Abstract
Taurine is known to be involved in many important physiological functions. Here we report that both in vivo andin vitro the taurine-synthesizing enzyme in the brain, namely cysteine sulfinic acid decarboxylase (CSAD), is activated when phosphorylated and inhibited when dephosphorylated. Furthermore, protein kinase C and protein phosphatase 2C have been identified as the enzymes responsible for phosphorylation and dephosphorylation of CSAD, respectively. In addition, the effect of neuronal depolarization on CSAD activity and 32P incorporation into CSAD in neuronal cultures is also included. A model to link neuronal excitation and CSAD activation by a Ca2+-dependent protein kinase is proposed.
Keywords: taurine, cysteine sulfinic acid decarboxylase, protein kinase C, protein phosphatase 2C, protein phosphorylation, protein dephosphorylation
Taurine, 2-aminoethanesulfonic acid, is one of the most abundant amino acids in the brain (Jacobsen and Smith, 1968). The physiological role of taurine has received much attention because of the reports that cats fed a taurine-deficient diet developed central retinal degeneration (Hayes et al., 1975), cardiomyopathy (Pion et al., 1987), and delay in neural development (Sturman, 1993). So far, taurine has been shown to be involved in various important physiological functions; e.g., serving as a trophic factor in the development of the CNS (Sturman, 1993), maintaining the structural integrity of the membrane (Pasantes-Morales and Cruz, 1985), regulating calcium homeostasis (Lazarewicz et al., 1985), modulating protein phosphorylation (Lombardini, 1992), and acting as an osmoregulator (Solis et al., 1988), a neuromodulator (Kuriyama, 1980), and a neurotransmitter (Okamoto et al., 1983; Taber et al., 1986).
Despite its importance, little is known about the mechanism of regulation of taurine biosynthesis in the brain. There has been controversy in the past regarding whether GABA and taurine are synthesized in the brain by the same enzyme (Blinderman et al., 1978). It is now well established that two distinctly different enzymes, namely, l-glutamate decarboxylase (GAD) and cysteine sulfinic acid decarboxylase (CSAD) are responsible for GABA and taurine synthesis in the brain, respectively (Wu, 1982). Recently, we have demonstrated that GAD activity is increased when GAD is dephosphorylated and inhibited when it is phosphorylated (Bao et al., 1994, 1995). Furthermore, protein kinase A (PKA) and calcineurin have been identified as the protein kinase and protein phosphatase (PrP) responsible for GAD phosphorylation and dephosphorylation, respectively (Bao et al., 1994).
In this communication, we report that unlike GAD, CSAD activity is enhanced under conditions favoring protein phosphorylation and is inhibited or inactivated when it is dephosphorylated. Furthermore, direct phosphorylation and concomitant increase of CSAD activity have been demonstrated in three different conditions: namely, in synaptosomal preparations, in purified CSAD, and in cultured neuronal system. In addition, PKC and PrP-2C have been identified as the enzymes involved in phosphorylation and dephosphorylation of CSAD, respectively. A model to link neuronal excitation to activation of CSAD by a Ca2+-dependent protein kinase is also included.
MATERIALS AND METHODS
Materials. Fresh porcine brains were obtained from a local slaughter house. Heat-inactivated fetal calf serum, poly-l-lysine, Triton X-100, 1.2-diolein,l-α-phosphatidyl-l-serine, cAMP-dependent protein kinase (PKA), PKA inhibitory peptide, and PKA catalytic subunit were from Sigma (St. Louis, MO). PKC and PKC inhibitory peptide were from Upstate Biotechnology (Lake Placid, NY). Okadaic acid was from Alexis (Laufelfingen, Switzerland). [I-14C]CSA was purchased through Research Products International (Santa Cruz, CA). All other radioisotopes were purchased from DuPont NEN (Boston, MA).
Nitrocellulose membranes (0.45 μm) were from Bio-Rad (Melville, NY). Tween-20 was from Fisher (Pittsburgh, PA). Goat anti-rabbit IgG conjugated with alkaline phosphatase and bromochloroindolyl phosphate/nitro blue tetrazolium (BCIP/NBT) color development substrate were from Promega (Madison, WI). Sepharose protein A resin and cyanogen bromide (CNBr)-activated Sepharose 4B resin were from Pharmacia (Piscataway, NJ). Complete Freund’s adjuvant, incomplete Freund’s adjuvant, Basal Medium Eagle, and glutamine were obtained from Life Technologies (Grand Island, NY).
Preparation of synaptosome. Preparation of crude synaptosomal fractions was conducted as described previously. Briefly, fresh porcine brains were homogenized in 0.32 m sucrose (w/v = 15 gm:100 ml) using a glass homogenizer. The homogenate was centrifuged at 1000 × g for 10 min, and the supernatant solution obtained was further centrifuged at 100,000 × g for 30 min. The resulting pellet was the crude synaptosomal preparation. The pellet was resuspended in Kreb’s–Ringer’s phosphate buffer, pH 7.2, containing 123 mm NaCl, 3 mm KCl, 0.4 mmMgCl2, 0.5 mmNaH2PO4, 0.25 mmNa2HPO4, and 1 mg/ml glucose, and divided into aliquots for further studies.
Extraction of CSAD from synaptosomal fractions in the presence of phosphatase or kinase inhibitors. Extraction of CSAD from synaptosomal fractions in the presence of PrP inhibitors was conducted as described previously for GAD (Bao et al., 1994, 1995). Briefly, fresh porcine brains were homogenized in 0.32 m sucrose, and synaptosomal fractions were prepared as described above. Aliquots of the synaptosomal fractions were centrifuged, and the pellets were resuspended in standard CSAD buffers [50 mm potassium phosphate, pH 7.2, 1 mm reduced glutathione (GSH), 2 mm 2-aminoethylisothiouronium bromide (AET), and 0.4 mm pyridoxal-5′-phosphate (PLP)] containing either phosphatase inhibitors or kinase inhibitors as indicated. The synaptosomes were then ruptured by sonication (3 × 1 sec). The suspensions obtained were kept at room temperature for 45 min with constant shaking. CSAD activity was then determined by the CSAD activity assay as described (Wu, 1982), except that a final concentration of 10 mm glutamate was included in the assay to block any CSAD activity attributable to GAD.
Phosphorylation of CSAD in synaptosomal fractions.Phosphorylation of CSAD by endogenous kinase in the presence of [γ-32P]ATP was performed as described previously (Bao et al., 1994, 1995). Synaptosomal fractions were lysed, and a phosphorylation reaction was performed under the following conditions: 50 mm Tris/citrate, pH 7.3, 1 mm AET, 2 mm GSH, 0.10 mm [γ-32P]-ATP (200 μCi/ml), and protein phosphatase inhibitors or protein kinase inhibitors as indicated. The reaction mixture was incubated at 37°C for 60 min and then centrifuged for 10 min at 14,000 rpm. The supernatant solution obtained was applied to an anti-CSAD IgG immunoaffinity column and eluted as described (Bao et al., 1994; Tang et al., 1996). For calf intestine phosphatase (CIP) treatment, 200 U CIP was added to the affinity column as described previously (Bao et al., 1994, 1995). The eluates were analyzed on a 10% SDS-PAGE. The gel was first stained for protein by the silver staining method, followed by autoradiographic visualization as described (Bao et al., 1994).
Phosphorylation and dephosphorylation of CSAD in purified preparations. Aliquots of purified CSAD were dialyzed at 4°C in 50 mm Tris/citrate buffer, pH 7.2, containing 1 mm GSH, 1 mm AET, and 0.2 mm PLP for 18 hr, with three changes. The CSAD samples were treated under the following conditions: (1) PKC buffer alone (containing 1 mmCaCl2, 5 mm MgCl2, 0.3 mg/ml l-α-phosphatidyl-l-serine, 0.06 mg/ml diolein, 0.03% Triton X-100, 0.1 mm ATP, and 100 μCi [γ-32P]-ATP); (2) PKC buffer plus 200 ng/ml PKC; (3) the same as (2), to be used later for CIP treatment; (4) the same as (2) plus 100 nm staurosporine; (5) the same as (2) plus 200 ng/ml PKC inhibitory peptide; (6) PKA buffer alone (containing 5 mm MgCl2, 0.1 mm cAMP, 0.1 mm ATP, and 100 μCi [γ-32P]-ATP); and (7) PKA buffer plus 150 U PKA catalytic subunit. The suspensions were incubated at 37°C for 45 min. The reactions were stopped by adding 5 × SDS sample loading buffer except for group (3), which was further incubated with 100 U CIP-agarose resin in the presence of 100 nm staurosporine for another 45 min at 37°C before SDS treatment. The samples were then subjected to SDS-PAGE, followed by autoradiography.
To determine the effect of kinase and phosphatase on CSAD activity, purified CSAD samples were treated under the same conditions as those described above, except that [γ-32P] ATP was omitted. At the end of treatment, the incubation mixture was transferred immediately for measurement of CSAD activity using the standard CSAD assay as described (Wu, 1982). In the case of the CIP groups, CIP-agarose resin or Sephadex G-25 resin was removed by brief centrifugation before assaying for CSAD activity. CSAD activity in each control group was used as the reference, 100%.
Phosphorylation of CSAD in cultured neurons. Whole-brain primary neuronal cultures were prepared from fetal rats (17–18 d gestation), using modifications of a previously published method (Lee et al., 1994). Briefly, culture medium was removed, and the cultures were washed and incubated with 1 ml of Earle’s balanced salt solutions (EBSS) (116.4 mm NaCl; 5.4 mm KCl; 0.8 mm MgSO4; 1.0 mmNaH2PO4; 26.2 mmNaHCO3; 1.8 mm CaCl2; 5.6 mmd-glucose, pH 7.4). [32P]-phosphate (85 μCi) was added to each dish and incubated for 1 hr in the incubator. The culture was treated for an additional 15 min by adding 1 ml of EBSS containing the following different substances: (1) EBSS alone; (2) EBSS plus 2 mmglutamate; (3) EBSS plus 2 mm glutamate and 50 mm taurine; or (4) high K+ EBSS (20.6 mm NaCl; 100.7 mm KCl; 0.8 mmMgSO4; 1.0 mmNaH2PO4; 26.2 mmNaHCO3; 1.8 mm CaCl2; 5.6 mmd-glucose, pH 7.4). The cells were harvested in CSAD buffer containing phosphatase inhibitor (0.2 mm vanadate), protease inhibitors (1 mmphenylmethylsulfonyl fluoride, 5 mm benzamidine), and 100 μm amino-oxyacetic acid (AOAA). The suspensions were then sonicated and centrifuged. The supernatants thus obtained were kept on ice for 1 hr to allow the removal of PLP by AOAA. Each supernatant fraction was then passed twice through an anti-CSAD affinity column and analyzed by immunoblotting and autoradiography.
For CSAD activity determination, a parallel experiment was conducted as described above except that no [32P]-phosphate was included. Cultures were harvested, sonicated in CSAD buffer containing protease and phosphatase inhibitors, and assayed for CSAD activity as described previously (Wu, 1982).
RESULTS
Effects of protein kinase and phosphatase inhibitors on CSAD activity in synaptosomal fractions
When CSAD was extracted under conditions favoring protein phosphorylation, e.g., in the presence of PrP inhibitors, CSAD activity was markedly increased (Fig. 1). When CSAD was extracted from synaptosomal fractions in a mixture of general PrP inhibitors, CSAD activity increased to 245 ± 20% of the control level. Vanadate alone increased CSAD activity to an extent of 259 ± 29% and hence can account for all the activation produced by the mixture of general PrP inhibitors mentioned above; however, okadaic acid, a potent PrP inhibitor, had no effect on CSAD activity even at 7.5 μm. Because PrP-2C is sensitive to vanadate but not to okadaic acid, whereas the other three types of PrPs, namely PrP-1, PrP-2A, and PrP-2B, are known to be highly sensitive to okadaic acid at the concentration used (7.5 μm), the results suggest that PrP-2C is probably involved in dephosphorylation of CSAD. On the other hand, CSAD activity decreased when it was extracted in the presence of protein kinase inhibitors. Among the kinase inhibitors used, only those that inhibited PKC activity decreased CSAD activity. PKC inhibitory peptide (100 ng/ml), staurosporine (1 μm), H-8 (10 μm), and chelerythrine (50 μm) decreased CSAD activity to 48 ± 9, 48 ± 2, 54 ± 6, and 57 ± 14% of the control level, respectively. No significant effect on CSAD activity was observed with PKA inhibitory peptide, indicating that PKC but not PKA is involved in the regulation of CSAD activity. In addition, when 5 mm ATP was included in the extraction, it increased CSAD activity to 172 ± 19% of the control level. In short, CSAD activity increased under conditions that favored protein phosphorylation, whereas CSAD activity decreased under the conditions that favored dephosphorylation of proteins.
Fig. 1.
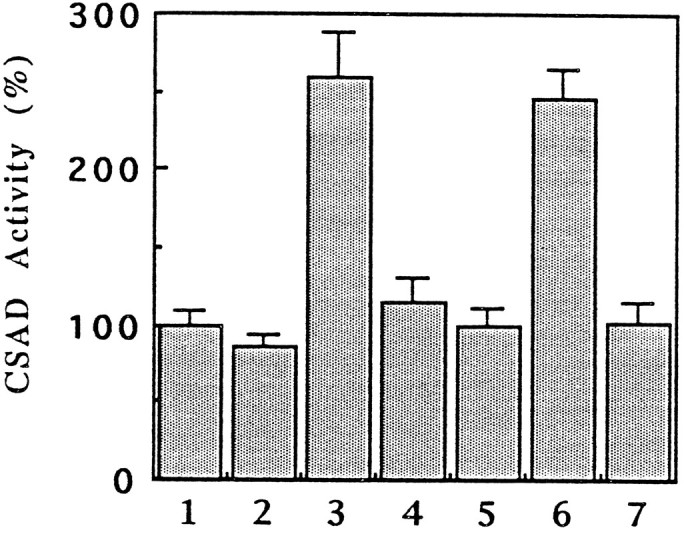
Relative CSAD activity in synaptosomal extracts in the presence of phosphatase inhibitors. CSAD was extracted from synaptosomal preparations in the presence of different protein phosphatase inhibitors. 1, Control; 2, 2 mm EDTA and 2 mm EGTA; 3, 0.2 mm sodium orthovanadate; 4, 2 mmsodium fluoride; 5, 0.2 mm sodium pyrophosphate; 6, mixture of2-5; 7, 7.5 μm okadaic acid. Each value is the mean ± SE (n = 4).
Effect of protein kinase and phosphatase activators and inhibitors on phosphorylation of CSAD in synaptosomal fractions
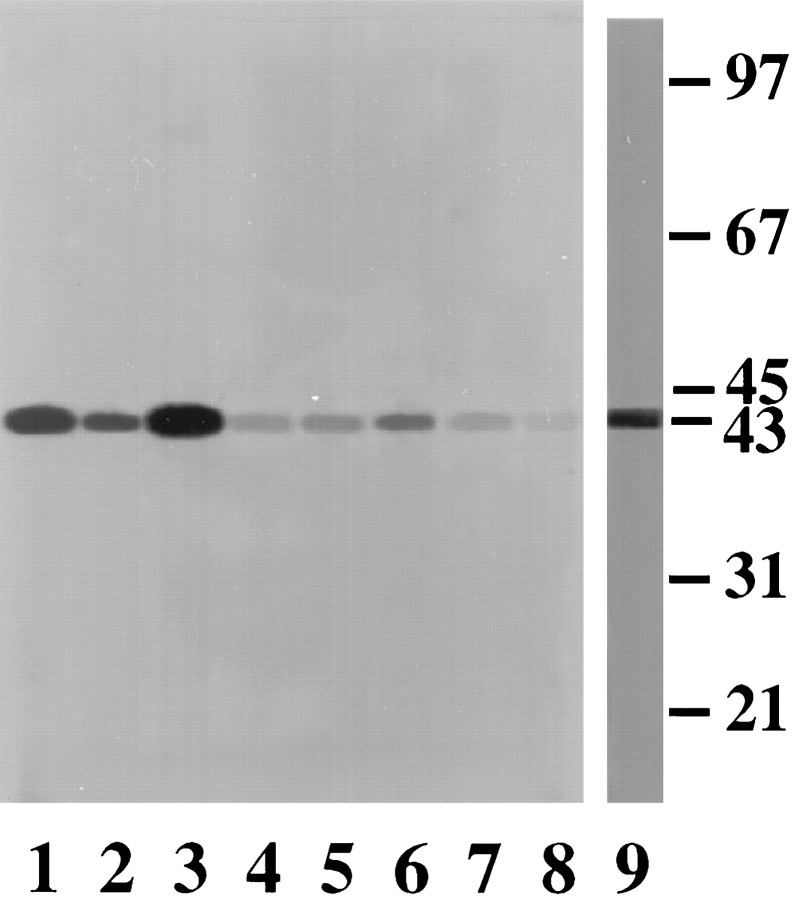
In addition to activity measurements, direct phosphorylation of CSAD by an endogenous kinase in the presence of [γ-32P]-ATP was demonstrated in lysed synaptosomal preparations. CSAD phosphorylation was enhanced in the presence of PKC activators (Fig. 2, lane 1) compared with the control (lane 2). PKC activators plus vanadate greatly increased CSAD phosphorylation (lane 3). Furthermore, CSAD phosphorylation was greatly reduced by PKC inhibitors, e.g., 100 ng/ml PKC inhibitory peptide (lane 4), 10 μm staurosporine (lane 5), 2 mm EGTA (lane 6), or 2 mmEDTA (lane 7). Incorporation of 32P into CSAD was also abolished by treatment with CIP (lane 8).
Fig. 2.
Autoradiograph of CSAD in synaptosomal preparations in the presence of protein kinase inhibitors, phosphatase inhibitors, and Ca2+-chelators. Incorporation of32P was performed in the lysed synaptosomal preparations under the condition indicated below. Lane 1: CSAD buffer + PKC activators (1 mm CaCl2, 5 mm MgCl2, 0.31 mg/ml,l-α-phosphatidyl-l-serine; 0.06 mg/ml 1.2-diolein); lane 2: CSAD buffer only; lane 3: CSAD buffer + PKC activators + 0.2 mm vanadate;lane 4: CSAD buffer + PKC activators + PKC inhibitory peptide (100 ng/ml); lane 5: CSAD buffer + PKC activators + 0.1 μm staurosporine; lane 6: CSAD buffer + PKC activators + 2 mm EGTA; lane 7: CSAD buffer + PKC activators + 2 mm EDTA;lane 8: CSAD buffer + PKC activators + 200 U CIP;lane 9: silver staining of immunoaffinity-purified CSAD.Arrow indicates the subunit of CSAD, a 43 kDa protein (Tang et al., 1996). The standard molecular weight markers (in kilodaltons) are also indicated.
Effect of PKA and PKC on phosphorylation and activity of CSAD in purified preparations
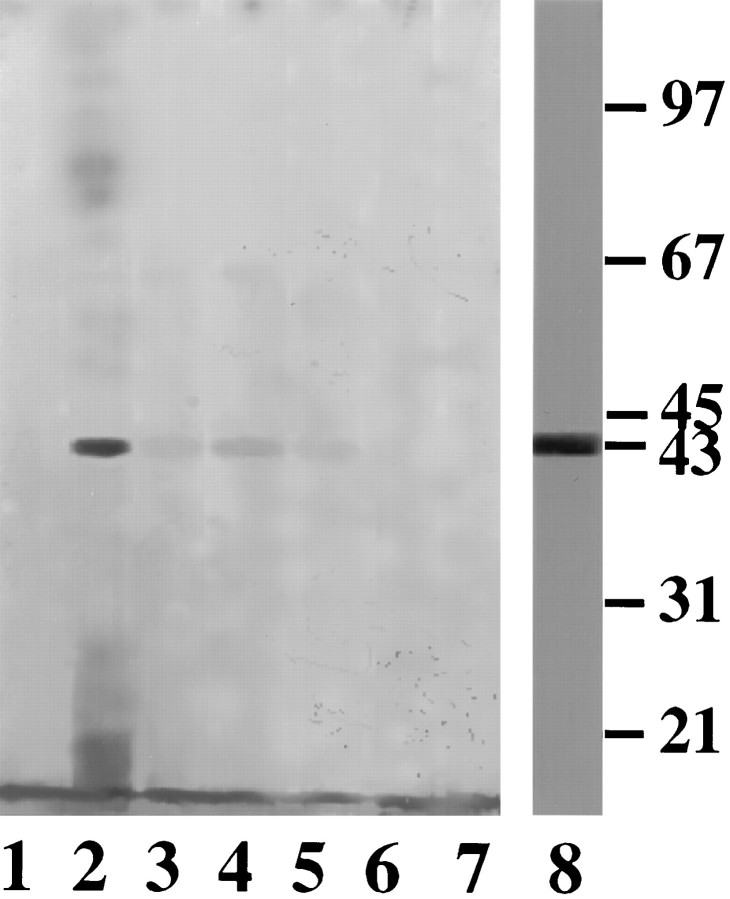
To ascertain that the above observations were not caused by interference from substances present in the crude extract, similar studies were conducted using a highly purified CSAD preparation. It was found that purified CSAD was phosphorylated by PKC (Fig.3, lane 2) but not PKA (lane 6). Incorporation of 32P into CSAD was greatly reduced in the presence of PKC inhibitors, e.g., staurosporine (lane 4) or PKC inhibitory peptide (lane 5). Furthermore, phosphorylated CSAD could be dephosphorylated by CIP (lane 3). In addition to [32P] incorporation experiments, a parallel group was treated under the same conditions as described above, except that [γ-32P]ATP was omitted and the preparation was assayed for CSAD activity. The activity of CSAD was found to correlate at least qualitatively with its state of phosphorylation as shown in Figure 3and Table 1. Activation of CSAD activity was observed by treatment with PKC but not PKA (Table 1), similar to the phosphorylation pattern of CSAD (Fig. 3). Furthermore, PKC-mediated activation of CSAD activity was abolished by CIP treatment (Table 1), corresponding to complete dephosphorylation of CSAD (Fig. 3, lane 3).
Fig. 3.
Autoradiograph of CSAD in highly purified preparations. Purified CSAD obtained as described (Tang et al., 1996) was phosphorylated using conditions similar to those described (Bao et al., 1994, 1995), except that exogenous kinase and CIP were included as indicated. Lane 1: control in PKC buffer; lane 2: PKC treatment; lane 3: PKC + CIP; lane 4: staurosporine + PKC; lane 5: PKC inhibitory peptide + PKC; lane 6: PKA; lane 7: control in PKA buffer; lane 8: Coomassie blue staining of the purified CSAD used for the above phosphorylation experiments.
Table 1.
Effect of kinase and phosphatase on CSAD activity in the purified preparation
| Treatment | CSAD activity (% of control) |
|---|---|
| PKC control | 100 ± 5 |
| PKC treatment | 352 ± 14 |
| PKC + staurosporine | 107 ± 6 |
| PKC + CIP | 102 ± 4 |
| CIP control | 100 ± 5 |
| CIP treatment | 6 ± 2 |
| PKA control | 100 ± 5 |
| PKA treatment | 85 ± 6 |
Purified CSAD was treated under the following conditions. For the PKA control, the same incubation conditions were used as in the group treated with PKA, except that ATP and the catalytic subunit of PKA were omitted. For the phosphatase (CIP) control, the conditions were the same as those for the group treated with CIP, except that no CIP was included. CSAD activity was determined by the radiometric method, as described (Wu, 1982). Each value is the mean ± SE (n = 4).
Effect of neuronal stimulation on phosphorylation of CSAD in cultured neurons
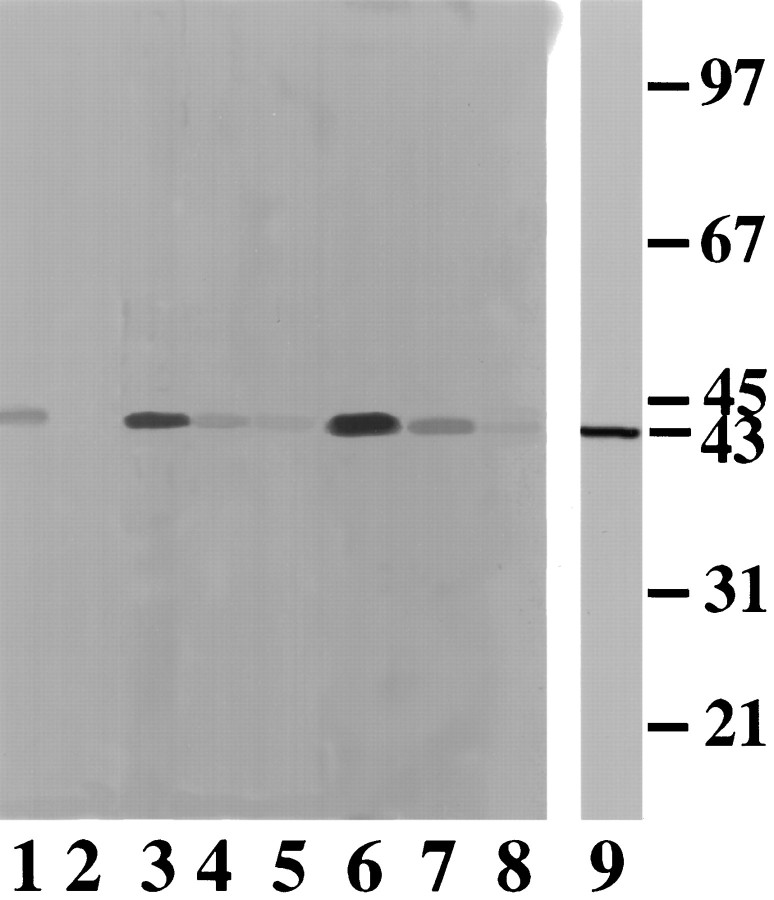
To demonstrate that CSAD activity is regulated through phosphorylation in vivo, and also to determine its physiological significance, neuronal primary cultures were used in these studies. Cultured neurons were first incubated with [32P]-phosphate, followed by treatment under various conditions as indicated: (1) control, medium alone, (2) medium plus 2 mm glutamate, (3) medium plus 2 mmglutamate and 25 mm taurine, and (4) medium containing high K+. As shown in Figure4, direct phosphorylation of CSAD by endogenous kinase was demonstrated in primary neuronal cultures (lane 1), and the degree of 32P- incorporation into CSAD was greatly reduced either by CIP treatment (lane 2) or in the presence of PKC inhibitors, e.g., H-8 (lane 5) and calphostin C (lane 8), suggesting that PKC is likely to be responsible for CSAD phosphorylation in vivo. Stimulation by either glutamate (lane 3) or high K+ (lane 6) enhanced32P incorporation into CSAD. Furthermore, glutamate- or high K+-induced increase of CSAD phosphorylation was greatly inhibited by taurine (lanes 4, 7).
Fig. 4.
Autoradiograph of CSAD in cultured neurons.Lane 1: control; lane 2: CIP treatment after stimulation; lane 3: 1 mm glutamate treatment; lane 4: 1 mm glutamate and 25 mm taurine; lane 5: 1 μm H-8;lane 6: high K+ treatment;lane 7: high K+ and 25 mmtaurine; lane 8: 1 μm calphostin C;lane 9: immunoblotting test of cultured neuronal preparation with anti-CSAD serum.
Effect of neuronal stimulation on CSAD activity in cultured neurons
In a parallel experiment, CSAD activity was measured under the same conditions as described above for CSAD phosphorylation except that no [32P]-phosphate was included. It was found that CSAD activity was increased to 187 and 150% by stimulation with glutamate and high K+, respectively. The control group, which had a specific activity of 7.14 nmol/mg protein/hour, was used as 100%. The potentiation of CSAD activity by stimulations with glutamate and high K+ was blocked by taurine as indicated by the decrease of CSAD activity to 70 and 83% of the control value, respectively.
DISCUSSION
Although CSAD was established as the taurine-synthesizing enzyme in the brain more than a decade ago (Wu, 1982), little progress has been made regarding its regulation. In the peripheral tissues, it has been reported that hepatic CSAD activity was decreased in female rats, adrenalectomy rats, and rats fed with l-methionine, and the decrease of CSAD activity was attributable to changes in CSAD protein (Jerkins and Steele, 1992). The same authors reported further that CSAD activity in liver of rats fed sulfur amino acids, e.g., cystine-, homocystine-, methionine-, or ethionine-supplemented diets, was reduced to 60, 40, 40 and 8%, respectively (Jerkins and Steele, 1991a), suggesting that CSAD activity may be specifically regulated by sulfur amino acids through the S-adenosylmethionine-dependent pathway of methionine metabolism. Recently, it has been shown that in hyperthyroidism the decrease in liver CSAD activity was caused by a decrease in CSAD mRNA (Jerkins and Steele, 1991b; Kaisakia et al., 1995). In this communication, we report that CSAD activity is activated when it is phosphorylated and inhibited when it is dephosphorylated. Furthermore, we suggest that PKC and PrP-2C are responsible for phosphorylation and dephosphorylation of CSAD, respectively. This conclusion is based on the following observations. (1) In crude synaptosomal preparations, CSAD activity is greatly enhanced when it is extracted in the presence of PrP inhibitors. Furthermore, only vanadate can account for all the activation of CSAD by PrP inhibitors. Okadaic acid seems to have no effect, even at high concentration. These results suggest that PrP-2C is probably responsible for CSAD dephosphorylation because PrP-2C is the only PrP that is sensitive to vanadate but not to okadaic acid. (2) Incorporation of [32P] into CSAD is enhanced by PKC activator and reduced by PKC inhibitors in crude synaptosomal preparations. In addition, there is a good correlation between the degree of [32P] incorporation into CSAD and CSAD activity. (3) Incorporation of [32P] into CSAD is also demonstrated by PKC but not PKA in purified CSAD preparations. Again CSAD activity is greatly enhanced under phosphorylation conditions mediated by PKC but not by PKA. All of these observations are compatible with the notion that PKC, but not PKA, is likely to be responsible for CSAD phosphorylation. It should be pointed out, however, that our results cannot rule out the possibility that other Ca2+-dependent protein kinases, e.g., calcium/calmodulin protein kinase II, may also be involved in phosphorylation and activation of CSAD. In addition to the mechanisms discussed above, CSAD activity seems to be regulated by taurine itself. This is supported by the observation that the enhanced [32P] incorporation and activation of CSAD activity by neuronal stimulation, e.g., l-glutamate or high K+, are inhibited in the presence of taurine. Although the concentration of taurine (25 mm) used in these studies seems to be high, it is still within physiological range of taurine in the brain. The concentration of taurine in the synaptosomes of the developing rat brain is reported to be ∼25–35 mmand is gradually decreased to ∼5–10 mm in adult rat brain (Lleu et al., 1992). It is conceivable that taurine is taken up into the cell where it regulates intracellular Ca2+, [Ca2+]i, by inhibiting the reverse mode of Na+/Ca2+exchanger. This will lead to a decrease in [Ca2+]i, resulting in the inhibition of Ca2+-dependent protein kinases such as PKC and the subsequent phosphorylation and activation of CSAD. Indeed, taurine has been shown to reduce Ca2+ influx into cells by inhibiting the Na+/Ca2+exchanger (Matsuda et al., 1995). Furthermore, it has been reported that taurine inhibits phosphorylation of certain proteins in the retina or in the heart (Lombardini, 1992).
In summary, it seems that there is a good correlation between changes of CSAD activity and the extent of CSAD phosphorylation in bothin vitro (purified preparation) and in vivo(cultured neurons) systems. For instance, an increase of CSAD activity under depolarization conditions is accompanied by an increase in32P incorporation into CSAD. Similarly, when CSAD activity is inhibited, such as in the presence of taurine or PKC inhibitors, the extent of 32P incorporation into CSAD is also reduced. On the basis of the above results, the following model is proposed, as shown in Figure 5. When neurons are stimulated, the arrival of action potential (step 1) will open the voltage-dependent Ca2+-channel (step 2), resulting in an increase of intracellular free Ca2+, [Ca2+]i. Elevation of [Ca2+]i will trigger release of taurine (step 3) as well as activation of PKC (step 4), which in turn activates CSAD through protein phosphorylation (step 5). The activated CSAD then synthesizes more taurine (step 6) to replenish that lost because of release by stimulation. When enough taurine has accumulated in the cells, it then inhibits the activation of PKC directly or indirectly (possibly through regulating Ca2+availability), thus shutting down activation of CSAD through inhibition of CSAD phosphorylation by PKC. The activated CSAD soon returns to its inactive state through the action of a protein phosphatase, most likely PrP-2C (step 7).
Fig. 5.
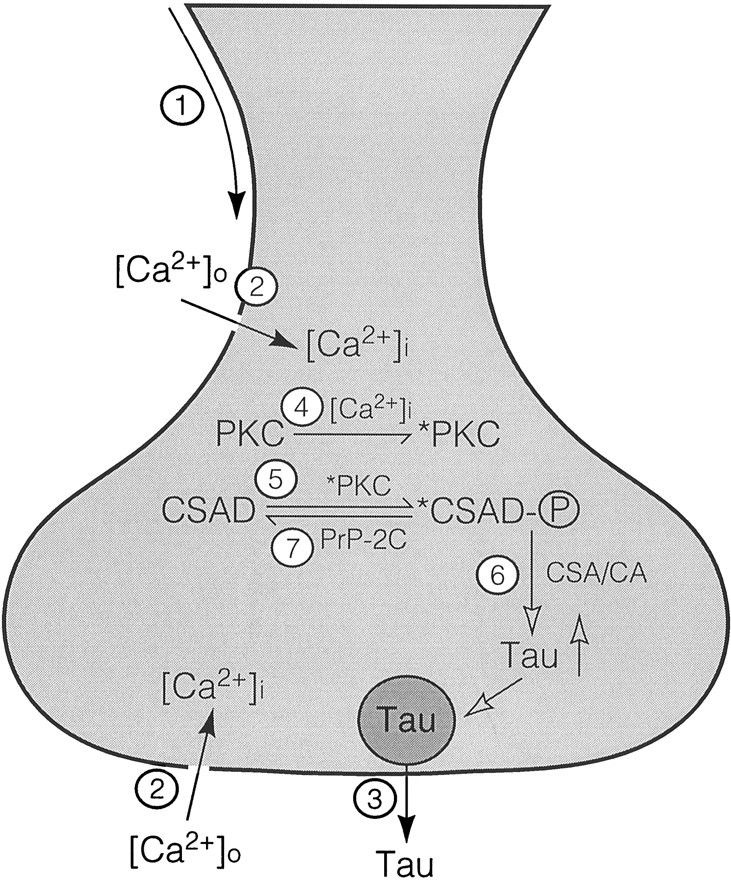
A proposed model on the role of protein phosphorylation in the regulation of taurine biosynthesis in the brain. The sequence of events leading from neuronal stimulation to increased synthesis of taurine is as follows: (1) arrival of action potential; (2) opening of voltage-dependent Ca2+ channels; (3) release of taurine; (4) activation of PKC by elevated [Ca2+]i; (5) activation of CSAD by PKC-mediated protein phosphorylation; (6) increase of taurine biosynthesis by activated CSAD; and (7) termination of taurine biosynthesis by inactivation of CSAD through PrP-2C-mediated dephosphorylation.
Footnotes
This work was supported by the National Science Foundation (IBN-9723079), the Office of Naval Research (N00014-94-1-0457), the University of Kansas General Research Fund, and National Institutes of Health (NS20978). We thank Drs. Erik Floor and James Orr for critical review of this manuscript. The expert typing of the manuscript by Sharon Lee Hopkins is greatly appreciated.
Correspondence should be addressed to Dr. Jang-Yen Wu, Department of Physiology and Cell Biology, University of Kansas, Lawrence, KS 66045-2106.
REFERENCES
- 1.Bao J, Nathan B, Wu JY. Role of protein phosphorylation in the regulation of brain l-glutamate decarboxylase activity. J Biomed Sci. 1994;1:237–244. doi: 10.1007/BF02253308. [DOI] [PubMed] [Google Scholar]
- 2.Bao J, Cheung WY, Wu JY. Brain l-glutamate decarboxylase: inhibition by phosphorylation and activation by dephosphorylation. J Biol Chem. 1995;270:6464–6467. doi: 10.1074/jbc.270.12.6464. [DOI] [PubMed] [Google Scholar]
- 3.Blinderman JM, Maitre M, Ossola L, Mandel P. Purification and some properties of l-glutamate decarboxylase from human brain. Eur J Biochem. 1978;86:143–152. doi: 10.1111/j.1432-1033.1978.tb12293.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayes KC, Carey RE, Schmidt SY. Retinal degeneration associated with taurine deficiency in the cat. Science. 1975;188:949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968;48:424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- 6.Jerkins AA, Steele RD. Dietary sulfur amino acid modulation of cysteine sulfinic acid decarboxylase. Am J Physiol. 1991a;261:551–555. doi: 10.1152/ajpendo.1991.261.5.E551. [DOI] [PubMed] [Google Scholar]
- 7.Jerkins AA, Steele RD. Cysteine sulfinic acid decarboxylase activity in response to thyroid hormone administration in rats. Arch Biochem Biophys. 1991b;286:428–432. doi: 10.1016/0003-9861(91)90061-m. [DOI] [PubMed] [Google Scholar]
- 8.Jerkins AA, Steele RD. Quantification of cysteine sulfinic acid decarboxylase in male and female rats: effect of adrenalectomy and methionine. Arch Biochem Biophys. 1992;294:534–538. doi: 10.1016/0003-9861(92)90721-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaisakia PJ, Jerkins AA, Goodspeed DC, Steele RD. Cloning and characterization of rat cysteine sulfinic acid decarboxylase. Biochim Biophys Acta. 1995;1262:79–82. doi: 10.1016/0167-4781(95)00058-o. [DOI] [PubMed] [Google Scholar]
- 10.Kuriyama K. Taurine as a neuromodulator. Fed Proc. 1980;39:2680–2684. [PubMed] [Google Scholar]
- 11.Lazarewicz JW, Noremberg K, Lehmann A, Hamberger A. Effects of taurine on calcium binding and accumulation in rabbit hippocampal and cortical synaptosomes. Neurochem Int. 1985;7:421–428. doi: 10.1016/0197-0186(85)90164-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Deupree DL, Chen SC, Kao LS, Wu JY. Role of Ca2+ in AMPA-mediated poly phosphoinositides turnover in primary neuronal cultures. J Neurochem. 1994;62:2325–2332. doi: 10.1046/j.1471-4159.1994.62062325.x. [DOI] [PubMed] [Google Scholar]
- 13.Lleu PL, Croswell S, Huxtable RJ. Phospholipids, phospholipid methylation and taurine content in synaptosomes of developing rat brain. Adv Exp Med Biol. 1992;315:221–228. doi: 10.1007/978-1-4615-3436-5_26. [DOI] [PubMed] [Google Scholar]
- 14.Lombardini JB. Taurine: nutritional value and mechanisms of action. Adv Exp Med Biol. 1992;315:309–318. doi: 10.1007/978-1-4615-3436-5_37. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda T, Takuma K, Azuma J, Baba A (1995) Protective effect of taurine against calcium paradox-induced injury in cultured rat astrocytes. Abstract presented at the International Taurine Symposium ’95, Osaka, Japan, June 27–July 1, 1995, p. 80.
- 16.Okamoto K, Kimura H, Sakai Y. Evidence for taurine as an inhibitory neurotransmitter in cerebellar stellate interneurons: selective antagonism by TAG (6-aminomethyl-3-methyl-4H, 1, 2, 4-benzothiadiazine-1, 1-dioxide). Brain Res. 1983;265:163–168. doi: 10.1016/0006-8993(83)91350-1. [DOI] [PubMed] [Google Scholar]
- 17.Pasantes-Morales H, Cruz C. Taurine and hypotaurine inhibit light-induced lipid peroxidation and protect rod outer segment structures. Brain Res. 1985;330:154–157. doi: 10.1016/0006-8993(85)90018-6. [DOI] [PubMed] [Google Scholar]
- 18.Pion PD, Kittleson MD, Rogers QR, Morris JG. Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy. Science. 1987;237:764–768. doi: 10.1126/science.3616607. [DOI] [PubMed] [Google Scholar]
- 19.Solis JM, Herranz AS, Herreras J, Lerma J, Del Rio RM. Does taurine act as an osmoregulatory substance in the rat brain? Neurosci Lett. 1988;91:53–58. doi: 10.1016/0304-3940(88)90248-0. [DOI] [PubMed] [Google Scholar]
- 20.Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- 21.Taber TC, Lin CT, Song GX, Thalman RH, Wu JY. Taurine in the rat hippocampus: localization and postsynaptic action. Brain Res. 1986;386:113–121. doi: 10.1016/0006-8993(86)90147-2. [DOI] [PubMed] [Google Scholar]
- 22.Tang XW, Hsu CC, Sun Y, Wu E, Yang CY, Wu JY. Multiplicity of brain cysteine sulfinic acid decarboxylase-purification, characterization and subunit structures. J Biomed Sci. 1996;3:442–453. doi: 10.1007/BF02258048. [DOI] [PubMed] [Google Scholar]
- 23.Wu JY. Purification and characterization of cysteic/cysteine sulfinic acids decarboxylase and l-glutamate decarboxylase in bovine brain. Proc Natl Acad Sci USA. 1982;79:4270–4274. doi: 10.1073/pnas.79.14.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]