Abstract
The Coolidge effect describes the reinitiation of sexual behavior in a “sexually satiated” animal in response to a novel receptive mate. Given the role of the mesolimbic dopamine (DA) system in the initiation and maintenance of motivated behavior, microdialysis was used to monitor nucleus accumbens (NAC) DA transmission during copulation, sexual satiety, and the reinitiation of sexual behavior. In agreement with earlier reports, the presentation of an estrous female behind a screen and copulation were associated with significant increases in NAC DA efflux. Return of NAC DA concentrations to baseline values coincided with a period of sexual satiety, although concentrations of the DA metabolites, dihydroxyphenylacetic acid and homovanillic acid, remained elevated. The presentation of a novel receptive female behind a screen resulted in a slight increase in NAC DA, which was augmented significantly during renewed copulation with the novel female. The present data suggest that the stimulus properties of a novel receptive female may serve to increase NAC DA transmission in a sexually satiated male rat, and this, in turn, may be related to the reinitiation of sexual behavior.
Keywords: sexual behavior, nucleus accumbens, microdialysis, dopamine (DA), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), rat, sexual satiation, Coolidge effect, incentive motivation, copulation, reward, appetitive, consummatory, mesolimbic, exhaustion, novelty
A male rat that has copulated to satiety can be induced to mate again if the initial female is replaced with a novel receptive female. This has come to be known as the Coolidge effect and has been observed in a number of mammalian species (Wilson et al., 1963). General factors such as fatigue or motoric depression are not sufficient to explain the apparent state of sexual satiation, because stimuli from a novel female can still induce copulation. Sexual satiety can also be “reversed” pharmacologically, to a significant degree, by the administration of a variety of drugs that can act on different neurotransmitter systems. These drugs include yohimbine, 8-OH-DPAT (Rodriguez-Manzo and Fernandez-Guasti, 1994, 1995a), nalaxone (Pfaus and Gorzalka, 1987; Rodriguez-Manzo and Fernandez-Guasti, 1995a,b), and apomorphine (Mas et al., 1995c). Although the peripheral action of these drugs cannot be ruled out (e.g., adrenergic effects on erectile function), effects on central mechanisms underlying sexual satiety have been proposed on the basis of selective central noradrenergic lesion experiments (Rodriguez-Manzo and Fernandez-Guasti, 1995a) and microdialysis experiments that monitored dopaminergic metabolism in the medial preoptic area (Mas et al., 1995a,b).
Given that central mechanisms may mediate the reinitiation of sexual behavior characteristic of the Coolidge effect, a likely candidate is the mesolimbic dopamine (DA) system, projecting from the ventral tegmental area to the NAC. Mesolimbic DA seems to act as a primary modulator in complex integrative processes that involve the evaluation of environmental stimuli, such as cues from a sexually receptive female, and the organization of goal-directed behaviors, including copulation (Fibiger and Phillips, 1986; Blackburn et al., 1992; Phillips et al., 1992; LeMoal, 1995; Salamone, 1996).
Although midbrain DA neurons respond to primary rewards and cues predictive of reward, novel or unpredictable environmental stimuli induce neuronal activation most robustly over repeated training sessions (Fabre et al., 1983; Schultz, 1992; Mirenowicz and Schultz, 1994). There is a great deal of evidence that supports an important facilitatory role for mesolimbic DA in the initiation and maintenance of rat sexual behavior (Pfaus and Everitt, 1995), and a number of microdialysis studies report increases in NAC DA efflux during appetitive and consummatory phases of male sexual behavior (Pfaus et al., 1990; Pleim et al., 1990; Damsma et al., 1992; Wenkstern et al., 1993; Fumero et al., 1994; Mas et al., 1995b). There are, however, relatively few data on the neurochemical correlates of sexual satiation and the reinitiation of sexual behavior. The application of in vivo microdialysis to monitor mesolimbic DA neurotransmission during the Coolidge effect provides a unique opportunity to examine the role of NAC DA in copulation, sexual satiety, and the reinitiation of copulation.
A microdialysis experiment was conducted to determine the following: (1) whether the onset of sexual satiety is accompanied by the return of extracellular DA concentrations in the NAC to precopulation values or below, and (2) whether the reinstatement of copulatory behavior in a “sexually satiated” male rat with a novel receptive female is correlated with increases in NAC DA efflux.
MATERIALS AND METHODS
Subjects. Male Sprague Dawley rats, obtained from the Animal Care Centre (at the University of British Columbia), and female Long–Evans rats, obtained from Charles River Canada (St. Constant, Quebec, Canada), were housed in wire mesh cages (18 × 25 × 65 cm; five per cage) in separate colony rooms. Colony rooms were maintained at a temperature of ∼20°C on a reverse 12 hr light/dark cycle. Rats had unlimited access to food (Purina Rat Chow) and water.
Surgery and behavioral testing before brain microdialysis.Female rats were ovariectomized bilaterally under halothane gas anesthesia (Fluothane, Ayerst Laboratories) at least 4 weeks before testing. Sexual receptivity in the stimulus females was induced by subcutaneous injections of estradiol benzoate (10 μg) and progesterone (500 μg), 48 and 4 hr, respectively, before each test session. Male rats were screened for sexual behavior on two occasions, 4 d apart, in Plexiglas chambers (35 × 35 × 40 cm) with wire mesh floors. Only male rats that reached a performance criterion, which included intromission within 5 min of the presentation of the female and ejaculation within 15 min of the first intromission, during the two screening tests were implanted with microdialysis probe guide cannulae.
Male rats (n = 5) were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) before stereotaxic surgery. Microdialysis probe guide cannulae (19 gauge) were implanted bilaterally over the NAC (coordinates from bregma: anterior, +1.7 mm; medial, ± 1.1 mm; ventral, −1.0 mm; flat skull) and were secured to the skull with dental acrylic and jeweler’s screws. Bilateral guide cannulae implants were used to maximize the opportunity for a successful microdialysis experiment. Fortunately, in the present experiment, only one cannula was needed for each rat. Male rats were housed individually in large plastic cages with corncob bedding for the remainder of the experiment. One week after surgery, rats were tested for sexual behavior. During this portion of training, the testing chamber was equipped with a sliding Plexiglas screen that divided the chamber into large and small compartments. Male rats were introduced into the large compartment and 15 min later, a female was placed behind the screen. After a 15 min preparatory period, the screen was removed, and the rats were allowed to copulate for 30 min. Three training sessions were conducted, one every 4 d. All rats reached the performance criterion during every session.
Coolidge effect experiment. Rats were implanted unilaterally with microdialysis probes 12–18 hr before the Coolidge effect experiment and placed in the large compartment of the testing chamber with free access to food and water. On the morning of the experiment, microdialysis samples were collected every 15 min. The experiment consisted of the following seven consecutive phases: (1) baseline (at least 60 min); (2) female 1 behind the screen (15 min); (3) copulation with female 1 until a 30 min period passed without a mount; (4) reintroduction of female 1 behind the screen (15 min); (5) access to female 1 for a 15 min period provided there was no mounting (if mounting did occur, this phase was treated as phase 3); (6) introduction of female 2 behind the screen (15 min); 7) copulation with female 2 for 60 min.
Behavior was filmed under low illumination using a JVC video system and observed on a video monitor located outside the testing room. Standard measures of sexual behavior were recorded using a computer and appropriate software (Holmes et al., 1987).
After the microdialysis experiment, animals were given an overdose of chloral hydrate and perfused intracardially with saline and formalin (4%). Brains were sliced and frozen, and, subsequently, coronal sections were stained with cresyl violet to determine the placement of microdialysis probes. Only rats with probe placements within the NAC were used for behavioral and neurochemical analyses.
Microdialysis and HPLC-electrochemical detection.Microdialysis probes were concentric in design with a semipermeable hollow fiber membrane (2 mm membrane exposed, 340 μm outer diameter, 65000 molecular weight cutoff, Filtral 12, Hospal) at the distal end. Probes were perfused at 1.0 μl/min with a modified Ringer’s solution (0.01 m sodium phosphate buffer, pH 7.4, 1.3 mmCaCl2, 3.0 mm KCl, 1.0 mmMgCl2, 147 mm NaCl) using a gastight syringe (Hamilton, Reno, NV) and a syringe pump (model 22, Harvard Apparatus, South Natick, MA). A microdialysis probe guide collar was used to secure the microdialysis probe inside the guide cannula. A steel coil, attached to a liquid swivel (Instech 375s) that was mounted on top of the testing chamber, was used to protect the probe tubing (Fiorino et al., 1993).
Microdialysate analytes, which included DA and its metabolites dihyroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), were separated by reverse-phase chromatography (Ultrasphere column; Beckman, Fullerton, CA, ODS 5 μm, 15 cm, 4.6 mm, inner diameter) using a 0.083m sodium acetate buffer, pH 3.5 (5% methanol). Analyte concentrations were quantified by electrochemical (EC) detection. The apparatus consisted of a Bio-Rad (Richmond, CA) pump, a Valco Instruments (Houston, TX) EC10W two-position injector, an ESA (Bedford, MA) Coulochem II EC detector, and a dual-channel chart recorder (Kipp and Zonen, Bohemia, NY). Electrochemical detector parameters were the following: electrode 1, +450 mV; electrode 2, −300 mV; and guard cell, −450 mV. Typical probe recoveries, conducted in vitro and at room temperature, were 22% for DA, 18% for DOPAC, and 18% for HVA.
RESULTS
Behavior
Behavioral measures from the Coolidge effect experiment are presented in Table 1. Latencies to mount, intromit, and ejaculate, as well as the postejaculatory interval after the first ejaculation were similar to those in the previous training session (data not shown). This suggests that the microdialysis procedure did not alter normal sexual behavior. The development of sexual satiation, as indicated by the mean number of ejaculations before the criterion was met (7.8 ± 0.5), a progressive decrease in the number of intromissions preceding each ejaculation, and a progressive increase in the postejaculatory interval (data not shown), was similar to that reported in previous studies (Beach and Jordan, 1956; Fowler and Whalen, 1961; Fisher, 1962; Bermant et al., 1966; Rodriguez-Manzo and Fernandez-Guasti, 1994; Mas et al., 1995d). Individual variability was observed with respect to the number of ejaculations achieved with female 1, the time spent copulating with female 1, and the number of presentations of female 1 required to reach the satiation criterion (Table 1, bottom). Some rats required numerous reintroductions of female 1 until phase 5 was complete (n = 3). The acts of placing female 1 behind the screen and the removal of the partition may have served as primary appetitive cues leading to copulation. It should also be noted that a satiation criterion of 30 min without a mount, although used previously (Beach and Jordan, 1965; Mas et al., 1995b), is arbitrary and does not guarantee that a rat would not have mounted given more time. Even so, delays or removal and replacement procedures did not result reliably in renewed copulation with female 1 (e.g., phases 4 and 5).
Table 1.
Behavior during the Coolidge effect experiment
| Measure of sexual behavior | Female 1 | Female 2 | |
|---|---|---|---|
| Mount latency | 31.2 ± 10.3 sec | 54.4 ± 30.3 sec | |
| Intromission latency | 31.2 ± 10.3 sec | 156.4 ± 75.6 sec | |
| Ejaculation latency | 534.4 ± 111.4 sec | 512.0 ± 195.9 sec, (n = 2) | |
| Postejaculatory interval | 414.8 ± 51.8 sec | 850.0 ± 53.0 sec, (n = 2) | |
| Number of mounts (1st hr) | 16.0 ± 2.1 | 11.4 ± 4.5 | |
| Number of intromissions (1st hr) | 37.0 ± 3.3 | 11.2 ± 4.5* | |
| Number of ejaculations (1st hr) | 4.2 ± 0.5 | 0.6 ± 0.41-160 | |
| Interaction with female 1: | |||
| Number of ejaculations (total) | 7.8 ± 0.5 (range, 6–11) | ||
| Time to sexual satiety | 144.0 ± 14.7 min (range, 105–195) | ||
| Number of presentations | 2.0 ± 0.5 (range, 1–4) | ||
Values are presented as means ± S.E.M. (n = 5, except where indicated). The mount latency, intromission latency, ejaculation latency, and postejaculatory interval are derived from the first copulatory bout with each female.
p < 0.05;
F1-160: p< 0.01.
All rats exhibited the Coolidge effect. The activity associated with placement of female 2 behind the screen and, in particular, the removal of the partition may have contributed to this result, but, again, these events were not by themselves sufficient to renew copulation earlier in the experiment. Comparisons between measures of sexual behavior with female 1 and female 2 were made using t tests with a Bonferroni correction. Although mount and intromission latencies in response to female 2 were not different significantly from those in the first copulatory bout with female 1, in general, sexual behavior with female 2 was less robust, as indicated by significantly fewer ejaculations (mean, 0.6 vs 4.2; F = 49.86;p < 0.01) and intromissions (mean = 11.2 vs 37.0;F = 20.17; p < 0.05) during the first hour. The numbers of mounts in the first hour with females 1 and 2 were not different significantly.
It is important to note that females used during the satiation portion of the experiment (i.e., female 1) still exhibited strong proceptive (i.e., hopping and darting) and receptive (i.e., lordosis) behavior for the complete duration of their contact with the male.
Neurochemistry
Basal nanomolar concentrations of DA and its metabolites in microdialysates, presented as the mean ± SEM, of the first three baseline samples were: DA, 3.0 ± 0.7; DOPAC, 619.1 ± 77.7; and HVA, 234.2 ± 49.0 (uncorrected for probe recovery;n = 5). These values represented 100% baseline scores.
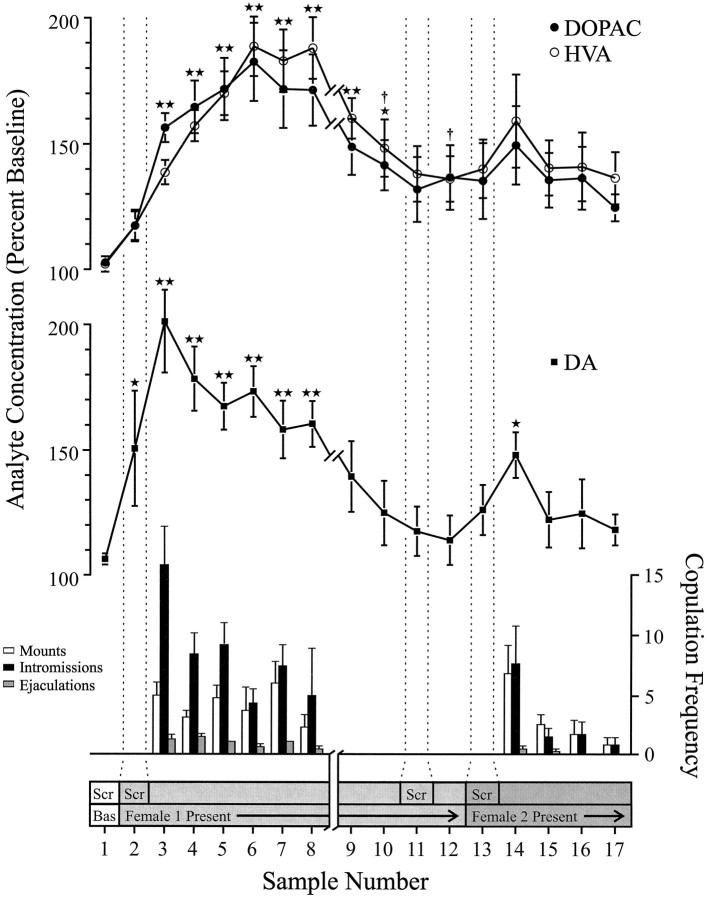
Behaviorally defined data points, corresponding to each phase of the experiment and common to every rat, were used for neurochemical analyses. These included the following: (1) seven samples after the first introduction of female 1, (2) four samples accompanying the absence of copulatory behavior with female 1, and (3) five samples after presentation of female 2. Figure 1 illustrates changes in concentrations of DA (line graph, middle) and DA metabolites (line graph, top) paralleling copulatory behavior (bar graph, bottom) during the test for the Coolidge effect.
Fig. 1.
Nucleus accumbens neurochemical correlates of sexual behavior during the Coolidge effect. The first eight samples represent chronologically continuous data points from phases 1 to 3. Sample 1 is the fourth and last precopulation baseline samples (Bas). Sample 2 represents introduction of female 1 behind the screen (Scr). After 15 min, the screen was removed, and rats were allowed to copulate (samples 3–8). Thebreak on the x-axis corresponds to the exclusion of data from three rats that copulated for extended periods with the initial female. The last nine samples were also continuous chronologically. Samples 9 and 10 correspond to the satiation period of phase 3 (i.e., 30 min without a mount). Female 1 was then reinserted behind the screen (sample 11) and, 15 min later, the screen was removed (sample 12). After 15 min devoid of copulation, female 2 was placed behind the screen (sample 13). Samples 14–17 correspond to copulation with female 2. The number of mounts, intromissions, or ejaculations associated with each 15 min microdialysis sample are shown in the bottom bar graph. Neurochemical data are expressed in terms of percentage of baseline concentrations. Changes in NAC DA (closed squares), DOPAC (closed circles), and HVA (open circles) efflux are presented as line graphs. The following comparisons were made: baseline sample 1 versus samples 2–10; new baseline sample 10 versus samples 11 and 12; new baseline sample 12 versus samples 13–17 (*p < 0.05; ** p < 0.01). Independent t tests were made between baseline values (samples 1, 10, and 12). For significant differences from the first baseline (sample 1), †p < 0.05.
Separate one-way, repeated-measures ANOVAs were performed on neurochemical data associated with female 1 (samples 1–12) and female 2 (samples 12–17). A priori comparisons were made using Dunn’s multiple comparison test (Bonferroni t). The following three main comparisons were made: (1) initial baseline (sample 1) versus samples 2–10 (first exposure to female 1), (2) second baseline (sample 10) versus samples 11 and 12 (reexposure to female 1), and (3) third baseline (sample 12) versus samples 13–17 (exposure to female 2).
There was a significant overall change in DA efflux in response to female 1 [F(11,44) = 8.48; p < 0.001] and female 2 [F(5,20) = 2.83;p < 0.05]. A significant increase in DA efflux was found when female 1 was present behind the screen (+44%,p < 0.05; sample 2). During copulation, DA concentrations increased further, reaching a maximum value (+95%;p < 0.01) during the first copulatory bout (sample 3). DA remained elevated throughout copulation and only returned to baseline concentrations in the 30 min period in which no mounting occurred (samples 9 and 10). Neither reintroduction of female 1 behind the screen (sample 11) nor the opportunity to interact physically, but without mounting (sample 12), elevated DA concentrations relative to the second baseline value (sample 10). The presence of female 2 behind the screen (sample 13) resulted in a small increase in DA efflux (12%) from the third baseline value (sample 12) that did not reach statistical significance. Renewed copulation with female 2 resulted in a significant (34%) increase (p < 0.05) in DA efflux during the first copulation sample (sample 14). Although weak copulatory behavior continued over the next three samples, DA concentrations decreased to baseline values (samples 15–17). Independent t tests conducted among “baseline” samples (i.e., 1, 10, and 12) demonstrated that these values were not significantly different.
In the three rats that resumed copulation when female 1 was reintroduced, NAC DA concentrations increased when female 1 was present behind the screen (range, 25–47%) and during copulation (range, 13–37%), relative to the sample just before the reintroduction of the female. These increases, however, only occurred when sexual behavior was vigorous and led to ejaculation.
Significant overall changes in DOPAC [F(11,44) = 9.57; p < 0.001] and HVA [F(11,44) = 12.47; p < 0. 001] concentrations were found in response to female 1, but not female 2. Metabolite concentrations increased slightly (+15% in both cases) during the presentation of female 1 behind the screen (sample 2), but this was not significant statistically. There were, however, significant increases in the concentrations of DOPAC and HVA during copulation (samples 3–8), reaching maximum values (+80 and +86%, respectively; p < 0.01) after 60 min (sample 6 in both cases). Although metabolite concentrations decreased during the period of sexual inactivity at the end of contact with female 1 (samples 9 and 10), concentrations still remained elevated with respect to baseline (p < 0.05 in both cases). Reintroduction of female 1 behind the screen (sample 11), access to female 1 after removal of the screen (sample 12), and the introduction of female 2 (sample 13) did not result in any changes in metabolite concentrations. Slight, but statistically insignificant, increases in DOPAC and HVA concentrations (+23% in both cases) relative to baseline (sample 12) corresponded to the first bout of copulation with female 2 (sample 14). This increase was short-lived, however, and declined to baseline values for the remaining three samples (15–17). Independent ttests conducted among “baseline” samples (i.e., 1, 10, and 12) indicated that the second and third baseline values (samples 10 and 12, respectively), although not different from each other, remained elevated significantly compared with the first baseline sample for DOPAC and HVA (p < 0.05 in both cases).
Histology
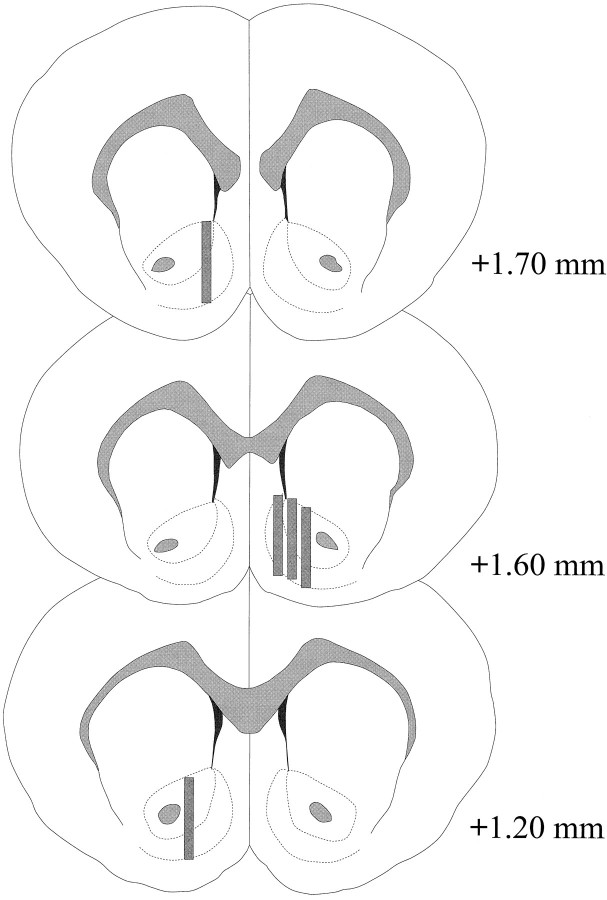
Microdialysis probes were located in the NAC (Fig.2) in a range extending +1.20 to +1.70 mm from bregma (flat skull). There was variability also in the mediolateral plane; data reflect sampling from the shell and core subregions of the NAC.
Fig. 2.
Location of microdialysis probes within the NAC of male rats used in the Coolidge effect experiment. Shaded rectangles correspond to the exposed membrane area of the microdialysis probes. Serial coronal brain sections were redrawn fromPaxinos and Watson (1986).
DISCUSSION
In agreement with earlier reports, the present results demonstrate enhanced mesolimbic DA transmission associated with appetitive and consummatory components of male rat sexual behavior as assessed byin vivo microdialysis (Mas et al., 1990; Pfaus et al., 1990;Pleim et al., 1990; Damsma et al., 1992; Wenkstern et al., 1993; Fumero et al., 1994; Mas et al., 1995a,b,d). In addition, these results provide a neurochemical correlate for sexual satiation and the subsequent reinitiation of copulation in response to a novel receptive female (the Coolidge effect). The present data suggest that the stimulus properties of a novel receptive female may serve to increase NAC DA transmission in a sexually satiated male rat that may, in turn, be related to the reinitiation of sexual behavior. This is first evident in the slight increase in NAC DA during the presentation of a novel female behind the screen and occurs most convincingly as a more pronounced increase during renewed copulation with female 2 (Fig.1).
The presence of the first receptive female behind the screen resulted in a robust appetitive increase in NAC DA efflux (44% from baseline) similar in magnitude to what was reported in previous experiments using a similar design (30%, Pfaus et al., 1990; 35%,Damsma et al., 1992). Also in agreement with these studies was the observation that NAC DA efflux was enhanced further during copulation (to >95% above baseline in the present experiment). Although we can view consummatory behaviors as being associated with enhanced NAC DA release (Wenkstern et al., 1993; Wilson et al., 1995), it is important to examine the terms “appetitive” and “consummatory” in the context of sexual behavior. Whereas the phase in which the female is present behind the screen is exclusively appetitive or preparatory, the behavior during the copulation phase cannot be considered purely consummatory. Because “appetitive” can be used to describe all behaviors leading to the consummation of a motivated behavior (copulation), the primary behavior the male exhibits while active in the “consummatory” phase is best described as appetitive; the male spends most of his time and effort pursuing the female to copulate. In this regard, we can correlate maximal NAC DA transmission with consummatory as well as intense appetitive components of male rat sexual behavior.
Access to the second, novel female resulted in renewed copulation in every subject. Previous studies have shown that the majority of rats allowed to copulate to satiety, using a similar behavioral protocol to the one used in the present experiment, did not resume mating when tested 24 hr later (Beach and Jordan, 1956). It is likely that the presence of the novel stimulus properties of female 2, which may have included olfactory as well as visual and auditory cues, resulted in renewed copulation. An interesting question, which remains to be answered, is by what mechanism a male rat distinguishes a novel female from a female with which he has mated recently. A site for that mechanism may lie in the main olfactory system. It has been reported that the integrity of this system is essential for the Coolidge effect in hamsters (Johnston and Rasmussen, 1984). The vomeronasal–accessory olfactory system, however, in which a pheromonal memory process was described recently in mice (Kaba et al., 1994), is also a prime candidate. In this regard, it is noteworthy that increases in NAC DA transmission were measured using in vivo voltammetry in male rats presented with bedding that was exposed to female rats in estrus (Louillot et al., 1991; Mitchell and Gratton, 1992). Furthermore, the application of K+ directly to the vomeronasal nerve layer of the accessory olfactory bulb, as well as to the accessory olfactory bulb itself, was sufficient to increase NAC DA transmission (Mitchell and Gratton, 1992).
The first 15 min bout of copulation with female 2 was associated with a significant increase in NAC DA. In contrast to female 1, interaction with female 2 did not produce increases in NAC DA of the same magnitude during either the appetitive (12%) or consummatory (34%) phases. These small increases in NAC DA, however, correlate well with the reduced level of sexual behavior displayed with female 2 compared with female 1. Metabolite concentrations remained elevated during the satiety phase, resulting in new baseline concentrations (samples 10 and 12) that were elevated significantly from the initial baseline value (sample 1).
The temporal lag in the increase in DOPAC and HVA concentrations during copulation is consistent with their formation as metabolites of the parent compound, DA. It has been suggested that microdialysis metabolite concentrations, at least during a natural behavior that is not pharmacologically driven, provide a useful index of neural activity (Damsma et al., 1992; Fumero et al., 1994). The fact that metabolite concentrations remained elevated even during periods of sexual inactivity in this experiment, when DA concentrations had returned to pretest baseline values, casts doubt on this suggestion.
The persistent elevation of DA metabolite concentrations seen in this experiment mirrors the medial preoptic area (mPOA) profile of DA metabolites observed in rats the first day after they had copulated to satiation (Mas et al., 1995a,b). Sustained elevations of DOPAC and HVA concentrations in the NAC or mPOA are not always observed when the mating period is of a fixed duration, much shorter than the time required to reach satiation. For example, many studies have shown that DOPAC concentrations were increased and remained elevated during copulation but declined to baseline values soon after the female was removed (Pfaus et al., 1990; Pleim et al., 1990; Damsma et al., 1992;Hull et al., 1993; Wenkstern et al., 1993; Hull et al., 1995). In the study by Mas et al. (1995b), basal extracellular concentrations of DOPAC and HVA in the mPOA remained elevated over 4 consecutive days corresponding to a period of sexual inactivity. By the fourth day, just before animals resumed copulation, the basal concentrations of the metabolites were close to presatiation values. The authors likened the pattern of neurochemical changes to those seen after the administration of DA receptor blockers (Zetterström et al., 1984; Imperato and DiChiara, 1985) and have suggested that the state of sexual inactivity may be mediated via prolactin release, which may act as an “endogenous neuroleptic” (Mas et al., 1995a,b,d). It is clear that neuroleptic administration is accompanied by increases in extracellular metabolite concentrations and DA efflux (Zetterström et al., 1984; Imperato and DiChiara, 1985). Unfortunately, Mas et al. (1995a,b) were not able to detect mPOA DA concentrations. In the present study, DA concentrations in the NAC returned to precopulation values, whereas DOPAC and HVA concentrations remained elevated. This pattern is inconsistent with a role for an endogenous neuroleptic acting in the NAC to induce sexual satiety.
Given the involvement of mesolimbic DA neurons in motivated behavior (Fibiger and Phillips, 1986; Blackburn et al., 1992; Kalivas et al., 1993; LeMoal, 1995) and their sensitivity to novel environmental stimuli (Fabre et al., 1983; Schultz, 1992; Mirenowicz and Schultz, 1994), the observed increases in extracellular concentrations of NAC DA in response to the novel female are consistent with the hypothesis that activity in this DA system is important for the reinitiation of sexual behavior. In addition, reports of appetitive and consummatory increases in DA transmission (Hull et al., 1993, 1995;Mas et al., 1995b; Sato et al., 1995) and neuronal activity (Shimura et al., 1994) in the mPOA of male rats during sexual behavior suggest that this structure may also contribute to renewed copulation characteristic of the Coolidge effect.
In keeping with a general role for the mesolimbic DA system in motivated behavior, it is well established that extracellular concentrations of DA also are elevated before, during, and immediately after consumption of a meal, with a return to baseline values ∼30 min later (Wilson et al., 1995). It is well known that satiety induced by food is influenced by its sensory properties. Humans and animals reject the food on which they were fed to satiety and ingest other foods that had not been eaten (Rolls, 1986). This raises the question as to whether extracellular DA efflux in the NAC would be increased selectively by the presentation of a novel type of food, but not by food consumed recently to satiety in a manner analogous to that reported in the present study in the context of sexual motivation. If confirmed, this general relationship between the sensory properties of natural rewards, satiety, and mesolimbic DA transmission would imply a critical role for this neural system in the regulation of motivational processes, the disruption of which may lead to serious disorders of eating and sexual function.
Footnotes
This work was supported by Group Program Grant PG-12808 from the Medical Research Council of Canada.
Correspondence should be addressed to Anthony G. Phillips, Department of Psychology, 2136 West Mall, University of British Columbia, Vancouver, British Columbia, Canada V6T 1Z4.
REFERENCES
- 1.Beach FA, Jordan L. Sexual exhaustion and recovery in the male rat. Q J Exp Psychol. 1956;8:121–133. [Google Scholar]
- 2.Bermant G, Lott DF, Anderson L. Temporal characteristics of the Coolidge effect in male rat copulatory behavior. J Comp Physiol Psychiatry. 1966;65:447–452. doi: 10.1037/h0025841. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- 4.Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Fabre M, Rolls ET, Ashton JP, Williams G. Activity of neurons in the ventral tegmental region of the behaving monkey. Behav Brain Res. 1983;9:213–235. doi: 10.1016/0166-4328(83)90129-8. [DOI] [PubMed] [Google Scholar]
- 6.Fibiger HC, Phillips AG. Reward, motivation, cognition: psychobiology of mesotelencephalic dopamine systems. In: Bloom FE, Geiger SD, editors. Handbook of physiology: the nervous system IV. American Physiology Society; Bethesda, MD: 1986. pp. 647–675. [Google Scholar]
- 7.Fiorino DF, Coury AG, Fibiger HC, Phillips AG. Electrical stimulation of reward sites in the ventral tegmental area increases dopamine transmission in the nucleus accumbens of the rat. Behav Brain Res. 1993;55:131–141. doi: 10.1016/0166-4328(93)90109-4. [DOI] [PubMed] [Google Scholar]
- 8.Fisher A. Effects of stimulus variation on sexual satiation in the male rat. J Comp Physiol Psychiatry. 1962;55:614–620. doi: 10.1037/h0042710. [DOI] [PubMed] [Google Scholar]
- 9.Fowler H, Whalen RE. Variation in incentive stimulus and sexual behavior in the male rat. J Comp Physiol Psychiatry. 1961;54:68–71. doi: 10.1037/h0046549. [DOI] [PubMed] [Google Scholar]
- 10.Fumero B, Fernendez-Vera JR, Gonzalez-Mora JL, Mas M. Changes in monoamine turnover in forebrain areas associated with masculine sexual behavior: a microdialysis study. Brain Res. 1994;662:233–239. doi: 10.1016/0006-8993(94)90817-6. [DOI] [PubMed] [Google Scholar]
- 11.Holmes GM, Holmes DG, Sachs BD. An IBM-PC based data collection system for recording rodent sexual behavior and for general event recording. Physiol Behav. 1987;44:825–828. doi: 10.1016/0031-9384(88)90070-4. [DOI] [PubMed] [Google Scholar]
- 12.Hull EM, Eaton RC, Moses J, Lorrain DS. Copulation increases dopamine activity in the medial preoptic area of male rats. Life Sci. 1993;52:935–940. doi: 10.1016/0024-3205(93)90528-b. [DOI] [PubMed] [Google Scholar]
- 13.Hull EM, Jianfang D, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imperato A, DiChiara G. Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J Neurosci. 1985;5:297–306. doi: 10.1523/JNEUROSCI.05-02-00297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston RE, Rasmussen K. Individual recognition of female hamsters by males: role of chemical cues and of the olfactory and vomeronasal systems. Physiol Behav. 1984;33:95–104. doi: 10.1016/0031-9384(84)90019-2. [DOI] [PubMed] [Google Scholar]
- 16.Kaba H, Hayashi Y, Higuchi T, Nakanishi S. Induction of an olfactory memory by the activation of a metabotropic glutamate receptor. Science. 1994;265:262–264. doi: 10.1126/science.8023145. [DOI] [PubMed] [Google Scholar]
- 17.Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- 18.LeMoal M. Mesocorticolimbic dopaminergic neurons. Functional and regulatory roles. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 283–294. [Google Scholar]
- 19.Louillot A, Gonzalez-Mora JL, Guadalupe T, Mas M. Sex-related olfactory stimuli induce a selective increase in dopamine release in the nucleus accumbens of male rats. Brain Res. 1991;553:313–317. doi: 10.1016/0006-8993(91)90841-i. [DOI] [PubMed] [Google Scholar]
- 20.Mas M, Gonzalez-Mora JL, Louillot A, Sole C, Guadalupe T. Increased dopamine release in the nucleus accumbens of copulating male rats as evidenced by in vivo voltammetry. Neurosci Lett. 1990;110:303–308. doi: 10.1016/0304-3940(90)90864-6. [DOI] [PubMed] [Google Scholar]
- 21.Mas M, Fumero B, Fernandez-Vera JR, Gonzalez-Mora JL. Neurochemical correlates of sexual exhaustion and recovery as assessed by in vivo microdialysis. Brain Res. 1995a;675:13–19. doi: 10.1016/0006-8993(95)00029-p. [DOI] [PubMed] [Google Scholar]
- 22.Mas M, Fumero B, Gonzalez-Mora JL. Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav Brain Res. 1995b;71:69–79. doi: 10.1016/0166-4328(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 23.Mas M, Fumero B, Perez-Rodriguez I. Induction of mating behavior by apomorphine in sexually sated rats. Eur J Pharmacol. 1995c;280:331–334. doi: 10.1016/0014-2999(95)00270-u. [DOI] [PubMed] [Google Scholar]
- 24.Mas M, Fumero B, Perez-Rodriguez I, Gonzalez-Mora JL. The neurochemistry of sexual satiety. An experimental model of inhibited desire. In: Bancroft J, editor. The pharmacology of sexual function and dysfunction. Raven; New York: 1995d. pp. 115–126. [Google Scholar]
- 25.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopaminergic neurons. J Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JB, Gratton A. Mesolimbic dopamine release elicited by activation of the accessory olfactory system: a high speed chronoamperometric study. Neurosci Lett. 1992;140:81–84. doi: 10.1016/0304-3940(92)90687-3. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates (2nd ed). Academic; San Diego: 1986. [Google Scholar]
- 28.Pfaus JG, Damsma G, Nomikos GG, Wenkstern D, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- 29.Pfaus JG, Everitt BJ. The psychopharmacology of sexual behavior. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 743–758. [Google Scholar]
- 30.Pfaus JG, Gorzalka BB. Opioids and sexual behavior. Neurosci Biobehav Rev. 1987;11:1–34. doi: 10.1016/s0149-7634(87)80002-7. [DOI] [PubMed] [Google Scholar]
- 31.Phillips AG, Blaha CD, Pfaus JG, Blackburn JR. Neurobiological correlates of positive emotional states: dopamine, anticipation and reward. In: Strongman, editor. International review of studies on emotion. Wiley; New York: 1992. pp. 31–50. [Google Scholar]
- 32.Pleim ET, Matochik JA, Barfield RJ, Auerbach SB. Correlation of dopamine release in the nucleus accumbens with masculine sexual behavior in rats. Brain Res. 1990;524:160–163. doi: 10.1016/0006-8993(90)90507-8. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Manzo G, Fernandez-Guasti A. Reversal of sexual exhaustion by serotonergic and noradrenergic agents. Behav Brain Res. 1994;62:127–134. doi: 10.1016/0166-4328(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Manzo G, Fernandez-Guasti A. Participation of the central noradrenergic system in the reestablishment of copulatory behavior of sexually exhausted rats by yohimbine, naloxone, and 8-OH-DPAT. Brain Res Bull. 1995a;38:399–404. doi: 10.1016/0361-9230(95)02007-e. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Manzo G, Fernandez-Guasti A. Opioid antagonists and the sexual satiation phenomenon. Psychopharmacol. 1995b;122:131–136. doi: 10.1007/BF02246087. [DOI] [PubMed] [Google Scholar]
- 36.Rolls BJ. Sensory-specific satiety. Nutr Rev. 1986;44:93–101. doi: 10.1111/j.1753-4887.1986.tb07593.x. [DOI] [PubMed] [Google Scholar]
- 37.Salamone JD. The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods. 1996;64:137–149. doi: 10.1016/0165-0270(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 38.Sato Y, Wada H, Horita H, Suzuki N, Shibuya A, Adachi H, Kato R, Tsukamoto T, Kumamoto Y. Dopamine release in the medial preoptic area during male copulatory behavior in rats. Brain Res. 1995;692:66–70. doi: 10.1016/0006-8993(95)00656-b. [DOI] [PubMed] [Google Scholar]
- 39.Schultz W. Activity of dopamine neurons in the behaving primate. Semin Neurosci. 1992;4:129–138. [Google Scholar]
- 40.Shimura T, Yamamoto T, Shimokochi M. The medial preoptic area is involved in both sexual arousal and performance in male rats: re-evaluation of neuron activity in freely moving animals. Brain Res. 1994;640:215–222. doi: 10.1016/0006-8993(94)91875-9. [DOI] [PubMed] [Google Scholar]
- 41.Wenkstern D, Pfaus JG, Fibiger HC. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 1993;618:41–46. doi: 10.1016/0006-8993(93)90426-n. [DOI] [PubMed] [Google Scholar]
- 42.Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JR, Kahn RE, Beach FA. Modification in the sexual behavior of male rats produced by changing the stimulus female. J Comp Physiol Psychiatry. 1963;56:636–644. doi: 10.1037/h0042469. [DOI] [PubMed] [Google Scholar]
- 44.Zetterström T, Sharp T, Ungerstedt U. Effect of neuroleptic drugs on striatal dopamine release and metabolism in the awake rat studied by intracerebral dialysis. Eur J Pharmacol. 1984;106:27–37. doi: 10.1016/0014-2999(84)90674-5. [DOI] [PubMed] [Google Scholar]