Abstract
We demonstrate that the NMDA receptor is involved in taste learning in the insular cortex of the behaving rat and describe two facets of this involvement. Blockage of the NMDA receptor in the insular cortex by the reversible antagonist APV during training in a conditioned taste aversion (CTA) paradigm impaired CTA memory, whereas blockage of the NMDA receptor in an adjacent cortex or before a retrieval test had no effect. When rats sampled an unfamiliar taste and hence learned about it, either incidentally or in the context of CTA training, the tyrosine phosphorylation of the NMDA receptor subunit 2B (NR2B) in the insular cortex was specifically increased. The level of tyrosine phosphorylation on NR2B was a function of the novelty of the taste stimulus and the quantity of the taste substance consumed, properties that also determined the efficacy of the taste stimulus as a conditioned stimulus in CTA; however, blockage of the NMDA receptor by APV during training did not prevent tyrosine phosphorylation of NR2B. We suggest that tyrosine phosphorylation of NR2B subserves encoding of saliency in the insular cortex during the first hours after an unfamiliar taste is sampled and that this encoding is independent of another, necessary role of NMDA receptors in triggering experience-dependent modifications in the insular cortex during taste learning. Because a substantial fraction of the NR2B protein in the insular cortex seems to be expressed in interneurons, saliency and the tyrosine phosphorylation of NR2B correlated with it may modulate inhibition in cortex.
Keywords: taste learning, conditioned taste aversion, insular cortex, latent inhibition, NMDA receptor subunit 2B, tyrosine phosphorylation
Information about taste in the rat converges on the central gustatory area (GC) in the insular cortex (Finger, 1987;Hettinger and Frank, 1992). Rats lacking GC can still react to gustatory input (Braun et al., 1982), but lesions in GC cause marked deficits in various aspects of taste learning (Braun et al., 1972; Dunn and Everitt, 1988; Bermudez-Rattoni and McGaugh, 1991; Gallo et al., 1992; Kiefer and Orr, 1992). Such results led to the hypothesis that the gustatory area in the insular cortex plays only a minor role in fundamental taste detection and reactivity but a major role in higher level processing of taste information (Braun et al., 1982).
A convenient paradigm for investigating taste learning is conditioned taste aversion (CTA) (Revusky and Garcia, 1970; Bures et al., 1988;Schafe et al., 1995). In CTA, organisms learn to avoid a novel taste if its ingestion is followed by transient poisoning. The behavioral manifestations of CTA have been investigated extensively over the years (Bures et al., 1988), but the neurobiological foundations of this experience-dependent behavior are not yet elucidated (Chambers, 1990;Yamamoto et al., 1994). We have approached the role of the insular cortex in taste learning in general, and in CTA in particular, by using local transient metabolic lesions and by correlating molecular alterations that are unveiled in insular cortex during learning and afterward with the behavioral change. Using such methods, we were able to show that taste memory requires protein synthesis (Rosenblum et al., 1993) and cholinergic activity (Naor and Dudai, 1996) in the insular cortex during training, and that sampling a novel taste, either incidentally or in the context of CTA training, specifically enhances tyrosine phosphorylation of a set of proteins in that cortex (Rosenblum et al., 1995).
The major protein whose tyrosine phosphorylation is modulated by taste learning in the insular cortex is a postsynaptic density (PSD) constituent of molecular weight (MW) 180 kDa (Rosenblum et al., 1995). The main 180 kDa tyrosine kinase substrate in the PSD is the 2B subunit of the NMDA receptor (NR2B) (Moon et al., 1994; Lau and Huganir, 1995). NR2B, which is expressed throughout the embryonic rat brain but becomes restricted to the forebrain in the adult (Mori and Mishina, 1995), is considered to play an essential role in neuronal pattern formation and plasticity (Kutsuwada et al., 1996). Tyrosine phosphorylation of NR2A or NR2B alters the NMDA receptor functional properties in vitro (Yu et al., 1997). Tyrosine phosphorylation of NR2B also was implicated recently in long-term potentiation (LTP) in the hippocampal formation in vivo (Rosenblum et al., 1996b; Rostas et al., 1996). In the present report we describe the participation of the NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the insular cortex and propose that cortical NMDA receptors play multiple roles in CTA, and specifically that tyrosine phosphorylation of NR2B is related to the encoding of sensory input saliency in cortex.
MATERIALS AND METHODS
Animals. Male Wistar rats (∼60 d old, 200–250 gm) were used. They were caged individually at 22 ± 2°C in a 12 hr light/dark cycle.
Reagents. Polyclonal antiphosphotyrosine (αPY) and αPY-agarose (monoclonal PY20) were from Zymed (San Francisco, CA). Horseradish peroxidase (HRP)-linked protein A and the enhanced chemiluminescence (ECL) kit were from Amersham (Buckinghamshire, UK). Protein A-Sepharose was from Pharmacia (Uppsala, Sweden), biotinylated goat anti-rabbit and Elite avidin–biotin complex were from Vector (Burlingame, CA), RNase A and diaminobenzidine (DAB) peroxidase substrate (SIGMAFAST) were from Sigma (St. Louis, MO), and MK-801 and APV were from RBI (Natick, MA). 35S-UTP was from Amersham, and NdeI was from New England Biolabs (Beverly, MA). All other chemicals were of analytical grade or the highest grade available.
Preparation of anti-NR2B (αNR2B). We have prepared polyclonal antibodies to NR2B using specific peptides as immunogens. Two polyclonal antibodies were generated: ab971 and ab013. For preparation of the first, three peptides from the C-terminal portion of NR2B were synthesized (Biological Services, The Weizmann Institute of Science): residues 1085–1101 (peptide A, NH2-YKDSLKKRPASAKSRRE-COOH), 1103–1118 (peptide B, NH2-DEIELAYRRRPPRSPD-COOH), and 1464–1482 (peptide C, NH2-NGSSNGHVYEKLSSIESDV-COOH). The mixture of the three peptide-KLH conjugates was injected into rabbits in complete Freund’s adjuvant, and one of the sera, ab971, was used in the preliminary set of experiments. Analysis of the binding of ab971 to the different peptides and their mixture in an ELISA test showed that >90% of the response was contributed by peptide B. Subsequently, a second antibody was raised against peptide B alone, ab013, and this was used throughout the rest of the study. Results obtained with ab971 and ab013 were identical. The sequence of peptide B is specific to NR2B (Ishii et al., 1993). The specificity of the antibodies was established by several tests: the reaction of ab013 with the 180 kDa polypeptide was not detected in the preimmune serum and was completely blocked by peptide B (10 μm). In addition, we expressed the NR2A and NR2B proteins in vitro, using the NR2A cDNA cloned in pCDNA3 and the NR2B cloned in pBluescript KS(−) (kindly provided by S. Nakanishi, Kyoto University) in the TnT-coupled reticulate lysate systems (Promega, Madison, WI); ab971 precipitated the NR2B protein (a 162 kDa protein in the reticulate lysate transcription and translation system) but not the NR2A protein (data not shown).
Behavioral procedures. CTA was performed as described inRosenblum et al. (1993), with minor modifications. Unless indicated otherwise, saccharin (0.1% w/v, sodium salt) was used as the unfamiliar taste in training [i.e., the conditioned stimulus (CS)], and injection of LiCl (0.15 m, 2% body weight, i.p.) as the malaise-inducing agent [unconditioned stimulus (UCS)]. At the beginning of the behavioral experiment, the rats were trained for 3 d to get their daily water ration once a day for 10 min from two pipettes each containing 10 ml of water. On the conditioning day, they were allowed to drink the saccharin solution instead of water from similar pipettes for 10 min, and 50 min later were injected with LiCl. Under these conditions, 3 d after training the conditioned rats preferred water to saccharin at a ratio of 9:1 in a multiple choice test situation (three pipettes with 5 ml of saccharin each, three with 5 ml of water each), whereas nonconditioned rats preferred saccharin to water. The behavioral data are presented below in terms of aversion index, defined as [ml water/(ml water + ml saccharin)] consumed in the test; 0.5 is chance level, and the higher the aversion index, the more the rats prefer water to the conditioned taste.
In some experiments, a latent inhibition procedure (Lubow, 1989) was combined with CTA to further isolate the effect of taste learning on cortical molecular mechanisms from the potential confounding effects of the UCS and the CS–UCS association. Latent inhibition is a process by which preexposure to a sensory stimulus diminishes the ability of that same stimulus to serve as an associated stimulus in subsequent learning. Thus, exposure of rats to an unfamiliar taste several days before this same taste serves as the CS in CTA training, significantly reduces the acquired aversion (Rosenblum et al., 1993). Under such conditions, the degree of aversion after CTA training is a measure of the memory of saccharin acquired incidentally (Hebb, 1949) in the pre-CTA trial. In latent inhibition experiments, the rats were exposed either to saccharin or water (control) for 10 min, in two pipettes of 10 ml each, 3 d before CTA training, as described above, in which saccharin was used as the CS. Testing was also as described above for the usual CTA procedure.
Surgery and microinjection. Rats were anesthetized with 5.6 ml/kg Equithesin (2.12% w/v MgSO4, 10% v/v ethanol, 39.1% v/v propylene glycol, 0.98% w/v sodium pentobarbitone, 4.2% w/v chloral hydrate), restrained in a stereotaxic apparatus (Kopf), and implanted bilaterally with a guide stainless steel cannula (23 gauge) aimed 1.0 mm above the gustatory neocortex [anteroposterior +1.2 mm, lateral ±5.5 mm, ventral 5.5 mm relative to bregma; according to Paxinos and Watson (1986)]. The cannulae were positioned in place with acrylic dental cement and secured by two skull screws. A stylus was placed in the guide cannulae to prevent clogging. Animals were allowed 1 week to recuperate before being subjected to experimental manipulations. The stylus was removed from the guide cannula, and a 28 gauge injection cannula, extending 1.0 mm from the tip of the guide cannula, was inserted. The injection cannula was connected via PE20 tubing to a Hamilton microsyringe driven by a microinfusion pump (CMA/100, Carnegie Medicin). Microinjection was performed bilaterally in a 1 μl vol/hemisphere delivered over 1 min. The injection cannula was left in position before withdrawal for an additional 1 min to minimize dragging of the injected liquid along the injection tract.
Homogenization and fractionation. Rats were decapitated either 60 min after the completion of the exposure to the unfamiliar taste (taste only groups), 10 min after the completion of CTA training (CTA groups), or 10 min after intraperitoneal injection of LiCl (LiCl only groups). The insular cortex containing the GC, or other brain areas indicated in Results, were dissected out. For the insular cortex, the crossing of the rhinal fissure and the medial cerebral artery was used as a reference point, and cortical tissue 1.0 mm rostral, 0.5 mm caudal, and 1.5 mm dorsal to it was excised. Two homogenization and processing protocols were used. In protocol A, the tissue was homogenized in a glass–Teflon homogenizer in SDS sample buffer containing 10% glycerol, 5% β-mercaptoethanol, and 2.3% SDS, in 62.5 mm Tris-HCl, pH 6.8. This type of homogenate was then subjected to SDS-PAGE and immunoblotting with αPY or αNR2B, as detailed below, for the determination of the level of protein tyrosine phosphorylation. Protocol B was used in experiments in which the level of phosphotyrosine on NR2B was determined and included affinity purification of the tyrosine phosphorylated protein fraction before SDS-PAGE and immunoblotting with αNR2B, as detailed below. In this protocol, the tissue from two to four rats from the same experimental group was combined and homogenized in 0.35 m sucrose, 0.5 mm EGTA, 2 mm EDTA, 2 mmNa3VO4, and 1 mm PMSF, in 10 mm Tris-HCl, pH 7.4. The samples were centrifuged for 1 min at 2000 × g, the supernatant was collected, the pellet was recentrifuged as above, and the supernatants were combined and diluted 1:1 with 2% SDS. The sample was then sonicated for 10 min and boiled for 5 min, followed by dilution of 1:10 in 100 mmNaCl, 5 mm EDTA, 50 mm NaF, 0.1% SDS, 0.1% Triton X-100, and 1 mmNa3VO4, in 50 mm Tris-HCl, pH 7.5. The resulting aliquots were subjected to affinity chromatography on αPY-agarose. After application and equilibration for 2 hr at room temperature, the resin was washed with 100-fold packed resin volume of the application buffer, and the tyrosine phosphorylated protein fraction was then eluted with 40 mmp-nitrophenyl phosphate (PNPP).
Western blot analysis. Aliquots in SDS sample buffer (equal amounts of protein) were subjected to SDS-PAGE (Laemmli, 1970) (7.5% polyacrylamide in the presence of SDS and β-mercaptoethanol) and Western blot analysis (Burnette, 1981). The amount of protein in each sample was always determined before loading, and the same amount of protein (range, 25–50 μg) was loaded in each lane (Lowry et al., 1951). After the run the lanes were also compared by Ponceau staining. After it was blocked with 1% BSA, the blot was reacted either overnight at 4°C or for 1 hr at room temperature with either αPY or αNR2B. The presence of the αPY or αNR2B was determined with HRP-linked protein A and the ECL kit. Quantification was performed using a computerized densitometer and image analyzer (Molecular Dynamics, Sunnyvale, CA).
Immunocytochemistry. Rats were anesthetized with Equithesin as above and perfused transcardially with 200 ml PBS at ambient temperature and then with 2.5% paraformaldehyde in cold PBS containing 5% sucrose, pH 7.4. The brain was removed immediately after the perfusion and placed overnight in a fixative containing 1% paraformaldehyde in PBS and 30% sucrose. Fifty-micrometer-thick sections were cut using a freezing microtome and put in 0.01% sodium azide in PBS in a 24-well plate and kept at 4°C until they were used. The sections were washed three times, 5 min each, in PBS and then treated with 0.9% H2O2 in 50% methanol in PBS for 30 min. The sections were washed five times in PBS as above and immersed for 15 min in 0.15 m glycine in PBS, pH 7.4. Then they were blocked with 20% normal goat serum (NGS) in PBS and 0.5% Triton X-100 for 1–3 hr at 37°C. After they were blocked, the sections were incubated with αNR2B (1:500 in 2% NGS in PBS) overnight at room temperature. For control, 2% NGS was used in the absence of αNR2B. Sections were washed three times and incubated for 1.5 hr with 1:200 biotinylated goat anti-rabbit in 2% NGS. Then they were washed three times as above and incubated for 1.5 hr with avidin–biotin complex. The sections were washed once in PBS and twice in 50 mm Tris-Cl, pH 7.4. Antibody binding was visualized using a DAB peroxidase substrate tablet set. The sections were mounted on gelatinized slides, dried, counterstained with hematoxylin, dehydrated in alcohol, and covered with Permount.
In situ hybridization. Levels of NR2B mRNA were examined in brain sections using in situ hybridization of a35S-labeled cRNA probe complementary to residues 4069–4560 of the C terminal of NR2B mRNA. The antisense cRNA was transcribed from a NdeI-linearized pBluescript KS−/NR2B (S. Nakanishi, Kyoto University), with T7 RNA polymerase in the presence of 35S-UTP. The brain was removed rapidly and frozen on dry ice. Brain sections (30 μm) mounted on gelatinized slides were dried for 2 min at 52°C and then fixed in 4% paraformaldehyde for 20 min at room temperature, rinsed in PBS for 5 min, and reacted with 0.25% acetic anhydride in 0.1 mtriethanolamine for 10 min at room temperature. The sections were rinsed in PBS and dehydrated by successive rinses in 70%, 80%, 95%, and 100% ethanol. The slides were then dried for 1 hr at room temperature. The sections were hybridized with the35S-labeled NR2B cRNA in hybridization solution at 47°C for 12 hr. After hybridization, the sections were incubated in the following solutions: 5× SSC, 10 mm DTT at 50°C for 30 min; 50% formamide, 2× SSC, 100 mm DTT at 65°C for 20 min and ×3 in washing solution (5 mm EDTA, 0.4m NaCl in 10 mm Tris-Cl, pH 7.5) at 37°C for 15 min each. This was followed by incubation with 20 mg RNase A in washing solution at 37°C for 30 min, washing solution at 37°C for 15 min, then 2× SSC at 37°C for 15 min, and finally 0.1× SSC at 37°C for 15 min. The sections were dehydrated in ethanol/0.3m ammonium acetate series: 30%, 60%, 80%, and 95%. Slides were washed in 100% ethanol, dried, dipped in Kodak NTB-2 emulsion at 45°C, and stored for 7 d at 4°C in a light-tight box. Developing was in Kodak D-19 for 2 min, followed by incubation in 1% acetic acid for 1 min, Kodak X-ray fixer (AL4) for 4 min, and water for 5 min at 16°C followed by 6 × 5 min at room temperature. The slides were lightly stained with hematoxylin/eosin as a counterstain.
RESULTS
The effect of taste learning on the tyrosine phosphorylation of NR2B in the insular cortex
In rats sampling an unfamiliar taste and hence learning about it, either incidentally or in the context of CTA, tyrosine phosphorylation of a set of proteins in the insular cortex but not in other brain areas is specifically increased (Rosenblum et al., 1995). The major modulated proteins are of MW 100, 115, and 180 kDa (ibid) (Fig.1A). We now have established that a 180 kDa phosphoprotein, which is the most prominent member of the aforementioned set, is NR2B. This was determined by subjecting rats to taste learning and then quantitatively immunoblotting the tyrosine phosphorylated fraction, isolated from the insular cortex by affinity chromatography on an αPY-resin, with αNR2B (Fig.1B). Mere intraperitoneal injection of LiCl, i.e., the UCS used in CTA training, had no effect (Fig.1A–C). The effect of taste experience on the tyrosine phosphorylation of the 180 kDa polypeptide was similar regardless of whether αPY or αNR2B was used on the blot. In the set of experiments summarized in Figure 1C, the ratio of tyrosine phosphorylation in saccharin-experienced rats to that in water-experienced rats (S/W) was 1.60 ± 0.11 (n = 7) using αPY and 1.63 ± 0.15 (n = 11) using αNR2B. The total level of NR2B in the insular homogenate before purification on the αPY affinity resin was not altered by experience: S/W = 1.05 ± 0.06 (n = 8). Assuming that the αPY affinity resin retains tyrosine-phosphorylated NR2B whether it is phosphorylated on one or more tyrosine residues, one may infer from the above data that the increase in tyrosine phosphorylation is attributable to an increase in the number of phosphorylated receptor molecules rather than merely an increase of phosphorylation on already phosphorylated molecules.
Fig. 1.
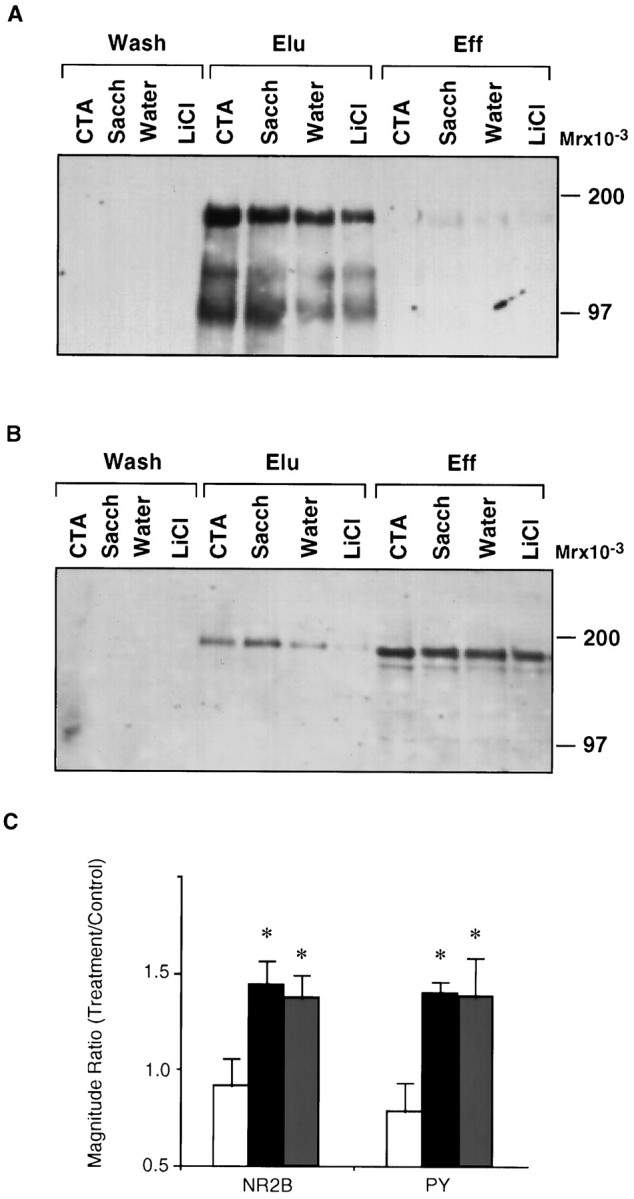
The effect of gustatory experience on the tyrosine phosphorylation of NR2B in the insular cortex. A, Electrophoretogram of fractions from the insular cortex of rats subjected to gustatory and/or visceral experience, eluted from αPY affinity resin and immunoblotted with αPY. Eff, Fraction washed from the resin in the application buffer;Elu, fraction eluted with PNPP; Wash, fraction washed before PNPP elution. For experimental procedures see Materials and Methods. LiCl, Sample from the insular cortex of rats injected intraperitoneally with LiCl, without previous exposure to a novel taste; Water, rats drinking water (used as for determining basal level of tyrosine phosphorylation after drinking a familiar solution); Sacch, rats drinking an unfamiliar taste, saccharin; CTA, rats trained on CTA using saccharin as the unfamiliar taste. B, Same as inA but immunoblotted with αNR2B; C, summary of data from experiments like those depicted inA (PY) and B(NR2B) above. Open bar,LiCl; black bar, CTA;gray bar, Sacch. Water was used as control. Values are mean ± SEM; n = 3–6 each; * p < 0.04.
The effect of the unfamiliar taste on tyrosine phosphorylation was not confined to saccharin. A similar increase in tyrosine phosphorylation of NR2B in the insular cortex was detected after a drink for the first time of a solution of 0.6% NaCl (1.65-fold increase in tyrosine phosphorylation of NR2B relative to water intake, two experiments) or 1.0% monosodium glutamate (1.56-fold increase, one experiment).
CTA and tyrosine phosphorylation of NR2B as a function of the amount of unfamiliar taste consumed
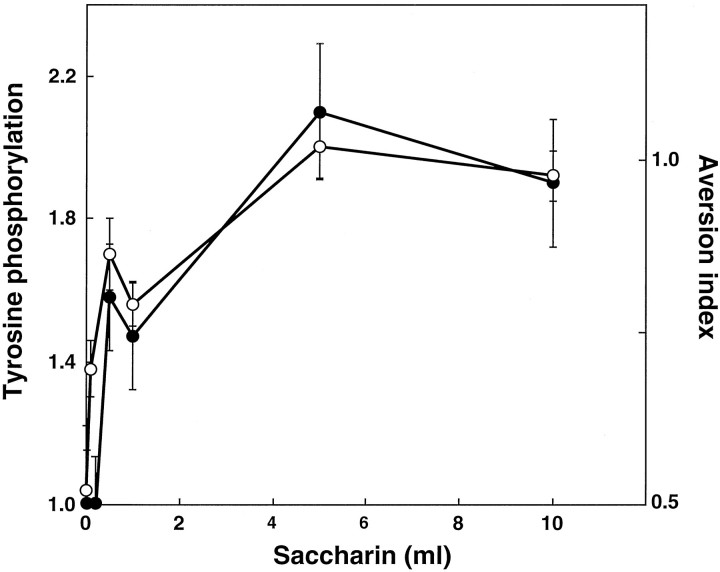
The effect of experiencing the unfamiliar taste on the tyrosine phosphorylation of NR2B was found to depend on the dose of the taste consumed: maximal phosphorylation was obtained after a consumption of ∼5 ml of saccharin (Fig. 2). A similar dose–response curve was obtained for the dependence of CTA behavior on the amount of saccharin consumed in training (Fig. 2).
Fig. 2.
Tyrosine phosphorylation on NR2B and CTA memory as a function of the amount of saccharin (i.e., the unfamiliar taste) consumed during CTA training. Open circles, Aversion index; closed circles, fold increase in tyrosine phosphorylation at 10 min after the completion of CTA training. A different group of animals was used for each data point (n = 6–10 each).
Tyrosine phosphorylation of NR2B as a function of familiarity with the taste
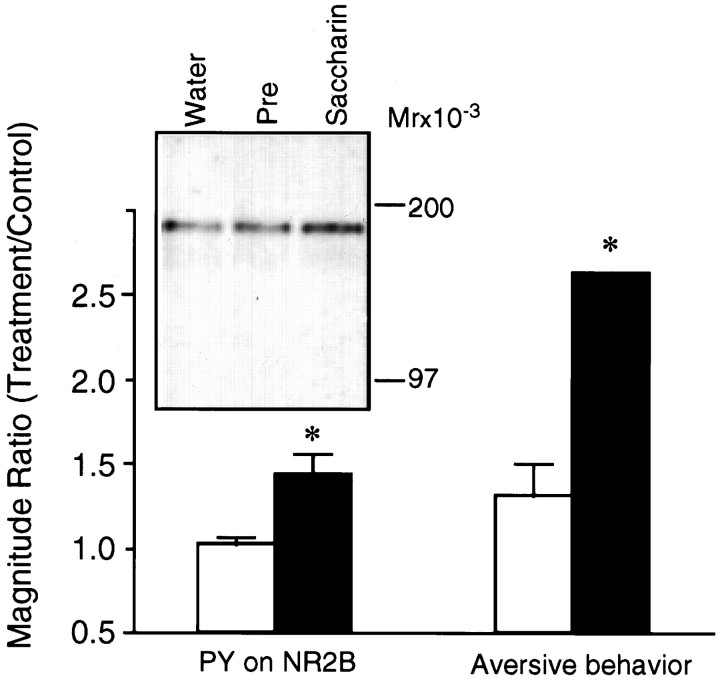
The question was asked whether familiarity with the taste decreases the effect of drinking it on tyrosine phosphorylation. From behavioral experiments it is known that familiarity with a taste diminishes the ability of that same taste to serve as the conditioned stimulus in CTA. This is an instance of the more general phenomenon of latent inhibition, in which memory of a sensory stimulus diminishes the ability of that same stimulus to serve as associative stimulus in subsequent learning (Lubow, 1989). A single preexposure to saccharin indeed significantly decreased the effectiveness of subsequent CTA training with saccharin; the same preexposure also significantly decreased the effect of drinking the saccharin solution on tyrosine phosphorylation of NR2B in the insular cortex (Fig.3).
Fig. 3.
Tyrosine phosphorylation on NR2B (PY on NR2B) and CTA memory (Aversive behavior) as a function of the familiarity of the taste consumed during training.Left, inset, A representative blot of samples immunoblotted with αNR2B. Water, Rats receiving water only; Pre (open bar), rats preexposed once to 15 min of saccharin drinking, 24 hr before the experiment; Saccharin (closed bar), rats sampling saccharin for the first time. Magnitude was the intensity of ECL reaction on the immunoblot; control was drinking water instead of saccharin. Values are mean ± SEM; * p < 0.01 (n = 5 experiments). Right, Rats were trained on CTA using saccharin either as a novel taste (closed bar) or 24 hr after a 15 min preexposure to it (open bar). Magnitude was the aversion index. Control was substitution of saccharin with water on the preexposure day. Values are mean ± SEM; * p < 0.01 (n = 4 animals).
The effect of an NMDA receptor antagonist in the insular cortex
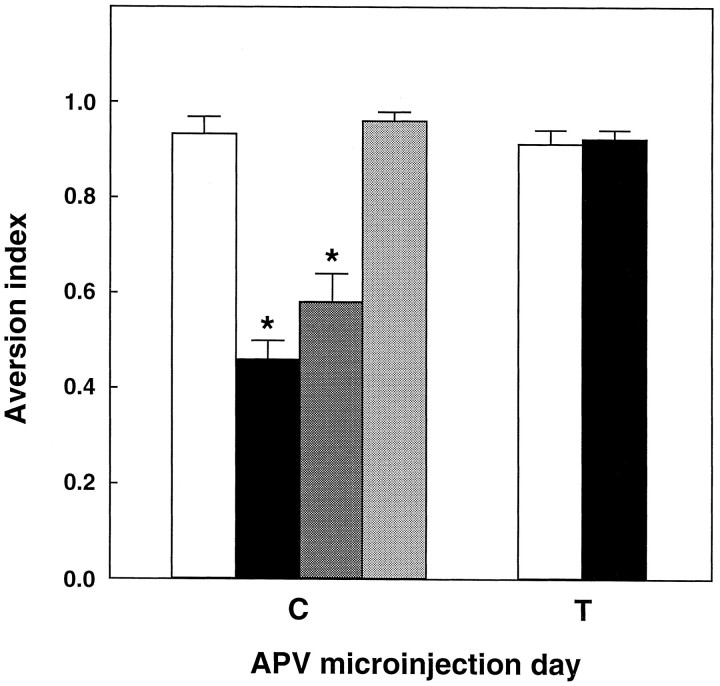
To determine whether the NMDA receptor in the insular cortex is necessary for taste learning, we locally microinjected an NMDA receptor antagonist, APV (Watkins and Olverman, 1987), in that cortex either before exposure to the novel taste or before intraperitoneal injection of the LiCl in CTA training. APV in the insular cortex during training impaired subsequent CTA memory, without impairing sensory, motivational, or motor abilities required for proper reaction to the taste solution and for expression of memory once it has been formed (Fig. 4). Furthermore, the effect on CTA was not observed when the APV was injected 2 mm above the GC (Fig. 4).
Fig. 4.
Effect of the NMDA receptor antagonist APV in the insular cortex on CTA memory. The antagonist was microinjected into the cortex as described in Materials and Methods before or in CTA training (C) or before testing, 3 d after training (T). Open bar, Microinjection of ACSF as control; black bar, 10 μg APV, 30 min before exposure to saccharin;dark shaded bar, 10 μg APV, 30 min before LiCl injection in CTA training. Light shaded bar, APV microinjected 2 mm above the coordinates used for injection into the insular cortex. Values are mean ± SEM; n = 8; * p < 0.01.
Although APV in the insular cortex impaired CTA when injected into the insular cortex before and during training as above, it had no effect on the tyrosine phosphorylation of NR2B induced by the novel taste (S/W = 2.0 ± 0.1 vs 1.8 ± 0.2 in control vs APV-injected rats, respectively; n = 4 in each of the four groups of rats used in this comparison).
Localization of NR2B mRNA and protein in the insular cortex
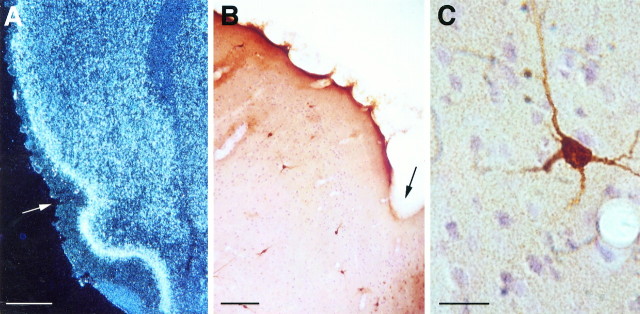
We used an RNA-labeled probe complementary to the C-terminal sequence of the NR2B to identify the anatomical localization of the NR2B message in cortex. Confirming earlier observations (Monyer et al., 1994), the mRNA was observed all over the cortex, with layers II–III displaying especially heavy staining. A continuum was observed from the piriform cortex dorsally throughout the cortex, including the insular and taste areas (Fig. 5A). To obtain information on the localization of the NR2B-expressed polypeptide, we performed an immunocytochemical analysis. Variability was revealed between different cortical regions, with heavier staining in the cingulate, retrosplenial, and motor areas (data not shown). In the insular cortex there was a low number of heavily stained neurons, especially in layers II–III (Fig. 5B). Many of these were multipolar, possibly GABAergic interneurons (Fig. 5C), similar to the situation observed in the hippocampal formation (K. Rosenblum, G. Richter-Levin, and Y. Dudai, unpublished observations). In addition, there was staining of large pyramidal cells in layer V, sometimes including their apical dendrites, with the number of these neurons increasing after moving rostrally from the agranular cortex to the granular insular cortex (data not shown).
Fig. 5.
Localization of NR2B in the insular cortex.A, Dark-field picture of a coronal section showingin situ hybridization with an RNA-labeled probe complementary to a C-terminal sequence of NR2B. Scale bar, 500 μm.B, A coronal section of the insular cortical area of rat brain after immunocytochemistry performed with αNR2B. In bothA and B, the arrowindicates the rhinal fissure. Scale bar, 150 μm. C, A typical multipolar neuron stained for αNR2B in the insular cortex. Scale bar, 20 μm.
DISCUSSION
In this work we demonstrate that the NMDA receptor is involved in taste learning in the insular cortex of the behaving rat and describe two facets of this involvement. The first is the need for NMDA receptor activity in CTA learning (but not in retrieval of CTA memory once it has been formed), and the second is the correlation of the tyrosine phosphorylation of NR2B with the novelty of the learned taste stimulus.
Previous authors differ with respect to their conclusions on the role of NMDA receptors in central gustatory plasticity (Welzl et al., 1990;Willner et al., 1992; Aguado et al., 1994; Caramanos and Shapiro, 1994;Fanslow et al., 1994). These studies used either intraperitoneal or intracerebroventricular administration of NMDA ligands, which are problematic as far as the delineation of the target area is concerned, whereas we have used direct, local application of the antagonist into the insular cortex. Furthermore, NMDA receptors are expected to be required for some but not other facets of learning, even within a single paradigm, and hence their unveiled role may differ according to the protocol used and the variables measured (Caramanos and Shapiro, 1994; Fanslow et al., 1994; Bannerman et al., 1995). Our data show that the function of the NMDA receptor in the insular cortex is obligatory for taste learning and furthermore that it may also contribute to a representation of the UCS or to an interaction of the representations of the CS and UCS in cortex. An association of the CS and UCS in CTA can happen in the absence of a functional insular cortex and probably takes place in subcortical areas (Bures et al., 1988; Yamamoto et al., 1994; Schafe et al., 1995), but this does not contradict the possibility that under normal conditions the cortex does foster an effective interaction of the representations of taste and malaise.
The present report extends our previous observation that the sampling of a novel taste, either incidentally or in the context of CTA training, is correlated with tyrosine phosphorylation in the insular cortex but not in other brain areas (Rosenblum et al., 1995), and establishes that the major protein so modulated is NR2B. Furthermore, our results demonstrate that tyrosine phosphorylation of NR2B is a function of the novelty and intensity, hence saliency, of the taste stimulus, properties that also determine the efficacy of a taste stimulus as a conditioned stimulus in CTA. NR2B has previously been shown to undergo tyrosine phosphorylation (Moon et al., 1994; Lau and Huganir, 1995). Our data thus establish that this post-translational modification takes place in brain in vivo, in the behaving rat, in response to a physiological stimulus within the context of a natural learning situation, in a cortical area that is expected to process the relevant sensory stimulus and subserve its storage.
Protein tyrosine phosphorylation has been implicated in short-term neuronal plasticity (O’Dell et al., 1991; Grant et al., 1992; Abe and Saito, 1993). The enhancement in tyrosine phosphorylation induced by taste in the insular cortex was previously shown by us to occur within minutes and last for hours (Rosenblum et al., 1995); it is hence a candidate molecular mechanism for short-term memory and for processes that subserve consolidation of short- into long-term taste memory (Dudai, 1996). This mechanism is apparently related to the memory of taste but not of the taste-malaise association, because the UCS did not significantly augment the tyrosine phosphorylation after the CS. Hippocampal LTP, a candidate cellular plasticity mechanism in the CNS, is also correlated with an increase of tyrosine phosphorylation of NR2B that lasts for several hours and may subserve short and intermediate maintenance of LTP (Rosenblum et al., 1996b; Rostas et al., 1996). Therefore it is tempting to suggest that mechanisms similar to those operating in LTP also take place in the insular cortex in response to a novel taste stimulus.
In cultured mammalian neurons, the function of the NMDA receptor channel is regulated by protein tyrosine kinases (Wang and Salter, 1994), e.g., src (Yu et al., 1997), and by protein phosphatases (Wang et al., 1994; Tong et al., 1995). Köhr and Seeburg (1996) suggest that the increase in glutamate-activated currents induced by src and fyn kinases in cultured HEK 293 embryonic kidney cells is mediated via tyrosine phosphorylation of NR2A and not NR2B, but even if this proves to hold for cortical neurons in vivo, the possibility should be considered that the effect of tyrosine phosphorylation of NR2B is not necessarily reflected in channel properties, but rather in altered function of an intracellular signal transduction machinery interfacing with the receptor (e.g., Niethammer et al., 1996; see discussion inRosenblum et al., 1996b). Altered interaction with other PSD proteins may play a role in such regulation (Kornau et al., 1995; Gomperts, 1996; Muller et al., 1996; Niethammer et al., 1996). Some of the components of such multiprotein functional conglomerates may be the 100 and 115 kDa polypeptides, whose tyrosine phosphorylation is modulated in cortex and hippocampus together with that of NR2B (Rosenblum et al., 1995, 1996b).
Although the NMDA receptor in the insular cortex was found necessary for taste learning, its function was not obligatory for the tyrosine phosphorylation of the 2B subunit. On the basis of this functional dissociation, the correlation between novelty and amount of taste and the tyrosine phosphorylation of NR2B, and the time course of the effect (Rosenblum et al., 1995), we propose that tyrosine phosphorylation of NR2B is related to the encoding of saliency in the cortex during the first hours after an unfamiliar taste is sampled and that this encoding is independent of another, necessary role of NMDA receptors, probably reliant on receptor-mediated channel activity, in triggering experience-dependent modifications in the insular cortex during taste learning. The saliency or contextual encoding is expected to involve non-NMDA glutamatergic receptors or other neuromodulatory receptors. Appealing candidates are cholinergic receptors in cortex, because transient impairment of cholinergic function in the insular cortex disrupts the encoding of information on novel tastes (Naor and Dudai, 1996), and microinjection of carbachol into the insular cortex enhances tyrosine phosphorylation of NR2B (Rosenblum et al., 1996a). A plausible model is thus that activation of the cholinergic system by contextual saliency leads to acetylcholine receptor-induced increase in Ca2+ influx in cortical neurons (Cox et al., 1994;Lev et al., 1995), resulting in activation of protein kinase(s) such as members of the src family mentioned above (Kohr and Seeburg, 1996) or the fak family (Lev et al., 1995), and culminating in enhanced phosphorylation of a set of substrates, including NR2B. This might be a molecular manifestation of the cross-talk of the glutamatergic and cholinergic systems in cortex, a cross-talk that is proposed to play a prominent function in learning and attention (Aigner, 1995).
It is not unlikely that the two roles of NMDA receptor in CTA described in the present report involve two subtypes of receptor, only one of which may contain the post-translationally modified NR2B. In this respect it is of interest to note that our immunocytochemical analysis unveils the presence of NR2B in cortical interneurons and that in the dentate gyrus, the NR2B protein is preferentially located in GABAergic interneurons and may play a role in modulating local circuit inhibition (K. Rosenblum, G. Richter-Levin, and Y. Dudai, unpublished observations). A previous immunocytochemical analysis of the distribution of the NMDA receptor protein in the rat brain identified the NR2B in cortex, especially in outer layers, but no information was provided on higher resolution of cortical tissue and on the identity of stained cells (Wenzel et al., 1995). The issue of discrepancies between NR2B mRNA and NR2B protein localization in the brain (Wenzel et al., 1995) (K. Rosenblum, D. E. Berman, and Y. Dudai, unpublished observations), and the possibility of post-transcriptional regulation of expression such as that suggested for NR2A (Wood et al., 1996), await further investigation. Nevertheless, if experience-dependent tyrosine phosphorylation of NR2B in insular cortex takes place on interneurons, the possibility arises that saliency may regulate inhibition of cortical circuits.
Footnotes
The support of the Carl Dominic Center for Brain Research is gratefully acknowledged. We thank E. Soriano for advice on the immunocytochemical analysis and T. V. P. Bliss for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Yadin Dudai, Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel.
REFERENCES
- 1.Abe K, Saito H. Tyrosine kinase inhibitors, herbimycin A and lavendustin A, block formation of long-term potentiation in the dentate gyrus in vivo. Brain Res. 1993;621:167–170. doi: 10.1016/0006-8993(93)90315-e. [DOI] [PubMed] [Google Scholar]
- 2.Aguado L, Antionio AS, Perez L, del Valle R, Gomez J. Effects of the NMDA receptor antagonist ketamine on flavor memory, conditioned aversion, latent inhibition, and habituation of neophobia. Behav Neural Biol. 1994;61:271–281. doi: 10.1016/s0163-1047(05)80010-x. [DOI] [PubMed] [Google Scholar]
- 3.Aigner TG. Pharmacology of memory: cholinergic-glutamatergic interactions. Curr Opin Neurobiol. 1995;5:155–160. doi: 10.1016/0959-4388(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- 6.Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiol Behav. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- 7.Braun JJ, Lasiter PS, Kiefer SW. The gustatory neocortex of the rat. Physiol Psychol. 1982;10:13–45. [Google Scholar]
- 8.Bures J, Buresova O, Krivanek J. Brain and behavior: paradigms for research in neural mechanisms. Wiley; New York: 1988. [Google Scholar]
- 9.Burnette WW. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 10.Caramanos Z, Shapiro ML. Spatial memory and N-methyl-d-aspartate receptor antagonists APV and MK-801: memory impairments depend on familiarity with the environment, drug dose, and training duration. Behav Neurosci. 1994;108:30–43. doi: 10.1037//0735-7044.108.1.30. [DOI] [PubMed] [Google Scholar]
- 11.Chambers KC. A neural model for conditioned taste aversions. Annu Rev Neurosci. 1990;13:373–385. doi: 10.1146/annurev.ne.13.030190.002105. [DOI] [PubMed] [Google Scholar]
- 12.Cox CL, Metherate R, Ashe JH. Modulation of cellular excitability in neocortex: muscarinic receptor and second messenger-mediated action. Synapse. 1994;16:123–136. doi: 10.1002/syn.890160206. [DOI] [PubMed] [Google Scholar]
- 13.Dudai Y. Consolidation: fragility on the road to the engram. Neuron. 1996;17:367–370. doi: 10.1016/s0896-6273(00)80168-3. [DOI] [PubMed] [Google Scholar]
- 14.Dunn LT, Everitt BJ. Double dissociation of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav Neurosci. 1988;102:3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Fanslow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-d-aspartate antagonist dl-2-amino-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behav Neurosci. 1994;108:235–240. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- 16.Finger TE. Gustatory nuclei and pathways in the central nervous system. In: Finger TE, Silver WL, editors. Neurobiology of taste and smell. Wiley; New York: 1987. pp. 331–353. [Google Scholar]
- 17.Gallo M, Roldan G, Bures J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res. 1992;52:91–97. doi: 10.1016/s0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- 18.Gomperts SN. Clustering membrane proteins: it’s all coming together with the PSD-95/SAP90 protein family. Cell. 1996;84:659–662. doi: 10.1016/s0092-8674(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 19.Grant SGN, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in the fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 20.Hebb DO. The organization of behavior: a neuropsychological theory. Wiley; New York: 1949. [Google Scholar]
- 21.Hettinger TP, Frank ME. Information processing in mammalian gustatory cortex. Curr Opin Neurobiol. 1992;2:469–478. doi: 10.1016/0959-4388(92)90182-k. [DOI] [PubMed] [Google Scholar]
- 22.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 23.Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- 24.Köhr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol (Lond) 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 26.Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Masaaki A, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lau L-F, Huganir RL. Differential tyrosine phosphorylation of N-methyl-d-aspartate receptor subunits. J Biol Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- 29.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Lubow RE. Latent inhibition and conditioned attention theory. Cambridge UP; London: 1989. [Google Scholar]
- 32.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 33.Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-d-aspartate receptor subunit 2B. Proc Natl Acad Sci USA. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori M, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- 35.Muller BM, Kistner U, Kindler S, Chung WJ, Kuhlendhal S, Fenster SD, Lau L-F, Veh RW, Huganir RL, Gundelfinger ED, Garner CC. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 36.Naor C, Dudai Y. Transient impairment of cholinergic function in the rat insular cortex disrupts the encoding of taste in conditioned taste aversion. Behav Brain Res. 1996;79:61–67. doi: 10.1016/0166-4328(95)00262-6. [DOI] [PubMed] [Google Scholar]
- 37.Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Dell TJ, Kandel ER, Grant SGN. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd Ed. Academic; New York: 1986. [Google Scholar]
- 40.Revusky S, Garcia J. Learned associations over long delays. Psychol Learn Motiv. 1970;4:3–84. [Google Scholar]
- 41.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 42.Rosenblum K, Schul R, Meiri N, Hadari YR, Zick Y, Dudai Y. Modulation of protein tyrosine phosphorylation in rat insular cortex after conditioned taste aversion learning. Proc Natl Acad Sci USA. 1995;92:1157–1161. doi: 10.1073/pnas.92.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenblum K, Berman DE, Hazvi S, Dudai Y. Carbachol mimics effects of sensory input on tyrosine phosphorylation in cortex. NeuroReport. 1996a;7:1401–1404. doi: 10.1097/00001756-199605310-00015. [DOI] [PubMed] [Google Scholar]
- 44.Rosenblum K, Dudai Y, Richter-Levin G. Long-term potentiation increases tyrosine phosphorylation of the N-methyl-d-aspartate receptor subunit 2B in rat dentate gyrus in vivo. Proc Natl Acad Sci USA. 1996b;93:10457–10460. doi: 10.1073/pnas.93.19.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rostas JAP, Bernt VA, Voss K, Errington ML, Bliss TVP, Gurd JW. Enhanced tyrosine phosphorylation of the 2B subunit of the N-methyl-d-aspartate receptor in long term potentiation. Proc Natl Acad Sci USA. 1996;93:10452–10456. doi: 10.1073/pnas.93.19.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schafe GE, Seeley R, Bernstein IL. Forebrain contribution to the induction of a cellular correlate of conditioned taste aversion in the nucleus of the solitary tract. J Neurosci. 1995;15:6791–6796. doi: 10.1523/JNEUROSCI.15-10-06789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- 48.Wang L-Y, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369:230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y-Y, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 50.Watkins JC, Olverman HJ. Agonists and antagonists for excitatory amino acid receptors. Trends Neurosci. 1987;10:265–272. [Google Scholar]
- 51.Welzl H, Alessandri B, Battig K. The formation of a new gustatory memory trace in rats is prevented by the noncompetitive NMDA receptor antagonist ketamine. Psychobiology. 1990;18:43–47. [Google Scholar]
- 52.Wenzel A, Scheurer L, Kunzi R, Fritschy JM, Mohler H, Benke D. Distribution of NMDA receptor subunit proteins NR2A, 2B, 2C and 2D in rat brain. NeuroReport. 1995;7:45–48. [PubMed] [Google Scholar]
- 53.Willner J, Gallagher M, Graham PW, Crooks GB. N-methyl-d-aspartate antagonist d-APV selectively disrupts taste-potentiated odor aversion learning. Behav Neurosci. 1992;106:315–323. doi: 10.1037//0735-7044.106.2.315. [DOI] [PubMed] [Google Scholar]
- 54.Wood MW, VanDongen HMA, VanDongen AMJ. The 5′ untranslated region of the N-methyl-d-aspartate receptor NR2A subunit controls efficiency of translation. J Biol Chem. 1996;271:8115–8120. doi: 10.1074/jbc.271.14.8115. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65:123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 56.Yu X-M, Askalan R, Keil GJ, II, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]