Abstract
The effect of superfused serotonin (5-HT; 50 μm) on the synaptic responses of the lateral giant (LG) interneuron in crayfish was found to depend on the social status of the animal. In socially isolated animals, 5-HT persistently increased the response of LG to sensory nerve shock. After social isolates were paired in a small cage, they fought and determined their dominant and subordinate status. After 12 d of pairing, 5-HT reversibly inhibited the response of LG in the social subordinate and reversibly increased the response of LG in the social dominant crayfish. The effect of 5-HT changed approximately linearly from response enhancement to inhibition in the new subordinate over the 12 d of pairing. If, after 12 d pairing, the subordinate was reisolated for 8 d, the response enhancement was restored. If the subordinate, instead, was paired with another subordinate and became dominant in this new pair, the inhibitory effect of 5-HT changed to an enhancing effect over the next 12 d of pairing. If, however, two dominant crayfish were paired and one became subordinate, the enhancing effect of 5-HT persisted in the new subordinate even after 38 d pairing. These different effects of serotonin result from the action of two or more molecular receptors for serotonin. A vertebrate 5-HT1 agonist had no effect on social isolates but reversibly inhibited the response of LG in both dominant and subordinate crayfish. The inhibitory effects of the agonist developed approximately linearly over the first 12 d of pairing. A vertebrate 5-HT2 agonist persistently increased the response of LG in isolate crayfish and reversibly increased the response of the cell in dominant and subordinate crayfish. Finally, although neurons that might mediate these effects of superfused 5-HT are unknown, one pair of 5-HT-immunoreactive neurons appears to contact the LG axon and initial axon segment in each abdominal ganglion in its projection caudally from the thorax.
Keywords: serotonin, 5-HT receptors, agonistic behavior, escape, lateral giant interneuron, social dominance
Behavioral differences associated with dominant and subordinate animals often appear during the first agonistic encounter, when one animal begins to win and the other to lose the first fight. Subsequent fights are usually decided more quickly, until fighting is avoided altogether by the retreat of the subordinate in the face of the dominant’s advance. The transition between the similar behavior of two strangers to the bipolar difference in the behavior of the new dominant and subordinate can occur very quickly, within minutes or hours of their first encounter. These different behavioral states can persist for variable periods of time, from a few minutes or hours to a lifetime.
The nervous system of an animal that participates in a plastic social hierarchy must be able to mediate rapid behavioral transitions in either direction as well as to mediate indefinite periods of behavior appropriate to each social status. The neural mechanisms for change and maintenance of social status are unknown, but neuromodulator substances have been linked to both. Immediate changes in social behavior have been seen in some animals after injection with a neuromodulator. Flank marking, a sign of social dominance in hamsters, occurs within moments of the injection of arginine vasopressin in the superior chiasmastic nucleus of the hypothalamus of freely behaving hamsters (Ferris et al., 1986; Albers and Rawls, 1989). Similarly, injected serotonin evokes a dominant posture and promotes aggression in crayfish and lobsters (Livingstone et al., 1980; Kravitz, 1988; Huber, 1995). Slower changes in social behavior and the maintenance of social status have been correlated with changed serum levels of androgens, serotonin, and serotonin metabolites. Elevated levels of serotonin or serotonin metabolites have been found to correlate with dominance in some primates (Steklis et al., 1986; Brammer et al., 1987; Raleigh et al., 1991; Shively et al., 1991) and with aggression in mice (Cases et al., 1995; Nelson et al., 1995). Reductions in serotonin metabolites have been correlated with dominance or aggression in fish, rodents, and other primates, and with violent aggression in humans (Yodyingyuard et al., 1985; Bronson and Winter, 1992; Coccaro, 1992; Linnoila and Virkkunen, 1992; Winberg et al., 1992, 1993; Brunner et al., 1995).
These results indicate that one mechanism of neural modulation of social behavior controls the levels of neuromodulatory substances available to interact with neuronal targets. Another possible mechanism would control the receptors for neuromodulators in the target neurons. Here we present evidence that the modulatory effect of serotonin on a command neuron for escape in crayfish changes in response to new social experience and changes in social status. These slow but reversible modulatory changes appear to result from changes in the population of serotonin receptors. The modulatory and receptor changes appear to be part of a larger but reversible process of neuronal and behavioral adaptation to persistent changes in social status (Krasne and Shamasian, 1996). A preliminary report of some of this work has appeared previously (Yeh et al., 1996).
MATERIALS AND METHODS
Dominant and subordinate crayfish. Juvenile (1–3 cm) crayfish (Procambarus clarkii) were hatched and raised in the laboratory. The animals were isolated for >1 month after they became free-swimming by placing them individually in 6-cm-diameter cylindrical plastic mesh cages within a larger aquarium. Socially dominant and subordinate animals were created by pairing previously isolated crayfish without regard to sex for 12 d or longer. Pairs were placed in 6-cm-diameter mesh cages in groups of 8 in a 20 gallon aquaria. Crayfish fight immediately after pairing, and the fighting results in establishment of a stable dominant/subordinate relationship. Dominant/subordinate relationships are dependent on size and experience, rather than on sex (Bovbjerg, 1953). The two animals are both active and move about the cage, frequently climbing over each other. The dominant animal initiates most and wins all of the agonistic interactions between the pair. During these interactions, the subordinate avoids contact with the dominant, either by retreating when approached or by staying as far away from the dominant as possible, often up on the wall of the container.
Experimental animals were chosen from among the population of crayfish that had been individually housed for 1 month or longer or from the pairs of animals that had been together for at least 12 d. Each pair was observed for 10–15 min, the social dominant and social subordinate were identified, and one was selected for experimentation, whereas the other was not used.
Dissection and experimental procedure. A crayfish of known social status (isolate, subordinate, or dominant) was chilled to immobility, and the abdomen was removed, pinned out in a Petri dish, and dissected dorsally to expose the ventral nerve cord. The nerve cord and isolated abdomen preparation was maintained at room temperature in 10 ml of crayfish saline continuously replaced at ∼1.2 ml/min (Van Harreveld, 1936). The axon of the lateral giant (LG) interneuron was identified visually in the terminal connective and penetrated proximally (Fig. 1). Four shocks [0.3 msec, 90 sec interstimulus interval (ISI)] at each of four stimulus levels between 3 and 7.5 V were applied to the ipsilateral third and fourth nerves of the terminal ganglion and evoked both sub- and superthreshold LG EPSPs. The stimulus series was presented once, and then the stimuli at the two lowest levels were repeated. The preparation was then superfused with serotonin (100 μm in 7 preparations at the outset of these experiments, and 50 μm for the remaining 93 preparations) or with a 5-HT agonist [α-methyl-5-HT or 1-(3-chlorophenyl)piperazine (mCPP) at 50 μm, 67 preparations], all at 1.2 ml/min. If perfect mixing had occurred, the continuous replacement of the bath with 50 μm 5-HT would have brought the bath concentration to 95% of 50 μm in ∼25 min. Stimuli at a constant subthreshold voltage level were presented at 3 min ISI during the first 45 min of superfusion. The previous stimulus series was then repeated during continued superfusion. The effects on the LG response produced by the two concentrations of serotonin were indistinguishable. A saline wash followed; responses to a final set of stimuli were recorded after 1 hr wash. Although the electrophysiology was performed with knowledge of the animal’s dominance status, the effects of serotonin on the responses of all three types of animals were unmistakable and immediately distinguishable: the responses of isolate and dominant animals were always increased from control levels, whereas those of subordinates were always decreased.
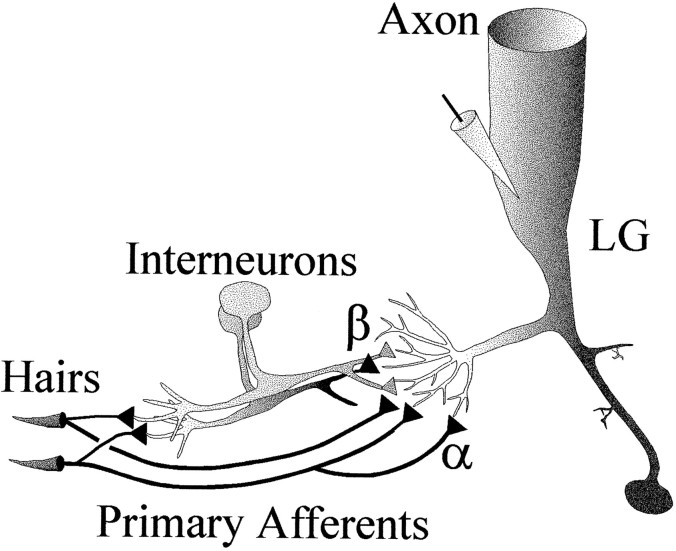
Fig. 1.
The tailflip circuit in crayfish, showing convergence of mechanosensory afferents and interneurons on the LG command neuron for tailflip. Hairs and other mechansoreceptors on the abdominal surface project primary afferents into the ventral nerve cord where they excite the LG directly (α) and indirectly through mechanosensory interneurons (β). EPSPs were recorded in the initial axon segment of the LG neuron.
The same experiments were performed on a set of adult (8–12 cm) crayfish that were obtained commercially and maintained communally until they were separated into pairs for at least 2 weeks. The effects of serotonin and serotonin agonists on the responses of the LG neurons in these adult dominant and subordinate animals were no different from the responses of the dominant and subordinate juveniles.
5-HT immunocytochemistry and Lucifer yellow (LuY) injection.Crayfish were anesthetized by cooling them gradually over 20 min to 4°C. The complete ventral nerve cord was removed under cold saline and pinned out in a Petri dish lined with SYLGARD (Dow Corning, Midland, MI), and the cord was desheathed in one segment to expose the LG axon. LG was penetrated with a micropipette filled with LuY, which was injected iontophoretically with −2 nA continuous current for 1 hr. The nerve cord was transferred to another dish, repinned, and fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (PB) for 6 hr at room temperature. The tissue was then rinsed in 0.1 mPB containing 0.4% Triton X-100, 0.2% bovine serum albumin (BSA), and 0.1% sodium azide over 6 hr at 4°C on a shaker. Six solution changes occurred over this time. Published immunocytochemical procedures to stain for serotonin in the crayfish CNS were then followed (Beltz and Kravitz, 1983; Real and Czternasty, 1990), using a secondary antibody conjugated to Texas Red.
RESULTS
Effect of 5-HT on LG responses in isolate, subordinate, and dominant juvenile crayfish
The LG neurons form a tightly coupled network of interneurons that together evoke an upward-directed tailflip escape response when any one of them fires a single action potential (Wiersma, 1947). An LG neuron is present in each abdominal hemiganglion where it is excited by local mechanosensory afferents and segmental and multisegmental interneurons (Fig. 1) (Krasne, 1969; Kennedy et al., 1971; Zucker, 1972a). Stimulation of sensory afferents in nerves 3 and 4 of the terminal abdominal ganglion (A6) evokes a biphasic EPSP in the LG neuron. The first depolarizing wave (α) is produced by monosynaptic connections from primary afferents, whereas the second wave (β) results from disynaptic inputs from mechanosensory interneurons (MSIs). Similar responses are seen in juvenile (2 cm) and adult (>8 cm) crayfish (Edwards et al., 1994).
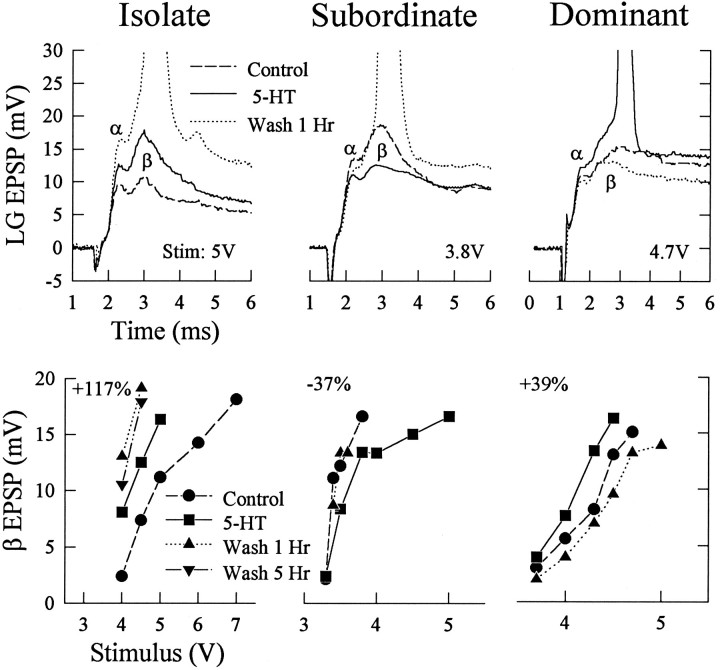
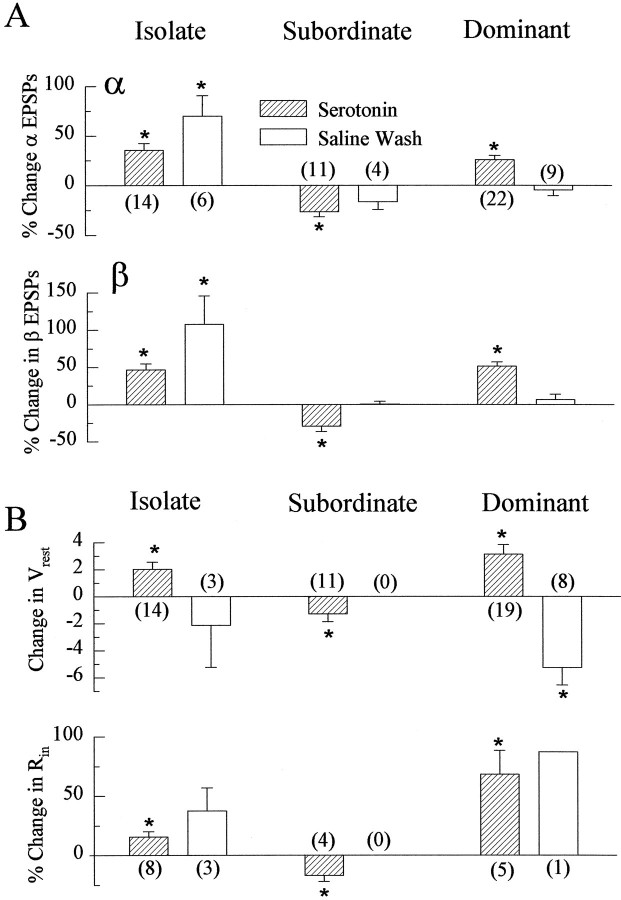
The responses of the LG neuron to nerve shock in social isolates increased after 45 min superfusion of the exposed ventral nerve cord in the isolated abdomen with 50 or 100 μm serotonin (Figs.2, 3A). Amplitudes of both α and β EPSPs became larger, and this enhancement persisted, and even increased, after washing the preparation with saline for 1 hr. The increase in response occurred at all stimulus levels and caused previously subthreshold stimulus levels to become superthreshold (Figs.2B, 4). The response increase was accompanied by a small, persistent depolarization of LG, along with a small increase in the input resistance of the cell, measured at the initial axon segment (Figs. 1, 3B).
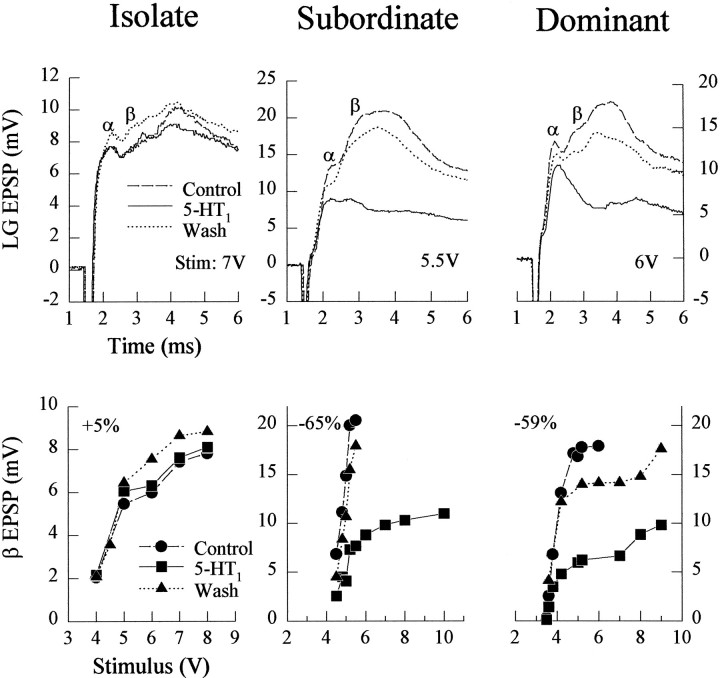
Fig. 2.
Responses of isolate, subordinate, and dominant juvenile crayfish to sensory nerve shock before (Control) and during bath application of 50 μm5-HT and after 1 hr saline wash (Wash1 Hr; see Materials and Methods). Top, EPSPs and spikes evoked by the stimulus level indicated and recorded under the three conditions, with the α and β EPSP components labeled. Bottom, β EPSP amplitudes evoked by a range of stimulus intensities delivered to the same animals as in the top. Each value is the average of at least four responses to one stimulus level; the bars indicating ±1 SEM are obscured by the symbols. The Isolate crayfish was tested over the range of stimulus intensities after 1 hr and after 5 hr of saline wash; the other animals were tested only after 1 hr wash. The average percent change in the response for each animal is given in the top left of each panel. The value is the percent change in response produced by 5-HT from the control response, averaged over all subthreshold stimulus levels tested.
Fig. 3.
Changes in EPSPs, resting membrane potential, and input resistance of LG produced by superfused 5-HT and after 1 hr saline wash. A, Mean ± SEM percent change of the α (top) and β (bottom) LG EPSPs produced by 5-HT (at either 50 or 100 μm) and by 1 hr saline wash in isolate (left), subordinate (middle), and dominant (right) juvenile crayfish. B, Mean ± SEM change in resting membrane potential (top) and the percent change in input resistance (bottom) produced by 5-HT in isolate (left), subordinate (middle), and dominant (right) crayfish. The number of animals in each experiment is given in parentheses; the same animals were used for α and β measurements in A. Responses that are significantly different than zero are indicated with anasterisk. Those differences are significant atp < 0.05, as indicated by the fact that the difference between the absolute value of the response mean and the absolute value of the 95% confidence limit is greater than zero.
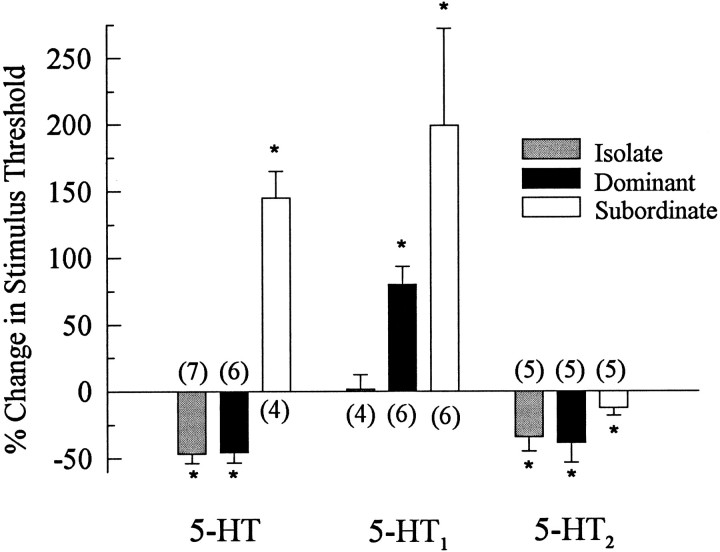
Fig. 4.
Effect of 5-HT, 5-HT1 agonist, and 5-HT2 agonist on the stimulus threshold of LG in isolate, dominant, and subordinate juvenile crayfish. The percent change in stimulus threshold was measured as the difference between the stimulus voltage necessary to fire LG in the presence of the agonist and the stimulus voltage required before the drug was applied. Mean ± SEM percent change are shown; responses that are significantly different than zero are indicated with an asterisk. Those differences are significant at p < 0.05, as indicated by the fact that the difference between the absolute value of the response mean and the absolute value of the 95% confidence limit is greater than zero. The number of animals used is given inparentheses.
The responses of the LG neuron in social subordinates were reduced by serotonin superfusion (Figs. 2, 3). α and β EPSPs were reduced at all stimulus levels and caused previously superthreshold stimulus levels to become subthreshold (Figs. 2, 4). The reduction in response was accompanied by a small decrease in resting membrane potential and a decrease in input resistance. Saline wash (1 hr) eliminated the response reduction that occurred in the presence of 5-HT.
The responses of the LG in social dominants were increased by serotonin at all stimulus levels but, unlike in the isolates, the response increase was eliminated by saline wash (Figs. 2, 3). The stimulus threshold of the LG neuron was reduced during serotonin superfusion in these animals (Fig. 4). As in the isolates, the response increase in the dominants was accompanied by a small depolarization and an increase in input resistance. The depolarization was removed by saline wash.
Concentration dependence of superfused serotonin on the response of the LG neuron
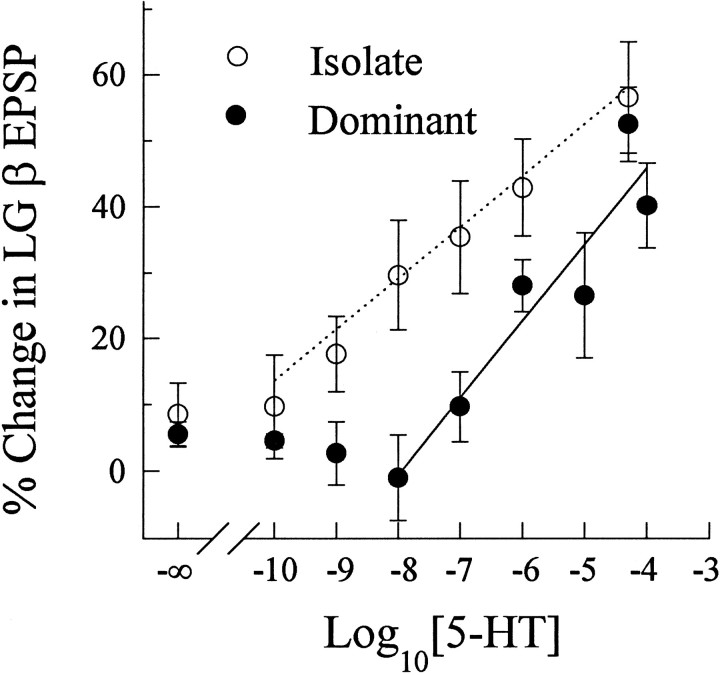
The effect of superfused serotonin on the responses of the LG neuron was tested over a range of concentrations from 10−10 to 10−3m in isolate and dominant juvenile crayfish (Fig. 5). A small increase in LG responsiveness (+10%) during the experiment was revealed by a second exposure to saline alone (−∞ on the abscissa in Fig. 5). The LG response of the isolate animal increased in a log/linear manner from between 10−10 and 10−9m. This apparent concentration threshold for isolates was below the range of hemolymph concentrations of monoamines found in the lobster (Livingstone et al., 1981). The effect of serotonin on the response of the LG in dominant crayfish remained at control levels for concentrations below 10−7m, above which it also increased linearly with the log of the applied 5-HT concentration. An analysis of covariance indicated that the the dominant and isolate regression curves were different at the p = 0.012 level (F(1,60) = 6.70).
Fig. 5.
Concentration dependence of 5-HT effect on LG EPSPs in social isolate and dominant crayfish. Mean ± SEM percent changes in the β EPSPs in dominant (filled circles) and isolate (open circles) crayfish were calculated from the difference between EPSPs recorded before (control) and after superfusion with either saline (0 m5-HT, −∞ on the abscissa) or a known concentration of 5-HT (from 10−10 to 10−4m).Solid and dashed lines are linear regressions of the β EPSPs from dominant and isolate crayfish, respectively, against the log10[5-HT] concentration from 10−8 to 10−4m (dominant) and 10−10 to 10−4m (isolate).
Time course and reversibility of the change in the neuromodulatory effect of 5-HT after a change in social status
To determine how the effect of serotonin on the LG neuron’s response changes after a change in social status, we repeated the experiments described above at intervals from 1 to 12 d after the agonistic interaction that determined the animal’s new social status. As in the isolates, serotonin increased the response of the LG in the new subordinates in the first few days after pairing. The enhancing effect of serotonin on both the α and the β EPSPs decreased in new subordinates over the first week after the animals were paired, and it became increasingly inhibitory in the second week (Fig.6).
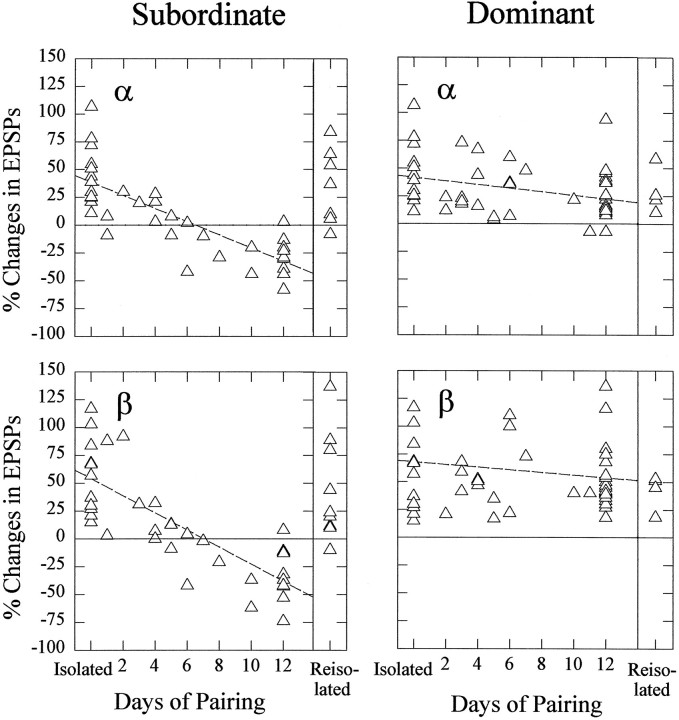
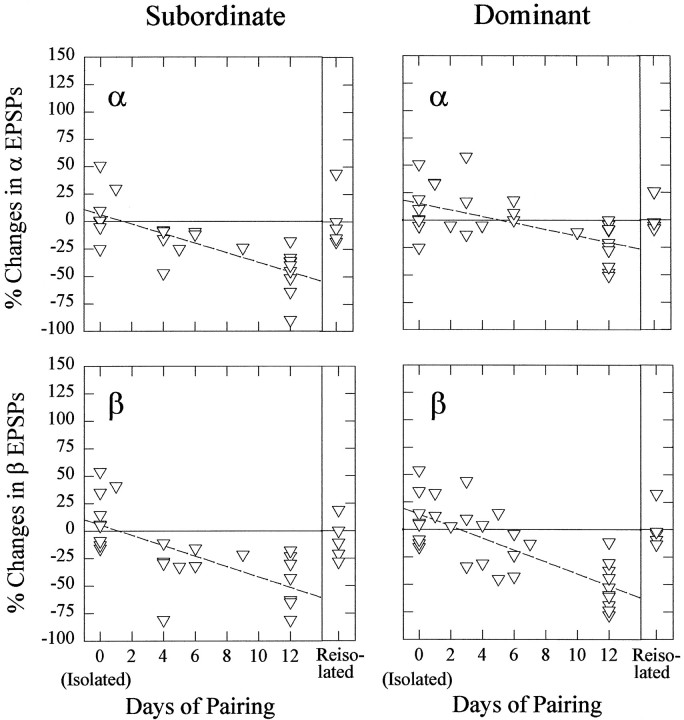
Fig. 6.
Change in the effect of 5-HT on LG EPSP amplitude in isolate, new subordinate, and new dominant crayfish with the duration of subordinate/dominant pairing, and in reisolated crayfish. Left, Percent change in α EPSPs (top) and β EPSPs (bottom) from control levels produced by bath application of 50 μm 5-HT in new subordinates. Change in EPSPs in isolates is shown on theleft of each panel (Isolated); change in EPSPs in animals that have been paired for 12 d or longer and then reisolated for 8 d is shown on theright of each panel (Reisolated).Right, Similar plots of data from new dominant crayfish. Each triangle represents data from one animal.
The excitatory effect of serotonin was restored in new subordinates that were reisolated. When animals were paired for 12 d and the subordinates were then reisolated for 8 d, serotonin increased the responses of the LG neuron in these reisolated animals to the same degree as occurred in the isolates. This result indicates that the development of serotonergic inhibition in new subordinates is reversible after reisolation of the animal.
The enhancing effect of serotonin on both α and β LG responses in social isolates was maintained in new dominant crayfish, but became “washable” after 12 d of pairing. Development of the “washability” of the serotonin effect was not traced over the 12 d period of pairing. The responses of dominant animals that were reisolated for 8 d after 12 d of pairing were also increased by serotonin.
Casualty rates among paired animals depend on their social status
The reversal of serotonin’s effect on the response of LG after reisolation of subordinate crayfish suggested that the modulatory effects of serotonin might change with subordinate-to-dominant transitions and vice versa. To test this, we took subordinate animals from existing pairs and paired them to form new dominant/subordinate pairs, and we took dominant animals from other existing pairs and formed new dominant/subordinate pairs. These animals suffered higher casualty rates than the pairs of former isolates, in which only 35% of the new subordinates were killed. New subordinates in pairs of former subordinates experienced a casualty rate near 50%, and new subordinates in pairs of former dominants suffered a 72% casualty rate. In those new pairs of either type in which both animals survived, the new dominant and subordinate crayfish displayed behavior patterns that were indistinguishable from those of dominants and subordinates created by pairing former isolates.
The modulatory effect of serotonin follows a change in social status from subordinate to dominant
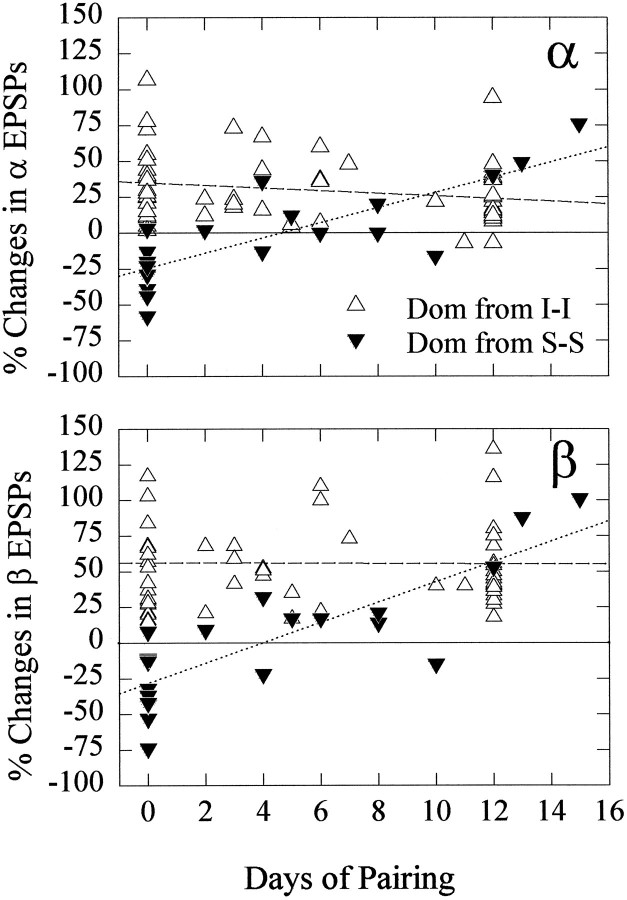
After two former subordinates were paired and the new dominant had been identified, that animal was tested for the effect of serotonin on the response of the LG neuron. We found that the inhibitory effect of serotonin that is typical of subordinates gradually changed to the enhancing effect typical of dominants over a 2 week period (Fig.7).
Fig. 7.
Effect of 5-HT on α (top) and β (bottom) LG EPSPs in new dominants produced by pairing subordinate crayfish (Dom from S-S) for periods of time between 2 and 15 d (filled inverted triangles). The change in EPSPs in the 12 d subordinates is replotted at left (0 d of pairing) from Figure 6. The effect of 5-HT on LG EPSPs in dominants derived from pairing isolate crayfish (Dom from I-I) is replotted from Figure 6 for comparison (open triangles). The dottedand dashed lines are linear regressions of thefilled inverted triangles and open triangles, respectively.
Effect of 5-HT depends on social history and on social status
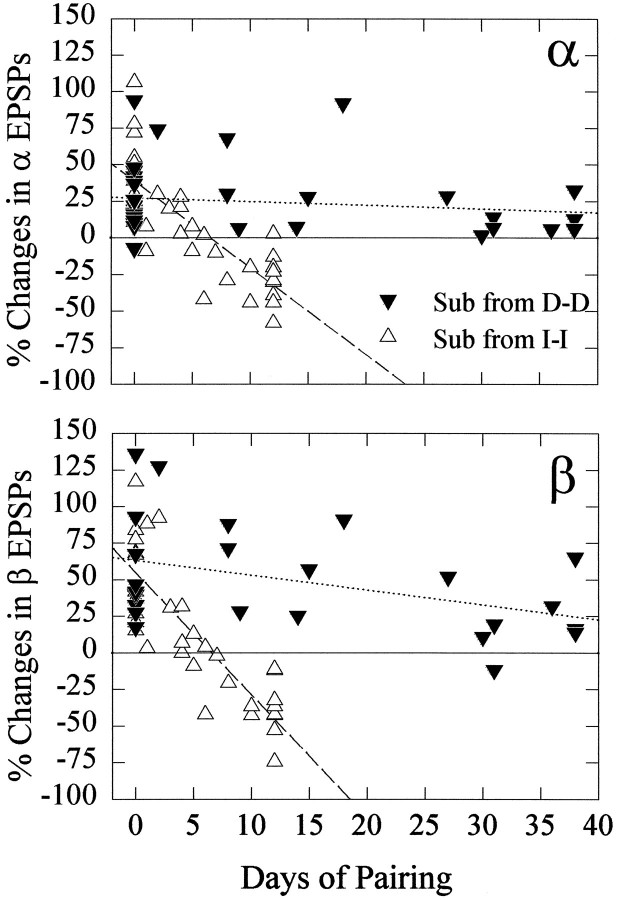
When we tested new subordinates made from pairing dominant crayfish, we found that the effect of serotonin on the response of the LG neuron depended on the social history of the animal as well as on its current social status. Instead of becoming inhibitory after 2 weeks of pairing, serotonin continued to increase the responses of the LG neuron in the new (formerly dominant) subordinates even after 38 d pairing (Fig. 8). Despite this “dominant-like” effect of serotonin on their LG responses, the social behavior of these subordinates was similar to that of new subordinates made from isolate pairs: throughout the 38 d period, the subordinate avoided the dominant during interactions and retreated or tailflipped at its approach.
Fig. 8.
Effect of 5-HT on α (top) and β (bottom) LG EPSPs in new subordinates produced by pairing dominant crayfish (Sub from D-D) for periods of time between 2 and 38 d (filled inverted triangles). The change in EPSPs in the 12 d dominants is replotted at left (0 d of pairing) from Figure 6. The effect of 5-HT on LG EPSPs in subordinates derived from pairing isolate crayfish (Sub from I-I) is replotted from Figure 6 for comparison (open triangles). The dottedand dashed lines are linear regressions of thefilled inverted triangles and open triangles, respectively.
Effects of vertebrate 5-HT1 and 5-HT2agonists in animals of different social status
The different effects of serotonin on the LG neuron responses of animals of different social status suggested to us that changes in social status may cause changes in serotonin receptors or in their transduction mechanisms. To test for different serotonin receptors, we repeated the experiments described above with one of two vertebrate serotonin agonists substituted for serotonin. When we substituted 50 μm mCPP Cl2, a vertebrate 5-HT1agonist, for serotonin, we found that it had no effect on the responses of the LG neuron in isolate animals but that it inhibited LG neuron responses in both dominant and subordinates (Fig. 9). These effects occurred at all LG stimulus levels and were reversible with a saline wash.
Fig. 9.
Effect of vertebrate 5-HT1 agonist mCPP-Cl2 on LG responses to sensory nerve shock in isolate, subordinate, and dominant juvenile crayfish. The experiments and the figure are organized as in Figure 2, with 50 μm5-HT1 agonist substituted for 5-HT.
Like the effect of serotonin, the full inhibitory effect of the 5-HT1 agonist developed over a 12 d period after the isolates were paired (Fig. 10). As stated above, the 5-HT1 agonist had no effect on the responses of the LG neuron in isolates. In the new subordinate crayfish, the 5-HT1 agonist became inhibitory after 4 d, and the inhibition became stronger over the next week. α and β EPSPs were similarly affected. The inhibitory effect of the 5-HT1agonist developed in the new dominants in a similar manner. Reisolation of the new dominants and subordinates removed the inhibition so that the 5-HT1 agonist once again had no effect on the response of the LG neuron.
Fig. 10.
Change in the effect of the 5-HT1 agonist mCPP-Cl2 on the amplitudes of α (top) and β (bottom) LG EPSPs in isolate crayfish and in newly paired subordinate (left) and dominant (right) crayfish. Responses of animals that were reisolated for 8 d after 12 d of dominant or subordinate status are shown to the right of each panel. The experiments and the figure are organized as in Figure 6, with 50 μm 5-HT1 agonist substituted for 5-HT.
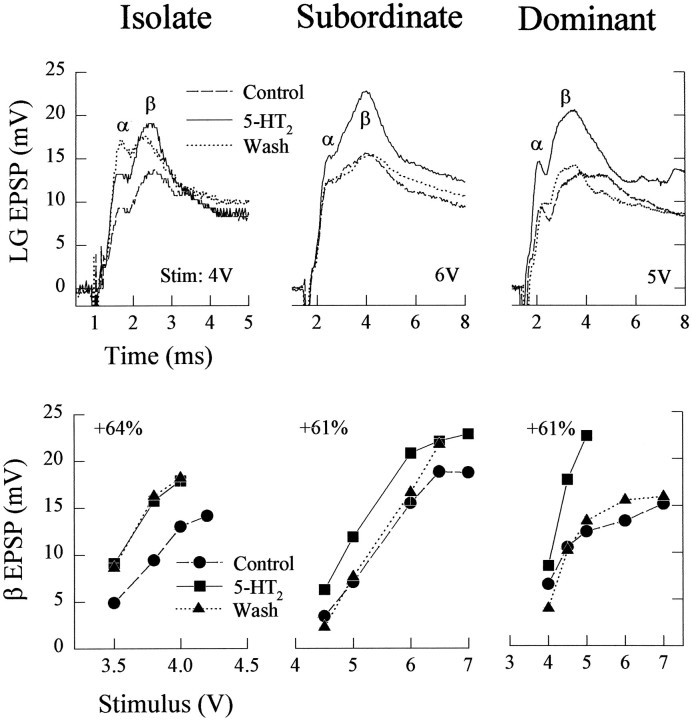
Substitution of 50 μm α-CH3 5-HT, a vertebrate 5-HT2 agonist, for serotonin had completely different effects (Fig. 11). In socially isolate crayfish, α-CH3 5-HT increased the responses of the LG neuron and continued to do so after 1 hr saline wash, much as serotonin had done (see Fig. 2). In both subordinate and dominant crayfish, the 5-HT2 agonist also increased the response of the LG neuron, but it did so reversibly: the increase was removed after a 1 hr saline wash (Fig. 11). Response increases occurred at all stimulus levels.
Fig. 11.
Effect of 5-HT2 agonist α-methyl-5-HT on LG responses to sensory nerve shock in isolate, subordinate, and dominant juvenile crayfish. The experiments and the figure are organized as in Figure 2, with 50 μm5-HT2 agonist substituted for 5-HT.
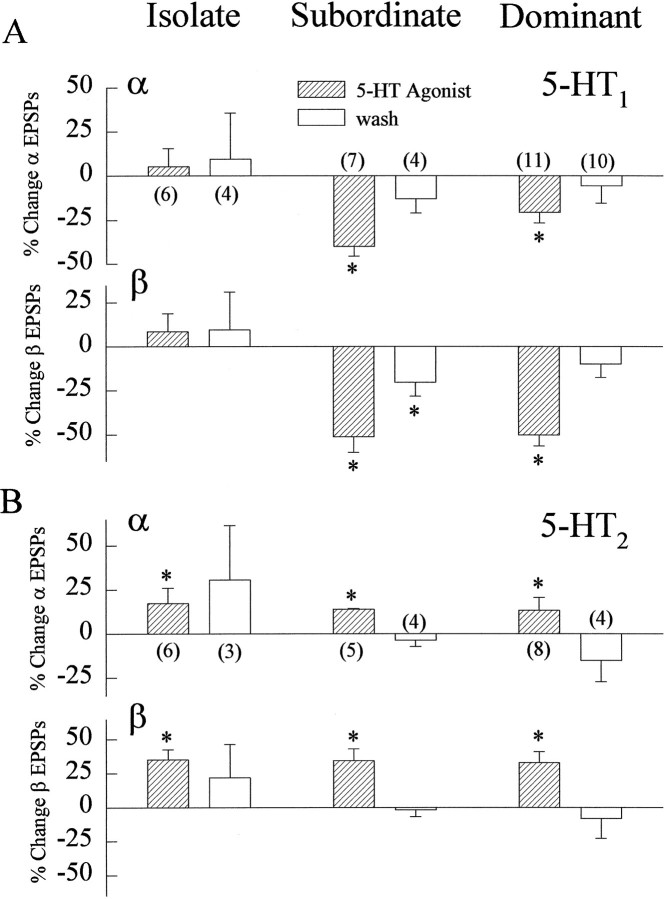
The opposing effects of the vertebrate 5-HT1 and 5-HT2 agonists were seen in all of the animals examined (Fig. 12). The 5-HT1 agonist reduced the β EPSPs of the LG neuron by an average of nearly 50% in dominant and subordinate animals, whereas the 5-HT2 agonist increased β EPSPs by an average of between 32 and 35% in isolate, dominant, and subordinate crayfish. The effects on α EPSPs were similar but usually smaller.
Fig. 12.
Effect of 5-HT1 and 5-HT2agonists on LG neuron responses. A, Percent change in α and β LG EPSPs in isolate, subordinate, and dominant crayfish before and during superfusion of 50 μm 5-HT1agonist mCPP-Cl2 and after saline wash for 1 hr.B, Percent change in α and β LG EPSPs in isolate, subordinate, and dominant crayfish before and during superfusion of 50 μm 5-HT2 agonist α-CH3 5-HT and after saline wash for 1 hr. The number of animals used in both sets of experiments is given in parentheses in the top frame of each panel.
5-HT-like-immunoreactive terminals and the LG axon
Approximately 100 neurons in the crayfish CNS display 5-HT-like immunoreactivity (Real and Czternasty, 1990). Many of these crayfish neurons appear homologous to the 5-HT-immunoreactive neurons in the CNS of lobster (Beltz and Kravitz, 1983). Stimulation of one of these lobster neurons (the large cells in the first abdominal ganglion) has been shown to mimic the modulatory effect of superfused serotonin on command-activated abdominal postural flexion (Ma et al., 1992). We wished to determine whether any of these or other 5-HT-like-immunoreactive processes appear to innervate the LG neuron.
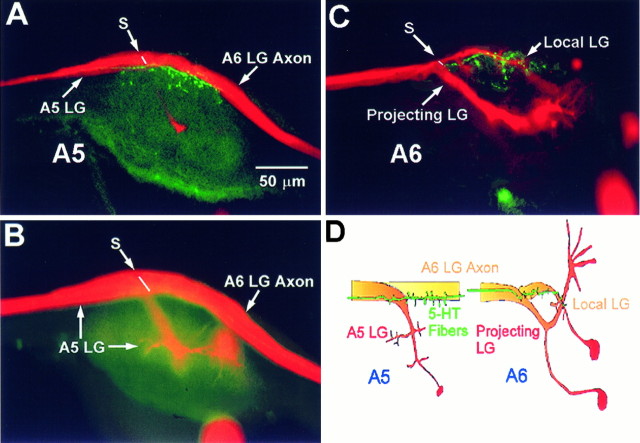
In socially isolate juvenile crayfish, we first injected the axon of the LG neuron with LuY and then processed the entire CNS for 5-HT-like immunoreactivity, using a secondary antibody conjugated with Texas Red (see Materials and Methods). We found a pattern of 5-HT-like immunostaining that was very similar to that reported previously for crayfish (Real and Czternasty, 1990). We also found a pair of stained fibers, each of which projected along the lateral and dorsal margins of one side of the ventral nerve cord and gave rise to a set of terminal varicosities in each abdominal ganglion that were in close proximity to ventral surface of the LG axon (Fig. 13). In abdominal ganglia 1–5 (A1–A5), the terminal varicosities were adjacent to the distal end of the axon of the next caudal LG (Fig. 13A), where it makes output connections with both the segmental giant interneuron and the fast flexor motor neurons, and where it makes a septate junction with the axon of the ganglionic LG (Watanabe and Grundfest, 1961). A few 5-HT varicosities appear on the anterior side of the septate junction, adjacent to the initial axon segment of the ganglionic LG (Fig. 13A). Curiously, the LG dendrites appear to have no 5-HT-like-immunoreactive fibers or terminals near them (Fig.13B). In the terminal abdominal ganglion, A6, there are two LG neurons, a local LG (lLG) and a projecting LG (pLG) (Kondoh and Hisada, 1983). Immunostained varicosities are largely confined to the axon and proximal dendrite of the local LG neuron, although a few span the septate junction between the lLG and the pLG and appear to make contact with the pLG at its initial axon segment (Fig. 13C). There are no immunostained varicosities or fibers near the distal dendrites of the lLG or anywhere on the dendrites of projecting LG, where it receives inputs from primary afferents and interneurons (Fig.13D). We have traced the lateral/dorsal 5-HT-immunostained fiber rostrally through the thoracic ganglia, but we have not yet identified its cell body.
Fig. 13.
5-HT-like-immunoreactive terminals and the LG neurons. A, The LG neuron in ganglion A5 stained with LuY (false red) and photographed with a confocal attachment (Newport Instruments) laterally in the focal plane of the LG axon, and 5-HT-like-immunoreactive axonal terminals labeled with Texas Red (false green) photographed in the same focal plane as the LG image. The 5-HT-like terminals are restricted to the vicinity of the initial axon segment of the ganglionic LG and to the anterior end of the axon of the next caudal LG (from A6). The septate junction between the two is indicated (S). Similar immunoreactivity is seen in all five anterior abdominal ganglia.B, The same preparation as in A, photographed in the focal plane of LG’s major dendrites in A4. No 5-HT immunoreactivity above background is seen in this plane.C, The local and projecting LG neurons in A6 (red), with the 5-HT-like-immunoreactive terminals (green) on the proximal segment of the both neurons. No label appears near the dendrites of the projecting LG, which receive inputs from mechanosensory interneurons and primary afferents (D). D, Diagram showing the relationship of the LG neurons to the 5-HT neuron in A5 and A6.
DISCUSSION
The effects of 5-HT vary with social status and social history
We have found that the effect of serotonin on a central synapse depends on the social status and the social history of the animal. Like other neuromodulators in many animals, serotonin sets the gain of synapses and circuits in crayfish and, in this way, appears to modify or select those circuits for a greater or lesser role in controlling the behavior of the animal (Kravitz, 1988; Katz, 1995). In the present instance, the modulatory effect of serotonin is itself modulated by the social status and social history of the crayfish, so that in dominant crayfish the LG response is transiently increased, in subordinates it is transiently inhibited, and in isolates it is persistently increased.
Although crayfish adopt dominant or subordinate patterns of behavior immediately after their first fight, the changes in the effect of serotonin take much longer to develop. The changes only follow persistent changes in social status and develop more or less linearly over an ∼2 week period after the change in social status. The slow change in the effect of serotonin may reflect the time it takes to turn the population of serotonin receptors over, but it may also reflect the undesirability of changing the receptor population merely because the animal had a bad day. After the changes in the effects of serotonin are established, they can be reversed if the change in social status is reversed in a persistent manner, such as when a subordinate animal becomes dominant. However, we also found that some changes in the modulatory effect of serotonin occur more easily than changes in the reverse direction: changes in the effect of serotonin that follow a subordinate-to-dominant social promotion follow much more quickly than changes that follow a dominant-to-subordinate social demotion.
It is unclear what signals the abdominal nervous system to begin to change the effect of serotonin on the response of LG after a change in social status. Presumably, social status is recognized in the brain or anterior nervous system, and a persistent signal alerts LG and perhaps other parts of the nervous system to the nature of the change.
Changes in social status have also been found to influence cell size and the level of expression of gonadotropin-releasing hormone (GnRH) in a population of hypothalamic neurons in an African cichlid fish. The cells are larger and the expression levels are greater in territorial (dominant) males, and both are smaller in nonterritorial (subordinate) males (Francis et al., 1993; White and Fernald, 1993). Like the modulatory effects of serotonin described above, the effects of social promotion (size increases) on the GnRH neurons occur more readily than the do the effects of social demotion (size decreases).
5-HT receptors
The changes in the effects of serotonin that we report in crayfish appear to result from changes in the effects of different serotonin receptor subtypes that have opposing effects on the response of the LG neuron. The inhibition of the response of the LG neuron by a 5-HT1 agonist appears to result from a crayfish serotonin receptor subtype that is distinct from the receptor subtype that was preferentially excited by a 5-HT2 agonist and facilitated the responses of LG to its inputs. Each of these effects lasted only as long as the agonist was present. The persistent excitatory effect of both serotonin and the 5-HT2 agonist on the response of the LG neuron in isolated crayfish may result from activation of a third receptor subtype or from what might be a modified version of the second receptor subtype. At present, however, both the nature of the different 5-HT receptor subtypes and the mechanisms of their effects, including their second messengers, are unknown.
The changes in the effects of serotonin with social pairing may reflect differential changes in the receptor subtypes or in their downstream mechanisms. The lack of an inhibitory effect of 5-HT or the 5-HT1 agonist in social isolate crayfish and the subsequent appearance of the effect in new dominant and subordinate crayfish suggest that the inhibitory receptor mechanism is absent or disabled in isolate crayfish and is increasingly activated or restored in new dominant and subordinate animals. As this happens, the persistently excitatory effect of 5-HT in isolates may be either replaced by or transformed into the transiently excitatory 5-HT effect in the same new dominant and subordinate crayfish. Under this hypothesis, the transient excitatory effect of one receptor becomes preeminent in new dominant crayfish, whereas the inhibitory receptor becomes preeminent in new subordinate animals. At present, these hypotheses await testing by detailed analysis of the different 5-HT receptor subtypes, their second messengers, and their physiological mechanisms.
The crayfish serotonin receptors may differ from vertebrate serotonin receptors. The 5-HT1 agonist that we used, mCPP Cl2, has its greatest effects on vertebrate 5-HT1B and 5-HT1C receptors, but also has some agonistic effect on 5-HT1A receptors (Julius, 1991;Leonard, 1992; Humphrey and Hartig, 1993). It is unclear whether the crayfish receptors that respond to this agonist, or the receptors that respond to the vertebrate 5-HT2 agonist, are homologous to their counterparts in vertebrates. Recent work in crab has shown that crustacean serotonin receptors may be distinct from those of vertebrates (Zhang and Harris-War- rick, 1994) .
The reason for the difference in threshold concentration for the effects of serotonin in isolate and dominant animals (Fig. 5) is also unknown. It may reflect either a difference in the sensitivity of serotonin receptors in the two types of animals or the coactivation of excitatory and inhibitory effects in the dominant animal that would increase its threshold concentration.
Most of the serotonin receptors that affect the response of the LG neuron appear to act on the LG neuron itself, but some may also be presynaptic to LG. Serotonin depolarized LG in isolate (Glanzman and Krasne, 1983) and dominant crayfish and hyperpolarized it in subordinate crayfish. Serotonin had similar effects on α and β EPSPs in LG but had no significant effect on the responses of mechanosensory interneurons that contribute to the β EPSP, which suggests that serotonin acts directly on LG. The lack of serotonergic neurons near LG dendrites (Fig. 13) is consistent with this. However, serotonin can act neurohumorally in lobsters, and crayfish possess homologous serotonergic neurosecretory neurons in the abdominal nervous system (Beltz and Kravitz, 1983; Real and Czternasty, 1990). Because serotonin has been well established as a presynaptic modulator in bothAplysia (Brunelli et al., 1976; Bernier et al., 1982; Klein, 1993; Zhu et al., 1995) and at the crayfish neuromuscular junction (Dixon and Atwood, 1989), it is possible that the humoral effects are mediated presynaptically.
Serotonin immunoreactivity and the LG neuron
The LG neurons appear to be contacted in each abdominal hemiganglion by a serotonergic neuron that projects caudally along the nerve cord from the thorax. The location of the serotonergic varicosities on the LG axon is where LG makes connections to fast flexor (FF) motorneurons (Krasne, 1969; Kennedy et al., 1971; Zucker, 1972a,b; Roberts et al., 1982; Fraser and Heitler, 1989; Edwards et al., 1991; Heitler et al., 1991). Some of the axonal terminals of the serotonin fiber are just rostral to the septate junction between LG axons, on the initial axon segment of the ganglionic LG, where they could affect EPSPs and the threshold for spike initiation in the ganglionic LG neuron (Vu and Krasne, 1993).
Serotonin, aggression, social status, and the escape circuit
Serotonergic systems have been implicated in the release of aggressive behavior in humans, mice, fish, and crayfish and lobsters (Winberg et al., 1991; Brunner et al., 1995; Cases et al., 1995; Huber, 1995; Huber and Kravitz, 1995). Injection of serotonin into freely behaving subordinate crayfish evokes a sudden increase in fighting behavior affecting both the duration and the intensity of fighting (Huber, 1995; Huber and Kravitz, 1995). These results suggest that the onset of aggression is accompanied by the release of endogenous serotonin. If this occurs, then the threshold for LG-mediated tailflip escape should go up in subordinate crayfish and down in dominants and isolates. Experiments on pairs of freely behaving dominant and subordinate crayfish have shown that the LG stimulus threshold increased greatly in the subordinate member of the pair during a fight and slightly or not at all in the dominant member (Krasne et al., 1997). Although the relative difference in the change of LG threshold in the two crayfish is consistent with the effect of serotonin reported here, it is not yet known whether endogenous serotonin is released during a fight.
Adaptive significance of status-related changes in LG excitability
LG is part of a reflexive escape circuit and is known to be activated only by sharp, sudden taps on the tail, not voluntarily by the animal. The tailflip pitches the animal upside down and forward, away from the source of the tap. During a fight, such a tailflip would pitch a subordinate animal upside down and toward its opponent. Suppression of the response of LG during a fight might help a retreating subordinate that bumps its abdomen prevent an unwanted tailflip. An attacking dominant crayfish would be most likely to experience an LG tailflip triggered by the sudden attack of a third party so that, under these conditions, it may be advantageous for the threshold of LG to be reduced.
Footnotes
This work was supported by National Institutes of Health Research Grant RO1-NS26457 and National Science Foundation Research Grant IBN-9423846. We thank C. D. Derby, M. Hörner, F. B. Krasne, and E. A. Kravitz for many helpful discussions of this work.
Correspondence should be addressed to Dr. Donald H. Edwards, Department of Biology, Georgia State University, Atlanta, GA 30302-4010.
REFERENCES
- 1.Albers HE, Rawls S. Coordination of hamster lordosis and flank marking behavior: role of arginine vasopressin within the medial preoptic-anterior hypothalamus. Brain Res Bull. 1989;23:105–109. doi: 10.1016/0361-9230(89)90168-8. [DOI] [PubMed] [Google Scholar]
- 2.Beall SP, Langley DJ, Edwards DH. Inhibition of escape tailflip in crayfish during backward walking and the defense posture. J Exp Biol. 1990;152:577–582. doi: 10.1242/jeb.152.1.577. [DOI] [PubMed] [Google Scholar]
- 3.Beltz BS, Kravitz EA. Mapping of serotonin-like immunoreactivity in the lobster nervous system. J Neurosci. 1983;3:585–602. doi: 10.1523/JNEUROSCI.03-03-00585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltz BS, Kravitz EA. Aminergic and peptidergic neuromodulation in crustacea. J Exp Biol. 1986;124:115–141. [Google Scholar]
- 5.Bernier L, Castellucci VF, Kandel ER, Schwarz JH. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3,5-monophosphate in sensory neurons mediating the gill- and siphon-withdrawal reflex in Aplysia. J Neurosci. 1982;2:1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovbjerg RV. Dominance order in the crayfish Orconectes virilis (Hagen). Physiol Zool. 1953;26:173–178. [Google Scholar]
- 7.Brammer GL, McGuire MT, Raleigh MJ. Similarity of 5-HT2 receptor sites in dominant and subordinate vervet monkeys. Pharmacol Biochem Behav. 1987;27:701–705. doi: 10.1016/0091-3057(87)90197-3. [DOI] [PubMed] [Google Scholar]
- 8.Bronson KR, Winter JC. Reversal of testosterone-induced dominance by the serotonergic agonist quipazine. Pharmacol Biochem Behav. 1992;42:809–811. doi: 10.1016/0091-3057(92)90034-d. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 10.Brunner HG, Nelen M, Breakefield XO, Robers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1995;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 11.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, DeMaeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coccaro EF. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. Int Clin Psychopharmacol. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dixon D, Atwood HL. Phosphatidylinositol system’s role in serotonin-induced facilitation at the crayfish neuromuscular junction. J Neurophysiol. 1989;62:239–246. doi: 10.1152/jn.1989.62.1.239. [DOI] [PubMed] [Google Scholar]
- 14.Edwards DH, Heitler WJ, Leise EM, Fricke RA. Postsynaptic modulation of rectifying electrical synaptic inputs to the LG escape command neuron in crayfish. J Neurosci. 1991;11:2117–2129. doi: 10.1523/JNEUROSCI.11-07-02117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards DH, Yeh S-R, Barnett LD, Nagappan PR. Changes in synaptic integration during the growth of the lateral giant neuron of crayfish. J Neurophysiol. 1994;72:899–908. doi: 10.1152/jn.1994.72.2.899. [DOI] [PubMed] [Google Scholar]
- 16.Ferris CF, Meenan DM, Axelson JF, Albers HE. A vasopressin antagonist can reverse dominant/subordinate behavior in hamsters. Physiol Behav. 1986;38:135–138. doi: 10.1016/0031-9384(86)90143-5. [DOI] [PubMed] [Google Scholar]
- 17.Francis RC, Soma K, Fernald RD. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci USA. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser K, Heitler WJ. Thoracic output of crayfish giant fibers. II. The segmental giant neurone. J Comp Physiol [A] 1989;166:125–132. [Google Scholar]
- 19.Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish’s lateral giant escape reaction. J Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitler WJ, Fraser K, Edwards DH. Different types of rectification at electrical synapses made by a single crayfish neurone investigated experimentally and by computer simulation. J Comp Physiol [A] 1991;169:707–718. doi: 10.1007/BF00194899. [DOI] [PubMed] [Google Scholar]
- 21.Huber R. Serotonin controls decision making aspects of fighting in crayfish. In: Burrows ML, Matheson T, Newland PL, Schuppe H, editors. Nervous systems and behavior. Proceedings of the 4th International Congress of Neuroethology. Thieme; New York: 1995. p. 168. [Google Scholar]
- 22.Huber R, Kravitz E. A quantitative analysis of agonistic behavior in juvenile amercian lobsters (Homarus americanus L.). Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- 23.Humphrey PPA, Hartig P. A proposed new nomenclature for 5-HT receptors. Trends Pharmacol Sci. 1993;14:233–236. doi: 10.1016/0165-6147(93)90016-d. [DOI] [PubMed] [Google Scholar]
- 24.Julius D. Molecular biology of serotonin receptors. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- 25.Katz PS. Intrinsic and extrinsic neuromodulation of motor circuits. Curr Biol. 1995;5:799–808. doi: 10.1016/0959-4388(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy D, Zucker RS, Selverston AI. Neuronal circuit mediating escape responses in crayfish. Science. 1971;173:645–650. doi: 10.1126/science.173.3997.645. [DOI] [PubMed] [Google Scholar]
- 27.Klein M. Differential cyclic AMP dependence of facilitation at Aplysia sensorimotor synapses as a function of prior stimulation: augmentation versus restoration of transmitter release. J Neurosci. 1993;13:3793–3801. doi: 10.1523/JNEUROSCI.13-09-03793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondoh Y, Hisada M. Intersegmental to intrasegmental conversion by ganglionic fusion in lateral giant neurones of crayfish. J Exp Biol. 1983;107:515–519. [Google Scholar]
- 29.Krasne FB. Excitation and habituation of the crayfish escape reflex: the depolarizing response in lateral giant fibres of the isolated abdomen. J Exp Biol. 1969;50:29–46. doi: 10.1242/jeb.50.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Krasne FB, Lee SC. Response-dedicated trigger neurons as control points for behavioral actions: selective inhibition of lateral giant command neurons during feeding in crayfish. J Neurosci. 1988;8:3703–3712. doi: 10.1523/JNEUROSCI.08-10-03703.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krasne FB, Shamasian A, Kulkarni R. Altered excitability of the crayfish lateral giant escape reflex during agonistic encounters. J Neurosci. 1997;17:692–699. doi: 10.1523/JNEUROSCI.17-02-00709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kravitz EA. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- 33.Leonard BE. Sub-types of serotonin receptors: biochemical changes and pharmacological consequences. Int Clin Psychopharmacol. 1992;7:13–21. doi: 10.1097/00004850-199200710-00002. [DOI] [PubMed] [Google Scholar]
- 34.Linnoila VM, Virkkunen M. Aggression, suicidality and serotonin. J Clin Psychol [Suppl] 1992;53:46–51. [PubMed] [Google Scholar]
- 35.Livingstone MS, Harris-Warrick RM, Kravitz EA. Serotonin and octopamine produce opposite postures in lobsters. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- 36.Livingstone MS, Schaeffer SF, Kravitz EA. Biochemistry and ultrastructure of serotonergic nerve endings in the lobster: serotonin and octopamine are contained in different nerve endings. J Neurobiol. 1981;12:27–54. doi: 10.1002/neu.480120104. [DOI] [PubMed] [Google Scholar]
- 37.Ma PM, Beltz BS, Kravitz EA. Serotonin-containing neurons in lobsters: their role as “gain-setters” in postural control mechanisms. J Neurophysiol. 1992;68:36–54. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- 38.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 39.Raleigh MJ, Brammer GL, Yuwiler A, Flannery JW, McGuire MT, Geller E. Serotonergic influences on the social behavior of vervet monkeys (Cercopithecus aethiops sabaeus). Exp Neurol. 1980;68:322–334. doi: 10.1016/0014-4886(80)90089-8. [DOI] [PubMed] [Google Scholar]
- 40.Raleigh MJ, Brammer GL, McGuire MT, Yuwiler A. Dominant social status facilitates the behavioral effects of serotonergic agonists. Brain Res. 1985;348:274–282. doi: 10.1016/0006-8993(85)90445-7. [DOI] [PubMed] [Google Scholar]
- 41.Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- 42.Real D, Czternasty G. Mapping of serotonin-like immunoreactivity in the ventral nerve cord of crayfish. Brain Res. 1990;521:203–212. doi: 10.1016/0006-8993(90)91544-q. [DOI] [PubMed] [Google Scholar]
- 43.Roberts A, Krasne FB, Hagiwara G, Wine JJ, Kramer AP. Segmental giant: evidence for a driver neuron interposed between command and motor neurons in the crayfish escape system. J Neurophysiol. 1982;47:761–781. doi: 10.1152/jn.1982.47.5.761. [DOI] [PubMed] [Google Scholar]
- 44.Shively CA, Brammer GL, Kaplan JR, Raleigh MJ, Manuck SB. The complex relationship between behavioral attributes, social status, and whole blood serotonin in male Macaca fascicularis. Am J Primatol. 1991;23:99–112. doi: 10.1002/ajp.1350230204. [DOI] [PubMed] [Google Scholar]
- 45.Steklis HD, Raleigh MJ, Kling A, Tachiki K. Biochemical and hormonal correlates of dominance and social behavior in all-male groups of squirrel monkeys (Saimiri sciureus). Am J Primatol. 1986;11:133–146. doi: 10.1002/ajp.1350110206. [DOI] [PubMed] [Google Scholar]
- 46.Van Harreveld AA. A physiological solution for freshwater crustaceans. Proc Soc Exp Biol Med. 1936;344:428–432. [Google Scholar]
- 47.Vu ET, Krasne FB. Crayfish tonic inhibition: prolonged modulation of behavioral excitability by classical GABAergic inhibition. J Neurosci. 1993;13:4394–4402. doi: 10.1523/JNEUROSCI.13-10-04394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe A, Grundfest H. Impulse propagation at the septal and commissural junctions of crayfish lateral giant axons. J Gen Physiol. 1961;45:267–308. doi: 10.1085/jgp.45.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White SA, Fernald RD. Gonadotropin-releasing hormone-containing neurons change size with reproductive state in female Haplochromis burtonii. J Neurosci. 1993;13:434–441. doi: 10.1523/JNEUROSCI.13-02-00434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiersma CAG. Giant nerve fiber system of the crayfish: a contribution to comparative physiology of synapse. J Neurophysiol. 1947;10:23–38. doi: 10.1152/jn.1947.10.1.23. [DOI] [PubMed] [Google Scholar]
- 51.Winberg S, Nilsson GE, Olsen KH. Social rank and brain levels of monoamines and monoamine metabolites in arctic charr, Salvelinus aopinus. J Comp Physiol [A] 1991;168:241–246. [Google Scholar]
- 52.Winberg S, Nulsson GE, Olsen KH (1992) Changes in brain serotonergic activity during hierarchic behavior in arctic charr (Salvelinus alpinus L.) are socially induced J Comp Physiol [A] 170:93–99. [DOI] [PubMed]
- 53.Winberg S, Carter CG, McCarthy ID, Zhong-Yang H, Nilsson GE, Houlihan DF. Feeding rank and brain serotonergic activity in rainbow trout Onchorhynchus mykiss. J Exp Biol. 1993;179:197–211. [Google Scholar]
- 54.Yeh S-R, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- 55.Yodyingyuard U, de la Riva C, Abbott DH, Keverne EB. Relationship between dominance hierarchy, cerebrospinal fluid levels of amine transmitter metabolites (5-hydroxyindole acetic acid and homovanillic acid) and plasma cortisol in monkeys. Neuroscience. 1985;16:851–858. doi: 10.1016/0306-4522(85)90099-5. [DOI] [PubMed] [Google Scholar]
- 56.Zhang B, Harris-Warrick RM. Multiple receptors mediate the modulatory effects of serotonergic neurons in a small neural network. J Exp Biol. 1994;190:55–77. doi: 10.1242/jeb.190.1.55. [DOI] [PubMed] [Google Scholar]
- 57.Zhu H, Wu F, Schacher S. Change in expression and distribution of Aplysia cell adhesion molecules can influence synapse formation and elimination in vitro. J Neurosci. 1995;15:4173–4183. doi: 10.1523/JNEUROSCI.15-06-04173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zucker RS. Crayfish escape behavior and central synapses. I. Neural circuit exciting the lateral giant fiber. J Neurophysiol. 1972a;35:599–620. doi: 10.1152/jn.1972.35.5.599. [DOI] [PubMed] [Google Scholar]
- 59.Zucker RS. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972b;35:621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]