Abstract
To explore the origins and possible behavioral consequences of structural plasticity in an insect brain, we have taken advantage of the following: (1) the highly compartmentalized nature of the primary antenno-sensory centers (antennal lobes) of the brain, (2) the ease with which individual compartments, or glomeruli, within the antennal-lobe neuropil can be identified, and (3) the predictability of changes to readily identifiable glomeruli in the antennal lobes of the adult worker honey bee. Treatment with the juvenile hormone analog methoprene and hive manipulation techniques are used to induce precocious foraging behavior in young worker honey bees. The impact of these treatments on the ontogeny of olfactory learning performance and on the volumes of readily identifiable glomeruli in the antennal lobes of the bee brain are examined in parallel. The study reveals that (1) significant changes in glomerular volume are activity dependent and (2) associative learning of floral odors improves with experience. Improvements in associative learning performance coincide temporally with increases in glomerular volume. This raises an important question: are changes in glomerular volume that result from shifts in behavior simply a consequence of changes in the use of peripheral sensory pathways, or are they associated with events that underlie learning and the formation of long-term memories?
Keywords: Apis mellifera, structural plasticity, antennal lobes, juvenile hormone, learning, memory
The antenno-sensory system of the honey bee plays a key role in the life and survival of this highly social insect. The antennae of the bee house a diverse array of receptors that includes not only olfactory receptors but also receptors sensitive to touch, taste, temperature, and humidity (Esslen and Kaissling, 1976). Behavioral activities essential to the maintenance of a honey bee colony, including nursing, comb building, and foraging, all rely on sensory information from these multifunctional organs.
The principal termination sites within the insect brain of olfactory neurons housed in the antennae are the antennal lobes, structures that bear a strong morphological resemblance to the vertebrate olfactory bulb (Masson and Mustaparta, 1990; Boeckh and Tolbert, 1993). The antennal lobes are highly compartmentalized: each compartment, or glomerulus, consists of a small sphere of densely packed synaptic neuropil that contains the terminal arbors of primary sensory afferent neurons, processes of local interneurons, the dendrites of projection (output) neurons, and ramifications of centrifugal neurons from other regions of the brain (Christensen and Hildebrand, 1987). In the honey bee, many glomeruli can be identified on the basis of their size, location, and position with respect to anatomical “landmarks” in the antennal-lobe neuropil (Arnold et al., 1985; Flanagan and Mercer, 1989), and recent studies have shown that odors evoke specific patterns of activity across the glomeruli of the antennal lobes (Lieke, 1993;Joerges et al., 1997). During the lifetime of the adult worker bee, antennal-lobe glomeruli exhibit significant changes in volume (Withers et al., 1993; Winnington et al., 1996), but whether these structural changes are activity dependent or hormonally driven has yet to be resolved.
There is a shift with age in the activities that an adult worker bee is most likely to perform. These activities fall into four relatively distinct age-related categories: (1) cell cleaning, (2) brood and queen tending, (3) comb building and food handling, and (4) guarding and foraging (Winston, 1987). Honey bee behavior, however, is as plastic as it is predictable. In response to the needs of the colony, young bees exhibit precocious foraging behavior, and foragers will revert, if necessary, to tasks within the hive. The shift to duties outside the hive, such as guarding and foraging, is mediated by increasing juvenile hormone (JH) titers. Young bees treated with JH analogs or JH mimics begin foraging at an earlier age than normal, display premature regression of milk-producing (hypopharyngeal) glands, and prematurely produce and respond to alarm pheromones (Robinson, 1987a, 1992). These responses mimic those induced in young bees as a result of removing workers of normal foraging age from the colony.
It has been suggested that JH may act directly on the brain neuropil, giving rise to changes in brain volume in anticipation of shifts in behavior (Withers et al., 1995; Fahrbach and Robinson, 1996). An alternative explanation is that the behavioral changes themselves lead to structural changes in the bee brain (Withers et al., 1993, 1995;Durst et al., 1994; Winnington et al., 1996). Here we examine in parallel the effects of hive manipulation and treatment with the JH analog methoprene, not only on behavior but also on the structure of three readily identifiable glomeruli in the antennal lobes of the brain: glomerulus T1-44, a large glomerulus on the dorsal surface of the lobe (Fig. 1A), and two prominent glomeruli, T4-2(1) and T4-3(1), located at the posterior of the antennal-lobe neuropil (Fig. 1B). These glomeruli are among the easiest to identify in the antennal lobes of the bee (Arnold et al., 1985; Flanagan and Mercer, 1989), and each shows a unique pattern of growth during the lifetime of the adult worker (Winnington et al., 1996).
Fig. 1.
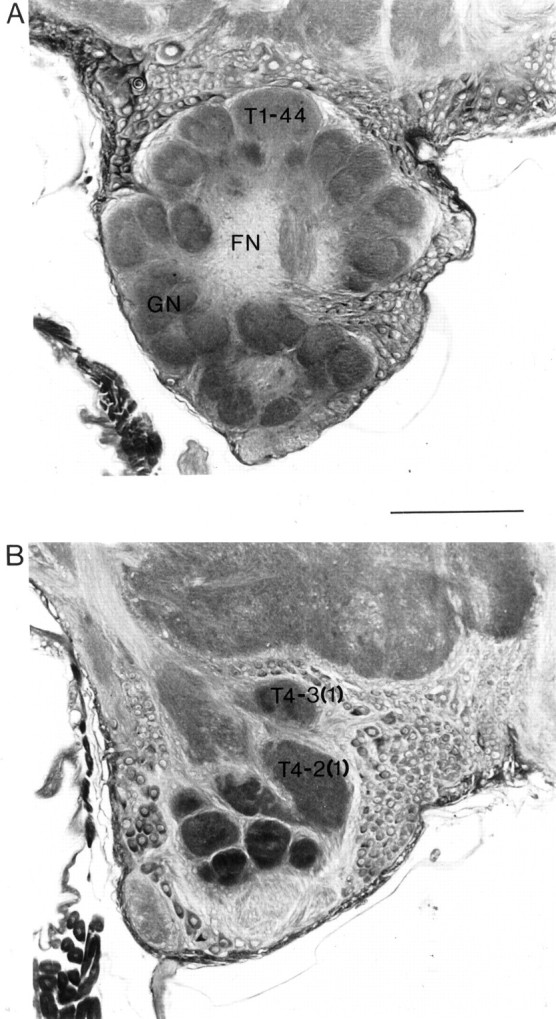
Frontal sections (5 μm thick) of the antennal lobe of a worker honey bee brain. A, Section 90 μm from the anterior surface of the antennal lobe showing the glomerular neuropil (GN), the fibrous (aglomerular) neuropil (FN), and glomerulus T1-44. B, Section 245 μm from the anterior surface of the antennal lobe showing the positions of the T4-2(1) and T4-3(1) glomeruli. Scale bar, 100 μm.
Our results suggest that although hormone treatment may contribute to structural plasticity of the antennal-lobe neuropil, significant changes in glomerular volume are activity dependent. Improvements in olfactory learning performance also appear to be experience-dependent and correlate temporally with changes in glomerular volume induced by precocious foraging behavior.
MATERIALS AND METHODS
Animals. The experiments described in this study were performed over two consecutive summers. Bees were collected from six hives located at the Department of Zoology, University of Otago. Each hive contained a naturally mated queen. For experiments in which bees of a known age were required, newly emerged bees were marked on the thorax with nontoxic paint. Bees were considered to be 0 d old for the first 24 hr after emergence, and their age was recorded in daily increments thereafter. For identification of individual foragers, numbered tags were glued to the thorax.
Induction of precocious foraging behavior. Two well established techniques were used to induce precocious foraging behavior: treatment with the JH analog methoprene (Robinson, 1985,1987b) and hive manipulation (Lindauer, 1961; Winston and Fergusson, 1985). Methoprene [isopropyl (2E,4E,7S)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate] was kindly donated by Sandoz Agro (Basel, Switzerland). After each use the methoprene was resealed under nitrogen gas and stored at −20°C. Methoprene (200 μg) dissolved in 5 μl of acetone was applied topically to the abdomen of 1-d-old bees. Bees of the same age treated with 5 μl acetone alone served as a control. A third group of bees received no treatment at all. For ease of identification, number tags of different colors were used for each of these three groups.
To induce precocious foraging behavior in a manner more closely resembling the effects of natural perturbations, three nucleus hives were established, and bees of normal foraging age (>3 weeks old) were removed from the colonies. Each of the nucleus hives established for this purpose contained two frames of sealed brood, one frame of unsealed brood, and one frame of honey. In addition, each hive was colonized with a queen, a small number of drones, and several thousand workers. The nucleus hives were placed some distance from the parent hive, and bees of foraging age were removed from the colony by lifting each frame above the nucleus hive and shaking it gently. The shaking action stimulated older (foraging age) bees to fly from the frames and return to the parent hive. Young bees would cling to the frames or fall into the nucleus box below. Newly emerging adults in the nucleus hives were tagged with numbered tags or marked with paint, as required.
Estimating age of first flight. The age at which control bees, bees treated with methoprene, acetone-treated bees, and bees from the manipulated hives first began foraging was estimated using methods similar to those described elsewhere (Jaycox, 1976; Robinson, 1985;Page et al., 1992). If weather conditions permitted flying activity, the entrance to each hive was observed for a 30 min period each day, and the number of each bee seen entering or leaving the hive was recorded, along with the time of the observation. Daily observations were continued until the tagged bees in the hives were between 19 and 31 d of age. The same colony was used, not only for the experiment in which bees of normal foraging age were removed, but also for observations of methoprene-treated, acetone-treated, and untreated control groups.
Hypopharyngeal gland measurements. Gland weights were used as an additional indicator of the impact of methoprene treatment and the behavioral activities of 2-, 4-, and 10-d-old bees in each of the four treatments. Hypopharyngeal glands are largest in nurse bees but regress once the bees switch to duties outside the hive, such as foraging (for review, see Winston, 1987). Methoprene-treated bees, acetone-treated bees, untreated controls, and bees from manipulated hives in which foraging-age bees had been removed were cold-anesthetized before the hypopharyngeal glands were dissected from the head capsule and weighed.
Histology and volumetric analysis. Bees (10 d old) from all four treatments that were collected for analysis of hypopharyngeal gland weights were used also for the stereological analysis of the primary antenno-sensory centers (antennal lobes) of the brain. Once the hypopharyngeal glands had been removed, the entire head capsule containing the brain was placed into aged alcoholic Bouin’s fixative for 3 d and then transferred for 2 d to 70% alcohol. The brain was then removed from the head capsule, dehydrated, and embedded in paraffin wax. Serial sections of 5 μm thickness were cut and mounted on gelatin-coated slides and stained using a modification of the Klüver-Barrera method (Klüver and Barrera, 1953) with luxol fast blue and cresyl fast violet. Although the original method calls for sections to be stained for 6 min in cresyl fast violet preheated to ∼57°C, it was found in this study that stronger contrast between the luxol fast blue and cresyl fast violet stains could be obtained if sections were placed in cresyl fast violet preheated to ∼30°C for 45–60 sec only.
Cavalieri’s direct estimator of volume (Gundersen et al., 1988), a technique that allows the volume of an object in serial histological sections to be estimated with an error of <5%, was used to estimate the volume of the antennal-lobe neuropil and three readily identifiable antennal-lobe glomeruli. Estimates were made of the total volume of the antennal-lobe neuropil and of its two major divisions, the outer glomerular layer and the central core (Fig. 1). The three glomeruli examined in this study have been identified elsewhere as T4-3(1), T4-2(1), and T1-44 (Flanagan and Mercer, 1989) (Fig.1A,B). Volumetric measurements were taken from the right antennal lobe wherever possible, although occasionally the left lobe was used. Winnington et al. (1996) found no significant difference between the volume of the left and right antennal-lobe neuropil of the bee brain.
Images of frontal sections of the antennal lobe were projected from an Olympus BHS system microscope to a Panasonic WV-CM140 video monitor using a Panasonic WV-CL500 video camera. An acetate sheet with a grid pattern of known dimensions was taped to the monitor at an angle specified by random number tables and a protractor. The number of grid intersections falling on the structure of interest was counted. This was repeated at 25 μm intervals using a 25 mm2grid on sections magnified ∼690× for whole antennal-lobe volumes, and at 5 μm intervals using a 20 mm2 grid on sections magnified ∼1830× for the measurement of individual glomeruli. Measurements started at a random point within the first 25 μm of the beginning of the antennal-lobe neuropil for whole lobe measurements and at the first 5 μm section for individual glomeruli. In this way a minimum total of 100 points were counted over at least 10 serial sections for each brain structure in question (Winnington et al., 1996). Total grid counts for each structure were converted to estimates of volume using the following equation from Gundersen et al. (1988): Vol(object) = t ·a(p) · ∑P(object), where t is the distance between sections,a(p) the area associated with each point (grid size divided by the magnification, all squared), and ∑P(object) is the total number of grid points counted for the object.
In a first set of experiments, antennal-lobe volumes were examined in 10-d-old methoprene-treated, acetone-treated, and untreated controls collected from the same hive. It was not clear whether the methoprene-treated bees used in this experiment had been foraging precociously. At the same time, however, a group of 10-d-old precociously foraging bees from a hive in which bees of normal foraging age had been removed also was examined. The experiment was repeated the following summer to examine further the effects of foraging experience on glomerular volumes. In the second set of experiments, four groups of 10-d-old bees were examined: (1) untreated control bees that had never been observed foraging, (2) methoprene-treated bees that had never been observed foraging, (3) methoprene-treated bees that were prevented from foraging, and (4) methoprene-treated bees in which foraging behavior had been observed. To determine with reasonable certainty that bees from groups 1 and 2 had not been foraging, the bees used in this study were observed in the hive at 6, 8, and 10 d of age, and their position on the comb was recorded. Because forager bees tend to move to the outer frames, which are used for storing food (Jaycox et al., 1974), only bees observed on brood comb toward the center of the hive were collected. The methoprene-treated bees that were prevented from foraging were placed on a frame containing food as well as sealed and unsealed brood. The frame was then placed in a wire and mesh enclosure in the center of the hive. The mesh size was large enough to ensure that bees on either side of the enclosure could touch and feed one another, but small enough to prevent the bees within the enclosure from leaving. To obtain methoprene-treated foragers, the hive was monitored as described above to establish the identity of precociously foraging bees.
Olfactory conditioning. The effects of methoprene treatment and hive manipulation on olfactory learning behavior also were examined. Honey bees were tested for their ability to associate a floral odor with a food reward using the proboscis conditioning paradigm first described by Kuwabara (1957). Bees collected from the hive were chilled in a freezer for up to 10 min to cold-anesthetize them for ease of handling. Individual bees were set up in tubes and secured in place by a piece of tape placed between the head and thorax. Once the bees had recovered from the chilling and mounting procedure, they were held over a small container of 30% sugar solution and allowed to feed to satiation. They were then left in their tubes overnight. The following day, between 10 A.M. and 4 P.M., a single conditioning trial was used to condition the bees to lavender.
Essential oil of lavender (2 μl) was pipetted onto a 1 cm2 piece of filter paper, which was then placed in a 60 ml syringe. The syringe was used to apply a puff of lavender-scented air (the conditioned stimulus) directly onto the antennae of the bee. Approximately 3 sec later, a droplet of 30% sugar solution (the unconditioned stimulus) was touched to the antennae, inducing the bee to extend its proboscis to feed. The bee was then allowed to feed from the sugar solution for ∼6 sec while still being exposed to the scent of lavender. Each bee was tested 18 min after the single conditioning trial to determine whether it had learned to associate lavender scent with a reward. The percentage of bees displaying the conditioned response (proboscis extension) when presented with the lavender scent in the absence of a food reward was recorded.
Any bee exhibiting proboscis extension in response to the conditioned stimulus previous to conditioning was discarded. Bees that failed to exhibit proboscis extension in response to sugar-water stimulation of the antennae also were not used.
The percentage of bees displaying the conditioned response after a single conditioning trial was examined in the following: (1) methoprene-treated bees, (2) acetone-treated bees, (3) untreated controls, and (4) bees from a manipulated hive in which normal-age foragers had been removed. Experiments were performed on 2-, 4-, and 10-d-old bees. Bees belonging to the first three treatment groups were conditioned over the same time period. Up to six groups of 18 bees were conditioned each day, and equal numbers of bees from each treatment were spread across these groups. Because sample sizes in the original study for 2-d-old methoprene-treated, acetone-treated, and untreated controls were low, the experiment was repeated with 2-d-old bees the following summer.
Statistical analysis. A modified Kolmogorov–Smirnov test (Koziol and Byar, 1975) of the empirical cumulative distribution functions of age at first flight was used to analyze differences over time in the numbers of bees flying in the four treatment groups. Because <100% of bees from each group were observed foraging when observations ceased, the test was modified for use with data that were truncated in time. Each bee was counted only once, on the first day it was observed flying. Multiple two-sample tests were performed, comparing all combinations of the four treatment groups.
Logistic regression analysis was used to examine changes with age in the percentage of bees exhibiting associative learning. χ2 analysis was used to reveal any significant differences between treatments in the learning levels of bees of the same age. Where a significant difference between groups was identified, multiple planned pairwise χ2 tests were performed to determine where the differences lay. SEs of the proportion of bees exhibiting the conditioned response were calculated by taking the square root of p(1 − p)/n, wherep is the proportion of bees showing a response andn is the number of bees tested.
One-way ANOVA was used to compare the hypopharyngeal gland weights of same-aged bees from different treatments. In cases in which a significant difference between treatments was identified, post hoc Tukey’s tests were used to determine where the differences lay. Two-way ANOVA was used to examine the effects of age and treatment on gland weights and to look for age-treatment interactions. One-way ANOVA and post hoc Tukey’s tests were used also to compare volumetric estimates of the antennal-lobe neuropil and identifiable antennal-lobe glomeruli in bees receiving different treatments.
Statistical analyses were performed using the Minitab software package (Minitab 8.2, State College, PA, 1991), and a level of significance of 5% was accepted for all tests. A Minitab macro program for the logistic regression analysis, written by Mr. Raymond Webster (University of Otago), was kindly supplied by Mr. John Harraway (Mathematics and Statistics Department, University of Otago) (Harraway, 1995).
RESULTS
Precocious behavioral development
Both treatment with the JH analog methoprene and removal of normal foraging-age bees from the colony induced young bees to begin foraging at an earlier than normal age. The empirical cumulative distribution functions of the age of first flight are presented in Figure2A. Individual two-sample Kolmogorov–Smirnov tests reveal that the first-flight distribution of methoprene-treated bees differs significantly from both that of the untreated controls (K = 1.98;p < 0.001) and that of bees treated with acetone (K = 1.82; p < 0.001). The flight distribution of bees from the manipulated hive also differs significantly from that of untreated controls (K = 2.95; p < 0.001) and acetone-treated bees (K = 2.53; p < 0.001). There is no significant difference, however, between the untreated control and acetone-treated groups (K = 0.40; p > 0.90), nor is there a difference between the first-flight distributions of methoprene-treated bees and bees from the manipulated hive (K = 1.20; p > 0.05). Overall, bees treated with methoprene and bees exposed to hive manipulation began flying at an earlier age than either of the two control groups.
Fig. 2.
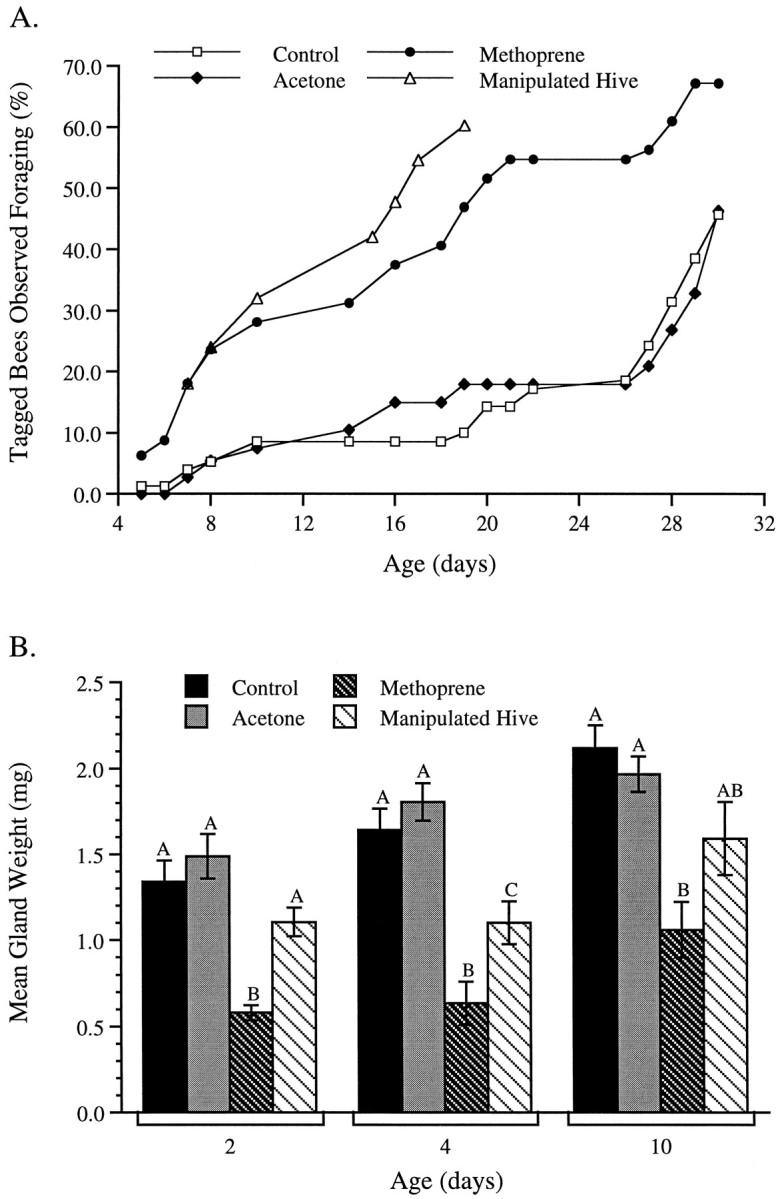
A, Cumulative distribution of the percentage of marked bees seen foraging. Each bee was counted only once, on the first day it was seen flying from the hive. The first-flight distributions of methoprene-treated bees and bees from the manipulated hive differed significantly from the first-flight distributions of the acetone-treated and control groups (p < 0.001). There were no significant differences between the two control groups or between the methoprene-treated bees and bees from the manipulated hive, respectively. Sample sizes: 100 bees from the manipulated hive and 80 bees for each of the other three treatment groups. B, Mean (±SE) wet hypopharyngeal weights in 2-, 4-, and 10-d-old bees. At each age, bars marked with differentletters differ significantly. Letters do not represent significant differences across age groups. The hypopharyngeal glands of methoprene-treated bees were significantly reduced in all three age groups (p < 0.001). In the 2- and 4-d-old groups, the hypopharyngeal gland weights of bees from the manipulated hive were significantly larger than those of the methoprene-treated bees. Hypopharyngeal gland weights of 4-d-old bees from the manipulated hives were also significantly smaller than those of the control groups. Sample sizes: 11 bees in the 2-d-old acetone-treated group and 12 bees for all other groups.
Hypopharyngeal gland weights: a useful indicator of methoprene effects
In all age groups examined in this study the hypopharyngeal glands of untreated control bees and controls treated with acetone were at least twice as heavy as those of methoprene-treated bees (Fig.2B). Gland weights of workers collected from hives in which bees of normal foraging age had been removed (the manipulated hives) were intermediate between these two extremes. One-way ANOVA reveals significant differences between the gland weights of bees in the four treatment groups at 2 d (F(3,43) = 15.70; p < 0.001), 4 d (F(3,44) = 19.65; p < 0.001), and 10 d of age (F(3,44) = 8.86;p < 0.001). Furthermore, post hoc Tukey’s tests show that at all ages, the gland weights of bees treated with methoprene are significantly lower than those of controls (untreated and acetone-treated bees), whereas the gland weights of 2- and 10-d-old bees from the manipulated hive are not significantly different from same-age control values. In 4-d-old bees, the mean gland weight of bees from the manipulated hive is significantly smaller than the gland weights of controls, but significantly greater than the mean gland weight of methoprene-treated bees. A similar trend is apparent in 10-d-old bees, but in this age group differences between the gland weights of bees from the manipulated hive are not significantly different from those recorded in bees belonging to the other three treatments.
Two-way ANOVA revealed that the mean gland weights of bees from all four treatment groups increased significantly with age (F(2,131) = 19.50; p < 0.001). There was also a significant interaction between age and treatment on the gland weights of bees from all four treatment groups (F(6,131) =15.14; p < 0.001). The interaction suggests that although the mean gland weights increased significantly with age in all four treatments, the rate of increase was not the same for the four groups.
Structural plasticity of the antennal lobes
The structure of the antennal-lobe neuropil and the positions of the three readily identifiable antennal-lobe glomeruli examined in this study are shown in Figure 1. Figure3A shows the mean volumes of the antennal-lobe neuropil of 10-d-old methoprene-treated, acetone-treated, and untreated bees, as well as the mean antennal-lobe volume of 10-d-old bees from a manipulated hive in which bees of normal foraging age had been removed. One-way ANOVA revealed no significant differences between these treatments in the volume of the glomerular neuropil (F(3,24) = 0.67; p > 0.5), the central fibrous core (F(3,24) = 0.61;p > 0.6), or the antennal-lobe neuropil as a whole (F(3,24) = 1.12; p > 0.3). Analysis of identifiable antennal-lobe glomeruli, however, revealed site-specific effects of hive manipulation in the glomerular layer of the antennal lobes (Fig. 3B).
Fig. 3.
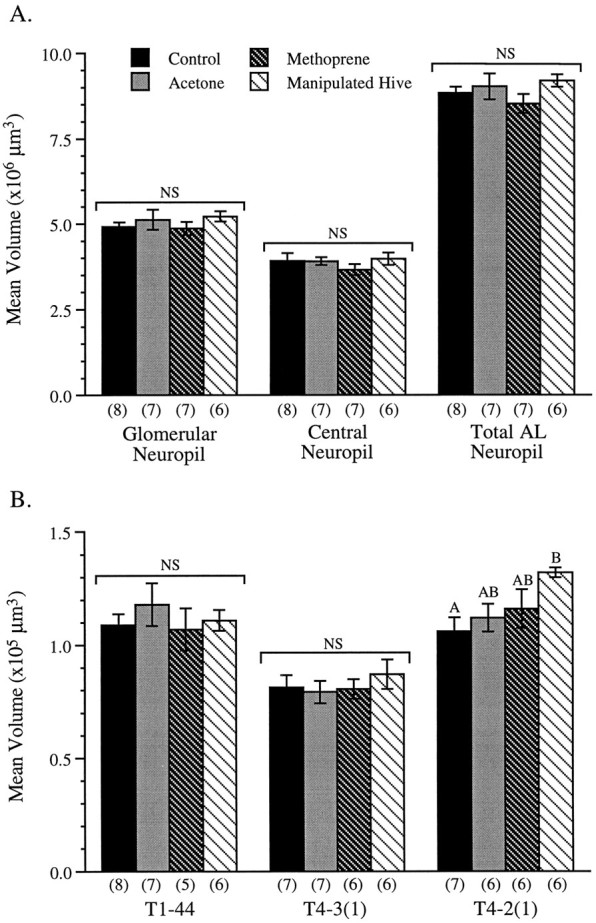
A, Mean (±SE) antennal-lobe neuropil volumes for 10-d-old bees from each of the four treatment groups. Together, the glomerular neuropil and the fibrous (central, aglomerular) neuropil comprise the total antennal-lobe neuropil. There were no significant differences (NS) between treatments for each of the three neuropil measurements. Numbers inparentheses are sample sizes. B, Mean (±SE) glomerular volumes for 10-d-old bees from each of the four treatment groups. Bars with differentletters differ significantly. There were no significant differences (NS) between treatment groups in the volumes of either the T1-44 or the T4-3(1) glomerulus. The mean volume of glomerulus T4-2(1), however, was significantly smaller in the untreated control group than in bees from the manipulated hive (p < 0.05). Numbers inparentheses are sample sizes.
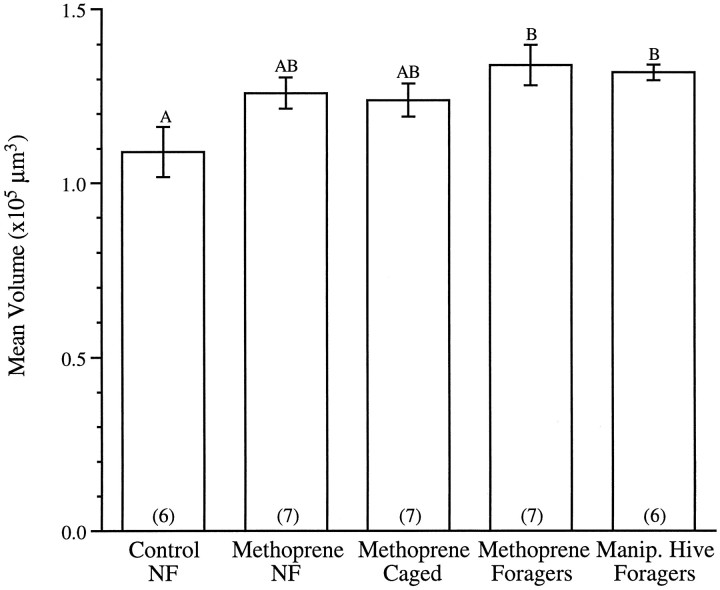
Although there is no significant difference between the mean volumes of glomerulus T1-44 recorded in the four treatment groups (F(3,22) = 0.40; p > 0.7) or T4-3(1) (F(3,22) = 0.40; p > 0.7), the volume of glomerulus T4-2(1) is significantly larger in precocious foragers from the manipulated hive than in untreated controls (F(3,21) = 3.29; p < 0.05). In this first set of experiments, the mean volume of T4-2(1) in bees treated with methoprene did not differ significantly from the volumes of this glomerulus recorded in bees receiving other treatments. It was not clear in this experiment, however, whether bees treated with methoprene had been foraging precociously. For this reason, the experiment was repeated the following summer so that the volume of glomerulus T4-2(1) in 10-d-old methoprene-treated bees exhibiting foraging behavior could be compared directly with volumes of the same glomerulus in methoprene-treated bees that were never observed foraging, as well as in bees treated with methoprene that were prevented from foraging (Fig. 4). For purposes of comparison, Figure 4 includes also the mean T4-2(1) volume of precociously foraging bees from the manipulated hive recorded the previous summer (also see Fig. 3B).
Fig. 4.
Mean (±SE) volumes of the T4-2(1) glomerulus for (1) nonforaging untreated bees (Control NF), (2) nonforaging methoprene-treated bees (Methoprene NF), (3) methoprene-treated bees prevented from foraging (Methoprene Caged), and (4) methoprene-treated bees observed foraging (Methoprene Foragers). The mean glomerular volumes of bees from the manipulated hive observed foraging (Manip. Hive Foragers) has been included for purposes of comparison (also see Fig. 3B). Bars with different letters differ significantly. The mean volume of the T4-2(1) glomerulus was significantly larger in both groups of precocious foragers when compared with the mean volume of the control group (p < 0.02). Glomerular volumes of the two groups of methoprene-treated bees that did not forage did not differ significantly from those of either the control group or the two groups of precocious foragers. Numbers inparentheses are sample sizes.
ANOVA reveals that the T4-2(1) volumes recorded in the two groups of precocious foragers (methoprene-treated bees and bees from the manipulated hive) are significantly larger than those of nonforaging controls (F(4,28) = 3.55; p < 0.02) and that there is no significant difference between the T4-2(1) volumes of methoprene-treated foragers and precocious foragers from the manipulated hive. Methoprene-treated bees that did not take part in foraging activities, or were prevented from doing so, exhibited T4-2(1) volumes not significantly different from those of precocious foragers or controls.
Influences on associative learning behavior
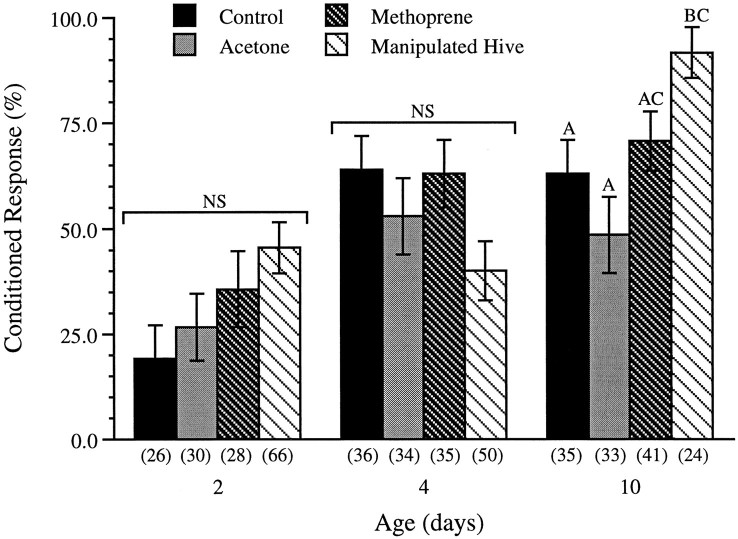
Figure 5 shows the percentage of 2-, 4-, and 10-d-old bees in each of the four treatment groups (untreated controls, controls treated with acetone, methoprene-treated bees, and bees exposed to hive manipulation) that displayed a conditioned response to lavender after a single conditioning trial. χ2 tests reveal no significant differences between the four treatments in the response levels of 2- and 4-d-old bees (χ23 = 6.90, p > 0.07; χ23 = 6.46, p > 0.09, respectively). Multiple pairwise χ2 tests of 10-d-old bees, however, show that the learning levels of bees from the manipulated hive are significantly higher than those of the untreated bees and acetone-treated controls (χ23= 12.24; p < 0.007). Logistic regression analysis reveals that the overall increase in learning levels with age also is highly significant (χ21 = 25.09;p < 0.0001).
Fig. 5.
Percentage of 2-, 4-, and 10-d-old bees showing the conditioned response (±SE of the proportions) from the four treatment groups. Bars with differentletters differ significantly. There were no significant differences between treatments in the levels of conditioned responses recorded at 2 or 4 d of age. At 10 d of age, however, bees from the manipulated hive exhibited a significantly higher level of responses than bees from either of the two control groups (p < 0.007). Numbers inparentheses are sample sizes. NS, Not significantly different.
Although not statistically significant, there appeared to be a strong trend in the learning levels of 2-d-old bees across the four treatment groups (Fig. 5). To determine whether the lack of a statistically significant difference between the four groups of 2-d-old bees could be attributable to the small sample sizes, the experiment was repeated the following summer using larger numbers of bees. Once again, however, χ2 analysis revealed no significant differences (χ23 = 1.68; p > 0.6) between the levels of conditioned responses observed in 2-d-old methoprene-treated bees (49.2%; n = 59), acetone-treated bees (37.7%; n = 61), untreated control bees (44.8%; n = 67), and bees from a hive manipulated as described above (45.5%; n = 66).
DISCUSSION
Significant increases in glomerular volume are activity dependent
The results of this study provide strong evidence for activity-dependent enhancement of glomerular volumes in the antennal lobes of the brain of the adult worker honey bee. We show for the first time that precocious foraging behavior in young worker bees is accompanied by premature enlargement of the T4-2(1) glomerulus and that activity-dependent changes in the volume of this glomerulus are site-specific and highly predictable. Bees that failed to show precocious foraging behavior, or were prevented from performing such tasks, exhibited T4-2(1) volumes that were not significantly different from those of controls.
Under normal colony conditions, the rate of growth of glomerulus T4-2(1) in bees 4–10 d of age is slow, but rapid increases in the volume of this glomerulus occur later in life and are correlated temporally with a shift to foraging duties (Winnington et al., 1996). If the volume of T4-2(1) is enhanced as a consequence of foraging activities, precocious foraging behavior should be accompanied by premature enlargement of this glomerulus. As is evident in the present study (Fig. 2A), worker bees normally begin foraging at ∼3 weeks of age (for review, see Seeley, 1982; Winston, 1987); however, treatment with JH analogs, such as methoprene (Robinson, 1985,1987c; Robinson et al., 1989), or removal of foraging-age bees from the colony (Lindauer, 1961; Winston and Fergusson, 1985; Robinson et al., 1989) induces bees to begin foraging precociously (Fig.2A). Here we show that there is a significant and premature enlargement of glomerulus T4-2(1) in 10-d-old precocious forager bees (Figs. 3, 4). This premature increase in glomerular volume seems not to be driven by JH titers alone but to be dependent on shifts in behavior.
Evidence that the growth of glomerulus T4-2(1) is enhanced by activities performed while foraging is supported by behavioral reversion experiments. Foragers forced to revert to nursing duties exhibit significantly smaller T4-2(1) volumes than bees that continue to forage (Winnington et al., 1996). Interestingly, the hive manipulations used in these earlier experiments, like those described in the present study, had no effect on the volumes of either T1-44 or T4-3(1) (Fig. 3B). In striking contrast, removal of the queen from a colony early in the adult life of a worker has a significant impact on T1-44 and T4-3(1) volumes, but no effect on the volume of the T4-2(1) glomerulus (Morgan et al., 1997).
On the basis of the earlier study by Winnington et al. (1996), which revealed that T1-44 grows rapidly during the first 6–10 d of adult life but shows little change in volume thereafter, we predicted that the volume of T1-44 would be similar in all 10-d-old bees, regardless of whether they were foraging. This proved to be correct; however, the impact of precocious foraging behavior on the T4-3(1) glomerulus was less predictable. T4-3(1) is remarkably similar in volume in nectar foragers, nurses, comb builders, and normal 10-d-old bees, but the volume of this glomerulus in pollen foragers is significantly larger than in all other behavioral groups (Winnington et al., 1996). The precocious foragers examined in the present study showed no evidence of premature enlargement of glomerulus T4-3(1), suggesting that methoprene treatment induced nectar-foraging rather than pollen-foraging activities in these young bees. Bees that forage for nectar and pollen are generally thought to belong to the same age class (Winston, 1987), but the glomerular volumes of these two behavioral castes suggest that pollen foragers may belong to a slightly older age class than nectar foragers.
Are there also nonactivity-dependent changes?
Trends toward increasing T4-2(1) volumes in methoprene-treated bees that did not exhibit precocious foraging behavior (Figs.3B, 4) should not be ignored. Direct hormonal actions have been proposed as a mechanism underlying structural plasticity in the mushroom bodies of the bee brain (Fahrbach et al., 1995; Withers et al., 1995; Fahrbach and Robinson, 1996) and may complement the indirect effects of JH that result from behavior-related modifications of the brain neuropil. Preliminary observations in our laboratory suggest, however, that bees that fail to show precocious foraging behavior as a result of methoprene treatment nonetheless exhibit behaviors that differ markedly in some cases from those of untreated controls. The contribution of these less dramatic shifts in behavior to activity-related changes in glomerular volume requires further investigation before it can be concluded that hormonal treatment has a direct impact on the antennal-lobe neuropil of the bee.
A role for the antennal lobes in memory formation?
Significant changes in T4-2(1) volume were apparent only in those bees that had shifted to duties outside the hive. How could such shifts in behavior lead to structural changes in the antennal-lobe neuropil? One possibility is that the pattern of neuronal activity across the diverse array of sensory neurons housed in the antennae is altered by shifts in behavior leading to activity-dependent changes in the structure of the antennal-lobe neuropil (Winnington et al., 1996). This would be reminiscent of structural plasticity reported in the primary somatosensory cortex of the vertebrate brain, the dynamic properties of which reflect changes in the use of peripheral sensory pathways (Merzenich et al., 1983 a,b; Jenkins et al., 1990). Experience-dependent plasticity has been demonstrated recently in an elegant study of the optic lobes of the fruitfly Drosophila melanogaster (Barth et al., 1997), and deprivation experiments in bees support the view that changes in the use of peripheral sensory pathways can have a significant impact also on the antennal lobes of the insect brain (Gascuel and Masson, 1987). Volume changes in the antennal-lobe neuropil, however, could also reflect the formation of long-term memories (Bailey and Kandel, 1993), events associated more commonly with mushroom bodies of the insect brain (Hammer and Menzel, 1995).
Involvement of the antennal lobes in olfactory learning is well established, but their role in memory formation has yet to be resolved (Hammer and Menzel, 1995). Although most honey bees learn rapidly to associate floral odors with a food reward, an ability that is fundamental to their success as foragers (Mauelshagen and Greggers, 1993; Menzel, 1993; Hammer and Menzel, 1995; Menzel and Müller, 1996), young adult workers 0–3 d of age have been found to be very poor learners (Fig. 5) (Morgan et al., 1997). Improvements in olfactory learning performance during the first week of adult life correlate temporally with rapid increases in the volume of the glomerular neuropil of the antennal lobes (Withers et al., 1993; Winnington et al., 1996; Morgan et al., 1997). It is possible that early maturation of the brain neuropil is a prerequisite for the expression of some associative learning behaviors and that events underlying learning and memory continue to have an impact on the structure of the antennal-lobe neuropil throughout the lifetime of the adult worker bee. Associative learning levels in 10-d-old precocious foragers were significantly higher than in bees of the same age performing duties within the hive, suggesting that associative learning of floral odors improves with experience. It is interesting to note, in addition, that bees showing significantly higher than normal learning levels (Fig. 5) also exhibited T4-2(1) volumes significantly larger than those of controls (Fig. 3B). The possibility that learning and memory contribute to the structural plasticity that is so prevalent in the antennal lobes of the bee brain clearly warrants further attention.
Underlying mechanisms
The development of olfactory learning performance in honey bees is significantly altered in mutant bees that exhibit abnormal biogenic amine synthesis (Lopatina et al., 1985). Furthermore, if dopamine is applied to the antennal lobes of the honey bee, before or after one-trial conditioning, the percentage of bees that respond to a conditioned olfactory stimulus is significantly reduced (Macmillan and Mercer, 1987). Recent studies have revealed also that biogenic amines modulate the excitability (Kloppenburg and Hildebrand, 1995; Mercer et al., 1995, 1996a), as well as the growth (Mercer et al., 1996b; Kirchhof and Mercer, unpublished data), of insect antennal-lobe neurons. Taken together, these results suggest that in insects, as in vertebrates (Keverne and de la Riva, 1982; Brennan et al., 1990;Kendrick et al., 1992; McLean et al., 1993; Moriizumi et al., 1994;Jiang et al., 1996), biogenic amines contribute to the structural and functional plasticity of primary olfactory centers of the brain. The recent identification and pharmacological characterization of two distinct dopamine-receptor subtypes in the brain of the bee (Kokay and Mercer, 1996) and analyses of their expression in situ(Kokay and Mercer, 1995) and in antennal-lobe neurons in vitro (Kirchhof and Mercer, 1997) provide important first steps toward defining more clearly the roles played by biogenic amines in the brain of the bee.
The highly compartmentalized nature of the antennal lobes, the ease with which individual compartments can be identified, and the predictability of changes to this highly structured neuropil make this an ideal system for examining the mechanisms and behavioral consequences of structural plasticity in the brain.
Footnotes
A.M. was supported by Otago Research Grant MFZ B06. We particularly thank Vivian Butz Huryn for her assistance and advice and Dr. Ruth Napper for supplying equipment used for stereology. We are grateful also to Gerald Stokes and Ken Miller for their technical assistance and to Barbara Kirchhof and Ilona Kokay for their helpful comments and suggestions.
Correspondence should be addressed to Dr. Alison R. Mercer, Department of Zoology, University of Otago, Dunedin, New Zealand.
REFERENCES
- 1.Arnold G, Masson C, Budharugsa S. Comparative study of the antennal lobes and their afferent pathway in the worker bee and the drone (Apis mellifera). Cell Tissue Res. 1985;242:593–605. [Google Scholar]
- 2.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 3.Barth M, Hirsch HVB, Meinertzhagen IA, Heisenberg M. Experience-dependent developmental plasticity in the optic lobe of Drosophila melanogaster. J Neurosci. 1997;17:1493–1504. doi: 10.1523/JNEUROSCI.17-04-01493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeckh J, Tolbert LP. Synaptic organization and development of the antennal lobe in insects. Microsc Res Tech. 1993;24:260–280. doi: 10.1002/jemt.1070240305. [DOI] [PubMed] [Google Scholar]
- 5.Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- 6.Christensen TA, Hildebrand JG. Functions, organization and physiology of the olfactory pathways in the lepidopteran brain. In: Gupta AP, editor. Arthropod brain: its evolution, development, structure and functions. Wiley; New York: 1987. pp. 457–484. [Google Scholar]
- 7.Durst C, Eichmüller S, Menzel R. Development and experience lead to increased subcompartments of the honeybee mushroom body. Behav Neural Biol. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. [DOI] [PubMed] [Google Scholar]
- 8.Esslen J, Kaissling KE. Zahl und Verteilung antennaler Sensillen bei der Honigbiene. Zoomorphologie. 1976;83:227–251. [Google Scholar]
- 9.Fahrbach SE, Robinson GE. Juvenile hormone, behavioral maturation, and brain structure in the honey bee. Dev Neurosci. 1996;18:102–114. doi: 10.1159/000111474. [DOI] [PubMed] [Google Scholar]
- 10.Fahrbach SE, Giray T, Robinson GE. Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol Learn Memory. 1995;63:181–191. doi: 10.1006/nlme.1995.1019. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan D, Mercer AR. An atlas and 3-D reconstruction of the antennal lobes in the worker honey bee, Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol. 1989;18:145–159. [Google Scholar]
- 12.Gascuel J, Masson C. Influence of olfactory deprivation on synapse frequency in developing antennal lobe of the honey bee Apis mellifera. Neurosci Res Commun. 1987;1:173–180. [Google Scholar]
- 13.Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Versterby A, West MJ. The new stereological tools: dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 14.Hammer M, Menzel R. Learning and memory in the honeybee. J Neurosci. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harraway J. Regression methods applied. University of Otago; Dunedin, New Zealand: 1995. [Google Scholar]
- 16.Jaycox ER. Behavioral changes in worker honey bees (Apis mellifera L.) after injection with synthetic juvenile hormone (Hymenoptera: Apidae). J Kans Entomol Soc. 1976;49:165–170. [Google Scholar]
- 17.Jaycox ER, Skowronek W, Guynn G. Behavioral changes in worker honey bees (Apis mellifera) induced by injections of a juvenile hormone mimic. Ann Entomol Soc Am. 1974;67:529–534. [Google Scholar]
- 18.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci. 1996;16:6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joerges J, Küttner A, Galizia CG, Menzel R. Representation of odours and odour mixtures visualized in the honeybee brain. Nature. 1997;387:285–288. [Google Scholar]
- 21.Kendrick KM, Levy F, Keverne EB. Changes in the sensory processing of olfactory signals induced by birth in sheep. Science. 1992;256:833–836. doi: 10.1126/science.1589766. [DOI] [PubMed] [Google Scholar]
- 22.Keverne EB, de la Riva C. Pheromones in mice: reciprocal interaction between the nose and brain. Nature. 1982;296:148–150. doi: 10.1038/296148a0. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof BS, Mercer AR. Antennal-lobe neurons of the honey bee, Apis mellifera, express a D2-like dopamine receptor in vitro. J Comp Neurol. 1997;383:189–198. doi: 10.1002/(sici)1096-9861(19970630)383:2<189::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Kloppenburg P, Hildebrand JG. Modulatory effects of 5-hydroxytryptamine on interneurons in the antennal lobe of the sphinx moth, Manduca sexta. J Exp Biol. 1995;198:603–611. doi: 10.1242/jeb.198.3.603. [DOI] [PubMed] [Google Scholar]
- 25.Klüver H, Barrera E. A method for the combined staining of cells and fibres in the nervous system. J Neuropathol Exp Neurol. 1953;12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kokay IC, Mercer AR. Ontogeny of dopamine receptors in insect (Apis mellifera) brain. Soc Neurosci Abstr. 1995;21:632. doi: 10.1016/0006-8993(95)01179-x. [DOI] [PubMed] [Google Scholar]
- 27.Kokay IC, Mercer AR. Characterisation of dopamine receptors in insect (Apis mellifera) brain. Brain Res. 1996;706:47–56. doi: 10.1016/0006-8993(95)01179-x. [DOI] [PubMed] [Google Scholar]
- 28.Koziol JA, Byar DP. Percentage points of the asymptotic distributions of one and two sample K-S statistics for truncated or censored data. Technometrics. 1975;17:507–510. [Google Scholar]
- 29.Kuwabara M. Bildung des bedingten Reflexes von Pavlovs Typus bei der Honigbiene (Apis mellifica). J Fac Sci Hokkaido Univ. 1957;13:458–467. [Google Scholar]
- 30.Lieke E. Optical recording of neuronal activity in the insect central nervous system: odorant coding by the antennal lobes of honeybees. Eur J Neurosci. 1993;5:49–55. doi: 10.1111/j.1460-9568.1993.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 31.Lindauer M. Communication among social bees. Harvard UP; Cambridge, MA: 1961. [Google Scholar]
- 32.Lopatina NG, Chesnokova EG, Dolotovskaya LZ, Medvedeva AV. Conditioning and ontogenesis of honey bee mutants defective in tryptophan metabolism via kynurenine pathway. Ontogenez. 1985;16:616–619. [Google Scholar]
- 33.Macmillan CS, Mercer AR. An investigation of the role of dopamine in the antennal lobes of the honeybee, Apis mellifera. J Comp Physiol [A] 1987;160:359–366. [Google Scholar]
- 34.Masson C, Mustaparta H. Chemical information processing in the olfactory system of insects. Physiol Rev. 1990;70:199–245. doi: 10.1152/physrev.1990.70.1.199. [DOI] [PubMed] [Google Scholar]
- 35.Mauelshagen J, Greggers U. Experimental access to associative learning in honeybees. Apidologie. 1993;24:249–266. [Google Scholar]
- 36.McLean JH, Darby-King A, Sullivan RM, King SR. Serotonergic influence on olfactory learning in the neonate rat. Behav Neural Biol. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- 37.Menzel R. Associative learning in honey bees. Apidologie. 1993;24:157–168. [Google Scholar]
- 38.Menzel R, Müller U. Learning and memory in honeybees: from behavior to neural substrates. Annu Rev Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- 39.Mercer AR, Hayashi JH, Hildebrand JG. Modulatory effects of 5-hydroxytryptamine on voltage-activated currents in cultured antennal-lobe neurons of the sphinx moth, Manduca sexta. J Exp Biol. 1995;198:613–627. doi: 10.1242/jeb.198.3.613. [DOI] [PubMed] [Google Scholar]
- 40.Mercer AR, Kloppenburg P, Hildebrand JG. Serotonin-induced changes in the excitability of cultured antennal-lobe neurons of the sphinx moth, Manduca sexta. J Comp Physiol [A] 1996a;178:21–31. doi: 10.1007/BF00189587. [DOI] [PubMed] [Google Scholar]
- 41.Mercer AR, Kirchhof BS, Hildebrand JG. Enhancement by serotonin of the growth in vitro of antennal lobe neurons of the sphinx moth, Manduca sexta. J Neurobiol. 1996b;29:49–64. doi: 10.1002/(SICI)1097-4695(199601)29:1<49::AID-NEU4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3B and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983a;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 43.Merzenich MM, Kaas JH, Wall J, Sur M, Nelson RJ, Felleman D. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983b;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- 44.Morgan SM, Butz Huryn V, Downes SR, Mercer AR (1997) The effects of queenlessness on the maturation of the honey bee olfactory system. Behav Brain Res, in press. [DOI] [PubMed]
- 45.Moriizumi T, Tsukatani T, Sakashita H, Miwa T. Olfactory disturbance induced by deafferentation of serotonergic fibers in the olfactory bulb. Neuroscience. 1994;61:733–738. doi: 10.1016/0306-4522(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 46.Page RE, Robinson GE, Britton DS, Kim Fondrk M. Genotypic variability for rates of behavioral development in worker honeybees (Apis mellifera L.). Behav Ecol. 1992;3:173–180. [Google Scholar]
- 47.Robinson GE. Effects of a juvenile hormone analogue on honey bee foraging behavior and alarm pheromone production. J Insect Physiol. 1985;31:277–282. [Google Scholar]
- 48.Robinson GE. Hormonal regulation of age polyethism in the honeybee, Apis mellifera. In: Menzel R, Mercer A, editors. Neurobiology and behavior of honeybees. Springer; Berlin: 1987a. pp. 266–279. [Google Scholar]
- 49.Robinson GE. Modulation of alarm pheromone perception in the honey bee: evidence for division of labor based on hormonally regulated response thresholds. J Comp Physiol [A] 1987b;160:613–619. [Google Scholar]
- 50.Robinson GE. Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol. 1987c;20:329–338. [Google Scholar]
- 51.Robinson GE. Regulation of division of labour in insect societies. Annu Rev Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- 52.Robinson GE, Page RE, Strambi C, Strambi A. Hormonal and genetic control of behavioral integration in honey bee colonies. Science. 1989;246:109–113. doi: 10.1126/science.246.4926.109. [DOI] [PubMed] [Google Scholar]
- 53.Seeley TD. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol. 1982;11:287–293. [Google Scholar]
- 54.Winnington A, Napper RM, Mercer AR. Structural plasticity of the antennal lobes of the brain of the adult worker honey bee. J Comp Neurol. 1996;365:479–490. doi: 10.1002/(SICI)1096-9861(19960212)365:3<479::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 55.Winston ML. The biology of the honey bee. Harvard UP; Cambridge, MA: 1987. [Google Scholar]
- 56.Winston ML, Fergusson LA. The effect of worker loss on temporal caste structure in colonies of the honeybee. Can J Zool. 1985;63:777–780. [Google Scholar]
- 57.Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- 58.Withers GS, Fahrbach SE, Robinson GE. Effects of experience and juvenile hormone on the mushroom bodies of honey bees. J Neurobiol. 1995;20:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]