Abstract
Changes in the human neuromagnetic alpha rhythm were monitored during an object detection task to study the effects of visual shape processing on the parieto-occipital activity. Pictures of coherent meaningful objects, which the observers had to detect, and of disorganized meaningless non-objects were presented briefly between masks. The non-objects were systematically followed by a higher level of alpha than the objects, the difference emerging on average 400 msec after the stimulus, with a median delay of 130 msec after evoked response onsets in the occipital, temporal, and parietal cortices. Without attention to visual shape, the alpha levels did not differ between objects and non-objects. The alpha level was higher after non-objects than missed objects, and higher after missed than correctly detected objects, suggesting that the alpha level is inversely related to saliency or familiarity of the object and does not directly reflect visual awareness.
The reactive alpha rhythm was generated in the parieto-occipital sulcus, which in several primate species includes areas belonging to the dorsal visual pathway. According to current views, the parietal cortex produces attentional signals that filter out irrelevant information in the ventral visual stream. Our results reinforce the idea of bidirectional interaction: information derived from visual shape can rapidly modify activity in the parieto-occipital region. The synchronized alpha oscillations may reflect attenuation of occipito-parietal information transfer and disengagement of parietal cortex from object selection.
Keywords: object recognition, attention, magnetoencephalography, brain rhythms, human, parieto-occipital sulcus, V6, V6A, PO, ventral visual stream, dorsal visual stream
Prompt detection of new visual objects in the ever changing environment requires fast shifts of attention, effortlessly achieved by the visual system of the brain. The primate visual system can be divided, on anatomical and functional grounds, into ventral occipitotemporal and dorsal occipitoparietal streams (Ungerleider and Mishkin, 1982; Felleman and Van Essen, 1991;Goodale and Milner, 1992). The ventral stream is assumed to be mainly responsible for discrimination and recognition of visual objects, whereas the dorsal stream is involved in object localization, visual guidance of motor behavior, and attention. The two streams are interconnected at several levels of cortical hierarchy and evidently cooperate in a dynamic fashion (Van Essen et al., 1992).
The posterior parietal cortex, the pulvinar, and the superior colliculus have been suggested to form a posterior attentional network that shifts visual attention to different spatial locations (Petersen et al., 1987; Posner and Petersen, 1990); the spatial attention may then modify activity of the ventral visual stream (Moran and Desimone, 1985; Motter, 1993). According to current views (LaBerge and Brown, 1989; Posner and Petersen, 1990; Corbetta et al., 1991;LaBerge, 1995), the parietal attentional system dynamically gates input to the ventral visual stream and helps to choose appropriate targets for object recognition. Up to now, the parietal attentional system has been studied mainly during spatial tasks, whereas less is known about its behavior during shape-based discrimination (Desimone and Duncan, 1995).
Here we report on the reactivity of magnetoencephalographic (MEG) parieto-occipital alpha rhythm during a difficult visual discrimination task. The MEG alpha rhythm originates mainly near the parieto-occipital sulcus (POS), with only minor contribution from the calcarine sulcus (Williamson and Kaufman, 1989; Salmelin and Hari, 1994; Salenius et al., 1995); the rhythm may reflect activity in the human dorsal visual stream (Hari and Salmelin, 1997). Our previous analysis of evoked fields, recorded during the same task (Vanni et al., 1996b), revealed activation of the lateral occipital and temporal cortices, probable parts of the human ventral stream (Corbetta et al., 1991; Malach et al., 1995), and of the left parietal cortex; only the right lateral occipital cortex displayed activity that correlated with the proportion of correct object detections.
The goal of the present study was to determine whether visual shape can modify the parieto-occipital alpha rhythm and whether the alpha level covaries more closely with the stimulus features or with the conscious perception. We also tried to determine the temporal relation between changes in the evoked and spontaneous cortical activity.
A preliminary report of part of this study has been published previously in abstract form (Vanni et al., 1996a).
MATERIALS AND METHODS
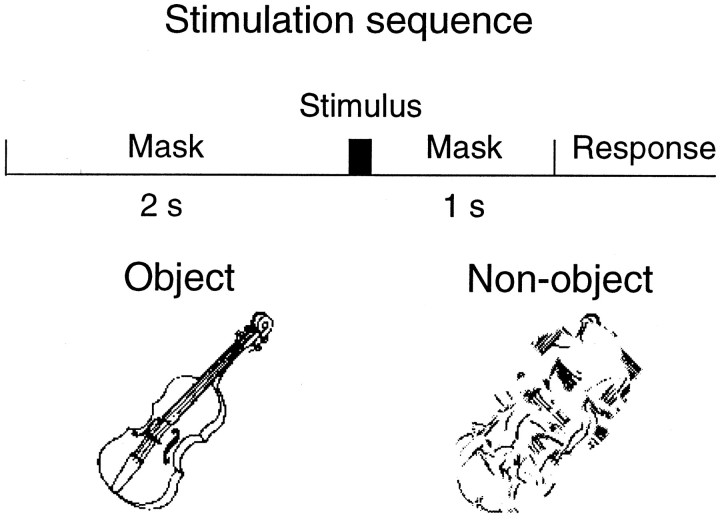
Eight right-handed healthy adults (four females, four males; mean age 24.9 years, range 21–28 years) participated in the study. During the recordings they sat in a magnetically shielded room, with the head supported against the bottom surface of the helmet-shaped magne-tometer. Line drawings of 200 animate or inanimate objects (Snodgrass and Vanderwart, 1980) and of corresponding non-objects were used as stimuli. The non-objects were generated by randomly rotating circular areas with a radius of 0.5° within the object drawings until there were no areas with recognizable features. The stimuli were presented between backward and forward masks once every 3–5 sec in a randomized order (Fig. 1). The mask was a line drawing (mean luminance 12 cd/m2) 7.9 × 7.9° in size. The black (0.4 cd/m2) and white (25 cd/m2) stimuli were presented to the area covered by the mask. Observers fixated a point in the middle of the mask. Three different stimulus durations (means 30, 46, and 106 msec) were used in consecutive sessions to vary the rate of object detection and to examine the corresponding changes in brain activation. Because of different screen refresh rates in the stimulus computer and Sony VPL-350QM data projector, each stimulus sequence contained 20–30% of stimuli with duration deviating by one screen refreshing time from the desired. We do not consider this a serious drawback, however, because after 100 averages in each stimulus category, the SEM of the stimulus duration was <0.8 msec for the 30, 46, and 106 msec stimuli. A control study aimed at comparing activity related to correct and missed object detections was run 1 year later; seven observers were presented with stimuli at a duration that resulted in ∼50% correct detections. During this session the actual stimulus durations were measured with an optical fiber, and only responses to stimuli with identical durations were averaged off-line.
Fig. 1.
Stimulation sequence with examples of object and non-object stimuli.
One second after the stimulus had disappeared, the observers were requested to respond by lifting the right index finger if they had perceived a coherent and meaningful object and the left index finger if nothing at all or nothing coherent and meaningful was perceived. The purpose was to reach high specificity in object detection. On average only 7, 8, and 3% of non-objects were detected as objects (false positives) at the 30, 46, and 106 msec stimulus durations, respectively. The proportion of correctly detected objects increased with stimulus duration, being 48 ± 9, 72 ± 7, and 95 ± 2% (mean ± SEM) for the 30, 46, and 106 msec stimuli, respectively.
Magnetic signals were measured with a Neuromag-122 whole-scalp neuromagnetometer containing 122 planar SQUID gradiometers at 61 locations (Ahonen et al., 1993). The two sensors at each location measure two orthogonal tangential derivatives of the magnetic field component perpendicular to the surface of the sensor array. The planar gradiometers measure the strongest signals directly above local cortical currents. Signals were bandpass-filtered (0.03–90 Hz) and digitized at 297 Hz.
Amplitude spectra were calculated for all channels from 0.86 sec poststimulus periods (resolution 1.2 Hz), triggered from the stimulus onset. The temporal spectral evolution (TSE) method (Salmelin and Hari, 1994) was used to determine the average amplitude level of alpha as a function of time, with respect to stimulus onset. The signals were first filtered through the 8–13 Hz passband and then rectified, and the absolute values were averaged over ∼100 trials, time-locked to stimulus onset. The averaged signals were finally low pass-filtered at 15 Hz to smooth the TSE curve. The difference between TSE signals for objects and non-objects was estimated to begin when the non-object minus object subtraction curve exceeded 2 SD of its prestimulus (−2000–0 msec) baseline signal variation. Similarly, the evoked responses were estimated to begin when the source strength exceeded 2 SD of the prestimulus (−200–0 msec) signal variation for a minimum duration of 50 msec. Only the 106 msec stimulus duration, resulting in the best signal-to-noise ratio, was used for the latency comparisons between evoked responses and alpha rhythm. This comparison includes some inaccuracy attributable to noise affecting the onset latency determinations and to the narrow-band signal filtering used to calculate the TSE curves.
To locate sources generating the reactive alpha rhythm, twelve 2 sec epochs (six after objects and six after non-objects in alternate order) were selected from the spontaneous signals filtered through 8–13 Hz. Equivalent current dipoles were automatically localized with a least-squares search at 1 msec intervals, with a fixed selection of 50 channels covering the whole occipital lobe and most of the parietal cortex but excluding the rolandic region. This channel selection covered the strongest parieto-occipital alpha signals in all observers. From each oscillatory cycle only one dipole with the best explanation of the field variance (minimum 89% over the 50 channels) was accepted.
RESULTS
The level of the parieto-occipital alpha rhythm is higher after non-objects than objects
Figure 2 (left) shows the poststimulus amplitude spectra from one parieto-occipital channel for two observers. The ∼10 Hz activity is more abundant after non-objects (dashed lines) than objects in both observers; the difference is strongest in parieto-occipital midline (Fig. 2,middle). For more detailed analysis, four channel pairs above the POS were selected for each observer on the basis of the individual magnetic resonance images. These locations typically covered the maximum difference, as illustrated in Figure 2 (middle) for Observers 4 and 5. An areal mean signal across these four sensor pairs was then used for comparisons of alpha reactivity quantified by means of the TSE analysis. In Observer 4 (Fig. 2, top right), the alpha was suppressed after both stimulus categories, more strongly for objects than non-objects, and it returned earlier back to the baseline level after non-objects; the difference started at 190 msec and peaked at 560 msec. For Observer 5 (Fig. 2, bottom right), the suppression was negligible, and the non-objects evoked an alpha increase above the prestimulus level; the difference between the categories started at 390 msec and peaked at 890 msec. Despite individual variations in alpha reactivity, the difference waveforms between TSE curves to non-objects versus objects were similar (Fig. 2,bottom right).
Fig. 2.
Left, Frequency spectra of MEG signals, triggered from the stimulus initiation, from one channel over the parieto-occipital region. Middle, The helmet-shaped sensor array. Each location (square) contains two planar gradio-meters measuring the orthogonal derivatives of the magnetic field gradient (Ahonen et al., 1993). The contours show the distribution of the maximum difference between non-objects and objects at 10 Hz. Right, Temporal spectral evolution (TSE) curves showing the level of alpha oscillations as a function of time. The signals are averages across eight channels (in the four shaded locations in the middle). Mean differences during the integration period (300–1000 msec,shaded areas) were used as the measures of the poststimulus alpha difference.
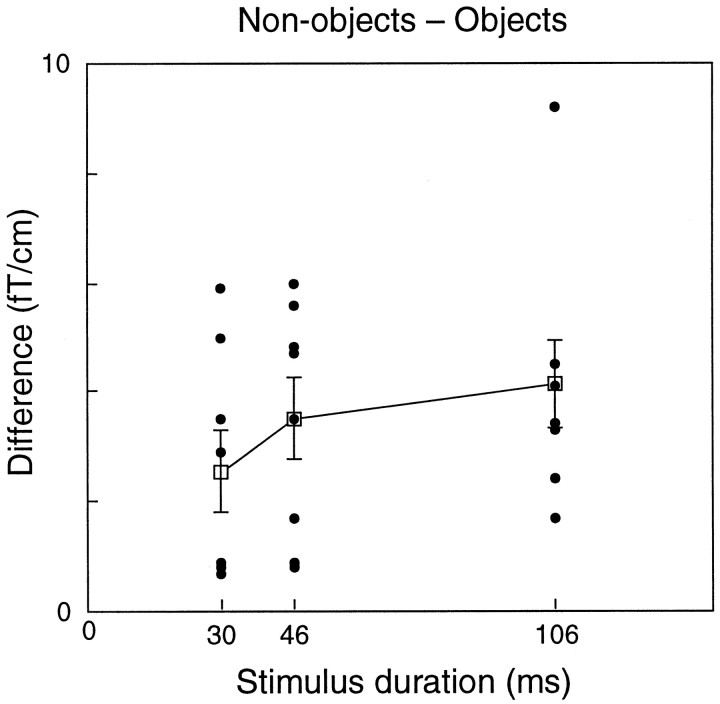
Figure 3 shows the mean differences (non-object minus object) in the alpha level between 300 and 1000 msec after the stimulus onset for all observers and all stimulus durations. The level was systematically higher after non-objects than objects (p < 0.005 for each stimulus duration; binomial test) as reflected by the consistently positive values in Figure 3. The mean (± SEM) differences were 2.6 ± 0.7, 3.5 ± 0.7, and 4.1 ± 0.8 fT/cm for the 30, 46, and 106 msec stimulus durations, respectively (difference between durations, NS).
Fig. 3.
The mean differences (between 300 and 1000 msec after the stimulus) of non-object minus object alpha levels for all observers and stimulus durations. Each dot represents one observer. Mean (±SEM) values for all observers are shown.
The alpha rhythm modulation starts soon after the beginning of evoked cortical activity
The evoked magnetic fields were modeled with four to seven dipoles, as has been described previously in detail (Vanni et al., 1996b). Strong signals were first searched during 0–1 sec after stimulus presentation. The signal patterns were then modeled, during discrete time points, with equivalent current dipoles, found with a least-squares search, and using a spherical volume conductor model. Contamination from other active areas was reduced by restricting the search to 14–38 channels over the local signal maxima.
At least six of eight observers showed significant responses in the lateral occipital cortices (starting at 145 ± 13 msec in the left and 219 ± 8 msec in the right hemisphere; p < 0.01 for the latency difference), in the superior temporal areas (starting at 241 ± 23 msec in the left and 213 ± 10 msec in the right hemisphere; NS), and in the left parietal cortex (starting at 216 ± 24 msec). The source locations of individual observers varied in different brain regions on average by 17–34 mm with respect to external landmarks.
Most sources showed dissimilar activity for objects and non-objects, but in only 18 of 31 sources the strength difference exceeded sustainedly 2 SD of baseline signal variation. The non-object versus object difference in the alpha level (called “alpha effect” below) started at 370 ± 50 msec for the 106 msec stimuli; the mean across all durations was 400 ± 20 msec, with no significant difference between the stimulus durations.
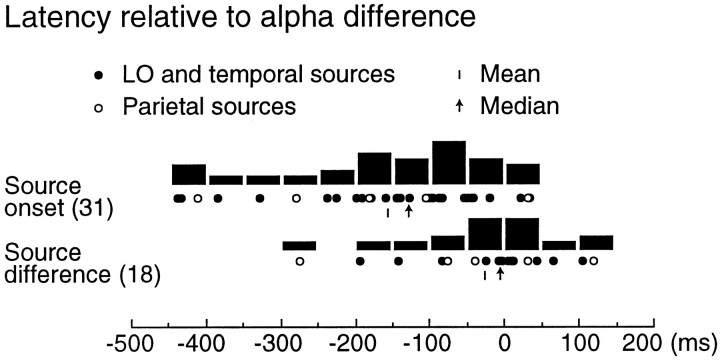
Figure 4 shows the evoked response onset latencies relative to the individual alpha effect onsets. In most cases the evoked responses started before the alpha effect (median difference 130 msec; p < 0.02; paired two-tailed ttest with each observer’s average source activation latency compared with the alpha effect onset). The amplitude difference between objects and non-objects emerged at about the same time in the evoked responses and in the alpha level (median time interval 10 msec; NS). The onset latencies of parietal and other evoked responses did not differ systematically.
Fig. 4.
Temporal relations between evoked responses and alpha effect for the 106 msec stimuli. Top, The evoked response onset (31 sources in 8 observers) are presented relative to the emergence of each observer’s alpha difference between non-objects and objects (at 0 msec). Bottom, The non-object versus object strength difference onset latencies are compared between the evoked responses and alpha rhythm. The black columnsshow the number of sources within one 50 msec time interval.
The alpha level is higher after non-objects than missed object detections
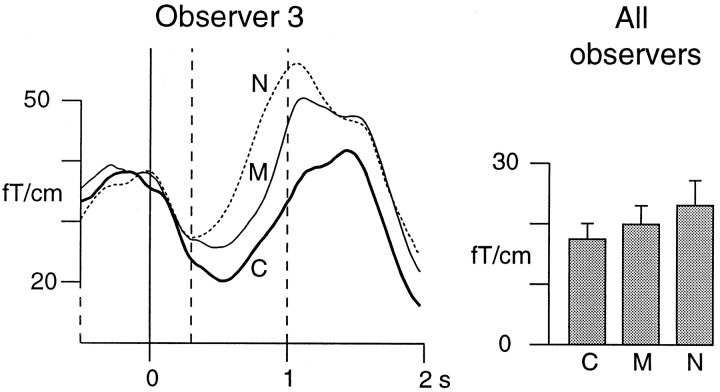
Figure 5 shows the TSE curves of Observer 3 for the correct (64%) and missed (36%) objects and for the non-objects, all with 30 msec duration. The mean alpha level between 300 and 1000 msec is lowest with correct object detections (24.1 fT/cm), higher after missed objects (30.1 fT/cm), and highest after non-objects (37.9 fT/cm). The corresponding alpha levels for all observers were 17.7 ± 2.6, 20.2 ± 3.1, and 23.4 ± 4.1 fT/cm (Fig. 5, right); the differences between both correct and missed objects, and between missed objects and non-objects, were significant (p < 0.05; paired two-tailedt test).
Fig. 5.
TSE curves for correct (C) and missed (M) object and for non-object (N) detections for Observer 3, all with 30 msec stimulus duration. The shaded bars show the mean (±SEM) alpha levels for all observers during the 300–1000 msec integration period.
The reactive alpha originates in the POS
Figure 6 shows the sources of the reactive, poststimulus parieto-occipital alpha for Observer 1. In agreement with previous studies (for review, see Hari and Salmelin, 1997), the sources cluster in the POS close to the midline. The sources of prominent alpha oscillations were identified for three other observers with essentially the same results. Only very few sources were found close to the calcarine sulcus.
Fig. 6.
The sources of alpha rhythm superimposed on the MR image of Observer 1. The white dots andbars indicate the locations and directions of the source currents. The thin white lines mark the section level in the other plane.
Without discrimination task the alpha rhythm reacts similarly to both object and non-object stimuli
Control experiments were performed with Observers 1 and 3, both with abundant alpha rhythm, to further clarify the role of the object categorization task for the poststimulus alpha reactivity. Instead of discriminating the (106 msec) stimuli to objects and non-objects, the task was to indicate detection of any stimulus with right index finger lift. Figure 7 shows that during this task the alpha reactivity did not differ between objects and non-objects.
Fig. 7.
The TSE curves from the control experiment with no visual discrimination task.
DISCUSSION
Our results show that the level of parieto-occipital alpha rhythm is higher after meaningless non-objects than after meaningful objects, which the observer had to detect. The alpha level difference is obviously related to the attention-to-shape demand of the task: when no discrimination was required, the object versus non-object alpha difference vanished. The increasing alpha level from correct to missed objects and from missed objects to non-objects may be inversely related to the saliency or familiarity of the stimulus. Evidently, the level does not directly reflect the contents in the visual awareness because the non-objects and missed objects, both resulting in identical behavioral response (“non-object”), still differed in the alpha level. The non-object versus object difference emerged roughly simultaneously in the alpha level and in the evoked responses. Provided that the visual cortical areas are similarly organized in humans and other primates, these results suggest that visual targets, defined by shape, promptly modify activity in the human dorsal visual stream.
The behavior and origins of the alpha rhythm
The electroencephalographic alpha rhythm (for review, seeNiedermeyer, 1993) is suppressed by opening the eyes (Berger, 1929) and with increased attentiveness (Pollen and Trachtenberg, 1972b; Ray and Cole, 1985), and enhanced by viewing a uniform visual field (Lehtonen and Lehtinen, 1972). Although visual imagery attenuates both electric (Slatter, 1960) and magnetic alpha (Kaufman et al., 1990;Salenius et al., 1995), the most effective alpha suppressor is the external visual stimulation (Pollen and Trachtenberg, 1972a; Ray and Cole, 1985). Despite the well characterized behavior of the occipital alpha rhythm, its connections to cortical processes are less well known.
In line with earlier findings (Williamson and Kaufman, 1989; Salmelin and Hari, 1994; Salenius et al., 1995; Hari et al., 1997), alpha oscillations recorded in the present study were generated at or near the POS. Hari and Salmelin (1997) recently suggested that the MEG alpha might be generated in the human homolog of monkey V6 complex. Area V6, also known as area PO in macaque, is located in the anterior bank of the POS and is strongly interconnected with area V6A, which abuts it dorsally (Galletti et al., 1996). V6/PO receives connections from other visual cortices (V1, V2, V3, V3A, V4, and V5/MT), with relative emphasis on the visual field periphery, and it is widely and reciprocally connected to several parietal areas (Colby et al., 1988;Cavada and Goldman-Rakic, 1989a; Blatt et al., 1990; Felleman and Van Essen, 1991) and to the dorsal premotor cortex (Tanné et al., 1995). V6/PO provides a relatively direct route from striate and prestriate cortices to the parietal cortex (Colby et al., 1988) and has been suggested to participate both in the encoding of visual space and in the control of gaze and arm reaching movements (Galletti et al., 1991; Galletti et al., 1995). Interestingly, the spontaneous activity recorded with microelectrodes from the V6A is inhibited during increased attentiveness (Galletti et al., 1996), thereby resembling the behavior of the human alpha rhythm.
Interplay between the parietal attentional and ventral stream object recognition systems
The posterior parietal attentional system (Posner et al., 1984;Steinmetz and Constantinidis, 1995) may dynamically gate the input to the ventral visual stream and help to choose the appropriate targets for object recognition (LaBerge and Brown, 1989; Posner and Petersen, 1990; Corbetta et al., 1991; Van Essen et al., 1992; LaBerge, 1995). The pulvinar nucleus of thalamus probably mediates the object selection effects on the basis of both spatial location and object features (Desimone et al., 1990; LaBerge and Buchsbaum, 1990; Corbetta et al., 1991; Robinson and Petersen, 1992). Like most visual areas in primates, the POS region also has connections with the pulvinar (Trojanowski and Jacobson, 1976; Graham et al., 1979; Zeki, 1986). In dog, both pulvinocortical and corticocortical connections seem to be important for the generation of the cortical alpha rhythm (Lopes da Silva et al., 1980). Because the attentional modulation may be restricted to a part of the pulvinar (Petersen et al., 1985), it would be interesting to know, for the interpretation of the present results, whether the pulvinar subnuclei exerting and not exerting attentional effects have similar POS connections.
Both inferotemporal and prefrontal cortices in monkeys participate in shape-based discrimination (Fuster et al., 1985; Chelazzi et al., 1993). In addition, parts of the inferior parietal lobule, which receive extensive input from area PO (Andersen et al., 1990; Boussaoud et al., 1990), react to unattended stimuli during attentive fixation (Mountcastle et al., 1981). Similarly, the human parietal cortex is activated during tasks based on object features (Roland et al., 1990;Fletcher et al., 1995). Interestingly, patients with lesion in the parieto-occipital cortex may suffer from simultanagnosia, the inability to consciously see more than one object at a time; although they easily recognize the targets they perceive, they probably have an inability to shift attention from one stimulus to another, that is, a failure in the cooperation between the parietal attentional and the object recognition systems (Farah, 1990). With brief displays, simultaneous object detection is limited also in healthy humans, supporting the view that objects, like locations, may serve as sequentially processed attentional units (Duncan, 1980, 1984).
Our results suggest that both objects and non-objects first evoke similar activity and within the next 450 msec the distinction between the stimuli emerges, rather simultaneously, in several cortical areas, belonging possibly to both the ventral and dorsal visual streams. Interestingly, the ∼400 msec onset latency of our alpha effect resembles the duration of an “attentional blink” during which subsequent visual targets do not reach awareness (Luck et al., 1996).
The two most plausible causal explanations for the shape-based POS reactivity are that (1) the shape recognition (“ventral stream”) information is relayed to POS via corticocortical or pulvinar connections or (2) objects are discriminated independently in the ventral stream and POS. Although V6/PO contains a high proportion of orientation-selective neurons, these are most likely used for locating objects in space (Galletti et al., 1991) rather than for shape discrimination. Thus it is more likely that the ventral stream is the source of the object-discriminating signals in POS. This would imply that the communication between the dorsal and ventral streams is bidirectional: in addition to the dorsal-to-ventral stream effects, the information derived from ventral stream processes may modify the dorsal stream activity. Such bidirectionality is expected, given the abundant cross-connections between the visual cortices (Felleman and Van Essen, 1991).
Does the parieto-occipital alpha rhythm reflect a functional state?
During sleep, a tonic hyperpolarization of the cellular membrane leads to a burst firing mode of thalamic neurons and, because of synchronous thalamocortical firing, to 7–14 Hz sleep spindles in the cortex (Steriade and Llinas, 1988). During this oscillatory mode, information transfer is reduced at the thalamic relay neurons: the cortex is disconnected from peripheral sensory inputs (Glenn and Steriade, 1982; Steriade and Llinas, 1988). According to Lopes da Silva (1991), gating, a change in the functional state of a neural assembly leading to changes in the information transfer, might be one of the main roles of the oscillatory mode. In this context, the strong alpha oscillations after non-objects (non-targets) might reflect functional disengagement of the POS, or of the parietal regions connected to POS, from the low level visual cortices.
The receptive fields in monkey inferotemporal cortex are smallest during attentive fixation (Richmond et al., 1983), suggesting decreased sensitivity to new targets. Assuming that the parietal cortex, at this stage, supports object selection to the ventral stream, the POS alpha increase after non-objects might reflect enhanced ventral stream sensitivity attributable to lack of target.
The monkey inferior parietal lobule and prefrontal cortices are densely connected (Cavada and Goldman-Rakic, 1989b) and activated during both spatial- and object-feature-related working memory tasks (Friedman and Goldman-Rakic, 1994). One plausible explanation for the present results, in addition to varying attentive demands, is that the salient objects activate this working memory network to a greater extent than the non-objects. In this case either corticocortical or common pulvinar connections with POS could mediate the modulation of alpha rhythm.
Some earlier and the present findings could be summarized as follows to form a framework for future experiments. The POS cortex is an essential part of a network, which during attentive fixation is searching for subsequent targets. If the system is damaged, as in simultanagnosia, the nonattended objects disappear from visual awareness. When the normally functioning visual system is engaged with an object, the POS is in an asynchronous transfer mode and mediates information to the parietal cortex about the next form or location to be attended to. Detection of simultaneous objects requires shift of attention; this dorsal stream preprocessing is detectable as “interference” between simultaneous visual targets. If the visual system is not engaged with a target, the POS cortex is resting and the alpha rhythm appears.
Footnotes
This research was supported by the Academy of Finland and Sigrid Jusélius Foundation. We thank Jukka Saarinen for participation in a part of this study; Gabriel Curio, Karin Portin, Stephan Salenius, and Heidi Schlitt for comments on this manuscript; and Veikko Jousmäki, Lauri Parkkonen, Mika Seppä, and Kimmo Uutela for expert technical assistance.
Correspondence should be addressed to Dr. Simo Vanni, Brain Research Unit, Low Temperature Laboratory, Helsinki University of Technology, P.O. Box 2200, FIN-02015 HUT, Espoo, Finland.
REFERENCES
- 1.Ahonen AI, Hämäläinen MS, Kajola MJ, Knuutila JET, Laine PP, Lounasmaa OV, Parkkonen LT, Simola JT, Tesche CD. 122-channel SQUID instrument for investigating the magnetic signals from the human brain. Physica Scripta. 1993;T49:198–205. [Google Scholar]
- 2.Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- 3.Berger H. Über das Elektroenkephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- 4.Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- 5.Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- 6.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellations of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 7.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 8.Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in the inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- 9.Colby CL, Gattas R, Olson CR, Gross CR. Topographical organization of cortical afferents to extrastriate area PO in the macaque: a dual tracer study. J Comp Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- 10.Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 12.Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harbor Symp Quant Biol. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- 13.Duncan J. The locus of interference in the perception of simultaneous stimuli. Psychol Rev. 1980;87:272–300. [PubMed] [Google Scholar]
- 14.Duncan J. Selective attention and the organization of visual information. J Exp Psychol Gen. 1984;113:501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- 15.Farah MJ. Visual agnosia: disorders of object recognition and what they tell us about normal vision. MIT; Cambridge, MA: 1990. [Google Scholar]
- 16.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ. The mind’s eye-precuneus activation in memory related imagery. NeuroImage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- 18.Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuster JM, Bauer RH, Jervey JP. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- 20.Galletti C, Battaglini PP, Fattori C. Functional properties of neurons in the anterior bank of the parieto-occipital sulcus of the Macaque monkey. Eur J Neurosci. 1991;3:452–461. doi: 10.1111/j.1460-9568.1991.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Galletti C, Battaglini PP, Fattori P. Eye position influence on the parieto-occipital area PO (V6) of the Macaque monkey. Eur J Neurosci. 1995;7:2486–2501. doi: 10.1111/j.1460-9568.1995.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 22.Galletti C, Fattori P, Battaglini PP, Shipp S, Zeki S. Functional demarcation of a border between areas V6 and V6A in the superior parietal gyrus of the Macaque monkey. Eur J Neurosci. 1996;8:30–52. doi: 10.1111/j.1460-9568.1996.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 23.Glenn LL, Steriade M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci. 1982;2:1387–1404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 25.Graham J, Lin C-S, Kaas JH. Subcortical projections of six visual cortical areas in the owl monkey, Aotus trivirgatus. J Comp Neurol. 1979;187:557–580. doi: 10.1002/cne.901870307. [DOI] [PubMed] [Google Scholar]
- 26.Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- 27.Hari R, Salmelin S, Mäkelä JP, Salenius S, Helle M. Magnetoencephalographic cortical rhythms. Int J Psychophysiol. 1997;26:51–62. doi: 10.1016/s0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman L, Schwartz B, Salustri C, Williamson SJ. Modulation of spontaneous brain activity during mental imagery. J Cogn Neurosci. 1990;2:124–132. doi: 10.1162/jocn.1990.2.2.124. [DOI] [PubMed] [Google Scholar]
- 29.LaBerge D. Attentional processing. The brain’s art of mindfulness. Harvard UP; Cambridge, MA: 1995. [Google Scholar]
- 30.LaBerge D, Brown V. Theory of attentional operations in shape identification. Psychol Rev. 1989;96:101–124. [Google Scholar]
- 31.LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehtonen JB, Lehtinen I. Alpha rhythm and uniform visual field in man. Electroencephalogr Clin Neurophysiol. 1972;32:139–147. doi: 10.1016/0013-4694(72)90136-8. [DOI] [PubMed] [Google Scholar]
- 33.Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr Clin Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- 34.Lopes da Silva FH, Vos JE, Mooibroek J, Rotterdam AV. Relative contribution of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50:449–456. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- 35.Luck SJ, Vogel EK, Shapiro KL. Word meanings can be accessed but not reported during the attentional blink. Nature. 1996;383:616–618. doi: 10.1038/383616a0. [DOI] [PubMed] [Google Scholar]
- 36.Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RBH. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 38.Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2 and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 39.Mountcastle VB, Andersen RA, Motter BC. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci. 1981;1:1218–1235. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography. Basic principles, clinical applications and related fields, 3rd edition. Williams & Wilkins; Baltimore: 1993. pp. 131–152. [Google Scholar]
- 41.Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- 42.Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- 43.Pollen DA, Trachtenberg MC. Alpha rhythm and eye movements in eidetic imagery. Nature. 1972a;237:109–112. doi: 10.1038/237109a0. [DOI] [PubMed] [Google Scholar]
- 44.Pollen DA, Trachtenberg MC. Some problems of occipital alpha block in man. Brain Res. 1972b;41:303–314. doi: 10.1016/0006-8993(72)90504-5. [DOI] [PubMed] [Google Scholar]
- 45.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 46.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray WJ, Cole H. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228:750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- 48.Richmond BJ, Wurtz RH, Sato T. Visual responses of inferior temporal neurons in awake rhesus monkey. J Neurophysiol. 1983;50:1415–1432. doi: 10.1152/jn.1983.50.6.1415. [DOI] [PubMed] [Google Scholar]
- 49.Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- 50.Roland PE, Gulyás B, Seitz RJ, Bohm C, Stone-Elander S. Functional anatomy of storage, recall and recognition of a visual pattern in man. NeuroReport. 1990;1:53–56. doi: 10.1097/00001756-199009000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Salenius S, Kajola M, Thompson WL, Kosslyn S, Hari R. Reactivity of magnetic parieto-occipital alpha rhythm during visual imagery. Electroencephalogr Clin Neurophysiol. 1995;95:453–462. doi: 10.1016/0013-4694(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 52.Salmelin R, Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr Clin Neurophysiol. 1994;91:237–248. doi: 10.1016/0013-4694(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 53.Slatter KH. Alpha rhythms and mental imagery. Electroencephalogr Clin Neurophysiol. 1960;12:851–859. [Google Scholar]
- 54.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn Mem] 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 55.Steinmetz MA, Constantinidis C. Neurophysiological evidence for a role of posterior parietal cortex in redirecting visual attention. Cereb Cortex. 1995;5:448–456. doi: 10.1093/cercor/5.5.448. [DOI] [PubMed] [Google Scholar]
- 56.Steriade M, Llinas R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 57.Tanné J, Boussaoud D, Noëlle B-Z, Rouiller EM. Direct visual pathways for reaching movements in the macaque monkey. NeuroReport. 1995;7:267–272. [PubMed] [Google Scholar]
- 58.Trojanowski JQ, Jacobson S. Areal and laminar distribution of some pulvinar cortical efferents in rhesus monkey. J Comp Neurol. 1976;169:371–392. doi: 10.1002/cne.901690307. [DOI] [PubMed] [Google Scholar]
- 59.Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Mansfield RJW, Goodal MD, editors. The analysis of visual behavior. MIT; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- 60.Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- 61.Vanni S, Revonsuo A, Saarinen J, Hari R. Meaningful images enhance activation in occipital and left temporal and parietal cortices. Soc Neurosci Abstr. 1996a;22:1616. [Google Scholar]
- 62.Vanni S, Revonsuo A, Saarinen J, Hari R. Visual awareness of objects correlates with activity of right occipital cortex. NeuroReport. 1996b;8:183–186. doi: 10.1097/00001756-199612200-00037. [DOI] [PubMed] [Google Scholar]
- 63.Williamson S, Kaufman L. Advances in neuromagnetic instrumentation and studies of spontaneous brain activity. Brain Topogr. 1989;2:129–139. doi: 10.1007/BF01128850. [DOI] [PubMed] [Google Scholar]
- 64.Zeki S. The anatomy and physiology of area V6 of macaque monkey visual cortex. J Physiol (Lond) 1986;381:62P. [Google Scholar]