Abstract
In the majority of developing neurons, GABA can exert depolarizing actions, thereby raising neuronal Ca2+. Ca2+elevations can have broad consequences during development, inducing gene expression, altering neurite outgrowth and growth cone turning, activating enzyme pathways, and influencing neuronal survival. We used fura-2 and fluo-3 Ca2+ digital imaging to assess the effects of inhibiting or activating the cAMP signal transduction pathway on GABA activity mediating Ca2+ rises during the early stages of in vitro hypothalamic neural development. Our experiments stemmed from the finding that stimulation of transmitter receptors shown to either activate or inhibit adenylyl cyclase activity caused a rapid decrease in Ca2+ rises mediated by synaptically released GABA.
Both the adenylyl cyclase activator forskolin and the inhibitor SQ-22,536 reduced the Ca2+ rise elicited by the synaptic release of GABA. Bath application of the membrane-permeable cAMP analogs 8-bromo-cAMP (8-Br-cAMP) or 8-(4-chlorophenylthio)-cAMP (0.2–5 mm) produced a rapid, reversible, dose-dependent inhibition of Ca2+ rises triggered by synaptic GABA release. Potentiation of GABAergic activity mediating Ca2+ rises was observed in some neurons at relatively low concentrations of the membrane-permeable cAMP analogs (20–50 μm). In the presence of tetrodotoxin (TTX), postsynaptic Ca2+ rises triggered by the bath application of GABA were only moderately depressed (13%) by 8-Br-cAMP (1 mm), suggesting that the inhibitory effects of 8-Br-cAMP were largely the result of a presynaptic mechanism.
The protein kinase A (PKA) inhibitors H89 and Rp-3′,5′-cyclic monophosphothioate triethylamine also caused a large reduction (>70%) in Ca2+ rises triggered by synaptic GABA release. Unlike the short-term depression elicited by activation of the cAMP signal transduction pathway, Ca2+ depression elicited by PKA inhibition persisted for an extended period (>30 min) after PKA inhibitor washout. Postsynaptic depression of GABA-evoked Ca2+ rises triggered by H89 (in the presence of TTX) recovered rapidly, suggesting that the extended depression observed during synaptic GABA release was largely through a presynaptic mechanism. Long-term Ca2+ modulation by cAMP-regulating hypothalamic peptides may be mediated through a parallel mechanism.
Together, these results suggest that GABAergic activity mediating Ca2+ rises is dependent on ongoing PKA activity that is maintained within a narrow zone for GABA to elicit a maximal Ca2+ elevation. Thus, neuromodulator-mediated changes in the cAMP-dependent signal transduction pathway (activation or inhibition) could lead to a substantial decrease in GABA-mediated Ca2+ rises during early development.
Keywords: mediobasal hypothalamus, calcium, GABA, GABA excitation, protein kinase A, cAMP, development, digital imaging
The role of GABA as an excitatory neurotransmitter during the early stages of neural development has been documented over the past several years (for review, see Cherubini et al., 1991). Several studies have shown that GABA triggers neural excitability by activating the GABAA receptor (Ben-Ari et al., 1989; Chen et al., 1996). Because of a relatively depolarized Cl−reversal potential (Chen et al., 1996), opening of the GABAA receptor allows Cl− to efflux from the neuron, triggering membrane potential depolarization, activation of voltage-sensitive Ca2+ channels, and, as a result, an increase in intracellular Ca2+ (Yuste and Katz, 1991;Yamashita and Fukuda, 1993; Obrietan and van den Pol, 1995). Although glutamate receives a lot of attention regarding its ability to elevate cytosolic Ca2+ levels in developing neurons, we found that many developing neurons show a greater Ca2+ elevation in response to GABA than to an equimolar concentration of glutamate (Obrietan and van den Pol, 1995). As neurons mature, GABA becomes a predominantly inhibitory transmitter that decreases cytosolic Ca2+ levels (Obrietan and van den Pol, 1995). GABA has been shown to possess many of the effects of other, better characterized, fast excitatory neurotransmitters known to increase intracellular Ca2+ during development. For example, GABAAreceptor activation can trigger BDNF induction (Berninger et al., 1995) and alter the neural phenotype (Marty et al., 1996). In addition, DNA synthesis in cortical neural progenitor cells can be blocked by specifically inhibiting GABAA receptor activity (LoTurco et al., 1995). GABA can increase Ca2+ levels in neurites and growth cones (Obrietan and van den Pol, 1996a), which may alter growth cone motility and the rate of neurite extension. In addition, GABA-mediated Ca2+ influx in growth cones leads to an increase in GAP43 and MARCKS protein phosphorylation (Fukura et al., 1996). These results suggest that the ability of GABA to raise intracellular Ca2+ levels may be central to its functional role during development.
The cAMP signal transduction pathway is a potent regulator of synaptic neural physiology (for review, see Anholt, 1994; Cooper et al., 1994). A primary mechanism by which cAMP regulates neural excitability is through the activation of cAMP-dependent protein kinase A (PKA). The neuromodulatory actions of PKA have been documented extensively and include altering ion channel activity (Nagel et al., 1992; Johnson et al., 1994; Surmeier et al., 1995), gene expression (Impey et al., 1996), and neurotransmitter release (Sciancalepore and Cherubini, 1995;Huang et al., 1996). Although a considerable amount of information is known about the effects of PKA at the molecular and single channel levels, much remains to be determined about how its actions at a variety of sites may affect cytosolic Ca2+ regulation in synaptically active neurons. For example, PKA has been shown to reduce the amplitude of the GABAA receptor conductance (Moss et al., 1992), to decrease voltage-gated Na+ channel currents (Smith and Goldin, 1996), and to potentiate kainate-induced currents (Gu and Moss, 1996). A question of particular interest is how GABAergic activity mediating Ca2+ elevations may be regulated during early development by PKA.
Similar to GABA, PKA has also been shown to play an important role during development. The cAMP signal transduction pathway has been implicated in a variety of developmentally regulated processes, including cell cycle regulation (Grieco et al., 1996), neural migration (Behar et al., 1995), and gene induction (Zhang et al., 1993). Additionally, many neurotransmitters and neuropeptides that affect neural physiology through the regulation of adenylyl cyclase activity, including neuropeptide Y (NPY), dopamine, glutamate, and serotonin, are expressed in early development and could serve to modulate the Ca2+-elevating actions of GABA (Rajaofetra, 1989; Belin et al., 1991; Marti et al., 1992; van den Pol et al., 1995, 1996a; Lieb et al., 1996). Based on these findings, we studied the modulation of GABAergic Ca2+ elevating activity by the cAMP-mediated signal transduction pathway.
Here we report that Ca2+ rises regulated by synaptic GABA release during early development are dramatically influenced by activation or inhibition of the cAMP-dependent signal transduction cascade.
MATERIALS AND METHODS
Tissue culture. The mediobasal hypothalamus was removed from embryonic day 18 Sprague Dawley rats. The tissue was enzymatically digested in a mild protease solution (10 U/ml papain and 0.2 mg/ml l-cysteine in Earl’s balanced salt solution) for 30 min. Next, the tissue was pelleted, and the protease solution was removed. Tissue was then suspended in standard tissue culture medium (glutamate- and glutamine-free DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin/streptomycin, and 6 gm/l glucose) and then triturated into a single-cell suspension. Cells were washed and pelleted an additional three times. The single-cell suspension was plated onto 22 mm2 glass coverslips that had been coated with high-molecular-weight (540,000 Da) poly-d-lysine. High-density cultures (200,000/cm2) were used for all experiments. Hypothalamic neural cultures were maintained in a Napco 3600 incubator (37°C and 5% CO2) until they were ready for use. To limit non-neuronal cell proliferation, cytosine arabinofuranoside (1 μm) was added to the tissue culture medium 1 d after plating.
Fura-2 and fluo-3 Ca2+ digital imaging. Cells were loaded for 20 min with either 5 μm fura-2 AM or fluo-3 AM in standard perfusion solution (137 mm NaCl, 25 mm glucose, 10 mm HEPES, 5 mm KCl, 1 mm MgCl2, 3 mm CaCl2, pH 7.4). The cells were then washed and allowed to recover for 15 min before the start of the experiment. Coverslips were then loaded into a laminar style perfusion chamber. Solutions rapidly moved as a straight wave through the perfusion chamber, and complete washout of the chamber occurred in ∼5 sec. For fura-2, neurons were imaged using a 40× Olympus objective with high 340/380 nm transmittance on a Nikon Diaphot 300 inverted microscope. Fluo-3 experiments were performed using a 100× Olympus objective. Unless noted otherwise, Ca2+digital recordings were made from the cell soma. All experiments were performed at room temperature.
A 486 PC clone was used to collect data, run Ca2+ analysis software (Fluor; Universal Imaging Corporation, West Chester, PA), and control the Lambda-10-filtered wheel driver (Sutter Instruments). Sixteen (500 msec) digital frames of data were collected every 3 sec. Excitation light came from a 150 W Xenon lamp. The equation [Ca2+]i =Kd(R −Rmin)/(Rmax −R) was used to convert fura-2 ratiometric fluorescent Ca2+ values to free Ca2+ concentrations.R is the ratio of the two fluorescence intensities,Rmin is the ratio in the absence of Ca2+, and Rmax is the ratio in a saturating concentration of Ca2+. TheKd for binding of Ca2+ to fura 2 was taken to be 224 nm (Grynkiewicz et al., 1985). Data for fluo-3 fluorescence are represented on a 0–255 U scale. As standard protocol for both fura-2 and fluo-3 imaging, background fluorescence values were subtracted.
To determine the effects of different receptor agonists or signal transduction modulators on Ca2+ rises triggered by the synaptic release of GABA, the mean Ca2+ rise from the Ca2+ level in the presence of the GABAAreceptor antagonist bicuculline was determined over a 15 sec period just before the application of a receptor agonist or signal transduction modulator. The mean Ca2+ rise was then determined over a 15 sec period 90 sec after administration of the receptor agonist or signal transduction modulator. Data for Ca2+ rises for the two conditions are reported as a mean (pooled) Ca2+ rise of all responsive neurons ± SEM. Thirty to 50% of the neurons exhibited a Ca2+ rise on bicuculline removal. For assays that evoked a Ca2+response, either through agonist administration to the perfusion solution or through electrical stimulation, the maximal Ca2+ rise for individual neurons was determined by subtracting the mean basal Ca2+ level for a 15 sec period just before stimulation from the peak-evoked Ca2+ rise. Ca2+ responses from a total of 1165 neurons were recorded in the course of these experiments. Modulation of GABA-related activity could refer to either a presynaptic or postsynaptic site of action, whereas modulation of GABA-evokedCa2+ rises refers to a postsynaptic site of action.
Electrical stimulation. Electrodes from a Grass SD9 stimulator were placed at both ends of the perfusion chamber. Ca2+ rises were stimulated by passing 2–5 V/cm2, at 20 Hz frequency and 2 msec duration, through the chamber for 6 sec. Ca2+ rises were visible immediately and usually peaked 4 sec after current application. At these voltages, Ca2+ rises were inhibited by either the application of the voltage-dependent Na+ channel blocker tetrodotoxin (TTX; 1 μm) or the administration of fast excitatory neurotransmitter antagonists. At voltages higher than those used in this paper (>6 V/cm2), TTX did not block the Ca2+ response, suggesting an additional nonsynaptic mechanism for the induction of Ca2+ rises.
Immunostaining. Neurons were fixed for 1 hr in cold (−20°C) methanol and then treated for 30 min with 3% BSA and 0.1% Triton X-100 in PBS. Neurons were then washed and incubated with mouse anti-synapsin 1 antibody (1:100) (Chemicon, Temecula, CA). The synapsin antiserum recognized a band of the correct weight on Western blots, and preabsorption with synapsin antigen blocked staining (Smith et al., 1994). Rat anti-α-tubulin antibody (Sera Lab) was used at 1:200 for 30 min. Neurons were washed and incubated with FITC- and Texas Red-labeled secondary antibodies (Jackson Laboratory, Bar Harbor, ME) for 30 min. After washing, cell fluorescence was visualized with the appropriate filter sets. In the fluorescent microscope, synapsin-immunostained boutons were green, and tubulin immunoreactivity was red.
Reagents. SKF-38393, McN-A-343, cytosine arabinofuranoside, GABA, cAMP, 8-bromo-cAMP (8-Br-cAMP), 8-(4-chlorophenylthio)-cAMP (4-Cl-cAMP), and poly-d-lysine were acquired from Sigma (St. Louis, MO). SQ-22,536, forskolin, (±)−2-amino-5-phosphonopentanoic acid (AP5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), bicuculline, nimodipine, and TTX were acquired from Research Biochemicals (Natick, MA). NPY was acquired from Peninsula Labs. Papain was acquired from Worthington (Freehold, NJ); DMEM was from Life Technologies (Gaithersburg, MD); and fura-2 AM and fluo-3 AM were from Molecular Probes (Eugene, OR).
RESULTS
cAMP signal transduction pathway activation
To address the possibility that activating the cAMP signal transduction pathway affects GABA activity that mediates Ca2+ elevations, membrane-permeable cAMP analogs that mimic the cellular effects of cAMP were administered to synaptically active hypothalamic neurons. Figure 1A shows that the withdrawal of the GABAA receptor antagonist bicuculline (20 μm) from the perfusion solution elicited a rapid and stable Ca2+ rise, indicating that cytosolic Ca2+ was raised by synaptically released GABA. Addition of 8-Br-cAMP (1 mm) caused a rapid reduction in Ca2+ levels. The mean ± SEM Ca2+ rise after removal of bicuculline was 73 ± 6 nm. After the addition of 8-Br-cAMP the Ca2+ rise decreased to 28 ± 5 nm, representing a statistically significant (p < 0.0001, two-tailed t test) reduction in GABA-mediated Ca2+ rise of 62% (n = 68). Figure 1B shows that mean inhibition of GABA activity regulating Ca2+ rises by 8-Br-cAMP was dose-dependent. GABA-mediated Ca2+ levels were increased in some neurons, but only at low 8-Br-cAMP concentrations. Specifically, the Ca2+ level in 6 of 20 neurons was raised by 50 μm extracellular 8-Br-cAMP, and at 150 μm 8-Br-cAMP, the Ca2+ level was raised in 4 of 30 neurons. However, at higher concentrations (500 μm and 1 and 5 mm) 8-Br-cAMP only depressed Ca2+ levels and did not elevate Ca2+ levels in any neurons (n = 90).
Fig. 1.
Membrane-permeable analogs of cAMP trigger reversible Ca2+ depression. A, The removal of bicuculline (BIC, 20 μm) from the perfusion solution triggered a rapid and sustained Ca2+rise in the two representative neurons. Repeated application of 8-Br-cAMP (1 mm) induced a rapid depression of the GABA-related Ca2+ rises that lasted only as long as 8-Br-cAMP was applied. B, Dose–response effect of 8-Br-cAMP on the synaptically released GABA-mediated Ca2+level. The Ca2+ rise from a bicuculline-defined baseline was determined over a 15 sec period just before and 90 sec after 8-Br-cAMP application. The white bar (set at 100%) is the normalized Ca2+ rise just before 8-Br-cAMP application. Error bars indicate SEM. N, Total number of neurons assayed. C, 8-Br-cAMP had no effect on neuronal Ca2+ levels if synaptic GABA release was inhibited by the administration of the Na+ channel blocker TTX (1 μm). Neurons with only small baseline Ca2+fluctuations were shown, so that any effect of 8-Br-cAMP on basal Ca2+ might be observed. D, Membrane-impermeable cAMP (1 mm) had little extracellular effect on GABA-mediated Ca2+ levels. All experiments were performed in the presence of AP5 (100 μm) and CNQX (10 μm). All synaptic release experiments were performed with embryonic day 18 neurons after 5 DIV. In A, C, D, thebar along the x-axis shows time in min, and the bar to the left of each Ca2+ trace is the calibrated cytosolic Ca2+value for each neuron.
Another membrane-permeable cAMP analog, 4-Cl-cAMP (2 mm), also triggered a rapid Ca2+ depression (Fig.2A). The effectiveness of 4-Cl-cAMP was also dose-dependent; at 2 mm, 4-Cl-cAMP caused a statistically significant (p < 0.0001, two-tailed t test) 81% Ca2+ depression (n = 29), whereas 200 μm 4-Cl-cAMP reduced the GABA-mediated Ca2+ rise by a statistically significant (p < 0.001, two-tailed ttest) 39% (n = 25). Figure 2B shows that the coadministration of the potent PKA inhibitor Rp-3′,5′-cyclic monophosphothioate triethylamine (Rp-cAMPS) largely blocked the effects of 4-Cl-cAMP, suggesting that the actions of membrane-permeable cAMP analogs are the result of PKA activation. Application of 4-Cl-cAMP (1 mm) triggered a 78% decrease in GABA-mediated Ca2+ levels, whereas 4-Cl-cAMP (1 mm) administration, in the presence of Rp-cAMPS (200 μm), resulted in a 46% Ca2+ decrease (n = 42). As with low concentrations of 8-Br-cAMP, the administration of relatively low levels of 4-Cl-cAMP triggered Ca2+ rises that were not observed at higher concentrations. Figure 2Cshows two neurons treated to both low (20 μm) and high (1 mm) concentrations of 4-Cl-cAMP. 4-Cl-cAMP at a concentration of 20 μm triggered an enhancement in both the mean Ca2+ concentration (Ca2+concentrations shown for each bracketed region ± SEM) and, in some neurons, the size of GABA-mediated Ca2+ transients. By comparing the mean Ca2+ rise during bracketed region B (after bicuculline withdrawal) with the mean Ca2+ rise during bracketed region C (during 20 μm 4-Cl-cAMP), we found that 4-Cl-cAMP triggered a >20% increase in GABA-mediated Ca2+ rises in 17 of 57 neurons. This effect is in striking contrast to the large Ca2+ decrease observed on administration of 1 mm 4-Cl-cAMP. These neurons were cultured on embryonic day 18 and maintained for 5 d in vitro (DIV). Figures often show data records from two or more neurons recorded simultaneously. This serves both to validate subtle, but consistent, effects of pharmacological manipulations and to show the heterogeneity of neural response characteristics.
Fig. 2.
Modulation of GABA activity mediating Ca2+ rises. A, The membrane-permeable analog of cAMP, 4-Cl-cAMP (2 mm), elicited a rapid and reproducible Ca2+ depression. B, Coadministration of the protein kinase A inhibitor Rp-cAMPS (200 μm) largely blocked the Ca2+ depression elicited by 4-Cl-cAMP (1 mm). C, Two representative neurons show that administration of a low concentration of 4-Cl-cAMP (20 μm) triggered a Ca2+increase, whereas a high concentration of 4-Cl-cAMP (1 mm) triggered a Ca2+ depression. Numbers under each bracket refer to the mean ± SEM Ca2+ concentration for the period within the bracketed region. Twenty time points (3 sec interval) were used to determine each Ca2+ concentration, except for the beginning of the experiment [bicuculline (BIC) administration], at which 10 time points were used. Letters underbrackets identify each bracketed region.
To remove any complicating effects of synaptically released glutamate on Ca2+ rises, neurons were constantly perfused with the ionotropic glutamate receptor antagonists AP5 (100 μm) and CNQX (10 μm). In the presence of glutamate receptor blockers AP5 and CNQX, basal Ca2+ levels did not change when neurons were switched between perfusion solutions containing bicuculline (20 μm) or the Na+ channel blocker TTX (1 μm), suggesting that in the absence of glutamatergic neurotransmission, GABA was the sole transmitter responsible for increasing neuronal Ca2+ levels (data not shown).
The Na+ channel blocker TTX (1 μm) inhibited Ca2+ transients mediated by the synaptic release of GABA (Fig. 1C). 8-Br-cAMP (1 mm) had no independent effect on basal Ca2+ levels (Fig.1C). As an additional control we added cAMP (1 mm) to the perfusion solution. Because cAMP is membrane-impermeable, it should not mimic the intracellular effects of 8-Br-cAMP or 4-Cl-cAMP. Figure 1D shows that cAMP had very little effect on GABA-mediated Ca2+ rises, suggesting that 8-Br-cAMP and 4-Cl-cAMP were acting primarily through an intracellular mechanism to depress Ca2+ levels rather than at an extracellular receptor. The Ca2+ rise before the addition of cAMP was 101 ± 10 nm; during cAMP application, the Ca2+ rise decreased to 87 ± 10 nm, representing a statistically insignificant (p > 0.05, two-tailed t test) 14% cAMP-dependent decrease (n = 38).
8-Br-cAMP inhibits GABA activity through presynaptic and postsynaptic mechanisms
To determine whether activation of the cAMP-dependent signal transduction cascade inhibits GABA-related Ca2+ rises through a presynaptic or postsynaptic mechanism, we compared the effects of 8-Br-cAMP on postsynaptic Ca2+ rises elicited by bath application of GABA with the effects of 8-Br-cAMP on electrically stimulated Ca2+ rises dependent on presynaptic GABA release. Figure 3A shows Ca2+rises elicited by bath application of GABA (10 μm). Interestingly, Ca2+ rises were either inhibited (top trace) or potentiated (bottom trace) by 8-Br-cAMP (1 mm). Relative to the control Ca2+ rise immediately preceding 8-Br-cAMP administration, 8-Br-cAMP depressed the Ca2+ rise by >20% in 32 of 109 neurons and potentiated the GABA-evoked Ca2+ rises in 19 of 109 neurons by >20%. In the absence of 8-Br-cAMP, repeated peak Ca2+ rises evoked by GABA did not vary by >20% in any of the 109 neurons assayed. Figure 3C is a scatter plot analysis of the modulatory effects of 8-Br-cAMP administration on GABA-evoked Ca2+ rises. Each point (neuron) is a representation of the first Ca2+ rise in the presence of 8-Br-cAMP divided by the control Ca2+ rise immediately preceding 8-Br-cAMP administration for each of the 109 neurons assayed. Data are displayed as percentages. Zero percent on the y-axis signifies that the peak rise in the presence of 8-Br-cAMP was equivalent to the control peak Ca2+ rise (no modulation); values <0% represent depression, and values >0% represent response potentiation. Of note is the dual effects (both potentiation and depression) of 8-Br-cAMP on GABA-evoked Ca2+ rises. To ensure that the GABA-evoked Ca2+ rise was purely postsynaptic in nature, the neurons were continuously perfused with TTX (1 μm) to block action potential-dependent synaptic release of neurotransmitters.
Fig. 3.

8-Br-cAMP reduces presynaptic release of GABA.A, Brief (15 sec) repeated exogenous administrations of GABA (10 μm) (arrows) to the perfusion solution elicited rapid and reproduciblepostsynaptic Ca2+ rises. 8-Br-cAMP (1 mm) coadministration was capable of either enhancing or depressing the amplitude of GABA-evoked Ca2+ rises (see text). To ensure that GABA-evoked Ca2+ rises were exclusively postsynaptic in nature, the neurons were continuously perfused with TTX (1 μm) to block action potential-dependent presynaptic transmitter release. B, Repeated electrical stimulation (E.S.) triggered reproducible Ca2+ rises triggered bypresynaptic GABA release. Administration of TTX (1 μm) or bicuculline (BIC, 20 μm) blocked the Ca2+ rise, suggesting that electrical stimulation triggered action potential-dependent synaptic release of GABA. Administration of 8-Br-cAMP (1 mm) largely blocked the GABA activity. This experiment was performed in the constant presence of AP5 (100 μm) and CNQX (10 μm) to block the Ca2+ rise elicited by glutamate receptor activation. C, D, Analysis of the modulatory effects of 8-Br-cAMP administration on GABA-evoked Ca2+ rises (C) and on electrically evoked Ca2+ rises (D). Circlesrepresent the first Ca2+ rise in the presence of 8-Br-cAMP divided by the control Ca2+ rise immediately preceding 8-Br-cAMP administration; each circle represents the response of a single neuron. Results are displayed as percentages of the control Ca2+ rise. The 0% point on they-axis signifies that the rise in the presence of 8-Br-cAMP was equivalent to the control Ca2+ rise. Values >0% represent response potentiation; values <0% represent depression. E, The effects of 8-Br-cAMP on bath application of GABA and electrically stimulated synaptic release of GABA on Ca2+ rises were compared. Only neurons with control evoked Ca2+ rises (second Ca2+ rise, just before 8-Br-cAMP) from 120 to 140 nm were used to allow for an equitable comparison. See Results for details. Error bars indicate SEM. The mean GABA-evoked Ca2+ rise was slightly depressed by the administration of 8-Br-cAMP (postsynaptic effect), whereas the electrically evoked rise was dramatically depressed by 8-Br-cAMP administration (presynaptic plus postsynaptic). The electrically evoked Ca2+ rise was virtually abolished by TTX or bicuculline. Electrical stimulation experiments were performed after 4 DIV, a period when synaptic connections are found in most neurons.
To determine that GABA was released by an action potential-dependent mechanism at this early stage of development, a series of experiments was performed on neurons at 4 DIV. Electrical stimulation caused a large Ca2+ rise in both neural cell bodies and neurites. Neurites loaded with fluo-3 (Fig. 4A) show a large electrically evoked Ca2+ rise localized to small regions, suggesting dendrite segments postsynaptic to axonal boutons (Fig. 4D). This Ca2+ rise was reduced by bath application of either TTX (Fig. 4B) or bicuculline (Fig. 4C). These results suggest that action potential-mediated presynaptic release of GABA was responsible for the Ca2+ rise. Ca2+ rises occurred in the presence of the glutamate receptor antagonists AP5 (100 μm) and CNQX (10 μm), ruling out synaptic glutamate release as the cause of the Ca2+ rise. To demonstrate further that synapses were formed at this developmental stage, cells were immunostained with synapsin antiserum. Highly localized, punctate synapsin staining is shown in Figure 4E. For control purposes, these cells were also immunostained for the structural protein α-tubulin (Fig. 4F). In contrast to the punctate synapsin staining, tubulin immunoreactivity was found throughout neurons and glia.
Fig. 4.

Electrical stimulation triggers GABA-mediated Ca2+ rises in neurites. A, Fluo-3-loaded neurites are shown under control conditions (no electrical stimulation). B, In the presence of TTX (1 μm), electrical stimulation does not elicit a Ca2+ rise. C, Electrically induced Ca2+ rise is also largely inhibited by bath application of bicuculline (20 μm). D, In the absence of bicuculline and TTX, large and localized (red arrows) Ca2+ rises were triggered by electrical stimulation. These experiments were performed in the presence of AP5 (100 μm) and CNQX (10 μm).A′–D′, High magnification of areas fromA–D shown by the box inA. The color bar shows color codes of low and high Ca2+ levels. Scale bar, 2.5 μm.E, Neurons immunostained for synapsin I. Blue arrows indicate punctate staining, corresponding to synapse location. F, Same region immunostained for α tubulin.Yellow arrows identify the same neurite in both micrographs. Scale bar, 8 μm.
Figure 3B shows that electrical stimulation triggered reproducible Ca2+ rises in the neural cell soma. The addition of either TTX (1 μm) or bicuculline (20 μm) to the perfusion solution blocked the rise. As in neurites, Ca2+ rises occurred in the presence of the glutamate receptor antagonists AP5 (100 μm) and CNQX (10 μm). Under this condition, the addition of 8-Br-cAMP (1 mm) caused a large depression in the electrically evoked Ca2+ rise. Relative to the control electrically stimulated Ca2+ rise immediately preceding 8-Br-cAMP administration, 8-Br-cAMP depressed the Ca2+ rise by >20% in 40 of 49 neurons. No neurons exhibited a >20% potentiation of the peak evoked Ca2+ response. A scatter plot analysis of the effects of 8-Br-cAMP on electrically evoked Ca2+ rises in 49 neurons is shown in Figure 3D. Of note is the largely inhibitory actions of 8-Br-cAMP on electrically evoked Ca2+ rises. This is in contrast with data for purely postsynaptically evoked Ca2+ rises (Fig. 3C), suggesting that 8-Br-cAMP also affects GABA activity mediating Ca2+ rises through presynaptic regulation of GABA release.
Figure 3E compares the modulatory effects of 8-Br-cAMP on Ca2+ rises elicited by bath-applied GABA with electrically evoked release of presynaptic GABA. For a comparison of effectiveness of 8-Br-cAMP on postsynaptic (GABA-evoked) and presynaptic plus postsynaptic (electrically induced GABA release) responses, data for Figure 3E were collected from neurons that exhibited a peak Ca2+ rise from 120 to 140 nm during the second (control) GABA-evoked Ca2+rise. Ca2+ rises of approximately equivalent levels were used so that a presynaptic component of 8-Br-cAMP-mediated Ca2+ modulation could be fairly subtracted from the purely postsynaptic modulation of GABA-evoked Ca2+ rises. Of interest was the finding that the mean Ca2+ rise evoked by bath application of GABA was only slightly depressed (13%; statistically insignificant, P > 0.05, two-tailedt test) by 8-Br-cAMP, whereas electrically induced Ca2+ rises dependent on synaptic GABA release were highly significantly (p < 0.0001, two-tailedt test) reduced (61%). This difference in 8-Br-cAMP depression of electrically versus GABA-evoked Ca2+ rises (48%) was probably the result of inhibited GABA release. Unlike the other experiments, which used 5 DIV cultures, 4 DIV cultures were used for electrical stimulation experiments. At this time point during development, neurons were synaptically connected, yet the Ca2+-elevating action mediated by the spontaneous release of GABA was relatively low. Taken together, these data suggest that cAMP-dependent processes regulate Ca2+ rises by decreasing the amount of presynaptic GABA release and to a lesser extent by altering postsynaptic Ca2+ responsiveness to GABA.
Modulation of adenylyl cyclase activity
To determine whether altering endogenous cAMP levels would modulate GABA synaptic activity mediating Ca2+ rises, neurons were treated either with forskolin (an adenylyl cyclase activator) to increase cAMP levels or with SQ-22,536 (an adenylyl cyclase inhibitor) to decrease cAMP levels. Figure5A shows two representative neurons exhibiting four Ca2+ rises: two Ca2+ rises before and two Ca2+ rises after a 15 min application of forskolin (20 μm). The administration of forskolin greatly depressed (>70%) the Ca2+ rises elicited by bicuculline withdrawal. The effects of forskolin on GABA-mediated Ca2+ rises are quantified in Figure 5B;1st and 2nd refer to the two Ca2+rises elicited by bicuculline withdrawal before forskolin administration, whereas 3rd and 4th refer to the Ca2+ rises after forskolin administration. A comparison of the first two with the second two Ca2+ rises shows that the effects of forskolin were statistically significant (p < 0.0001, two-tailed t test).
Fig. 5.
Adenylyl cyclase modulation alters GABA Ca2+ rises. A, Before the addition of adenylyl cyclase modulators, withdrawal of bicuculline (BIC, 20 μm) elicited rapid, reproducible Ca2+ responses. A, After a 15 min administration of the adenylyl cyclase activator forskolin (20 μm), GABA-mediated Ca2+ rises elicited by the removal of bicuculline from the perfusion solution were significantly depressed, relative to Ca2+ rises elicited before forskolin administration. B, Bar graph representation of the mean Ca2+ rises triggered by the four bicuculline withdrawals;1st and 2nd refer the Ca2+rises before forskolin administration, and 3rd and4th refer to the Ca2+ rises after forskolin administration. C, Relative to the initial two Ca2+ rises, the Ca2+ rises elicited after the administration of the adenylyl cyclase inhibitor SQ-22,536 (100 μm) were significantly depressed. The dashed line is meant to approximate the mean GABA-mediated Ca2+ rise before adenylyl cyclase modulators were added.D, Graphical representation of the mean Ca2+rises triggered by the four bicuculline withdrawals; 1stand 2nd refer to the two Ca2+ rises before SQ-22,536 administration, and 3rd and 4threfer to the two Ca2+ rises after SQ-22,536 administration. Error bars indicate SEM. All experiments were performed in the presence of AP5 (100 μm) and CNQX (10 μm).
Interestingly, SQ-22,536 (100 μm) also depressed the Ca2+ rise elicited by bicuculline withdrawal (Fig.5C). As with forskolin, a 15 min pretreatment caused a statistically significant (p < 0.05, two-tailedt test) decrease (>29%) in the mean Ca2+ rise initiated by bicuculline withdrawal (Fig. 5D). Together, these results indicate that both increasing and decreasing cAMP levels through modulating adenylyl cyclase activity reduces GABA-mediated Ca2+ rises.
Protein kinase A inhibition
To determine whether a basal level of PKA-mediated phosphorylation plays a role in maintaining the level of GABA Ca2+ rises, we assessed the effect of the potent PKA inhibitors H89 and Rp-cAMPS. Figure 6Ashows that the administration of Rp-cAMPS (200 μm) to synaptically active neurons initiated a rapid Ca2+depression. The removal of bicuculline caused a mean Ca2+rise of 70 ± 6 nm. Addition of Rp-cAMPS decreased the Ca2+ rise to 18 ± 2 nm, representing a statistically significant (p < 0.0001, two-tailed t test) 74% decrease in Ca2+activity (n = 45). Of interest was the finding that the Ca2+ depression triggered by Rp-cAMPS persisted for an extended period even after the Rp-cAMPS was washed out. Fifty-eight percent of the neurons assayed (26 of 45) did not recover >50% of their pre-Rp-cAMPS Ca2+ level during any time after Rp-cAMPS withdrawal. In control experiments, none of 35 unstimulated neurons showed >50% reduction in GABA Ca2+ rises over an identical period.
Fig. 6.
GABA Ca2+ levels are reduced by protein kinase A inhibitors. A, Administration of the protein kinase A inhibitor Rp-cAMPS (200 μm) caused a rapid reduction in the Ca2+ level. In thetop neuron, the Ca2+ level remained depressed for an extended period after the removal of Rp-cAMPS from the perfusion solution. B, Pretreating neurons with Rp-cAMPS (200 μm) blocked GABA-mediated Ca2+ rise induction elicited by bicuculline (BIC, 20 μm) removal. C, Another protein kinase A inhibitor, H89 (15 μm), also rapidly depressed GABA-mediated Ca2+ levels. As with Rp-cAMPS, the Ca2+ depression triggered by H89 persisted for an extended period after H89 withdrawal. D, The administration of H89 (15 μm) depressed Ca2+ rises triggered by electrical stimulation (E.S., arrows) of GABA release. Electrically induced Ca2+ rises could be blocked by tetrodotoxin or bicuculline (not shown here). As with its effects on spontaneous GABA release, the effects of H89 persisted even after it was withdrawn from the perfusion solution.
Rp-cAMPS also inhibited the induction of Ca2+ rises mediated by the synaptic release of GABA (Fig. 6B). The two representative neurons in Figure 6B initially exhibited three robust Ca2+ rises, whereas after Rp-cAMPS pretreatment, bicuculline withdrawal did not initiate a Ca2+ rise. As did Rp-cAMPS, a short, 2 min application of H89 (15 μm) triggered a rapid Ca2+ depression (Fig. 6C). H89 reduced the GABA-mediated Ca2+rise from 44 ± 5 to 12 ± 1 nm(n = 14). The level of H89-mediated depression was statistically significant (p < 0.0001, two-tailed t test). As a measure of the long-term effectiveness of H89, only 4 of the 14 neurons assayed recovered >50% of their pre-H89 Ca2+ level 30 min after H89 withdrawal.
H89 also depressed GABA activity triggered by electrical stimulation. Figure 6D shows that the brief administration of H89 (15 μm) resulted in a statistically significant (p < 0.0001, two-tailed t test) depression in the Ca2+ rise. Electrical stimulation triggered a mean Ca2+ rise of 441 ± 26 nm. Addition of H89 reduced the Ca2+ rise to 170 ± 12 nm (n = 78). As in the endogenous activity assays described above, inhibition initiated by H89 persisted long after it was washed from the perfusion chamber in some neurons. The addition of TTX or bicuculline largely blocked the electrically evoked Ca2+ rise.
PKA and voltage-dependent Ca2+ channels
In these experiments, we addressed the mechanism of postsynaptic PKA actions. In the constant presence of TTX (1 μm), the bath application of GABA (10 μm) triggered a rapid Ca2+ rise that was depressed by the H89 (15 μm) administration (Fig. 7A). After H89 withdrawal from the perfusion solution, neuronal Ca2+ responsiveness to GABA slowly recovered toward pre-H89 levels. Because GABA elicits a Ca2+ rise through the activation of voltage-activated Ca2+ channels (VACCs), the direct effect of H89 on high K+ (15 mm)-induced Ca2+ rises triggered by VACC activation was assessed. Similar to its effects on GABA, H89 administration rapidly depressed Ca2+ rises mediated by VACCs (Fig. 7B). After H89 withdrawal the recovery of Ca2+ responsiveness had a general appearance that was very similar to the recovery of GABA responsiveness, suggesting that the H89-mediated depression of GABA responses may result, in part, from VACC inhibition. Figure 7,C and D, shows scatter plot analyses of the effects of H89 on GABA-evoked (n = 105) or high K+-evoked (n = 104) Ca2+ rises, respectively. Single-cell values were determined by dividing the first evoked Ca2+ rise in the presence of H89 by the control-evoked Ca2+ rise immediately preceding H89 administration. Values are expressed as percentages of the control Ca2+ rise. A Ca2+ rise in the presence of H89 that was larger than control rises was >0%, whereas a rise of smaller peak height than the control rise was <0%; rises of equal peak height were 0%. Of note, the overall level of Ca2+ depression elicited by H89 was greater for K+-evoked Ca2+rises than for GABA-evoked Ca2+ rises. Figure 7Eshows that high K+-induced Ca2+ rises were largely suppressed by the administration of the L-type Ca2+channel blocker nimodipine (1 μm). A graphical representation of mean Ca2+ rises in response to GABA and high K+ before and during H89 or nimodipine application is shown in Figure 7F. The mean Ca2+ depressions triggered by H89 and nimodipine were statistically significant (p < 0.0001, two-tailed t test). These findings suggest that tonic PKA-mediated Ca2+ channel phosphorylation is required for GABA to elicit a robust Ca2+ rise.
Fig. 7.

PKA inhibition reduces evoked Ca2+ responsiveness. A, GABA (10 μm) (arrows) elicited rapid Ca2+ rises that were depressed by the administration of the PKA inhibitor H89 (15 μm) in two neurons.B, H89 also depressed Ca2+ rises initiated by high K+ (15 mm) (arrows) administration. C, D, Analysis of the modulatory effects of H89 on GABA-evoked Ca2+ rises (C) or high K+-evoked Ca2+ rises (D).Circles are percentage representations of the first Ca2+ rise in the presence of H89 divided by the control Ca2+ rise immediately preceding H89 administration; eachcircle represents the response of a single neuron. The 0% point on the y-axis signifies that the evoked rise in the presence of H89 was equivalent to the evoked Ca2+rise. Values >0% represent potentiation; values <0% represent depression. E, The L-type Ca2+ channel blocker nimodipine (1 μm) largely blocked Ca2+ rises elicited by high K+. Both GABA and high K+ were applied for 15 sec. F, Graphical representation of the mean Ca2+ rises elicited either by GABA or high K+ before (white bars) or during the coadministration of H89 (black bars) or nimodipine (striped bar).N, Total number of neurons assayed. Error bars indicate SEM.
Modulation of GABA Ca2+ rises in adenylyl cyclase-coupled neurotransmitter receptor systems
The experiments above used pharmacological tools to directly alter different stages of intracellular pathways that affect kinase-mediated phosphorylation. We include the experiments below to demonstrate that activating neurotransmitter receptors that have previously been shown to modulate cAMP levels (either increase or decrease) exert actions parallel to those occurring when agents that act downstream of the receptor are activated (i.e., experiments described above).
We tested whether transmitter receptors shown to be either positively or negatively coupled to cAMP production alter GABA Ca2+rises. Toward this end, we chose the well characterized dopamine D1 receptor. The D1 receptor has been shown to be coupled to an increase in cAMP levels (Shultz et al., 1987; Steffey et al., 1991; Liu et al., 1992; Lovenberg et al., 1991). Administration of the dopamine D1 receptor-specific agonist SKF-38393 (5 μm) triggered a rapid and reproducible depression in the GABA-mediated Ca2+rise (Fig. 8A). The withdrawal of bicuculline from the perfusion solution triggered a mean Ca2+ rise of 86 ± 4 nm from the basal Ca2+ level in the presence of bicuculline. Addition of SKF-38393 caused the Ca2+ rise to decrease to 56 ± 3 nm, representing a statistically significant (p < 0.0001, two-tailed t test) 35% depression in the GABA-mediated Ca2+ rise (n = 79). Previously, we found that NPY (100 nm) caused a large (>70%) depression in the Ca2+ rise elicited by synaptically released GABA (Obrietan and van den Pol, 1996b). An example of the Ca2+-depressing actions of NPY is shown in Figure 8B. Several studies have shown that NPY receptor stimulation triggers inhibitory G-protein activation, leading to a decreased cAMP level (McAuley et al., 1991;Bleakman et al., 1992; Larhammar et al., 1992; Zhu et al., 1992). Additionally, administration of the muscarinic acetylcholine receptor agonist McN-A-343 (100 μm) triggered a Ca2+depression (Fig. 8C). The withdrawal of bicuculline from the perfusion solution elicited a mean Ca2+ rise of 96 ± 7 nm from the basal Ca2+ level. McN-A-343 administration caused the Ca2+ rise to decrease to 65 ± 5 nm, representing a statistically significant (p < 0.0001, two-tailed t test) 32% depression in the GABA-mediated Ca2+ rise (n = 52). A large number of studies have shown that the muscarinic acetylcholine receptors modulate cAMP levels (McKinney et al., 1991; Schwarz et al., 1993; Burford et al., 1995; Migeon et al., 1995). Interestingly, in 13 of 58 neurons, a lower McN-A-343 concentration (15 μm) increased GABA-mediated Ca2+ levels by >10%. In contrast, only 1 of 52 neurons treated with the higher concentration of McN-A-343 (100 μm) showed a >10% increase in the GABA-mediated Ca2+ rise. These results support the hypothesis that receptor systems coupled to either an increase or a decrease in cAMP production may alter GABA Ca2+ rises significantly.
Fig. 8.

Ca2+ rises mediated by GABA activity are depressed by selective activation of neurotransmitter receptors coupled to adenylyl cyclase regulation. The removal of bicuculline (BIC, 20 μm) from the perfusion solution triggered a rapid and sustained Ca2+ rise in the three representative neurons. A, Stimulation of the D1 receptor by the administration of SKF-38393 (5 μm) reduced the amplitude of the spontaneous GABA-mediated Ca2+rise. B, NPY (100 nm) triggered a rapid Ca2+ depression. Blockade of the GABAA receptor with bicuculline at the end of the experiment reduced Ca2+to basal levels. C, Stimulation with the muscarinic acetylcholine receptor agonist McN-A-343 (100 μm) also triggered Ca2+ depression. The ionotropic glutamate receptor antagonists AP5 (100 μm) and CNQX (10 μm) were perfused throughout the experiment to block synaptically released glutamate from triggering a Ca2+rise.
DISCUSSION
Activation of the GABAA receptor, either through exogenous agonist application or synaptic GABA release, elicits a Ca2+ rise in the majority of developing neurons from many brain regions (Obrietan and van den Pol, 1995). In the present study, we characterized the functional role of the cAMP signal transduction pathway in terms of its ability to regulate GABA-related Ca2+ rises. Our data suggest that either activating or inhibiting the cAMP signal transduction pathway significantly depressed the GABA activity detected with Ca2+ imaging. The results of affecting the cAMP signal transduction pathway were similar to the effects of stimulating transmitter receptors thought to be coupled to either a decrease or an increase in cAMP levels.
PKA activation or inhibition, as described in this paper, may cause a variety of different mechanisms to work together or in opposition to one another, with the general effect being a decrease in the Ca2+ rise initiated by GABA-mediated neurotransmission. To our knowledge, ours is the first report characterizing the regulation of GABA-mediated Ca2+ rises by direct modulation of the cAMP signal transduction pathway. These effects were observed with agents that either activated (membrane-permeable cAMP analogs and forskolin) or inhibited (PKA inhibitors and SQ-22536) the cAMP signal transduction pathway at several different points along the pathway.
Postsynaptic effects
Blocking PKA activity with H89 or Rp-cAMPS depressed peak Ca2+ rises elicited by the bath application of GABA. Because the presynaptic release of GABA-containing vesicles was blocked with TTX, the observed inhibitory effect of PKA was postsynaptic in nature. A number of possible mechanisms could account for the depression of GABA-mediated Ca2+ levels observed when the cAMP signal transduction cascade was altered. A decrease in GABAA receptor activity resulting from an inhibition of steady-state GABAA receptor phosphorylation could explain the depression resulting from PKA inhibition. Along these lines, several studies have shown that GABAA receptor-mediated activity is regulated by phosphorylation. GABAA currents were decreased by >90% if cells were ATP-depleted (Chen et al., 1990), suggesting that a basal level of phosphorylation was required for maintenance of optimal GABAA receptor channel function. In addition, GABAA receptor conductance has been shown to be directly affected by PKA-mediated phosphorylation (Moss et al., 1992). In the hippocampus, the frequency of GABA-mediated giant depolarizing potentials is modulated by 8-Br-cAMP or forskolin and inhibited by Rp-cAMPS (Strata et al., 1995). Within this context, our results suggest that tonic postsynaptic PKA-mediated phosphorylation is required for optimal Ca2+ responsiveness.
Ca2+ responses triggered by the direct activation of VACCs (largely L-type) were also suppressed by H89. Because the ability of GABA to elicit a Ca2+ rise in hypothalamic neurons is dependent on L-type Ca2+ channel activation (Obrietan and van den Pol, 1995), these results suggest that H89-mediated depression of GABA-evoked Ca2+ rises may result, in part, from the inhibition of L-type VACC activity. A basal level of phosphorylation may be required for optimal L-type Ca2+ channel activity; Ca2+ currents in HEK-293 cells transfected with the L-type Ca2+ channel gene were depressed by administration of PKA inhibitors (Perez-Reyes et al., 1994). Additionally, forskolin increased the L-type Ca2+ current in ferret ventricular myocytes, and this increase was blocked by H89 (Yuan and Bers, 1995). Our results in neurons are consistent with these findings. This does not rule out a possible additional effect of phosphorylation on K+ channels that could, in turn, influence VACCs.
Presynaptic effects
The cAMP signal transduction pathway appeared to act at both presynaptic and postsynaptic sites to affect GABA-related Ca2+ rises. Ca2+ rises triggered by electrical stimulation of presynaptic GABA release were depressed to a much greater extent by 8-Br-cAMP than postsynaptic Ca2+ rises elicited by the bath application of GABA. Additionally, postsynaptic Ca2+ rises in a subpopulation of neurons were potentiated by 8-Br-cAMP, whereas potentiation was not observed when Ca2+ rises were elicited by electrically induced presynaptic GABA release. These differential effects suggest that the cAMP signal transduction pathway may inhibit presynaptic GABA release, as well as modulate postsynaptic GABA responsiveness. This conclusion is consistent with the view that PKA has a spatially limited action and may exert independent or opposing effects in presynaptic axons compared with the postsynaptic somatodendritic complex.
PKA activators 8-Br-cAMP or 4-Cl-cAMP decreased GABA-related Ca2+ rises, although we also noted that low concentrations of 8-Br-cAMP or 4-Cl-cAMP increased Ca2+ levels in a subpopulation of neurons during endogenous activity assays. These Ca2+ modulatory effects of 8-Br-cAMP and 4-Cl-cAMP were largely through a presynaptic mechanism. For a presynaptic receptor coupled to adenylyl cyclase activation (which would result in increased PKA activity), there have been a variety of reports describing either potentiation or inhibition of transmitter release. For example, activation of the dopamine D1 receptor (coupled to cAMP production) facilitates neurotransmission in the hippocampus (Imperato et al., 1993) and in the ventral tegmetal area (Cameron and Williams, 1993), whereas D1 receptor stimulation has been shown to decrease neurotransmission in the basal forebrain (Momiyama et al., 1996) and in the shell region of the nucleus accumbens (Pennartz et al., 1992).
The exclusive effect of PKA inhibitors on Ca2+ rises elicited by presynaptic GABA release was inhibitory. Receptors coupled to adenylyl cyclase inhibition (which would result in decreased PKA activity), including the GABAB receptor (Dittman and Regehr, 1996), the adenosine A1 receptor (Potier and Dutar, 1993), and the NPY receptor (Bleakman et al., 1992), have been shown to decrease transmitter release. These findings suggest that the effects of PKA on transmitter release may depend on the presynaptic expression of a variety of phosphorylation targets that may be differentially expressed in different brain regions, developmental stages, or neural phenotypes. This is consistent with the presynaptic localization of many hypothalamic neuromodulatory receptors that act through G-proteins to regulate cAMP (Chen and van den Pol, 1996).
Long-term Ca2+ depression
In assays in which Ca2+ rises were elicited via synaptic GABA release, inhibition of PKA by H89 or Rp-cAMPS triggered a rapid and long-term Ca2+ depression (>30 min) in a large number of neurons (58%). Based on the differential postsynaptic effects of PKA inhibitors, long-term Ca2+ depression seems to be mediated through a largely presynaptic mechanism. Interestingly, membrane-permeable cAMP analogs triggered short- but not long-term Ca2+ depression also through a largely presynaptic mechanism. These results suggest that the cellular mechanisms that inhibit PKA-mediated phosphorylation may be uniquely positioned to depress neural activity over extended periods.
NPY has both presynaptic and postsynaptic actions on GABA activity in developing hypothalamic neurons. Whereas NPY triggered a predominately brief postsynaptic depression of GABA-evoked Ca2+ rises (Obrietan and van den Pol, 1996b), presynaptic actions of NPY on transmitter release were often long-term (Obrietan and van den Pol, 1996b; van den Pol et al., 1996c). In addition, NPY receptor stimulation depressed the Ca2+ rise in a similar percentage of neurons and for a similar duration as did direct PKA inhibition. NPY has been shown both to decrease cAMP levels in neurons (Harfstrand et al., 1987; McAuley et al., 1991) and to act through a Gi/Go-protein-coupled mechanism to reduce Ca2+ rises elicited by synaptic GABA release (Bleakman et al., 1992; Obrietan and van den Pol, 1996b; van den Pol et al., 1996c). Activation of a Gi-protein-coupled mechanism could result in decreased adenylyl cyclase activity and, in turn, decreased PKA activity, an effect similar to adding Rp-cAMPS or H89 to the perfusion solution. These results indicate that the long-term effects of NPY receptor activation on GABA-mediated Ca2+ rises may be the result of decreased PKA activity. Other receptor systems coupled to adenylyl cyclase regulation have been shown to trigger extended depression of neural activity, including the GABABreceptor (Yang et al., 1994; Wagner and Alger, 1995) and metabotropic glutamate receptor (Bolshakov and Siegelbaum, 1994;O’Mara et al., 1995). Immunohistochemical analysis has revealed a high level of adenylyl cyclase expression both in postsynaptic densities and in presynaptic axon terminals (Mons and Cooper, 1995), suggesting that adenylyl cyclase may play an important role both as a regulator of postsynaptic membrane ion conductance and presynaptic neurotransmitter release.
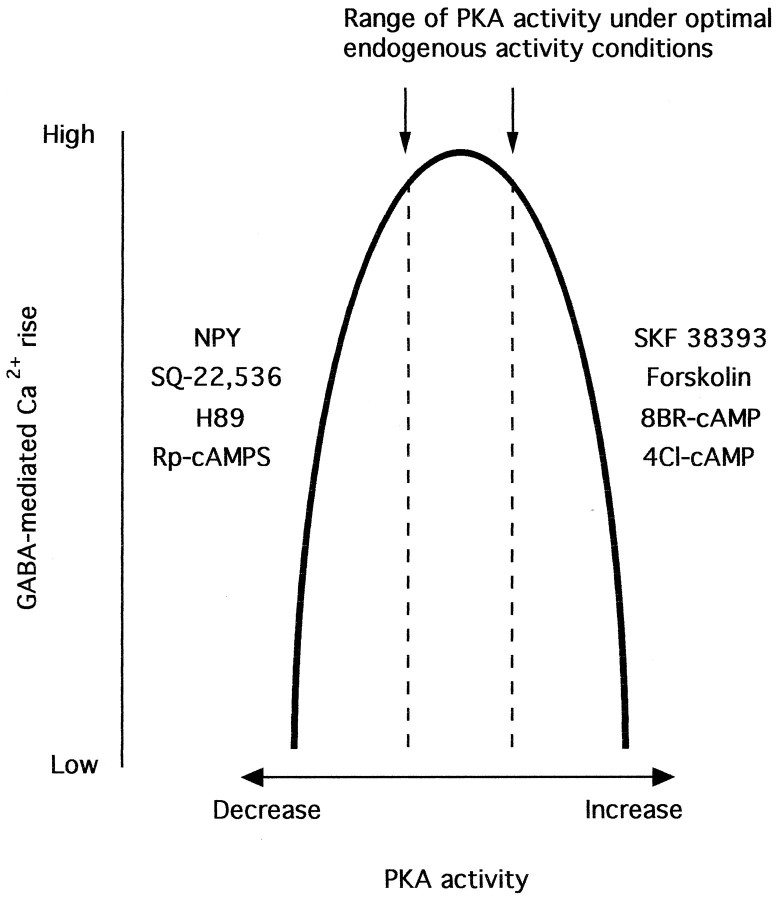
Biphasic effects of phosphorylation on GABA transmission
GABA-related Ca2+ rises were depressed by both strong activation and inhibition of the cAMP signal transduction system. This biphasic response could be explained if the effects of PKA on GABA-mediated Ca2+ rises were characterized by an inverted U-shape function (Fig. 9). During GABA neurotransmission, a basal level of tonic PKA-mediated phosphorylation results in a near maximal Ca2+ level. This condition would put PKA activity near the top of the inverted U-shape function. When PKA activity is radically altered, either through large increases (D1 receptor activation, forskolin, and cAMP analogs) or decreases (NPY receptor activation, SQ-22,536, Rp-cAMPS, and H89), PKA activity moves out of the region of the inverted U-shape function that provides maximal GABA-mediated Ca2+ rises.
Fig. 9.
Model of biphasic effect of PKA activity on GABA transmission. Proposed model describing how increases or decreases in PKA activity result in a reduction in GABA-mediated Ca2+rises. Arrows and dashed lines indicate the range of endogenous PKA activity required for maximal GABA-mediated Ca2+ rises. See Discussion for details.
Low extracellular concentrations of 8-Br-cAMP (50–150 μm) or 4-Cl-cAMP (20 μm) increased GABA-mediated activity in some neurons, whereas high concentrations of 8-Br-cAMP (≥500 μm) or 4-Cl-cAMP (≥200 μm) only decreased Ca2+ levels. This is consistent with the hypothesis that the basal level of PKA activity in some neurons is just below that needed to provide a maximal level of GABA-mediated Ca2+ rises. As described by an inverted U-shape response, this effect would result in a movement toward the apex of the inverted U. The data fitted to this model indicate that GABA-mediated Ca2+ rises depend on ongoing PKA activity, and that PKA activity must be maintained within a narrow zone for GABA to elicit a maximal Ca2+ rise.
Functional role of GABA-mediated Ca2+ increases during early development
Changes in cytosolic Ca2+ levels affect a variety of developmentally regulated neural processes. Induction of Ca2+ influx was primarily thought to be through the activation of classic membrane-depolarizing transmitters such as glutamate. Recent data have shown that GABA can exert a similar depolarizing action in developing neurons. This Ca2+-elevating action of GABA is not restricted to hypothalamic neurons but was found in the majority of developing neurons from eight brain regions, including hippocampus, spinal cord, cortex, olfactory bulb, and striatum (Reichling et al., 1994; Obrietan and van den Pol, 1995), and after severe neural injury (van den Pol et al., 1996b). The ability of GABA to increase Ca2+ levels suggests that it may have an important role during nervous system development. Along these lines, the GABA agonist muscimol can upregulate BDNF mRNA expression in rat hippocampal neurons, and this effect is blocked by the L-type Ca2+ channel blocker nifedipine (Berninger et al., 1995). GABA secretion decreased DNA synthesis in cortical progenitor cells (LoTurco et al., 1995). GABA induces motility of embryonic cortical neurons through an increase in intracellular Ca2+ (Behar et al., 1996). Other GABA-mediated actions during neural development include altering neurite outgrowth (Barbin et al., 1993), triggering chemokinesis (Behar et al., 1994), inducing GABA receptor expression (Meier et al., 1984), and regulating neural phenotype (Marty et al., 1996). We have recently reported that GABA triggers localized Ca2+ increases in developing neurites and growth cones (Obrietan and van den Pol, 1996a), suggesting a possible Ca2+-dependent role for GABA in altering the directionality of growth cone motility and the rate of neurite outgrowth. Based on these reports and the findings in our paper, during early development the activation of neurotransmitter receptors (such as NPY, glutamate, dopamine, serotonin, melatonin, and acetylcholine) coupled to the cAMP signal transduction pathway could substantially alter GABA-related Ca2+ rises. This, in turn, could affect the myriad of Ca2+-dependent developmental processes described above.
Depression of Ca2+ rises attributable to an inhibitory action of presynaptic neuromodulators on GABA release is only found during early development. Because GABA does not raise Ca2+in older neurons, but may instead decrease it (Obrietan and van den Pol, 1995), neuromodulatory inhibition of GABA release in mature neurons would be expected to exert the opposite effect on Ca2+. Thus, in developing neurons, modulatory inhibition of GABA release reduces cytosolic Ca2+ but in mature neurons would raise Ca2+.
Footnotes
This work was supported by National Institutes of Health Grants NS10174 and NS34887, the National Science Foundation, and Air Force Office of Scientific Research.
Correspondence should be addressed to Anthony N. van den Pol, Department of Neurosurgery, Yale University, School of Medicine, 333 Cedar Street, New Haven, CT 06520.
REFERENCES
- 1.Anholt RR. Signal integration in the nervous system: adenylate cyclases as molecular coincidence detectors. Trends Neurosci. 1994;17:37–41. doi: 10.1016/0166-2236(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 2.Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993;152:150–152. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 3.Behar TN, Schaffner AE, Colton CA, Somogyi R, Olah Z, Lehel C, Barker JL. GABA-induced chemokinesis and NGF-induced chemotaxis of embryonic spinal cord neurons. J Neurosci. 1994;14:29–38. doi: 10.1523/JNEUROSCI.14-01-00029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behar TN, Schaffner AE, Tran HT, Barker JL. GABA-induced motility of spinal neuroblasts develops along a ventrodorsal gradient and can be mimicked by agonists of GABA-A and GABA-B receptors. J Neurosci Res. 1995;42:97–108. doi: 10.1002/jnr.490420111. [DOI] [PubMed] [Google Scholar]
- 5.Behar TN, Li Y-X, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belin MF, Fevre-Montange M, Reboul A, Didier-Bazes M, Ehret M, Maitre M, Tardy M. Primary dissociated cell culture of embryonic rat metencephalon: presence of GABA in serotonergic neurons. Neurosci Lett. 1991;125:101–106. doi: 10.1016/0304-3940(91)90001-a. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol (Lond) 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development (Camb) 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- 9.Bleakman D, Harrison NL, Colmers WF, Miller RJ. Investigation into neuropeptide Y-mediated presynaptic inhibition in cultured hippocampal neurones of the rat. Br J Pharmacol. 1992;107:334–340. doi: 10.1111/j.1476-5381.1992.tb12747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 11.Burford N, Tobin A, Nahorski S. Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3′,5′-cyclic monophosphate accumulation in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1995;274:134–142. [PubMed] [Google Scholar]
- 12.Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, van den Pol AN. NPY Y1- and Y2-like receptors coexist in pre- and postsynaptic sites: inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in rat developing hypothalamic neurones. J Physiol (Lond) 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen QX, Stelzer A, Kay AR, Wong RKS. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurons. J Physiol (Lond) 1990;420:207–222. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 17.Cooper DM, Mons N, Fagan K. Ca2+-sensitive adenylyl cyclases. Cell Signal. 1994;6:823–840. doi: 10.1016/0898-6568(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 18.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukura H, Komiya Y, Igarashi M. Signaling pathway downstream of GABAA receptor in growth cone. J Neurochem. 1996;67:1426–1434. doi: 10.1046/j.1471-4159.1996.67041426.x. [DOI] [PubMed] [Google Scholar]
- 20.Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271:1718–1723. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 22.Gu Q, Moss RL. 17-β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harfstrand A, Fredholm B, Fuxe K. Inhibitory effects of neuropeptide Y on cyclic AMP accumulation in slices of the nucleus tractus solitarius region of the rat. Neurosci Lett. 1987;76:185–190. doi: 10.1016/0304-3940(87)90713-0. [DOI] [PubMed] [Google Scholar]
- 24.Huang C-C, Hsu K-S, Gean P-W. Isoproterenol potentiates synaptic transmission primarily by enhancing presynaptic calcium influx via P- and/or Q-type calcium channels in the rat amygdala. J Neurosci. 1996;16:1026–1033. doi: 10.1523/JNEUROSCI.16-03-01026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imperato A, Obinu MC, Gessa GL. Stimulation of both dopamine D1 and D2 receptors facilitates in vivo acetylcholine release in the hippocampus. Brain Res. 1993;618:341–345. doi: 10.1016/0006-8993(93)91288-4. [DOI] [PubMed] [Google Scholar]
- 26.Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BD, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;24:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larhammar D, Blomqvist A, Yee F, Jazin E, Yoo H, Wahlested C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J Biol Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- 29.Lieb K, Andersen C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre- and postnatal development of dopaminergic neuron numbers in the male and female mouse midbrain. Dev Brain Res. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- 30.Liu YF, Civelli O, Zhou QY, Albert PR. Cholera toxin-sensitive 3′,5′-cyclic adenosine monophosphate and calcium signals of the human dopamine-D1 receptor: selective potentiation by protein kinase A. Mol Endocrinol. 1992;6:1815–1824. doi: 10.1210/mend.6.11.1282671. [DOI] [PubMed] [Google Scholar]
- 31.LoTurco JJ, Owens DF, Heath MJS, Davis MBE, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 32.Lovenberg TW, Roth RH, Nichols DE, Mailman RB. D1 dopamine receptors of NS20Y neuroblastoma cells are functionally similar to rat striatal D1 receptors. J Neurochem. 1991;57:1563–1569. doi: 10.1111/j.1471-4159.1991.tb06352.x. [DOI] [PubMed] [Google Scholar]
- 33.Marti E, Biffo S, Fasolo A. Neuropeptide Y m-RNA and peptide are transiently expressed in the developing rat spinal cord. NeuroReport. 1992;3:401–404. doi: 10.1097/00001756-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Marty S, Berninger B, Carroll P, Thoenen H. GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron. 1996;16:565–570. doi: 10.1016/s0896-6273(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 35.McAuley MA, Macrae IM, Farmer R, Reid JL. Effects of neuropeptide Y on forskolin, α 2- and β-adrenoceptor-regulated cAMP levels in the rat brain slice. Peptides. 1991;12:407–412. doi: 10.1016/0196-9781(91)90077-3. [DOI] [PubMed] [Google Scholar]
- 36.McKinney M, Miller J, Gibson V, Nickelson L, Aksoy S. Interactions of agonists with M2 and M4 muscarinic receptor subtypes mediating cyclic AMP inhibition. Mol Pharmacol. 1991;40:1014–1022. [PubMed] [Google Scholar]
- 37.Meier E, Drejer J, Schousboe A. GABA induces functionally active low-affinity GABA receptors on cultured cerebellar granule cells. J Neurochem. 1984;43:1737–1744. doi: 10.1111/j.1471-4159.1984.tb06102.x. [DOI] [PubMed] [Google Scholar]
- 38.Migeon J, Thomas S, Nathanson N. Differential coupling of m2 and m4 muscarinic receptors to inhibition of adenylyl cyclase by Giα and G(o)α subunits. J Biol Chem. 1995;270:16070–16074. doi: 10.1074/jbc.270.27.16070. [DOI] [PubMed] [Google Scholar]
- 39.Momiyama T, Sim JA, Brown DA. Dopamine D1-like receptor-mediated inhibition of excitatory transmission onto rat magnocellular basal forebrain neurones. J Physiol (Lond) 1996;495:97–106. doi: 10.1113/jphysiol.1996.sp021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mons N, Cooper DMF. Immunohistochemical localization of adenylyl cyclase in rat brain indicates a highly selective concentration at synapses. Proc Natl Acad Sci USA. 1995;92:8473–8477. doi: 10.1073/pnas.92.18.8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- 42.Nagel G, Hwang TC, Nastiuk KL, Nairn AC, Gadsby DC. The protein kinase A-regulated cardiac Cl− channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992;360:81–84. doi: 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- 43.O’Mara SM, Rowan MJ, Anwyl R. Metabotropic glutamate receptor-induced homosynaptic long-term depression and depotentiation in the dentate gyrus of the rat hippocampus in vitro. Neuropharmacology. 1995;34:983–989. doi: 10.1016/0028-3908(95)00062-b. [DOI] [PubMed] [Google Scholar]
- 44.Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental transition from Ca2+ excitatory to inhibitory. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obrietan K, van den Pol AN. Growth cone calcium elevation by GABA. J Comp Neurol. 1996a;372:167–175. doi: 10.1002/(SICI)1096-9861(19960819)372:2<167::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Obrietan K, van den Pol AN. Neuropeptide Y depresses GABA-mediated Ca2+ transients in developing suprachiasmatic nucleus neurons: a novel form of Ca2+ long-term depression. J Neurosci. 1996b;16:3521–3533. doi: 10.1523/JNEUROSCI.16-10-03521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pennartz CMA, Dolleman-Van Der Weel MJ, Kitai ST, Silva FHLD. Presynaptic dopamine d1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Reyes E, Yuan W, Wei X, Bers DM. Regulation of the cloned L-type cardiac calcium channel by cyclic-AMP-dependent protein kinase. FEBS Lett. 1994;342:119–123. doi: 10.1016/0014-5793(94)80484-2. [DOI] [PubMed] [Google Scholar]
- 49.Potier B, Dutar P. Presynaptic inhibitory effect of baclofen on hippocampal inhibitory synaptic transmission involves a pertussis toxin-sensitive G-protein. Eur J Pharmacol. 1993;231:427–433. doi: 10.1016/0014-2999(93)90120-7. [DOI] [PubMed] [Google Scholar]
- 50.Rajaofetra N. Pre-natal and post-natal ontogeny of serotonergic projections to the rat spinal cord. J Neurosci Res. 1989;22:305–321. doi: 10.1002/jnr.490220311. [DOI] [PubMed] [Google Scholar]
- 51.Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol (Lond) 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sciancalepore M, Cherubini E. Protein kinase A-dependent increase in frequency of miniature GABAergic currents in rat CA3 hippocampal neurons. Neurosci Lett. 1995;187:91–95. doi: 10.1016/0304-3940(95)11348-7. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz R, Davis R, Jaen J, Spencer C, Tecle H, Thomas A. Characterization of muscarinic agonists in recombinant cell lines. Life Sci. 1993;52:465–472. doi: 10.1016/0024-3205(93)90303-k. [DOI] [PubMed] [Google Scholar]
- 54.Shultz PJ, Sedor JR, Abboud HE. Dopaminergic stimulation of cAMP accumulation in cultured rat mesangial cells. Am J Physiol. 1987;253:H358–H364. doi: 10.1152/ajpheart.1987.253.2.H358. [DOI] [PubMed] [Google Scholar]
- 55.Smith RD, Goldin AL. Phosphorylation of brain sodium channels in the I-II linker modulates channel function in Xenopus oocytes. J Neurosci. 1996;16:1965–1974. doi: 10.1523/JNEUROSCI.16-06-01965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith TW, Nikulasson S, De Girolami U, De Gennaro LJ. Immunohistochemistry of synapsin I and synaptophysin in human nervous system and neuroendocrine tumors: applications in diagnostic neuro-oncology. Clin Neuropathol. 1994;12:335–342. [PubMed] [Google Scholar]
- 57.Steffey ME, Snyder GL, Barrett RW, Fink JS, Ackerman M, Adams P, Bhatt R, Gomez E, MacKenzie RG. Dopamine D1 receptor stimulation of cyclic AMP accumulation in COS-1 cells. Eur J Pharmacol. 1991;207:311–317. doi: 10.1016/0922-4106(91)90005-3. [DOI] [PubMed] [Google Scholar]
- 58.Strata F, Sciancalepore M, Cherubini E. Cyclic AMP-dependent modulation of giant depolarizing potentials by metabotropic glutamate receptors in the rat hippocampus. J Physiol (Lond) 1995;489:115–125. doi: 10.1113/jphysiol.1995.sp021035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surmeier DJ, Bargas J, Hemmings HCJ, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 60.van den Pol AN, Obrietan K, Cao V, Trombley PQ. Embryonic hypothalamic expression of functional glutamate receptors. Neuroscience. 1995;67:419–439. doi: 10.1016/0306-4522(95)96912-w. [DOI] [PubMed] [Google Scholar]
- 61.van den Pol AN, Cao V, Belousov AB. Dopamine enhancement and depression of glutamate-regulated calcium and electrical activity in hypothalamic neurons. J Neurophysiol. 1996a;46:3934–3948. doi: 10.1152/jn.1996.76.6.3934. [DOI] [PubMed] [Google Scholar]
- 62.van den Pol AN, Obrietan K, Chen G. Excitatory actions of GABA after neuronal trauma. J Neurosci. 1996b;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Pol AN, Obrietan K, Chen G, Belousov AB. Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J Neurosci. 1996c;16:5883–5895. doi: 10.1523/JNEUROSCI.16-18-05883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner JJ, Alger BE. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J Neurosci. 1995;15:1577–1586. doi: 10.1523/JNEUROSCI.15-02-01577.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamashita M, Fukuda Y. Calcium channels and GABA receptors in the early embryonic chick retina. J Neurobiol. 1993;24:1600–1614. doi: 10.1002/neu.480241205. [DOI] [PubMed] [Google Scholar]
- 66.Yang XD, Connor JA, Faber DS. Weak excitation and simultaneous inhibition induce long-term depression in hippocampal CA1 neurons. J Neurophysiol. 1994;71:1586–1590. doi: 10.1152/jn.1994.71.4.1586. [DOI] [PubMed] [Google Scholar]
- 67.Yuan W, Bers DM. Protein kinase inhibitor H-89 reverses forskolin stimulation of cardiac L-type calcium current. Am J Physiol. 1995;268:C651–C659. doi: 10.1152/ajpcell.1995.268.3.C651. [DOI] [PubMed] [Google Scholar]
- 68.Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H, Li Y-C, Young AP. Protein kinase A activation of glucocorticoid-mediated signaling in the developing retina. Proc Natl Acad Sci USA. 1993;90:3880–3884. doi: 10.1073/pnas.90.9.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu J, Li W, Toews ML, Hexum TD. Neuropeptide Y inhibits forskolin-stimulated adenylate cyclase in bovine adrenal chromaffin cells via a pertussis toxin-sensitive process. J Pharmacol Exp Ther. 1992;263:1479–1486. [PubMed] [Google Scholar]