Abstract
The primary sensory Rohon–Beard (R–B) neurons ofXenopus larvae are highly analogous to the C fibers of the mammalian pain pathway. We explored the actions of 5-HT by studying the modulation of Ca2+ currents. In ∼80% of the acutely isolated R–B neurons, 5-HT inhibited the high voltage-activated (HVA) currents by 16% (n = 29) and the T-type currents by 24% (n = 41). The modulation of the T-type and the HVA currents was mimicked by selective 5-HT1A and 5-HT1D agonists: 8-OH-DPAT and L-694,247. The effects of the agonists were blocked by their respective 5-HT1A or 5-HT1D antagonists:p-MPPI and GR127935, suggesting that both 5-HT1A and 5-HT1D receptors were involved. Approximately 70% of the actions of 5-HT on HVA currents was occluded by ω-conotoxin-GVIA (N-type channel blocker), whereas the rest of the modulation (∼30%) was occluded by <100 nmω-agatoxin-TK (P/Q-type channel blocker). This suggests that 5-HT acts on N- and P/Q-type Ca2+ channels. Neither the modulation of the T-type nor that of the HVA currents was accompanied by changes in their voltage-dependent kinetics. Cell-attached patch-clamp recordings suggest that the modulation of the T-type channel occurs through a membrane-delimited second messenger. We have studied the functional consequences of the modulation of T-type Ca2+ channels and have found that these channels play a role in spike initiation in R–B neurons. Modulation of T-type channels by 5-HT therefore could modulate the sensitivity of this sensory pathway by increasing the thresholds of R–B neurons. This is a new and potentially important locus for modulation of sensory pathways in vertebrates.
Keywords: 5-HT1A receptor, 5-HT1D receptor, T-type Ca2+ currents, ω-conotoxin-GVIA, ω-agatoxin-TK, spike initiation, Xenopus, Rohon–Beard neurons
Serotonin (5-HT) released from the descending fibers of the raphe nucleus plays an important role in limiting the access of nociceptive information from the spinal cord to higher centers (Millan, 1995). The receptors and mechanisms by which 5-HT exerts this antinociceptive action in the spinal cord have been studied incompletely. 5-HT1A (Eide et al., 1990; Crisp et al., 1991; Lucas et al., 1993; Del Mar et al., 1994), 5-HT1B (Eide et al., 1990) and 5-HT2A/2C (Xu et al., 1994) receptors have been implicated in the modulation of nociception. However, the roles of 5-HT1D receptors in the modulation of sensory transmission remain unknown.
The cellular actions of serotonin on sensory neurons also are understood incompletely. One possible action is to inhibit Ca2+ entry into sensory neurons (Del Mar et al., 1994). In other CNS neurons N-type or P/Q-type high voltage-activated (HVA) calcium channels can be modulated by serotonin, mostly via 5-HT1A receptor and membrane-delimited G-protein pathways (Pennington et al., 1991; Koike et al., 1994; Bayliss et al., 1995; Foehring et al., 1996). These calcium channels are known to be involved in triggering synaptic transmission (Luebke et al., 1993; Luebke and Dunlap, 1994; Wall and Dale, 1994; Wheeler et al., 1994) (for review, see Dunlap, 1997). By contrast, T-type channels are not involved in transmitter release but, instead, influence the firing properties of CNS neurons (Llinás and Yarom, 1981; Crunelli et al., 1989;Suzuki and Rogawski, 1989; White et al., 1989; Zhang et al., 1993). The effects of 5-HT on T -type channels are variable. In some CNS neurons 5-HT has no action on T-type channels (e.g., motoneurons; Bayliss et al., 1995), whereas in others it acts to increase the T-type current (Berger and Takahashi, 1990; Fraser and MacVicar, 1991). However, neither the functions of T-type channels in sensory transmission nor its modulation by 5-HT has been described.
Previous reports have demonstrated that 5-HT mediates presynaptic inhibition of transmitter release from Xenopus primary sensory neurons: Rohon–Beard (R–B) neurons (Sillar and Simmers, 1994). We therefore examined the effects of 5-HT on voltage-dependent Ca2+ channels in acutely isolated R–B neurons. Our aims were to characterize the voltage-dependent Ca2+channels modulated by 5-HT, identify the types of receptors involved, and explore some of the possible functional consequences. We found that 5-HT inhibits both the N-, P/Q-type HVA currents and the T-type currents. Although the modulation of HVA currents could contribute to the presynaptic inhibition of transmitter release from R–B neurons (Sillar and Simmers, 1994), the modulation of T-type currents suggests an additional and important locus for modulation of sensory pathways.
MATERIALS AND METHODS
Preparation of the acutely isolated spinal neurons
Acutely isolated spinal neurons were prepared by methods based on those described by Dale (1991). In accordance with the United Kingdom Animals (Scientific Procedure) Acts of 1986, stage 40–42Xenopus larvae (Nieuwkoop and Faber, 1956) were anesthetized in a solution of MS222 (0.5 mg/ml; Tricaine, Sigma, Poole, UK); pinned to a rotatable SYLGARD table in HEPES saline with the following composition (in mm): 117.4 Na+, 3 K+, 1 Mg2+, 2 Ca2+, 2 NO3−, 2.4 HCO3−, 124 Cl−, 10 HEPES, and 10 glucose at pH 7.4; and their spinal cords were removed carefully, transferred to a dish containing 0.1–0.3 mg/ml DNase in HEPES saline, and incubated at room temperature for 3 min. After this, the spinal cords were placed in a dish containing 8 mg/ml Pronase (Sigma) in a low chloride trituration saline, composed of (in mm) 117.4 Na+, 115 MeSO3−, 3 K+, 1 Mg2+, 2 Ca2+, 2 NO3−, 2.4 HCO3−, 9 Cl−, 10 HEPES, and 10 glucose at pH 7.4 and incubated at room temperature for 2 min. They were transferred to a dish of dissociation saline composed of (in mm) 115 Na+, 115 MeSO3−, 3 K+, 2 EDTA, 3 Cl−, 10 HEPES, 10 glucose, and 10 piperazine-N, N′-bis (2-ethanesulfonic acid, PIPES) at pH 7.0 for 1 min and then to a dish of PIPES saline composed of (in mm) 115 Na+, 115 MeSO3−, 3 K+, 0.1 Mg2+, 0.1 Ca2+, 3 Cl−, 20 glucose, and 10 PIPES at pH 7.0 for 3 min. The spinal cords were triturated gently in a saline containing 3 mg/ml DNase in a microfuge tube until the cords had dissociated. Finally, the cells were transferred to 35 mm poly-d-lysine-coated dishes in HEPES saline and allowed to settle and stick to the substrate for at least 1 hr before recording.
Patch-clamp recordings
Owing to their unique morphological characteristics, Rohon–Beard neurons were readily identifiable under phase-contrast microscopy, based on the criteria of Dale (1991): a large spherical soma (mean diameter 23 μm), a large nucleus (mean diameter 12 μm), and dark nucleolus. Whole-cell calcium currents and unitary calcium channel recordings were obtained as described by Hamill et al. (1981). Electrodes were fabricated with a Sutter Instrument P97 puller (Novato, CA) from capillary glass obtained from World Precision Instruments (TW 150F; Sarasota, FL) and Clark Electromedical Instruments (GC150F-10; Pangbourne, UK), coated with SYLGARD, and fire-polished. A List L/M-PC amplifier together with a DT2831 interface (Data Translation) was used to record and digitize the voltage and current records. Data were acquired to the hard disk of an IBM-compatible PC, although an optical disk was used for long-term storage of experimental records. The whole-cell recordings had access resistances ranging from 4 to 12 MΩ. Between 70 and 85% of this access resistance was compensated for electronically. The adequacy of the voltage clamp was accessed by studying the I–V relations obtained from a series of voltage steps separated by 5 mV. The criterion for effective space clamp was a smoothly activating current. For the recording of Ca2+ currents, external solutions were composed of (in mm) 57.5 Na+, 57.5 TEA, 2.4 HCO3−, 3 K+, 10 Ca2+, 1 Mg2+, 10 HEPES, 1 4-aminopyridine (4-AP), pH 7.4, adjusted to 260 mOsm/l, and TTX (140 nm). The pipette solution contained (in mm) 100 Cs+, 1 Ca2+, 6 Mg2+, 20 HEPES, 5 ATP, and 10 EGTA, pH 7.4, adjusted to 240 mOsm/l. Unitary channel recordings (cell-attached mode) were made by methods described by Chen and Hess (1990); the membrane potential outside the bath was zeroed with the following external solution: 110 mm K-MeSO3, 10 mm EGTA, and 10 mm HEPES, titrated to pH 7.4 with KOH. The pipette solution contained 110 mmBaCl2 and HEPES 10 mm, pH 7.5. Leak subtraction was performed on unitary channel and whole-cell recordings by either of two methods. For one method the current of interest was blocked (Y3+ 30 μm or Cd2+120 μm), and the remaining leak currents were subtracted from the equivalent experimental records from the same cell. In the other method a scaled negative version of the experimental pulse protocol was given to the same cell. This subsequently was scaled up and added to the experimental records. In both cases the leak currents were obtained immediately before or after each set of experimental records. Drugs were applied through a multibarreled microperfusion pipette that was positioned within 1 mm of the cell. All experiments were performed at room temperature, 18–22°C. Experiments using nifedipine were performed in dark conditions.
Chemicals used
Serotonergic agonists and antagonists. The following serotonergic agonists and antagonists were used: 7-trifluoromethyl-4-(methyl-1-piperazinyl) pyrrolo[1, 1-a]-quinoxaline dimaleate (CGS12066B, Tocris Cookson, Bristol, UK), 5-carboxyamidotryptamine (5-CT, RBI, Natick, MA), 5-hydroxytryptamine (5-HT, RBI), N-[4-methoxy-3-(4-methyl-1-piperazinyl) phenyl]-2′-methyl-4′-(5-methyl-1, 2, 4-oxadiazol-3-yl) [1, 1-biphenyl]-4-carboxamide (GR127935, GlaxoWellcome Research and Development, Research Triangle Park, NC), R(+)-8-OH-DPAT (DPAT, RBI), ketanserin tartrate (RBI), α-methyl-5-hydroxytryptamine (α-M-5-HT, Tocris Cookson), 2-[5-[3-(4-methylsulphonylamino) benzyl1,2,4-oxadiazol-5-yl]-1 H-indole-3-yl]ethanamine (L-694,247, Tocris Cookson), 1-(2-methoxyphenyl)-4-[4-(2-phthalimido) utyl] piperazine (NAN-190, RBI), 4-[Iodo-N-[2-[4-(methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-benzamide (p-MPPI, RBI), and N-desmethylclozapine (RBI). 5-CT, L-694,247, clozapine, and NAN-190 were dissolved initially by a few drops of dimethylsulphoxide and stored in a freezer.
Ion channel blockers. The following ion channel blockers were used: tetrodotoxin (TTX, Sigma), ω-agatoxin IVA and ω-agatoxin-TK (agatoxin, Alomone Labs, Jerusalem, Israel), ω-conotoxin GVIA (ω-CgTx, Bachem, Torrance, CA), ω-conotoxin MVIIC (Alomone Labs), nifedipine (Sigma), tetraethylammonium chloride (TEA, Aldrich), and yttrium nitrate (Y3+, Sigma).
Statistics
All data are presented as mean ± SD unless otherwise stated. Analysis by Student’s t test was performed for paired and unpaired observations. Differences in frequency of occurrence were assessed by using a 2 × 2 contingency table and χ2 parameter. p values of <0.05 were considered as a significant level.
Fitting
The Levenberg–Marquardt algorithm was used to fit the Hill equation to dose–response data. This gave the best-fitting parameters and their SEs. For all other fitting procedures, the simplex algorithm was used.
RESULTS
Serotonin reversibly reduced both the HVA and T-type Ca2+ currents
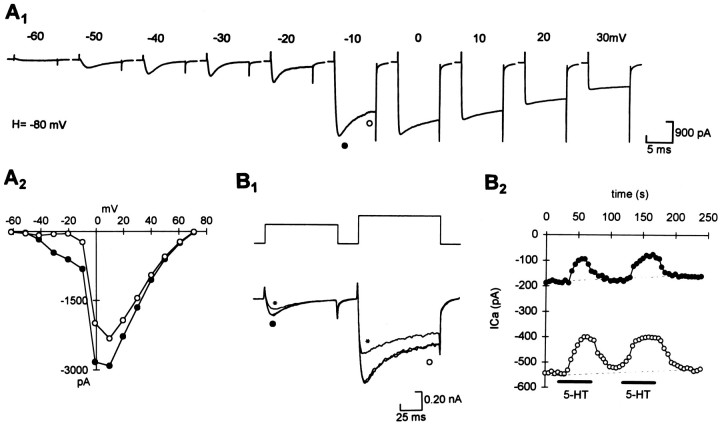
Whole-cell Ca2+ currents recorded from acutely isolated R–B neurons possess both T-type and HVA currents. These can be distinguished by their voltage dependence of activation and inactivation. T-type currents were elicited at test potentials above −60 mV and almost totally inactivated in a 100 msec test pulse. HVA currents were evoked at potentials of −20 mV or more and came to dominate the whole-cell current at potentials above −10 mV (Fig.1A). Using a twin pulse from a holding potential of −90 mV, we elicited both the T-type and the HVA currents, and we examined the effects of 5-HT on both. In ∼80% of the neurons examined, 5-HT reversibly reduced both the T-type and the HVA Ca2+ currents (Fig.1B).
Fig. 1.
Both the HVA and the T-type Ca2+ currents were reduced by 5-HT in acutely isolated R–B neurons. A, R–B neurons possess both the HVA and the T-type Ca2+ currents.A1, Whole-cell Ca2+ currents were recorded by using steps from a holding potential of −80 mV to test potentials between −60 and +30 mV in a stage 42 Xenopus R–B neuron.A2, I/V curve measured from the peak (showing the T-type and HVA currents) and end (showing only the HVA currents) of the Ca2+ currents (symbols correspond to measurements inA1).B1, T-type and HVA currents were elicited in the same neuron by test steps of −30 and +10 mV, respectively, from a holding potential of −90 mV. Both were reduced by 1 μm 5-HT (shown by asterisk); unlabeled traces are control and wash. B2, Courses of the effects of 5-HT showing that they were totally reversible on both currents in the same neuron (symbolscorrespond to measurements inB1).
Inhibition of the HVA currents by 5-HT was not voltage-dependent
The modulation of the HVA currents by serotonin was examined in R–B neurons. 5-HT never caused the slowing of activation of the Ca2+ currents (Fig.2A1) that usually occurs during direct G-protein modulation (for review, seeHille, 1994). In five neurons there was no voltage sensitivity to the block by 5-HT (Fig. 2A2). In most cases, voltage-dependent G-protein inhibition can be relieved by giving a positive prepulse immediately preceding the test pulse. We therefore tested whether prepulses could lessen the block of HVA currents by 5-HT. In five of five neurons tested, the inhibition of HVA currents by 5-HT was not, even partially, relieved by a very positive prepulse (Fig. 2B), suggesting that the inhibition of HVA currents by 5-HT occurred only via voltage-independent mechanisms.
Fig. 2.
Voltage-independent inhibition of the HVA Ca2+ currents by 5-HT.A1, Ca2+currents elicited by steps from a holding potential of −80 mV (asterisk = 1 μm 5-HT).A2, The inhibition of HVA currents by 5-HT (1 μm) was independent of membrane potential (n = 5);I5-HT, current in presence of 5-HT;ICon, current in the control.B1, Example showing that the inhibition of the HVA currents in RB neurons was not changed by applying a +120 mV prepulse (asterisk = trace elicited by test pulse with 120 mV prepulse in 1 μm5-HT). B2, Summary showing no significant difference between the mean inhibition of the HVA currents before and after applying a 120 mV prepulse in five R–B neurons.
5-HT receptor subtypes involved in the inhibition of the HVA currents
To identify the serotonin receptors involved in the modulation of HVA calcium currents, we applied selective agonists and antagonists. The effects of 8-OH-DPAT (100 nm), a selective 5-HT1A agonist (Middlemiss and Fozard, 1983), were fully blocked by the selective 5-HT1A antagonistp-MPPI (100 nm; Kung et al., 1994) in six examined neurons (Fig.3A2,B). The actions of L-694,247, a specific 5-HT1D agonist (Beer et al., 1993), were not blocked by the selective 5-HT1Aantagonists NAN-190 (100 nm) and p-MPPI (100 nm) but were blocked by GR127935 (100 nm), a selective 5-HT1D antagonist (Skingle et al., 1993) in six examined neurons (Fig.3A1,B). This suggests that both 5-HT1A and 5-HT1D receptors were involved in the inhibition of the HVA Ca2+current.
Fig. 3.
The inhibition of HVA Ca2+currents by 5-HT is dose-dependent and mediated via 5-HT1Aand 5-HT1D receptors.A1, The selective 5-HT1D agonist L-694,247 (100 nm) inhibited HVA currents. The effects of L-694,247 (100 nm) were blocked by the selective 5-HT1D antagonist GR127935 (100 nm). A2, The selective 5-HT1A agonist 8-OH-DPAT (100 nm) also inhibited the HVA currents. The effects of 8-OH-DPAT (100 nm) were totally blocked by the selective 5-HT1A antagonist p-MPPI (100 nm). B, Summary of the antagonist effects on the agonists of 8-OH-DPAT and L-694,247 (*p < 0.05 vs agonists alone). C, The inhibition of HVA currents by 5-HT was dose-dependent (n = 7–39 for each dose). The smooth line is the best-fitting Hill equation.
In those neurons that responded, the addition of 5-HT produced a dose-dependent reversible reduction of HVA currents with a half-block concentration (IC50) of 40.8 ± 20.4 nm (Fig. 3C). The 5-HT1D agonist L-694,247 had a similar IC50 (38.1 ± 10.4 nm, n = 16) for the inhibition of HVA currents. The observed maximum reduction of HVA currents was 29%, whereas the mean maximum inhibition by 5-HT (1 μm) was 16.2 ± 2.4% (n = 39). Recovery from the effects of 5-HT on the HVA currents was rather slow on washout. In six cells the time course for wash was fit with a single exponential curve. This gave a mean time constant of 22.5 ± 3.4 sec (n = 6) for the recovery of the HVA current from inhibition by 5-HT.
HVA calcium channel identity
To identify further the HVA channel types possessed byXenopus R–B neurons, we used ω-conotoxin, fraction GVIA (ω-CgTx), which is a selective N-type Ca2+ channel blocker (Feldman et al., 1987), ω-agatoxin-TK, which is a P/Q-type channel blocker (Teramoto et al., 1995), and nifedipine, a selective blocker of L-type channels. ω-CgTx (1 μm) irreversibly blocked 70.4 ± 2.8% (n = 16) of the HVA currents (Fig. 4A,B). Thus in R–B neurons the HVA calcium currents were carried mostly through ω-CgTx-sensitive (N-type) channels. ω-Agatoxin-TK (200 nm) irreversibly blocked 25.5 ± 3.2% (n = 6) of the total HVA currents (Fig. 4A). The actions of ω-agatoxin-TK on HVA currents probably were saturated at ∼40 nm, because the higher dose of 200 nm did not produce further block. The ω-agatoxin-sensitive current did not show significant inactivation (e.g., Fig. 4A2), suggesting that R–B neurons possess many more P-type channels than Q-type channels (Teramoto et al., 1995). Nifedipine (10 μm) did not block the HVA currents in three of eight R–B neurons and blocked only very small amounts of the HVA current in the other five neurons (mean block 5.5 ± 1.8%, p > 0.1 vs control; Fig. 4A), suggesting that R–B neurons possess only a very small number of L-type channels. In five neurons examined, 100 μm Cd2+ blocked the remainder of the HVA current after combined treatment with ω-CgTx and ω-agatoxin (∼5%; Fig. 4A), suggesting that R–B neurons also possess a very small amount of R-type channels (Birnbaumer et al., 1991). Thus the calcium influx via HVA currents was carried mostly by N- and P-type channels in the sensory neurons ofXenopus.
Fig. 4.
The identity of the calcium channels inhibited by 5-HT in RB neurons. A1, Time courses of the effects of calcium channel blockers ω-agatoxin-TK (100 nm), ω-CgTx (1 μm), nifedipine (10 μm), and Cd2+ (100 μm) on the HVA currents. A2, Calcium currents elicited by steps to 10 mV from a holding potential of −50 mV during the application of the blockers (same neuron as inA1).A3, Summary of the effects of calcium channel blockers on the HVA currents in R–B neurons.B1, Recording showing that the inhibition of HVA currents by 5-HT (1 μm) was mostly occluded by ω-conotoxin (1 μm) in a R–B neuron.B2, Recording showing that the inhibition of HVA currents by 5-HT (1 μm) was partially occluded by ω-agatoxin-TK (100 nm), whereas the remaining inhibition was totally occluded by 1 μm ω-conotoxin (asterisk = HVA currents recorded in 5-HT on top of Ca2+ channel blockers).B3, Summary of the inhibition of the HVA currents by 5-HT alone and additional inhibition on top of calcium channel blockers (*p < 0.05 and **p < 0.01 vs 5-HT alone).
We next characterized the identity of the HVA channels modulated by 5-HT in R–B neurons by applying 5-HT alone and in the presence of ω-CgTx (1 μm) or ω-agatoxin-TK (100 nm) to determine whether the blocking action of the two drugs was additive or occlusive. 5-HT inhibited the HVA current by 18.4 ± 1.4% (n = 18; p < 0.01 vs control) when applied alone. In the presence of ω-CgTx (1 μm), 5-HT produced only 6.2 ± 1.3% further inhibition (n = 16; p < 0.01 vs 5-HT alone; Fig.4B1,B3). Therefore, ∼65% of the inhibition of HVA currents was occluded by ω-CgTx. This substantial occlusion suggests that N-type Ca2+ channels were the predominant target of 5-HT. In six neurons the effects of 5-HT also were attenuated significantly by 100 nm agatoxin: 5-HT on top of agatoxin only blocked a further 11.6 ± 1.2% inhibition of HVA currents, which is significantly less than 5-HT alone (p < 0.05 vs 5-HT alone; Fig.4B2,B3), suggesting that P/Q-type channels account for ∼30% of the inhibition of HVA currents by 5-HT. On top of both ω-CgTx and ω-agatoxin-TK, 5-HT produced almost no further inhibition (2.1 ± 0.4%,n = 5; Fig.4B2,B3). This suggests that N- and P/Q-type calcium channels together account for almost all of the inhibition of HVA currents by 5-HT.
The downstream functional consequences and the signaling pathways for the modulation of the HVA channels, such as N-type and P/Q-type channels, by G-protein-coupled receptors have been widely described (for review, see Hille, 1994; Wickman and Clapham, 1995; Dunlap, 1997). The most novel aspects of our results are the modulation of neuronal T-type channels, which differ in their kinetic properties from the HVA channels. These differences mean that HVA and T-type channels perform different roles in the control of neuronal excitability. We therefore have concentrated on characterizing modulation of the T-current in more detail.
Serotonin inhibits T-type Ca2+ currents via 5-HT1A and 5-HT1D receptors
The effect of 5-HT on T-type Ca2+ current was examined by using a repeated pulse protocol with a test potential of −30 mV (or more) from a holding potential of −90 mV. In acutely isolated R–B neurons, the addition of 5-HT produced dose-dependent inhibition (Fig.5A1). The maximum inhibition was 54% of the control T-type Ca2+ current, whereas the mean maximum inhibition for 5-HT (1 μm) was 24.0 ± 2.2% (n= 41).
Fig. 5.
Inhibition of the T-type calcium currents by serotonergic agonists. A, Representative traces (asterisk represents recordings in 100 nmselective agonists; unlabeled traces are control and wash) and dose–response relations showing the block of T-type currents by selective serotonergic agonists: 5-HT (A1), 8-OH-DPAT (A2), and L-694,247 (A3); n = 7–41 for each point. The solid line is the best fit of the Hill equation.A4, The inhibition of T-type currents by L-694,247 (100 nm) and 8-OH-DPAT (100 nm) was additive. B, Summary of the mean inhibition of T-type currents by selective serotonergic agonists at maximum dose in R–B neurons: 5-HT (1 μm,n = 41), 8-OH-DPAT (100 nm,n = 23), L-694,247 (100 nm,n = 22), CGS-12066B (1 μm,n = 8), α-M-5-HT (1 μm,n = 7), and 5-CT (100 nm,n = 5) (**p < 0.01 vs control).
The time course for wash of the serotonergic modulation of T-type currents could be fit with a single exponential curve. This gave a mean time constant of 9.8 ± 2.6 sec (n = 6) for recovery from inhibition by 5-HT, which was one-half of that for the recovery time course seen during modulation of the HVA currents measured in the same neurons. This large difference in the speed of recovery from inhibition for the T-type and HVA currents suggests that different underlying second messengers may be involved. To identify the 5-HT receptors involved in modulation of the T-type current, we applied specific agonists and antagonists to the R–B neurons.
5-HT agonists
A range of selective 5-HT1 agonists—including 8-OH-DPAT, L-694,247, 5-CT (Beer et al., 1992; Hoyer et al., 1994), and a 5-HT1/2 agonist, α-methyl-5-HT (Ismaiel et al., 1990)—reversibly inhibited the T-type Ca2+ currents (Table 1, Fig. 5A,B). However, the 5-HT1B receptor agonist CGS-12066B (Neale et al., 1987), from 10 nm to 1 μm (n= 8), had almost no effect on T-type Ca2+ currents (Fig. 5B, Table 1). The effects of 5-HT, 8-OH-DPAT, and L-694,247 were very potent with IC50 values <1 nm (Fig. 5A, Table 1). Thus, a diversity of agonists that act on 5-HT1 receptors (except 5-HT1B) inhibited T-type Ca2+currents by similar amounts at very low concentrations (<10 nm). In three of three examined R–B neurons, the actions of saturating doses of L-694,247 (100 nm) and 8-OH-DPAT (100 nm) were additive (Fig.5A4), strongly suggesting that they act on different receptors.
Table 1.
Effects of selective serotonergic agonists and antagonists on the T-type Ca2+ currents in acutely isolatedXenopus R–B neurons
| Agonist | Current inhibition | IC50 (nm) | 5-HT1Aantagonist1-a (% block of agonist) | 5-HT1Dantagonist1-b (% of block of agonist) | 5-HT2A/2C antagonist1-c(% of block of agonist) |
|---|---|---|---|---|---|
| 5-HT (1 μm) | 24.0 ± 2.2% (n = 41)1-160 | 0.6 ± 0.4 | 44.6 ± 5.2% (n = 6)1-160 | 40.5 ± 4.7% (n = 5)1-160 | −6.7 ± 3.4 (n = 9) |
| 5-CT (100 nm) | 22.6 ± 3.4% (n = 5)1-160 | – | 49.4 ± 7.1% (n = 5)1-160 | – | – |
| 8-OH-DPAT (100 nm) | 21.2 ± 1.8 (n = 23)1-160 | 0.3 ± 0.1 | 72.5 ± 4.7% (n = 6)1-160 | −4.4 ± 5.4% (n = 6) | – |
| L-694,247 (100 nm) | 18.4 ± 1.8% (n = 22)1-160 | 0.1 ± 0.1 | 4.9 ± 6.2% (n = 4) | 74.5 ± 3.9% (n = 6)1-160 | – |
| CGS12066B (1 μm) | 4.1 ± 3.8 (n = 8) | – | – | – | – |
| α-M-5HT (100 nm) | 19.7 ± 4.4 (n = 5)1-160 | – | 53.6 ± 3.3% (n = 5)1-160 | – | 7.5 ± 4.4% (n = 5) |
5-HT antagonists
To confirm the identities of the serotonin receptor subtypes involved in the inhibition of the T-type Ca2+current, we used specific antagonists (Fig.6, Table 1). Our results are consistent with an involvement of 5-HT1A and 5-HT1Dreceptors. In brief, the effects of 5-HT, 8-OH-DPAT, 5-CT, and α-methyl-5-HT were blocked by the 5-HT1A antagonistp-MPPI (Kung et al., 1994) (Fig. 6, Table 1). In addition, a second 5-HT1A antagonist, NAN-190 (100 nm; Liau et al., 1991), also reduced the effect of 8-OH-DPAT and 5-CT by similar amounts (68.5 ± 5.6%, n = 5 and 52.1 ± 3.3%, n = 6, respectively) but, strangely, had no effect on the actions of 5-HT (data not shown). Neitherp-MPPI nor NAN-190 blocked the effects of L-694,247 (Fig.6B, Table 1). However, 5-HT1D antagonist GR127935 (Skingle et al., 1993) blocked the effects of 5-HT and L-694,247 without affecting 8-OH-DPAT (Fig. 6B, Table1). 5-HT2 receptors were unlikely to be involved because neither ketanserin nor clozapine, which are both 5-HT2A/2Cantagonists (Awouters, 1985; Kuoppamaki et al., 1993), had any effects on 5-HT or α-methyl-5-HT. Thus both 5-HT1A and 5-HT1D receptors are involved in the inhibition of T-type current, a conclusion further strengthened by the additive effects of the specific antagonists (Fig.6A3,B3).
Fig. 6.
Effects of selective antagonists on the inhibition of T-type calcium currents by selective agonists inXenopus R–B neurons. Both p-MPPI (100 nm, A1) and GR127935 (100 nm, A2) partially blocked the effect of 5-HT (1 μm).A3, p-MPPI (100 nm) and GR127935 (100 nm) produced an additive block of the effect of 5-HT (1 μm). p-MPPI blocked the effect of 8-OH-DPAT (100 nm,A4), whereas GR127935 (100 nm) blocked the effect of L-694,247 (100 nm,A5).B1, Summary of the block by the selective antagonists on 8-OH-DPAT (100 nm) (**p < 0.01 vs 8-OH-DPAT).B2, Summary of the block by antagonists on L-694,247 (100 nm) (**p< 0.01 vs L-694,247). B3, Summary of the block by selective antagonists on 5-HT (1 μm) (**p < 0.01 vs 5-HT).
Modulation of T-type channel does not occur through a freely diffusible second messenger pathway
To test whether the modulation of T-type channels was mediated via a freely diffusible second messenger or by a membrane-delimited pathway, we examined the effects of 5-HT on T-type channel activity recorded in the cell-attached mode. The membrane potential was zeroed with high potassium extracellular saline. In 16 of 55 patches recorded, T-type unitary channel activities were elicited by repeated test steps of −50 or −60 mV from holding potentials of 50–90 mV. The high Ba2+ levels will screen surface charge on the membrane; therefore, the absolute membrane potential experienced by the channels is likely to be shifted to more negative potentials by as much as 20–30 mV (Hille, 1992). Thus the test protocol is similar, but not be exactly equivalent, to those used to evoke T-type currents in the earlier whole-cell recordings performed with normal levels of divalent cations. In eight patches located near the neuronal process, but not the nucleus, quasi-macroscopic T-type currents were elicited by almost every test pulse (Fig. 7A), suggesting that these patches contained many T-type channels. In patches from six neurons that had a large number of T-type channels, the averaged currents were not modulated by 5-HT (Fig. 7B). Given the reliability of the modulation of the whole-cell T-type currents by 5-HT (nearly 90% of the cells responded to 5-HT), we would expect to see at least five of these patches being modulated if 5-HT were acting via a freely diffusible second messenger pathway. The lack of response therefore suggests that 5-HT cannot act via a freely diffusible second messenger but may instead use a membrane-delimited pathway, such as direct modulation by G-proteins.
Fig. 7.
Effects of 5-HT on T-type unitary channel recordings. A1, Consecutive unitary T-type Ba2+ currents were elicited by steps from potentials equivalent to the membrane potential of approximately −90 to −30 mV (after allowing for the screening effect of high Ba2+ levels on membrane surface charge) in a cell-attached patch. All traces are leak-subtracted.A2, Average unitary T-type Ba2+ current record obtained by averaging of 50 consecutive recordings in the same patch. The solid lineis the best fit of the single exponential equation; time constant (τ) = 23.5 msec. B1, 5-HT (1 μm) did not modulate the average (50–100 consecutive traces) unitary T-type Ba2+ currents recorded in cell-attached patches. In Cell A there were no changes in the T-type current. In Cell B, however, the averaged currents were reduced, but this did not reverse on washing, suggesting that the reduction may result from a “run down” of channel activity. B2, Summary of the effects of 5-HT (1 μm) on T-type unitary channel recordings in six R–B neurons.
5-HT did not change the kinetics of the T-type currents
We explored whether the inhibition of T-type channels by 5-HT might be voltage-dependent. The amount of inhibition of T-type currents remained constant from membrane potentials of −50 mV (mean inhibition 26.0 ± 4.2%, n = 4) to −20 mV (mean inhibition 25.2 ± 3.7). In five cells in which T-type tail currents were reduced, 5-HT did not change the voltage dependence of T-type current activation (Fig. 8A). Similarly, the voltage dependence of steady-state inactivation remained unchanged (Fig. 8B). In six of six neurons, when the reduced T-type currents were scaled up to the same magnitude as control, the currents elicited by test potentials from −50 to −30 mV in 5-HT overlapped with their corresponding control traces (Fig.8C1). However, at membrane potentials more positive than −30 mV at which the HVA currents start to develop, the scaled traces did not overlap in three neurons (e.g., Fig.5A). This probably was attributable to contamination of the T-type currents by the HVA currents and was not seen in neurons that had been pretreated with 1 μm ω-conotoxin-GVIA (data not shown). This therefore suggests that 5-HT reduced the T-type currents without altering the macroscopic kinetics. This was confirmed by looking at the time course of inactivation at a range of voltages. Once again 5-HT had no effect on the kinetics of inactivation (Fig.8C). Thus, unlike most examples of G-protein-mediated modulation of HVA channels, serotonin reduced the T-type currents without significantly altering the voltage or time dependence of channel gating.
Fig. 8.
5-HT did not alter the kinetic characteristics of the whole-cell T-type calcium currents.A1, Leak-subtracted T-type tail currents (shown by arrow) were elicited by a series of test pulses (−80 to −20 mV in 5 mV steps) from a holding potential of −100 mV in control. A2, Summary showing that 5-HT (1 μm) had no effect on the activation of the T-type currents in five R–B neurons. The data were fit with the Boltzmann relation (solid line):I/Imax = {1 + exp[(V +V½)/K]}−1.Squares, Control (V½= −37.2 mV; K = −7.6); filled circles, 5-HT (1 μm) (V½ = −38.8 mV;K = −7.3).B1, The T-type currents, measured at the peak, undergo steady-state inactivation.B2, 5-HT (1 μm) had no effect on the steady-state inactivation of the T-type currents in four R–B neurons. The data were fit with the Boltzmann relation (solid line). Squares, Control (V½ = −74.7 mV;K = 5.2); filled circles, 5-HT (1 μm) (V½ = −75.2 mV;K = 5.4). C1,Top left, T-type currents recorded in control and 1 μm 5-HT (shown by asterisk). Top right, The T-type currents recorded in 5-HT were scaled up to the same peak magnitude as control to show that the two traces overlap almost completely. The time-dependent inactivation of the T-type currents was measured by fitting with a single exponential (solid line). C2, 5-HT (1 μm) had no effect on the time-dependent inactivation in three R–B neurons. Squares, Control;filled circles, 5-HT (1 μm).
The reduction of T-type currents by serotonin increased R–B neuron firing threshold
We next explored the function roles of the T-type currents and the consequences of modulation of this current by 5-HT for sensory transmission. The trivalent cations, Y3+, La3+, and other lanthanides, have been reported to block differentially the T-type and HVA Ca2+channels. The smaller cations are more potent T-type antagonists, and the IC50 for Y3+ blocking of T-type channels is ∼0.1 nm (Mlinar and Enyeart, 1993). We tested the dose–response of Y3+ on T-type and HVA currents. At doses of 10 nm or less, Y3+could reversibly block the T-type currents while having no effect on the HVA currents in R–B neurons (Fig.9). We therefore used Y3+ (1–10 nm) as a selective T-type channel blocker to test possible functional roles of T-type currents.
Fig. 9.
Differential block of the T-type and HVA Ca2+ currents by Y3+ in R–B neurons. A, Time series measurements in a R–B neuron showing the dose–response for Y3+ on T-type currents (A1) and HVA currents (A2) recorded in the same neuron.A3, Example of the HVA and T-type currents recorded simultaneously in the same neuron, as shown inA1 andA2 (asterisks= 10 nm Y3+). B, Summary of the effects of Y3+ (10 nm) on the T-type currents and HVA currents (**p < 0.01 vs control).
Under current clamp, R–B neurons were injected with repeated 5 msec depolarizing command currents (365.5 ± 15.9 pA at a resting potential of approximately −50 mV, n = 10) to evoke repeated action potentials (Fig.10A1). To examine whether the T-type channels could be involved in setting spike threshold, we carefully measured the threshold current that could just evoke spikes in the R–B neurons and observed the effects of Y3+ (1–10 nm) and 5-HT (10 nm) at this threshold level of current injection. This low concentration of 5-HT greatly blocks the T-type current but has little effect on the HVA current (compare Fig.5A1 with 3C). At a resting membrane potential of −48.5 ± 6.4 mV, n = 10, at which T-type channels were inactivated (see Fig. 8B), neither Y3+ nor 5-HT had any effects on the neuron firing (Fig. 10B1). By injecting a negative holding current (−22.5 ± 4.2 pA,n = 10), we set the membrane potentials of these neurons to a more negative value of approximately −90 mV (−90.5 ± 4.8 mV, n = 10). At these membrane potentials Y3+ (10 nm) clearly reduced the R–B neuron firing probability (Fig.10B2,C), suggesting that the T-type channels played a role in spike initiation. 5-HT also reduced the probability of R–B neuron firing (Fig.10B2,C). Therefore modulation of T-type channels by 5-HT could increase the firing threshold of R–B neuron and potentially modulate the sensitivity of this sensory pathway.
Fig. 10.
Effects of the selective T-type Ca2+ channel blockers and 5-HT on R–B neuron firing. A1, Current-clamp recording in a R–B neuron, using steps of current injection to detect the threshold current that evoked an action potential.A2, Y3+ (10 nm; asterisk), which selectively blocked T-type currents, blocked the action potential evoked at threshold current injection. B1, Current-clamp recording from a R–B neuron showing that injection of repeated current pulses just above the threshold caused reliable firing. At resting membrane potentials more positive than −50 mV, neither Y3+ (10 nm) nor 5-HT (10 nm) had any effects on neuron firing.B2, At more negative resting membrane potentials (−90 mV), both Y3+ (10 nm) and 5-HT (10 nm) reversibly reduced the probability of firing in the neuron. C, Summary of the effects of Y3+ (10 nm) and 5-HT (10 nm) on the probability of R–B neuron firing in response to repetitive threshold current injection at a resting membrane potential of −90 mV, where the probability was measured as the number of pulses that evoked a spike/total number of pulses during control, drug treatment, and wash (**p < 0.01 vs control or wash).
DISCUSSION
The larva of the South African amphibian, Xenopus laevis, is a simple model for studying the spinal mechanisms of sensory modulation. Around the time of hatching, the spinal cord contains only eight classes of differentiated neuron (Roberts and Clarke, 1982). The trunk skin of the tadpole is innervated by the free nerve endings of a single, homogeneous population of mechanosensory afferents called Rohon–Beard neurons (Hughes, 1957). The R–B neurons are highly analogous to human C fibers. Like C fibers, R–B neurons are unmyelinated and possess free nerve endings, use glutamate as a transmitter (Sillar and Roberts, 1988) and substance P as a cotransmitter (Clarke et al., 1984), and have capsaicin receptors (Kuenzi and Dale, 1996). The acutely isolated R–B neurons have a distinctive morphology, maintain similar membrane properties to theirin vivo counterparts, and can be recognized easily in vitro (Dale, 1991). The R–B neurons therefore can be used as a highly advantageous model for studying the neuromodulation of sensory pathways.
Identity of serotonergic receptors
We found that T-type currents were blocked by the 5-HT1A agonist 8-OH-DPAT and the 5-HT1D agonist L-694,247. The IC50 values for both agonists were very similar to those reported in mammals (Middlemiss and Fozard, 1983; Beer et al., 1993). The effect of the 5-HT1A agonist was blocked only by the 5-HT1A antagonist p-MPPI (Kung et al., 1994), whereas the effect of L-694,247 was blocked only by the 5-HT1D antagonist GR127935 (Skingle et al., 1993). Therefore, these receptors have a very similar pharmacology to the mammalian 5-HT1A and 5-HT1D receptors, and we propose that they are the amphibian equivalents of these receptors.
5-HT1A receptors have been described previously in amphibian spinal cord (Holohean et al., 1992; Tan and Miletic, 1992). The 5-HT1A receptor can inhibit Ca2+entry through HVA channels into afferent terminals of nociceptors in mammalian spinal cord (Del Mar et al., 1994). 5-HT1Areceptors also have been reported to be involved in the voltage-dependent G-protein-coupled inhibition of the HVA currents in other CNS neurons (Pennington et al., 1991; Koike et al., 1994; Bayliss et al., 1995; Foehring, 1996). Unlike these previous reports, the inhibition of the HVA currents by 5-HT1A receptors inXenopus R–B neurons is not voltage-dependent.
5-HT1D receptors have been found mostly in the mammalian brain and in pigeon (Bruinvels et., 1992; Hoyer et al., 1992) (for review, see Waeber and Palacios, 1990) but not yet in the amphibian nervous system. 5-HT1D receptors seem to exist in those species in which the 5-HT1B receptor is absent and may play a role in these species equivalent to that of the 5-HT1B receptor in rat and mouse (for review, see Zifa and Fillion, 1992). Our findings that 5-HT1D receptors are present in Xenopus are the first to suggest that 5-HT1D receptors exist in the amphibian nervous system and are consistent with previous reports that the 5-HT1Breceptors are absent in frog (for review, see Zifa and Fillion, 1992).
The 5-HT1D receptor inhibits synaptic transmission (Jones et al., 1995) and acts as an autoreceptor in the mammalian raphe nucleus (Davidson and Stamford, 1995). The actions of 5-HT1D receptor on Ca2+ currents and its role in the sensory transmission have not been reported; therefore, our results are the first to show that 5-HT1D receptors also are involved in limiting sensory transmission by inhibiting T-type and HVA Ca2+ channels. In the Xenopus spinal cord 5-HT1D receptors also are found in a class of glycinergic inhibitory premotor interneurons (Q. Q. Sun and N. Dale, unpublished results) that contribute to the control of locomotion in Xenopus. Therefore, the 5-HT1D receptor may play a important role both in the descending supraspinal control of sensory transmission and in the regulation of locomotion patterns inXenopus.
Modulation of the T-type currents
Although some neurotransmitters have been documented to inhibit T-type currents, presumably via G-protein-mediated processes (Formenti and Sansone, 1991), serotonin has been reported to enhance rather than inhibit T-type currents in CNS neurons (Berger and Takahashi, 1990; Fraser and MacVicar, 1991). In contrast to this, we found that serotonin reversibly inhibited T-type currents in R–B neurons.
5-HT has been reported previously to mediate presynaptic inhibition of transmitter release from R–B neurons inXenopus (Sillar and Simmers, 1994). However, T-type channels generally are not thought to be involved in supporting synaptic transmission. The modulation of T-type currents therefore suggests an additional and important locus of modulation: initiation of R–B neuron discharge in response to sensory stimuli. R–B neurons have a very negative resting potential (approximately −90 mV; Spitzer and Lamborghini, 1976), so the T-type calcium current is unlikely to be in an inactivated state in these neurons at rest. Because the T-type currents are the only voltage-gated inward currents activated in these neurons between −60 and −30 mV, they may play an important role in triggering action potentials. Small reductions of the T-type calcium current therefore could modulate the responsiveness of Rohon–Beard neuron to sensory stimuli. We found that both selective T-type channel blockers and low doses of 5-HT greatly reduced the probability of R–B neuron firing in response to threshold current injection at resting membrane potentials of −90 mV but not at more positive membrane potentials of −50 mV at which the T-type channels were inactivated. This strongly supports our hypothesis that T-type channels play a role in the spike initiation in R–B neurons and that the modulation of T-type channels may raise the R–B firing threshold. We also found that the T-type currents were much more sensitive to serotonin agonists (IC50 = 0.1 nm for 5-HT) than HVA currents (IC50 = 40 nm for 5-HT). During 5-HT release the T-type channels are thus likely to be modulated first when the concentrations of 5-HT are low. In our cell-attached recordings we found that in patches located near the neuronal process, but not the nucleus, quasi-macroscopic T-type can be elicited. The most effective locus for the T-type channels to influence spike initiation would be in the peripheral neurites close to the site of mechanotransduction. We therefore propose that both the 5-HT receptors and T-type channels are in the peripheral neurites and that modulation of these channels alters the sensitivity of the sensory pathway.
Modulation of the N- and P/Q-type HVA Ca2+ currents
5-HT inhibits N- and P/Q-type HVA calcium currents in neocortical pyramidal neurons (Foehring, 1996), in rat motor neurons (Bayliss, 1995), or N-type currents in sensory neurons (Del Mar et al., 1994) and other CNS neurons (Pennington et al., 1991; Koike et al., 1994). Consistent with these reports, our results showed that N- and P/Q-type HVA Ca2+ current was inhibited by 5-HT in R–B neurons.
N- and P/Q-type calcium channels are involved in supporting synaptic transmission in the CNS and peripheral nervous systems (Luebke et al., 1993; Luebke and Dunlap, 1994; Wall and Dale, 1994; Wheeler et al., 1994) (for review, see Dunlap, 1997). Therefore, the reduction of N- and P-type currents could contribute to the previously reported presynaptic inhibition of transmitter release from R–B neurons (Sillar and Simmers, 1994).
The modulation of N-type and P/Q-type currents in R–B neurons was not accompanied with slowing of activation and was voltage-independent, which is different from most of the examples of G-protein-mediated modulation (for review, see Hille, 1994; Wickman and Clapham, 1995) in which a kinetic-slowing is always accompanied with the reduction of HVA currents. The difference in voltage dependence for modulatory components may have different functional implications under various physiological conditions. The voltage-dependent modulation, which can be removed by preceding stimulation, would be phasic in nature, whereas the voltage-independent modulation would persist under conditions of repetitive firing.
Distinctive biochemical pathways may underlie the modulation of T-type and HVA currents
In R–B neurons both HVA and T-type currents were inhibited by 5-HT in a voltage-independent manner via the same receptors. However, the modulation of T-type channels differed from the HVA channels in that it had ∼100 times greater sensitivity to 5-HT and that the time course for wash was twice as fast. These differences may reflect the involvement of distinctive biochemical mechanisms. Our results suggest that the reduction of T-type currents occurred through a membrane-delimited pathway, possibly a direct G-protein–channel interaction. In the mammalian CNS the most common signal transduction pathway for the modulation of N- and P/Q- channels is via a voltage-dependent interaction between the calcium channels and the G-protein βγ subunits (Ikeda, 1996; Waard et al., 1997) (for review, see Dunlap, 1997). However, in those cases in which the modulation is voltage-independent, it may be mediated by protein kinase C (Diverse-Pierluissi et al., 1995). Further studies to address the participation of various signal transduction pathways, such as whether the G-proteins involved are PTX-sensitive and if cAMP/PKA or PKC is involved in the modulation, certainly will be very useful.
Footnotes
We are grateful to the Royal Society (UK) for generous support and to the Committee of Vice Chancellors and Principals for an Overseas Research Studentship award to Q.Q.S. We also thank Dr. F. M. Kuenzi for helpful comments on an earlier version of this paper and GlaxoWellcome for the kind gift of GR127935.
Correspondence should be addressed to Dr. Nicholas Dale at the above address.
REFERENCES
- 1.Awouters F. The pharmacology of ketanserin, the first selective serotonin S2-antagonist. Drug Dev Res. 1985;6:263–300. [Google Scholar]
- 2.Bayliss DA, Umemiya M, Berger AL. Inhibition of N- and P-type calcium currents and after-hyperpolarization in rat motoneurones by serotonin. J Physiol (Lond) 1995;485:635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer MS, Stanton JA, Bevan Y, Chauhan NS, Middlemiss DN. An investigation of the 5-HT1D receptor binding affinity of 5-hydroxytryptamine, 5-carboxyamidotryptamine, and sumatriptan in the central nervous system of seven species. Eur J Pharmacol. 1992;213:193–197. doi: 10.1016/0014-2999(92)90681-s. [DOI] [PubMed] [Google Scholar]
- 4.Beer MS, Stanton JA, Bevan Y, Heald A, Reeve AJ, Street LJ, Matassa VG. L-694,247: a potent 5-HT1D receptor agonist. Br J Pharmacol. 1993;110:1196–1200. doi: 10.1111/j.1476-5381.1993.tb13941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofman F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. The naming of voltage-gated calcium channels. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 7.Bruinvels AT, Lery H, Nozulak J, Palacios JM, Hoyer N. 5-HT1D binding sites in various species: similar pharmacological profile in dog, monkey, calf, guinea-pig, and human brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:243–248. doi: 10.1007/BF00173535. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Hess P. Mechanisms of gating of T-type calcium channels. J Gen Physiol. 1990;96:603–630. doi: 10.1085/jgp.96.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke JWD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy, and immunohistochemistry of Rohon–Beard neurones in embryos of Xenopus laevis. J Physiol (Lond) 1984;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisp T, Stafinsky JL, Spanos LJ, Uram M, Perni VC, Donepudi HB. Analgesic effects of serotonin and receptor-selective serotonin agonists in the rat spinal cord. Gen Pharmacol. 1991;22:247–251. doi: 10.1016/0306-3623(91)90441-8. [DOI] [PubMed] [Google Scholar]
- 11.Crunelli V, Lightowler S, Pollard C. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol (Lond) 1989;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale N. The isolation and identification of spinal neurones that control movement in the Xenopus embryo. Eur J Neurosci. 1991;3:1025–1035. doi: 10.1111/j.1460-9568.1991.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 13.Davidson C, Stamford JA. Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B, and 5-HT1D autoreceptors. Br J Pharmacol. 1995;114:1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Mar LP, Cardenas CG, Scroggs RS. Serotonin inhibits high-threshold Ca2+ channel currents in capsasin-sensitive acutely isolated adult-rat DRG neurons. J Neurophysiol. 1994;72:2551–2554. doi: 10.1152/jn.1994.72.5.2551. [DOI] [PubMed] [Google Scholar]
- 15.Diverse-Pierluissi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 16.Dunlap K. Integration hot-spot gets hotter. Nature. 1997;385:394–396. doi: 10.1038/385394a0. [DOI] [PubMed] [Google Scholar]
- 17.Eide PK, Joly NM, Hole L. The role of spinal cord 5-HT1A and 5-HT1B receptors in the modulation of a spinal nociceptive reflex. Brain Res. 1990;536:195–200. doi: 10.1016/0006-8993(90)90025-7. [DOI] [PubMed] [Google Scholar]
- 18.Feldman DH, Olivera BM, Yoshikami D. Omega Conus geographus toxin: a peptide that blocks calcium channels. FEBS Lett. 1987;2:295–300. doi: 10.1016/0014-5793(87)80073-x. [DOI] [PubMed] [Google Scholar]
- 19.Foehring RC. Serotonin modulates N- and P-type calcium currents in neocortical pyramidal neurons via a membrane-delimited pathway. J Neurophysiol. 1996;75:648–659. doi: 10.1152/jn.1996.75.2.648. [DOI] [PubMed] [Google Scholar]
- 20.Formenti A, Sansone V. Inhibitory action of acetylcholine, baclofen, and GTP-γ-S on calcium channels in adult rat sensory neurons. Neurosci Lett. 1991;131:267–272. doi: 10.1016/0304-3940(91)90630-c. [DOI] [PubMed] [Google Scholar]
- 21.Fraser DD, MacVicar BA. Low-threshold transient calcium current in rat hippocampal lacunosum moleculare interneurons: kinetics and modulation by neurotransmitters. J Neurosci. 1991;11:2812–2820. doi: 10.1523/JNEUROSCI.11-09-02812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 23.Hille B. Ion channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 24.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 25.Holohean AM, Hackman JC, Shope SB, Davidoff RA. Serotonin1A facilitation of frog motoneuron responses to afferent stimuli and to N-methyl-d-aspartate. Neuroscience. 1992;48:469–477. doi: 10.1016/0306-4522(92)90506-w. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer D, Lery H, Waeber C, Bruinvels AT, Nozulak J, Palacios JM. “5-HT1R” or 5-HT1D sites? Evidence for 5-HT1D binding sites in rabbit brain. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:249–254. doi: 10.1007/BF00173536. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PA. VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Res. 1994;46:157–203. [PubMed] [Google Scholar]
- 28.Hughes AFW. The development of the primary sensory system in Xenopus laevis (Daudin). J Anat. 1957;91:323–338. [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 30.Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents α-methylserotonin and 2-methylserotonin. J Med Chem. 1990;33:755–758. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- 31.Jones JFX, Martin GR, Ramage AG. Evidence that 5-HT1D receptors mediate inhibition of sympathetic ganglionic transmission in anesthetized cats. Br J Pharmacol. 1995;116:1715–1717. doi: 10.1111/j.1476-5381.1995.tb16651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koike H, Saito H, Matsuki N. 5-HT1A receptor-mediated inhibition of N-type calcium current in acutely isolated ventromedial hypothalamic neuronal cells. Neurosci Res. 1994;19:161–166. doi: 10.1016/0168-0102(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 33.Kuenzi FM, Dale N. Effects of capsaicin and analogues on potassium and calcium currents and vanilloid receptors in Xenopus embryo spinal neurons. Br J Pharmacol. 1996;119:81–90. doi: 10.1111/j.1476-5381.1996.tb15680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kung HF, Kung M, Clarke W, Mayani S, Zhuang Z. A potential 5-HT1a receptor antagonist: p-MPPI. Life Sci. 1994;55:1459–1462. doi: 10.1016/0024-3205(94)00686-5. [DOI] [PubMed] [Google Scholar]
- 35.Kuoppamaki M, Syvalahti E, Hietala J. Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists. Eur J Pharmacol. 1993;245:179–182. doi: 10.1016/0922-4106(93)90126-t. [DOI] [PubMed] [Google Scholar]
- 36.Liau LM, Sleight AJ, Pitha J, Peroutka SJ. Characterization of a novel and hydroxytryptamine1A receptor antagonist. Pharmacol Biochem Behav. 1991;38:555–559. doi: 10.1016/0091-3057(91)90013-r. [DOI] [PubMed] [Google Scholar]
- 37.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurons in vitro: different types of voltage-dependent ionic conductances. J Physiol (Lond) 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas JJ, Mellstrom B, Colado MI, Naranjo JR. Molecular mechanisms of pain: serotonin1A receptor agonists trigger transactivation by c-fos of the prodynorphin gene in spinal cord neurons. Neuron. 1993;10:599–611. doi: 10.1016/0896-6273(93)90163-l. [DOI] [PubMed] [Google Scholar]
- 39.Luebke JI, Dunlap K. Sensory neuron N-type calcium currents are inhibited by both voltage-dependent and independent mechanisms. Pflügers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 40.Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 41.Middlemiss DN, Fozard JH. 8-Hydroxy-2-(di-N-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- 42.Millan MJ. Serotonin (5-HT) and pain—a reappraisal of its role in the light of receptor multiplicity. Semin Neurosci. 1995;7:409–419. [Google Scholar]
- 43.Mlinar B, Enyeart JJ. Block of current through T-type calcium channels by trivalent cations and nickel in neural rat and human cells. J Physiol (Lond) 1993;469:639–652. doi: 10.1113/jphysiol.1993.sp019835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal RF, Fallon SL, Boyer WC, Wasley JWF, Martin LL, Stone GA, Glaeser BS, Sinton CM, Williams M. Biochemical and pharmacological characterization of CGS12066B, a selective serotonin-1B agonist. Eur J Pharmacol. 1987;136:1–9. doi: 10.1016/0014-2999(87)90772-2. [DOI] [PubMed] [Google Scholar]
- 45.Nieuwkoop PD, Faber J. Normal tables of Xenopus laevis (Daudin). North-Holland; Amsterdam: 1956. [Google Scholar]
- 46.Pennington NJ, Kelly JS, Fox AP. A study of the mechanism of Ca2+ current inhibition produced by serotonin in rat dorsal raphe neurons. J Neurosci. 1991;11:3594–3609. doi: 10.1523/JNEUROSCI.11-11-03594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts A, Clarke JDW. The neuroanatomy of an amphibian embryo spinal cord. Philos Trans R Soc Lond [Biol] 1982;296:195–212. doi: 10.1098/rstb.1982.0002. [DOI] [PubMed] [Google Scholar]
- 48.Sillar KT, Roberts A. Unmyelinated cutaneous afferent neurons activate two types of excitatory amino acid receptor in the spinal cord of Xenopus laevis embryos. J Neurosci. 1988;8:1350–1360. doi: 10.1523/JNEUROSCI.08-04-01350.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sillar KT, Simmers AJ. Presynaptic inhibition of primary afferent transmitter release by 5-hydroxytryptamine at a mechanosensory synapse in the vertebrate spinal cord. J Neurosci. 1994;14:2636–2647. doi: 10.1523/JNEUROSCI.14-05-02636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skingle M, Skopes DIC, Feniuk W, Connor HE, Carter MC, Clitherow MC. GR127935: a potent orally active 5-HT1D receptor antagonist. Br J Pharmacol. 1993;110:9P. [Google Scholar]
- 51.Spitzer NC, Lamborghini JE. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci USA. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki S, Rogawski MA. T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc Natl Acad Sci USA. 1989;86:7228–7232. doi: 10.1073/pnas.86.18.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan H, Miletic V. Diverse actions of 5-hydroxytryptamine on frog spinal dorsal horn neurons in vitro. Neuroscience. 1992;49:913–923. doi: 10.1016/0306-4522(92)90367-b. [DOI] [PubMed] [Google Scholar]
- 54.Teramoto T, Niidome T, Miyagawa T, Nishizawa Y, Katayama K, Sawada K. Two types of calcium channels sensitive to omega-agatoxin-TK in cultured rat hippocampal neurons. NeuroReport. 1995;6:1648–1688. doi: 10.1097/00001756-199508000-00022. [DOI] [PubMed] [Google Scholar]
- 55.Waard MD, Liu H, Walker D, Scott VES, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 56.Waeber C, Palacios JM. 5-HT1 receptor binding sites in the guinea pig superior colliculus are predominantly of the 5-HT1D class and are presynaptically located on primary retinal afferents. Brain Res. 1990;528:207–211. doi: 10.1016/0006-8993(90)91659-5. [DOI] [PubMed] [Google Scholar]
- 57.Wall MJ, Dale N. GABAB receptors modulate an ω-conotoxin-sensitive calcium current that is required for synaptic transmission in the Xenopus embryo spinal cord. J Neurosci. 1994;14:6248–6255. doi: 10.1523/JNEUROSCI.14-10-06248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 59.White G, Lovinger DM, Weight FF. Transient low-threshold Ca2+ current triggers burst firing through an afterdepolarizing potential in an adult mammalian neuron. Proc Natl Acad Sci USA. 1989;86:6802–6806. doi: 10.1073/pnas.86.17.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wickman K, Clapham DE. Ion channels regulation by G-proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 61.Xu W, Qiu XC, Han JS. Serotonin receptor subtypes in spinal antinociception in the rat. J Pharmacol Exp Ther. 1994;269:1182–1189. [PubMed] [Google Scholar]
- 62.Zhang L, Valiante TA, Carlen PL. Contribution of the low-threshold T-type calcium current in generating the post-spike depolarizing afterpotential in dentate granule neurons of immature rats. J Neurophysiol. 1993;70:223–231. doi: 10.1152/jn.1993.70.1.223. [DOI] [PubMed] [Google Scholar]
- 63.Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992;44:401–457. [PubMed] [Google Scholar]