Abstract
Protein tyrosine phosphorylation is a key event in diverse intracellular signaling pathways and has been implicated in modification of neuronal functioning. We investigated the role of tyrosine phosphorylation in regulating type A GABA (GABAA) receptors in cultured CNS neurons. Extracellular application of genistein (50 μm), a membrane-permeable inhibitor of protein tyrosine kinases (PTKs), produced a reversible reduction in the amplitude of GABAAreceptor-mediated whole-cell currents, and this effect was not reproduced by daidzein (50 μm), an inactive analog of genistein. In contrast, intracellular application of the PTK pp60c-src (30 U/ml) resulted in a progressive increase in current amplitude, and this potentiation was prevented by pretreatment of the neurons with genistein. Immunoprecipitation and immunoblotting of cultured neuronal homogenates indicated that the β2/β3 subunit(s) of the GABAA receptor are tyrosine phosphorylated in situ. Moreover, genistein (50 μm) was found to be capable of decreasing GABAA currents in human embryonic kidney 293 cells transiently expressing functional GABAA receptors containing the β2 subunit. Thus, the present work provides the first evidence that native GABAA receptors are phosphorylated and modulated in situ by endogenous PTKs in cultured CNS neurons and that phosphorylation of the β subunits may be sufficient to support such a modulation. Given the prominent role of GABAA receptors in mediating many brain functions and dysfunctions, modulation of these receptors by PTKs may be important in a wide range of physiological and pathological processes in the CNS.
Keywords: GABAA receptor, protein tyrosine phosphorylation, protein tyrosine kinase, cultured neurons, recombinant GABAA receptor, HEK 293 cell
Protein tyrosine phosphorylation is considered a key biochemical event in numerous cellular processes, including proliferation, growth, and differentiation. In addition, it has also been implicated in modification of neuronal functions in physiological processes such as synaptogenesis (Catarsi and Drapeau, 1993) and long-term potentiation (Terlau and Seifert, 1989; O’Dell et al., 1991) and in pathological conditions such as ischemia (Kindy, 1993; Yokota et al., 1994) and epilepsy (Stratton et al., 1991). The mechanisms by which protein tyrosine phosphorylation affects neuronal functioning in the mammalian CNS remain unclear, but they may involve the modulation of both voltage and ligand-gated channel function (Raymond et al., 1993; Levitan, 1994; Wang and Salter, 1994; Chen and Leonard, 1996;Holmes et al., 1996; Wang et al., 1996).
GABA is the principal inhibitory neurotransmitter in the CNS, and it binds to three distinct receptor subtypes: GABAA, GABAB, and GABAC. Because they open bicuculline-sensitive Cl− channels, GABAA receptors are responsible for most of the fast inhibitory synaptic transmission in the brain (Sivilotti and Nistri, 1991; Mody et al., 1994). Structurally, GABAA receptors are presumably heteropentameric structures and are assembled by combining homologous subunits. Molecular cloning has thus far revealed a multiplicity of different GABAA receptor subunits divided into five different classes: α (1–6), β (1–4), γ (1–3), δ, and ρ (1–2) (Macdonald and Olsen, 1994; Smith and Olsen, 1995). The precise subunit composition and stoichiometry of native GABAA receptors are currently unknown, but the most abundant population of native GABAA receptors in the mammalian brain is believed to be the α1β2γ2 subunit combination (Benke et al., 1991; McKernan and Whiting, 1996). Each of the GABAA receptor subunits is a 40–60 kDa polypeptide containing four transmembrane regions. The putative intracellular domain between the third and fourth membrane-spanning regions contains numerous potential consensus sites for protein phosphorylation by various protein kinases (Macdonald and Olsen, 1994; McKernan and Whiting, 1996), suggesting that these receptors may be phosphorylated and modulated by protein kinases. Thus, modulation of GABAAreceptors by protein phosphorylation has been a major focus of recent studies.
In common with studies of modulation by protein phosphorylation of other ligand-gated channels, most of the previous investigations have focused on modulation of GABAA receptors by serine/threonine-specific phosphorylation (Browning et al., 1993;Raymond et al., 1993; Levitan, 1994). In contrast, modulation of GABAA receptors by tyrosine-specific phosphorylation has been studied only recently. The function of GABAA receptors has been shown to be subject to modulation by factors affecting protein tyrosine phosphorylation in mouse brain membrane vesicles (Valenzuela et al., 1995) and in cultured sympathetic neurons (Moss et al., 1995), suggesting that GABAA receptors are dynamically regulated by a balance between activities of endogenous protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). There has been no evidence, however, for in situ tyrosine phosphorylation of any subunit of native GABAA receptors by endogenous PTKs, and consequently the molecular substrate(s) for functional modulation of the receptor by protein tyrosine phosphorylation in the CNS remains unknown.
Given the prominent role of GABAA receptors in brain functions and dysfunctions and the ubiquitous signaling pathways using PTKs and PTPs in the brain, in the present study we set out to examine whether the native GABAA receptor expressed in CNS neurons is functionally modulated and phosphorylated by endogenous PTKs, and if so, to determine which subunit(s) is the most likely substrate.
Parts of this paper have been published previously in abstract form (Wang and Wang, 1995; Wan et al., 1996).
MATERIALS AND METHODS
Preparation of neuronal cultures. Methods for preparing cultures from embryonic rat spinal dorsal horn have been described in detail (Salter and Hicks, 1994). For primary cultures of dorsal medulla neurons, fetal Wistar rats (embryonic day 17–19) were decapitated, and their brainstems were removed surgically under a dissection microscope. The dorsal part of the medulla containing the solitary complex was dissected out using block dissection (Yu, 1989). The tissue was treated with trypsin and triturated using a Pasteur pipette. The cells were then plated onto collagen-coated 25 mm glass coverslips and set into a standard 35 mm culture dish. The cultures were maintained in minimum essential medium supplemented with 10% fetal bovine serum, 10% heat-inactivated horse serum, and 1 U/ml insulin. Cells were used for recording 1–3 weeks after plating.
Plasmids and transient transections. All of the GABAA receptor subunit cDNAs were gifts of Drs. C. Kaufman and D. Gunnersen (Laboratory of Neuroscience, National Institute of Diabetes and Digestive and Kidney Diseases). CMVα1 containing the rat α1 cDNA was cloned into the expression vector pRc/CMV (Invitrogen, San Diego, CA); CMVβ2, the rat β2 subunit cDNA, was cloned into pcDNAI (Invitrogen); and CMVγ2, the short form of the rat γ2 subunit, was cloned into pcDNA3 (Invitrogen). To facilitate identification of the transfected cells for electrophysiological recordings, a cDNA encoding the jellyfish green fluorescent protein (GFP) inserted into pcDNA3 (Marshall et al., 1995) (a gift from Drs. J. R. Howe and T. E. Hughes, Yale University) was used as an expression marker and cotransfected with GABAA receptor subunit cDNAs. Human embryonic kidney (HEK) 293 cells were plated onto collagen-coated 22 mm glass coverslips set in a standard 35 mm culture dish and maintained in minimum essential medium α (αMEM) supplemented with 10% fetal calf serum (Life Technologies, Gaithersburg, MD). Plasmid transfections were performed using Lipofectamine (Life Technologies) according to the protocol provided by the manufacturer. Each 35 mm dish of HEK 293 cells was transfected with 1 μg of each GABAAreceptor subunit plasmid plus 0.5 μg of GFP plasmids and 5–10 μl of Lipofectamine for 4–5 hr at 37°C in Opti-MEM (Life Technologies). Dishes were then maintained in regular culture media. Recordings were performed 30–48 hr after transfection.
Electrophysiological recordings. For electrophysiological recordings, coverslips containing cultured neurons or HEK 293 cells were transferred into a glass-bottomed chamber and visualized under differential interference contrast and epifluorescent video microscopy. Cells were bathed in an extracellular recording solution composed of (in mm): NaCl 140, KCl 5.4, HEPES 25, CaCl21.3, glucose 33, and tetrodotoxin 0.001, pH 7.35; osmolarity, 310–320 mOsm. Recordings were made with pipettes (resistance 2–5 MΩ) filled with intracellular solution that contained (in mm): CsCl 140, HEPES 10, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid 10, pH 7.25; osmolarity, 300–315 mOsm. Na2-ATP (4 mm) and MgCl2 (2 mm) were included in the intracellular recording solution to support the process of protein phosphorylation, thereby preventing current rundown during a prolonged period of whole-cell recording (Chen et al., 1990). Currents were recorded under standard whole-cell voltage-clamp configuration using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). GABAA receptors were activated by pressure ejection of GABA (100 μm, in extracellular recording solution) at 1 min intervals from a micropipette with its tip located 20–50 μm from the cell. The holding potential of the patch was −60 mV, unless indicated otherwise. Current recordings were sampled onto an IBM-PC compatible computer by using pClamp software (pClamp6, Axon Instruments).
Immunoprecipitation and immunoblotting. After a 10 min incubation in either extracellular recording solution or extracellular solution supplemented with 100 μm genistein, cultured neurons were homogenized in 10 mm sodium phosphate buffer containing 5 mm EDTA, 5 mm EGTA, 50 mm sodium fluoride, 50 mm sodium chloride, 1 mm orthovanadate, 5 mm sodium pyrophosphate, 0.1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg pepstatin, 0.1 mg aprotinin, 2% Triton X-100, and 0.5% SDS and boiled for 5 min. GABAA receptor subunits were isolated by immunoprecipitating the homogenate (containing 200 μg of protein) with 10 μg of mouse monoclonal antibody (bd-17; Boehringer Mannheim Biochem, Mannheim, Germany) immobilized on protein G-Sepharose beads (Sigma, St. Louis, MO). The bd-17 antibody recognizes both β2 and β3 subunits of the rat GABAA receptor (Ewert et al., 1990; Benke et al., 1991). Tyrosine-phosphorylated proteins were isolated by immunoprecipitating homogenate (containing 200 μg protein) with 1 μl polyclonal rabbit antiphosphotyrosine antibody (Transduction Laboratories, Lexington, KY) immobilized on protein A-Sepharose beads (Sigma). For Western blotting, whole homogenates (50 μg/lane) or products of the immunoprecipitation were separated on 10% SDS-PAGE mini gels and transferred to nitrocellulose membrane. Membranes were then probed with either mouse monoclonal anti-β2/β3 antibody (15 μg/ml) or rabbit polyclonal anti-phosphotyrosine antibody (1:200; Upstate Biotech, Lake Placid, NY) followed by a horseradish peroxidase-conjugated secondary antibody (Amersham Life Science, Buckinghamshire, UK). Protein-antibody complex was then visualized with enhanced chemiluminescence reagents (Amersham, Arlington Heights, IL).
Statistical analysis. All values are shown as the mean ± SE. Statistical analysis was performed using Student’s ttest with significance defined as p < 0.05.
RESULTS
Modulation of GABAA receptor-mediated currents by endogenous PTK activity
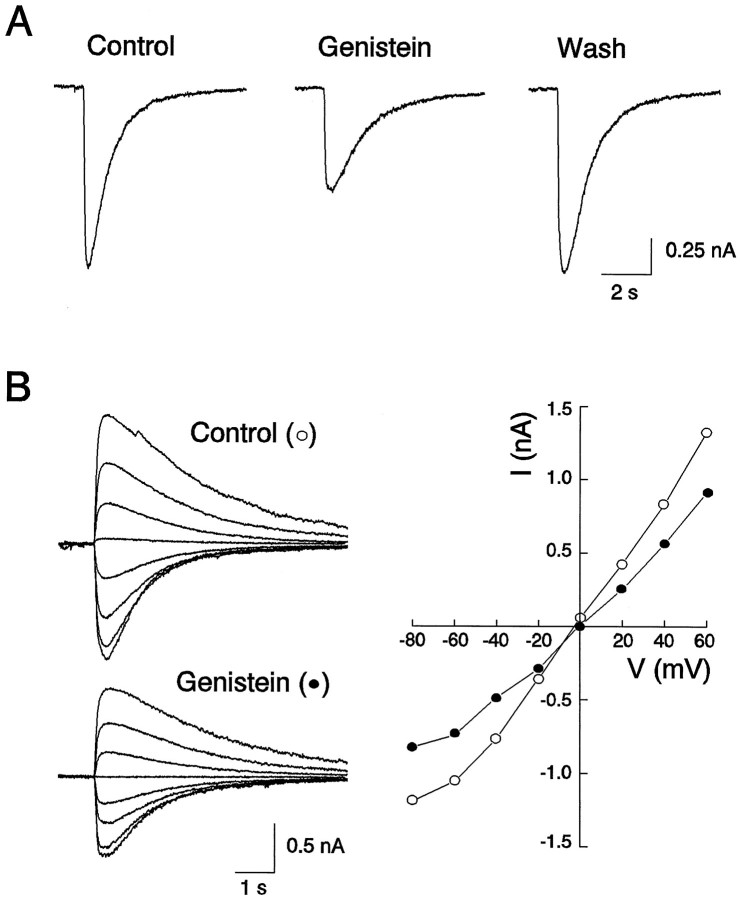
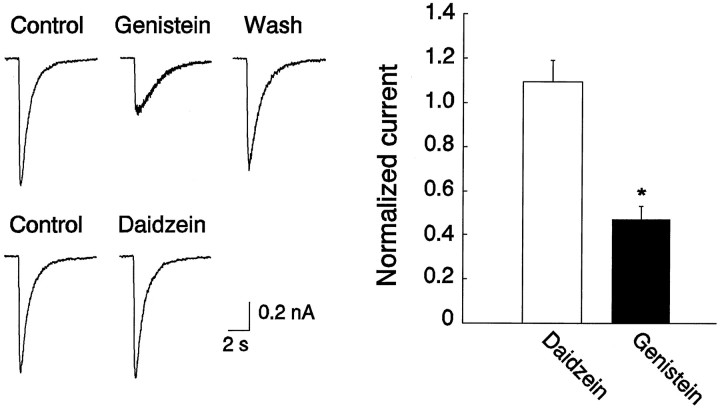
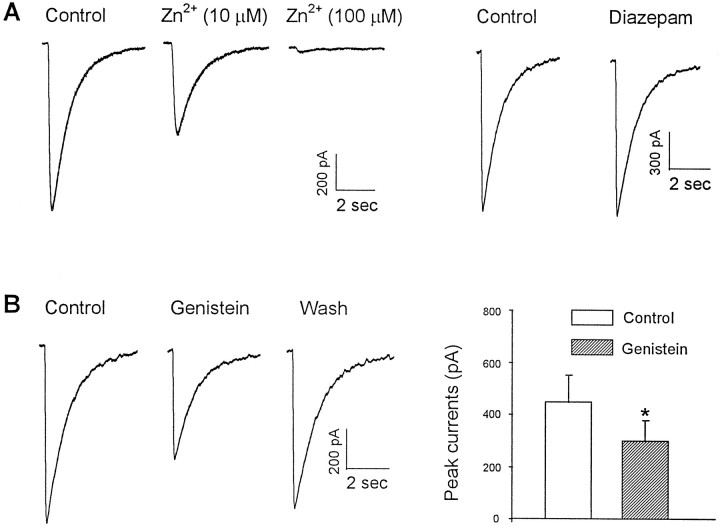
To investigate the role of protein tyrosine phosphorylation in regulating the function of GABAA receptors in the CNS, our initial experiments focused on endogenous PTKs and were performed using cultured spinal dorsal horn neurons. As shown in Figure1, pressure ejection of GABA (100 μm) produced an inward current response at a holding membrane potential of −60 mV. The currents had a reversal potential of ∼0 mV, with a slightly inward rectified current–voltage (I–V) relationship within the range of holding membrane potential from −100 to +60 mV (Fig. 1) and were blocked by bath application of GABAA receptor antagonist bicuculline (20 μm; data not shown), consistent with GABAA receptor mediation of these currents (Macdonald and Olsen, 1994). Extracellular application of genistein (50 μm), a membrane-permeable inhibitor of PTKs (Akiyama et al., 1987; O’Dell et al., 1991), produced a reversible reduction in the amplitude of the GABAA currents without altering theI–V curve or the reversal potential, suggesting that the reduction of the current by genistein is attributable to a change in channel conductance rather than an alteration of driving force. On average, currents were reduced to 0.44 ± 0.05 times control within 5 min after the drug application (n = 9). We next investigated the specificity of genistein as a PTK inhibitor by examining the effect of daidzein, an inactive analog of genistein (Akiyama et al., 1987; Wang and Salter, 1994), on the GABA currents. As shown in Figure 2, in contrast to genistein, bath perfusion of the same cells with daidzein (50 μm) produced no change in the amplitude of the currents (1.09 ± 0.11;n = 4). To determine whether the modulation is unique to GABAA receptors in dorsal horn neurons, we also investigated effects of genistein on GABA currents in cultured dorsal medulla neurons. Bath application of genistein (50 μm) reduced the amplitude of currents by 0.45 and 0.51% of control, respectively, in two neurons tested.
Fig. 1.
Genistein, an inhibitor of PTKs, suppresses GABAA receptor-mediated currents in a cultured spinal dorsal horn neuron. A, Genistein (100 μm) applied in the bath medium inhibited currents evoked by pressure application of GABA (100 μm) from a micropipette whose tip was positioned within 50 μm of the neuron. Currents were recorded under whole-cell configuration at a holding membrane potential of −60 mV in all figures, unless specifically indicated otherwise. B, Genistein reduced the slope conductance but not the reversal potential of the GABA currents in the same neuron. On the left are individual current traces evoked at membrane potentials from −80 to +60 with a step of 20 mV in the absence (○) and presence (•) of genistein (100 μm) in the bath medium. On the right are theI–V curves constructed from recordings shown on theleft.
Fig. 2.
Genistein produced a reduction in the amplitude of GABA currents by inhibiting activity of PTKs. Daidzein, an inactive analog of genistein, does not mimic the effect of genistein on GABAA currents. The left panel shows representative GABAA current traces obtained from the same neuron in the presence or absence of genistein (100 μm) and daidzein (100 μm), respectively. Thegraph on the right shows averaged currents from four spinal dorsal horn neurons for each treatment. Currents were normalized by taking currents in the presence of the respective drug over control.
Modulation of GABAA receptor-mediated currents by activity of an exogenous PTK
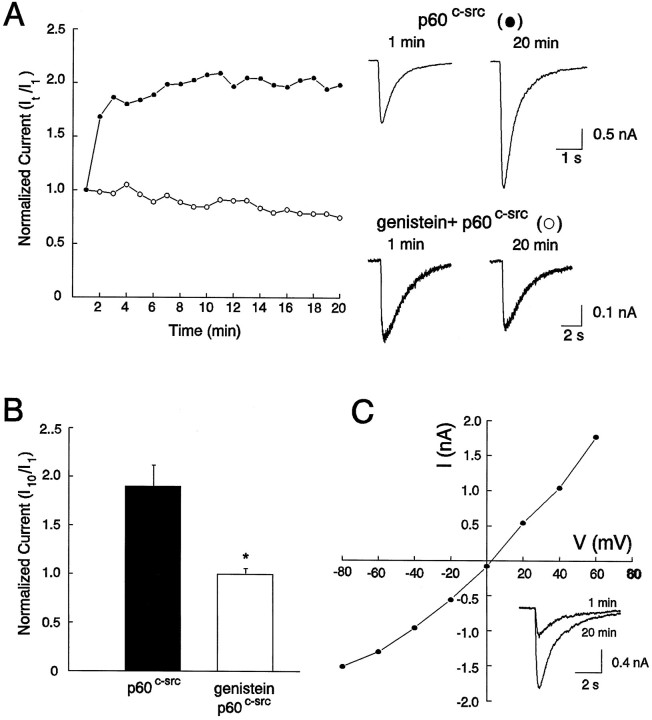
To examine the effect of exogenous PTK on the function of GABAA receptors, we applied the cytosolic PTK pp60c-src (30 U/ml) directly into cultured spinal dorsal horn neurons via the recording pipette (Wang and Salter, 1994). This resulted in a progressive increase in the current amplitude, which reached a steady level within 5–10 min. On average, the current amplitude increased to 1.86 ± 0.23 times the initial level after 10 min (n = 4) (Fig. 3A,B). As with genistein, pp60c-src did not affect theI–V relationship or the reversal potential (Fig.3C). To confirm that the effect of pp60c-src is caused by its tyrosine kinase activity, we then applied this enzyme to neurons that had been incubated with genistein (50 μm) for 10 min. In all cases, pretreatment with genistein prevented the potentiation of GABAA current by pp60c-src (Fig. 3B) (1.01 ± 0.06; n = 4). GABA-activated currents were also found to be potentiated by pp60c-src in two cultured dorsal medulla neurons tested.
Fig. 3.
Recombinant pp60c-srcpotentiated GABA currents in cultured neurons. A, Intracellular perfusion of the PTK pp60c-src (30 U/ml, •) potentiated GABA currents in control neurons but not in neurons that had been treated with genistein (100 μm, ○) 10 min before the start of whole-cell recordings.B, Graph of normalized currents recorded in the presence of pp60c-src alone (n = 4) and pp60c-src plus genistein (n = 4). Currents were normalized by taking currents recorded at 10 min (I10) over those recorded at 1 min (I1) after the start of whole-cell recordings. C, I–V relationship constructed from individual currents evoked at holding membrane potentials from −80 to +60 after the potentiation by pp60c-src has been established.
Tyrosine phosphorylation of GABAA receptor subtypes in cultured neurons in situ
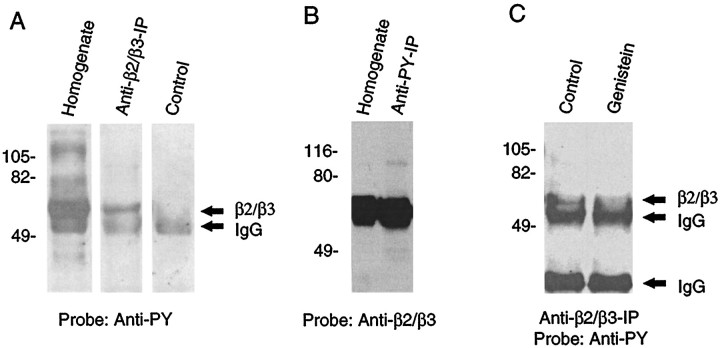
Because potential tyrosine phosphorylation sites are present in the major intracellular domains of GABAA subunits and some of these sites can be phosphorylated in vitro (Valenzuela et al., 1995), the functional modulation of GABAA receptors may be a result of direct phosphorylation and dephosphorylation of the GABAA receptor subunits. To test this hypothesis, whole homogenates of the cultured spinal dorsal horn neurons were immunoprecipitated with monoclonal antibody recognizing β2/β3 subunits of the rat GABAA receptors, the most common subunits of native CNS GABAA receptors (Benke et al., 1991;Fritschy et al., 1992). Proteins were resolved on SDS-PAGE and probed with a polyclonal antiphosphotyrosine antibody. As shown in Figure4A, the whole homogenate contains many tyrosine-phosphorylated proteins, consistent with the presence of endogenously active PTKs in these cells. The GABAA receptor antibody isolated a tyrosine-phosphorylated protein band that migrates at ∼58 kDa, a predicted molecular weight for β subunits of GABAA receptors (Benke et al., 1991), indicating that the β2/β3 subunits are tyrosine phosphorylated. In other experiments, we immunoprecipitated the whole homogenate with the antiphosphotyrosine antibody and similarly probed the resulting blot with anti-β2/β3 antibody. This anti-β2/β3 blot revealed an immunoreactive band migrating at the predicted molecular weight of β subunits in both the whole homogenate and the antiphosphotyrosine immunoprecipitate (Fig.4B). Thus, these results demonstrate that β2/β3 subunits of the GABAA receptor in neuronal cultures are phosphorylated at tyrosine residues by endogenous PTKs. Furthermore, treatment of the neurons with genistein (100 μm; 10 min), before immunoprecipitation with anti-β2/β3 antibody, caused a reproducible decrease in antiphosphotyrosine immunoreactivity of the GABAA receptor subunits (n = 2) (Fig.4C). These results suggest that the level of tyrosine phosphorylation of the β2/β3 subunits of the GABAAreceptor is modulated by genistein treatment.
Fig. 4.
Tyrosine phosphorylation of the β2/β3 subunit(s) of the GABAA receptor in cultured neurons by endogenous PTKs. A, Phosphotyrosine blotting shows that the immunoprecipitated β2/β3 subunit(s) is tyrosine phosphorylated. Whole homogenate of cultured spinal dorsal horn neurons was immunoprecipitated using a monoclonal antibody recognizing both β2/β3 GABAA receptor subunits. Both whole homogenate (Homogenate) and immunoprecipitate (Anti-β2/β3-IP) were then resolved on 10% SDS-PAGE and electrotransferred to nitrocellulose membrane. The same amount of anti-β2/β3 antibody was also loaded on a separate lane as an IgG control (Control). The resulting membrane was probed with a rabbit polyclonal antiphosphotyrosine antibody. Anti-β2/β3 antibody isolated a tyrosine-phosphorylated protein, which has the predicted molecular weight (58 kDa) of native GABAA receptor β subunits and corresponds to a major phosphotyrosine-containing band seen in the whole homogenate lane. B, Anti-β2/β3 subunit(s) blotting confirms that the subunit protein is among phosphotyrosine-containing proteins. Whole homogenate was immunoprecipitated with a rabbit polyclonal anti-PY antibody, and the whole homogenates and immunoprecipitates (Anti-PY-IP) were then resolved on 10% SDS-PAGE and subjected to immunoblotting using mouse monoclonal anti-β2/β3 antibody (Anti-β2/β3). The anti-β2/β3 antibody reacts with a protein band (β2/β3) at a molecular weight of ∼58 kDa in both homogenates and anti-PY immunoprecipitates. C, Genistein decreases the tyrosine phosphorylation level of β2/β3 subunits in cultured spinal dorsal horn neurons. The homogenate of control neurons (Control) or neurons treated with genistein (100 μm, 10 min; Genistein) was immunoprecipited using the anti-β2/β3 antibody and probed with the anti-phosphotyrosine antibody.
Modulation of recombinant GABAA receptors by endogenous PTKs
To investigate the contribution of the β subunit to tyrosine phosphorylation modulation of the receptor function, we next examined effects of genistein on GABAA currents in HEK 293 cells transiently expressing recombinant GABAA receptors consisting of various combinations of rat α1, β2, and γ2 subunits. To identify the transfected cells for electrophysiological studies, cDNA encoding GFP (Marshall et al., 1995) was used as a gene marker and cotransfected with GABAA receptor subunit cDNAs. We first studied the modulation in cells expressing the α1β2γ2 subunit combination.
Figure 5A is an example of transfected cells visualized under epifluorescent illumination with a standard FITC filter. Under standard whole-cell recording configuration, all fluorescent cells tested expressed functional GABAAchannels, as evidenced by their current responses to pressure ejections of GABA (100 μm) (Fig. 5B), confirming the utility of cotransfection of GFP cDNA as a gene expression marker in electrophysiological studies of recombinant GABAAreceptors. The induced GABA currents were potentiated by diazepam (5 μm) but were insensitive to inhibition by Zn2+ (100 μm), consistent with the classic pharmacology of recombinant GABAA receptors containing αβγ subunits (Angelotti et al., 1993a; Macdonald and Olsen, 1994; Connolly et al., 1996a). As illustrated in Figure5C, application of genistein led to a reversible reduction of the current amplitude, suggesting a tonic modulation of the receptor function by endogenous PTKs.
Fig. 5.
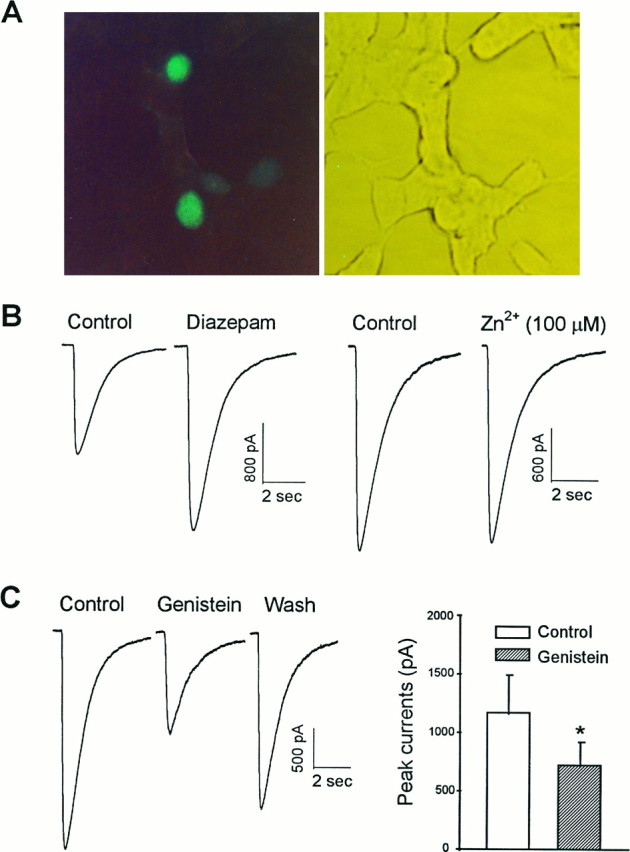
Inhibition of PTK activity suppressed GABA currents in HEK 293 cells expressing GABAA receptors with a combination of α1β2γ2 subunits. A, An example of cells cotransfected with plasmids encoding GFP and the GABAA receptor subunits. Cells in the same field were visualized under FITC fluorescent (left) and phase-contrast (right) illumination, respectively.B, Whole-cell currents evoked by pressure ejection of GABA (100 μm) in one of the fluorescent cells. These currents were potentiated by bath application of diazepam (5 μm; left) but not notably affected by extracellular Zn2+ at a concentration of 100 μm (right). C, Genistein (50 μm) reversibly reduced the peak GABA currents in these cells. On the left are individual current traces recorded before (Control), 5 min after application of drug (Genistein), and 10 min after change of the bathing medium (Wash). Graph on the right summarizes data from eight individual cells.
To determine whether the presence of β2 subunit in the GABAA receptor complex is sufficient for the receptor modulation, we next attempted to examine the modulation in cells transfected with either α1β2 or α1γ2 subunits. To our surprise, although functional channels were detected in all fluorescent cells transfected with the α1β2 subunit combination, no current response to GABA was recorded in any fluorescent cells transfected with α1γ2 subunits. GABA currents induced in cells expressing the combination of α1β2 subunits were considerably smaller than those recorded in cells expressing α1β2γ2 subunits (448 ± 103 pA,n = 7, vs 1173 ± 317 pA, n = 8). These currents were substantially inhibited by a low concentration of Zn2+ (10 μm) but not notably affected by diazepam, consistent with the pharmacology of GABAAreceptors lacking a γ subunit (Fig.6A). As shown in Figure6B, bath application of genistein (50 μm) inhibited the GABAA currents in all cells transfected with α1β2 subunits to a degree similar to that of cells expressing α1β2γ2 subunits, in spite of their striking differences in channel conductance and sensitivity to modulation by diazepam and Zn2+. These results suggest that the presence of β2 subunit is sufficient to render functional GABAA receptors sensitive to modulation by protein tyrosine phosphorylation.
Fig. 6.
Inhibition of PTKs reduced GABA currents in cells expressing GABAA receptors consisting of the α1β2 subunits. A, Pharmacological characteristics of GABA-induced currents in a cell transfected with α1β2 subunits. GABA currents were inhibited in a dose-dependent manner by Zn2+ but were unaffected by diazepam (5 μm), consistent with the absence of a γ subunit in the functional GABAA receptors expressed in this cell.B, Application of genistein (50 μm) in the same cell produced a reversible reduction of the amplitude of the GABA currents. Graph on the right represents data from seven individual cells.
DISCUSSION
In the present work, we have observed that the GABAA receptor-mediated currents in cultured spinal and brainstem neurons were inhibited by bath application of genistein. This effect is likely attributable to specific inhibition of PTK activity because daidzein, which is structurally similar to genistein but has no effect on PTK activity, did not affect the GABA current (Fig. 1). These results suggest that the endogenous PTKs may play an important role in maintaining the function of native GABAA receptors in these neurons. This hypothesis is further supported by the demonstrated effects of intracellular application of the exogenous PTK pp60c-src. Application of pp60c-src potentiated the GABAAcurrents, and the effect is mediated through its kinase activity: it was prevented by pretreatment of the neurons with genistein. Thus, the present work strongly suggests that native GABAA receptors in the CNS are potentiated by endogenous PTKs. This is in contrast, in most cases, to the modulation of the receptor by serine/threonine-specific phosphorylation. Several GABAAreceptor subunits have been shown to be phosphorylated and modulated by cAMP-dependent protein kinase A, protein kinase C, or the type II calcium/calmodulin-dependent protein kinase (Browning et al., 1993;Raymond et al., 1993; Levitan, 1994; Macdonald and Olsen, 1994). Generally serine/threonine phosphorylation of GABAAreceptors has been found to reduce GABAA receptor activity, and conversely, dephosphorylation of the receptor is often associated with the enhanced receptor function (Raymond et al., 1993; Levitan, 1994; Macdonald and Olsen, 1994) (but see Angelotti et al., 1993b;Leidenheimer et al., 1993; Lin et al., 1996).
One simple explanation to account for the effect of PTKs on the receptor function is the direct phosphorylation of the receptor subunits at their tyrosine residues: most of the GABAAreceptor subunits contain tyrosine residues (Macdonald and Olsen, 1994;McKernan and Whiting, 1996). By using immunoprecipitation with a subunit-specific antibody, we identified a major antiphosphotyrosine-reactive band to be the β subunit(s), providing the first evidence for in situ phosphorylation of native GABAA receptor subunit(s) at the tyrosine residues by endogenous PTKs. Given that this antibody reacts with both β2 and β3 subunits (Ewert et al., 1990) and that because the two proteins are similar in size they are recognized as a single band (Benke et al., 1991), it is not possible using this protocol to determine which of these two β subunits is responsible for the observed tyrosine phosphorylation. It should be noted, however, that to date there has been no evidence for any population of native CNS GABAAreceptors containing more than one type of β subunit and that the β2 subunit is by far the most abundant β subunit of native GABAA receptors in the mammalian CNS (McKernan and Whiting, 1996). Moreover, the β2 subunit of purified native GABAAreceptors has been reported to be tyrosine phosphorylated in vitro by the recombinant PTK v-src (Valenzuela et al., 1995). In addition to the β subunits, Valenzuela et al. (1995) found that the γ2 subunit of the purified GABAA receptors can be phosphorylated by v-src. In the present study, we did not observe any detectable tyrosine-phosphorylated protein band that corresponds to the predicted molecular weight of γ subunits (between 41–43 kDa) (Benke et al., 1991; Moss et al., 1995; McKernan and Whiting, 1996). This result argues against in situ tyrosine-specific phosphorylation of γ subunits of the native GABAAreceptor by endogenous PTKs in our preparation. Alternatively, because the γ subunits have been suggested to be sensitive to protease activity (Moss et al., 1992), the failure to detect the phosphorylated band corresponding to γ subunits could simply be attributable to the low level of the intact γ subunit proteins on the Western blots.
The role of β2 and/or β3 subunit phosphorylation in functional modulation of the GABAA receptors by protein tyrosine phosphorylation has not been studied previously. Valenzuela et al. (1995) found that inhibiting PTK activity reduces GABA currents inXenopus oocytes expressing either α1β1 or α1β1γ2 subunit combinations. Because the α subunit thus far has not been shown to be tyrosine phosphorylated, these results would argue for a contribution of β1 subunit phosphorylation to the functional modulation of the receptor. In contrast, Moss et al. (1995) have reported that it is the γ2, but not the β1, subunit that is fully accountable for the functional modulation of the receptor function by protein tyrosine phosphorylation. They used transient transfection of A293 cells with cDNAs encoding subunit α1β1γ2 along with site-directed mutagenesis and found that both β1 and γ2 subunits are tyrosine phosphorylated in cells cotransfected with thev-src cDNA; however, phosphorylation of the γ2 subunit alone affects receptor function (Moss et al., 1995). With respect to the effect of β1 phosphorylation on GABAA receptor function, the different conclusions of Valenzuela and Moss may stem from differences in the expression systems (oocytes verses mammalian cells). Neither of these studies examined a contribution of β2 and/or β3 subunits to the modulation.
In the present work we have demonstrated that inhibition of PTK activity with genistein reduces both the amplitude of GABAAcurrents and the level of tyrosine phosphorylation of β2/β3 subunits of the GABAA receptor in cultured neurons. Moreover, we also observed a reduction of GABA currents in cells expressing either α1β2 or α1β2γ2 subunits. Together, these results suggest that GABAA receptors are functionally regulated by the state of protein tyrosine phosphorylation in these cells. As mentioned previously, the α1 subunit has not been found to be tyrosine phosphorylated in either the present work or any previous work (Moss et al., 1995; Valenzuela et al., 1995). Thus, an involvement of phosphorylation of this subunit in the observed modulation by endogenous PTK is unlikely. The ability of genistein to inhibit GABA currents in cells expressing only α1 and β2 subunits and lacking the γ2 subunit suggests that the presence of the γ2 subunit is also not required for the modulation. One may still argue for a contribution from an endogenously expressed γ subunit; potential expression of some endogenous GABAA receptor subunits in HEK cells has recently been proposed (Ueno et al., 1996). This possibility, however, can be ruled out because GABAA currents recorded from cells transfected with the α1β2 combination have pharmacological characteristics of currents gated through GABAA receptors lacking a γ subunit; the currents are highly sensitive to Zn2+ inhibition but resistant to modulation by benzodiazepines (Macdonald and Olsen, 1994a; Connolly et al., 1996a). Thus, the present work strongly supports the importance of the role of β subunits in the modulation of the GABAA receptor function by endogenous PTKs. We should point out, however, that the sufficient role of the β2 subunit in the functional modulation of the GABAA receptors does not exclude a possible contribution from a γ subunit to the functional modulation in γ subunit-containing receptors. The failure to produce a functional channel in cells transfected with the α1γ2 combination, lacking the β subunit, precludes a clarification of this issue in the present study.
Another pertinent point that we believe warrants a special comment is the apparent requirement for a β subunit to produce a functional GABAA receptor. Among the three combinations (α1β2, α1γ2, and α1β2γ2) tested, GABA currents can be recorded only in cells expressing GABAA receptors containing β2 subunits (α1β2 and α1β2γ2), suggesting a requirement for the β subunit in combination with the α1 subunit to form a functional GABAA channel. These observations are in agreement with those of Angelotti et al. (1993a) and Krishek et al. (1994). These authors found that no functional GABAA receptors can be detected by electrophysiological recording in L929 cells or A293 cells expressing α1γ2, indicating that this subunit combination fails to form functional receptors in mammalian expression systems (but seeVerdoorn et al., 1990). The failure of subunit combinations lacking a β subunit to produce functional GABAA channels may be attributable to the inability of the receptor complexes to access the cell surface (Q. Wan and Y. T. Wang, unpublished observation). This has also been suggested in a recent study, which reported that cell surface expression of GABAA receptors could be detected only in cells transfected with α1 and β2 subunit, regardless of the presence or absence of the γ2 subunit (Connolly et al., 1996a). Thus, these results suggest an important role for β subunits in targeting GABAA receptor complex to the membrane surface, which is a prerequisite for forming a functional GABAA channel. Because β2/β3 subunits are the most abundant subunits of the native GABAA receptors in the CNS (Benke et al., 1991) and may play an important role in relocating the receptors between distinct neuronal domains (Connolly et al., 1996b), phosphorylation of the β2/β3 subunits, and hence modulation of the receptor function, by endogenous PTKs may represent a novel mechanism by which plasticity of GABAA receptor-mediated synaptic inhibition is mediated in the mammalian CNS.
Footnotes
This work was supported by a grant from the Medical Research Council of Canada and by the Fealdman Memorial Fund to Y.T.W. Y.T.W is a Research Scholar of the Heart and Stroke Foundation of Canada/Ontario, and M.W.S is a Canadian Medical Research Council Scholar. We thank Drs. Claire Kaufman and Debra Gunnersen at the Laboratory of Neuroscience, National Institute of Diabetes and Digestive and Kidney Diseases for providing us with GABAA receptor subunit cDNAs, Drs. J. R. Howe and T. E. Hughes (Yale University) for GFP cDNAs, Apotex Inc. (Weston, Ontario) for diazepam, Drs. J. MacDonald and G. Keil for helpful comments on this manuscript, and Ms. J. L. Hicks for preparing and maintaining spinal dorsal horn neuronal cultures.
Correspondence should be addressed to Yu Tian Wang, McMaster Building, Room 5018F, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario M5G 1X8, Canada.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Angelotti TP, Uhler MD, Macdonald RL. Assembly of GABAA receptor subunits: analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J Neurosci. 1993a;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelotti TP, Uhler MD, Macdonald RL. Enhancement of recombinant gamma-aminobutyric acid type A receptor currents by chronic activation of cAMP-dependent protein kinase. Mol Pharmacol. 1993b;44:1202–1210. [PubMed] [Google Scholar]
- 4.Benke D, Mertens S, Trzeciak A, Gillessen D, Mohler H. GABAA receptors display association of γ2-subunit with α1- and β2/3-subunits. J Biol Chem. 1991;266:4478–4483. [PubMed] [Google Scholar]
- 5.Browning MD, Endo S, Smith GB, Dudek EM, Olsen RW. Phosphorylation of the GABAA receptor by cAMP-dependent protein kinase and by protein kinase C: analysis of the substrate domain. Neurochem Res. 1993;18:95–100. doi: 10.1007/BF00966927. [DOI] [PubMed] [Google Scholar]
- 6.Catarsi S, Drapeau P. Tyrosine kinase-dependent selection of transmitter responses induced by neuronal contact. Nature. 1993;363:353–355. doi: 10.1038/363353a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Leonard JP. Protein tyrosine kinase-mediated potentiation of currents from cloned NMDA receptors. J Neurochem. 1996;67:194–200. doi: 10.1046/j.1471-4159.1996.67010194.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen QX, Stelzer A, Kay AR, Wong RK. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol (Lond) 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996a;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Connolly CN, Wooltorton JRA, Smart TG, Moss SJ. Subcellular localisation of GABAA receptors is determined by receptor β subunits. Soc Neurosci Abstr. 1996b;22:326.19. [Google Scholar]
- 11.Ewert M, Shivers BD, Luddens H, Mohler H, Seeburg PH. Subunit selectivity and epitope characterization of mAbs directed against the GABAA/benzodiazepine receptor. J Cell Biol. 1990;110:2043–2048. doi: 10.1083/jcb.110.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes TC, Fadool DA, Levitan IB. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci. 1996;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindy MS. Inhibition of tyrosine phosphorylation prevents delayed neuronal death following cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:372–377. doi: 10.1038/jcbfm.1993.50. [DOI] [PubMed] [Google Scholar]
- 15.Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 16.Leidenheimer NJ, Whiting PJ, Harris RA. Activation of calcium-phospholipid-dependent protein kinase enhances benzodiazepine and barbiturate potentiation of the GABAA receptor. J Neurochem. 1993;60:1972–1975. doi: 10.1111/j.1471-4159.1993.tb13432.x. [DOI] [PubMed] [Google Scholar]
- 17.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 18.Lin YF, Angelotti TP, Dudek EM, Browning MD, Macdonald RL. Enhancement of recombinant alpha 1 beta 1 gamma 2L gamma-aminobutyric acid A receptor whole-cell currents by protein kinase C is mediated through phosphorylation of both beta 1 and gamma 2L subunits. Mol Pharmacol. 1996;50:185–195. [PubMed] [Google Scholar]
- 19.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 20.Marshall J, Molloy R, Moss GW, Howe JR, Hughes TE. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 21.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 22.Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 23.Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- 24.Moss SJ, Gorrie GH, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature. 1995;377:344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- 25.O’Dell TJ, Kandel ER, Grant SG. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- 26.Raymond LA, Blackstone CD, Huganir RL. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993;16:147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- 27.Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in cultured neurones and glia from the dorsal horn of the spinal cord. J Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- 29.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trend Pharmacol. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 30.Stratton KR, Worley PF, Litz JS, Parsons SJ, Huganir RL, Baraban JM. Electroconvulsive treatment induces a rapid and transient increase in tyrosine phosphorylation of a 40-kilodalton protein associated with microtubule-associated protein 2 kinase activity. J Neurochem. 1991;56:147–152. doi: 10.1111/j.1471-4159.1991.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 31.Terlau H, Seifert W. Influence of epidermal growth factor on long-term potentiation in the hippocampal slice. Brain Res. 1989;484:352–356. doi: 10.1016/0006-8993(89)90380-6. [DOI] [PubMed] [Google Scholar]
- 32.Ueno S, Zorumski C, Bracamontes J, Steinbach JH. Endogenous subunits can cause ambiguities in the pharmacology of exogenous γ-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1996;50:931–938. [PubMed] [Google Scholar]
- 33.Valenzuela CF, Machu TK, McKernan RM, Whiting P, VanRenterghem BB, McManaman JL, Brozowski SJ, Smith GB, Olsen RW, Harris RA. Tyrosine kinase phosphorylation of GABAA receptors. Mol Brain Res. 1995;31:165–172. doi: 10.1016/0169-328x(95)00048-w. [DOI] [PubMed] [Google Scholar]
- 34.Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- 35.Wan Q, Man HY, Braunton JL, Brown GM, Wang YT (1996) GABAA-receptor β2 subunit is sufficient for modulation of the receptor by tyrosine phosphorylation. Soc Neurosci Abstr 505.10.
- 36.Wang YT, Salter MW. Regulation of NMDA receptors by protein-tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 37.Wang YT, Wang W. Functional regulation of GABAA receptors by protein tyrosine phosphorylation. Soc Neurosci Abstr. 1995;21:242.12. [Google Scholar]
- 38.Wang YT, Yu X-M, Salter MW. Ca2+-independent reduction of NMDA receptor-mediated currents by protein tyrosine phosphorylation. Proc Natl Acad Sci USA. 1996;93:1721–1725. doi: 10.1073/pnas.93.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokota M, Saido TC, Miyaji K, Tani E, Kawashima S, Suzuki K. Stimulation of protein-tyrosine phosphorylation in gerbil hippocampus after global forebrain ischemia. Neurosci Lett. 1994;168:69–72. doi: 10.1016/0304-3940(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 40.Yu WA. Dissection of motor nuclei of trigeminal, facial, and hypoglossal nerves from fresh rat brain. In: Shahar A, deVellis A, Vernadakis B, Harber B, editors. Manual of the nervous system. Allan R. Liss; New York: 1989. pp. 30–39. [Google Scholar]