Abstract
Microglial cells are activated in response to brain insults; the mechanisms of this process are not yet understood. One of the important signaling mechanisms that might be involved in microglia activation is related to changes in the intracellular calcium concentration ([Ca2+]i). Using fluo-3 microfluorimetry, we have found that external application of the complement fragment C5a (4–10 nm) induced [Ca2+]ielevation in microglial cells in situ in corpus callosum slices. Similarly, application of complement fragments C5a (0.1–10.0 nm) or C3a (100 nm) generates biphasic [Ca2+]i transients composed of an initial peak followed by a plateau in cultured microglia. Incubation of microglial cells for 30 min with pertussis toxin (PTX; 1 μg/ml) inhibited both C5a- and C3a-triggered [Ca2+]iresponses, suggesting the involvement of PTX-sensitive G-proteins in the signal transduction chain. Removal of Ca2+ ions from the extracellular solution eliminated the plateau phase and limited the response to the initial peak. The restoration of the extracellular Ca2+ concentration within 30–60 sec after the beginning of the complement fragment-induced [Ca2+]ielevation led to the recovery of the plateau phase. Inhibition of the endoplasmic reticulum Ca2+ pumps with 500 nmthapsigargin transiently increased the [Ca2+]i and blocked the [Ca2+]i signals in response to subsequent complement fragment application. Our data suggest that complement factors induce [Ca2+]i responses by Ca2+ release from internal pools and subsequent activation of Ca2+ entry controlled by the filling state of the intracellular Ca2+ depots.
Keywords: microglia, complement fragments, [Ca2+]i, InsP3-induced Ca2+ release, G-proteins, capacitative calcium entry
Microglial cells are the resident brain macrophages that determine the immune responses of the CNS. Damage in the nervous system leads to activation of the resting microglia, resulting in a sequence of morphological, immunological, and functional changes (Thomas, 1992; Perry et al., 1993). The intimate nature of the signaling events underlying microglial activation is still unknown. Various pathological processes in the CNS (e.g., multiple sclerosis, inflammatory processes, Alzheimer’s disease) involve the activation of the complement signaling system (Morgan, 1994). The generation of complement fragments (anaphylatoxins) C3a and C5a is associated with complement activation, which plays an important role in immunological responses (for review, see Goldstein, 1988). Both C3a and C5a complement fragments act as specific chemotaxins for leukocytes and in addition may affect other immunocompetent cells, such as microglia. The action of anaphylatoxins is achieved via activation of specific receptors, coupled presumably with pertussis toxin (PTX)-sensitive G-proteins (Gerardy-Schahn et al., 1989; Gerard and Gerard, 1991,1994). Activation of C5a and C3a receptor subtypes evoked elevation of cytoplasmic free calcium concentration ([Ca2+]i) in neutrophiles (Monk and Partridge, 1993; Norgauer et al., 1993). The activation of C5a receptors was reported to initiate phospholipase C-controlled inositol (1,4,5)-trisphosphate (InsP3) production, with subsequent Ca2+ release from internal pools (Norgauer et al., 1993). The intimate mechanism underlying C3a-triggered [Ca2+]i elevation remains controversial: one study revealed the stimulation of InsP3 turnover with subsequent intracellular Ca2+ release (Klos et al., 1992), whereas other studies demonstrated that C3a activates plasmalemmal Ca2+ influx (Norgauer et al., 1993).
It is widely accepted that one of the important and ubiquitous determinants of intracellular signaling is closely associated with the fluctuations of [Ca2+]i (Berridge, 1993;Clapham, 1995a; Kostyuk and Verkhratsky, 1995). The mechanisms of calcium signal generation have been characterized in detail for various mammalian cells, including secretory cells, blood cells, myocytes, neurones, and various populations of glial cells (Kostyuk and Verkhratsky, 1994, 1995; Petersen et al., 1994; Pozzan et al., 1994;Verkhratsky and Kettenmann, 1996); however, the mechanisms of [Ca2+]i homeostasis in microglial cells are not well characterized. A limited number of observations demonstrate that some agonists, including ATP (Walz et al., 1993), carbachol (Whittemore et al., 1993), and bacterial endotoxins (Bader et al., 1995), elevate [Ca2+]i in cultured microglia, whereas platelet-activating factor was reported to induce [Ca2+]i elevation in both primary-cultured and immortalized microglial cells (Righi et al., 1995). Conceptually, Ca2+ ions might be delivered into the cytoplasm via (1) opening of ligand- or voltage-activated plasmalemmal Ca2+channels (Hofmann et al., 1994), (2) liberation of Ca2+from internal pools attributable to the activation of intracellular Ca2+ release channels (Kostyuk and Verkhratsky, 1994, 1995;Pozzan et al., 1994), and (3) activation of newly discovered plasmalemmal calcium permeability, controlled by the filling state of internal Ca2+ pools (“capacitative calcium entry”) (Putney, 1990; Penner et al., 1993; Fasolato et al., 1994).
In the present work we have used microfluorimetric [Ca2+]i recordings to examine the mechanisms involved in the agonist-induced [Ca2+]isignaling in microglial cells. We have found that complement fragment C5a transiently elevated [Ca2+]i in ameboid microglial cells on the surface of acutely prepared corpus callosum slices; the similar [Ca2+]i elevation was observed in microglial cells in culture that were challenged with complement fragments C5a and C3a. This complement fragmentsinduced [Ca2+]i signaling was composed of Ca2+ release from thapsigargin-sensitive internal calcium stores, and additional plasmalemmal Ca2+entry activated the depletion of internal Ca2+ depots.
Preliminary results have been published previously in abstract form (Möller et al., 1996)
MATERIALS AND METHODS
Culture of microglial cells. Microglia cells were prepared from cortex of newborn Naval Medical Research Institute-mice, essentially as described previously (Giulian and Baker, 1986; Frei et al., 1986). In brief, cortical tissue was carefully freed from major blood vessels and meninges. Tissue was trypsinized for 2 min, triturated with a fire-polished pipette, and washed twice. The cortical cells were cultured in DMEM supplemented with 10% fetal calf serum, with change of medium every third day. After 9–12 d, microglia were separated from the underlying astrocytic monolayer by gentle agitation, taking advantage of their differential adhesive properties. Microglial cells in the supernatant were washed once and plated on poly-l-lysine-coated glass coverslips at a density of 2 × 104 cells/cm2. Microglial cells were allowed to settle for 20 min. Nonadhesive cells were removed by washing in PBS, and cells were cultured in serum-free medium (Macrophage-SFM, Life Technologies, Gaithersburg, MD) supplemented with 20% astrocyte-conditioned medium. Astrocyte-conditioned medium was collected from astrocytic monolayers maintained in DMEM without serum for 3 d. Supernatant was pooled, centrifuged, filtered, and stored at −70°C until use. Plated cells were kept in the same medium 1–4 d before being used for experiments. To test for the purity of the cultures, the cells were stained with Griffonia simplicifolia isolectin-B4 (IL-B4) (Streit, 1990). Briefly, cultures were fixed in 4% paraformaldehyde in PBS for 5 min and after two washes in PBS were incubated with biotinylated IL-B4 for 20 min. Cell-bound lectin was visualized by streptavidin-bodipy. The staining showed that the cultures contained >98% microglia. As described previously (Giulian and Baker, 1986), two different morphological forms of microglial cells could be distinguished: ramified cells and ameboid cells with a flat halo around the soma.
[Ca2+]i measurements in cultured microglial cells. Cultured microglial cells were loaded with Ca2+ indicator by incubation of glass coverslips with adhered cells in normal physiological bathing solution supplemented with 5 μm fluo-3 acetoxymethylester and 0.02% pluronic F-127 for 30 min at room temperature. For measuring the intracellular calcium concentration, a confocal laser scanning microscope (Sarastro 2000; Molecular Dynamics, Sunnyvale, CA) was used. The scanner was mounted on the upright microscope (Axioscope, Zeiss, Oberkochen, Germany) equipped with a 40× magnification, numerical aperture 0.75, water immersion objective. Fluo-3 was excited at the 488 nm line of an argon laser, and the fluorescence was measured at emission wavelength above 510 nm selected with a longpass filter. Images were constructed from 128 × 128 pixels and were acquired every 3 sec. Fluo-3 is a nonratiometric Ca2+ indicator and does not provide absolute Ca2+ concentrations. Therefore, the Ca2+concentration changes are depicted as fluorescence intensity ratioF/F0. The resting fluorescence valueF0 was determined at the beginning of each experiment by averaging 10 images. To exclude possible influences associated with changes in cell volume and/or position of the cell, both of these parameters were monitored throughout the experiments. No obvious changes in shape and location of cells were detected within the time windows used for our experiments. Acquisition of the fluorescence data and image analysis were performed using the Imagespace (Molecular Dynamics) and standard PC evaluation software.
[Ca2+]i recordings from microglial cells in brain slices. The preparations for recordings from microglial cells in situ were prepared essentially as described by Brockhaus et al., 1993. Briefly, forebrain hemispheres were taken from 6- to 9-d-old mice, and coronal slices (200 μm thick) were cut using the vibratome (Vibracut, Plano). For loading the cells with Ca2+-sensitive dye, slices (1 hr after preparation) were incubated in carbogen (95% CO2/5% O2)-gassed bicarbonate-buffered physiological solution supplemented with 5 μm fluo-3 acetoxymethylester and 0.02% pluronic F-127 for 30 min at 37°C. For microfluorimetric recordings, slices were placed into chambers mounted on the stage of an upright microscope connected with a confocal laser scanner. The chamber was perfused continuously with bicarbonate-buffered solution; substances were introduced by changing the perfusate. Changes in fluo-3 fluorescence were measured as described above. Cells were visualized with a 40× magnification water immersion objective (numerical aperture 0.75). Microglial cells on the surface of the corpus callosum slice were identified by their morphological appearance, as described previously (Brockhaus et al., 1993).
Solutions and reagents. All solutions were freshly prepared from refrigerated stock solutions. The standard bathing solution for experiments with cultured cells was composed of (in mm): 150 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, adjusted with NaOH to pH 7.4. The bicarbonate-buffered solution for experiments with corpus callosum slices contained (in mm): 135 NaCl, 5.4 KCl, 2.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1.6 NaH2PO4, 10 glucose, pH 7.4, when continuously gassed with 5% CO2/95% O2. To obtain calcium-free solution, CaCl2 was omitted, MgCl2was increased to 2 mm, and 5 mm EGTA was added. Fluo-3/AM and pluronic F-127 were obtained from Molecular Probes (Eugene, OR). Recombinant human C5a complement fragment as well as all other chemicals were obtained from Sigma (St. Louis, MO).
Preparation of C3a complement fragment. C3a and its desaminated analog C3a-desaminoarginine (C3a-desArg) were purified from human plasma as described previously (Kreitzschmar et al., 1992). Briefly, fresh plasma containing 10 mm EGTA and 1.5 mmdl-2-mercaptomethyl-3-guanidinoethyl-thiopropanoic acid was incubated for 1 hr with zymosan (10 mg/ml) in the presence of 6-aminohexanoic acid (50 mm) and MgCl2 (10 mm) and then adjusted to pH 5.0 with 1 N HCl followed by the addition of the same volume of boiling NaCl solution (150 mm). After cooling, centrifugation, and cation exchange chromatography on S-Sepharose Fast Flow, the C3a/C3a-desArg-containing fractions were identified in a dot-blot assay using the C3a/C3a-desArg-specific monoclonal antibody (mAb) H13 (Burger et al., 1987) and pooled for further purification on a Mono S-column. The eluted fractions gave a single band with a molecular weight of 9 kDa in SDS-PAGE and Western blot. Biological activity was measured with guinea pig platelets in the ATP release assay using luciferin-luciferase in a luminometer. C3a activity was completely blocked by preincubation with mAb H13. C3a-desArg was prepared by the same procedure, but in the absence of the inhibitors, and caused no detectable ATP release. These preparations contained no detectable C5a signal as measured by an ELISA using a mAb to human C5a (kindly provided by Dr. Opperman, Göttingen).
RESULTS
Complement fragment C5a induces complex [Ca2+]i responses in microglia
We have investigated the effects of complement fragment C5a on [Ca2+]i in microglial cells using two experimental models, namely ameboid microglial cells on the surface of acutely prepared early postnatal brain slices and microglial cells in primary culture. In both experimental models, external application of C5a initiated transient increases in [Ca2+]i. In mice at postnatal days 6–10, microglial cells appear specifically at the surface of the corpus callosum slices in the region of the cingulum (Brockhaus et al., 1993). These cells on the surface were preferentially loaded after the slice was incubated with the membrane-permeable form of the Ca2+indicator fluo-3. Using a confocal scanning system, we could selectively record the fluorescence changes in individual microglial cells, because the cells sat on top of the slice surface (Fig.1A). The application of complement fragment C5a (4–10 nm) triggered a transient elevation in [Ca2+]i (Fig. 1B) in 9 of 48 cells studied. The C5a-induced [Ca2+]itransient was characterized by an initial fast peak followed by a slowly decaying plateau.
Fig. 1.

C5a-induced [Ca2+]isignals in microglial cells in brain slices. A, Phase-contrast (left) and fluorescence image (right) taken from the same microglial cell visualized on the surface of a brain slice. Note that the cells sit on top of the slice surface. The focal plane was chosen to selectively record the fluorescence from the microglial cells. B, Representative examples of [Ca2+]i recordings from two different microglial cells in the brain slices in response to bath application of 5 nm C5a.
In cultured microglial cells, C5a-triggered changes in cytoplasmic Ca2+ concentration were observed more frequently, namely in ∼85% of the cells (2 nm, 30 sec application;n = 451). In some cases (5 of 72 preparations), we observed that none of the cells within a given culture preparation responded to C5a; these cells also did not respond to any of the other the agonists that microglial cells commonly respond to, such as ATP (Walz et al., 1993), and were weakly stained with fluo-3. We suspected that these cultures were not in a healthy condition and excluded these data from our analysis. Figure 2Ashows a micrograph of a microglial culture as used for the experiments. Complement fragment-induced [Ca2+]itransients were observed regardless of the morphological variability of the microglial cells. Figure 2B shows a representative trace of the [Ca2+]i responses triggered by C5a in 80% of the cells (n = 451). It consisted of a fast initial increase followed by a second, plateau-like phase of [Ca2+]i elevation. A longer (60 sec) application of C5a substantially prolonged the plateau phase in 92% of the responding cells, whereas the characteristics of the initial peak component of [Ca2+]i response were not changed significantly (n = 63; 83% responding to C5a) (Fig. 2C).
Fig. 2.
Representative examples of C5a-induced [Ca2+]i signals as recorded from different cultured microglial cells. A, Morphological appearance of cultured mouse microglial cells. B, Application of 2 nm C5a for 30 sec induced a biphasic [Ca2+]i transient, with an initial peak [Ca2+]i increase and a subsequent plateau phase of the [Ca2+]i elevation.C, Prolonged [Ca2+]i elevation triggered by 60 sec application of 2 nm C5a, which elicited a sustained plateau phase that did not recover to the resting level during the 5 min recording time. D, Blockade of the 2 nm C5a-induced [Ca2+]i signal by preincubation of cells with PTX (1 μg/ml, 30 min). Note that application of 100 μm ATP was still able to induce a [Ca2+]i elevation.
These experiments demonstrate that microglial cells in situand in culture express receptors for complement fragments. In the following experiments, we studied the mechanism of the complement-induced Ca2+ response. We performed these experiments in culture only, because (1) this preparation allowed us to analyze several cells in one experiment, (2) the probability of cells to respond was higher, and (3) we were limited to our supply of C5a.
The C5a response is mediated by G-proteins and shows a steep concentration dependence
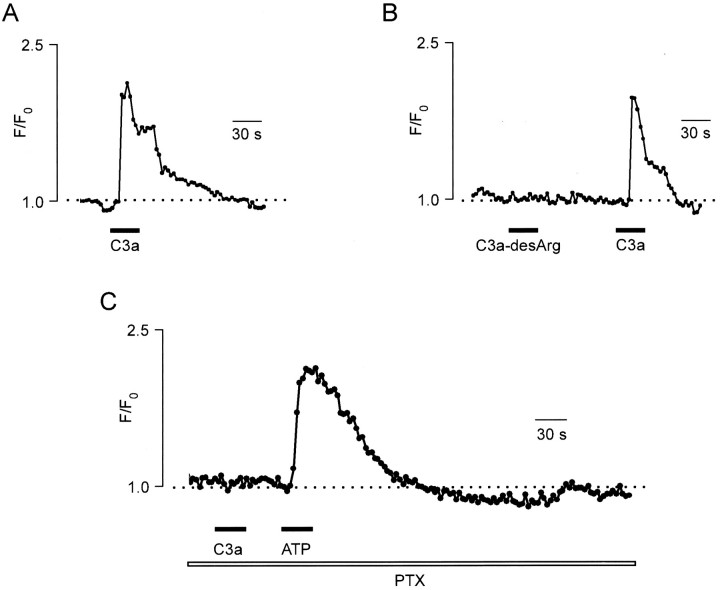
The incubation of microglial cells with PTX (1 μg/ml, 30 min) completely inhibited the generation of the C5a-triggered [Ca2+]i signal (n = 51), indicating the involvement of PTX-sensitive G-proteins. As a control for the responsiveness of the cell, we applied 100 μmATP, which is known to elevate [Ca2+]i in microglial cells via P2 purinoreceptors coupled with PTX-insensitive G-protein subtypes (Dubyak and El-Moatassim, 1993; Walz et al., 1993). As demonstrated in Figure 2D, PTX pretreatment completely abolished the [Ca2+]iresponse to C5a, whereas the ATP-induced [Ca2+]i elevation remained unaffected (n = 51).
The threshold concentration for C5a-induced [Ca2+]i elevation was found at 0.5 nm. Figure 3A shows the representative examples of the [Ca2+]itransients measured from the same cell in response to increasing C5a concentrations, and the dose–response curve is given in Figure3B. When the cells were challenged by low C5a concentrations (0.5–1.0 nm), oscillatory [Ca2+]i responses were often recorded (Fig.3C). The appearance of these [Ca2+]i oscillations in complement-stimulated microglial cells was reversely concentration dependent. At 0.5 nm C5a, the oscillatory [Ca2+]iresponses were predominant, being observed in 67% of the cells (n = 117); at 1 nm, C5a oscillatory [Ca2+]i responses were recorded in only 11% of cells (n = 96) and at 2 nm C5a in 5% of cells tested (n = 451). [Ca2+]i oscillations were never observed at C5a concentrations exceeding 2 nm (n = 137).
Fig. 3.
Concentration dependence of the C5a-mediated [Ca2+]i transients in cultured microglial cells. A, Examples of [Ca2+]itransients recorded from a microglial cell in response to application of 0.5 nm, 1 nm, 5 nm, and 10 nm C5a for 30 sec. B, Average values of the amplitudes of C5a-induced [Ca2+]i transients were obtained from nine experiments, similar to those described inA, and normalized to the amplitude of the response to 10 nm C5a. Error bars represent SD. C, Example of oscillatory [Ca2+]i response triggered by application of 0.5 nm C5a.
The complement factor C3a triggers a similar [Ca2+]i elevation
Application of 100 nm C3a complement fragment induced [Ca2+]i elevations (Fig. 4) in ∼80% of cultured microglial cells (n = 117; we were not able to test C3a effects on an in situ model because of the limited supply of the substance). The [Ca2+]i elevation was specific to C3a; the external application of 100 nm C3a-desArg (the form of C3a with reduced biological activity) did not change [Ca2+]i (n = 34; Fig.4B). Similar to C5a, application of C3a induced a complex [Ca2+]i response showing an initial peak followed by a plateau phase. The C3a-induced [Ca2+]i elevation involved the G-protein signal transduction step as revealed by its sensitivity to PTX (n = 29; Fig. 4C).
Fig. 4.
C3a-induced [Ca2+]isignals in cultured microglial cells. A, Application of 100 nm C3a for 30 sec induced [Ca2+]i elevation composed of an initial peak [Ca2+]i increase and a subsequent plateau phase of the [Ca2+]i elevation.B, Similar to A, [Ca2+]i was recorded from a single microglial cell in response to bath application of 100 nm C3a-desArg (nonactive analog of C3a) and 100 nm C3a. C, Blockade of the C3a-induced [Ca2+]i signal after preincubation of cells with PTX (1 μg/ml, 30 min). Control application of 100 μm ATP still induced [Ca2+]i elevation.
C5a- and C3a-triggered [Ca2+]i signals are modified by external Ca2+
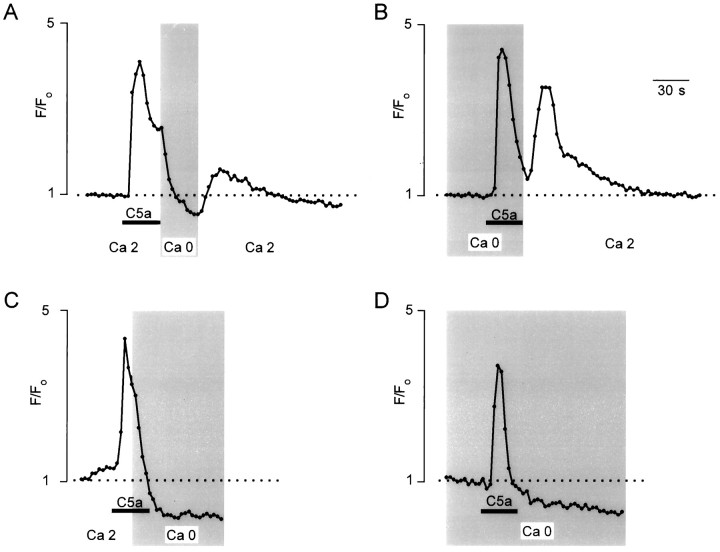
Removal of extracellular calcium (Ca2+o) substantially modified the kinetics of the C3a- and C5a-triggered [Ca2+]i response: the application of both ligands in Ca2+-free external solution still evoked an increase in [Ca2+]i, but it was limited only to the initial peak response (Fig. 5B,C). This was observed in all experiments when complement fragments were applied in Ca2+-free extracellular solution (n = 67 for C5a; n = 21 for C3a). The fast peak in [Ca2+]i elevation recorded in Ca2+-free external solution was usually followed by a long-lasting “undershoot” when [Ca2+]idropped to a subresting level (Figs. 5, 6). Furthermore, in Ca2+-free extracellular solution, complement fragments were able to induce [Ca2+]i elevation only with the first application; each successive application of either C5a or C3a was ineffective (Fig. 5B,C; the control [Ca2+]i transients, in response to successive application of C5a in Ca2+-containing external solution, are shown on Fig. 5A).
Fig. 5.
Complement fragments induced [Ca2+]i elevation in Ca2+-free external solution. A, C5a (2 nm)-induced [Ca2+]i transients were recorded in control conditions (left) and after removal of Ca2+ions from the bath (right). Note that in Ca2+-free extracellular solution, C5a was able to induce [Ca2+]i transients only once.B, The representative example of C3a (100 nm)-induced [Ca2+]i elevation as recorded in Ca2+-free external solution.
Fig. 6.
Modification of the C5a (2 nm)-induced [Ca2+]i signal by external Ca2+.A, Brief removal of the external Ca2+immediately after C5a application leads to an instant drop in [Ca2+]i; reestablishing the external Ca2+ caused the recovery of the sustained component of [Ca2+]i signal. B, Restoration of the physiological external Ca2+ concentration immediately after C5a application in Ca2+-free medium leads to the generation of the plateau phase. C, Persistent removal of the external Ca2+ after C5a application totally abolished the development of the plateau phase of [Ca2+]i signal. D, Application of C5a in Ca2+-free medium evoked only the peak component of the [Ca2+]i signal.
To characterize the Ca2+o-dependence of the plateau phase of the [Ca2+]i transients, we removed Ca2+ ions from the bath at different time points with respect to C5a application (Fig. 6). The brief removal of Ca2+ ions from the external solution immediately after C5a application terminated the development of the plateau phase of the response; reintroducing physiological Ca2+o concentration led to the restoration of the sustained component of the [Ca2+]iresponse (n = 67; Fig. 6A). Similarly, if the Ca2+ ions were removed from the extracellular space before C5a application and external Ca2+ concentration was restored immediately after the end of C5a application, we observed the plateau phase (n = 56; Fig. 6B). In contrast, if Ca2+ ions were removed during C5a application and the external solution was kept Ca2+-free, or if C5a was applied while the cell was bathed continuously in Ca2+-free conditions, we always observed only the peak [Ca2+]i increase (n = 54, Fig. 6C; n = 67, Fig. 6D). It has to be noted that removal of external Ca2+ by itself for 2–5 min sometimes (<10% of observations) caused a small decrease in basal fluo-3 fluorescence; however, this decrease never exceeded 10% in theF/F0 ratio, and therefore it cannot be responsible for the phenomena described above. After the physiological Ca2+ concentration was reestablished, fluo-3 fluorescence returned to the basal level; no obvious increase in [Ca2+]i after Ca2+-free periods were observed.
Thapsigargin blocked the effects of complement fragments and induced plasmalemmal Ca2+ influx
To clarify the mechanisms responsible for the plateau phase of complement fragment-triggered [Ca2+]iresponses, we used thapsigargin to selectively suppress the [Ca2+]i signal component associated with Ca2+ release from intracellular stores. Figure7A illustrates the effect of thapsigargin on complement fragment-induced [Ca2+]i responses in microglial cells. Bath application of 500 nmthapsigargin induced [Ca2+]i elevations with both a fast initial rise and a prominent plateau phase. After the recovery to the basal level, applications of 100 nm C3a (n = 32) or 2 nm C5a (n = 56) were not able to induce [Ca2+]iresponses, indicating that both the peak and the plateau phase of complement-triggered [Ca2+]i elevation are crucially dependent on Ca2+ release from internal stores. Furthermore, manipulation with extracellular Ca2+ modified thapsigargin-triggered [Ca2+]i transients in a similar way as described above for C5a. When thapsigargin was applied in Ca2+-free external solution (Fig. 7B), only a small transient [Ca2+]i elevation was observed (n = 52). Moreover, brief removal of extracellular Ca2+ during the development of thapsigargin-triggered [Ca2+]i transient led to an immediate drop in [Ca2+]i, whereas restoration of external Ca2+ concentration caused the recovery of the plateau phase of the [Ca2+]itransient (n = 73) (Fig. 7C). These results indicate that the plateau phase of both thapsigargin and complement fragment-induced [Ca2+]i responses might be associated with plasmalemmal Ca2+ influx activated by, or as a consequence of, the depletion of internal calcium stores.
Fig. 7.

Thapsigargin inhibits [Ca2+]i mobilizing effects of C5a.A, In control conditions, external application of 2 nm C5a evoked a [Ca2+]i transient in a microglia cell. Subsequently, the cell was incubated with 500 nm thapsigargin. Application of thapsigargin induced [Ca2+]i elevation that returned to the basal level. The succeeding application of 2 nm C5a failed to induce the [Ca2+]i response.B, Similar to A, C5a and thapsigargin were applied while the cell was bathed in Ca2+-free extracellular solution. C, Effect of brief removal of extracellular Ca2+ on a thapsigargin (500 nm)-induced [Ca2+]i transient. Note that changing to the Ca2+-free extracellular media caused an immediate drop in the [Ca2+]ilevel, whereas restoration of the external Ca2+concentration to physiological levels resulted in recovery of the plateau phase of a thapsigargin-triggered [Ca2+]i transient.
DISCUSSION
Microglial cells express complement anaphylatoxin receptors linked to second-messenger pathways
Here we show that the anaphylatoxins C3a and C5a induce the generation of cytoplasmic calcium elevation in microglial cells. The complement fragments C5a and C3a triggered [Ca2+]i in cultured microglial cells; similarly, C5a induced [Ca2+]i elevationin situ. The considerably smaller proportion of cells responding to C5a in situ might reflect differences in experimental techniques, e.g., limitations for bath application attributable to irregular slice surface or C5a inactivation by factors released from the tissue. Alternatively, differences in the activation stages of the cells in situ versus in vitro could determine their sensitivity to C5a. Nevertheless, our study demonstrated clearly that microglial cells in situ are capable of expressing C5a receptors that may carry relevant functions in brain microglia.
Receptors for C5a and C3a anaphylatoxins have been found previously and characterized molecularly in various immune cells, vascular endothelial cells, hepatocytes, epithelial cells, lung vascular smooth muscle cells, etc. (for review, see Wetsel, 1995). The molecular cloning of the C5a receptor revealed its similarity with the superfamily of seven-transmembrane segment G-protein-coupled metabotropic receptors (Gerard and Gerard, 1994). Recently, the expression of C5a receptors was demonstrated in human astroglial and microglial cells (Lacy et al., 1995), and a possible functional significance was revealed, based on C5a-induced changes in microglial motility (Yao et al., 1990; Nolte et al., 1996). It was shown previously that the activation of complement fragment receptors triggers [Ca2+]i responses in both neutrophiles (Monk and Partridge, 1993; Norgauer et al., 1993) and eosinophils (Elsner et al., 1994). These [Ca2+]i signals resulted from either InsP3-mediated intracellular Ca2+ release or plasmalemmal Ca2+ influx (cf. Klos et al., 1992; Norgauer et al., 1993). In this paper, we demonstrate that microglial cells express functionally active receptors for C5a and C3a linked to the generation of intracellular Ca2+ signals. In concordance with previous observations in immune cells (Gerard and Gerard, 1994;Vanek et al., 1994), the complement fragment-induced [Ca2+]i response in microglia is preceded by the activation of PTX-sensitive G-proteins. Our data suggest that both the C5a and the C3a receptors converge to the same intracellular signaling pathway and initiate cytoplasmic Ca2+ signaling. Similar to that described for purino- and adrenoreceptors in astrocytes (Shao and McCarthy, 1995), the C5a- or C3a-induced Ca2+signal in the microglial cells is characterized by a steep concentration dependence.
Complement fragments trigger two distinct mechanisms for [Ca2+]i elevation
The two components of the complement fragment-induced [Ca2+]i signals, namely the initial peak and the plateau phase, are distinguished by their sensitivity to extracellular [Ca2+]. The plateau phase depended on the presence of Ca2+ ions in the external milieu in contrast to the initial peak. This implies that complement fragments activate two distinct mechanisms for Ca2+delivery into the microglial cytoplasm: the peak response is associated with Ca2+ release from internal structures, and the plateau phase depends on plasmalemmal Ca2+ entry. The thapsigargin sensitivity of the initial peak indicates that it is attributable to Ca2+ release from InsP3-sensitive Ca2+ stores, as described for many types of nonexcitable cells (Petersen et al., 1994). The transient nature of this Ca2+ response presumably reflects the rapid depletion of the internal stores. Indeed, only the first application of C3a or C5a in a Ca2+-free external solution induced a Ca2+transient, whereas subsequent ligand applications were ineffective, indicating the exhaustion of the stores.
The second component, the plateau phase, was dependent on extracellular Ca2+, suggesting that the [Ca2+]i increase was caused by an influx through the plasma membrane. Two possible signals could trigger the plasmalemmal Ca2+ conductance: (1) the complement receptors could directly activate Ca2+- channels or carriers, or (2) the depleted Ca2+ stores could release a signal to activate a plasmalemmal Ca2+ influx. Our data support the second mechanism. (1) The intracellular Ca2+ release is a prerequisite for the generation of the plateau, and (2) the plateau phase could be transiently interrupted by removal of extracellular Ca2+. The entire response was prolonged by the time of Ca2+ removal, indicating that a defined amount of Ca2+ is required to refill the stores, and (3) a Ca2+ increase with a similar behavior could be elicited by depleting stores with thapsigargin. Similarly, transient removal of extracellular Ca2+ prolonged the thapsigargin response.
Agonist-induced [Ca2+]i responses consisting of initial transient peak followed by a plateau-like phase have been found in various nonexcitable cells. Several lines of evidence have demonstrated that the first phase of the [Ca2+]i response is determined by Ca2+ release from internal stores. The depletion of the internal Ca2+ pools, in turn, activates the Ca2+ entry pathway (capacitative Ca2+ entry) (Putney, 1990). Ca2+ channels controlled by the filling state of intracellular Ca2+ pools have been identified recently in various nonexcitable cells (Hoth and Penner, 1992, 1993). These Ca2+ release-activated membrane Ca2+channels (code named CRAC channels) represent a family of channels with conductances varying from tens of femtosiemens (Lückhoff and Clapham, 1994) to several picosiemens (Vaca and Kunze, 1994). The signaling pathway between the depleted stores and the CRAC channels is still conjectural. Several possible mechanisms have been hypothesized, including release of a specific diffusable messenger from the depleted stores (Randriamampita and Tsien, 1993; Clapham, 1995b), Ca2+ release-dependent activation of cytoplasmic NO synthase with subsequent generation of cGMP (Xu et al., 1994), or a direct interaction between InsP3-gated Ca2+release channels and plasma membrane (Fasolato et al., 1994). Other, so far unknown mechanisms, however, might be responsible for the generation of the Ca2+ entry activated by the depletion of the intracellular stores. Whatever the mechanisms are, we assume that microglial cells also possess the capacitative Ca2+ entry pathway that plays an important role in shaping the cytoplasmic Ca2+ signals.
Possible functional significance of the complement anaphylatoxin receptors in microglia
The activation of microglia plays a crucial role in the reaction of the brain tissue to damage (Streit et al., 1988;Gehrmann et al., 1995). This activation from the resting form is a multi-step process resulting at the end point in a cytotoxic, phagocytotic cell (Gehrmann and Kreutzberg, 1995). Complement anaphylatoxins may play a role as early activating messengers in brain pathologies, including damage occurring during multiple sclerosis or Alzheimer’s disease (Morgan, 1994). Interestingly, β-amyloid, which accumulates in the plaques during Alzheimer’s disease, activates the complement system in vitro, and there are indications that such activation indeed occurred in this pathology (Rogers et al., 1992;Schultz et al., 1994). Microglial cells accumulate at the plaques, and complement factors could trigger this activation. Components of the complement system can be produced directly by CNS cells, mainly by astrocytes (Barnum, 1995). Microglial cells, in turn, secrete factors such as cytokines, which affect astrocytes. Thus, the complement factors could be a part of the complex paracrine system, which will come into play during pathological events or during immune responses in the CNS.
Footnotes
This research was supported by Deutsche Forschungsgemeinschaft. We are grateful to Dr. R. Penner and Dr. S. Lyons for helpful comments on this manuscript. We thank R. Krauß for excellent technical assistance.
Correspondence should be addressed to Dr. H. Kettenmann, Max-Delbrück Center of Molecular Medicine, Robert-Rössle Strasse 10, 13122 Berlin-Buch, Germany.
REFERENCES
- 1.Bader M-F, Taupenot L, Ulrich G, Aumis D, Ciesielski-Treska J. Bacterial endotoxin induces [Ca2+]i transients and changes the organization of actin in microglia. Glia. 1995;11:336–344. doi: 10.1002/glia.440110406. [DOI] [PubMed] [Google Scholar]
- 2.Barnum SR. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med. 1995;6:132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 4.Brockhaus J, Ilschner S, Banati RB, Kettenmann H. Membrane properties of ameboid microglial cells in the corpus callosum slice from early postnatal mice. J Neurosci. 1993;13:4412–4421. doi: 10.1523/JNEUROSCI.13-10-04412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger R, Bader A, Kirschfink M, Rotter U, Schrod L, Wörner I, Zilow G. Functional analysis and quantification of the complement C3 derived anaphylatoxin C3a with a monoclonal antibody. Clin Exp Immunol. 1987;68:703–709. [PMC free article] [PubMed] [Google Scholar]
- 6.Clapham DE. Calcium signaling. Cell. 1995a;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 7.Clapham DE. Intracellular calcium. Replenishing the stores. Nature. 1995b;375:634–635. doi: 10.1038/375634a0. [DOI] [PubMed] [Google Scholar]
- 8.Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 9.Elsner J, Oppermann M, Czech W, Dobos G, Schopf E, Norgauer J, Kapp A. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. Eur J Immunol. 1994;24:518–522. doi: 10.1002/eji.1830240304. [DOI] [PubMed] [Google Scholar]
- 10.Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Neurosci. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 11.Frei K, Bodmer S, Schwerdel C, Fontana A. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol. 1986;137:3521–3527. [PubMed] [Google Scholar]
- 12.Gehrmann J, Kreutzberg GW. Microglia in experimental pathology. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford UP; New York: 1995. pp. 883–904. [Google Scholar]
- 13.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 14.Gerard C, Gerard N. C5a anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 15.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 16.Gerardy-Schahn R, Ambrosius D, Saunders D, Casaretto M, Mittler C, Karwarth G, Gorgen S, Bitter-Suermann D. Characterization of the C3a receptor-proteins on guinea-pig platelets and human polymorphonuclear leukocytes. Eur J Immunol. 1989;19:1095–1102. doi: 10.1002/eji.1830190620. [DOI] [PubMed] [Google Scholar]
- 17.Giulian D, Baker T. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2187. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein IM. Complement: biologically active products. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation. Raven; New York: 1988. pp. 55–74. [Google Scholar]
- 19.Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 20.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 21.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol (Lond) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klos A, Bank S, Gietz C, Bautsch W, Kohl J, Burg M, Kreitzschmar T. C3a receptor on dibutyryl-cAMP-differentiated U937 cells and human neutrophils: the human C3a receptor characterized by functional responses and 125I-C3a binding. Biochemistry. 1992;31:11274–11282. doi: 10.1021/bi00161a003. [DOI] [PubMed] [Google Scholar]
- 23.Kostyuk P, Verkhratsky A. Calcium stores in neurones and glia. Neuroscience. 1994;63:381–404. doi: 10.1016/0306-4522(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 24.Kostyuk PG, Verkhratsky AN. Calcium signalling in the nervous system. Wiley; Chichester, UK: 1995. [Google Scholar]
- 25.Kreitzschmar T, Pohl M, Gasaretto M, Przewosny M, Bautsch W, Klos A, Saunders D, Köhl J. Synthetic peptides as antagonists of the anaphylatoxin C3a. Eur J Biochem. 1992;210:185–191. doi: 10.1111/j.1432-1033.1992.tb17407.x. [DOI] [PubMed] [Google Scholar]
- 26.Lacy M, Jones J, Wittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 1995;61:71–78. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 27.Lückhoff A, Clapham DE. Calcium channels activated by depletion of internal calcium stores in A431 cells. Biophys J. 1994;67:177–182. doi: 10.1016/S0006-3495(94)80467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Möller T, Nolte C, Verkhratsky A, Kettenmann H (1996) Agonist-induced calcium signalling in cultured mouse microglia: intracellular Ca2+ release and capacitative Ca2+ entry. J Physiol (Lond) 493.P:81P.
- 29.Monk PN, Partridge LJ. Characterization of a complement-fragment-C5a-stimulated calcium-influx mechanism in U937 monocytic cells. Biochem J. 1993;295:679–684. doi: 10.1042/bj2950679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan BP. Complement in diseases of the nervous system. In: Whaley K, Loos M, Weiler JM, editors. Complement in health and disease. Kluwer Academic; Nowell, MA: 1994. pp. 353–375. [Google Scholar]
- 31.Nolte C, Möller T, Walter T, Kettenmann H. Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin-cytoskeleton. Neuroscience. 1996;73:1091–1107. doi: 10.1016/0306-4522(96)00106-6. [DOI] [PubMed] [Google Scholar]
- 32.Norgauer J, Dobos G, Kownatzki E, Dahinden C, Burger R, Kupper R, Gierschik P. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin-sensitive G protein. Eur J Biochem. 1993;217:289–294. doi: 10.1111/j.1432-1033.1993.tb18245.x. [DOI] [PubMed] [Google Scholar]
- 33.Penner R, Fasolato C, Hoth M. Calcium influx and its control by calcium release. Curr Opin Neurobiol. 1993;3:368–374. doi: 10.1016/0959-4388(93)90130-q. [DOI] [PubMed] [Google Scholar]
- 34.Perry VH, Andersson PB, Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993;16:268–273. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- 35.Petersen OH, Petersen CCH, Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- 36.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 37.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 38.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 39.Righi M, Letari O, Sacerdote P, Marangoni F, Miozzo A, Nicosia S. myc-immortalized microglial cells express a functional platelet-activating factor receptor. J Neurochem. 1995;64:121–129. doi: 10.1046/j.1471-4159.1995.64010121.x. [DOI] [PubMed] [Google Scholar]
- 40.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao Y, McCarthy KD. Receptor-mediated calcium signals in astroglia: multiple receptors, common stores and all-or-nothing responses. Cell Calcium. 1995;17:187–196. doi: 10.1016/0143-4160(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 42.Schultz J, Schaller J, McKinley M, Bradt B, Cooper N, May P, Rogers J. Enhanced cytotoxicity of amyloid beta-peptide by a complement dependent mechanism. Neurosci Lett. 1994;175:99–102. doi: 10.1016/0304-3940(94)91088-x. [DOI] [PubMed] [Google Scholar]
- 43.Streit WJ. An improved staining method for rat microglial cells using a lectin from Griffonia simplicifolia (GSA-I-B4). J Histochem Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- 44.Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- 45.Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Rev. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- 46.Vaca L, Kunze DL. Depletion of intracellular Ca2+ stores activates a Ca2+-selective channel in vascular endothelium. Am J Physiol. 1994;267:C920–C925. doi: 10.1152/ajpcell.1994.267.4.C920. [DOI] [PubMed] [Google Scholar]
- 47.Vanek M, Hawkins LD, Gusovsky F. Coupling of the C5a receptor to Gi in U-937 cells and in cells transfected with C5a receptor cDNA. Mol Pharmacol. 1994;46:832–839. [PubMed] [Google Scholar]
- 48.Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 49.Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol. 1995;7:48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 51.Whittemore ER, Korotzer AR, Etebari A, Cotman CW. Carbachol increases intracellular free calcium in cultured rat microglia. Brain Res. 1993;621:59–64. doi: 10.1016/0006-8993(93)90297-z. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Star RA, Tortorici G, Muallem S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J Biol Chem. 1994;269:12645–12653. [PubMed] [Google Scholar]
- 53.Yao J, Harvath L, Gilbert DL, Colton CA. Chemotaxis by a CNS macrophage, the microglia. J Neurosci Res. 1990;27:36–42. doi: 10.1002/jnr.490270106. [DOI] [PubMed] [Google Scholar]