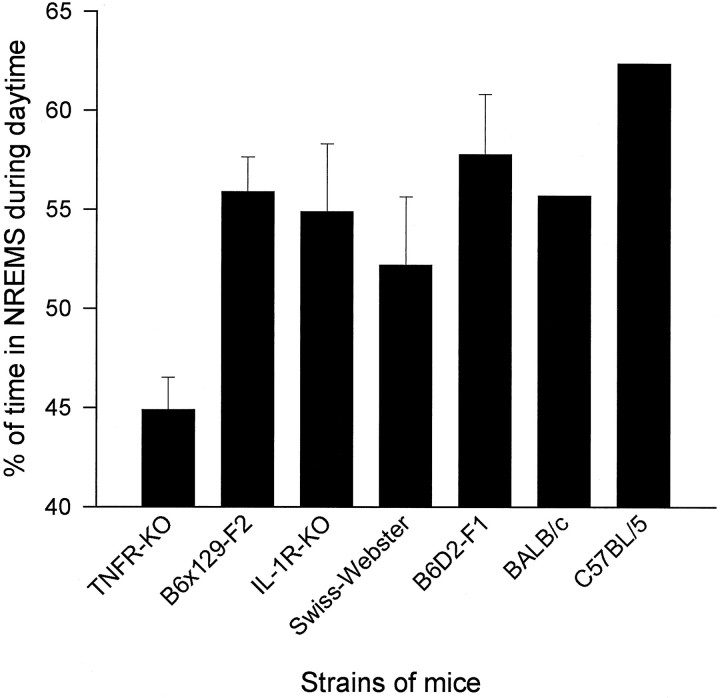
The finding that the TNFR-KO mice have less NREMS than their strain controls (44.9 vs 55.9%) provides further evidence that endogenous TNF is involved in physiological sleep regulation. The B6 × 129-F2 mice were chosen as a control strain, because this is the background on which the KO strain was developed. However, the genetic mix produced when selecting for a KO is different from a random mix produced by crossing two lines (Gerlai, 1996). Results, therefore, need to be interpreted with caution. Unfortunately, there is no ideal control strain; nevertheless, comparisons to a variety of strains of mice support the hypothesis that the TNFR-KO mice have a deficit in NREMS during daylight hours. Thus, other strains of mice recorded from in our laboratory [Swiss-Webster (Fang et al., 1996a), B6D2F1 (Zhang et al., 1996) and an IL-1 receptor KO strain (Fang et al., 1996b)] all have more NREMS during daylight hours than the TNFR-KO strain (Fig.5). Furthermore, the TNFR-KO mice have less NREMS than C57BL/6 and BALB/c mice (Roussal et al., 1984) (Fig. 5). The observation that TNFR-KO mice slept less than the controls primarily during the light period is consistent with the findings that the levels of TNFα mRNA (Bredow et al., 1996) and TNFα (Floyd et al., 1996) in the hypothalamus and hippocampus are greater during the light period than during the dark period in rats. Rats, like mice, also spend most of their time sleeping during daylight hours. Nevertheless, the results of the current experiment cannot exclude the possibility that the reduced sleep in TNFR-KO mice might be attributable to other abnormalities in these mice that manifest themselves during daylight hours.
Fig. 5.
Percent of time spent in NREMS in seven different strains of mice. TNFR-KO mice have less NREMS than six other strains during daylight hours. Sleep data for TNFR-KO, B6 × 129-F2, IL-1 type I receptor KO (IL-1R-KO), Swiss–Webster, and B6D2-F1 mice are from our laboratory. Data for C57BL/6 and BALB/c mice were from another laboratory (Roussel et al., 1984), and the SE were not available.
The decrease of REMS in TNFR-KO mice was not expected, because REMS was not changed after low doses of TNFα and was actually inhibited after high doses of TNFα in controls. Administration of high doses of exogenous TNFα also inhibits REMS in rabbits (Kapas et al., 1992), whereas inhibition of endogenous TNF using a soluble TNF receptor fragment inhibits NREMS but enhances REMS after sleep deprivation in rabbits (Takahashi et al., 1996c). It is not clear whether the lower amount of REMS in TNFR-KO mice is an indirect result of deficits in NREMS thereby limiting the normal access route to REMS via NREMS or is attributable to the lack of direct TNF actions on REMS-promoting mechanisms.
EEG slow-wave amplitudes decreased after TNFα (in control mice) and IL-1β (in TNFR-KO mice) treatments during the first 6 hr, after injection. In rats (Tobler et al., 1984; Opp et al., 1991) and rabbits (Krueger et al., 1984) these cytokines induce increases in EEG slow-wave activity after intracerebroventricular injections. Furthermore, during NREMS after sleep deprivation EEG slow-wave activity increases (Pappenheimer et al., 1975); this latter result is interpreted to indicate that EEG slow-wave activity is a measure of sleep intensity (Borbély and Tobler, 1989). Current results could be attributable to the route of administration. For example, we found recently that intraperitoneal injections of IL-1β into rats also decreased EEG slow-wave activity, although it did induce increases in NREMS duration (Hansen and Krueger, unpublished observation). Species differences may also be important. For example, although administration of exogenous TNFα induces fever in rats and rabbits, it actually decreases body temperature in mice (Kozak et al., 1995). Because EEG amplitudes increase with ambient temperature and brain temperature, it is possible that TNFα may suppress EEG amplitudes by decreasing body temperature. EEG slow-wave activity in mice is greater during the dark period, when mice spend most of their time awake, than in the light period when mice spend most of their time asleep (Tobler et al., 1996). Furthermore, cytokines can have divergent effects on NREMS duration and EEG slow-wave activity depending on dose and time of day. In rats, high doses of IL-1β reduce EEG slow-wave activity during the dark period and increase it during the light period, whereas low doses of IL-1β increase EEG slow-wave activity during both dark and light periods (Opp et al., 1991). Additional data also indicate that duration of NREMS and EEG slow-wave amplitudes are separable. Lesions of the hypothalamic preoptic area reduce NREMS duration and EEG slow-wave amplitude (Shoham et al., 1987b); after 8 d of recovery NREMS duration recovers, but EEG slow-wave activity remains depressed. Similar results were obtained after immunotoxin lesions of basal forebrain cholinergic neurons (Kapás et al., 1996). Rats fed a cafeteria diet increase duration of NREMS but decrease EEG slow-wave activity (M. Hansen, L. Kapás, and J. M. Krueger, unpublished observation). Finally, in rats, restricting food availability to daytime hours reverses the circadian rhythm of NREMS duration but does not affect the circadian rhythm of EEG slow-wave activity (Roky et al., 1995).
Sleep is regulated by multiple substances, and there are complex interactions among these humoral agents (Krueger et al., 1994). IL-1β and TNFα are two of the best characterized sleep-regulatory substances. IL-1β and TNFα induce the production of each other (Bachwich et al., 1986; Dinarello et al., 1986; Philip and Epstein, 1986). Inhibition of IL-1β attenuates TNFα-induced sleep, whereas inhibition of TNFα attenuates IL-1β-induced sleep (Takahashi et al., 1996a). However, it is not clear whether TNFα is necessary for the sleep-promoting effects of IL-1β and vice versa. The result that recombinant murine IL-1β induces robust increases in NREMS in TNFR-KO mice indicates that the TNF 55 kDa receptor is not necessary for the sleep-promoting effects of IL-1β. It is also unlikely that the actions of TNF via its 75 kDa receptor are involved in IL-1β-induced sleep, because the present results indicate that the TNF 75 kDa receptor is not involved in sleep regulation. Therefore, IL-1β is capable of inducing NREMS independently of TNF. We have also shown recently that IL-1 type I receptor KO mice do not have sleep responses to IL-1β but exhibit increased NREMS after TNFα (Fang et al., 1996b). Therefore, the IL-1 type I receptor is not necessary for the sleep-promoting effect of TNFα. Another related question is whether one sleep regulatory substance can compensate for the deficit of another. The result that TNFR-KO mice have less sleep than controls indicates that the deficit in TNF function is not, or at least not completely, developmentally compensated for by other sleep-promoting substances. In addition, we observed that IL-1 type I receptor KO mice have less NREMS compared with strain control mice during the dark period (Fang et al., 1996c), indicating that a deficit in IL-1 function is also not developmentally compensated. The IL-1 type I receptor KO mice also had significantly more REMS during the light period. The results from these two types of KO mice suggest that IL-1 and TNF promote sleep during different times of the day. Alternatively, IL-1β may contribute to physiological sleep both during light and dark periods, but the developmental compensation for the loss of TNFα and IL-1β is different. This latter interpretation is supported by the observation that temporary inhibition of IL-1 with anti-IL-1 antibodies inhibits spontaneous sleep during the light period in rats (Opp and Krueger, 1994). In conclusion, current results strongly support the notion that TNFα is involved in sleep regulation.
Footnotes
This work was supported, in part, by National Institutes of Health Grants NS-31453 and NS-25378 and the Office of Naval Research Grant N00014–90-J-1069. We thank Dr. Peggy Danneman for breeding the TNF-KO mice and Dr. W. Lesslauer for providing them. We also thank Mrs. Maria Swayze-Nations for her assistance in the preparation of this manuscript.
Correspondence should be addressed to Dr. James M. Krueger, Department of Physiology and Biophysics, The University of Tennessee, Memphis, 894 Union Avenue, Memphis, TN 38163.
REFERENCES
- 1.Bachwich PR, Chensue SW, Larric JW, Kunkel SL. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986;136:94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- 2.Borbély AA, Tobler I. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev. 1989;69:605–670. doi: 10.1152/physrev.1989.69.2.605. [DOI] [PubMed] [Google Scholar]
- 3.Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. Distribution and characterization of tumor necrosis factor-α-like immunoreactivity in the murine central nervous system. J Comp Neurol. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- 4.Bredow S, Obál F, Jr, Guha-Thakurta N, Taishi P, Krueger JM. Hypothalamic GHRH mRNA and TNFα mRNA levels are higher during the day than night. Soc Neurosci Abstr. 1996;22:146. [Google Scholar]
- 5.Darko DF, Miller JC, Gallen C, White W, Koziol J, Brown SJ, Hayduk R, Atkinson JH, Assmus J, Munnell DT, Naitoh P, McCutchen JA, Mitler MM. Sleep electroencephalogram delta frequency amplitude, night plasma levels of tumor necrosis factor α and human immunodeficiency virus infection. Proc Natl Acad Sci USA. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, Jr, O’Connor JV. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang J, Tooley D, Gatewood C, Renegar KB, Majde JA, Krueger JM. Differential effects of total and upper airway influenza viral infection on sleep in mice. Sleep. 1996a;19:337–342. [PubMed] [Google Scholar]
- 8.Fang J, Renegar KB, Kapás L, Wang Y, Krueger JM. The IL-1 type I receptor and the TNF 55 kDa receptor are involved in sleep regulation. J Sleep Res [Suppl] 1996b;5:62. [Google Scholar]
- 9.Fang J, Wang Y, Krueger JM. The TNF 55 kDa receptor and the IL-1 type receptor are involved in physiological sleep regulation. Soc Neurosci Abstr. 1996c;22:147. [Google Scholar]
- 10.Floyd RA, Krueger JM (1997) Diurnal variations of TNFa in the brain. NeuroReport, in press. [DOI] [PubMed]
- 11.Gerlai R. Gene targeting in neuroscience: the systemic approach. Trends Neurosci. 1996;19:188–189. [Google Scholar]
- 12.Hohagen F, Timmer J, Weyerbrock A, Fritsch-Montero R, Ganter U, Krieger S, Berger M, Bauer J. Cytokine production during sleep and wakefulness and its relationship to cortisol in healthy humans. Neuropsychobiology. 1993;28:9–16. doi: 10.1159/000118993. [DOI] [PubMed] [Google Scholar]
- 13.Hohmann H-P, Remy R, Brockhaus M, van Loon APGM. Two different cell types have different major receptors for human tumor necrosis factor (TNFα). J Biol Chem. 1989;264:14927–14934. [PubMed] [Google Scholar]
- 14.Hohmann H-P, Brockhaus M, Bauerle PA, Remy R, Kolbeck R, van Loon APGM. Expression of the type A and B tumor necrosis factor (TNF) receptor is independently regulated, and both receptors mediate activation of the transcription factor NF-kB. J Biol Chem. 1990;265:22409–22417. [PubMed] [Google Scholar]
- 15.Hunt JS, Chen HL, Hu XL, Chen TY, Morrison DC. Tumor necrosis factor-alpha gene expression in the tissue of normal mice. Cytokine. 1992;4:340–346. doi: 10.1016/1043-4666(92)90076-4. [DOI] [PubMed] [Google Scholar]
- 16.Kapás L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer JM, Krueger JM. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-α and TNF-α fragments. Am J Physiol. 1992;263:R708–R715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 17.Kapás L, Obál F, Jr, Book AA, Schweitzer JB, Wiley RG, Krueger JM. The effects of immunolesions of nerve growth factor-receptive neurons by 192 IgG-saporin on sleep. Brain Res. 1996;712:53–59. doi: 10.1016/0006-8993(95)01431-4. [DOI] [PubMed] [Google Scholar]
- 18.Kozak W, Conn CA, Klir JJ, Wong GH, Kluger MJ. Soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol. 1995;269:R23–R29. doi: 10.1152/ajpregu.1995.269.1.R23. [DOI] [PubMed] [Google Scholar]
- 19.Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984;246:R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- 20.Krueger JM, Toth LA, Floyd R, Fang J, Kapás L, Obál F., Jr Sleep, microbes and cytokines. Neuroimmunomodulation. 1994;1:100–109. doi: 10.1159/000097142. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neutrotopic virus. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nistico G, DeSarro G, Rotiroti D. Behavioral and electrocortical spectrum poer changes of interleukins and tumor necrosis factor after microinjection into different areas of the brain. In: Smirne, et al., editors. Sleep, hormones and immunological system. Masson; Milan: 1992. pp. 11–22. [Google Scholar]
- 23.Opp MR, Krueger JM. Anti-interleukin-1β reduces sleep and sleep rebound after sleep deprivation in rats. Am J Physiol. 1994;266:R688–R695. doi: 10.1152/ajpregu.1994.266.3.R688. [DOI] [PubMed] [Google Scholar]
- 24.Opp MR, Obál F, Jr, Krueger JM. Interleukin-1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991;260:R52–R58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- 25.Pappenheimer JR, Koski G, Fencyl V, Karnovsky ML, Krueger JM. Extractions of sleep-promoting factors from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 26.Philip R, Epstein LB. Tumor necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986;323:86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- 27.Puccioni-Sohler M, Rieckmann P, Kitze B, Lange P, Albrecht M, Flegenhauer K. A soluble form of tumor necrosis factor receptor in cerebrospinal fluid and serum of HTLV-1-associated myelopathy and other neurological diseases. Neurology. 1995;242:239–242. doi: 10.1007/BF00919597. [DOI] [PubMed] [Google Scholar]
- 28.Roky R, Kapás L, Fang J, Krueger JM. Restricted feeding to the light period affects the circadian rhythm of sleep and brain temperature in the rat. Sleep Res. 1995;24A:540. [Google Scholar]
- 29.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel R, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumor necrosis factor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 30.Roussel B, Turrillot P, Kitahama K. Effects of ambient temperature on the sleep-waking cycle in two strains of mice. Brain Res. 1984;294:67–73. doi: 10.1016/0006-8993(84)91310-6. [DOI] [PubMed] [Google Scholar]
- 31.Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, Wong GHW, Gatanaga T, Granger GA, Lentz R, Raab H, Kohr WJ, Goeddel DV. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- 32.Schutze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leukoc Biol. 1994;56:533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 33.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin-1 enhances slow-wave sleep. Am J Physiol. 1987a;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- 34.Shoham S, Blatteis CM, Krueger JM. Effects of preoptic area lesions on muramyl dipeptide-induced sleep and fever. Brain Res. 1987b;476:396–399. doi: 10.1016/0006-8993(89)91267-5. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi S, Kapás L, Fang J, Krueger JM. An anti-tumor necrosis factor antibody suppresses sleep in rats and rabbits. Brain Res. 1995a;690:241–244. doi: 10.1016/0006-8993(95)00609-t. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi S, Tooley DD, Kapás L, Fang J, Seyer JM, Krueger JM. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Pflügers Arch. 1995b;431:155–160. doi: 10.1007/BF00410186. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Kapás L, Fang J, Seyer JM, Krueger JM. Somnogenic relationships between interleukin-1 and tumor necrosis factor. Sleep Res. 1996a;25:31. [Google Scholar]
- 38.Takahashi S, Kapás L, Krueger JM. A tumor necrosis factor (TNF) receptor fragment attenuates TNFα and muramyl dipeptide-induced sleep and fever in rabbits. J Sleep Res. 1996b;5:106–114. doi: 10.1046/j.1365-2869.1996.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S, Kapás L, Seyer JM, Wang Y, Krueger JM. Inhibition of tumor necrosis factor attenuates physiological sleep in rabbits. NeuroReport. 1996c;7:642–646. doi: 10.1097/00001756-199601310-00063. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi S, Kapás L, Seyer JM, Wang Y, Krueger JM (1997) Inhibition of tumor necrosis factor prevents warming-induced sleep responses in rabbits. Am J Physiol, in press. [DOI] [PubMed]
- 41.Tchelingerian J, Vignais LL, Jacque C. TNF alpha gene expression is induced in neurons after a hippocampal lesion. NeuroReport. 1994;5:585–588. doi: 10.1097/00001756-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Tobler I, Borbély AA, Schwyzer M, Fontana A. Interleukin-1 derived from astrocytes enhances slow-wave activity in sleep EEG of the rat. Eur J Pharmacol. 1984;104:191–192. doi: 10.1016/0014-2999(84)90391-1. [DOI] [PubMed] [Google Scholar]
- 43.Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicker T, Moser M, Oesch B, McBride PA, Manson JC. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- 44.Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on production of cytokines in humans. Psychosom Med. 1994;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Vilcek J, Lee TH. Tumor necrosis factor. J Biol Chem. 1991;266:7313–7316. [PubMed] [Google Scholar]
- 46.Yamasu K, Shimada Y, Sakaizumi M, Soma G, Mizuno D. Activation of the systemic production of tumor necrosis factor after exposure to acute stress. Eur Cytokine Netw. 1992;3:391–398. [PubMed] [Google Scholar]
- 47.Zhang J, Obál F, Jr, Fang J, Collins BJ, Krueger JM. Sleep is suppressed in transgenic mice with a deficiency in the somatotropic system. Neurosci Lett. 1996;220:97–100. doi: 10.1016/s0304-3940(96)13232-8. [DOI] [PubMed] [Google Scholar]