Abstract
Basic fibroblast growth factor (bFGF) has been shown to induce neural fate in dissociated animal cap (AC) cells or in AC explants cultured in low calcium and magnesium concentrations. However, long-term disclosure of the cap may cause diffusion of the secreted molecule bone morphogenetic protein 4 (BMP-4), a neural inhibitor present in the AC. This may contribute to the subsequent neurogenesis induced by bFGF. Here we used conjugated and aged blastula AC to avoid diffusion of endogenous molecules from the AC. Unlike noggin, bFGF failed to induce neural tissue in this system. However, it enhanced neuralization elicited by a dominant negative BMP receptor (DN-BR) that inhibits the BMP-4 signaling. Posterior neural markers were turned on by bFGF in AC expressing DN-BR or chordin. Blocking the endogenous FGF signal with a dominant negative FGF receptor (XFD) mainly inhibited development of posterior neural tissue in neuralized ACs. Thesein vitro studies were confirmed in vivoin embryos grafted with XFD-expressing ACs in the place of neuroectoderm. Expression of some regional neural markers was inhibited, although markers for muscle and posterior notochord were still detectable in the grafted embryos, suggesting that XFD specifically affected neurogenesis but not the dorsal mesoderm. The use of these in vitro and in vivo model systems provides new evidence that FGF, although unable to initiate neurogenesis on its own, is required for neural induction as well as for posteriorization.
Keywords: FGF, BMP, neurogenesis, anteroposterior patterning, Xenopus, embryo
Neural induction occurs in the dorsal ectoderm during gastrulation when the dorsal mesoderm involutes beneath the ectoderm. Three neural inducers, noggin, follistatin, and chordin, have been identified in Xenopus (Lamb et al., 1993;Hemmati-Brivanlou et al., 1994; Sasai et al., 1995). All are secreted proteins expressed in the right place (dorsal mesoderm) and at the right time (during gastrulation) to exert neurogenic effect (Smith and Harland, 1991; Hemmati-Brivanlou et al., 1994; Sasai et al., 1994). However, evidence has shown that both noggin and chordin physically bind bone morphogenetic protein 4 (BMP-4) ligand to block BMP-4 signaling (Piccolo et al., 1996; Zimmerman et al., 1996). Therefore, BMP-4 is a crucial molecule for maintaining the ectodermal fate. Neuralization is a default state of the ectodermal cells that occurs only when the BMP-4 signaling is inhibited by these “neural inducers.”
Additionally another secreted molecule, fibroblast growth factor (FGF), a well recognized mesoderm inducer, has been suggested to be a neural inducer (for review, see Doniach, 1995). First, Kengaku and Okamoto (1993, 1995) found that basic FGF (bFGF) induces dissociated cells of gastrula animal caps (ACs) to differentiate to neurons and melanophores with expression of both anterior and posterior markers. Second, Lamb and Harland (1995) found that bFGF induces expression of posterior neural markers in the AC that are excised at stage 9 and aged in low calcium and magnesium concentrations (LCMR) before bFGF treatment is started at the gastrula stage. However, the above experimental systems did not eliminate the possibility that the induced neurogenesis might partly result from dilution of the neural inhibitor BMP-4 because of the AC cell dissociation or long-term disclosure of the AC.
In this study, we used a conjugated and aged blastula AC system to avoid diffusion of endogenous molecules from the explants. Unlike noggin, bFGF failed to induce neural tissue in this system. However, it enhanced neuralization elicited by a dominant negative BMP receptor (DN-BR) and induced posterior neural tissue in AC-expressing DN-BR or chordin, in agreement with the report by Cox and Hemmati-Brivanlou (1995). However, although they demonstrated that bFGF induces posterior neural markers in anterior neural tissues (excised at stage 13.5–14) or AC (excised at stage 10.5–11), these explants might have received zygotic neuralizing signals such as noggin, chordin, and follistatin, as well as embryonic FGF, before dissection (Smith and Harland, 1991;Isaacs et al., 1992; Hemmati-Brivanlou et al., 1994; Sasai et al., 1994). Then, these signals would synergize with exogenous FGF in the induction of posterior neural tissue. In contrast, we used ACs dissected at stage 8.5 that should be exempted from the effects of these zygotic neuralizing signals. Furthermore, “loss-of-function” studies are necessary to confirm the requirement of endogenous FGF activity for neurogenesis. Therefore, we used a dominant negative FGF receptor (XFD) (Amaya et al., 1991) to inhibit FGF signaling and found that XFD mainly inhibited development of posterior neural tissue. This was verified in embryos grafted with XFD-expressing AC in the place of neuroectoderm.
MATERIALS AND METHODS
Agents. DN-BR, noggin, chordin, and β-galactosidase (β-gal) cDNAs were in pSP64T vector. They were linearized and used for in vitro synthesis of capped mRNA using a transcription kit in accordance with the manufacturer’s instructions (Ambion) (Xu et al., 1997). The synthetic RNA was quantitated by ethidium bromide staining in comparison with a standard RNA. Xenopus noggin protein was a kind gift from Dr. R. M. Harland (University of California, Berkeley, CA). Human bFGF protein was obtained from the Biological Resources Branch of the Frederick Cancer Research and Development Center (Frederick, MD).
Embryo injection and explant culture. Xenopus laevis embryos were obtained by in vitro fertilization after induction of females with 500 U of human chorionic gonadotropin. Developmental stages were designated according to the stages described by Nieuwkoop and Faber (1967). At the two-cell stage, each blastomere was injected with synthetic RNA as described in the figure legends. ACs were dissected from the injected embryos at stage 8.5. In some experiments, the ACs from two embryos were sandwiched immediately after explantation and cultured until the equivalent of stage 10.5 when the AC conjugates were opened, separated, and cultured individually in the presence of bFGF or noggin before being harvested at stage 24 (see Fig.1A). In other experiments, the injected ACs were cultured alone and harvested at the tadpole stage. Explants were generally cultured at 22°C in 67% Leibovitz’s L-15 medium (Life Technologies, Bethesda, MD) supplemented with 7 mmTris-HCl, pH 7.5, and gentamicin at 50 μg/μl (referred to below as L-15 medium). In some experiments, AC explants were cultured in LCMR (Lamb et al., 1993) to keep them open until bFGF was added at stage 10.5 to serve as a positive control (Lamb and Harland, 1995). All of the explants were harvested for analysis of molecular markers by reverse transcription-PCR (RT-PCR).
Fig. 1.
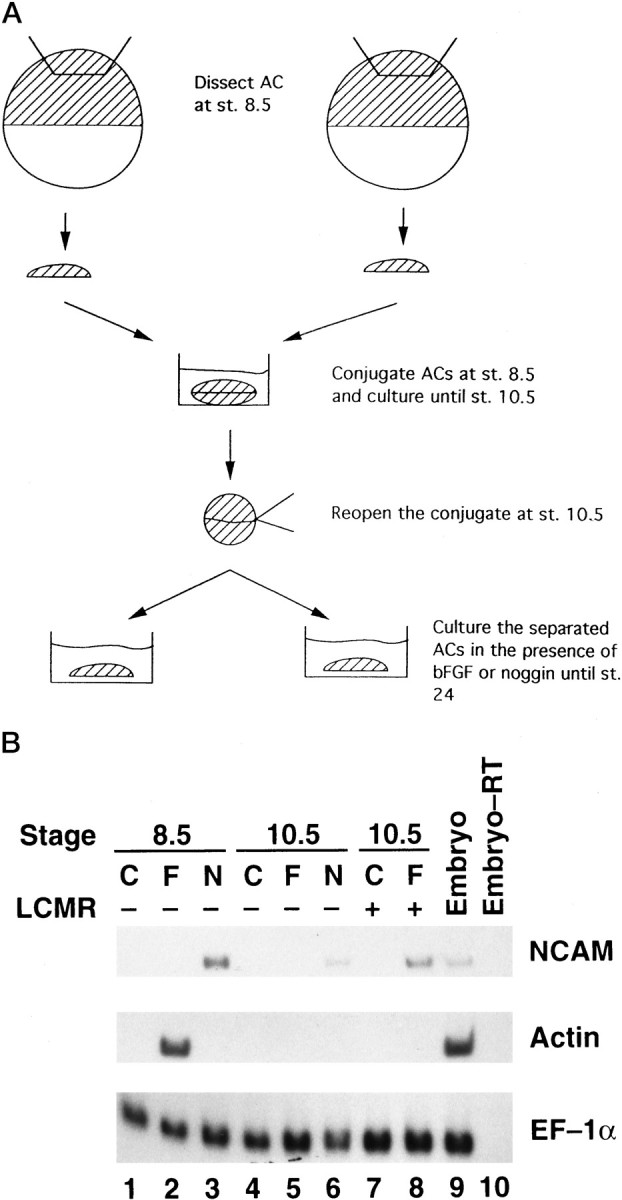
A, Scheme for the AC conjugation system (st., stage). B, Analysis of neuralization. ACs were dissected at stage 8.5 and cultured in the absence (C) or presence of bFGF (F) or noggin (N) at 0.1 μg/ml (lanes 1–3). Some ACs were conjugated at stage 8.5, manually opened at the equivalent of stage 10.5, and then cultured in L-15 medium alone or with bFGF or noggin (lanes 4–6). Alternatively, ACs were cultured in LCMR medium to remain open for control or bFGF treatment at stage 10.5 (lanes 7 and8). Whole embryos at stage 24 were used as a positive control (lane 9). The same sample used in lane 8 was processed for RT-PCR in the absence of RT to serve as a negative control (lane 10). ACs were cultured until stage 24 and analyzed for expression of NCAM and actinby RT-PCR. The expression of EF-1α was detected as an internal control for equal RNA loading.
The conjugated and aged AC explants have been tested for neuralization competence. Sharpe et al. (1987) found that Xenopus dorsal and ventral ectoderms have a different competence to be induced toward neural tissue; i.e., dorsal mesoderm induced neural markers more strongly in dorsal ectoderm than in ventral ectoderm. Otte and Moon (1992) reported that protein kinase C-α (PKC-α) is predominantly localized in dorsal ectoderm and responsible for the higher neuralization competence of the dorsal ectoderm. We tested the PKC-α expression in dorsal ectoderm and ventral ectoderm excised at stage 10, as well as in dorsal ectoderm or ventral ectoderm conjugates aged for 2 hr until stage 10.5. More PKC-α transcripts were detected in dorsal ectoderm than in ventral ectoderm whether conjugated and aged or not, indicating the retention of a dorsoventral (D-V) axis in our explant system. As for the anteroposterior (A-P) axis, it remains in stage 10.5 AC because anterior neural marker Otx2 and posterior neural marker HoxB9 are expressed on either end of the AC cotreated with noggin and FGF (Lamb and Harland, 1995).
Embryonic transplantation. Two animal blastomeres of donor embryos were injected with 2 ng of XFD or control β-gal RNA at the two-cell stage. ACs (approximately the central one-third of the whole animal hemisphere tissue) were dissected from the injected embryos at stage 8. Meanwhile tissues of the dorsal ectoderm, which is the prospective neuroectoderm (from 30° dorsal of the intermediate vertical line of the animal pole to just above the dorsal lip), were removed from recipient stage 10 embryos. The vacant area in the recipient embryos was covered immediately with the AC from the injected embryos. The grafted embryos were allowed to develop to stage 30 before being harvested for photography or RT-PCR analysis. Some injected embryos were not dissected but were allowed to develop to stage 30 to serve as nontransplantation controls.
RT-PCR. Total RNA was extracted from cultured explants with TRIzol reagent (Life Technologies) in accordance with the manufacturer’s instructions and subsequently was digested with DNase to remove genomic DNA. RT-PCR was performed using a Superscript preamplification system (Life Technologies). Primer sets and PCR conditions for molecular markers, i.e., neural cell adhesion molecule (NCAM), actin, XK81, chordin,CG13, Otx2, En2, Krox20,HoxB9, and EF-1α, have been described elsewhere (Hemmati-Brivanlou and Melton, 1994; Sasai et al., 1995; Wilson and Hemmati-Brivanlou, 1995; Xu et al., 1997). Although data from individual experiments are shown, the results were confirmed independently in all cases.
RESULTS
bFGF alone does not induce neural fate in gastrula stage AC
In the study of the role of FGF in neurogenesis, a conjugated AC system was used to avoid diffusion of endogenous molecules from the explants that is likely to occur in the LCMR culture system (Lamb and Harland, 1995) and in the cell dissociation system (Kengaku and Okamoto, 1993, 1995). ACs were dissected and conjugated at stage 8.5 and cultured until the equivalent of stage 10.5 in L-15 medium. Then, they were manually opened and exposed to bFGF or noggin at 0.1 μg/ml and cultured until stage 24 (Fig.1A). Expression of pan-neural marker NCAM (Kintner and Melton, 1987) was detected in noggin-treated caps but not in bFGF-treated caps (Fig.1B, lanes 5,6). To confirm that the bFGF used was biologically active, we treated some ACs from stage 8.5 (just after explantation) with bFGF or noggin at the same concentration (0.1 μg/ml). bFGF again failed to induce NCAM expression but did induce expression of muscle actin (Fig.1B, lane 2), which is consistent with its mesoderm-inducing activity. In contrast, noggin induced expression of NCAM but not of actin, which is in agreement with its neurogenic effect in the ectoderm. A much lower level of NCAM expression was induced by noggin when added to the AC culture at stage 10.5 than when added at stage 8.5 (Fig. 1B,lanes 3,6). This might be caused by a decrease in AC competence to neural induction or by accumulation of neural inhibitors such as BMP-4 in the stage 10.5 AC. Some ACs were cultured in LCMR to keep them open until bFGF was added at stage 10.5, and NCAM but not actin transcripts were detected in the bFGF-treated caps (Fig.1B, lane 8). These ACs served as a positive control to ensure that stage 10.5 ACs were competent for neurogenesis in LCMR medium (Lamb and Harland, 1995) in response to FGF, although these ACs failed to generate neural tissue in L-15 medium. The L-15 medium contains calcium and magnesium ions at normal concentrations and allows explants to heal rapidly. It is possible that the difference in neuralization competence might result from the diffusion of the neural inhibitor BMP-4 from the open AC cultured at low calcium and magnesium concentrations. This was based on the report that dispersed AC cells cultured in calcium- and magnesium-free medium undergo a cell-autonomous neuralization instead of epidermalization, and the neuralization can be reversed by addition of BMP-4 to the culture (Wilson and Hemmati-Brivanlou, 1995). Although ACs cultured in LCMR did not express NCAM (Fig. 1B, lane 7), dilution of BMP-4 in this medium might facilitate the neuralization triggered by bFGF treatment. To test this possibility, we used DN-BR in the subsequent experiments.
bFGF enhances neural induction by DN-BR
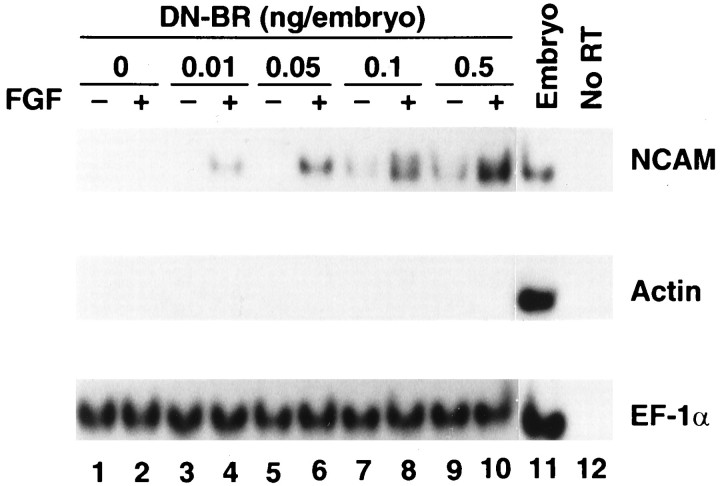
We used DN-BR to inhibit endogenous BMP-4 signaling (Graff et al., 1994; Maeno et al., 1994; Suzuki et al., 1994; Xu et al., 1996), a consequence similar to that resulting from dilution of BMP-4 concentration in ACs. DN-BR RNA at increasing doses (0, 0.01, 0.05, 0.1, and 0.5 ng/embryo) was injected into the animal pole area of the two-cell-stage embryos. ACs were dissected and conjugated at stage 8.5 and were cultured until the equivalent of stage 10.5 in L-15 medium. Then, they were manually opened and exposed to medium with bFGF at 0.1 μg/ml or to medium alone and cultured until stage 24 (Fig.1A). Without bFGF treatment, NCAM expression was absent in ACs injected with 0, 0.01, and 0.05 ng of DN-BR RNA but was present in ACs injected with 0.1 and 0.5 ng of DN-BR RNA (Fig.2). In conjunction with bFGF treatment, DN-BR RNA at as low as 0.01 ng was able to induce NCAM. The sensitivity increased 10-fold. At higher doses such as 0.1 and 0.5 ng of DN-BR RNA, NCAM expression was remarkably enhanced by bFGF treatment. Muscle actin was not detected in any of these ACs with or without bFGF treatment, thus excluding mesoderm involvement during neural induction. These data demonstrate that (1) neurogenesis does not occur in response to bFGF unless BMP-4 signaling is blocked; and (2) bFGF enhances neurogenesis induced by DN-BR. Therefore, we postulate that dilution of BMP-4 may be responsible for neurogenesis in ACs cultured in LCMR medium in the presence of bFGF.
Fig. 2.
FGF synergizes with DN-BR in neural induction. DN-BR RNA at increasing doses (0, 0.01, 0.05, 0.1, and 0.5 ng/embryo) was injected into the animal pole area of the two-cell-stage embryos. ACs were dissected and conjugated at stage 8.5 and were cultured until the equivalent of stage 10.5 in L-15 medium. Then they were manually opened and exposed to medium with bFGF at 0.1 μg/ml (+) or to medium alone (−) and cultured until stage 24 (see Fig.1A). The treated ACs were harvested for RT-PCR analysis to detect expression of NCAM, actin, and EF-1α.
bFGF posteriorizes neural tissue
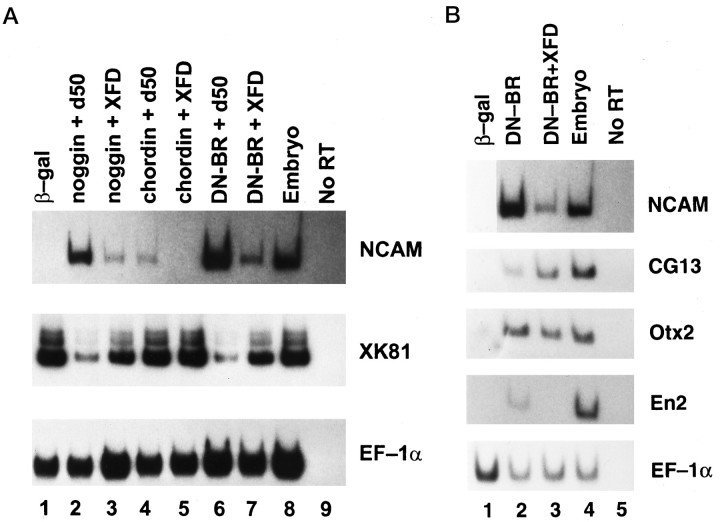
Next, we tested what kind of neural tissue was induced in AC treated with both DN-BR and bFGF, because DN-BR induces only the anterior type of neural tissue (Hawley et al., 1995; Xu et al., 1995). Injection of RNA encoding either DN-BR (1 ng) or chordin (1.5 ng) induced the anterior neural marker Otx2 (Blitz and Cho, 1995; Pannese et al., 1995) but not the hindbrain marker Krox20 (Bradley et al., 1993) nor the spinal cord marker HoxB9 (known previously as XlHbox6) (Wright et al., 1990) in AC cultured until stage 24 (Fig.3, lanes 2,4). However the injected AC, when treated with bFGF at 0.1 μg/ml starting at stage 10.5, expressed the posterior markers Krox20 and HoxB9 (Fig. 3, lanes 3,5). Again, muscle actin was not induced after the bFGF treatment (Fig. 3), nor was the early mesodermal marker Xbra when tested on AC treated with bFGF until stage 12 (data not shown), thus excluding the involvement of mesoderm induction. As a control, β-gal RNA-injected ACs expressed none of these markers (Fig. 3,lane 1). Based on these data, we propose that bFGF is able to posteriorize neural tissue induced by DN-BR or chordin. This is in agreement with the observation by Cox and Hemmati-Brivanlou (1995)that bFGF induces posterior neural fate in AC neuralized by a dominant negative activin receptor and follistatin. Both of these induce neural tissue of anterior type in ACs (Hemmati-Brivanlou and Melton, 1994;Hemmati-Brivanlou et al., 1994). However, the posteriorization they observed was on AC excised from embryos during gastrulation when neurogenesis had started. Therefore, the A-P axis in the prospective neuroectoderm might have been prepatterned before dissection, making it difficult to attribute posteriorization to bFGF alone. In contrast, we used ACs dissected from blastula stage embryos. They were conjugated and aged until the equivalent of gastrula stage and then opened with exposure to bFGF. Thus, posteriorization observed in this system can be directly credited to bFGF.
Fig. 3.
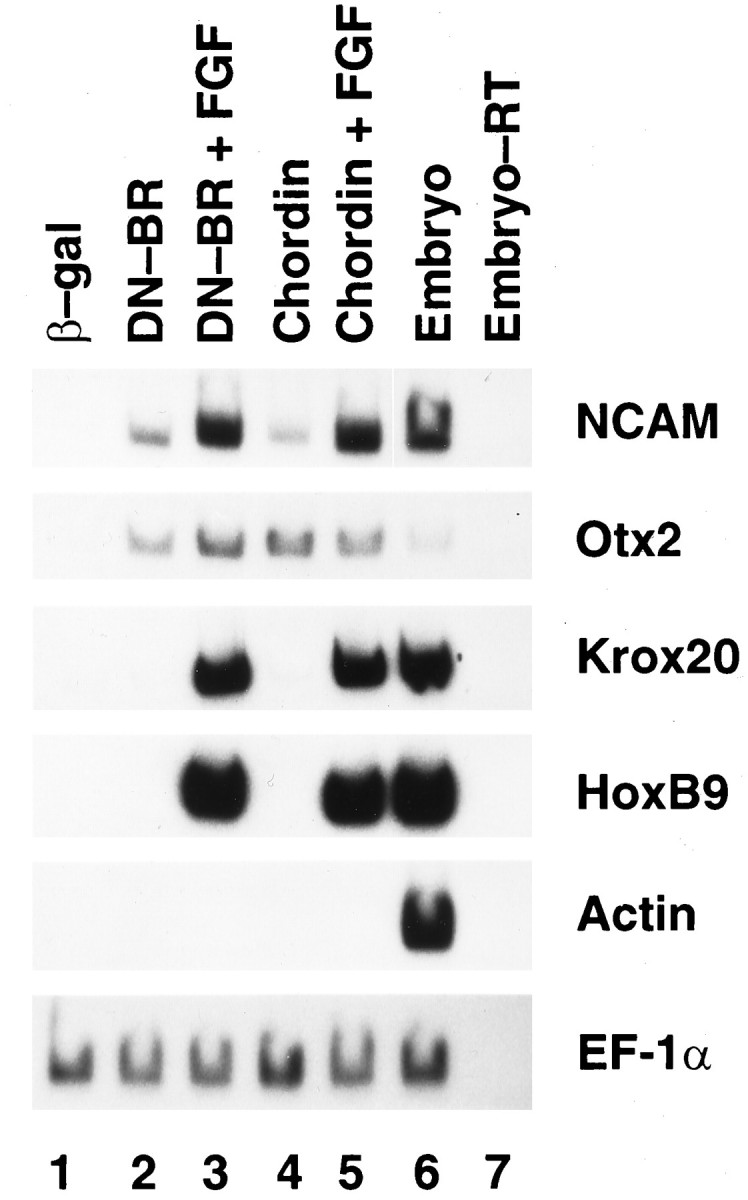
FGF posteriorizes anterior neural tissue induced by DN-BR or chordin. RNA encoding DN-BR (1 ng) or chordin (1.5 ng) was injected into the animal pole area of the two-cell-stage embryos. ACs were dissected, treated with FGF, and processed as described in Figure2. Expression of NCAM, Otx2,Krox20, HoxB9, actin, andEF-1α was tested by RT-PCR.
XFD inhibits neurogenesis
To elucidate the role of endogenous FGF in neurogenesis, we used XFD to block the FGF signaling in ACs expressing noggin, chordin, or DN-BR. Coinjection of XFD RNA downregulated NCAM expression induced by each of these neuralizing agents, whereas coinjection of d50, a defective FGF receptor mutant, failed to do this (Fig.4A). Injection with a mixture of RNAs encoding a wild-type FGF receptor, XFD, and DN-BR rescued the NCAM expression from the XFD inhibition (data not shown). All of these neural-inducing agents have been known to elicit neural tissue of an anterior type in AC cells (Lamb et al., 1993;Hemmati-Brivanlou et al., 1994; Hawley et al., 1995; Sasai et al., 1995; Xu et al., 1995). To address the fate of cells that were inhibited from neuralization by XFD, we tested for expression of the keratin marker gene XK81 in the above samples. As shown in Figure 4A, the neuralized explants expressed more NCAM and less XK81 whereas cotreatment with XFD reversed the ratio of NCAM to XK81. This suggests that XFD simply inhibits the neural fate and allows the cells to commit to the alternative epidermal fate, action similar to that of the neural inhibitor BMP-4 (Wilson and Hemmati-Brivanlou, 1995).
Fig. 4.
A, XFD inhibits neurogenesis while enhancing epidermalization in AC cells induced by noggin, chordin, and DN-BR. Various RNAs were injected into the animal pole area of the two-cell-stage embryos. ACs were dissected at stage 8.5 from the injected embryos and cultured until stage 24 before being harvested for RT-PCR analysis to detect expression of NCAM,XK81, and EF-1α. Lane 1, AC derived from embryo injected with RNA-encoding β-gal (3 ng);lane 2, noggin (0.5 ng) and d50 (2 ng); lane 3, noggin (0.5 ng) and XFD (2 ng);lane 4, chordin (1.5 ng) and d50 (2 ng); lane 5, chordin (1.5 ng) and XFD (2 ng); lane 6, DN-BR (1 ng) and d50 (2 ng); and lane 7, DN-BR (1 ng) and XFD (2 ng). B, XFD inhibits expression of midbrain–hindbrain boundary marker En2. Lane 1, ACs were dissected from embryos injected with RNA-encoding β-gal (3 ng); lane 2, DN-BR (1 ng); and lane 3, DN-BR (1 ng) and XFD (2 ng). ACs were cultured until stage 24, and expression of NCAM, CG13,Otx2, En2, and EF-1α was detected by RT-PCR.
Analysis of molecular markers in AC coexpressing DN-BR and XFD (Fig.4B) showed that expression of the most anterior ectodermal marker, CG13, which is expressed in cement gland and induced by neighboring neural or dorsal mesodermal tissue (Jamrich and Sato, 1989), was upregulated by XFD, whereas expression ofEn2, a marker of the midbrain–hindbrain boundary (Hemmati-Brivanlou et al., 1991), was totally inhibited by XFD. Similar results were observed also in AC coexpressing XFD and noggin or chordin (data not shown). However, XFD had no clear effect on Otx2expression (Fig. 4B), which is contradictory to the report that XFD totally inhibits Otx2 expression in neuralized AC (Launay et al., 1996). This difference may be explained as follows. According to the two-step model proposed for neurogenesis by Nieuwkoop et al. (1952) and Saxén and Toivonen (1961), the CNS development may include an activation (neural induction) step and a transformation (posteriorization) step. Low FGF may cooperate with the activation step (then higher XFD is required to block it), whereas higher FGF may mimic the transformation signal (then lower XFD is required to block it). Therefore, various amounts of XFD protein derived from the RNA injection may result in differential effects on neural induction and posteriorization. We might have used a low dose of XFD that only inhibited the posteriorization step, thus leaving Otx2 expression less affected. Nevertheless, both the gain-of-function (Fig.3) and loss-of-function (Fig. 4) experiments agree on the posteriorizing role of FGF in AC neurogenesis.
FGF signaling is required for posteriorization of the CNS in embryo
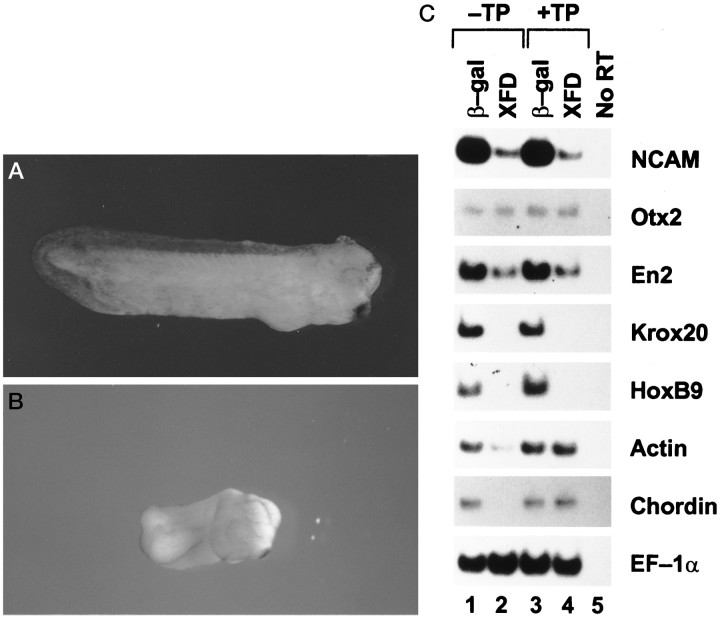
To advance our understanding of the role of FGF in neurogenesis that was obtained from the above explant systems to a living embryo, we used an AC transplantation system. Tissues of the dorsal ectoderm, which is the prospective neuroectoderm (from 30° dorsal of the intermediate vertical line of the animal pole to just above the dorsal lip), were removed from stage 10 embryos. The vacant area in the embryos was covered immediately with the AC expressing XFD or β-gal. The grafted embryos were allowed to develop to stage 30. This system avoided the influence of XFD on the dorsal mesoderm (the source of neural inducers in vivo). Some injected embryos were not dissected but were allowed to develop as nontransplantation controls. The embryos grafted with β-gal-expressing ACs (Fig.5A) developed in a similar manner to β-gal RNA-injected embryos (data not shown). The XFD-AC-grafted embryos completed gastrulation normally with a closed blastopore at neurula stage, whereas the XFD-injected embryos failed to complete gastrulation with an open blastopore (data not shown) as described by Amaya et al. (1991). At tail bud stage, the XFD-AC-grafted embryos lacked the posterior trunk (Fig. 5B), similar to XFD-injected embryos (Amaya et al., 1991). This suggests that XFD selectively expressed in the transplanted neuroectoderm also leads to posterior body truncation similar to that of XFD expressed ectopically. However, analysis of molecular markers suggested that muscle tissue still developed in the XFD-AC-grafted embryos. As shown in Figure5C, expression of NCAM and posterior neural makersKrox20 and HoxB9 (but not anterior neural markersOtx2 and En2) was attenuated by XFD in both AC-grafted and nongrafted embryos, whereas expression of the dorsal marker muscle actin (Stutz and Spohr, 1986) and the posterior mesodermal marker chordin that is expressed exclusively in the posterior notochord and tailbud hinge (Sasai et al., 1994) was inhibited only in XFD-injected embryos but not in XFD-AC-grafted embryos. This implies that a graft of the XFD AC did not affect dorsal and posterior mesoderm development in the embryo, and posterior body axis truncation only resulted from the inhibition of posterior neural tissue by XFD. This model, therefore, may best reflect the physiological role of FGF in the neuroectoderm: posteriorization of the CNS.
Fig. 5.
XFD inhibits posterior neural tissue in vivo. Two animal blastomeres of donor embryos were injected with 2 ng of XFD or control β-gal RNA at the two-cell stage. ACs were dissected from the injected embryos at stage 8 and transplanted to the site of neuroectoderm in recipient stage 10 embryos (+TP), which were allowed to develop to stage 30. Some XFD or β-gal RNA-injected embryos were not dissected and were allowed to develop to stage 30 as nontransplantation controls (−TP). Then, all embryos were harvested for photography (A, B) or RT-PCR analysis to detect expression of NCAM, Otx2, En2,Krox20, HoxB9, actin,chordin, and EF-1α(C). A, β-Gal and +TP;B, XFD and +TP. Photographs for XFD and −TP and for β-gal and −TP are not shown.
DISCUSSION
FGF is required for neural induction and posteriorization
To date, all known neural-inducing agents, including DN-BR, noggin, follistatin, and chordin, only induce an anterior type of neural tissue in ectodermal explants (Lamb et al., 1993;Hemmati-Brivanlou et al., 1994; Hawley et al., 1995; Sasai et al., 1995; Xu et al., 1995). The mechanism by which the posterior neural tissue is formed remains primarily unknown. Although bFGF does not induce neural tissue in AC cells, blockage of FGF signaling by XFD attenuated neurogenesis induced by noggin and DN-BR (Fig.4A). But we failed to see inhibition of expression of anterior neural marker Otx2 by XFD (Fig.4B), a result different from that reported by Launay et al. (1996). A likely explanation, as suggested above, attributes this controversy to different XFD doses injected by different investigators. FGF signaling may generally be required for neural induction as well as for posteriorization. Theoretically, cells of an anterior neural type if not posteriorized should remain in the anterior type. However, expression of the epidermal marker XK81 but not the anterior neural marker Otx2 was enhanced by XFD at the expense of the posterior neural tissue (Figs. 4A,5C), suggesting that FGF-posteriorized cells can be derived from a different group of cells rather than directly from the anterior cells; they are epidermalized if not instructed otherwise.
Additional evidence of the posteriorizing activity of FGF comes from the findings by Taira et al. (1997) that the embryonic FGF-inducible gene Xbra (Smith et al., 1991; Isaacs et al., 1994) can posteriorize the anterior neural tissue induced by the activated form of the organizer homeobox gene Xlim-1 (Taira et al., 1994). Thus, although FGF alone does not initiate neurogenesis, it may work in concert with neural inducers to induce posterior neural tissue. Factors with this posteriorizing activity have long been sought since the proposition of the two-step neural-patterning model (Nieuwkoop et al., 1952; Saxén and Toivonen, 1961), for which FGF has been suggested as a top candidate (Doniach, 1995; Sasai and De Robertis, 1997). However, opposite results negating the role of FGF in neural development have been reported by Kroll and Amaya (1996); they found that transgenic embryos with XFD contain well patterned nervous systems despite the severe defect of mesoderm development. This controversy may be (1) because the XFD expression by a transgene may not be sufficient to inhibit neuralization; or (2) because of different sensitivities to XFD between neuralization and mesodermalization (the latter may be more sensitive to XFD).
FGF posteriorizes neural tissue independent of mesoderm
FGF has been found to signal through proto-oncogenes Ras, Raf-1, MAPK, and AP-1 and to be responsible for the formation of the posterior mesoderm (Whitman and Melton, 1992; MacNicol et al., 1993; Isaacs et al., 1994; LaBonne et al., 1995; Umbhauer et al., 1995; Dong et al., 1996). It has been shown that posterior dorsal mesoderm has caudalizing activity on prospective anterior neural tissue (Cox and Hemmati-Brivanlou, 1995). Therefore, demonstration of whether FGF caudalizes the anterior neural tissue requires a mesoderm-free system.
By using the conjugated and aged AC explants, we have shown that XFD can inhibit expression of the midbrain–hindbrain boundary markerEn2 (Fig. 4B). In the in vivosystem, XFD selectively expressed in ACs grafted in place of neuroectoderm also inhibited expression of En2 as well as of hindbrain marker Krox20 and spinal cord markerHoxB9, leaving mesodermal development unaffected (Fig. 5). On the other hand, addition of bFGF into cultures of stage 10.5 ACs expressing DN-BR or chordin induced expression of Krox20 andHoxB9 but not of the dorsal mesodermal marker muscleactin (Fig. 3). These results suggest that FGF acts directly on neural tissue to promote posteriorization.
New models used in these studies
As mentioned above, we used conjugated blastula stage ACs and cultured them to the gastrula stage before exposing them to bFGF to reduce the possibility of BMP-4 diffusion from the explants. The use of this system is therefore a more direct approach to addressing the FGF effect than is the use of slow-healing AC explants or dispersed AC cells. More importantly, we extended our study from the in vitro system to an in vivo system by using a novel AC transplantation system, which seemed to be physiologically significant in elucidating the role of endogenous FGF signaling.
There are only a few reported embryonic systems used for the study of neural development. The AC explant culture and dispersed AC cell culture are two frequently used in vitro systems. However, results derived from these model systems are often not very conclusive. It is difficult to address the physiological significance of the results because of the existence of complex interactions between the neuroectoderm and its neighboring tissues, such as dorsal mesoderm, ventral ectoderm, ventral mesoderm, and endoderm. In these in vitro systems, not only are all of these interactions ignored, but the vertical and planar signals present within the neural tissue are also aberrant (in AC explants) or absent (in dissociated AC cells). Therefore, the need for establishment of an in vivoembryonic system to study neurogenesis has become important.
One major concern for establishing an in vivo system is to exclude the influence of tested agents on the development of dorsal mesoderm, because defect of the dorsal mesoderm can also lead to abnormality of neural induction and patterning. One way is injection of a single target cell in 32-cell-stage embryos to specifically express a gene of interest in a neural progenitor cell such as A1. However, not all A1 daughter cells will differentiate to neurons; some, although a small fraction, will participate in the formation of dorsal mesodermal tissues (Dale and Slack, 1987; Moody, 1987). Therefore, the involvement of the dorsal mesoderm cannot be completely eliminated. Another classic method for in vivo studies is implantation of ectodermal folds into the dorsal ectoderm of gastrula embryos in which the folds are induced to form neural tissue and can be assayed for their A-P properties (Nieuwkoop et al., 1952). However, there is not full contact between the implanted ectoderm explant (AC) and the dorsal mesoderm; therefore, it is hard to mimic all functions of neural-inducing signals traveling between the tissues.
In our studies, ACs were transplanted flatly to the site of the neuroectoderm, coming into full contact with the involuting dorsal mesoderm, and thus should best reflect the physiological process. This system also avoided the influence of XFD on the dorsal mesoderm, providing a more specific and direct approach to understanding the role of FGF in neural differentiation. Therefore, with this in vivo system, as well as with the conjugated and aged AC system, our paper demonstrates advances in techniques for studyingXenopus neurogenesis in addition to the role of FGF in neurogenesis.
Footnotes
This work was partly supported by the Shiffman Program for Clinical and Basic Research between Bar Ilan University of Israel and the National Cancer Institute of the United States. We thank Dr. R. M. Harland for the noggin cDNA and protein and Drs. Y. Sasai and E. M. De Robertis for the chordin cDNA. We also thank Dr. D. L. Newton for synthesis of the oligonucleotides used in the RT-PCR experiments and Dr. Magaret Beckwith and Mrs. Annie Rogers for editing this manuscript.
Correspondence should be addressed to Dr. Hsiang-fu Kung, Laboratory of Biochemical Physiology, Building 567, Room 152, Frederick, MD 21702-1201.
REFERENCES
- 1.Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in the Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 2.Blitz IL, Cho KWY. Anterior neuroectoderm is progressively induced during gastrulation: the role of the Xenopus homeobox gene orthodenticle. Development. 1995;121:993–1004. doi: 10.1242/dev.121.4.993. [DOI] [PubMed] [Google Scholar]
- 3.Bradley L, Snape A, Bhatt S, Wilkinson D. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech Dev. 1993;40:73–84.7. doi: 10.1016/0925-4773(93)90089-g. [DOI] [PubMed] [Google Scholar]
- 4.Cox WG, Hemmati-Brivanlou A. Caudalization of neural fate by tissue recombination and bFGF. Development. 1995;121:4349–4358. doi: 10.1242/dev.121.12.4349. [DOI] [PubMed] [Google Scholar]
- 5.Dale L, Slack JMW. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- 6.Dong Z, Xu R-H, Kim J, Zhan S-N, Ma W-Y, Colburn NH, Kung HF. AP-1/Jun is required for early Xenopus development and mediates mesoderm induction by fibroblast growth factor but not by activin. J Biol Chem. 1996;271:9942–9946. doi: 10.1074/jbc.271.17.9942. [DOI] [PubMed] [Google Scholar]
- 7.Doniach T. Basic FGF as an inducer of anteroposterior neural pattern. Cell. 1995;83:1067–1070. doi: 10.1016/0092-8674(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 8.Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 9.Hawley SHB, Winnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KWY. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- 10.Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 11.Hemmati-Brivanlou A, de la Torre JR, Holt C, Harland RM. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991;111:715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- 12.Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs HV, Tannahill D, Slack JMW. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development. 1992;114:711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs HV, Pownall ME, Slack JMW. eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J. 1994;13:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamrich M, Sato S. Differential gene expression in the anterior neural plate during gastrulation of Xenopus laevis. Development. 1989;105:779–786. doi: 10.1242/dev.105.4.779. [DOI] [PubMed] [Google Scholar]
- 16.Kengaku M, Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993;119:1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- 17.Kengaku M, Okamoto H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development. 1995;121:3121–3130. doi: 10.1242/dev.121.9.3121. [DOI] [PubMed] [Google Scholar]
- 18.Kintner CR, Melton DA. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development. 1987;99:311–325. doi: 10.1242/dev.99.3.311. [DOI] [PubMed] [Google Scholar]
- 19.Kroll K, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 20.LaBonne C, Burke B, Whitman M. Role of MAP kinase in mesoderm induction and axial patterning during Xenopus development. Development. 1995;121:1475–1486. doi: 10.1242/dev.121.5.1475. [DOI] [PubMed] [Google Scholar]
- 21.Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior–posterior pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- 22.Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- 23.Launay C, Fromentoux V, Shi D-L, Boucaut J-C. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- 24.MacNicol AM, Muslin AJ, Williams LT. Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell. 1993;73:571–583. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- 25.Maeno M, Ong RC, Suzuki A, Ueno N, Kung HF. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci USA. 1994;91:10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin). North-Holland; Amsterdam: 1967. [Google Scholar]
- 28.Nieuwkoop PD, Bloemsma FFSN, Boterenbrood EC, Hoessels ELMJ, Kremer A, Meyer G, Verheyen FJ. Activation and organization of the central nervous system in amphibians. J Exp Zool. 1952;120:83–108. [Google Scholar]
- 29.Ott AP, Moon RT. Protein kinase C isozymes have distinct roles in neural induction and competence in Xenopus. Cell. 1992;68:1021–1029. doi: 10.1016/0092-8674(92)90074-m. [DOI] [PubMed] [Google Scholar]
- 30.Pannese M, Polo C, Andreazzoli M, Vignali R, Kablar B, Barsacchi G, Boncinelli E. The Xenopus homologue of Otx-2 is a maternal homeobox gene that demarcates and specifies anterior body regions. Development. 1995;121:707–720. doi: 10.1242/dev.121.3.707. [DOI] [PubMed] [Google Scholar]
- 31.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- 33.Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- 35.Saxén L, Toivonen S. The two-gradient hypothesis in primary induction. The combined effect of two types of inductors mixed in different ratios. J Embryol Exp Morphol. 1961;9:514–533. [PubMed] [Google Scholar]
- 36.Sharpe CR, Fritz A, De Robertis EM, Gurdon JR. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987;50:749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- 37.Smith JC, Price BMJ, Green JBA, Weigel D, Hermann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- 38.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryo. Cell. 1991;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 39.Stutz F, Spohr G. Isolation and characterization of sarcomic actin genes in Xenopus laevis embryogenesis. J Mol Biol. 1986;187:349–361. doi: 10.1016/0022-2836(86)90438-9. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal–ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci USA. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taira M, Otani H, Saint-Jeannet J-P, Dawid IB. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature. 1994;372:677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- 42.Taira M, Saint-Jeannet J-P, Dawid IB. Role of the Xlim-1 and Xbra genes in anteroposterior patterning of neural tissue by the head and trunk organizer. Proc Natl Acad Sci USA. 1997;94:895–900. doi: 10.1073/pnas.94.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature. 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- 44.Whitman M, Melton DA. Involvement of p21ras in Xenopus mesoderm induction. Nature. 1992;357:252–254. doi: 10.1038/357252a0. [DOI] [PubMed] [Google Scholar]
- 45.Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by BMP-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 46.Wright CV, Morita EA, Wilkin DJ, De Robertis EM. The Xenopus XlHbox6 homeo protein, a marker of posterior neural induction, is expressed in proliferating neurons. Development. 1990;109:225–234. doi: 10.1242/dev.109.1.225. [DOI] [PubMed] [Google Scholar]
- 47.Xu R-H, Kim J, Taira M, Zhan S, Sredni D, Kung HF. A dominant negative bone morphogenetic protein 4 receptor causes neurolization in Xenopus ectoderm. Biochem Biophys Res Commun. 1995;212:212–219. doi: 10.1006/bbrc.1995.1958. [DOI] [PubMed] [Google Scholar]
- 48.Xu R-H, Dong Z, Maeno M, Kim J, Suzuki A, Ueno N, Sredni D, Colburn NH, Kung HF. Involvement of Ras/Raf/AP-1 in BMP-4 signaling during Xenopus embryonic development. Proc Natl Acad Sci USA. 1996;93:834–838. doi: 10.1073/pnas.93.2.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu R-H, Kim J, Taira M, Lin J-J, Zhang C-h, Sredni D, Evans T, Kung HF. Differential regulation of neurogenesis by the two GATA-1 genes. Mol Cell Biol. 1997;17:436–443. doi: 10.1128/mcb.17.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;66:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]