Abstract
Coexpression of the cloned voltage-dependent Ca2+ channel α2δ subunit with the pore-forming α1 subunit results in a significant increase in macroscopic current amplitude. To gain insight into the mechanism underlying this interaction, we have examined the regulatory effect of either the α2δ complex or the δ subunit on the Ca2+ channel α1 subunit. Transient transfection of tsA201 cells with the cardiac L-type α1Csubunit alone resulted in the expression of inward voltage-activated currents as well as measurable [3H]-PN200-110 binding to membranes from transfected cells. Coexpression of the α2δ subunit significantly increased the macroscopic current amplitude, altered the voltage dependence and the kinetics of the current, and enhanced [3H]-PN200-110 binding. Except for the increase in amplitude, coexpression of the δ subunit reproduced entirely the effects of the full-length α2δ subunit on the biophysical properties of the α1Ccurrents. However, no effect on specific [3H]-PN200-110 binding was observed on δ subunit coexpression. Likewise, profound effects on current kinetics of the neuronal α1A subunit were observed on coexpression of the α2δ complex in Xenopus oocytes. Furthermore, by using a chimeric strategy, we localized the region involved in this regulation to the transmembrane domain of the δ subunit. These data strongly suggest that the molecular determinants involved in α2δ regulation are conserved across L-type and non-L type Ca2+ channels. Taken together, our results indicate that the region of the α2δ subunit involved in the modulation of the gating properties of the high voltage-activated calcium channels is localized in the δ domain of the protein. In contrast, the level of membrane expression of functional channels relies on the presence of the α2 domain of the α2δ complex.
Keywords: L-type Ca channel, P/Q-type Ca channels, α2δ subunit, δ subunit, transient expression, tsA201 cells, dihydropyridine binding
Voltage-gated Ca2+ channels are multisubunit protein complexes that control the entry of Ca2+ions across the membrane of excitable cells and play a major role in several physiological processes, including neurotransmission, muscle contraction, hormone secretion, and gene expression. Five classes of voltage-gated Ca2+channels have been described so far on the basis of their biophysical and pharmacological properties (T-, L-, N-, P/Q-, and R-types). Functional differences among Ca2+ channel types are attributable to several factors, including the expression of distinct α1 pore-forming proteins and the selective association of β and α2δ regulatory subunits (for review, seeCatterall, 1995; Dunlap et al., 1995; De Waard et al., 1996).
According to available biochemical (Chang and Hosey, 1988; Schneider and Hofmann, 1988; Kuniyasu et al., 1992; Tokumaru et al., 1992) and molecular biological data (Mikami et al., 1989; Hullin et al., 1992;Perez-Reyes et al., 1992; Collin et al., 1993), Ca2+channels are composed of at least three subunits: α1, β, and α2δ. Expression of the cloned β subunit results in an increase in current amplitude and changes the biophysical properties of the α1 pore-forming subunit (Mori et al., 1991; Hullin et al., 1992; Perez-Reyes et al., 1992; Neely et al., 1993, 1995; Nishimura et al., 1993; Chien et al., 1995; Massa et al., 1995; Pérez-García et al., 1995; Kamp et al., 1996). Likewise, functional coexpression of the α2δ subunit, the product of a single gene that is post-translationally processed to yield separate subunits (α2 and δ) linked by disulfide bonds (De Jongh et al., 1990; Jay et al., 1991), also results in significantly increased macroscopic currents through α1/β recombinant calcium channels (Singer et al., 1991; Itagaki et al., 1992; Williams et al., 1992; Shistik et al., 1995; Bangalore et al., 1996; Wiser et al., 1996). Effects on dihydropyridine (DHP) binding also have been attributed to expression of the β and α2δ auxiliary subunits (Welling et al., 1993; Mitterdorfer et al., 1994; Wei et al., 1995).
Although these studies suggest multiple roles for the α2δ and β subunits in the processing and function of Ca2+ channels, little has been reported about the mechanisms of interaction between the α1 and α2δ subunits without the modulatory effect of the β subunits. Even less is known about the functional significance of the δ subunit. To begin to address how these proteins participate in channel function, we have studied the regulatory effects of both the α2δ ancillary complex and the δ subunit on the cardiac L-type and the neuronal class A α1 pore-forming subunits.
MATERIALS AND METHODS
Cell culture and transfection. Human embryonic kidney tsA201 cells (HEK293 cells transformed with SV40 large T antigen) were grown in DMEM–high glucose supplemented with 10% equine serum, 2 mml-glutamine, 110 mg/l sodium pyruvate, and 50 μg/ml gentamycin at 37°C in a 5% CO2 and 95% air humidified atmosphere. Transfections were performed with the calcium phosphate method (Ausubel et al., 1995) with 10 μg of plasmid cDNA encoding the rabbit L-type Ca2+ channel α1C pore-forming subunit (Wei et al., 1991) alone or in combination (molar ratio 1:1) with either plasmid cDNA encoding the rat full-length Ca2+ channel α2δ regulatory complex (Kim et al., 1992; Gurnett et al., 1996) or the δ subunit alone (Gurnett et al., 1996). The plasmid cDNA-encoding δ subunit was made by assembling a PCR fragment in the pcDNA3 mammalian expression plasmid (Invitrogen, San Diego, CA) after the α2δ signal sequence. The δN-myc plasmid used in Western blot analysis was made by using two sequential PCR reactions and ligating the product into the KpnI and EcoRI sites of the pcDNA3 vector. For electrophysiology, 3 μg of a plasmid DNA encoding the CD8 surface marker (EBO-pcD-Leu2; American Type Culture Collection, Rockville, MD) also was added to the DNA transfection mixture to select cells that expressed Ca2+ channels.
Immunoblotting and in vitro translation. tsA201 cells were harvested 2–3 d after transfection, and cell microsomes were prepared. Cells were lifted off plates into PBS, collected by centrifugation, and resuspended in lysis buffer (50 mmTris-HCl, pH 7.4) in the presence of protease inhibitors (in μm): 0.8 aprotinin, 640 benzamidine, 1.1 leupeptin, 0.7 pepstatin A, and 230 PMSF. Cells were homogenized and centrifuged at 1300 × g for 5 min. The microsomes in the supernatant were collected by centrifugation at 130,000 × g for 37 min, resuspended in 0.3 m sucrose, 20 mm Tris, and protease inhibitors, and stored at −80°C. Subsequently, equivalent amounts (200 μg of protein) of cell microsomes were electrophoresed on 5–16% gradient SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated overnight with a polyclonal antibody against α2δ (rabbit 136; 1:400) or a monoclonal antibody against the myc epitope (9E10; 1:1000), subsequently incubated with either horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Boehringer Mannheim, Indianapolis, IN) at 1:1000, and visualized with ECL. Ca2+ channel subunits also were synthesized by coupled in vitro transcription and translation with the TNT lysate system (Promega, Madison, WI) from the same plasmid cDNA used in transfections and analyzed by SDS-PAGE.
Electrophysiology. One day after transfection tsA201 cells were transferred to poly-l-lysine-coated coverslips and grown for 1–2 d until used for electrophysiology. To select Ca2+ channel-expressing cells, we incubated a coverslip immediately before recording in bathing solution (see below) containing 1–2 × 106/ml paramagnetic beads precoated with a monoclonal antibody (ITI-5C2) specific for the CD8 membrane antigen (Dynal, Lake Success, NY), and the positively transfected cells were subjected to the whole-cell mode of the patch-clamp technique (Hamill et al., 1981). Briefly, patch pipettes were pulled from borosilicate glass capillaries. Typical pipette resistances were 2–5 MΩ when filled with internal solution. Currents were recorded with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) and acquired on-line, using a TL-1 interface with pClamp 6 software (Axon). After the whole-cell mode had been established, capacitive transients were canceled with the amplifier. Currents were obtained from a holding potential of −80 mV by applying test pulses every 20 sec, and data were leak-subtracted on-line by a standard P/4 protocol. Current signals were filtered at 1–2 kHz (internal four-pole Bessel filter) and digitized at 20 or 1.67 kHz, depending on the duration of the voltage steps. The bath solution contained (in mm): 40 BaCl2, 125 TEA-Cl, 10 HEPES, and 5 glucose, pH 7.3. The internal solution consisted of (in mm): 135 CsCl, 5 MgCl2, 10 EGTA, 10 HEPES, 4 Mg-ATP, and 0.1 GTP, pH 7.3.
Electrophysiological analysis of calcium currents expressed inXenopus oocytes was performed as described previously (De Waard and Campbell, 1995). Briefly, the follicle membranes of stage V and VI isolated Xenopus laevis oocytes were digested enzymatically with 2 mg/ml collagenase IA (Sigma, St. Louis, MO) to facilitate injection and recording. cRNAs were transcribed in vitro, using T7 RNA polymerase or SP6 RNA polymerase in the case of α1A. Oocytes were injected with 50 nl of various subunit composition at the following cRNA concentrations: 0.7 μg/μl α1A, 0.1 μg/μl β4, and 0.7 μg/μl full-length α2δ subunit or α2Ad chimeric construct (Gurnett et al., 1996). Currents were recorded by the two-electrode voltage-clamp method with a TEV-200 amplifier (Dagan, Minneapolis, MN). Both voltage and current electrodes were filled with 3 m KCl and had resistances of ∼0.5 MΩ. Peak Ba2+ currents were measured for a test potential of 0 mV from a holding potential of −90 mV. Recordings were filtered at 0.5 kHz, sampled at 5 kHz, and analyzed by pClamp 6. Leak and capacitance currents were subtracted on-line by a P/6 protocol. The bath solution contained (in mm): 40 Ba(OH)2, 50 NaOH, 2 KCl, 1 niflumic acid, 0.1 EGTA, and 5 HEPES, pH 7.4.
Radioligand binding. The effect of the Ca2+ channel auxiliary subunits on the dihydropyridine binding of the α1C-transfected cells was characterized as follows. Aliquots (80 μg of protein) of cell microsomes prepared as mentioned above were resuspended in a total volume of 400 μl of buffer A (50 mm Tris, 0.1% BSA, and protease inhibitors) and incubated with increasing concentrations of (+)-[methyl-3H]-PN200-110 (Amersham, Arlington Heights, IL) in the dark at 37°C. After 60 min the receptor–ligand complexes were collected and washed with buffer A on Whatman GF/B filters with a cell harvester. Nonspecific binding was determined by the addition of 50 μm nitrendipine 10–15 min before the addition of [3H]-PN200-110. Specific binding was calculated by subtracting nonspecific from total binding.
Statistical analysis. The data are given as mean ± SE, and the number of experiments is indicated in the figure legends. Statistical differences between two means were determined by Student’st tests. Means were considered significantly different whenp < 0.05.
RESULTS
In vitro transcription–translation and cell expression of Ca2+ channel subunits
The plasmids used in the transfection initially were examined via a cell-free transcription–translation system. The α1C, α2δ, and δ cDNA clones directed the synthesis of three polypeptides of the expected molecular weight. Likewise, the expression of the α2δ complex and the δ subunit (containing the myc epitope fused to its N terminus) was demonstrated in tsA201 cells 48 hr post-transfection by Western blot analysis with either an antibody against the α2protein or the anti-myc antibody, respectively (data not shown). The expression of the α1C subunit (the pore-forming and DHP-sensitive component of the channel) was detected by electrophysiology and binding experiments, as detailed below.
Effect of the regulatory subunits on current amplitude
We initiated the study of the regulatory effects of the α2δ complex on the L-type Ca2+channel by comparing the fundamental biophysical properties between cells transiently transfected with constructs encoding α1C alone or cotransfected with the α2δ or δ subunits. Figure1A shows representative examples of mean current traces (average of three successive sweeps) obtained during 150 msec pulses in untransfected (control) and transfected cells at a test potential of +30 mV. The top trace reveals the absence of endogenous voltage-activated Ca2+channels in control cells. In contrast, transfection with the Ca2+ channel α1C subunit resulted in the expression of inward voltage-activated currents both in the presence and absence of the auxiliary subunits. A large increase in current amplitude was observed on coexpression of the full-length α2δ subunit: the peak current amplitude was increased approximately threefold at +30 mV (Fig. 1B, Table1). Although coexpression of the δ subunit did not modify the magnitude of the α1C currents, evident changes in voltage dependence and waveform were observed (see below).
Fig. 1.
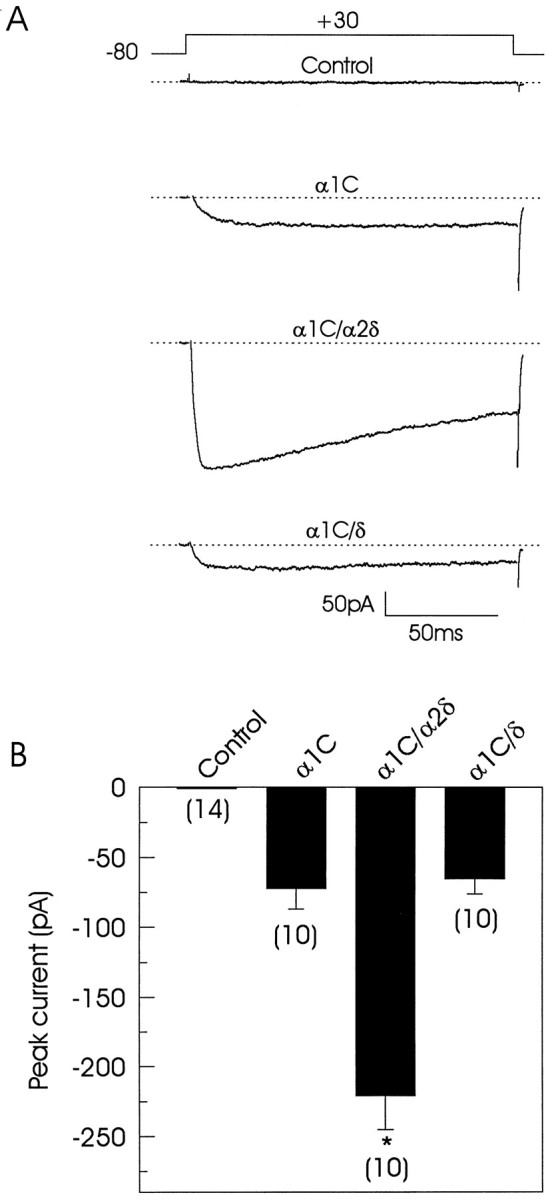
Whole-cell currents in tsA201 transiently transfected cells. A, Ba2+ currents induced by activating pulses in four different representative cells.Top trace corresponds to the current recorded in a control (untransfected) cell. Lower traces correspond to current records that were obtained in cells transfected with Ca2+ channel subunits in various combinations. The voltage protocol is shown above the traces, and thedotted line represents the baseline current.B, Comparison of peak current amplitudes at +30 mV in control and transfected cells. Data are expressed as mean ± SE, and the number of recorded cells is indicated inparentheses. Statistical significance of the difference between singly transfected and cotransfected cells was determined by Student’s t test (*p < 0.05).
Table 1.
Differential effects of α2δ and δ on the biophysical and binding properties of the α1C currents
| Properties | α1C | α1C/α2δ | α1C/δ |
|---|---|---|---|
| Peak current amplitude (pA) | −72 ± 15 | −221 ± 24 | −65 ± 11 |
| Apparent activation threshold (mV) | ≥−10 | ≥−20 | ≥−20 |
| Potential for half-activation (mV) | 19.6 | 8.7 | 8.5 |
| Potential for half steady-state inactivation (mV) | −6.8 | −15.9 | −16.4 |
| Decay of the current at 150 msec* (%) | 19.5 ± 4.2 | 39.2 ± 9.8 | 36.4 ± 6.2 |
| Rate of inactivation at +20 mV (μsec−1) | 2.5 ± 0.5 | 4.3 ± 0.4 | 4.6 ± 0.7 |
| Bmax (fmol/mg of protein) | 67 ± 29 | 141 ± 39 | 41 ± 10 |
| KD(nm) | 0.9 ± 0.5 | 0.1 ± 0.04 | 2.4 ± 0.9 |
Currents were recorded during depolarizations from −80 to +30 mV.
Bmax, Maximum binding capacity;KD, dissociation constant (mean ± SE; n = 3–5).
Effect of the auxiliary subunits on the voltage dependence of the expressed currents
Previous studies in our laboratory with neuronal class A recombinant Ca2+ channels expressed inXenopus oocytes demonstrated that coexpression of the δ subunit did not result in an enhancement of current amplitude (Gurnett et al., 1996). The question was raised, however, whether this protein directly interacted with the α1 subunit although it had no effect on the one parameter studied (current amplitude). In the present study a direct comparison of the electrophysiological properties of singly transfected and cotransfected cells showed that the concomitant expression of α2δ not only increased current amplitude but also influenced the voltage dependence of activation and inactivation. This allowed us then to study the effect of the δ subunit on these properties. As illustrated in Figure2A, in cells transfected with α1C only, the current begins to turn on at −10 mV and reaches the peak at +30 mV. When the peak currents measured at each test potential were normalized to the maximum current observed in each cell, averaged and plotted as a function of test potential, a ∼10 mV shift in the I–V curve in the hyperpolarizing direction was observed in the cells that expressed the α2δ subunit. Cotransfection of the δ subunit resulted in a similar shift in the voltage dependence of activation (Table1).
Fig. 2.
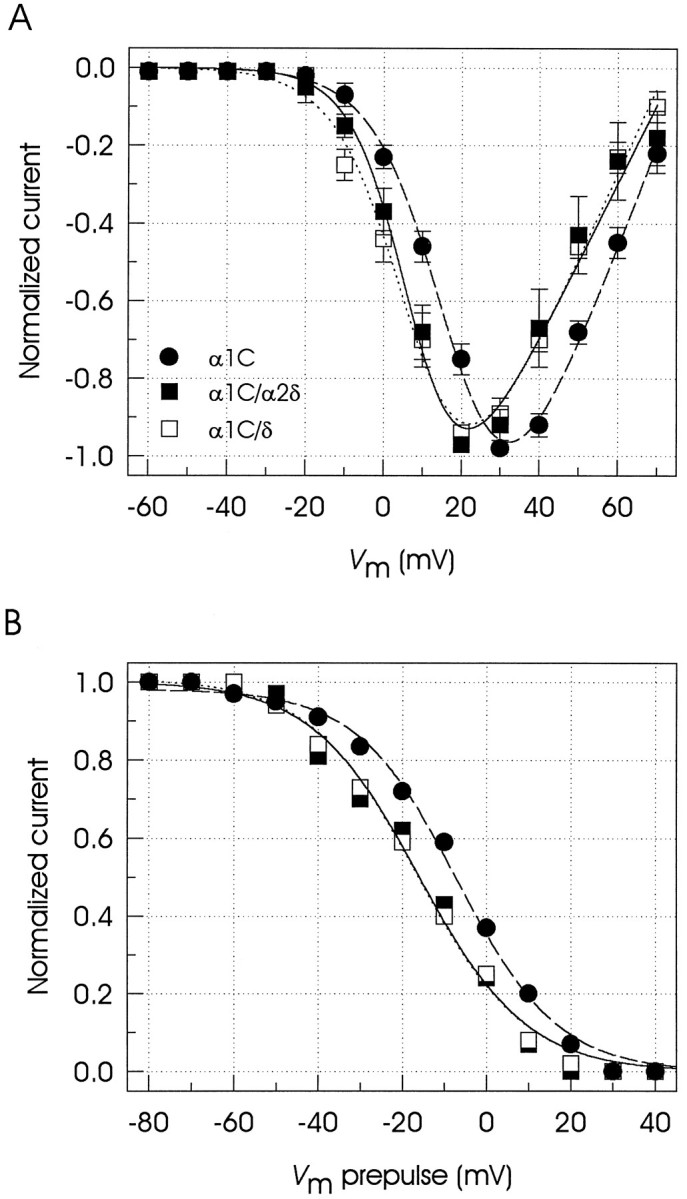
Voltage dependence of the currents through expressed Ca2+ channels. A, Plot of peak normalized current–voltage relationships in transiently transfected cells. Currents were recorded in response to 150 msec depolarizations from a holding potential of −80 mV with a 10 mV increase in the pulse amplitude per step. Symbolsrepresent mean ± SE values of five to eight cells in each condition. Fits of the I–V curves were obtained assuming an activation curve of a Boltzmann type:IBa = [g(Vm −Vrev)]/(1 + exp[−(Vm −V1/2)/s]), whereg is the conductance, Vmrepresents the test potential, Vrev is the apparent reversal potential, and s is the range of potential for an e-fold change aroundV1/2. B, Measurement of the voltage dependence of inactivation at steady state. The graph shows peak currents at +30 mV as a function of the prepulse potential for three different cells (α1C, •; α1C/α2δ, ▪; α1C/δ, □). A series of 13 different 1.2 sec prepulse potentials from −80 to +40 mV was applied first, and the inactivated currents were measured with 50 msec test pulses. The obtained inactivation curves were fit with a Boltzmann function of the form: IBa =Imax/(1 + exp[(Vm −V1/2)/s]), where the current amplitude IBa has decreased to a half-amplitude at V1/2 with ane-fold change over s mV. Pertinent parameters of the fits are given in Table 1.
The development of steady-state inactivation was studied by holding the cells for 1.2 sec at potentials ranging successively from −80 through +40 mV before a 50 msec step depolarization to a test potential of +30 mV. Figure 2B shows that the inactivation of the α1C currents occurs at relatively depolarized holding potentials; this has been reported also for other expressed high-voltage-activated channels (De Waard and Campbell, 1995). More importantly, coexpression of either α2δ or δ regulatory subunits induced a ∼10 mV hyperpolarizing shift in the voltage for 50% steady-state inactivation (Table 1).
Effect of the auxiliary subunits on current kinetics
We also investigated whether other properties of the expressed channels such as the inactivation kinetics could be modified by the presence of the regulatory subunits. Hence, the effect of the α2δ and δ subunits on inactivation was estimated from the percentage of current remaining after 150 msec activating pulses (Fig. 3A). The presence of either regulatory subunit decreased significantly the percentage of the remaining current at the end of the depolarizing pulse, indicating that both α2δ and δ subunits were able to accelerate inactivation of the α1C currents.
Fig. 3.
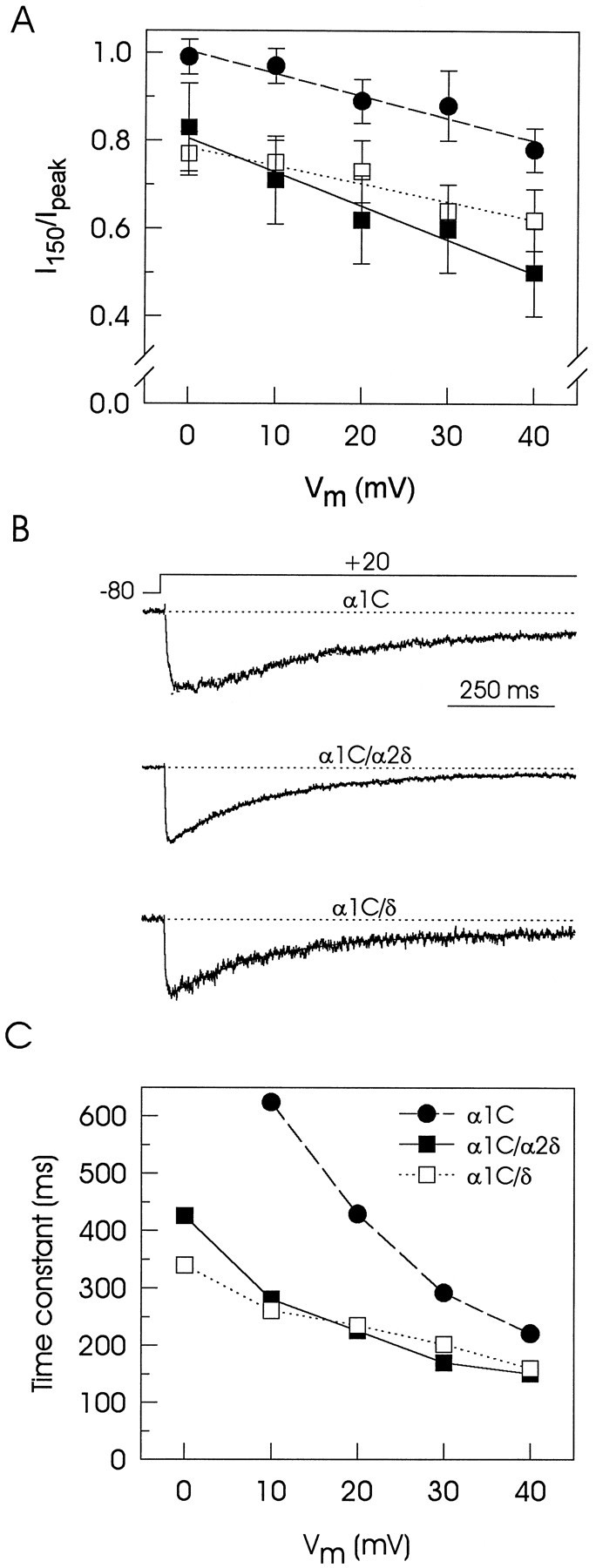
Effect of the α2δ complex on inactivation kinetics in transiently transfected tsA201 cells.A, The percentage of current remaining 150 msec into the depolarizing pulse is plotted at various membrane potentials in α1C (•), α1C/α2δ (▪), and α1C/δ (□) expressing cells. Astraight line provided a close fit to these data, and although of no theoretical significance, it was used to emphasize differences between singly transfected and cotransfected cells (n = 10). B, Representative records of currents obtained from three different transfected cells. The currents were recorded in response to 1 sec depolarizing pulses from −80 to +20 mV and ranged from −70 to −150 pA but are shown normalized to allow for comparison of kinetics. The inactivating phase of the currents was fit (superimposed lines) with a single exponential equation of the form: IBa= Aexp(−t/τ) + c, whereA is the initial amplitude, t is time, τ is the time constant for inactivation, and c is a constant. C, Time constants of α1Ccurrents are plotted at various membrane potentials. The same cells were used in B and C and represent typical values in each group (n = 3 cells). Mean ± SE values are given in Table 1.
To analyze this action in more detail, we estimated voltage-dependent inactivation from the time course of the currents during 1 sec activating pulses to +20 mV by fitting the decaying component with a single exponential equation. The use of Ba2+ as the charge carrier in these experiments minimized any Ca2+-dependent inactivation. Traces in Figure3B exemplify normalized representative records of membrane currents in singly transfected and cotransfected cells and show that variation in channel subunit composition results in different inactivation behavior. The decay of the macroscopic currents recorded from cells expressing α1C is slow as compared with those produced by coexpression of α1C with either the full-length α2δ subunit or the δ subunit. Figure3C compares the time constants of the currents expressed as a function of the step voltage and clearly indicates that the currents induced in the presence of the regulatory subunits inactivate faster than the currents recorded in cells transfected with only the α1C subunit.
To test the individual contribution of the α2 and the δ domains on the inactivation kinetics of the macroscopic currents and to determine whether the molecular determinants involved in the α2δ subunit regulation were conserved across L-type and non-L type Ca2+ channels, we performed additional electrophysiological recordings in Xenopus oocytes expressing the neuronal α1A subunit. Because the α2 domain is completely extracellular (Gurnett et al., 1996; Wiser et al., 1996) and does not bind directly to the α1 subunit in the absence of the δ domain (Gurnett et al., 1997), the interaction between these two proteins is difficult to assess. To overcome this problem, we created an α2δ chimeric subunit in which the transmembrane and the cytoplasmic regions of the δ subunit were substituted by equivalent sequences of adhalin, an unrelated type I transmembrane protein (α2Ad; Gurnett et al., 1996). This chimera has been shown to coimmunoprecipitate with the α1 subunit (Gurnett et al., 1997) and allowed us to test the participation of the α2 and the δ domains on the acceleration of the inactivation rate of the α1expressed currents.
In agreement with previous electrophysiological studies inXenopus oocytes (Mori et al., 1991; Williams et al., 1992;Ellinor et al., 1993), we observed that expression of the neuronal Ca2+ channel α1A pore-forming subunit alone generally resulted in small current density, although high concentrations of Ba2+ were used to increase the resolution of the currents. However, coexpression of the β4 subunit allowed to us to increase the expression of the α1A subunit to levels that permitted a systematic characterization of the biophysical properties of inactivation as detailed below.
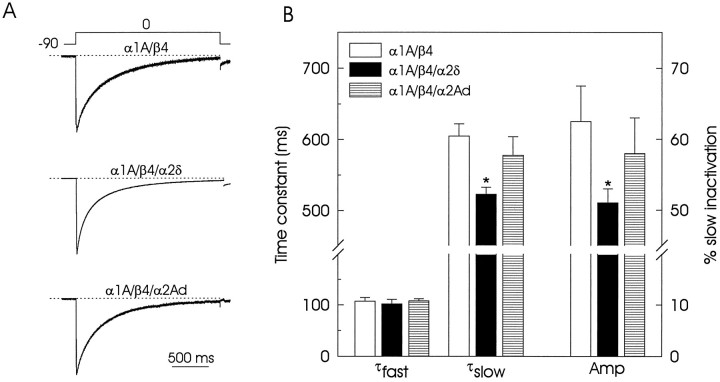
Figure 4A compares the time course of normalized representative traces during 2 sec depolarizing steps from −90 to 0 mV recorded in oocytes injected with various subunit combinations: α1A/β4; α1A/β4/α2δ, and α1A/β4/α2Ad. In the same manner as in tsA201 transfected cells, the α1currents in oocytes decayed during sustained depolarization as a result of the voltage-dependent inactivation of the channels. However, the time course of this inactivation consisted of two kinetic components: the first component followed a fast time constant and constituted ∼35% of the total inactivating current, and the second component was represented by a slower time constant and constituted the remaining ∼65%. As illustrated in Figure 4B, the average value of the time constant for the fast component (τfast) was approximately the same in the three sets of oocytes investigated. Conversely, the time constant for the slow component (τslow) was clearly faster in the α2δ subunit-expressing oocytes than in the other two groups of cells. In addition, the amplitude of the slow component (Amp) also was reduced greatly in the oocytes injected with the α2δ subunit. The expression of the chimera (α2Ad) did not affect the inactivation kinetics significantly, although there was a slight but not statistically significant reduction in both the time constant and the amplitude of the slow component of the current. These results suggest that the presence of an intact δ domain, including transmembrane sequence, is necessary for the acceleration in the inactivation rate of the α1 currents on α2δ subunit expression.
Fig. 4.
Time course of inactivation of the currents through recombinant calcium channels expressed inXenopus oocytes. A, Normalized representative currents for oocytes coexpressing the α1Aand the β4 subunits in the absence or in the presence of the full-length α2δ or the chimeric α2Ad subunits. In all cases the decay of the currents was poorly fit by a single exponential. The sum of two exponential functions was necessary:IBa = I∞+Af(exp(−t/τf) +As(exp(−t/τs) + c, where I∞ is the steady-state inward current, A is the amplitude,t is time, and τf and τs are time constants for the fast and slow components, respectively.B, Pooled data comparing the average time constants (τfast and τslow) and relative contribution of the slow component of inactivation amplitude (Amp) at 0 mV from various oocytes in the three groups (n = 6). Asterisks denote significant differences (p < 0.05).
Modulation of the DHP binding
To characterize the DHP-binding affinity of the α1C-transfected cells and its possible modulation by the α2δ subunit, we performed equilibrium radioligand binding experiments that used [3H]-PN200-110. In an initial series of experiments we observed that α1Ccurrents were sensitive to micromolar concentrations of DHPs (data not shown). Accordingly, Figure 5Ashows representative saturation isotherms of [3H]-PN200-110 binding to microsomes from α1C, α1C/α2δ, and α1C/δ-coexpressing cells. Specific binding was not observed in untransfected cells, consistent with the absence of endogenous Ca2+ currents and the absence of detectable levels of Ca2+ channel subunit protein expression. Scatchard analysis of the [3H]-PN200-110 saturation binding data revealed very similar values for the apparent dissociation constant (KD) and the total number of binding sites (Bmax) on both singly transfected cells and cells coexpressing the δ subunit. In marked contrast, when singly transfected cells and cells coexpressing the α2δ subunit were compared, a significant increase in both the number of binding sites and in affinity for the drug was observed (Table 1).
Fig. 5.
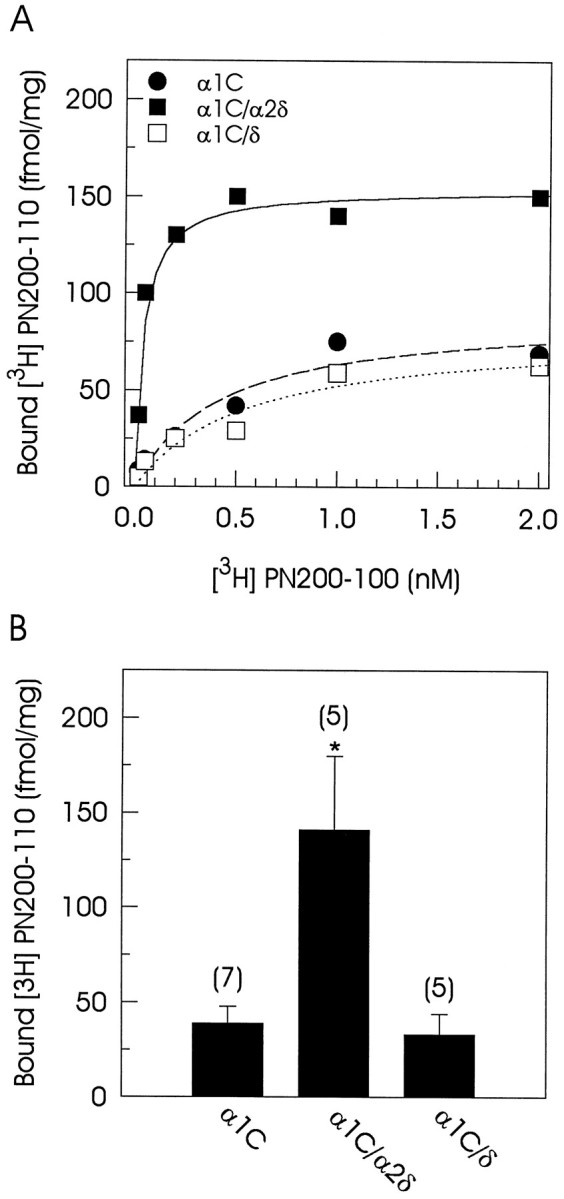
Dihydropyridine binding to the expressed Ca2+ channels. A, Equilibrium binding curves for [3H]-PN200-110 in cells transfected with α1C alone or in combination with the α2δ or the δ subunits. Symbolsrepresent mean values of specific DHP binding of duplicate samples in one representative experiment. Data were fit with a one-site ligand-binding equation of the form: y = [L] · Cap/(KD + [L]), where L is the concentration of free ligand, Cap is the maximum bound ligand, andKD is the dissociation constant.B, Comparison of specific [3H]-PN200-110 binding to microsomes of tsA201 cells transfected with α1C alone or in combination with α2δ or δ subunits. Binding assays were performed with 1 nm [3H]-PN200-110. Data are given as mean ± SE, and the number of separate experiments is indicated in parentheses. Asterisksdenote significant differences (p < 0.05).
To elucidate the mechanisms underlying the large α2δ-induced increase in α1C current amplitude, we compared current amplitude and DHP binding in both singly transfected and cotransfected cells. Because DHPs bind to the α1C subunit, an increase in the number of binding sites could account for the increase in current magnitude. Figure5B shows the comparison of specific DHP binding to microsomes from tsA201 transfected cells, using a near-saturating concentration of [3H]-PN200-110 (1 nm). Coexpression of α2δ subunit resulted in a ∼3.6-fold increase in the amount of [3H]-PN200-110 binding. When it is compared with the approximately threefold increase in current amplitude seen by coexpression of the α2δ subunit, this comparison suggests that there is an important correlation between the ability to increase current amplitude and the ability to enhance DHP binding. Consistent with this, the δ subunit was incapable of increasing either the current amplitude or the DHP binding (Table 1).
DISCUSSION
We have analyzed the effects induced by interaction of the α1C subunit with the α2δ and δ auxiliary subunits on the fundamental biophysical properties of the recombinant channels. Our data clearly show that the α2δ subunit significantly increased L-type calcium channel activity in transiently transfected tsA201 cells (Fig. 1). Likewise, as a result of this interaction, the α2δ subunit induced a hyperpolarizing shift in activation and steady-state inactivation (Fig. 2) of the expressed channels. Interestingly, we found that the δ subunit reproduced entirely the effects of the full-length α2δ subunit on the voltage dependence of the macroscopic α1C currents. These findings therefore provide evidence that the δ subunit per se can modulate the voltage-dependent behavior of the L-type Ca2+channel by interacting with the α1C subunit. Moreover, it may localize the region involved in modulation of voltage dependence to the δ domain of the α2δ complex such that the interaction between these two proteins may affect the S4 voltage sensor.
Another manifestation of the interaction between the α1Csubunit with the regulatory subunits is the acceleration of the inactivation rate. The traces in Figure 3B show that, during depolarization, inward currents in transfected tsA201 cells spontaneously decay in external Ba2+, indicating that the expressed calcium channels undergo voltage-dependent inactivation. The time course of the decaying component of these currents is well described with a single exponential equation. We found that expression of the α2δ subunit influenced the rate at which the currents inactivated. Again, the δ domain completely reproduced the effect of the full-length α2δ complex on inactivation kinetics (Table 1). Consistent with the above-mentioned findings, the percentage of decay of the α1C currents was increased significantly in the presence of either regulatory subunit when inactivation was estimated from the percentage of current remaining after 150 msec activating pulses (Fig. 3A, Table1).
In contrast with the monoexponential decay of the currents in the transfected tsA201 cells, the Ca2+ channels expressed in Xenopus oocytes inactivated in a biexponential manner (Fig. 4). Although many factors may give rise to two components, several experimental findings suggested to us that this result may reflect an intrinsic property of the neuronal expressed channels: the endogenous currents were negligible; the ratio of the slow and fast current amplitudes remained constant from oocyte to oocyte and was independent of the total current amplitude recorded. Moreover, our results indicate that the slow inactivating component of these currents was sensitive to the regulatory effect of the α2δ subunit. However, coexpression of the α2Ad chimera, in which the transmembrane domain of the α2δ complex (δ subunit) was replaced with that of adhalin, did not modify the inactivation kinetics of the α1A/β4channels. This suggests that the δ transmembrane domain may be the primary moiety involved in this regulation. Interestingly, because the molecular determinants of voltage-dependent inactivation in Ca2+ channels have been localized to the membrane-spanning segment S6 of the first repeat (IS6) of the α1 subunit (Zhang et al., 1994), this region also may be involved in the interaction with the δ subunit.
Another regulatory action of the α2δ complex was the drastic increase in α1C current amplitude. The mechanisms underlying this action may be better understood by comparing current amplitude and DHP binding in both singly transfected and cotransfected cells. Our results demonstrated that the total number of DHP binding sites increased on coexpression of the α2δ subunit. In addition, there was a large augmentation in the affinity for the DHP when α2δ was present (Fig. 5, Table 1). These results confirm that the α2δ subunit is crucial to the reconstitution of DHP binding (Wei et al., 1995), because the binding affinity and number of binding sites approached that of rabbit cardiac microsomes (Nishimura et al., 1993; Wei et al., 1995) only when the α2δ subunit was coexpressed. Furthermore, these observations suggest that the α2δ complex acts primarily by inducing important conformational changes in the pore-forming subunit. These changes in α1C conformation then would be responsible for an increase not only in the accessibility of the drug to its site but also in the opening probability of the channel, as it has been observed in Xenopus oocytes (Shistik et al., 1995). Because the binding site for DHPs has been localized to the IIIS5–S6 and IVS5–S6 regions (Grabner et al., 1996; Peterson et al., 1996), these sites also may have been involved in the interaction with the α2δ complex.
An alternative possibility to explain the results could be that the conformational changes induced by the α2δ complex may play an important role in the localization of the expressed channels on the cell surface. In support of this interpretation, Bangalore et al. (1996) have demonstrated that the coexpression of the α2δ complex increases the number of functional L-type calcium channels in the cell membrane as gauged by gating charge movement. In addition, Shistik et al. (1995) have shown that the α2δ complex triples the amount of α1Cprotein localized in the plasma membrane of Xenopus oocytes as detected by immunoprecipitation. As mentioned above, our results indicate that coexpression of the L-type calcium channel pore-forming subunit with α2δ increased both the number of the total binding sites and the affinity for the radiolabeled PN200-110. In contrast, coexpression with the δ subunit did not affect specific DHP binding (Fig. 5, Table 1). Interestingly, the chimeric α2Ad subunit has been shown to mimic the effects of the full-length α2δ subunit on PN200-110 binding when expressed in tsA201 cells (Gurnett et al., 1997). These findings indicate not only that expression of the α2 domain is necessary for the formation of a stable interaction capable of reconstituting normal DHP binding but also suggest that coexpression of this domain of the α2δ protein may facilitate proper insertion of channel proteins into the cell membrane. The mechanisms of the α2 subunit effect on the membrane trafficking of the α1 subunit require further investigation. Gating current studies will be needed to examine this issue.
Taken together, our findings indicate that the α2δ complex and the δ subunit interact in specific ways with the α1 subunit and participate in the functional regulation of the L-type and non-L type calcium channels. Our previous studies indicated that the extracellular α2 domain, which is particularly sensitive to structural modification, provides the elements required for channel stimulation (Gurnett et al., 1996). Here, we localize the region of the α2δ subunit involved in the shift in voltage-dependent activation and steady-state inactivation as well as the acceleration of the inactivation kinetics to the δ subunit, whereas the effects on increased currents and DHP binding affinity require the presence of the α2 domain of the α2δ complex.
Footnotes
R.F. is supported by a Human Frontier Science Program Organization postdoctoral fellowship. C.A.G. is supported by an American Heart Association predoctoral fellowship (Iowa affiliate). K.P.C. is an Investigator of the Howard Hughes Medical Institute. This work benefited from the use of the University of Iowa Diabetes and Endocrinology Research Center (National Institutes of Health DK25295). We thank Drs. X. Wei, T. P. Snutch, T. Tanabe, and L. Birnbaumer for providing the cDNA clones and Dr. A. George Jr for the tsA201 cell line. We are also grateful to H. Liu for experimental support and Drs. M. Henry and G. Biddlecome for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Kevin P. Campbell, Howard Hughes Medical Institute, Department of Physiology and Biophysics, University of Iowa College of Medicine, 400 Eckstein Medical Research Building, Iowa City, IA 52242.
REFERENCES
- 1.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Wiley; New York: 1995. [Google Scholar]
- 2.Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel α2δ subunit on ionic and gating current in transiently transfected HEK293 cells. Am J Physiol. 1996;270:H1521–H1528. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- 3.Catterall W. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 4.Chang FC, Hosey MM. Dihydropyridine and phenylalkylamine receptors associated with cardiac and skeletal muscle. J Biol Chem. 1988;263:18929–18937. [PubMed] [Google Scholar]
- 5.Chien A, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 6.Collin T, Wang JJ, Nargeot J, Schwartz A. Molecular cloning of three isoforms of the L-type voltage-dependent calcium channel β subunit from normal human heart. Circ Res. 1993;72:1337–1344. doi: 10.1161/01.res.72.6.1337. [DOI] [PubMed] [Google Scholar]
- 7.De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels: α2 and δ are encoded by same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- 8.De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel. J Physiol (Lond) 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Waard M, Gurnett CA, Campbell KP. Structural and functional diversity of voltage-activated calcium channels. In: Narahashi T, editor. Ion channels, Vol IV. Plenum; New York: 1996. pp. 41–87. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 11.Ellinor PT, Zhang JF, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 12.Grabner M, Wang Z, Hering S, Streissnig J, Glossmann H. Transfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 1996;16:207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- 13.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 14.Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. J Biol Chem. 1997;227:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- 15.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, Hofmann F, Flockerzi V. Calcium channel β subunit heterogeneity: functional expression of cloned cDNA from heart, aorta, and brain. EMBO J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itagaki K, Koch WJ, Bodi I, Klockner U, Slish DF, Schwartz A. Native-type DHP-sensitive calcium channel currents are produced by cloned rat aortic smooth muscle and cardiac α1 subunits expressed in Xenopus laevis oocytes and are regulated by α2- and β-subunits. FEBS Lett. 1992;297:221–225. doi: 10.1016/0014-5793(92)80542-o. [DOI] [PubMed] [Google Scholar]
- 18.Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 19.Kamp JT, Pérez-García MT, Marban E. Enhancement of ionic current and charge movement by coexpression of calcium channel β1A subunit with α1 subunit in a human embryonic kidney cell line. J Physiol (Lond) 1996;492:89–96. doi: 10.1113/jphysiol.1996.sp021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HL, Kim H, Lee P, King RG, Ching H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel α2 subunit. Proc Natl Acad Sci USA. 1992;89:3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuniyasu A, Oca K, Ide-Yamada T, Hatanaka Y, Abe T, Nakayama H, Kanaoka Y. Structural characterization of the dihydropyridine receptor-linked calcium channel from porcine heart. J Biochem. 1992;112:235–242. doi: 10.1093/oxfordjournals.jbchem.a123883. [DOI] [PubMed] [Google Scholar]
- 22.Massa E, Kelly KL, Yule DI, MacDonald RL, Uhler MD. Comparison of fura-2 imaging and electrophysiological analysis of murine calcium channel α1 subunits coexpressed with novel β2 subunit isoforms. Mol Pharmacol. 1995;47:707–716. [PubMed] [Google Scholar]
- 23.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 24.Mitterdorfer J, Froschmayr M, Grabner M, Striessnig J, Glossmann H. Calcium channels: the β-subunit increases the affinity of dihydropyridine and Ca2+ binding sites of the α1-subunit. FEBS Lett. 1994;352:141–145. doi: 10.1016/0014-5793(94)00938-4. [DOI] [PubMed] [Google Scholar]
- 25.Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 26.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Potentiation by the β subunit of the ratio of ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 27.Neely A, Olcese R, Baldelli P, Wei X, Birnbaumer L, Stefani E. Dual activation of the cardiac Ca2+ channel α1C subunit and its modulation by the β subunit. Am J Physiol. 1995;268:C732–C740. doi: 10.1152/ajpcell.1995.268.3.C732. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura S, Takeshima H, Hofmann F, Flockerzi V, Imoto K. Requirement of the calcium channel β subunit for functional conformation. FEBS Lett. 1993;324:283–286. doi: 10.1016/0014-5793(93)80135-h. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-García MT, Kamp TJ, Marban E. Functional properties of cardiac L-type calcium channels transiently expressed in HEK293 cells. Roles of α1 and β subunits. J Gen Physiol. 1995;105:289–306. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 31.Peterson BZ, Tanada TN, Catterall WA. Molecular determinants of high-affinity dihydropyridine binding in L-type calcium channels. J Biol Chem. 1996;271:5293–5296. doi: 10.1074/jbc.271.10.5293. [DOI] [PubMed] [Google Scholar]
- 32.Schneider T, Hofmann F. The bovine cardiac receptor for calcium channel blockers is a 195 kDa protein. Eur J Biochem. 1988;174:369–375. doi: 10.1111/j.1432-1033.1988.tb14107.x. [DOI] [PubMed] [Google Scholar]
- 33.Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contributions of changes in channel gating and α1 protein level. J Physiol (Lond) 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer D, Biel M, Lotan I, Flockerzi V, Hoffmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 35.Tokumaru H, Anzai K, Abe T, Kirino Y. Purification of the cardiac 1,4-dihydropyridine receptor using immunoaffinity chromatography with a monoclonal antibody against the α2δ subunit of the skeletal muscle dihydropyridine receptor. Eur J Pharmacol. 1992;227:363–370. doi: 10.1016/0922-4106(92)90152-l. [DOI] [PubMed] [Google Scholar]
- 36.Wei X, Perez-Reyes E, Lacerda AE, Schuster G, Brown AM, Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel α1 subunit by skeletal muscle β and γ subunits. J Biol Chem. 1991;266:21943–21947. [PubMed] [Google Scholar]
- 37.Wei X, Pan S, Lang W, Kim H, Schneider T, Perez-Reyes E, Birnbaumer L. Molecular determinants of cardiac Ca2+ channel pharmacology. Subunit requirement for the high affinity and allosteric regulation of dihydropyridine binding. J Biol Chem. 1995;270:27106–27111. doi: 10.1074/jbc.270.45.27106. [DOI] [PubMed] [Google Scholar]
- 38.Welling A, Bosse E, Cavalié A, Bottlendler R, Ludwig A, Nastainczyk W, Flockerzi V, Hoffmann F. Stable co-expression of calcium channel α1, β, and α2/δ subunits in a somatic cell line. J Physiol (Lond) 1993;471:749–765. doi: 10.1113/jphysiol.1993.sp019926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 40.Wiser O, Trus M, Tobi D, Halevi S, Giladi E, Atlas D. The α2/δ subunit of voltage-sensitive Ca2+ channels is a single transmembrane extracellular protein which is involved in regulated secretion. FEBS Lett. 1996;379:15–20. doi: 10.1016/0014-5793(95)01475-6. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Molecular determinants of voltage-dependent inactivation in calcium channels. Nature. 1994;372:97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]