Abstract
During development target-derived neurotrophins promote the survival of neurons. However, mature neurons no longer depend on the target for survival. Do target-derived neurotrophins retain retrograde signaling functions in mature neurons, and, if so, how are they executed? We addressed this question by using a phosphotyrosine-directed antibody to locate activated Trk receptors in adult rat sciatic nerve. We show that catalytically active Trk receptors are located within the axon of adult rat sciatic nerve and that they are distributed throughout the length of the axons. These catalytically active receptors are phosphorylated on tyrosine at a position that couples them to the signal-generating proteins Ras and PI3 kinase. Neurotrophin applied at sciatic nerve terminals increases both catalytic activity and phosphorylation state of Trk receptors at distant points within the axons. Trk activation initiated at the nerve terminals propagates through the axon toward the nerve cell body at an initial rate that exceeds that of conventional vesicular transport. However, our data suggest that this rapid signal is nevertheless vesicle-associated. Thus, in mature nerves, activated Trk receptors function as rapid retrograde signal carriers to execute remote responses to target-derived neurotrophins.
Keywords: neurotrophin, Trk, sciatic nerve, receptor tyrosine kinase, signal transduction
Target-derived neurotrophins evoke diverse responses in presynaptic neurons, including effects on survival, neurite outgrowth, and synaptic modulation (Levi-Montalcini and Angeletti, 1968; Levi-Montalcini, 1987; Barde, 1989; Snider and Johnson, 1989; Deckwerth and Johnson, 1993; Lai et al., 1993; Raffioni et al., 1993; Vogel, 1993; Mehler and Kessler, 1994). Some of these effects are local in nature (Campenot, 1982, 1987; Diamond et al., 1992b), whereas other responses require that neurotrophins, presented at the nerve terminals, initiate an intracellular signal that travels through the axon to the remote cell body (Hendry and Crouch, 1991;Campenot, 1994).
Both local and long distance neurotrophic functions are initiated by ligand binding and activation of receptor tyrosine kinases: TrkA for NGF, TrkB for BDNF, and neurotrophin (NT) NT4/5 and TrkC for NT3 (Bothwell, 1991; Barbacid, 1994). The activated receptors initiate local signaling at the membrane and also initiate signaling cascades that traverse the cytoplasm and culminate in the nucleus with transcriptional changes (Segal and Greenberg, 1996). Three models have been proposed to explain how signal transduction pathways convey a retrograde signal from a nerve terminal through an axon to a distant cell body (Hendry and Crouch, 1993; Campenot, 1994). The central feature of the first model is that target-derived neurotrophins are transported in vesicles from nerve terminals to cell bodies. On arrival at the cell body, neurotrophin receptors are activated and initiate signal transduction pathways. This model predicts that retrograde transport of neurotrophin to the cell body is sufficient for signaling and that neurotrophin receptors are activated exclusively within the cell body.
A second model is that neurotrophins bind and activate receptors at nerve terminals. Activated receptors then interact with and activate downstream mediators. In this model the axon could be considered an extension of the cell body cytoplasm, with active vesicular transport conveying downstream signaling molecules to the nucleus to complete the signal transduction cascade (Johanson et al., 1995). One prediction of this model is that neurotrophins activate receptors only at the nerve endings.
In a third model, receptors are activated by neurotrophins at nerve terminals and thereby initiate local signaling pathways. However, internalized activated receptors also are transported through the axon toward the nerve cell body. Activated receptors, alone or in a ligand–receptor complex, continue to activate downstream signal transduction pathways en route. Indeed, neurotrophin receptors are internalized after activation (Hosang and Shooter, 1987; Kahle et al., 1994) and undergo retrograde transport (Johnson et al., 1987; Raivich et al., 1991; Loy et al., 1994; Ehlers et al., 1995). Receptor internalization generally is considered a component of signal termination and receptor recycling rather than signal transduction (Sorkin and Waters, 1993). However, the possibility that Trk internalization might be part of a signaling pathway is bolstered by recent demonstrations that vesicle-associated Trk receptors in PC12 cells are phosphorylated (Grimes et al., 1996) and that receptor endocytosis actually stimulates some signaling molecules (Vieria et al., 1996). A prediction of this model for retrograde signaling is that activated receptors are distributed throughout the axon and are engaged in signal transduction.
Each model for retrograde signaling predicts a distinct distribution of activated neurotrophin receptors. To test these models, we used an antibody that recognizes phosphorylated Trks (Segal et al., 1996) to visualize activated receptors within adult rat sciatic nerve. We found that activated Trks are distributed along the length of sciatic nerve axons and that phosphorylation state and catalytic activity of axonal Trk receptors are regulated by neurotrophins applied at nerve terminals. The response to exogenous neurotrophin is surprisingly rapid. These data suggest that phosphorylated Trks propagate a retrograde signal in axons in the adult nervous system.
MATERIALS AND METHODS
Reagents
Anti-pY490 polyclonal antisera were raised against a phosphopeptide (VIENPQpYFGITNS) corresponding to the conserved Shc recognition site of all Trks (Segal et al., 1996). Anti-Trk and anti-TrkB were generous gifts from Dr. David Kaplan (Montréal Neurological Institute, Montréal, Québec, Canada). Anti-Trk is an antibody to the C-terminal tail of Trk (Hempstead et al., 1992). The TrkB-specific antibody was raised against a peptide in the intracellular domain of TrkB. The anti-phosphotyrosine antibody (4G10) (Druker et al., 1989) was a gift of Dr. Thomas Roberts (Dana-Farber Cancer Institute, Boston, MA). The Shc antibody was purchased from Upstate Biologicals (UBI, Lake Placid, NY). Recombinant human BDNF and NT3 were generous gifts of Dr. Andrew Welcher and Amgen (Thousand Oaks, CA). NGF was purchased from Life Technologies (Grand Island, NY). The peptides used for competition experiments were generated as described (Segal et al., 1996): the Y490 unphosphorylated peptide (VIENPQYFGITNS) and the NPXpY site of the erbB2 receptor (AENPEpYLGLDVPV).
Immunohistochemistry
Trk 3T3 cells were plated onto poly-d-lysine-coated coverslips in 24-well tissue culture plates. Quiescent cells were stimulated by the addition of 100 ng/ml neurotrophin or 0.1% BSA in PBS for 5 min; stimulation with vehicle alone served as a control. Untransfected NIH3T3 cells were stimulated with a cocktail of 50 ng/ml of each neurotrophin. The cells were fixed for 20 min in 4% paraformaldehyde in TBS and 1 mm sodium ortho vanadate, washed with TBS, and then permeabilized with 5% normal goat serum (NGS) and 0.5% NP-40 in TBS/vanadate for 1 hr. Cells were washed briefly and incubated with anti-pY490 (1:25) in 2% NGS in TBS/vanadate overnight at 4°C. For anti-pY490 staining and competition, the antibody was preincubated for 30 min at room temperature with a 100 nm concentration of the phosphopeptide immunogen, the corresponding unphosphorylated peptide, or a phosphopeptide representing the NPXY domain of the erbB2 receptor tyrosine kinase. Staining was visualized by avidin–biotin–HRP (Vector Labs, Burlingame, CA), followed by diaminobenzidine (DAB; Sigma, St. Louis, MO), according to the manufacturers’ procedures. Coverslips were mounted in Immumount (Shandon, Pittsburgh, PA).
Immunostaining of sciatic nerves. Anesthetized adult male Sprague Dawley rats (230–300 gm) were perfused intracardially with 4% paraformaldehyde in TBS/vanadate. Sciatic nerves were harvested and post-fixed for 6 hr. After incubation in 30% sucrose, nerves were embedded in Tissue Tek embedding media (Miles, Elkhart, IN) and frozen on dry ice. Cryostat sections (7.5–10 μm thickness) were cut and mounted onto coated slides (Fisher, Springfield, NJ). Then the sections were stained with the pY490 antibody, as described above. For double labeling, sections were incubated with both anti-pY490 and anti-α-acetylated tubulin (6-11-B1, Sigma) or anti-clathrin (ICN Biomedicals, Costa Mesa, CA) overnight at 4°C, followed by incubation with Cy3-conjugated goat anti-rabbit IgG and Cy2-conjugated goat anti-mouse IgG (Jackson Immunochemicals, West Grove, PA) in 2% NGS in TBS/vanadate for 1 hr.
Immunostaining of wild-type and BDNF −/− hippocampus. BDNF −/− and +/+ littermates were identified by PCR analysis. At postnatal day 14, anesthetized animals were perfused with 4% paraformaldehyde in PBS. Brains were removed, post-fixed in paraformaldehyde, and then infiltrated with sucrose. Coronal cryostat sections (15 μm) were cut and immunostained with pY490 and Cy3, as described above. To ensure specificity of staining, we performed peptide competitions with the phosphopeptide immunogen, the corresponding unphosphorylated peptide, and the erbB2 NPXpY peptide. Double staining with a monoclonal antibody to calbindin (Sigma) was done to facilitate identification of hippocampal structures.
Ligation
Adult male rats (Sprague Dawley; 230–300 gm) were anesthetized with Nembutal, and one of their sciatic nerves was exposed. The nerve was ligated ∼2 cm proximal to the gastrocnemius muscle. Two ligatures were made adjacent to each other with 6–0 silk. The animals were allowed to recover and were perfused 24 hr after ligation, as described above.
Protein lysates
NIH3T3 cells stably transfected with rat TrkA, rat TrkB, or porcine TrkC (Kaplan et al., 1991; Lamballe et al., 1991; Soppet et al., 1991) and PC12 cells that overexpress TrkA (Hempstead et al., 1992) were a gift of Dr. David Kaplan. The Trk 3T3 cells were grown in DMEM with 10% bovine calf serum, penicillin/streptomycin, glutamine, and 200 μg/ml Geneticin (G418). TrkPC12 cells were grown in the same media with 5% horse serum. TrkPC12 cells and TrkB 3T3 cells were changed to serum-free media 1 hr before stimulation by the addition of NGF or BDNF (50 ng/ml) for 5 min. Cells were harvested in lysis buffer (20 mm Tris, pH 8, 137 mm NaCl, 1% NP-40, 10% glycerol, 50 mm NaF, 10 mm NaPPi, 1 mm PMSF, 10 μg/ml aprotinin, 20 μmleupeptin, 2 mm Na orthovanadate, and 1 mmZnCl), mixed for 15 min at 4°C, and cleared by centrifugation.
Sciatic nerves were harvested from adult male rats (Sprague Dawley; 225–300 gm) anesthetized with Nembutal. Nerves were frozen rapidly in liquid nitrogen and stored at −80°C. For each sample, three whole nerves or eight nerve segments were pooled. Sciatic nerve extracts were made by mincing the nerve segments on dry ice and homogenizing them in a ground glass Dounce in lysis buffer. The extracts were incubated at 4°C for 15 min and cleared by centrifugation. Protein concentration in extracts was measured by Bio-Rad Protein Assay (Bio-Rad, Melville, NY). Extracts were stored at −80°C for up to 1 month before use.
Immunoprecipitation and immunoblot analysis
Extracts were immunoprecipitated with either anti-Trk or anti-pY490 or with no antibody. Sciatic nerve extracts (10–15 mg) and TrkB 3T3 cell extracts (0.5–1 mg) were immunoprecipitated with anti-Trk at a 1:100 dilution or with anti-pY490 at a 1:50–1:100 dilution. In competition experiments 100 nm peptide was preincubated with the pY490 antibody for 30 min at room temperature, and 100 nm peptide was added to the extracts. Extracts and antibody were incubated for 2 hr at 4°C. Protein A-Sepharose beads (50 ml) (Pharmacia Biotech, Uppsala, Sweden) in lysis buffer, preincubated with 10% BSA, were added to the extracts and incubated for 1 hr at 4°C. The beads were washed three times with lysis buffer, twice with 1 m LiCl in 20 mm Tris, pH 7, and twice with 20 mm Tris, pH 7. Washed beads were resuspended in 2× reducing sample buffer and boiled for 5 min before being size-fractionated on a 7.5% SDS-polyacrylamide gel. The gels were transferred to Immobilon P membranes (Millipore, Bedford, MA), and the blots were blocked in Blotto (5% nonfat dry milk in TBS) overnight at 4°C and then incubated overnight at 4°C with anti-pY490 (1:500), anti-Trk (1:2000), or anti-TrkB (1:1000) in Blotto. The blots were washed in Tris-buffered saline–Tween and incubated with a horseradish peroxidase-labeled goat anti-rabbit IgG (Bio-Rad), followed by visualization with enhanced chemiluminescence substrate (Amersham, Arlington Heights, IL). Alternatively, blots were incubated with anti-phosphotyrosine (4G10) for 1 hr at room temperature, followed by a horseradish peroxidase-labeled goat anti-mouse IgG (Bio-Rad), followed by enhanced chemiluminescence.
BDNF injections
Sciatic nerves of anesthetized adult male rats were exposed at the gastrocnemius muscle. Recombinant human BDNF (Amgen) or cytochrome C (Sigma) suspended in PBS was injected into the gastrocnemius muscle of separate rats at a dose of 5 μg/gm rat, using a Hamilton syringe (∼75–100 μl/muscle). In cut nerve experiments, sciatic nerves were cut near the gastrocnemius muscle before injection. At 10, 30, or 60 min after injection, sciatic nerves were harvested in three segments. One segment extended from 1 to 3 cm from the injection site (∼0.25 to 2.25 cm from the muscle belly). Two additional segments were taken from 3 to 5 cm and from 5 to 7 cm from the injection site. Samples were quick-frozen in liquid nitrogen and processed as described above.
In vitro kinase assay
Sciatic nerve extracts and TrkPC12 cells were immunoprecipitated with anti-Trk. For peptide competition experiments, the Trk antibody was incubated with 5 μm peptide immunogen. Immunoprecipitates were washed once with lysis buffer, twice with 1 m LiCl in 20 mm Tris, and once in kinase buffer (10 mm Tris, pH 7.4, and 10 mmMnCl2). The immunoprecipitates were incubated with ∼20 μCi γ-32P-ATP in 30 μl of kinase buffer for 15 min at room temperature, washed twice with cold PBS, and size-fractionated on a 7.5% SDS-polyacrylamide gel. For Trk kinase inhibition, 200 nm K252a (Kamiya Biomedical, Thousand Oaks, CA) was included in the kinase buffer. The gel was dried, and the bands were visualized with a PhosphoImager (Molecular Dynamics, Sunnyvale, CA).
Shc association
Sciatic nerve extracts were precleared with Protein A-Sepharose beads, immunoprecipitated with anti-Shc, and washed twice with lysis buffer and once with 20 mm Tris. Then immunoprecipitated proteins were size-fractionated and immunoblotted with anti-pY490 as described above.
Quantitation
TrkB proteins from pY490 immunoprecipitates were electrophoresed and immunoblotted with anti-phosphotyrosine or anti-TrkB. Films of the protein bands visualized by enhanced chemiluminescence were quantitated on an LKB Ultroscan II Enhanced Laser Densitometer. Three exposures from each experiment were scanned to ensure that quantitation was done in a linear range. The ratio between the BDNF-injected rats and cytochrome C-injected rats within each experiment was calculated for each protein band. The quantitative data from five or six independent experiments were analyzed. p values were calculated by using nonparametric statistics (one sample sign test for comparison, with an expected ratio of 1.0).
RESULTS
Antibody pY490 detects Trks phosphorylated at the Shc binding site
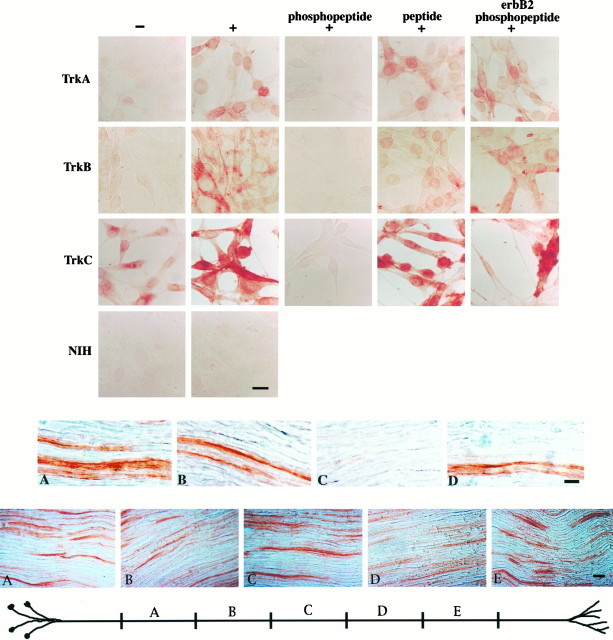
To determine where and when Trk receptors are activated in neuronsin vivo, we needed an immunochemical probe that could discriminate activated and inactivated receptor isoforms. In previous studies we described an antibody, pY490, directed against the phosphorylated epitope of an NPXY motif that serves as a Shc binding site in the neurotrophin receptors (Segal et al., 1996). To validate this antibody as an immunohistochemical reagent, we used NIH3T3 cells that had been transfected with the receptors for NGF, BDNF, or NT3 (TrkA, TrkB, or TrkC, respectively) (Kaplan et al., 1991; Lamballe et al., 1991; Soppet et al., 1991). As shown in Figure1, no pY490 immunostaining is observed in untransfected NIH3T3 cells. Control cells stimulated with vehicle alone show faint immunostaining, which reflects the fact that Trk 3T3 cells have a low level of constitutive receptor phosphorylation (Kaplan et al., 1991). Robust pY490 immunostaining is evident in these 3T3 cells after stimulation with the appropriate neurotrophin. Immunostaining is localized primarily to the plasma membrane of the cells, although occasionally staining is most intense in the vicinity of the nucleus. Peptide competition experiments were done to verify the specificity of the staining (Fig. 1). Immunostaining was abolished by preincubation of the antibody with its phosphopeptide immunogen. Preincubation of the antibody with the unphosphorylated peptide or a phosphopeptide corresponding to the NPXpY motif of the erbB2 receptor did not abolish the staining.
Fig. 1.
Top. Anti-pY490 detects activated TrkA, TrkB, and TrkC. TrkA-, TrkB-, and TrkC-expressing NIH3T3 cells were stimulated with neurotrophins and immunostained with the pY490 antibody, followed by avidin–biotin–HRP and DAB. TrkA, TrkB, and TrkC 3T3 cells stimulated with neurotrophin show increased pY490 immunostaining (+), as compared with control cells stimulated with vehicle alone (−). Immunostaining is abolished by preincubation of the antibody with its phosphopeptide immunogen (phosphopeptide). Preincubation of the antibody with the corresponding unphosphorylated peptide (peptide) or a phosphopeptide corresponding to the NPXY motif of the erbB2 receptor (erbB2 phosphopeptide) does not abolish the staining. No staining is observed in untransfected NIH3T3 cells (NIH). Scale bar, 10 μm.
The sensitivity of the pY490 antibody for detecting physiological changes in Trk phosphorylation has been tested in vivo. Hippocampal staining with anti-pY490 is reduced in BDNF −/− animals, as compared with wild-type controls (data not shown), whereas seizures increase pY490 immunostaining (J. Park and C. Stiles, unpublished observations). The pY490 antibody therefore can be used to detect activated TrkA, TrkB, and TrkC in immunocytochemistry as well as in biochemical analysis.
Phospho-Trk antibodies reveal activated Trks in rat sciatic nerve
As shown in Figure 2, activated Trk receptors can be found in the adult rat sciatic nerve. Figure2A shows longitudinal sections of adult rat sciatic nerve immunostained with anti-pY490. Peptide competition experiments (Fig. 2B–D) were done to establish that the antibody specifically recognizes phosphorylated Trks in the nerve. Immunostaining is abolished by preincubation of the antibody with its phosphopeptide immunogen, but not by preincubation of the antibody with the corresponding unphosphorylated peptide or a phosphopeptide corresponding to the NPXpY motif of the erbB2 receptor. The presence of activated receptors in sciatic nerve, as assayed with anti-pY490, indicates that neurotrophins are active in the adult peripheral nervous system.
Fig. 2.
Middle.Trk receptors are activated in mature sciatic nerve. Longitudinal sections of adult rat sciatic nerve were stained with anti-pY490, followed by avidin–biotin–HRP and DAB. Robust immunostaining is apparent in sections of nerve stained with anti-pY490 alone (A). Immunostaining is abolished by preincubation of the antibody with its phosphopeptide immunogen (C). Preincubation of the antibody with the corresponding unphosphorylated peptide (B) or a phosphopeptide corresponding to the NPXY motif of the erbB2 receptor (D) does not abolish the staining. Scale bar, 10 μm.
Activated Trks are present along the length of axons
Each of the models of neurotrophin signaling outlined above predicts a particular distribution of activated Trks within axons. To distinguish among the diverse models, we investigated the localization of activated Trks within the sciatic nerve. To determine how activated Trk receptors are distributed in the 6–7 cm of length between the cell bodies and the nerve terminals of the sciatic nerve, longitudinal sections from five sequential 1 cm segments were immunostained with anti-pY490. Representative pictures from each segment are shown in Figure 3. Activated Trks are evident in axons in panels A–E, suggesting that activated Trks are distributed uniformly within axons along the entire length of the sciatic nerve. A similar percentage of axons is stained in each segment. The uniform distribution of activated Trks along the length of axons is consistent with a model of retrograde signaling wherein Trk functions as a retrograde signal carrier.
Fig. 3.
Bottom. Activated Trk receptors are present along the length of sciatic nerve axons. Longitudinal sections from sequential segments along an adult rat sciatic nerve were immunostained with anti-pY490 and visualized with avidin–biotin–HRP and DAB. The diagram illustrates the approximate location of segments along the nerve. Representative pictures from each segment are shown. Immunostaining is evident in a subset of the axons in each segment. The staining in each segment is indistinguishable. Scale bar, 10 μm.
The appearance of pY490 immunostaining in Figures 2 and 3 suggests that activated receptors are found within axons. To confirm this impression, we costained sections of sciatic nerve with anti-pY490 and an antibody to acetylated α-tubulin, a marker of axons (Chitnis and Kuwada, 1990). Figure 4A shows that activated Trks are present in axons of the sciatic nerve. Further double-staining experiments were done with antibodies to α-tubulin, neurofilament proteins, and S-100 (data not shown). These costaining experiments confirm that activated Trks are confined to axons and are not present in Schwann cells or perineurial fibroblasts.
Fig. 4.
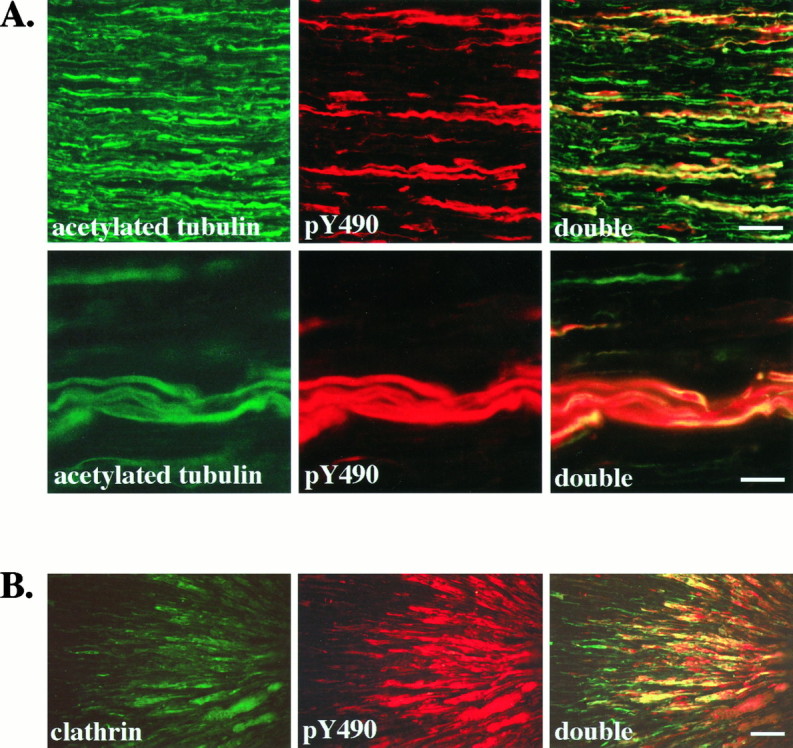
Activated Trk receptors are confined to axons in the sciatic nerve and accumulate distal to a ligation.A, Longitudinal sections of adult rat sciatic nerve were immunostained with anti-acetylated α-tubulin (acetylated tubulin) and anti-pY490 (pY490), as described, and viewed with fluorescence optics. Double labeling is shown in sections viewed with both fluorescein and rhodamine filters (double). All axons in the sciatic nerve are labeled by the acetylated tubulin antibody. In contrast, fewer axons are stained by anti-pY490. Colocalization is apparent as yellow in the double panels, demonstrating that pY490 labels only a subset of axons. Another field viewed at higher magnification (second row) shows that the anti-pY490 staining colocalizes with the acetylated tubulin staining, verifying that the pY490 immunostaining is present in axons of the sciatic nerve. Scale bars: First row, 50 μm; second row, 25 μm.B, The sciatic nerve of an adult rat was ligated ∼2 cm from the gastrocnemius muscle to interrupt vesicular transport. At 24 hr after ligation the nerve was harvested, and longitudinal sections of the nerve were immunostained with an antibody to the coated vesicle protein, clathrin and anti-pY490. The portion of nerve distal to the ligation site is shown with the ligation site to theright. Clathrin immunostaining shows accumulation of clathrin in axons on the distal side of the ligation (clathrin). Phosphorylated Trks, as shown by pY490 immunostaining, also accumulate on the distal side of the ligation (pY490). Costaining shows that phosphorylated Trks colocalize with clathrin (double). Scale bar, 50 μm.
These experiments also demonstrate that activated Trks are not found in all axons but are restricted to a subset of axons in the nerve. All of the pY490 immunoreactive axons also stained with the antibody to acetylated tubulin. However, only 10–40% of the axons in the nerve are stained with the pY490 antibody. The subset of axons that have detectable levels of activated Trk within them does not represent an obvious class of axons in the nerve (e.g., unmyelinated vs myelinated). Although virtually all neurons that travel in the sciatic nerve express one of the Trk species, it is important to note that we are visualizing phosphorylated Trks within axons at one instant in time. Therefore, the subset of axons that have detectable pY490 immunostaining may reflect transient phosphorylation of Trks rather than the segregation of activated Trks within one type of axon.
Activated Trks accumulate distal to a ligation
To determine whether phosphorylated Trk receptors in the axons are involved in retrograde signaling, we interrupted transport through the sciatic nerve by ligation. We then immunostained the nerves 24 hr after ligation (Fig. 4B). Activated Trk receptors, detected with anti-pY490, accumulate in axons on the distal side of the ligation, but not on the proximal side, indicating that phosphorylated Trks are moving from the synapse toward the cell body. Nerve ligation interrupts vesicular transport and results in an accumulation of vesicles at the ligation site but also might interrupt nonvesicular movement. Clathrin, a protein component of coated vesicles, accumulates in axons on the distal side of the ligation and colocalizes with phosphorylated Trks (Fig. 4B). These results suggest that phosphorylated Trk receptors are traveling by retrograde vesicular transport.
Anti-pY 490 recognizes activated Trks in nerve extracts
To verify that the immunohistochemical images displayed by pY490 in rat sciatic nerve correspond to activated Trks, we performed biochemical analyses. These verified the nature of anti-pY490 immunoreactive proteins and provided a quantitative method for analyzing responses to exogenous neurotrophin. In these and subsequent biochemical analyses we chose to focus on activation of TrkB, because recombinant neurotrophins can be administered readily as a bolus injection to gastrocnemius muscle, and the BDNF receptor TrkB is the most abundant receptor at motor nerve terminals.
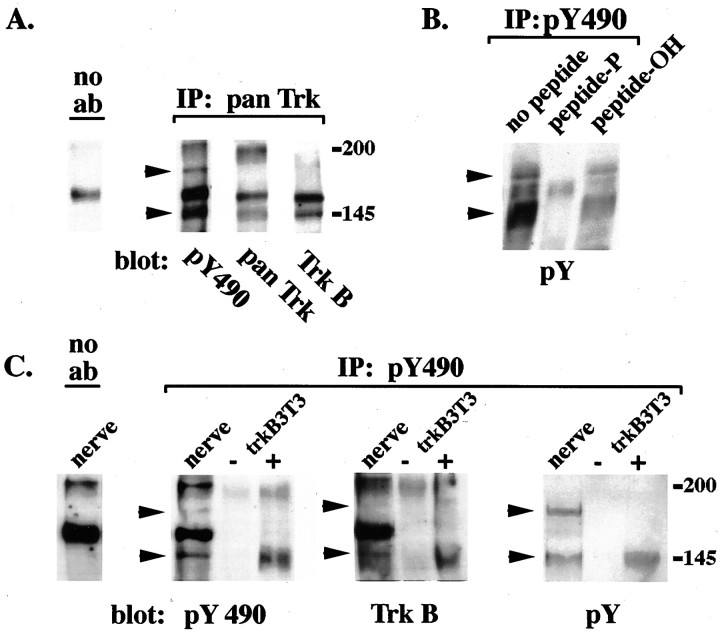
Sciatic nerve extracts were immunoprecipitated with a pan-Trk antibody that recognizes all full-length Trk species. These immunoprecipitates were size-fractionated and then immunoblotted with anti-pY490, anti-Trk, and anti-TrkB (Fig.5A). An artifactual band at 165 kDa is seen in all immunoprecipitates of sciatic nerve and represents a protein that is brought down by protein A-Sepharose beads. This band is variably recognized by anti-immunoglobulin secondary antibodies, as shown in the no antibody lanes. Anti-pY490 recognizes a diffuse band at 140–150 kDa and a single band with molecular mass of 180 kDa. The broad band at 140–150 kDa also is recognized by the pan-Trk antibody. The TrkB antibody recognizes a subset of protein within the broad band. Thus anti-pY490 recognizes activated TrkB from sciatic nerve lysates and also detects activated TrkA and/or TrkC immunoprecipitated by the pan-Trk antibody.
Fig. 5.
Anti-pY490 recognizes two forms of phosphorylated TrkB in the adult rat sciatic nerve. A, Extracts of adult rat sciatic nerve were immunoprecipitated with anti-Trk and immunoblotted with anti-pY490 (pY490), anti-Trk (pan Trk), or a TrkB-specific antibody (TrkB). Incubation of the samples with Protein A-Sepharose beads alone resulted in the binding of a nonspecific protein at ∼165 kDa (no ab). Anti-pY490 recognizes a diffuse band at 140–150 kDa as well as a distinct band at 180 kDa in the Trk immunoprecipitates. The broad band is recognized by anti-Trk, whereas anti-TrkB recognizes a subset within this band. The 180 kDa band is weakly recognized by anti-TrkB. B, Nerve extracts were immunoprecipitated by the pY490 antibody alone or preincubated with peptide and immunoblotted with anti-phosphotyrosine. Two proteins of 145 and 180 kDa are recognized by anti-phosphotyrosine (pY) in extracts immunoprecipitated with anti-pY490 alone (no peptide). Preincubation of the pY490 antibody with its phosphopeptide immunogen (peptide-P), but not preincubation of the antibody with the corresponding unphosphorylated peptide (peptide-OH), prevented immunoprecipitation of these two bands. C, Extracts of sciatic nerve (nerve) and unstimulated (−) or BDNF-stimulated (+) TrkB 3T3 cells were immunoprecipitated with pY490 antibody and immunoblotted with anti-pY490 (pY490), anti-TrkB (TrkB), and anti-phosphotyrosine (pY). Both the 145 and 180 kDa bands are immunoprecipitated from nerve extracts with the pY490 antibody. The TrkB antibody recognizes the 145 kDa band and weakly recognizes the 180 kDa band. Both bands are recognized by anti-phosphotyrosine.
Anti-pY490 also recognizes a 180 kDa protein within pan-Trk immunoprecipitates. This higher molecular weight species reacts weakly with the anti-TrkB antibody used here as well as a different antibody specific for TrkB (data not shown). On the basis of these results, the higher molecular weight protein may be a TrkB isoform analogous to higher molecular weight forms of TrkA reported in rat sciatic nerve (Ehlers et al., 1995).
In Figure 5C we show that anti-pY490 can be used to immunoprecipitate selectively the activated Trks from sciatic nerve lysates. Both the 145 and 180 kDa proteins are immunoprecipitated by pY490 antibody alone and are recognized by antibodies to TrkB or phosphotyrosine. These data confirm that anti-pY490 recognizes phosphorylated Trk receptors in sciatic nerve, including a predominant TrkB species of 145 kDa and a highly phosphorylated 180 kDa form of TrkB. To establish the specificity of pY490 antibody as an immunoprecipitating agent, we preincubated the antibody with the phosphorylated immunogen or the corresponding unphosphorylated peptide (Fig. 5B). As shown, the phosphopeptide preferentially inhibits immunoprecipitation of all activated Trk isoforms.
TrkB within axons responds to target-derived factors
Activated Trk receptors within the sciatic nerve could be regulated by neurotrophins synthesized by target cells, Schwann cells, and/or neurons. To determine whether the Trk receptors in sciatic nerve axons are activated by target-derived factors, we monitored the response to exogenous neurotrophin. A bolus of BDNF (5 μg/gm animal weight) was injected into the gastrocnemius muscle of adult rats. This large muscle is a target of sciatic nerve motor and sensory neurons. Control animals were injected with an equal amount of cytochrome C. At 10, 30, and 60 min after injection, we harvested the nerves in three segments. The distal segment begins 1 cm from the injection site and extends to 3 cm from this site, the middle segment extends from 3 to 5 cm from the injection, and the proximal segment extends from 5 to 7 cm from injection. Protein extracts of these segments were immunoprecipitated with anti-pY490 and immunoblotted with anti-phosphotyrosine and with anti-TrkB. For each experiment, sciatic nerves from eight BDNF-injected and eight cytochrome-C-injected control rats were harvested and pooled.
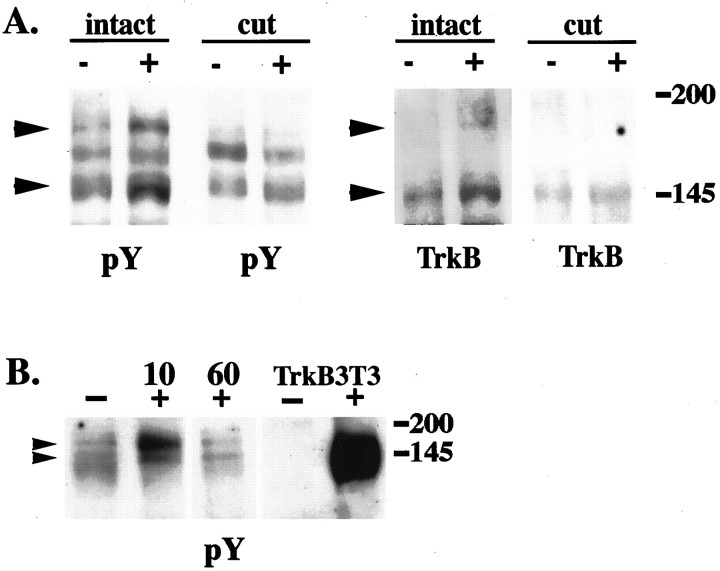
At 10 min after BDNF injection, there is an increase in the amount of activated TrkB in extracts of the distal nerve segments (Fig.6, Table1). Using anti-phosphotyrosine or anti-TrkB to blot the pY490 immunoprecipitates, we detected a twofold increase in the 145 kDa form and approximately a fivefold increase in the 180 kDa form (Table 1). Quantitative analysis of seven independent experiments confirmed that the increases in both protein species are reproducible and statistically significant.
Fig. 6.
TrkB receptors in sciatic nerve respond to target-derived BDNF. A, Cytochrome C (−) or BDNF (+) was injected into the gastrocnemius muscle of adult rats (intact). In cut nerve experiments (cut), sciatic nerves were cut near the gastrocnemius muscle before injection. Distal segments of sciatic nerves (1–3 cm from the injection site) were harvested 10 min after injection. Nerve extracts were immunoprecipitated with anti-pY490 and immunoblotted with anti-phosphotyrosine (pY) or anti-TrkB (TrkB). A nonspecific band is present in the blots at ∼165 kDa. Both the 145 and 180 kDa TrkB bands (arrows) are increased after BDNF injection (intact). When the nerve is cut before injection, no increase in these bands is observed with BDNF injection (cut). B, Cytochrome C (−) or BDNF (+) was injected into the gastrocnemius muscle of adult rats. Distal segments of sciatic nerves from these animals were harvested 10 and 60 min after injection. Nerve extracts and unstimulated (−) or BDNF-stimulated (+) TrkB 3T3 cells were immunoprecipitated with anti-pY490 and immunoblotted with anti-phosphotyrosine (P-tyr). There is an increase in both 145 and 180 kDa Trk bands within 10 min after BDNF injection. By 60 min after injection, the levels of Trk are equal to cytochrome C control (−).
Table 1.
BDNF applied at nerve endings causes an induction of TrkB phosphorylation in sciatic nerve axons
| TrkB species | Time after injection (minutes) | Fold induction: TrkB phosphorylation of BDNF injected/control (mean ± SE) |
|---|---|---|
| 145 kDa | 10 | 2.0 ± 0.4* |
| 30 | 2.3 ± 0.6 | |
| 60 | 1.1 ± 0.2 | |
| 180 kDa | 10 | 5.3 ± 1.7* |
BDNF or cytochrome C (control) was injected into the gastrocnemius muscle of adult rats, and the ipsilateral sciatic nerves were harvested 10, 30, or 60 min after injection. Sciatic extracts of eight animals were pooled for each condition and were immunoprecipitated with anti-pY490, immunoblotted with anti-phosphotyrosine, and visualized by enhanced chemiluminescence. The ratio between TrkB phosphorylation in BDNF-injected and control nerves within each experiment was calculated. Values shown represent the mean induction seen in eight experiments (145 kDa, 10 min time point), seven experiments (180 kDa, 10 min), five experiments (145 kDa, 30 min), or three experiments (145 kDa, 60 min).
Value is significantly greater than 1.0 (p < 0.05).
To ensure that the neurotrophin injected into the muscle remains localized within this target, we monitored the dispersion of tagged BDNF after injection. We injected biotinylated BDNF into the muscle, harvested both the muscle and the nerve after 10 min, and assayed each for the presence of biotinylated BDNF. Biotinylated BDNF is not detected in the nerve, but it is detected easily in muscle extract (data not shown). These data indicate that the increase in phosphorylated TrkB observed in sciatic nerve is a remote response to neurotrophin in the muscle target.
To confirm that the observed signal is traveling through the axon rather than extra-axonally, we cut the nerve at the usual site of excision (∼1 cm from the injection site) before injection and then repeated the experiment. No increase in Trk activation was observed when the continuity of the nerve was interrupted (Fig.6A). Collectively, these data indicate that the activation state of Trk receptors within the sciatic nerve is regulated, at least in part, by target-derived neurotrophins, and the response to target-derived factors travels through the nerve itself.
In these biochemical experiments we can analyze specifically the response of TrkB receptors to BDNF. Using immunohistochemistry, we cannot distinguish the identity of the activated Trk receptors. In immunohistochemical experiments with anti-pY490, we did not detect any increase in the number of axons containing activated Trk receptors in distal segments of BDNF injected nerves, as compared with cytochrome C-injected nerves and with control nerves (26% in control and in BDNF-injected). This result may indicate that the increase in TrkB phosphorylation after BDNF injections reflects an increase in the number of activated receptors per axon rather than an increase in the number of axons with activated receptors.
BDNF rapidly activates distant TrkB receptors
A surprising element of axonal TrkB activation in response to target-applied BDNF is that the increased activation in the distal segments is apparent within 10 min and remains apparent at 30 min after injection. Furthermore, Trk activation returns to the basal, unstimulated level by 60 min after the injection (Fig.6B, Table 1). The return to baseline is likely to reflect further propagation of the signal toward the cell body and receptor downregulation in response to the large bolus of neurotrophin. Thus, the response has traveled 1 cm from the injection site within 10 min and 3 cm from the injection site within 60 min. This corresponds to a rate of signal propagation of 8–16 μm/sec, a rate faster than conventional retrograde vesicular transport, previously reported as 2–5 μm/sec (Richardson and Riopelle, 1984; Sheetz et al., 1989;Vallee and Bloom, 1991). As expected, on the basis of the immunohistochemistry (Fig. 3) anti-pY490 also immunoprecipitated activated Trks from the middle and proximal segments of control and BDNF injected nerves. Thus far we have not detected any significant increase in Trk phosphorylation in these nerve segments at 10, 30, or 60 min after injection.
Axonal Trk has catalytic and signal-generating activities
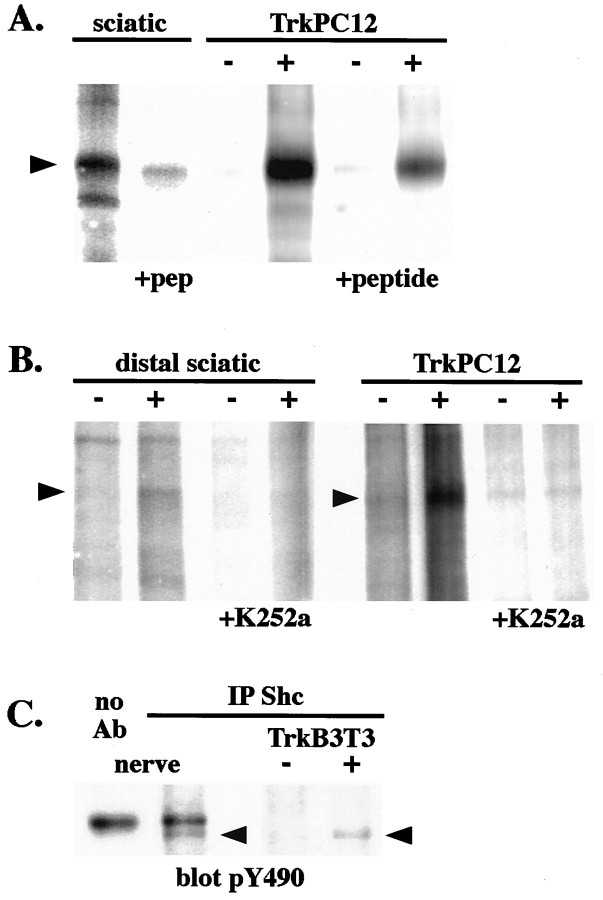
During neurotrophin signaling Trk receptors act as enzymes that catalyze protein phosphorylation, and they also act as a platform for the assembly of a multimeric signal-generating complex (Segal and Greenberg, 1996). To determine whether Trks within axons of sciatic nerve are enzymatically active, we performed an in vitroautophosphorylation assay on whole-nerve extracts. In anti-Trk immunoprecipitates we detect autophosphorylation of a broad band at 145 kDa, which corresponds in molecular mass to the major isoforms of TrkA, TrkB, and TrkC (Fig. 7A). The specificity of these kinase assays for detecting Trk kinase activity was assessed in two ways. Peptide competitions showed that this band is immunoprecipitated specifically by the anti-Trk antibody (Fig.7A). Furthermore, autophosphorylation was prevented by the Trk kinase inhibitor K252a (Fig. 7B).
Fig. 7.
Target-derived neurotrophin increases the catalytic activity of axonal Trk receptors. Extracts of normal whole sciatic nerves and TrkPC12 cells, either stimulated (+) or unstimulated (−) with NGF, were immunoprecipitated with anti-Trk or in the presence of peptide immunogen. An in vitro autophosphorylation assay was performed on the Trk immunoprecipitates. The immunoprecipitates were incubated with γ-32P-ATP. Thearrow indicates a broad band at 145 kDa, corresponding to autophosphorylation of Trk isoforms in the nerve. TrkA autophosphorylation is increased in TrkPC12 cells stimulated with NGF (+). The identities of the bands in these immunoprecipitates were confirmed by the peptide competition. B, Extracts of distal segments of cytochrome C-injected (−) or BDNF-injected (+) nerves and TrkPC12 cells, either control (−) or NGF-stimulated (+), were immunoprecipitated with anti-Trk. Note that the amount of protein used in each immunoprecipitation in this experiment is approximately one-half of that in A. The immunoprecipitates were incubated with γ-32P-ATP alone or in the presence of K252a (+K252a), a Trk kinase inhibitor. Thearrow indicates the 145 kDa species of Trk in the nerve. Trk autophosphorylation is increased in nerves injected with BDNF and in TrkPC12 cells stimulated with NGF. No kinase activity is apparent in extracts incubated with K252a. C, Extracts of normal rat sciatic nerves and TrkB 3T3 cells stimulated with BDNF (+) or unstimulated (−) were immunoprecipitated with anti-Shc and immunoblotted with anti-pY490. Precipitation with Protein A-Sepharose beads alone (no Ab) resulted in the binding of a nonspecific band at ∼165 kDa. A phosphorylated Trk band at 145 kDa is detected in the Shc immunoprecipitates, verifying that phospho-Trk is associated with Shc in nerve extracts.
To determine whether axonal Trk catalytic activity is regulated by target derived neurotrophins, we repeated this experiment on distal segments of sciatic nerve from rats that had been injected with BDNF or cytochrome C, as described above. The 145 kDa Trk kinase band can be seen in extracts from unstimulated distal segments, although we used approximately one-half the amount of protein used in whole-nerve experiments. At 10 min after BDNF injection, there is a fourfold increase in autophosphorylation of the 145 kDa Trk proteins within the distal nerve segment (Fig. 7B). These enzymatic studies provide further evidence that axonal Trks are regulated rapidly by target-derived neurotrophins.
To determine whether axonal Trks also function as platforms for the assembly of signal-generating molecules, we looked at receptor association with the crucial signal transduction molecule, Shc. Sciatic nerve extracts were immunoprecipitated with an antibody to Shc and immunoblotted with anti-pY490. Figure 7C shows that activated Trk is associated with Shc in the sciatic nerve. Taken together, our data indicate that, in axons, Trks are phosphorylated at the critical Shc recognition site, are catalytically active kinases, and are associated with Shc. These attributes are regulated by neurotrophin stimulation at the nerve terminals.
DISCUSSION
Functions of neurotrophins in the mature nervous system
Target-derived neurotrophins initiate a retrograde signal that is required for the survival of neurons in the developing peripheral nervous system. However, it has not been clear whether these molecules exert remote effects in the mature nervous system. Using a positionally specific phospho-Trk antibody, we have visualized Trk receptors phosphorylated at the Shc binding site in adult sciatic nerve axons. Tyrosine phosphorylation at this site is a particularly good indicator of the ability of Trk to act as a platform for assembly and activation of signaling molecules (Obermeier et al., 1994; Stephens et al., 1994). Furthermore, axonal Trk receptors are catalytically active and are associated with the signal-generating molecule Shc. The presence of activated receptors in sciatic nerve axons and the demonstration that the receptors are activated in response to neurotrophins applied at distant nerve terminals indicate that target-derived factors continue to exert remote effects on presynaptic neurons throughout life.
What are the functions of target-derived factors in mature neurons? Studies by Acheson et al. demonstrate that the survival of adult sensory neurons is dependent on autocrine neurotrophins rather than on factors produced by the targets (Acheson et al., 1995). However, focally applied neurotrophins can modulate transcriptional changes in response to nerve injury (Friedman et al., 1995). This raises the possibility that target-derived factors in the adult peripheral nervous system initiate retrograde signals necessary for nerve and synapse maintenance and repair. This is supported by data from the peripheral nervous system and CNS. In the peripheral nervous system NGF increases the caliber of axonal fibers (Gold et al., 1991), whereas NGF antibodies inhibit the sprouting of adult DRG neurons (Diamond et al., 1992a). In the CNS long-term potentiation is impaired in mice with targeted gene deletions of BDNF, and this defect is rescued by exogenous neurotrophin (Kang and Shuman, 1995; Korte et al., 1995;Figurov et al., 1996; Patterson et al., 1996).
What carries the retrograde signal?
Although the functions of target-derived neurotrophins in the developing and mature nervous system may differ, in both cases a neurotrophin-initiated signal must be propagated from the nerve terminal to the cell body. Three models for retrograde signaling have been proposed and are outlined above (Hendry and Crouch, 1993;Campenot, 1994). According to the first model, neurotrophin itself is the agent responsible for retrograde signaling. Although neurotrophinsin vivo are internalized specifically and transported retrogradely by responsive neurons, arriving intact at the neuronal cell body (Hendry, 1975; Johnson, 1978; DiStefano et al., 1992), intracellular injection of neurotrophins fails to elicit a response, and injected neurotrophin antibodies fail to inhibit a response (Heumann et al., 1981; Rohrer et al., 1982), casting doubt on the hypothesis that internalized neurotrophin is the signal carrier. Our data demonstrating that Trk receptors are activated throughout the axon provide further evidence against this model.
The distribution of activated Trk receptors within the sciatic nerve also provides evidence against the second model of signaling, because our data show that activated Trks are all along the axon and not only at the nerve terminal. Thus downstream signaling molecules, such as Ras or MAP Kinase, cannot be the only retrograde signal carriers.
Our studies support the hypothesis that Trk receptors function as retrograde signal carriers, as outlined in the third model. In response to neurotrophins delivered to the muscle target, there are rapid increases in phosphorylation state and catalytic activity of axonal Trk receptors located at least 1 cm from the nerve terminal. This response involves an intracellular signal that travels through the nerve, because BDNF-dependent Trk phosphorylation is prevented by nerve transection. Similarly, Ehlers et al. have demonstrated that target-derived NGF can cause, and antibodies to NGF can inhibit, the phosphorylation of TrkA receptors in a ligated nerve (Ehlers et al., 1995). Thus phosphorylation state and catalytic activity of Trk receptors within sciatic nerve axons reflect the level of neurotrophin at the nerve terminal. Because the phosphorylated Trk receptors within sciatic nerve are catalytically active and are associated with signal-generating molecules, they are ideal agents for propagating a biological response. Are these signal-generating particles associated with neurotrophins? No one has observed a population of neurotrophin that moves within the axon as fast as Trk phosphorylation (Fig. 6, Table 1). Thus, if ligand is associated with the signal-generating complex, it can amount to no more than a small fraction of the total transported neurotrophin within the axon.
The theory that membrane-bound receptors at the nerve terminals and internalized receptors within the axon, whether alone or as part of ligand–receptor complexes, are engaged in signal transduction all along the length of the axon is consistent with data from Campenot that NGF promotes axonal stability only between the site of NGF application and the cell body (Campenot, 1994). These en route retrograde effects could be explained by activated receptors that are distributed throughout the axon and are engaged in signal transduction.
Surprisingly, although activated receptors are detected all along the length of the nerve, thus far we have found a BDNF-induced increase in TrkB activation within a segment of sciatic nerve that extends from 1 to 3 cm from the injection site and not within the segments of the nerve farther from the injection site. This may reflect technical constraints, in that a smaller proportion of axons in the proximal nerve projects to the gastrocnemius muscle and so any increase may be below the level of detection. Another possibility is that we have not analyzed Trk phosphorylation in the middle and proximal segments at the critical time period. Alternatively, Trk receptors could be signal carriers only in the initial axonal segments. In the future it will be critical to link the neurotrophin-induced increase in axonal Trk phosphorylation to somatic responses to determine whether activated Trks are the initial or the sole signal carriers.
The mechanism of rapid signal propagation
Delineating the signal carrier is a first step toward elucidating the mechanism by which a retrograde signal is initiated and propagated through axons. Further studies will be needed to show definitively that phosphorylated Trk is associated with vesicles in neuronal axons, an association previously demonstrated in PC12 cells (Grimes et al., 1996). However, our data showing that phosphorylated Trk receptors accumulate distal to a ligation are consistent with a model that retrograde transport of vesicle-associated activated receptor propagates the signal.
The surprising finding is the rapidity of the response, which is not consistent with the speed of conventional retrograde vesicular transport. As shown (Fig. 6, Table 1), 10 min after injection of BDNF into the gastrocnemius muscle, we detect an increased level of activated TrkB in a segment of nerve that begins at 1 cm and extends to 3 cm from the injection site. Similarly, 10 min after neurotrophin injection we detect an increase in Trk catalytic activity in this nerve segment (Fig. 7). Thus the signal, as assessed by two independent criteria, has traveled 16 μm/sec into this initial nerve segment. If the subsequent return to basal levels of Trk phosphorylation 60 min after neurotrophin injection reflects continued retrograde movement of Trk receptors, then the signal has traveled through the segment at 8 μm/sec. Previous studies on neurotrophin transport in rat sciatic nerve have shown that in this system neurotrophins are transported to the cell body at 0.7–2 μm/sec (Richardson and Riopelle, 1984), which is similar to other in vivo and in vitromeasurements of vesicular transport and is lower than the rate of Trk signal propagation shown here (Richardson and Riopelle, 1984; Sheetz et al., 1989; Vallee and Bloom, 1991).
Two mechanisms by which activated Trk could propagate a retrograde signal are consistent with the data shown here. The first possibility is that Trk receptors are activated at the nerve terminals and then endocytosed alone or in a complex with ligand. Activated Trks travel from the nerve terminal by fast retrograde transport. As they move through the axon, activated Trk receptors remain catalytically active and continue to initiate signal transduction pathways by interacting with downstream signaling molecules. At some point, perhaps at the soma, the downstream effectors convey a signal from the vesicle-associated receptor to the nucleus.
An alternative hypothesis is that signal propagation in the initial nerve segment does not reflect the movement of individual molecules of phosphorylated Trk but, instead, represents a rapid change in phosphorylation state of Trk molecules that are distributed throughout the nerve terminal and distal axon. In the future in vitromodel systems (Campenot, 1982) will make it possible to determine whether Trk phosphorylation is propagated biochemically or physically through the axon and to ascertain the location and mechanism whereby a retrograde signal is converted from activated receptor to a nuclear response.
Footnotes
This work was supported by National Institutes of Health Grants NS35148 (R.A.S., C.D.S., A.B.) and NS27773 (S.L.P.) and by a Children’s Hospital core Grant (HD18655). A.B. was a postdoctoral fellow of the Robert Steel Foundation for Pediatric Cancer Research. R.A.S. is a Robert Ebert Clinical Fellow of the Klingenstein Foundation. We thank Dr. Andrew Welcher, Amgen Incorporated, for generously providing us with recombinant BDNF and Drs. David Kaplan (Montréal Neurological Institute) and Thomas Roberts (Dana-Farber Cancer Institute) for the generous gift of antibodies and transfected cell lines. We thank Abha Chandra, Lori Rua, and Ken Thress for technical assistance.
In compliance with Harvard Medical School guidelines on possible conflict of interest, we disclose that one of the authors (C.D.S.) has consulting relationships with Upstate Biotechnology and Sandoz Pharmaceuticals.
Correspondence should be addressed to Dr. Rosalind A. Segal, Department of Neurology, Harvard Institutes of Medicine, 77 Avenue Louis Pasteur, Boston, MA 02115.
REFERENCES
- 1.Acheson A, Conover J, Fandl J, DeChiara T, Russell M, Thadani A, Squinto S, Yancopoulos G, Lindsay R. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 2.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 3.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 4.Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991;65:915–918. doi: 10.1016/0092-8674(91)90540-f. [DOI] [PubMed] [Google Scholar]
- 5.Campenot RB. Development of sympathetic neurons in compartmentalized cultures. I. Local control of neurite growth by nerve growth factor. Dev Biol. 1982;93:1–12. doi: 10.1016/0012-1606(82)90232-9. [DOI] [PubMed] [Google Scholar]
- 6.Campenot RB. Local control of neurite sprouting in cultured sympathetic neurons by nerve growth factor. Brain Res. 1987;465:293–301. doi: 10.1016/0165-3806(87)90250-1. [DOI] [PubMed] [Google Scholar]
- 7.Campenot RB. NGF and the local control of nerve terminal growth: review. J Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- 8.Chitnis AB, Kuwada JY. Axonogenesis in the brain of zebrafish embryos. J Neurosci. 1990;10:1892–1905. doi: 10.1523/JNEUROSCI.10-06-01892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deckwerth TL, Johnson EJ. Neurotrophic factor deprivation-induced death. Ann NY Acad Sci. 1993;679:121–131. doi: 10.1111/j.1749-6632.1993.tb18293.x. [DOI] [PubMed] [Google Scholar]
- 10.Diamond J, Foerster A, Holmes M, Coughlin M. Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J Neurosci. 1992a;12:1467–1476. doi: 10.1523/JNEUROSCI.12-04-01467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992b;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 13.Druker B, Mamon H, Roberts T. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989;321:1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers M, Kaplan D, Price D, Koliatsos V. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figurov A, Pozzo-Miller L, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 16.Friedman B, Kleinfeld D, Ip N, Verge V, Moulton R, Boland P, Zlotchenko E, Lindsay R, Liu L. BDNF and NT4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci. 1995;15:1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold B, Mobley W, Matheson S. Regulation of axonal caliber, neurofilament content, and nuclear localization in mature sensory neurons by nerve growth factor. J Neurosci. 1991;11:943–955. doi: 10.1523/JNEUROSCI.11-04-00943.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimes M, Zhou J, Beattie E, Yuen E, Hall D, Valletta J, Topp K, LaVail J, Bunnett N, Mobley W. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hempstead BL, Rabin SJ, Kaplan L, Reid S, Parada LF, Kaplan DR. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992;9:883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- 20.Hendry IA. The response of adrenergic neurons to axotomy and nerve growth factor. Brain Res. 1975;94:87–97. doi: 10.1016/0006-8993(75)90879-3. [DOI] [PubMed] [Google Scholar]
- 21.Hendry IA, Crouch M. Retrograde axonal transport of the GTP-binding protein Giα: a potential neurotrophic intra-axonal messenger. Neurosci Lett. 1991;133:29–32. doi: 10.1016/0304-3940(91)90049-y. [DOI] [PubMed] [Google Scholar]
- 22.Hendry IA, Crouch M. Synergy, retrograde transport, and cell death. In: Loughlin S, Fallon J, editors. Neurotrophic factors. Academic; Boston: 1993. pp. 51–88. [Google Scholar]
- 23.Heumann R, Schwab M, Thoenen H. A second messenger required for nerve growth factor biological activity? Nature. 1981;292:838–840. doi: 10.1038/292838a0. [DOI] [PubMed] [Google Scholar]
- 24.Hosang M, Shooter EM. The internalization of nerve growth factor by high-affinity receptors on pheochromocytoma PC12 cells. EMBO J. 1987;6:1197–1202. doi: 10.1002/j.1460-2075.1987.tb02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johanson S, Crouch M, Hendry I. Retrograde axonal transport of signal transduction proteins in rat sciatic nerve. Brain Res. 1995;690:55–63. doi: 10.1016/0006-8993(95)00587-g. [DOI] [PubMed] [Google Scholar]
- 26.Johnson EM., Jr Destruction of the sympathetic nervous system in neonatal rats and hamsters by vinblastine; prevention by concomitant administration of nerve growth factor. Brain Res. 1978;141:105–118. doi: 10.1016/0006-8993(78)90620-0. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EM, Jr, Taniuchi M, Clark H, Springer J, Koh S, Tayrien M, Loy R. Demonstration of the retrograde transport of nerve growth factor receptor in the peripheral and central nervous system. J Neurosci. 1987;7:923–929. doi: 10.1523/JNEUROSCI.07-03-00923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahle P, Barker P, Shooter E, Hertel C. p75 nerve growth factor receptor modulates p140TrkA kinase activity, but not ligand internalization, in PC12 cells. J Neurosci Res. 1994;38:599–606. doi: 10.1002/jnr.490380512. [DOI] [PubMed] [Google Scholar]
- 29.Kang H, Shuman E. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol (Lond) 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:558–561. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 31.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai E, Clark KL, Burley SK, Darnell JE., Jr Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: a family of transcription factors of diverse biologic function. Proc Natl Acad Sci USA. 1993;90:10421–10423. doi: 10.1073/pnas.90.22.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamballe F, Klein R, Barbacid M. TrkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin 3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- 34.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 35.Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- 36.Loy R, Lachyankar M, Condon P, Poluha D, Ross A. Retrograde axonal transport and lesion-induced upregulation of the TrkA high-affinity NGF receptor. Exp Neurol. 1994;130:377–386. doi: 10.1006/exnr.1994.1217. [DOI] [PubMed] [Google Scholar]
- 37.Mehler MF, Kessler JA. Growth factor regulation of neuronal development. Dev Neurosci. 1994;16:180–195. doi: 10.1159/000112105. [DOI] [PubMed] [Google Scholar]
- 38.Obermeier A, Bradshaw RA, Seedorf K, Choidas A, Schlessinger J, Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/trk binding sites for SHC and PLC. EMBO J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson S, Abel T, Deuel T, Martin K, Rose J, Kandel E. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 40.Raffioni S, Bradshaw RA, Buxser SE. The receptors for nerve growth factor and other neurotrophins. Annu Rev Biochem. 1993;62:823–850. doi: 10.1146/annurev.bi.62.070193.004135. [DOI] [PubMed] [Google Scholar]
- 41.Raivich G, Hellweg R, Kreutzberger G. NGF receptor-mediated reduction in axonal NGF uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron. 1991;7:151–164. doi: 10.1016/0896-6273(91)90083-c. [DOI] [PubMed] [Google Scholar]
- 42.Richardson P, Riopelle R. Uptake of nerve growth factor along peripheral and spinal axons of primary sensory neurons. J Neurosci. 1984;4:1683–1689. doi: 10.1523/JNEUROSCI.04-07-01683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohrer H, Schafer T, Korsching S, Thoenen H. Internalization of nerve growth factor by pheochromocytoma PC12 cells: absence of transfer to the nucleus. J Neurosci. 1982;2:687–697. doi: 10.1523/JNEUROSCI.02-06-00687.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal R, Greenberg M. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 45.Segal R, Bhattacharyya A, Rua L, Alberta J, Stephens R, Kaplan D, Stiles C. Differential utilization of Trk autophosphorylation sites. J Biol Chem. 1996;271:20175–20181. doi: 10.1074/jbc.271.33.20175. [DOI] [PubMed] [Google Scholar]
- 46.Sheetz M, Steuer E, Schroer T. The mechanism and regulation of fast axonal transport. Trends Neurosci. 1989;12:474–478. doi: 10.1016/0166-2236(89)90099-4. [DOI] [PubMed] [Google Scholar]
- 47.Snider WD, Johnson EM. Neurotrophic molecules. Ann Neurol. 1989;26:489–506. doi: 10.1002/ana.410260402. [DOI] [PubMed] [Google Scholar]
- 48.Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 49.Sorkin A, Waters C. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 50.Stephens R, Loeb D, Copelan T, Pawson T, Greene L, Kaplan D. Trk receptors use redundant signal transduction pathways involving SHC and PLCγ1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 51.Vallee R, Bloom G. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- 52.Vieria A, Lamaze C, Schmid S. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 53.Vogel KS. Development of trophic interactions in the vertebrate peripheral nervous system. Mol Neurobiol. 1993;7:363–382. doi: 10.1007/BF02769183. [DOI] [PubMed] [Google Scholar]