Abstract
Cholecystokinin (CCK) is found co-localized with the inhibitory neurotransmitter GABA in interneurons of the hippocampus. Also, CCK receptors are found in abundance in this brain region. The possibility that CCK alters interneuron activity was examined using whole-cell current- and voltage-clamp recordings from visualized interneurons in the stratum radiatum of area CA1 in rat hippocampal slices. The effect of CCK on GABA-mediated IPSCs was also determined in pyramidal neurons. The sulfated octapeptide CCK-8S increased action potential frequency or generated inward currents in the majority of interneurons. These effects of CCK persisted in the presence of tetrodotoxin and cadmium, suggesting that they were direct. Current–voltage plots revealed that CCK-8S inhibited a conductance that was linear across command potentials and reversed near the equilibrium potential for K+ ions. The K+ channel blocker tetraethylammonium (10 mm) generated inward currents similar to those initiated by CCK, and it occluded the effect of the peptide. BaCl2 (1 mm) and 4-aminopyridine (2 mm) did not alter the effect of CCK. The CCKBreceptor antagonist PD-135,158 completely blocked the inward currents generated by CCK-8S. CCK also resulted in an increase in spontaneous action potential-dependent IPSC frequency, but no changes in action potential-independent miniature IPSCs or evoked IPSCs in pyramidal neurons. These results provide evidence that CCK can depolarize hippocampal interneurons through the inhibition of a resting K+ conductance, leading to increased tonic inhibition of pyramidal neurons. This action of CCK may contribute to its anticonvulsant properties, as observed in limbic seizure models.
Keywords: electrophysiology, epilepsy, hippocampus, leak conductance, neuropeptides, potassium channel
Cholecystokinin (CCK) was originally described as a gastrointestinal peptide (for review, see Mutt, 1988) and later identified in the CNS (Vanderhaeghen et al., 1975), where it can be found in high concentrations in the cerebral cortex, hippocampus, amygdala, septum, and hypothalamus (Somogyi et al., 1984; Crawley, 1985; Nunzi et al., 1985; Sloviter and Nilaver, 1987). The synthesis of CCK in the brain occurs through the enzymatic cleavage of the propeptide CCK-33 to several biologically active forms, including the sulfated octapeptide (CCK-8S), which is the most prevalent (Rehfeld et al., 1985). To date, two CCK receptors have been distinguished (designated CCKA and CCKB) based on their molecular characterization and different pharmacological profiles. Both receptors couple to G-proteins (Wank et al., 1994), and their relative distributions in the CNS and peripheral nervous system correlate well with CCK immunoreactivity (Dockray, 1987; Hays et al., 1980; Innis and Snyder, 1980). Cholecystokinin receptors have been shown to interact with several different cellular transduction systems in central and peripheral tissues, including the activation of phospholipase C (Kuwahara et al., 1993; Lee et al., 1993; Wu and Wang, 1996b), the modulation of potassium and calcium channels (Boden and Hill, 1988; Buckett and Saint, 1989; Miyoshi et al., 1991; Branchereau et al., 1993; Cox et al., 1995; Liu et al., 1995), and activation of nonselective cation channels (Dodd and Kelly, 1981; Jarvis et al., 1992).
Cholecystokinin is arguably the most abundant neuropeptide found in the CNS, and, as mentioned above, some of the highest levels of CCK immunoreactivity and CCK receptors are found in the hippocampal formation. Similar to other neuropeptides (e.g., vasoactive intestinal polypeptide, somatostatin, and neuropeptide Y), CCK is found co-localized with the inhibitory neurotransmitter GABA in local circuit interneurons within the hippocampus (Gulyas et al., 1993). In particular, moderate to high levels of CCK immunoreactivity can be found associated with interneurons in all of the major hippocampal strata; including lacunosum–moleculare, radiatum, pyramidale, and oriens (Greenwood et al., 1981; Somogyi et al., 1984; Kosaka et al., 1985; Nunzi et al., 1985). The axons of these CCK-positive neurons are known to terminate predominantly on pyramidal neuron somata in stratum pyramidale and on the proximal dendrites of these cells in stratum radiatum of the hippocampal CA1 and CA3 subfields (Freund and Buzsáki, 1996). In addition, CCK receptors are found in all of these regions of the hippocampus, with the densest concentration found in strata oriens and radiatum (Zarbin et al., 1983). Because each of these classes of interneuron provides inhibitory input to a large number of principal cells (pyramidal neurons) (Miles et al., 1996), they can exert powerful influence over principal cell excitability and ultimately hippocampal output to other brain regions (Cobb et al., 1995; Gulyas et al., 1996).
The reported physiological actions of CCK-8S in the CA1 region of the hippocampus are inconsistent. In some studies, the peptide had excitatory effects on extracellular and intracellular measures of pyramidal neuron activity (Jaffe et al., 1987; Boden and Hill, 1988;Bohme et al., 1988). However, other studies have shown that CCK-8S could either inhibit population spikes (MacVicar et al., 1987) or have no effect on these responses (Miller and Lupica, 1994). Given these inconsistencies and the observation that CCK-8S can increase GABA release in both the cerebral cortex and hippocampus (Sheehan and De Belleroche, 1985; Perez de la Mora et al., 1993), we hypothesized that at least some of its effects may be on local circuit inhibitory cells. This study was limited to interneurons in the stratum radiatum, because high levels of CCK are found there (Greenwood et al., 1981; Somogyi et al., 1984; Kosaka et al., 1985), and because these cells are easily distinguished from pyramidal neurons residing in stratum pyramidale. We demonstrate that CCK excited a majority of these interneurons, identify a likely mechanism for this excitation, and show that it leads to increased GABAergic inhibition of CA1 pyramidal neurons.
MATERIALS AND METHODS
Hippocampal slice preparation. Male Sprague Dawley rats (Sasco, Omaha, NE), 14–30 d old, were killed by decapitation, their brains rapidly removed, and placed in ice-cold, oxygenated artificial CSF (aCSF; see below). The brain was then blocked in a coronal plane approximately 2 mm anterior and 5 mm posterior to bregma using a razor blade. The posterior end of the tissue block was then glued to the stage of a vibrating tissue slicer (Technical Products International, St. Louis, MO) using cyanoacrylate. A midsaggital cut was then made with a scalpel blade to separate the two hemispheres, and brain slices were cut at a 300 μm nominal thickness. The slices were then transferred to a beaker containing aCSF aerated with 95% O2 and 5% CO2 at room temperature, where they were stored for at least 90 min before they were transferred to the recording chamber. Control aCSF consisted of (in mm): NaCl, 126; KCl, 3.0; MgCl2, 1.5; CaCl2, 2.4; NaH2PO4, 1.2; glucose, 11.0; and NaHCO3, 26; and saturated with 95% O2and 5% CO2.
Pyramidal neuron recording. Whole-cell “blind” patch-clamp recordings of spontaneous IPSCs (sIPSCs) and tetrodotoxin-resistant miniature IPSCs (mIPSCs) from pyramidal cells were obtained using methods described previously (Lupica, 1995). Briefly, cells were voltage-clamped at −60 to −90 mV using whole-cell electrodes containing (in mm): CsCl, 125.0; HEPES, 10.0; EGTA, 1.0; CaCl2, 0.1; Mg2+-ATP, 2.0; Na+-GTP, 0.2; and the quaternary lidocaine derivative QX-314, 2, pH 7.2–7.4. Only cells demonstrating <20 MΩ series resistance were used in these experiments. In most cases the series resistance did not change appreciably during the recording period. However, in those instances in which the series resistance increased, there was also an increase in the noise superimposed on the spontaneous events. When this occurred the cell was not used in further analyses. The glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μm) andd-(−)-2-amino-5-phosphonopentanoic acid (APV, 40 μm) were included in the extracellular medium to block excitatory postsynaptic potentials. Spontaneous IPSCs and mIPSCs were amplified 100-fold, filtered at 1–3 kHz, and recorded to FM tape for later analysis. Epochs of 1–2 min of data were digitized at 4–10 kHz and then analyzed using the Strathclyde analysis package (John Dempster, University of Strathclyde, Strathclyde, UK). During analysis, the software event detector was set to disregard events occurring within 5 msec of a previous one. Then, each of the detected events was visually inspected to ensure that near simultaneous or superimposed sIPSCs were not included in the analysis of event amplitude. Drug-induced changes in cumulative sIPSC and mIPSC amplitude distributions were analyzed for statistical significance using the Kolmogorov–Smirnov (K–S) test and a critical probability level ofp < 0.01.
Current-clamp recordings from pyramidal neurons were also performed using high-resistance (50–80 MΩ) micropipettes filled with 3m potassium acetate. Resting input resistance was measured by averaging the final 50 msec of the membrane response to a −0.5 nA, 300 msec current step. The slow afterhyperpolarization was measured at its peak after a 200 msec depolarizing current step (0.2–0.6 nA) that was set to elicit 6–10 action potentials during the control condition. Action potential frequency was measured from these same depolarizing current steps.
Interneuron recording. Whole-cell recordings from interneurons were obtained using an Axoclamp-2A amplifier (Axon Instruments, Burlingame, CA) and electrodes pulled from borosilicate thick-walled capillary tubing (inner diameter, 0.75 mm; outer diameter, 1.5 mm; Sutter Instrument Co., Novato, CA). These electrodes had resistances of 7–10 MΩ when filled with the following solution (in mm): K+-gluconate, 125.0; KCl, 10.0; HEPES, 10.0; EGTA, 1.0; CaCl2, 0.1; Mg2+-ATP, 2.0; and Na+-GTP, 0.2, adjusted to pH 7.2–7.4 with 1 m KOH, and brought to 270–280 mOsm with deionized water. In some of the experiments, 2% biocytin (Sigma, St. Louis, MO) was included in the internal solution so that the anatomical location of the interneurons could be verified, and their morphology could be compared with previous reports.
Stratum radiatum was initially identified using a low-power (4×) objective (40× total magnification) attached to an upright, fixed stage microscope (Carl Zeiss Inc., Thornwood, NY). Then, interneurons were visually identified using a 40× (400× total magnification) water immersion objective and differential interference contrast optics with infrared illumination (Dodt and Zieglgansberger, 1990). The positions of both the neuron and the whole-cell pipette were observed with the aid of a charge-coupled device video camera and television monitor. Positive pressure was maintained on the micropipette to keep its tip clear of debris. Once a neuron was identified, a pipette was directed toward its soma, and a stream of intracellular solution was ejected to clean the cell surface and to facilitate seal formation. The pipette was then advanced until the tip caused a visible indentation of the cell membrane. Positive pressure was then released, and seal formation between the pipette tip and the cell membrane usually began immediately. Otherwise, slight negative pressure was applied in an attempt to form a seal. Once the resistance of the seal reached ≥1 GΩ, the membrane patch was ruptured by applying additional negative pressure. Cells were then voltage-clamped at −55 mV or recorded at resting membrane potential during current-clamp experiments. Series resistance was compensated 70–80% using a bridge circuit and monitored throughout the experiments. Neurons were rejected if the series resistance was >30 MΩ. Voltage-clamp protocols were initiated using a pulse generator (Master 8; A.M.P.I., Jerusalem, Israel), and signals were acquired using a personal computer-based data acquisition system (NEUROPRO; R.C. Electronics, Goleta, CA). Data were also simultaneously monitored using a chart recorder (Gould Inc., Cleveland, Ohio).
During current-clamp experiments, input resistance was monitored using constant current pulses of −0.1–0.5 nA and 500 msec duration. Current-voltage curves were corrected for an empirically determined junction potential (−11.5 mV), which occurs when dissimilar conductors are in contact (Sherman-Gold, 1993). All results are expressed as mean ± SEM, and data were analyzed using ANOVA and appropriatepost hoc analyses or the Student’s t test. Statistical significance is indicated when p < 0.05.
Chemicals. Drugs were obtained from the following sources: CCK-8S, Abbott Laboratories (Chicago, IL), Bachem California (Torrance, CA), or Sigma (St. Louis, MO); PD-135,158 and PD-140,158, Research Biochemicals Inc. (Natick, MA); and tetrodotoxin (TTX), 4-aminopyridine (4-AP), barium chloride, tetraethylammonium (TEA), cadmium chloride, DNQX, and APV, Sigma. All drugs were made up at either 50 or 100 times the desired final concentration in deionized water and then added to the flow of the superfusion medium using a calibrated syringe pump (Razel Scientific Instruments Inc., Stamford, CT).
RESULTS
Physiology of stratum radiatum interneurons
Interneurons near the stratum radiatum–stratum pyramidale border and cells clearly within stratum lacunosum–moleculare were avoided to ensure that only interneurons residing in stratum radiatum were included in this study. The resting membrane potentials of these cells ranged between −39 and −64 mV, with an average of −49.9 ± 0.4 mV (n = 132). Of these neurons, two groups could be distinguished based on the presence or absence of spontaneous firing and resting membrane potential. The majority of cells spontaneously fired action potentials and had an average resting membrane potential of −48.0 ± 0.5 mV (n = 76; 81.7% total cells). The group of cells that did not exhibit spontaneous firing demonstrated a significantly hyperpolarized average resting membrane potential of −54.2 ± 1.7 mV (n = 17; 18.3% total cells;p < 0.001).
Excitatory effect of CCK-8S on stratum radiatum interneurons
Cholecystokinin-8S (500 nm) depolarized or caused inward currents in the majority of stratum radiatum interneurons (26 of 38, 68.4%). No obvious electrophysiological differences existed between the group of cells that responded to CCK-8S and those that did not. Current-clamp experiments indicated that perfusion of CCK-8S caused a rapid (1–1.5 min), reversible increase in the frequency of spontaneous action potentials in most of these cells (Fig.1A). In addition, many neurons that were quiescent during the control period began firing spontaneously in the presence of CCK-8S. An analysis of interneuron firing rate indicated that the frequency of action potential discharge increased by 176 ± 55.1% in the presence of CCK-8S (n = 4;p < 0.0001, paired t test). This increase in firing frequency was associated with a depolarization of the cell membrane and an increase in whole-cell input resistance (Fig.1B and inset).
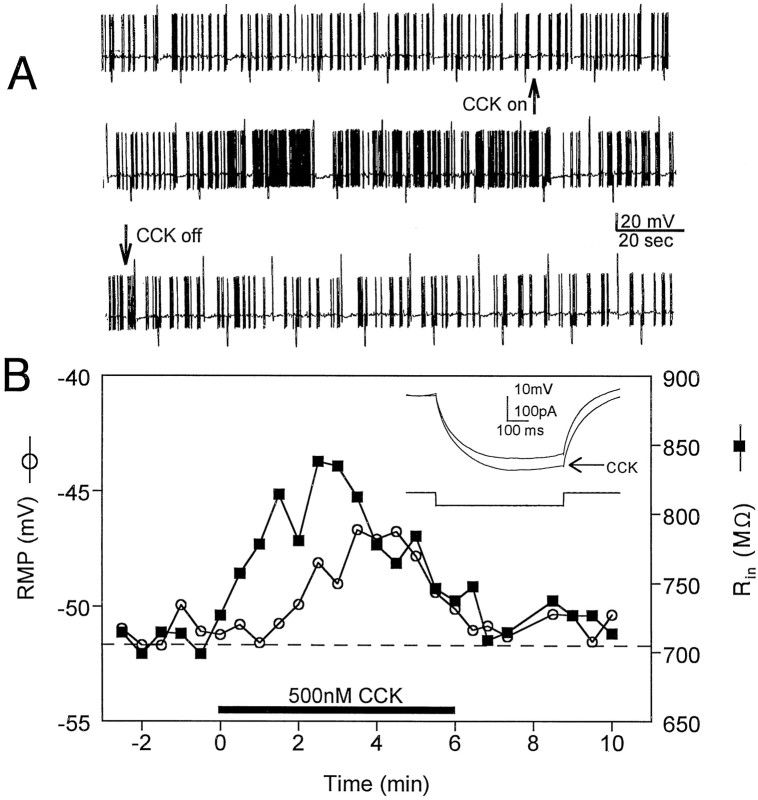
Fig. 1.
Whole-cell current-clamp recordings of the excitatory effects of CCK-8S (500 nm) on interneurons in the stratum radiatum of area CA1. A, Continuous chart record of the effect of CCK-8S on the action potential discharge rate in a spontaneously firing neuron. CCK-8S was bath-applied continuously beginning at the upward arrow and ending at thedownward arrow. The action potential amplitude is truncated by the slow frequency response of the chart recorder. The larger upward and downward deflections are membrane responses to depolarizing and hyperpolarizing current injection, respectively. The resting membrane potential of this cell was −54mV. B, Effects of CCK-8S (horizontal bar) on resting membrane potential (RMP, ○) and input resistance (Rin, ▪, inset) in a different stratum radiatum interneuron. Note that Rinbegins to increase just before the CCK-induced membrane depolarization. Also note that the effect of CCK-8S on firing rate (A), RMP, and Rin(B) is diminished in the continued presence of the peptide.
These initial current-clamp experiments indicated that CCK-8S could depolarize hippocampal interneurons and increase the frequency of spontaneous action potentials. This excitatory action of CCK-8S might be caused by the activation of an inward current with a reversal potential more depolarized than the resting membrane potential. However, because the depolarization and the change in firing rate were associated with an increase in whole-cell input resistance, we hypothesized that CCK inhibited a current with a reversal potential hyperpolarized to rest. We next conducted voltage-clamp experiments to test this possibility. Under these conditions, CCK-8S (500 nm) caused an inward change in the amount of current necessary to clamp the cell membrane at −55 mV (holding current, Fig.2A). Overall, CCK-8S caused a −10.0 ± 1.2 pA change in holding current (n = 25; p < 0.001 compared with baseline). In addition, this increase in holding current was associated with a decrease in whole-cell conductance (Fig. 2B), further suggesting that CCK-8S inhibited an ion channel that was active near the resting membrane potential.
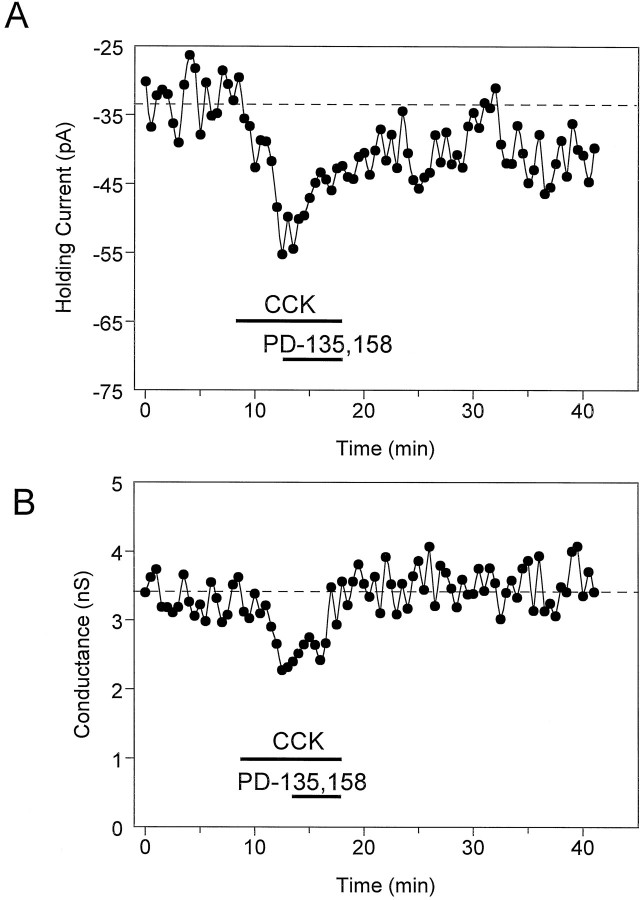
Fig. 2.
Effect of CCK-8S (500 nm) on holding current and whole-cell conductance in a CA1 stratum radiatum interneuron voltage-clamped at −55 mV. A, Time course illustrating the inward change in holding current with CCK-8S and the apparent reversal by the CCKB antagonist PD-135,158 (500 nm). B, Same cell as in A. Effect of CCK-8S on whole-cell steady-state membrane conductance in response to a brief hyperpolarizing voltage step (−10 mV, 300 msec). Note that the inward current caused by CCK-8S was temporally related to the decrease in whole-cell conductance. The delay in response onset was partly attributable to a 1–1.5 min lag time in the bath superfusion system.
Identification of the mechanism of the CCK-induced depolarization
To determine the mechanism of CCK’s effect on interneurons, current-voltage relationships were examined in the presence and absence of the peptide. In these experiments, TTX (500 nm) and CdCl2 (1 mm) were included in the bath to reduce the contribution of voltage-dependent conductances at depolarized membrane steps. Pilot experiments demonstrated that neither TTX nor CdCl2 altered the effects of CCK-8S on holding current (data not shown). As illustrated in Figure3A, 500 nm CCK-8S caused a decrease in the whole-cell slope conductance. When the control current–voltage curve was subtracted from that obtained during CCK-8S superfusion (Fig. 3A, ○), the CCK-sensitive current was found to be linear across the range of command voltages, with a reversal potential of −96.1 mV (n = 4; Fig.3A,B). This indicated that the CCK-8S-sensitive current was voltage-independent and that its reversal potential (Erev) was near that predicted for K+ ions by the Nernst equation when [K+]out = 3.0 mm(predicted Erev = −97 mV).
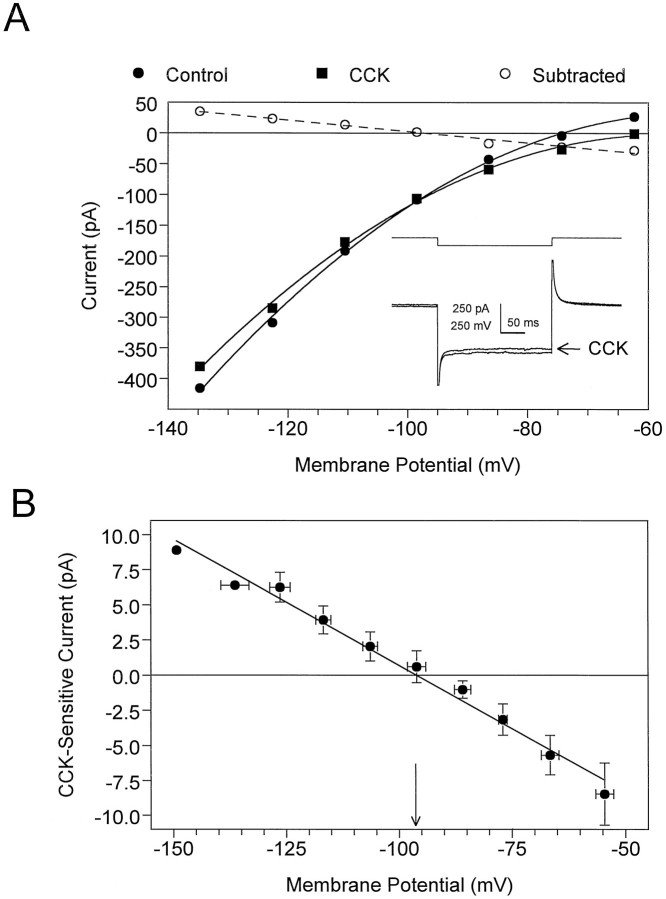
Fig. 3.
Current–voltage (I–V) relationship obtained from stratum radiatum interneurons indicates that CCK-8S (500 nm) inhibits a voltage-independent conductance.A, The neuron was voltage-clamped at −55 mV, and the membrane was stepped from −135 mV (inset traces) to approximately −62 mV using 250 msec voltage steps. The dashed line represents a linear regression fit (r2 = 0.98) to the data obtained when the I–V relationship observed during CCK-8S superfusion was subtracted (○) from that observed before adding CCK-8S. This current is subsequently referred to as the CCK-sensitive current. TheErev for the CCK-sensitive current in this cell was −97.3 mV. Inset, Control and CCK-8S (arrow) current responses obtained at the largest voltage step. Note the decrease in the slope conductance of the I–V curve and the reduced amplitude of the current response (inset) in the presence of CCK-8S. B, Average CCK-sensitive current from four neurons. The calculatedErev from this curve was −96.1 mV (arrow), which is similar to the calculated equilibrium potential for K+ ions (−97 mV) when [K+]out = 3.0 mm.
To confirm that the conductance altered by CCK-8S was mediated by potassium ions, additional current–voltage plots were generated in the presence of different extracellular concentrations of K+, and Erev was calculated for each curve. The experimentally obtained reversal potentials for 5 and 10 mm external potassium were in close agreement with those predicted from the Nernst equation (obtained, −78.7 ± 7.4 mV, n = 9; predicted, −84.0 mV; obtained, −61.6 ± 10.1 mV, n = 7; predicted, −66.4 mV, respectively). Collectively, these results support the hypothesis that CCK-8S inhibited a K+ conductance in these interneurons.
Effects of K+ channel blockers on the actions of CCK-8S
To examine the CCK-8S-sensitive conductance in stratum radiatum interneurons further, we tested the ability of several different K+ channel blockers to alter the effects of CCK-8S on holding current. In these experiments, one of the K+ channel blockers was applied to the slice via superfusion at the concentration indicated. Then, after any changes caused by the channel blocker had reached a stable plateau, CCK-8S was added to the preparation. As shown in Figure4A, the effect of CCK-8S (500 nm) on holding current (−10.0 ± 1.2 pA,n = 25) was insensitive to both barium (1 mm, −9.4 ± 2.4 pA, n = 9;p > 0.05) and 4-aminopyridine (2 mm, −8.4 ± 3.9 pA, n = 11; p > 0.05). However, in contrast to these channel blockers, the effect of CCK-8S was significantly reduced by TEA (10 mm, −2.5 ± 1.5 pA, n = 14; p < 0.001). This is further illustrated in Figure 4B, where it can be seen that TEA decreased the number of interneurons responding to CCK-8S, and inhibited the effect of CCK-8S in the cells that responded to the peptide. Also, by itself, TEA caused a significant (p < 0.05) inward change in holding current that was not significantly different in magnitude than that produced by CCK-8S alone (Fig. 4A; TEA = −10.4 ± 4.0 pA; n = 12; p > 0.05 compared with CCK-8S). In contrast, neither 4-aminopyridine nor barium significantly altered holding current (data not shown).
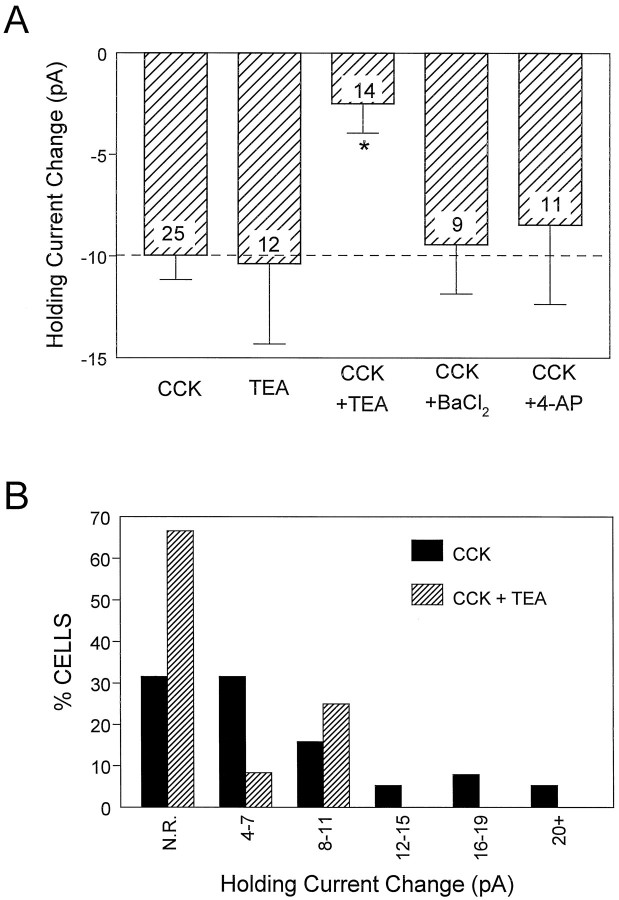
Fig. 4.
Effect of extracellular potassium channel blockers on the CCK-induced change in holding current in stratum radiatum interneurons. A, The average ± SEM effect of CCK-8S alone and during application of the K+channel blockers TEA (10 mm), BaCl2 (1 mm), and 4-AP (2 mm) is shown. The effect of CCK-8S was significantly inhibited only by TEA (*p< 0.001). Note that TEA alone also induced an inward change in holding current that was similar in amplitude to that of CCK-8S. Thenumber superimposed on each barrepresents the number of interneurons tested in each condition.B, Frequency histogram (using the data shown inA) demonstrating that TEA decreased not only the percentage of cells responding to CCK-8S but also the magnitude of the response of those cells that did respond. N.R., No response, defined as cells exhibiting a change in holding current of ≤3 pA.
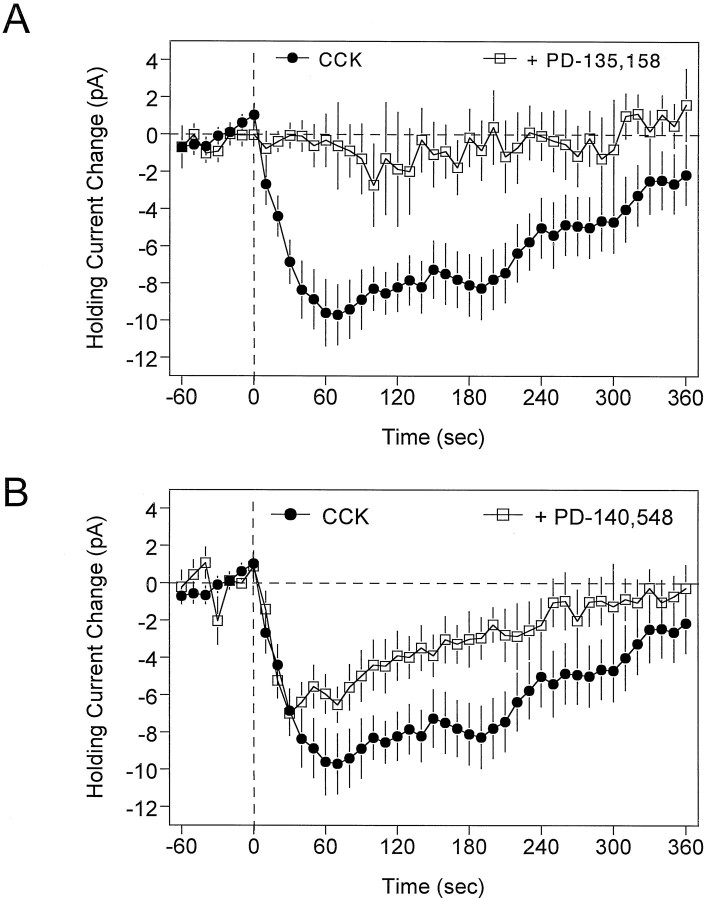
CCK receptor antagonists inhibit the effect of CCK-8S on interneurons
Several studies have shown that the effects of CCK in the hippocampus are mediated through the activation of the CCKBreceptor subtype (Bohme et al., 1988, 1989; Miller and Lupica, 1994). In support of this, PD-135,158 (100–500 nm), a highly selective CCKB receptor antagonist, completely blocked the change in holding current caused by 500 nm CCK-8S (Fig.5A; p < 0.0001). However, in contrast to the effect of the CCKB antagonist, the putative CCKA receptor antagonist PD-140,548 did not alter the inward current generated by CCK-8S at low concentrations (10–50 nm) and only partially inhibited the effect of CCK-8S at a higher concentration (200 nm; Fig. 5B;p < 0.05). Higher concentrations of PD-140,548 (300–500 nm) were also tested but were found to cause large inward shifts in the baseline holding current (data not shown).
Fig. 5.
Effect of CCK receptor antagonists on CCK-induced changes in holding currents. In these experiments, the antagonist was bath-applied for 10 min, and then CCK-8S (500 nm) was bath-applied for at least 6 min beginning at time 0 (vertical dashed line). The curves represent the time course of CCK-8S effects in the absence (•) or presence (□) of the antagonist, averaged (±SEM) across at least 11 cells in each group.A, The CCKB antagonist PD-135,158 (500 nm) caused a near complete block of the effects of CCK-8S (p < 0.001). B, The CCKA antagonist PD-140,548 (200 nm) only partially antagonized the effect of CCK-8S on interneuron holding current (p < 0.05). Note the decreased effect of CCK-8S over time in the control condition, and that the response had nearly returned to baseline by 6 min of CCK-8S application.
Effects of CCK-8S on GABA-mediated IPSCs
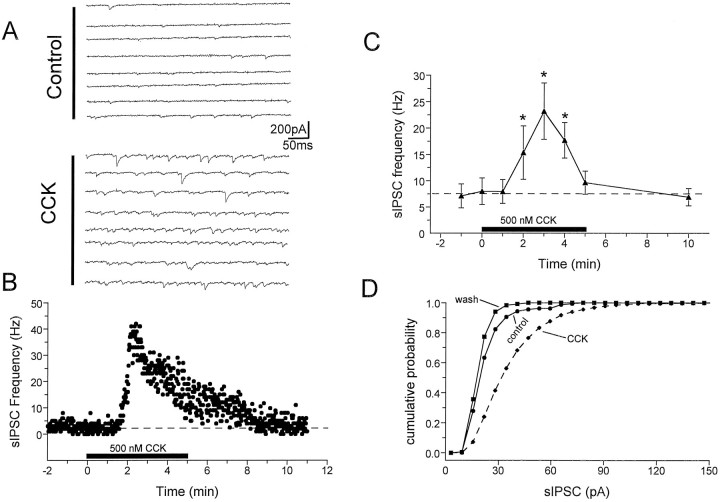
The observation that CCK-8S could excite interneurons located in area CA1 suggested that it might also increase the inhibitory signal to the postsynaptic targets of these cells, the CA1 pyramidal neurons. To test this hypothesis we measured the effects of CCK-8S on action potential-dependent sIPSCs in these neurons, using whole-cell pipettes containing CsCl. These recordings were performed in the presence of the glutamate receptor antagonists DNQX (10 μm) and APV (40 μm), added to block glutamate-mediated EPSCs. The inward currents recorded under these conditions reversed at approximately 0 mV and were completely blocked by the GABAA receptor antagonist bicuculline (10 μm). The baseline rate of sIPSCs varied widely among pyramidal neurons (2.6–17.3 Hz; average, 8.0 ± 2.5 Hz; n = 6). However, bath application of CCK-8S (100–500 nm) caused large, time-dependent increases (1.5- to 10-fold) in the frequency of sIPSCs in every cell tested (Fig. 6). During a 5 min CCK-8S application, the peak effect was seen during the third minute, when the average sIPSC frequency increased approximately threefold to 23.2 ± 5.3 Hz (p < 0.001). Continued application of CCK-8S resulted in the attenuation of its effect (Fig.6B,C), representing only 76% of the peak at the fifth minute of application (17.7 ± 3.4 Hz). Partly because of the desensitization, the effect of CCK-8S was fully reversed 5 min after cessation of peptide superfusion (6.8 ± 2.2 Hz; Fig.6B,C). Similar to sIPSC frequency, the average amplitude of these events varied widely among CA1 pyramidal neurons (range, 25.8–68.2 pA), with a group mean of 42.3 ± 8.4 pA (n = 6). CCK-8S significantly increased sIPSC amplitudes in four of six cells, as determined by cumulative amplitude histograms (Fig. 6D; p < 0.001, K–S test), and increased the group mean sIPSC amplitude to 53.3 ± 8.1 pA.
Fig. 6.
Effects of CCK-8S on spontaneous action potential-dependent IPSCs measured in CA1 pyramidal neurons.A, Consecutive digitized current traces before (Control) and during CCK-8S (500 nm) superfusion (CCK). Whole-cell recordings of sIPSCs were performed using CsCl-filled pipettes and ionotropic glutamate receptor antagonists (Lupica, 1995). Holding potential = −80 mV. B, Time course of CCK-8S effect on sIPSC frequency recorded from the same cell represented in A. CCK-8S was bath-applied for 5 min, beginning at time 0 (solid horizontal bar). C, Time course of the effect of CCK-8S on sIPSC frequency averaged (±SEM) across six pyramidal neurons. Each point represents the average sIPSC frequency during individual 1 min periods. The time of CCK-8S application is indicated by the solid horizontal bar. In each cell tested the sIPSP frequency was significantly increased by CCK-8S (*p < 0.01 compared with control, repeated measures ANOVA). D, Cumulative probability distribution of sIPSC amplitudes, derived from the same neuron described inA and B, demonstrating a significant increase in the average sIPSC amplitude (p< 0.001, K–S test). In this particular cell the average sIPSC amplitude increased from 25.8 pA (n = 156 events) during the control period to 39.7 pA (n = 1908 events) during the third minute of CCK-8S application. A total of four of six cells showed significant sIPSC amplitude increases. Note the diminished response in the continued presence of CCK-8S (B, C).
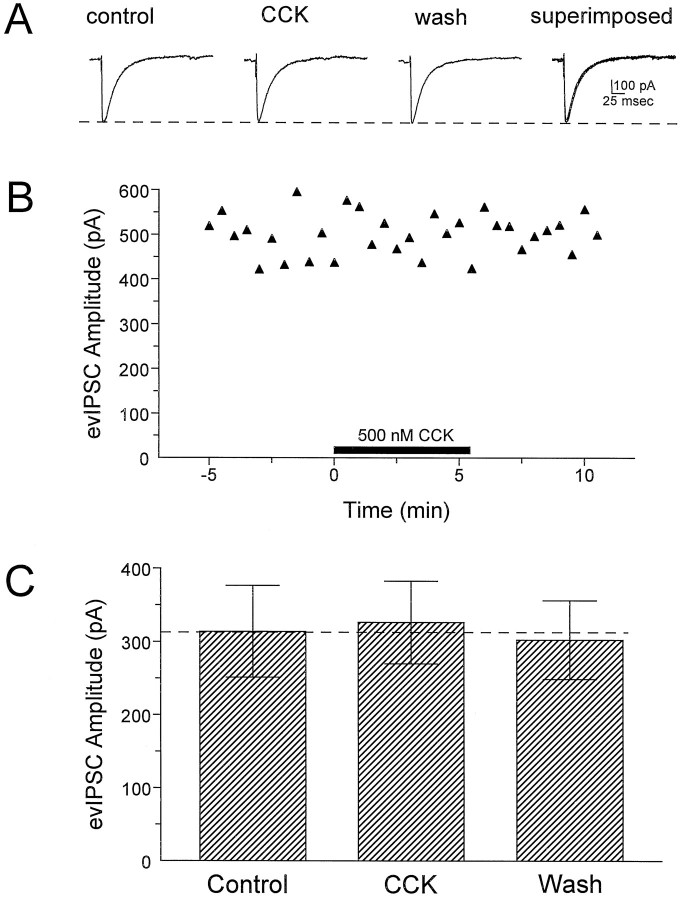
In addition to the sIPSCs, evoked monosynaptic IPSCs were recorded simultaneously during these experiments. However, in these same neurons, CCK-8S did not significantly alter the evoked IPSC amplitude (Fig. 7). Evoked monosynaptic IPSPs were also recorded under current-clamp conditions using conventional high-resistance micropipettes filled with potassium acetate (see Materials and Methods). Consistent with the results of the above whole-cell experiments, CCK-8S did not significantly alter the amplitude of these evoked IPSPs (n = 18; Table 1).
Fig. 7.
Effects of CCK-8S on evoked IPSCs (evIPSC) in CA1 pyramidal neurons. These evoked responses were recorded simultaneously with sIPSCs using CsCl-filled electrodes and glutamate receptor antagonists (Lupica, 1995).A, Digitized averages of at least five individual responses recorded during the indicated period throughout the representative experiment shown in B. The response labeled CCK represents the average of five consecutive responses beginning at 1.5 min into the CCK-8S application. Thedashed line indicates the amplitude of the control response. B, Plot of peak evoked IPSC amplitude for a single CA1 pyramidal neuron. The period of CCK-8S application is indicated by the solid horizontal bar. C, Mean ± SEM effect of CCK-8S (500 nm) on evoked IPSCs for all cells (n = 8). The effect of CCK-8S was determined as described for the response in B. Note that CCK-8S had no effect on evoked IPSC amplitudes despite the fact that it significantly increased the frequency and amplitude of sIPSCs recorded from these same neurons (Fig. 6).
Table 1.
Effects of cholecystokinin-8S (500 nm) on CA1 pyramidal neurons
| Control | CCK-8S | Wash | |
|---|---|---|---|
| RMP (mV) | −63.1 ± 1.5 | −63.3 ± 1.0 | −63.6 ± 1.7 |
| Rin(MΩ) | 32.6 ± 2.7 | 32.9 ± 3.4 | 31.6 ± 3.4 |
| AHP (mV) | 11.2 ± 1.4 | 11.1 ± 1.5 | 10.7 ± 1.9 |
| APF (Hz) | 33.2 ± 2.9 | 33.3 ± 2.4 | 31.2 ± 2.4 |
| IPSP (mV) | 8.9 ± 0.9 | 8.3 ± 0.8 | 8.1 ± 1.0 |
RMP, Resting membrane potential; Rin, input resistance; AHP, afterhyperpolarizing potential; APF, action potential frequency. All recordings were performed using high-resistance micropipettes; n = 18.
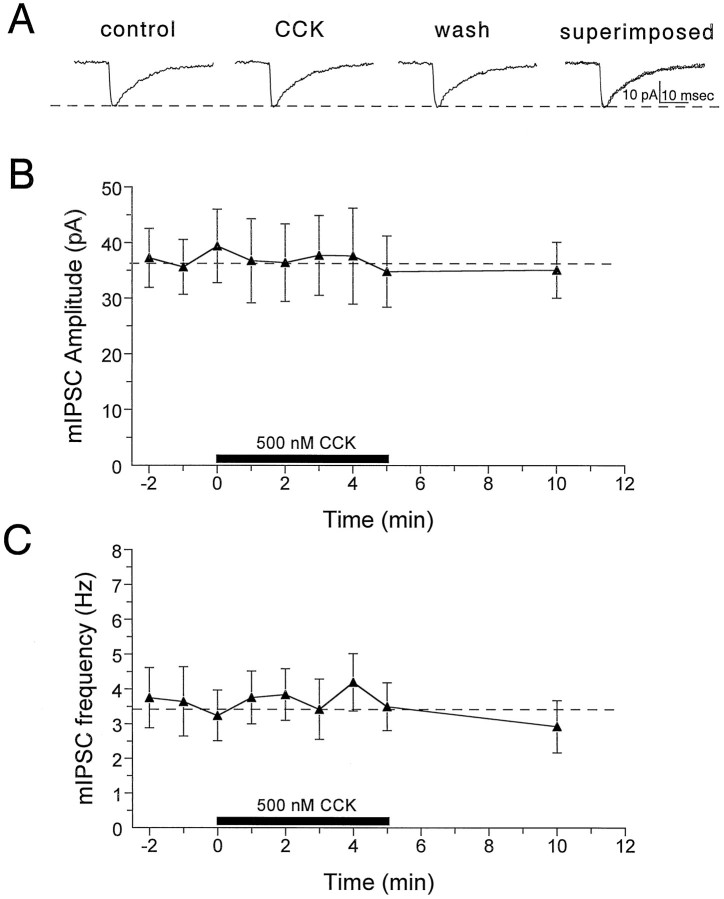
In a further attempt to determine whether the ability of CCK to increase tonic GABA-mediated inhibition could be attributed to actions of this peptide at inhibitory terminals, we examined its effects on action potential-independent mIPSCs in the presence of the voltage-dependent Na+ channel blocker TTX (1 μm). We have previously shown that mIPSCs recorded under these conditions reverse at approximately 0 mV and are completely blocked by bicuculline (Lupica, 1995). Similar to sIPSCs, the frequency of the mIPSCs varied dramatically among CA1 pyramidal neurons (range, 0.2–6.2 Hz; n = 8). However, the average control frequency of the mIPSCs was significantly lower than that of the sIPSCs (3.2 ± 0.7 vs 6.8 ± 2.2 Hz; p < 0.01). In contrast to the relatively large effect of CCK on action potential-dependent sIPSCs, CCK-8S did not alter mIPSC frequency or mIPSC amplitudes in any of the neurons tested (Fig.8). This result suggested that CCK-8S did not produce the changes in sIPSCs through actions at inhibitory terminals or by altering GABA receptor sensitivity.
Fig. 8.
Effects of CCK-8S on mIPSCs recorded from CA1 pyramidal neurons in the presence of TTX (1 μm).A, mIPSCs averaged during 1 min periods before CCK-8S application (control, n = 90), 2 min after CCK-8S application had begun (CCK,n = 85), and 5 min after drug application had been terminated (wash, n = 88). Thedashed horizontal line represents the amplitude of the control response. Note that CCK-8S did not alter the amplitude or the kinetics of the mIPSC responses. B, Time course of the effect of CCK-8S on mIPSC amplitude averaged (±SEM) across seven pyramidal neurons. Each point represents the average mIPSC amplitude calculated during individual 1 min periods, and the time of CCK-8S application is indicated by the solid horizontal bar. The mIPSC amplitude was not significantly altered by CCK-8S in any cell (p > 0.01, K–S test).C, Time course of the effect of CCK-8S on the frequency of mIPSCs for the same group of cells described in B. In each mIPSC experiment, TTX was applied to the slice for at least 15 min before recording control events, and the efficacy of the TTX blockade of Na+ channels was assessed by monitoring the disappearance of evoked IPSCs.
Absence of direct CCK-8S effects on CA1 pyramidal neurons
In contrast to several previous reports (Dodd and Kelly, 1981;Jaffe et al., 1987; Boden and Hill, 1988; Bohme et al., 1988), we did not observe CCK-induced changes in CA1 pyramidal neuron activity during our whole-cell recordings. However, because it was possible that the whole-cell configuration deprived pyramidal neurons of some intracellular constituent that was necessary to observe this response, we also examined the effects of CCK-8S on pyramidal neurons using conventional high-resistance intracellular recording techniques. Under these conditions, CCK-8S (500 nm) did not alter pyramidal neuron resting membrane potential, the slow afterhyperpolarizing response seen after a train of action potentials, input resistance, or action potential discharge rate (n = 18; Table 1). These results and those obtained with whole-cell microelectrodes suggest that CCK-8S had no direct effects on CA1 pyramidal neurons in these studies.
DISCUSSION
To gain insight into the functional importance of CCK in the hippocampus, we examined its effects on GABAergic interneurons, which are known to play important roles in modulating hippocampal activity and output. The present study demonstrated that CCK-8S could directly excite a population of interneurons, residing in the stratum radiatum of area CA1, through membrane depolarization associated with an increase in whole-cell input resistance and spontaneous action potential discharge rate. Under voltage-clamp conditions, it was determined that CCK-8S caused an inward shift in holding current and a decrease in conductance that were consistent with the excitatory actions of this peptide. To determine whether this increase in interneuron excitability was associated with increased inhibition of the targets of these GABAergic cells, we recorded IPSCs from CA1 pyramidal neurons. These experiments demonstrated that the functional consequence of increased interneuron excitability by CCK-8S was the augmentation of tonic inhibitory input to the pyramidal neurons. Furthermore, because CCK-8S did not alter the frequency or amplitude of action potential-independent mIPSCs or the amplitudes of evoked IPSCs, our results suggest that CCK-8S increased inhibitory tone by enhancing action potential-dependent release of GABA and not by acting either directly at inhibitory nerve terminals or at postsynaptic GABAA receptors.
Mechanism of the CCK excitation
The evidence that CCK-8S excited stratum radiatum interneurons via the suppression of a voltage-independent resting K+conductance was derived from the linear current–voltage relationship for the CCK-sensitive current and the ability of alterations in external potassium concentration to shift its reversal potential in a manner consonant with Nernst-predicted reversals. Also, the findings that the CCK-mediated current was occluded by TEA and that TEA alone generated similar inward currents further support the hypothesis that the effects of CCK-8S were attributable to a reduction in resting K+ channel activity. In contrast, both the inability of barium to block the effect of CCK-8S and the voltage independence of the CCK-sensitive current suggest that inward rectifier channels were not involved in this response. It is also unlikely that transient “A” currents were involved in the CCK response, because 4-aminopyridine did not occlude the CCK-induced change in holding currents (Rudy, 1988). Additionally, because these neurons were clamped near their resting membrane potential (−55 mV), it is not likely that delayed rectifier K+ channels were acted on by CCK-8S. The effects of CCK-8S on stratum radiatum interneurons also seemed to be direct and not caused by alterations in the release of other neurotransmitters, because its effects were found to persist in the presence of TTX. Similarly, the effect of CCK-8S was unaltered by cadmium, suggesting that voltage-dependent Ca2+ and Ca2+-dependent K+ channels were not permissive to the actions of CCK. Based on these findings we propose that CCK-8S directly excites interneurons in the stratum radiatum of area CA1 of the hippocampus, primarily through the suppression of a resting K+ leak conductance. A similar effect on a K+ leak conductance by α1-adrenergic agonists has recently been reported in these neurons (Bergles et al., 1996). Furthermore, this α1 effect was also associated with an increase in sIPSC frequency and amplitude recorded postsynaptically. These data, coupled with the findings of the present investigation, suggest that the inhibition of this K+ conductance may represent a common mechanism through which tonic inhibitory tone can be increased by different neuromodulators in the hippocampus.
The depolarization of neural membranes through the CCK-mediated inhibition of a resting K+ conductance has also been reported in brain areas other than the hippocampus. For example, Branchereau and colleagues (1993) demonstrated that the CCK-induced depolarization of neurons in the rat solitary nucleus reversed near the equilibrium potential for K+ ions and was associated with an increase in input resistance. Also, Cox et al. (1995) reported the inhibition of a voltage-independent K+ leak current by CCK-8S in rat reticular thalamic neurons. In addition to this mechanism, CCK can produce membrane depolarization, associated with a decrease in membrane resistance, through activation of a nonselective cation conductance (Dodd and Kelly, 1981; Jarvis et al., 1992; Wu and Wang, 1996a,b). However, in the present study we did not observe CCK-activated currents with reversal potentials consistent with this mechanism.
In a manner inconsistent with some previous reports (Dodd and Kelly, 1981; Jaffe et al., 1987; Boden and Hill, 1988; Bohme et al., 1988), we found no direct effects of CCK-8S on CA1 pyramidal neurons in the present intracellular experiments, or in our previous report using extracellular measures of pyramidal neuron activity (Miller and Lupica, 1994). The reason for this discrepancy is unclear at this time, although it may be attributable to the fact that, in many of these studies, CCK-8S was applied in small, concentrated quantities using micropipettes, whereas we have used bath application. It is possible that the more slowly bath-applied CCK-8S may cause desensitization of the pyramidal neuron response but leaves the interneuron response intact. This implies that both slowly and rapidly desensitizing responses to CCK-8S may be present in hippocampal neurons. In support of this, Dodd and Kelly (1981), using pipette application, reported that CCK-8S depolarized 85% of CA1 pyramidal neurons through the activation of a current associated with a decrease in input resistance and possessing a reversal potential of ∼−21mV. This suggests an underlying conductance much different from that seen in the interneurons. Additionally, Bohme et al. (1988), using bath application, demonstrated that significantly fewer (20%) CA1 pyramidal neurons were excited by CCK-8S. These results suggest that there may be two excitatory responses to CCK-8S in the hippocampus: one present only in interneurons, associated with a decrease in a K+leak conductance, which desensitizes relatively slowly (i.e., 3–6 min; Figs. 1, 5, 6); and another present in pyramidal neurons, which reverses near −20 mV and desensitizes more rapidly. Further experiments using faster application of CCK-8S will be needed to determine whether the more rapidly desensitizing response is also present in interneurons.
Pharmacology of CCK-8S actions on interneurons
Based on the ability of the selective CCKB receptor antagonist PD-135,158 to completely inhibit the effect of CCK-8S, and the significantly smaller effect of the putative CCKAreceptor antagonist PD-140,538, we hypothesize that the effect of CCK-8S on stratum radiatum interneurons was attributable to the activation of CCKB receptors. This is consistent with the CCK receptor identified in the modulation of [3H]GABA release in the hippocampus (Perez de la Mora et al., 1993), and with autoradiographic studies demonstrating that the CCKB receptor subtype is predominant in the hippocampal formation (Woodruff et al., 1991; Bohme et al., 1988). In addition, previous reports of CCK actions in the hippocampus suggest that they occur through activation of the CCKB receptor (Bohme et al., 1988, 1989; Migaud et al., 1994; Miller and Lupica, 1994), and this receptor has been implicated in most studies of excitatory effects of CCK throughout the CNS (Boden and Hill, 1988;Bohme et al., 1988; 1989; Branchereau et al., 1993). However, an exception to this conclusion can be found in the thalamus, where the excitatory effects of CCK were mediated by CCKA receptors (Cox et al., 1995). Responses mediated by the CCKB receptor are also known to desensitize (Dodd and Kelly, 1981; Boden and Hill, 1988). Consistent with these findings, we also observed a decreased response to CCK-8S in its continued presence. This can be seen both in the recordings of sIPSCs from pyramidal neurons (Fig.6B,C) and in the effects of CCK-8S on interneuron membrane potential, input resistance (Fig. 1B), and holding current (Figs. 2, 5). Furthermore, the time course of this agonist-induced desensitization was similar for interneuron and pyramidal cell effects, suggesting that the same receptor was involved.
Functional significance of CCK actions in the hippocampus
The demonstration of increased sIPSC frequency and amplitude recorded in CA1 pyramidal neurons suggests that CCK can increase tonic GABAergic inhibition by depolarizing interneurons in the hippocampus. Although it is impossible to conclude that the stratum radiatum interneurons that were depolarized by CCK-8S were responsible for the increase in tonic GABAergic output, many GABAergic cells located in this region of the hippocampus are known to form functional synapses with pyramidal neurons (Nunzi et al., 1985; Williams et al., 1994). Furthermore, our own preliminary anatomical reconstructions of biocytin-filled stratum radiatum interneurons suggest that axons from these cells ramify extensively throughout the CA1 pyramidal cell body layer (K. K. Miller, and C. R. Lupica, unpublished observations). A role for CCK may be suggested by the observation that glutamic acid decarboxylase (found in GABAergic neurons) and CCK are found co-localized in many of the interneurons in stratum radiatum (Greenwood et al., 1981; Kosaka et al., 1985; Nunzi et al., 1985). It is possible that endogenous CCK, subsequent to its release at interneuron–pyramidal neuron synapses, may regulate interneuron activity and GABA release by diffusing to CCK autoreceptors located on interneuron somata (Freund and Buzsáki, 1996). However, it is also possible that CCK may be released from interneurons to increase the excitability of CCK-negative cells, thereby increasing their inhibitory influence on hippocampal pyramidal neurons and hippocampal output. This latter point is supported by a report demonstrating CCK-immunoreactive terminals contacting both CCK-positive and -negative interneurons in the stratum radiatum (Nunzi et al., 1985).
Although the precise role CCK may play in regulating hippocampal activity has not been defined, it is known to act as an anticonvulsant and to delay the onset of generalized seizures in animals (Zetler, 1980; Kadar et al., 1984; Zhang et al., 1993). Also, CCK immunoreactivity and CCK mRNA levels are transiently increased in the cerebral cortex and the hippocampus after generalized seizures (Iadarola et al., 1986; Burazin and Gundlach, 1996; Zhang et al., 1996). Based on the results of the present study, we suggest that CCK may act as an endogenous anticonvulsant by promoting the release of GABA from inhibitory interneurons after generalized seizures, thereby increasing inhibitory tone. This may represent a compensatory change, possibly increasing the refractory period between ictal events. Further investigation into the conditions under which endogenous CCK can be released in these brain areas will be critical in confirming this hypothesis.
In conclusion, we have demonstrated for the first time that CCK can depolarize a population of interneurons located in the stratum radiatum of area CA1 of the hippocampus, through a reduction in a resting potassium conductance. This increase in interneuron excitability also resulted in enhanced tonic inhibition of CA1 pyramidal neurons, which would be expected to decrease the overall level of excitability of this brain area.
Footnotes
This work was supported by National Institutes of Health Grant DA 07725 from the United States Public Health Service.
Correspondence should be addressed to Dr. Carl R. Lupica, Department of Pharmacology, University of Colorado Health Sciences Center, Box C236, 4200 East Ninth Avenue, Denver, CO 80262.
REFERENCES
- 1.Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory action of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boden PR, Hill RG. Effects of cholecystokinin and pentagastrin on rat hippocampal neurons maintained in vitro. Neuropeptides. 1988;12:95–103. doi: 10.1016/0143-4179(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 3.Bohme G, Stutzmann J, Blanchard J. Excitatory effects of cholecystokinin in rat hippocampus: pharmacological response compatible with central- or B-type CCK receptors. Brain Res. 1988;451:309–318. doi: 10.1016/0006-8993(88)90776-7. [DOI] [PubMed] [Google Scholar]
- 4.Bohme G, Durieux C, Stutzmann J, Charpentier B, Roques BP, Blanchard J. Electrophysiological studies with new CCK analogs: correlation with binding affinity on B-type receptors. Peptides. 1989;10:407–414. doi: 10.1016/0196-9781(89)90051-x. [DOI] [PubMed] [Google Scholar]
- 5.Branchereau P, Champagnat J, Denavit-Saubie M. Cholecystokinin gated currents in neurons of the rat solitary complex in vitro. J Neurophysiol. 1993;70:2584–2595. doi: 10.1152/jn.1993.70.6.2584. [DOI] [PubMed] [Google Scholar]
- 6.Buckett K, Saint D. Cholecystokinin modulates voltage dependent K+ currents in cultured rat hippocampal neurons. Neurosci Lett. 1989;107:162–166. doi: 10.1016/0304-3940(89)90810-0. [DOI] [PubMed] [Google Scholar]
- 7.Burazin TCD, Gundlach AL. Rapid but transient increases in cholecystokinin mRNA levels in cerebral cortex following amygdaloid-kindled seizures in rats. Neurosci Lett. 1996;209:65–68. doi: 10.1016/0304-3940(96)12603-3. [DOI] [PubMed] [Google Scholar]
- 8.Cobb SR, Buhl ER, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;372:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 9.Cox CL, Hugenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol. 1995;74:990–1000. doi: 10.1152/jn.1995.74.3.990. [DOI] [PubMed] [Google Scholar]
- 10.Crawley JN. Comparative distribution of cholecystokinin and other neuropeptides. Ann NY Acad Sci. 1985;448:1–7. doi: 10.1111/j.1749-6632.1985.tb29900.x. [DOI] [PubMed] [Google Scholar]
- 11.Dockray GJ. Cholecystokinin in the brain. In: Martin JB, Brownstein MJ, Krieger DT, editors. Brain peptides update. Wiley; New York: 1987. pp. 127–140. [Google Scholar]
- 12.Dodd J, Kelly JS. The actions of cholecystokinin and related peptides on pyramidal neurons of the mammalian hippocampus. Brain Res. 1981;205:337–350. doi: 10.1016/0006-8993(81)90344-9. [DOI] [PubMed] [Google Scholar]
- 13.Dodt H-U, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC microscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- 14.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood RS, Godar SE, Reaves TA, Jr, Hayward JN. Cholecystokinin in hippocampal pathways. J Comp Neurol. 1981;203:335–350. doi: 10.1002/cne.902030303. [DOI] [PubMed] [Google Scholar]
- 16.Gulyas AI, Gorcs T, Freund TF. Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents. Neuroscience. 1993;37:31–44. doi: 10.1016/0306-4522(90)90189-b. [DOI] [PubMed] [Google Scholar]
- 17.Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hays SE, Beinfeld MC, Jensen RT, Goodwin FK, Paul SM. Demonstration of a putative receptor site for cholecystokinin in rat brain. Neuropeptides. 1980;1:53–62. [Google Scholar]
- 19.Iadarola MJ, Shin C, McNamara JO, Yang HY. Changes in dynorphin, enkephalin and cholecystokinin content of hippocampus and substantia nigra after amygdala kindling. Brain Res. 1986;365:185–191. doi: 10.1016/0006-8993(86)90738-9. [DOI] [PubMed] [Google Scholar]
- 20.Innis RB, Snyder SH. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci USA. 1980;77:6917–6922. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe D, Aitken P, Nadler J. The effects of cholecystokinin and cholecystokinin antagonists on synaptic function in the CA1 region of the rat hippocampal slice. Brain Res. 1987;415:197–203. doi: 10.1016/0006-8993(87)90288-5. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis CR, Bourque CW, Renaud LP. Depolarizing action of cholecystokinin on rat supraoptic neurones in vitro. J Physiol (Lond) 1992;458:621–632. doi: 10.1113/jphysiol.1992.sp019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadar T, Pesti A, Peuke B. Inhibition of seizures induced by picrotoxin and electroshock by cholecystokinin octapeptide and their fragments in rats after intracerebroventricular administration. Neuropharmacology. 1984;23:955–961. doi: 10.1016/0028-3908(84)90010-8. [DOI] [PubMed] [Google Scholar]
- 24.Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yanaihara N, Wu J-Y, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J Comp Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- 25.Kuwahara T, Nagase H, Takamiya M, Yoshizaki H, Kudoh T, Nakano A, Arisawa M. Activation of CCK-B receptors elevates cytosolic Ca++ levels in a pituitary cell line. Peptides. 1993;14:801–805. doi: 10.1016/0196-9781(93)90117-y. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y, Beinborn M, McBride EW, Lu M, Kolakowski LF, Kopin AS. The human brain cholecystokinin-B/gastrin receptor. J Biol Chem. 1993;268:8164–8169. [PubMed] [Google Scholar]
- 27.Liu N, Xu T, Xu C, Li C, Yu Y, Kang H, Han J-S. Cholecystokinin octapeptide reverses mu opioid receptor mediated inhibition of calcium current in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1995;275:1293–1299. [PubMed] [Google Scholar]
- 28.Lupica CR. δ and μ Enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995;15:737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacVicar BA, Kerrin J, Davison J. Inhibition of synaptic transmission in the hippocampus by cholecystokinin (CCK) and its antagonism by a CCK analog (CCK-27–33). Brain Res. 1987;406:130–135. doi: 10.1016/0006-8993(87)90777-3. [DOI] [PubMed] [Google Scholar]
- 30.Migaud M, Roques BP, Durieux C. Effects of cholecystokinin octapeptide and BC264, a potent and selective CCK-B agonist on aspartate and glutamate release from rat hippocampal slices. Neuropharmacology. 1994;33:737–743. doi: 10.1016/0028-3908(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 31.Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 32.Miller KK, Lupica CR. Morphine-induced excitation of pyramidal neurons is inhibited by cholecystokinin in the CA1 region of the rat hippocampal slice. J Pharmacol Exp Ther. 1994;268:753–761. [PubMed] [Google Scholar]
- 33.Miyoshi R, Kito S, Nomoto T. Cholecystokinin increases intracellular Ca++ concentration in cultured striatal neurons. Neuropeptides. 1991;18:115–119. doi: 10.1016/0143-4179(91)90102-o. [DOI] [PubMed] [Google Scholar]
- 34.Mutt V. Secretin and cholecystokinin. In: Mutt V, editor. Gastrointestinal hormones. Academic; San Diego: 1988. pp. 251–320. [Google Scholar]
- 35.Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and nonpyramidal neurons in the hippocampus. J Comp Neurol. 1985;237:485–505. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- 36.Perez de la Mora M, Hernandez-Gomez AM, Menendez-Franco J, Fuxe K. Cholecystokinin-8 increases K+-evoked [3H]γ-aminobutyric acid release in slices from various brain areas. Eur J Pharmacol. 1993;250:423–430. doi: 10.1016/0014-2999(93)90029-h. [DOI] [PubMed] [Google Scholar]
- 37.Rehfeld JF, Hansen HF, Marley PD, Stengaard-Pederson K. Molecular forms of cholecystokinin in the brain and relationship to neuronal gastrins. Ann NY Acad Sci. 1985;448:11–12. doi: 10.1111/j.1749-6632.1985.tb29902.x. [DOI] [PubMed] [Google Scholar]
- 38.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan MJ, De Belleroche JS. Facilitation of GABA release by cholecystokinin and caerulein in rat cerebral cortex. Neuropeptides. 1985;3:429–434. doi: 10.1016/0143-4179(83)90033-1. [DOI] [PubMed] [Google Scholar]
- 40.Sherman-Gold R. The axon guide for electrophysiology & biophysics laboratory techniques. Axon Instruments; Foster City, CA: 1993. [Google Scholar]
- 41.Sloviter RS, Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J Comp Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- 42.Somogyi P, Hodgson AJ, Smith AD, Nunzi MG, Gorio A, Wu J-Y. Different population of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984;4:2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderhaeghen JJ, Signeau JC, Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975;257:604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- 44.Wank SA, Pisegna JR, deWeerth A. Cholecystokinin receptor family: molecular cloning, structure, and functional expression in rat, guinea pig, and human. Ann NY Acad Sci. 1994;713:49–66. doi: 10.1111/j.1749-6632.1994.tb44052.x. [DOI] [PubMed] [Google Scholar]
- 45.Williams S, Samulack DD, Beaulieu C, Lacaille JC. Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol. 1994;71:2217–2235. doi: 10.1152/jn.1994.71.6.2217. [DOI] [PubMed] [Google Scholar]
- 46.Woodruff GN, Hill DR, Boden PR, Pinnock R, Singh L, Hughes J. Functional role of brain CCK receptors. Neuropeptides [Suppl] 1991;19:45–56. doi: 10.1016/0143-4179(91)90082-t. [DOI] [PubMed] [Google Scholar]
- 47.Wu T, Wang H. The excitatory effect of cholecystokinin on rat striatal neurons: ionic and cellular molecular mechanisms. Eur J Pharmacol. 1996a;307:125–132. doi: 10.1016/0014-2999(96)00213-0. [DOI] [PubMed] [Google Scholar]
- 48.Wu T, Wang H. Gαq/11 Mediates cholecystokinin activation of the cationic conductance in rat substantia nigra dopaminergic neurons. J Neurochem. 1996b;66:1060–1066. doi: 10.1046/j.1471-4159.1996.66031060.x. [DOI] [PubMed] [Google Scholar]
- 49.Zarbin MA, Innis RB, Wamsley JK, Snyder SH, Kuhar MJ. Autoradiographic localization of cholecystokinin receptors in rodent brain. J Neurosci. 1983;3:877–906. doi: 10.1523/JNEUROSCI.03-04-00877.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zetler G. Anticonvulsant effects of caerulin and cholecystokinin octapeptide compared with those of diazepam. Eur J Pharmacol. 1980;65:297–300. doi: 10.1016/0014-2999(80)90405-7. [DOI] [PubMed] [Google Scholar]
- 51.Zhang LX, Zhou Y, Du Y, Han JS. Effect of CCK-8 on audiogenic epileptic seizures in P77PMC rats. Neuropeptides. 1993;25:73–76. doi: 10.1016/0143-4179(93)90072-i. [DOI] [PubMed] [Google Scholar]
- 52.Zhang LX, Smith MA, Kim SY, Rosen JB, Weiss SRB, Post RM. Changes in cholecystokinin mRNA expression after amygdala kindled seizures: an in situ hybridization study. Mol Brain Res. 1996;35:278–284. doi: 10.1016/0169-328x(95)00230-p. [DOI] [PubMed] [Google Scholar]