Abstract
Taste and tactile fibers in the facial nerve of catfish innervate extraoral taste buds and terminate somatotopically in the facial lobe (FL)—a medullary structure crucial for gustatory-mediated food search. The present study was performed to determine the neural linkages between the gustatory input and the spinal motor output. Spinal injections of horseradish peroxidase (HRP) label spinopetal cells in the octaval nuclei, the nucleus of the medial longitudinal fasciculus, and reticulospinal neurons (Rsps) in the brainstem medial reticular formation (RF), including the Mauthner cell. A somatotopically organized, direct faciospinal system originating from superficial cells scattered in the lateral lobule of the facial lobe (ll) is also labeled. The brainstem reticulospinal cells are segmentally organized into 14 clusters within eight segments of the reticular formation and includes one cluster (RS5) directly ventral to the FL. Injections of HRP or fluorescent tracers into the medial lobule of the FL label a facioreticular projection terminating around the Rsps of RS5. DiI injections into this area of the RF retrogradely label deeply situated bipolar neurons, especially in the medial and intermediate lobules of the FL. Electrophysiological recordings in and around RS5 show units with large receptive fields and with responses to chemical and tactile stimulation. The FL projects to the spinal cord via two pathways: (1) a topographically organized direct faciospinal pathway, and (2) an indirect facioreticulospinal pathway in which reticular neurons process and integrate gustatory information before influencing spinal circuitry for motor control during food search.
Keywords: facial lobe, nucleus of the solitary tract, reticular formation, taste, reticulospinal, feeding
Many animal species possess elaborate sensory systems specialized for locating food in their environment. For example, bats use a highly developed audiovocal system to hunt insects via echolocation (Galambos, 1942; Griffin, 1958), toads have a visual system specialized for detecting worm-like movements (Ewert, 1970), pit vipers use infrared receptors for tracking their prey (Molenaar, 1974;Gruberg et al., 1979), and electric fish use electroreception for detecting and locating food in their environment (Bullock, 1982). Catfish have a highly sensitive, large gustatory sense that plays a critical role in the search for food in muddy waters (Herrick, 1901,1904, 1905; Bardach et al., 1967; Atema, 1971; Caprio et al., 1993;Valentincic and Caprio 1993). Catfish can detect concentration differences between their maxillary barbels and make turning movements appropriate to locate food (Johnsen and Teeter, 1980). The extraoral (facial nerve-innervated) taste receptors are highly sensitive to amino acids (Caprio, 1975, 1978) and are mapped spatially within the facial lobe (FL), the primary gustatory nucleus for external taste (Finger, 1976; Marui and Caprio 1982; Hayama and Caprio, 1989). Neuroethological studies show that the FLs are necessary for food localization (Atema, 1971). These studies suggest that catfish use the facial gustatory sense to detect and orient to chemical stimuli at a distance. Gustatory information must, therefore, reach motor or premotor centers within the CNS to modulate orientation and swimming, which are two essential behavioral components of food search.
Previous anatomical studies (Herrick, 1905; Finger, 1978; Morita and Finger, 1985) have established that the FL gives rise to three major projection systems: (1) an ascending lemniscal pathway reaching diencephalic levels directly and via a pontine relay, (2) a descending system ending in the funicular nuclei and spinal cord (Sp) dorsal horn, and (3) local reflex connections to brainstem reticular formation. The ascending system does not reach the optic tectum (TeO), often a site of multimodal sensory integration into a unified spatial map of the surroundings (Hartline et al., 1978; Knudsen, 1982). Although the FL maintains a highly ordered somatotopic map of gustatory space, this map is not retained in any of the higher lemniscal nuclei (Lamb and Caprio, 1992; Lamb and Finger, 1996). Thus, spatial information about gustatory stimuli must be relayed to premotor centers from the FL and not from higher-order gustatory nuclei.
The present study was initiated to identify premotor gustatory centers and descending gustatomotor pathways in the channel catfish,Ictalurus punctatus. In an effort to localize these pathways, we describe here the reticulospinal system, because it has not been described adequately in siluroid fishes. Electrophysiological investigation of the relevant areas of the reticular formation were performed to determine whether neurons located there respond to chemical as well as tactile cues, and whether the receptive fields of the reticular neurons are well defined and punctate as in the FL, or relatively nonspecific and broad as in higher-order gustatory nuclei (Lamb and Caprio, 1993).
MATERIALS AND METHODS
Animal acquisition and maintenance. Channel catfish,Ictalurus punctatus, (weighing 50–150 gm) were obtained from a local fish farm (Cline’s Trout Farm, Boulder, CO). The fish were maintained in aquaria kept at a 12 hr light/dark cycle. Animals were generally used for electrophysiological studies within 2–3 weeks after being transported to laboratory aquaria. Recordings from the reticular formation were obtained from >15 animals. In a few cases, the recording was followed by iontophoretic injection of HRP at the recording site. All studies were approved by the University of Colorado Health Science Center Institutional Animal Use and Care Committee.
Neuroanatomical studies. Connections of the FL and reticular formation (RF) were examined by the use of two in vivotracers, HRP and dextran amines, and by postmortem diffusion of the carbocyanine dye, diI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate). Neuroanatomical results from in vivo tracer studies are based on horseradish peroxidase (HRP; Sigma, St. Louis, MO; Type VI) injections into the Sp of 12 animals and into the FL or reticular formation of 14 animals. Formaldehyde-fixed brains of additional animals were used to obtain Nissl-stained sections in the transverse and horizontal planes for studying the normal pattern of nuclear organization and for postmortem tracing. For postmortem studies, small crystals of diI were applied to the FL in previously fixed (4% buffered paraformaldehyde) brains. After 2–8 weeks, the tissue was sectioned on a vibratome and examined with a Zeiss (Thornwood, NY) epifluorescent microscope.
Surgery. The animals were anesthetized by transferring them to a tank containing tricaine methane sulfonate (MS) 222 (∼150 mg/l). The anesthetized animals were positioned over a Plexiglas base and respired artificially by water containing MS 222 (∼90 mg/l). The actual dose of the anesthetic varied with the size and the physiological condition of the animal and was adjusted to eliminate respiratory movements. The appropriate portion of the cranium was removed by means of a dental drill and the cerebrospinal fluid and mesenchymal tissues were aspirated from the surface of the brain.
Single-label HRP studies. HRP injections were accomplished by an insect pin coated with a paste of HRP (Finger, 1976) or delivered centrally using iontophoresis (Kanwal et al., 1988). For small iontophoretic injections of HRP in the reticular formation, a train of positive current pulses of 3–5 μA (duration of pulse and interstimulus interval: 5 or 10 sec) was applied for 10–15 min using a A360, WPI constant current stimulator. HRP was applied to the FL (primary gustatory nucleus) in the channel catfish to identify the location of descending pathways. Unilateral and bilateral injections of HRP were also made in the ventral horn and dorsomedial regions of the Sp. HRP was injected at either of two anteroposterior levels of the Sp; anterior injections were made immediately caudal to the dorsal fin, and posterior injections were made caudal to the adipose fin and immediately anterior to the tail. A longitudinal incision, parallel and dorsal to the lateral line, was made in separate animals for injection of HRP into the Sp. The muscle tissue was separated gently with a pair of blunt forceps until the vertebral column was visible. The vertebral cartilage was nipped with a pair of fine scissors and HRP crystals were inserted into the Sp and canal. At the same time, the Sp was pinched at the intended site of injection to facilitate uptake of HRP. After allowing 3–7 d for transport of HRP (3–4 d for FL injection and 4–7 d for Sp injections), the animal was perfused with 4% glutaraldehyde solution. Frozen sections were collected in cold 0.1 mphosphate buffer and treated according to either a modified Hanker-Yates protocol (Bell et al., 1981) or the tetramethylbenzidine method (Mesulam, 1978).
Single-label DiI studies. The carbocyanine dye, DiI was used as a postmortem retrograde tracer to determine the nature and distribution of the cells that give rise to the facioreticular projection. For these experiments, the brain was removed from two catfish that had been perfused with 4% buffered paraformaldehyde, as above. After the brains were fixed for ≥2 d, a 1–2 mm slab of the medulla was prepared by making transverse cuts rostral and caudal to the FL. The tissue, except for the medial reticular formation (RF) was covered with 2% agar to prevent inadvertent application of the dye to nontargeted areas. Small crystals of DiI were then inserted bilaterally into the RF in the vicinity of cluster RS5, i.e., the area in receipt of facioreticular fibers. The tissue was then covered completely in agar and placed into fresh fixative at room temperature. After 2 weeks or 4 months, the agar was removed and the tissue was sectioned on a vibratome at 40–50 μm. The sections were coverslipped with fluoromount and were viewed with a Zeiss epifluorescent microscope equipped with rhodamine filter cube.
Double-label studies. In two cases, tracers were applied to both the Sp and FL. In one of these, two different fluorescent 10K dextran amines were used; in the other, biotinylated dextran was applied to the Sp and HRP was applied to the FL. In the former case, fixation was with 4% paraformaldehyde in phosphate buffer and in the latter case, 2% paraformaldehyde and 0.2% glutaraldehyde in buffer. The tissues were embedded in egg yolk, postfixed for an additional 2.5 hr, then transferred to sucrose buffer for cryoprotection. The next day, 60 μm transverse sections were cut on a cryostat.
For dual fluorescence, the sections were mounted and coverslipped in fluoromount. For the biotin-HRP label, free-floating sections were reacted in a metal-intensified DAB solution containing 25 mg DAB, 20 mg ammonium chloride, 0.5 ml of 1% cobalt chloride, 0.8 ml of 1% nickelous ammonium sulfate, and 25 μl of glucose oxidase (Sigma Type V; 4 mg/ml in acetate buffer) in 50 ml of 0.1 m phosphate buffer. The reaction was started with the addition of 2 ml of 1% β-d-glucose and monitored visually. This reaction produced a blue–black precipitate at the sites of peroxidase activity. After completion of the reaction, the sections were rinsed in phosphate buffer and placed overnight in avidin–biotin complex in PBS plus 0.3% Triton X-100 per standard ABC protocols. The sections were reacted with nonintensified DAB (25 mg/50 ml buffer plus 30 μl of 3% hydrogen peroxide) the next day after rinsing in buffer, thereby producing a brown reaction product at the sites of ABC binding.
Three-dimensional reconstruction of the reticulospinal system. Large unilateral injections of HRP were made in the ventral horn of the Sp and the tissue was processed for visualization of the labeled cells as explained in the previous section. For purposes of reconstruction, horizontal sections of the brainstem were positioned above a light box and an image was stored electronically on the hard drive of a Macintosh IIx (Apple, Inc.) using the IMAGE program. The serial sections were positioned sequentially and lined up on the monitor using the lacuna of the fourth ventricle and large blood vessels as fiducial marks. Each stored image was examined for labeled cell bodies with a nucleolus and X-Y coordinates of these were recorded by clicking a cross-hair on the screen. In case of ambiguity, the corresponding original section was examined under the microscope before registering a labeled cell on the captured image. TheX-Y coordinates of labeled cells in each section were transferred to a computer-aided design software package. This enabled the integration of all sections into a three-dimensional image, which could be viewed and plotted from any specified azimuth and elevation.
In addition, direct electronic image composites were prepared by scanning the microscopic sections at 1700 dpi with a Nikon Coolscan slide scanner. The images were aligned by rotation and translation in Photoshop running on a Quadra 800 Macintosh computer. Intrinsic blood vessels were used as fiducial structures.
Electrophysiological recordings
Surgery. The fish were immobilized with Flaxedil (∼0.1 ml of a 20% solution) and prepared for surgery in a Plexiglas holder. The head was kept in position by small brazing rods inserted through vertical Plexiglas plates so that minor opercular movements would not affect the stability of the recordings. A local anesthetic was applied over the skull before surgery by injecting ∼0.1 ml of Xylocaine (1% lidocaine hydrochloride) under the skin or by rubbing in the anesthetic with a cotton swab. A flap of the epithelium was incised, flipped back, and the skull was opened with a pair of rongeurs or a Dremel drill.
Data acquisition and analysis. For purposes of recording, a borosilicate glass micropipette filled with 2 m NaCl was lowered into the brain with a Narashige hydraulic microdrive. In some cases, the micropipette was back-filled with a dilute (∼10%) filtered solution of HRP in 0.1 m phosphate buffer, pH 7.2, for marking the recording site. Beyond a depth of 2.0 or 2.5 mm (depending on the size of the fish) the movement of the electrode was paused and the epithelium was stimulated by gentle rubbing with a sable-hair brush. The extent of the tactile receptive field was determined by touching various areas of the fish with the brush or by directing a gentle stream of water (through a PE20 tube) at different areas of the skin, mouth, or gills. In the event of a tactile response, a fine water stream was directed to an area within the receptive field and chemical stimulation was accomplished by injecting a bolus of stimulus (millimolar concentrations of l-alanine,l-arginine, or l-proline, a mixture of all three amino acids, or a complex food stimulus consisting of filtered beef liver extract; Kanwal and Caprio, 1987) into a stream of water flowing constantly. In this way, application of the chemical produced essentially no change in the flow characteristics of the ongoing stimulus stream, i.e., this procedure permits the separation of chemical from mechanical cues. The exact concentration of the applied chemicals cannot be determined exactly with this system but was estimated to be ∼50% of the injected concentration (Kanwal and Caprio, 1987). Occasionally, chemical stimuli were tested even in the absence of a unit response to mechanical stimulation. No units were found that responded only to chemical stimulation. The recorded neural activity was stored along with voice data on separate channels of magnetic tape via a video cassette recorder (JVC, model HR-D470U). The tape was played back to an oscilloscope for photographing impulse response patterns onto photographic film in a kymograph (Nihon-Koden).
RESULTS
Prespinal brainstem neurons
Small unilateral injections of HRP were restricted to the ventral horn of the Sp, whereas large injections extended either unilaterally to the lateral and dorsomedial region or bilaterally to the ventral horn region of the opposite side. Only quantitative differences were observed between the labeling after similar injections of HRP into the rostral and the caudal levels of the Sp. The results described in the following sections pertain primarily to spinal injections at the level of the dorsal fin. Any differences observed in the labeling from spinal injections made at caudal levels are indicated in the appropriate sections of the text.
In general, after spinal injections of HRP, retrogradely filled cells occurred (Fig. 1) ipsilaterally in the ll, and occurred bilaterally in the primary octaval nuclei (n. Oct.), the reticular formation (RF), and the nucleus of the mlf (data not shown). Fiber bundles labeled within the Sp were restricted mostly to the ventral and lateral funiculi of the Sp. Within the reticular core of the brainstem, the majority of labeled neurons resided in the ventromedial RF (Figs.1, 2). Smaller neurons were also labeled in the lateral RF and the ventromedial RF (Fig. 1J, small triangles). In addition, anterogradely filled dense fibers terminals were observed in the ventrolateral RF of the medulla (Fig. 1H–J, dots) as described for other species byHayle (1973). The facial and reticulospinal projections are described in greater detail below.
Fig. 1.
Chartings of HRP-labeled cell bodies in transverse sections through the Sp and medulla of the catfish brain after a large unilateral injection of HRP in the Sp at the level of the anterior dorsal fin. A dorsal view of the catfish brain indicating the levels of the transverse sections is shown in the top right corner. Triangles denote labeled cell bodies;dots represent labeled fibers.
Fig. 2.
A, Photomicrograph of a horizontal section through the FL of the right side showing labeled cell bodies (arrowheads) in the lateral lobule of the FL after spinal injection of HRP. Anterior is toward the top; medial is at left. B, Higher magnification view showing faciospinal cell morphology.
Direct faciospinal projections
Large injections in the Sp that included the dorsomedial quadrant also labeled medium-size (20–30 μm) cells situated superficially in the ll, ipsilateral to the site of injection (Fig.2A). Approximately 20 neurons were labeled after injections at the level of the first dorsal fin, whereas more caudal injections (caudal to the second dorsal fin) labeled only five to seven neurons. Filling of the dendrites of these neurons is generally incomplete (Fig. 2B). The large cell bodies, however, were filled densely with the reaction product and were scattered within the lateral lobule. In one case, longer survival times resulted in filling of most of the dendritic arbor of a few cells. Observations under higher magnification showed that although these neurons had an expansive dendritic arbor, the dendritic fields of neighboring neurons were largely nonoverlapping.
Reticulospinal projections
Retrograde labeling of reticular cells after spinal injections of HRP was examined in transverse, horizontal, and sagittal sections of the brain. Horizontal sections provided a convenient way to study the axial organization of the reticulospinal system, whereas transverse sections were useful to examine the dorsoventral separation of clusters of labeled neurons. The location, clustering patterns, and cellular morphology, however, differed significantly between the caudal medullary, rostral medullary, and pontine and mesencephalic RF. As described previously (Lee et al., 1993), the RF is divisible into eight segments (RS 1–8). We find that many of the segments can be further divided into cell clusters that share similar morphologies and position (Fig. 3C).
Fig. 3.
Labeled cell bodies of the reticular formation in horizontal sections after a large bilateral injection of HRP in the Sp at the level of the anterior dorsal fin. A, Image of a single horizontal section as processed with the “NW Gradient” kernel in the image to yield a pseudo-Nomarski effect.Asterisk indicates vertically oriented blood vessel used for alignment of photocomposite sections shown in (B). Arrow indicates Mauthner cell. B, Electronic photocomposite of four horizontal sections including that shown in A. As inA, the asterisk indicates the fiduciary blood vessel and the arrow indicates the Mauthner cell. Relevant reticulospinal (RS) groups are indicated bynumber. C, Diagrammatic representation of the reticulospinal system from a computer-generated three-dimensional reconstruction of serial horizontal sections from the whole brainstem of a different animal. The injection in this case entirely covered the right side of the partially transected Sp and also diffused to the opposite side. The 14 clusters that were distinguished on the basis of spatial rotations of the image using a computer are shown bydashed boundaries. These are presumed to extend over eight rostrocaudal segments, RS1–RS8, based on the terminology adopted by Lee et al. (1993). Note the location and extent of the cluster (RS5) that receives gustatory projections from the FL (circle). The thickness of the reconstructed brainstem containing labeled cells is 900 μm.
Caudal medullary reticular formation
At levels caudal to the vagal lobe (VL), large spinal injections of HRP labeled a continuous row of medium-size (10–20 μm) cells on both sides of the medial longitudinal fasciculus (mlf; including segments RS 7 and 8). A heavier label and a larger number of cells were found ipsilateral to the site of injection. The cell labeling on the contralateral side was also continuous, but the labeled cells were scattered loosely in this region. This pattern is quite similar to what is seen in the central gray of rostral regions of the Sp (Fig. 3A).
Rostral medullary and pontine reticular formation
At the level of the FL and VL, three distinct clusters (RS5, RS6, RS7) of reticulospinal cells can be distinguished in the ventromedial RF (Figs. 1, 3). These clusters consist of 15–20 μm cells and are bilaterally symmetric in the degree of labeling. Of these three clusters, the middle one contains relatively large cells (∼20 μm). The most caudal of these clusters contained a compact group of ∼15 cells, whereas the most rostral cluster was divisible into two laterally placed subgroups.
These cell clusters were separated by decussating fiber bundles. Some of the larger cells had dendrites that extended into the lateral RF. The most rostral cluster corresponded to the level of the facial motor nucleus.
Lateral to the facial motor nucleus, several medium-size (∼15 μm) cells were labeled bilaterally in the octaval nuclear complex (Fig.1K–L). In horizontal sections, these cells appeared as a narrow band at the lateral margins of the brainstem. Immediately rostral and dorsolateral to the facial motor nucleus, the Mauthner neurons were labeled heavily bilaterally if the injection site encroached even partially to the opposite side, because the large-diameter axons of the Mauthner neurons are situated medially in the Sp. In this respect, the Mauthner neuron provided a good way to confirm the unilaterality of the injection site.
Mesencephalic reticular formation
The mesencephalic prespinal neurons are similar to those described in other species (Prasada Rao et al., 1987; Lee and Eaton, 1991) and will not be described in detail here. Briefly, clusters of labeled cells extended laterally and rostrally on both sides of the mlf. In the caudal region of the mesencephalic reticular formation, one lateral cell group was also labeled bilaterally. The labeling in this nucleus was heavier on the contralateral side compared with the side ipsilateral to the injection site.
Three-dimensional arrangement of Rsps
Serial reconstructions of horizontal sections of spinally injected brains revealed that the HRP-labeled neurons are organized in a segmental manner along the anteroposterior axis and extend over the length of the medulla (Fig. 3). In a parasagittal view, Rsps are arranged as a punctuated longitudinal column of cells that is inclined dorsally toward rostral levels of the ventral brainstem. Also, cells at rostral levels are located more laterally than those in the caudal medulla (Fig. 3C).
A serial reconstruction was prepared from one fish with a partly bilateral (i.e., covering all of one side and encroaching into the medial half of the other side) injection of HRP into the rostral Sp (Fig. 3C). Altogether, 324 neurons are labeled contralateral to the injection site in the fully reconstructed set. Of these, 306 cells were localized to 14 clusters in the ventromedial RF, whereas 18 neurons are located too laterally to be included in any cluster. Clusters in segments RS5–RS8 show an ipsilateral bias in labeling, whereas the Mauthner neurons and clusters rostral to them are more heavily labeled on the side contralateral to the main injection site. Thus, the total number of neurons projecting to any one side of the Sp is estimated at ∼360. The clusters are postulated to lie in eight metameric segments (RS1–RS8), with RS4 containing the Mauthner neurons as suggested by Lee et al. (1993). The rostral segments RS1–RS5 contain two clusters each (medial and dorsolateral), whereas each of the remaining caudal segments contain one cluster organized relatively loosely.
The three-dimensional reconstruction identified two clusters of cells at the level of segment RS5 in the ventromedial RF. This corresponds to the mid to caudal region of the FL (as shown in Figs.1J, 4) and matches the level at which facioreticular projections were observed after injections of tracer into the FL (Fig.4B).
Fig. 4.

Photomicrographs of the right side of the ventral half of transverse sections through the brainstem at the level of the FL (approximate level of Fig. 1J).A, Reticulospinal neurons (Rsps) in segment RS5 labeled retrogradely by spinal injection of HRP.B, Approximately the same field of view showing projections from the FL as revealed by diI tracing.
FL injections
The surface of the FL was divided approximately into quadrants. Small, superficial injections of HRP were made at various loci in the FLs of separate animals. In general, the results of ascending and descending projections from the FLs were similar to those reported earlier (Finger, 1976, 1978; Morita and Finger, 1985). For the present study, only new findings related to the projections to premotor areas such as the RF are described.
Connections of the lateral lobule
Injection of HRP into the ll-labeled fibers terminating in the immediate vicinity of the injection site and also labeled several fibers terminating sparsely in the medial and intermediate lobules. The most heavily labeled fiber groups included the primary inputs to the FL and the large-diameter fibers exiting the FL in the ipsilateral secondary gustatory tract. A few (2–3) fibers were also seen to decussate via the internal arcuate fibers (iaf) and travel in a rostro–dorsal direction before terminating in superficial regions of the lateral lobule of the opposite side.
A separate group of fibers continued in a ventromedial direction after exiting the FL. Some of these fibers seemed to originate from retrogradely filled neurons located adjacent to the mlf, both ipsilateral and contralateral to the site of injection (see also Morita and Finger, 1985, their Figs. 4H, 8B). These neurons (∼15 μm in width and 50 μm in length) were similar to one another in terms of their bipolar shape, situation between the medial RF and the intermediate nucleus of the FL, and orientation of dendrites directed ventrally into the medial RF. A second pair of filled reticular cells were more triangular and located medial to the medial RF and adjacent to the mlf. In this case, the filling of the cell ipsilateral to the site of injection was more intense than the one on the contralateral side. In addition, as reported elsewhere (Finger, 1978; Morita and Finger, 1985), retrograde labeling was observed in cells of the nucleus lobobulbaris (nLB), whereas labeled fibers and terminals occurred in the parvocellular portion of nLB and preoptic nucleus.
Connections of the medial lobule
Injections of HRP into the medial lobule labeled local areas and fibers similar to those observed for the lateral lobule. Injections into the caudomedial quadrant, however, labeled a dense axonal projection of anterogradely filled fibers (Fig. 5). These connections were further attributed to one of two descending routes. Along one route, bipolar neurons in the dorsomedial reticular formation connected to the FL dorsally and to the ventromedial reticular formation ventrally. Along the other route, a slender facioreticular tract (FRt) (Fig. 5) originated from neurons in the caudomedial region of the FL (including the medial lobule and the medial region of the intermediate lobule), and projected directly to the ipsilateral ventromedial reticular formation. At higher magnifications, terminal swellings were observed adjacent to the cell bodies in the medial reticular cells (Fig.6A). Several fibers also cross the midline between fascicles of the mlf and the reticulospinal tract to terminate in the vicinity of the medial reticular neurons of the contralateral side. A few cell bodies were also labeled in the dorsomedial reticular formation.
Fig. 5.
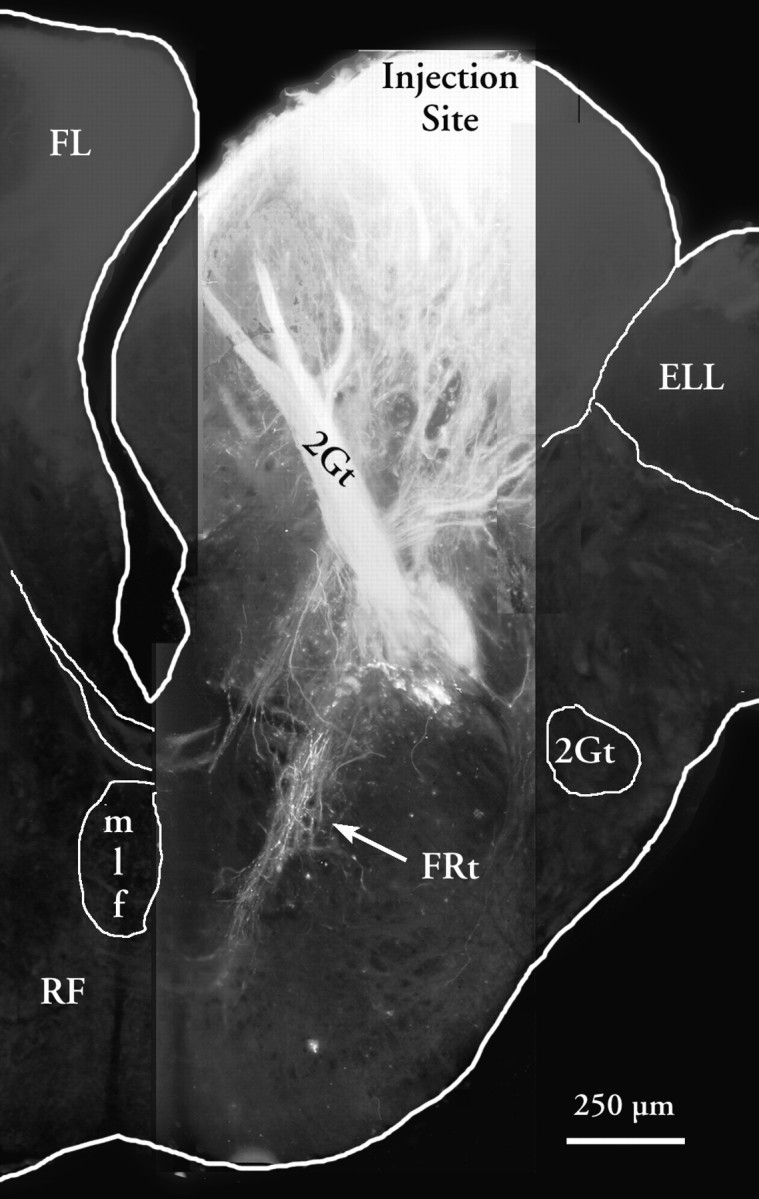
Low-power composite photomicrograph of projections of the FL as shown by diI tracing. Three high-power, high resolution fluorescent images were composited and scaled onto a low resolution image for orientation. The arrow shows the facioreticular fibers (FRt). A higher magnification view of the area of termination of this fiber system in an adjacent section is shown in Figure 4B.
Fig. 6.
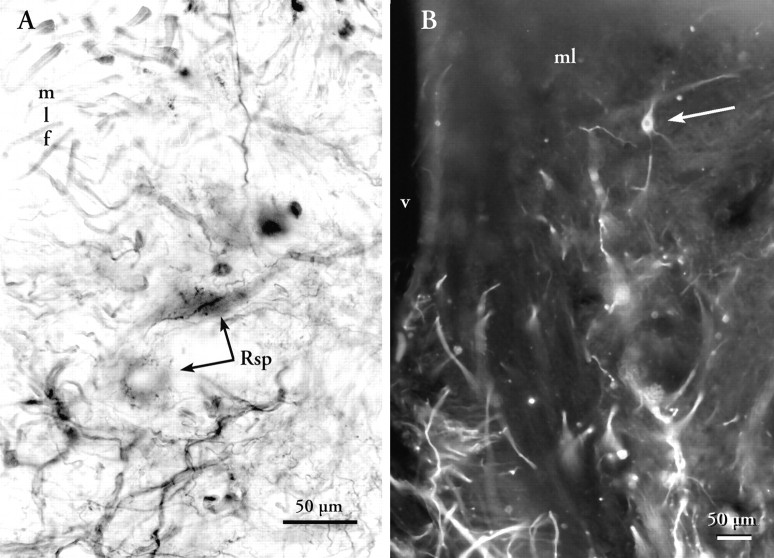
A, Photomicrograph of the RS5 group from a double-label preparation. HRP was injected in the Sp and appearsgray in this picture taken with ared–orange filter. Biotinylated dextran amine was injected in the FL and reacted to appear black in this micrograph. Note the black varicosities associated closely with the somata and proximal dendrites of two labeled reticulospinal neurons (Rsps). B, Photomicrograph of the ventral part of the medial lobule of the FL after a diI injection into the medial reticular formation. A retrogradely labeled neuron deep in the lobule is indicated by thearrow. Fourth ventricle is at left;arrow indicates retrogradely filled cell.
Deep medial injections of HRP into the FL also labeled a tract of fibers decussating at the level of the mlf and caudal to the level of the iaf. These fibers terminate mostly in the intermediate nucleus of the FL (niF) of the opposite side. Electrophysiological recordings from this region of the FL show that neurons in the niF have large receptive fields that are sometimes bilateral (Hayama and Caprio, 1989).
To rule out the possibility that the FRt might be entirely attributable to collaterals of retrogradely labeled RF neurons, diI was applied to the medial RF. Such application results in retrograde labeling of large (20 × 40 μm) bipolar neurons and medium-sized (12 × 20 μm −15 × 25 μm) bipolar neurons situated deep in the FL (Fig. 6B). The majority of these neurons lie within or adjacent to the fiber bundles lying along the ventral margin of the intermediate and medial lobules. A few scarce facioreticular cells can be found in association with fiber bundles of the lateral lobule.
Responses of single reticular neurons
The neurophysiological data included in this study are presented only in so far as to establish whether: (1) chemical or tactile stimuli influence RF neurons of RS5, and (2) receptive fields of the neurons of RS5 are similar to those of the FL in maintaining a fine-grain somatotopically order map of the body surface. In addition to multiunit mapping studies (results not presented here), we obtained single-unit data from >60 neurons in the medullary RF. Neurons in this region of the RF were generally divisible into three categories depending on their rate of spontaneous activity. Thirty-two percent of neurons exhibited very low rates (<1 spike/sec) or no spontaneous activity. This group of neurons showed an irregular spontaneous discharge rate of 0.64 ± 0.56 (mean ± SD) spikes/sec and an excitatory response averaging 12.1 ± 8.2 spikes/sec. The majority (50% of the population) of neurons showed a moderate rate (2–5 spikes/sec) of spontaneous activity. This group, with a mean rate of 2.72 ± 1.45 spikes/sec, showed a response, either excitatory or inhibitory, to chemical and/or tactile stimulation. Finally, several neurons (18% of the population) showed high rates of spontaneous activity and responded to taste and tactile stimulation either with excitation or inhibition. One such unit that was excited by tactile stimulation and inhibited by chemical stimulation is shown in Figure 7B. Several of these neurons fired rhythmically in synchrony with respiratory movements of the operculum. These neurons in the dorsomedial RF may belong to the “respiratory center” in the medulla. As shown for unit 3 in Figure 7A, some of the rhythmically bursting neurons respond with an increase in the number of spikes per burst, whereas for other units, chemical stimulation disrupted the bursting pattern of activity (Fig.7A, unit 4). The other two types of units, those with slow to medium rates of spontaneous activity, were intermingled within the ventromedial region of the RF. Neurons responsive to tactile stimulation of the extraoral surface generally showed little or no spontaneous activity. The spontaneous patterns of activity and responses to taste and tactile stimuli of a few neurons are shown in Figure 7.
Fig. 7.

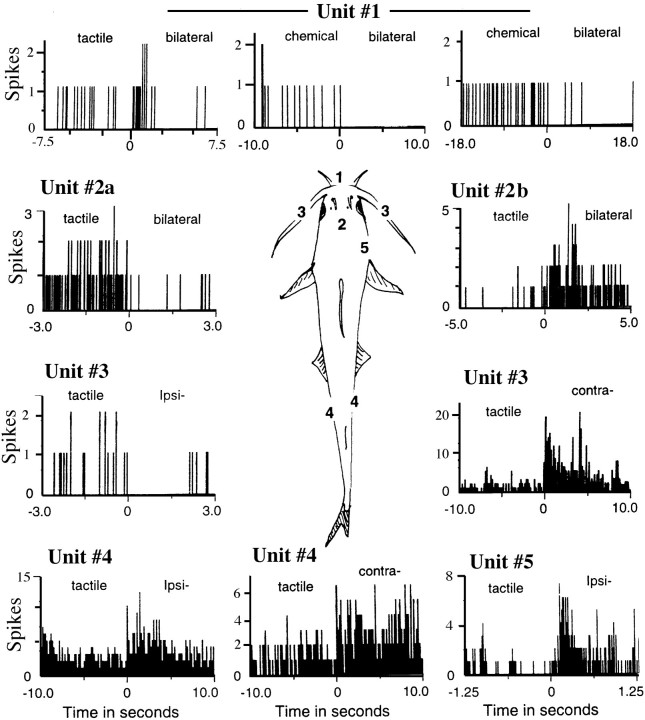
A, Multiunit, and B, single unit recordings to show the different patterns of spontaneous activity and responses of taste and touch sensitive reticular neurons at five different recording sites from a single animal. Thenumerical labels indicate recording site and thelower case alphabetic labels indicate responses to stimulus applications at different parts of the receptive field and/or to different stimuli. A1 shows a strong phasic response to tactile stimulation of the proximal portion of the maxillary barbel ipsilaterally and to the flank bilaterally and a weak response to stimulation of the head region on either side. Tactile stimulation of the contralateral maxillary barbel did not produce an obvious response. Cells at 2 show little spontaneous activity but responded vigorously to tactile stimulation of the head region. Cells at 3 and 4 exhibit a rhythmic bursting pattern (∼1 spike/sec) of spontaneous activity and respond to chemical stimulation with either a higher firing rate within each burst (3) or with a tonic discharge and transient disruption of the rhythmic pattern (4). The single unit shown in 5 is bimodal and responds with excitation and inhibition to tactile and taste stimuli, respectively. The receptive field of this unit is shown as stippled areas mapped on a dorsal view of the catfish on the left.Arrows indicate stimulus onset. All recordings, except4, have a common time scale.
The majority of neurons responded to punctate or tactile stimulation consisting of gliding movements of a soft brush. Both adaptive and nonadaptive units were present and stimuli as short as 200 msec in duration were sufficient to produce a response. As in the facial and vagal gustatory lobes, it was relatively difficult to elicit and quantify a response to a chemical stimulus, perhaps because of complexities of generating natural stimulus profiles. A mixture of amino acids (l-isomers of alanine, arginine, and proline at 10−3m concentration) and the liver extract were sometimes effective in producing a response.
With regard to the spatial organization of receptive fields, bilateral receptive fields extending over the whole flank region were commonly observed (Fig. 8, unit 4). Units with receptive fields in the head region tended to have smaller receptive fields, typically restricted only to the proximal or distal portion of the maxillary barbels (Fig. 8, units 1, 2, and 3). These receptive fields are much larger than the receptive fields of FL neurons (Hayama and Caprio, 1989).
Fig. 8.
PST histograms to show the variety of response properties exhibited by six different reticular units. The receptive fields of reticular neurons ranged from those restricted to the snout region to those covering most of the extraoral surface and/or oral cavity (see text). Stimuli were applied at time 0. Stimulus type is indicated at the top of each PST along with the location of the stimulus application as shown on a dorsal view of the catfish. Neuron labeled as 1 is bimodal and exhibits a biphasic excitatory/inhibitory response to tactile stimulation and tonic inhibition to taste stimuli. Activity can be suppressed for ≤18 sec after application of millimolar concentrations of amino acids and a commercially obtained bait mixture. 2a and2b show an excitatory versus inhibitory response of two neurons at the same recording site. The cell shown in 3responds with excitation and inhibition to ipsi- and contralateral receptive fields, whereas the cell shown in 4 responds similarly to ipsi- and contralateral mechanical stimulation. These data were obtained with computer-controlled application of mechanical and chemical stimuli.
In a few cases, iontophoretic injections of HRP through the recording electrode confirmed that these recordings were obtained from the vicinity of dendrites or cell bodies of neurons in the RS5 segment of the RF. As shown in Figure 9, we were successful in labeling a few or single cells at the recording site using this technique. This type of labeling from extracellular recordings was possible because of the typically large size of reticular neurons.
Fig. 9.
A, PST to show the response of a reticular neuron to tactile stimulation of the mandibular barbels. Bin width is 150 msec and the total time is 13 sec. B, Schematic cross-section through the level of the FL (approximate level of Fig. 1J) to show the location of recording site at a depth of 2.88 mm within the medulla.C, HRP-filled neuron after iontophoretic injection of a 10% solution of HRP at one of its dendrites from location of recordings shown in A.
DISCUSSION
Medullary gustatospinal projections
Our results show that FL neurons have both direct and indirect connections with the Sp. The direct connections and pathways constitute a monosynaptic route from the FL to the ipsilateral dorsal horn (Finger, 1978), whereas the indirect facioreticulospinal system seems to involve premotor pathways. The facioreticular projection is quite circumscribed, involving only RF neurons of segment RS5. Recordings from neurons in this area show that these cells respond to chemical and tactile stimuli applied to large areas of the body or gills. Thus, chemosensory information necessary for directed food search has access to these prespinal neurons. Further, the reticular neurons exhibit relatively large receptive fields more similar to those of the niF or secondary gustatory nucleus (Hayama and Caprio, 1989; Lamb and Caprio, 1993) than of the main portion of the FL. Thus, how the information encoded in the fine-grain somatotopic map in the FL is used by other areas of the brain remains enigmatic because none of the higher-order projection systems seems to retain this information.
This situation may be analogous to the remapping of visual information between the TeO and the midbrain premotor nuclei (Masino, 1992). In that system, the retinotopically organized representation of visual space in the tectum is transformed to orthogonal movement coordinates in the mesencephalic tegmentum. In this tectotegmental system, as in the facioreticular system examined in this work, no simple anatomical remapping occurs between the primary sensory map and the premotor control centers. That is, a small tracer injection in the primary sensory area does not yield a discrete, limited projection to a portion of the premotor center. Rigorous analysis of the midbrain premotor area has shown that it is organized in a movement coordinate system. Similarly, more rigorous study of the RS5 complex may reveal a map of orienting movements.
Whereas the facioreticular projection system affects premotor neurons of the reticular formation, the direct faciospinal system does not. This direct system extends from the ll to terminate in the spinal dorsal horn (Finger, 1978; this study). That only the lateral lobule gives rise to this pathway is noteworthy in that this lobule contains the representation of the territory innervated by the recurrent facial nerve, i.e., the same skin areas innervated by the segmental spinal nerves. Thus each dermatome receives sensory innervation from two sources: the segmental spinal nerve and from segmental branches of the recurrent facial nerve (Herrick, 1901). Taste buds within a dermatome are innervated by the recurrent facial fibers, whereas general somatosensory receptors in the same dermatome presumably are innervated by spinal nerves. Despite this seeming separation of modalities by nerve, the recurrent facial nerve exhibits both tactile and chemosensory responses (Davenport and Caprio, 1982). Thus, the specific contribution of the facial and spinal nerves to overall detection of cutaneous stimuli remains unclear. The direct projection from the ll to the spinal dorsal horn is organized somatotopically, i.e., neurons in the tail representation of the FL project to the caudalmost levels of the Sp. Accordingly, the direct facioreticular system may be more involved in correlation of the facial and spinal nerve sensory inputs from a single region of skin rather than in spinal motor control.
The major route by which gustatory input is likely to influence spinal motor activity seems to be via the Rsps residing in segment RS5 (Lee et al., 1993) of the RF of the brainstem. One important finding of this study is that a localized subset of reticulospinal cells, those of RS5, receive functional input from a gustatory lobe. The gustato–reticulospinal projection therefore represents a small part of the extensive reticulospinal system, which has been studied in aquatic species such as the lamprey (Rovainen, 1967, 1974), zebrafish (Kimmel et al., 1982; Mendelson, 1986; Mendelson and Kimmel, 1986; Metcalfe et al., 1986), and more recently goldfish (Prasada Rao et al., 1987; Lee and Eaton, 1991; Lee et al., 1993) and dogfish (Timerick et al., 1992). The current study allowed us to define the origin and level of the gustatory projections in relation to the entire reticulospinal system in a catfish.
Only one reticulospinal cluster receives input from the FL. Possibly, different clusters in the reticulospinal system receive major inputs from different sensory modalities. Further, within a cluster, each Rsp may respond preferentially to stimuli presented at one locus in space (Bosch and Paul, 1993).
Gustatory convergence and modulation of spinal motoneurons
We observed large tactile and chemosensory receptive fields of reticular neurons in the catfish. These receptive fields are quite different from the discrete, small receptive fields in the FL (Marui and Caprio, 1982). The receptive fields for reticular formation neurons extend typically either bilaterally over the anterior or posterior half of the body surface or unilaterally over the entire flank region, and may involve both excitatory and inhibitory effects. A few fibers that decussate transversely across bundles of the reticulospinal tract and terminate in the vicinity of ventromedial reticular neurons of the opposite side may account for the bilateral receptive fields of some reticular neurons. In addition, RF interneurons may relay information to the contralateral side.
Our neuroanatomical results and the neurophysiological evidence for functional synaptic efficacy between facial inputs to reticular neurons suggest that facial gustatory information may have an important influence on the premotor (reticulospinal) centers that control swimming. Our results outline an important neural pathway mediating food search behavior, which is under the direct influence of the highly sensitive extraoral taste system of the channel catfish. Behavioral experiments have already shown that denervation of taste buds from one side of the flank results in circling toward the side of greater sensory input and locating the release point of a chemical stimulus such as dilute liver extract (Bardach et al., 1967).
The role of Rsps in vertebrate locomotion is well established (Grillner et al., 1987, 1995). Experiments involving electrical stimulation of Rsps also support a role of Rsps in the regulation of the pattern of locomotion and swimming movements. For example, in cats, electrical stimulation of the Rsps increased the amplitude, but not the stepping rate (Orlovsky, 1970). In goldfish, the circuitry for controlling escape trajectories, which can be considered a special case of swimming, has been analyzed carefully using intracellular and optical recording techniques (Fetcho and Faber 1988; Fetcho and Svoboda, 1993;Fetcho and O’Malley 1995). In catfish, knowledge of the neural mechanisms that mediate sensorimotor coordination of swimming is lacking. A subset of gustatoreticular neurons, however, did show a monosynaptic projection to the Sp as evidenced by a short latency (<1 msec) response and lack of habituation to repetitive antidromic stimulation by a pair of electrodes inserted in the ventrolateral portion of the Sp (Kanwal, unpublished observation).
Gustatoreticular systems
Connections from a primary gustatory nucleus to the premotor reticular formation have been described also in rodents. The rostral, gustatory portion of the nucleus of the solitary tract maintains direct and indirect projections to the parvocellular lateral reticular formation of the medulla (Beckman and Whitehead, 1991). The portion of the reticular formation receiving the gustatory inputs has connections to cranial motor nuclei implicated in mouth and tongue movements (Beckman and Whitehead, 1991; Ter Horst et al., 1991). Thus in mammals, too, a gustatoreticular system seems involved in gustatory modulation of motor activity. In the case of rodents, food acquisition entails tongue and mouth movements; the reticulomotor connection accordingly involves brainstem motor nuclei. In catfish, food acquisition entails modulation of body position and swimming orientation; thus, the reticulomotor connection involves spinal motor networks. Whether any gustatory information reaches prespinal reticular neurons in rodents is, however, unclear. Certainly, the region of the reticular formation receiving input from the nucleus of the solitary tract contains neurons that project to rostral spinal levels (Jones and Yang, 1985). Conceivably, such reticulocervical neurons might be involved in gustatory-evoked reflexive head turning in humans (Steiner, 1979) as well as rodents (Grill and Norgren, 1978).
In summary, two gustato–spinal pathways arise from the FL in the channel catfish. The direct faciospinal pathway may be involved in coordinated processing of taste and somatic sensory inputs from the same site on the body surface. In contrast, the indirect, facioreticulospinal pathway may coordinate navigation toward food, i.e., the pattern of swimming movements, by modulating the activity of the spinal locomotor network.
Footnotes
This work was supported by National Institutes of Health Grant DC00147 (T.E.F.) and National Institutes of Health Training Grant T32 NS 07083. We thank Bärbel Böttger for technical help with many of the neuroanatomical procedures and Steve Singer for assistance in preparation of the electronic images.
Correspondence should be addressed to Thomas E. Finger, Department of Cellular and Structural Biology, University of Colorado School of Medicine, 4200 East Ninth Avenue, Denver, CO 80262.
Jagmeet Kanwal’s present address: Department of Neurology, Georgetown University Medical Center, 3970 Reservoir Road NW, Washington, DC 20007.
REFERENCES
- 1.Atema J. Structures and functions of the sense of taste in the catfish (Ictalurus natalis). Brain Behav Evol. 1971;4:273–294. doi: 10.1159/000125438. [DOI] [PubMed] [Google Scholar]
- 2.Bardach JE, Todd JH, Crickmer R. Orientation by taste in fish of the genus Ictalurus. Science. 1967;155:1276–1278. doi: 10.1126/science.155.3767.1276. [DOI] [PubMed] [Google Scholar]
- 3.Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res. 1991;557:265–279. doi: 10.1016/0006-8993(91)90143-j. [DOI] [PubMed] [Google Scholar]
- 4.Bell CC, Finger TE, Russell C. Central connections of the posterior lateral line lobe in mormyrid fish. Exp Brain Res. 1981;42:9–22. doi: 10.1007/BF00235724. [DOI] [PubMed] [Google Scholar]
- 5.Bosch TJ, Paul DH. Differential responses of single reticulospinal cells to spatially localized stimulation of the optic tectum in a teleost fish, Salmo trutta. Eur J Neurosci. 1993;5:742–750. doi: 10.1111/j.1460-9568.1993.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 6.Bullock TH. Electroreception. Annu Rev Neurosci. 1982;5:121–170. doi: 10.1146/annurev.ne.05.030182.001005. [DOI] [PubMed] [Google Scholar]
- 7.Caprio J. High sensitivity of catfish taste receptors to amino acids. Comp Biochem Physiol. 1975;52A:247–251. doi: 10.1016/s0300-9629(75)80160-5. [DOI] [PubMed] [Google Scholar]
- 8.Caprio J. Olfaction and taste in the channel catfish: an electrophysiological study of the responses to amino acids and derivatives. J Comp Physiol. 1978;123:357–371. [Google Scholar]
- 9.Caprio J, Brand JG, Teeter JH, Valentincic T, Kalinoski DL, Kohbara J, Kumazawa T, Wegert S. The taste system of the channel catfish: from biophysics to behavior. Trends Neurosci. 1993;16:192–197. doi: 10.1016/0166-2236(93)90152-c. [DOI] [PubMed] [Google Scholar]
- 10.Davenport CJ, Caprio J. Taste and tactile recordings from the ramus recurrens facialis innervating flank taste buds in the catfish. J Comp Physiol. 1982;147A:217–229. [Google Scholar]
- 11.Ewert J-P. Neural mechanisms of prey-catching and avoidance behavior in the toad (Bufo bufo L.). Brain Behav Evol. 1970;3:36–56. doi: 10.1159/000125462. [DOI] [PubMed] [Google Scholar]
- 12.Fetcho JR, O’Malley DM. Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophysiol. 1995;73:399–406. doi: 10.1152/jn.1995.73.1.399. [DOI] [PubMed] [Google Scholar]
- 13.Fetcho JR, Faber DS. Identification of motoneurons and interneurons in the spinal network for escapes initiated by the Mauthner cell in goldfish. J Neurosci. 1988;8:4192–4213. doi: 10.1523/JNEUROSCI.08-11-04192.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fetcho JR, Svoboda KR. Fictive swimming elicited by electrical stimulation of the midbrain in goldfish. J Neurophysiol. 1993;70:765–780. doi: 10.1152/jn.1993.70.2.765. [DOI] [PubMed] [Google Scholar]
- 15.Finger TE. Gustatory pathways in the bullhead catfish. I. Connections of the anterior ganglion. J Comp Neurol. 1976;165:513–526. doi: 10.1002/cne.901650407. [DOI] [PubMed] [Google Scholar]
- 16.Finger TE. Gustatory pathways in the bullhead catfish. II. Facial lobe connections. J Comp Neurol. 1978;180:691–706. doi: 10.1002/cne.901800404. [DOI] [PubMed] [Google Scholar]
- 17.Galambos R. Cochlear potentials elicited from bats by supersonic sounds. J Acoust Soc Am. 1942;14:41–49. [Google Scholar]
- 18.Griffin D. Listening in the dark. Yale UP; New Haven, CT: 1958. [Google Scholar]
- 19.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 20.Grillner S, Wallén P, Dale N, Brodin L, Buchanan J, Hill RH. Transmitters, membrane properties and network circuitry in the control of locomotion in lamprey. Trends Neurosci. 1987;10:34–41. [Google Scholar]
- 21.Grillner S, Deliagina T, Ekeberg O, El Manira A, Hill RH, Lansner A, Orlovski GN, Wallén P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 22.Gruberg ER, Kicliter E, Newman EA, Kass L, Hartline PH. Connections of the tectum of the rattlesnake Crotalus viridus: an HRP study. J Comp Neurol. 1979;188:31–42. doi: 10.1002/cne.901880104. [DOI] [PubMed] [Google Scholar]
- 23.Hartline PH, Kass L, Loop MS. Merging modalities in the optic tectum: infrared and visual interaction in rattlesnakes. Science. 1978;199:1225–1229. doi: 10.1126/science.628839. [DOI] [PubMed] [Google Scholar]
- 24.Hayama T, Caprio J. Lobule structure and somatotopic organization of the medullary facial lobe in the channel catfish, Ictalurus punctatus. J Comp Neurol. 1989;285:9–17. doi: 10.1002/cne.902850103. [DOI] [PubMed] [Google Scholar]
- 25.Hayle TH. A comparative study of spinal projections to the brain (except cerebellum) in three classes of poikilothermic vertebrates. J Comp Neurol. 1973;149:477–496. doi: 10.1002/cne.901490405. [DOI] [PubMed] [Google Scholar]
- 26.Herrick CJ. The cranial nerves and cutaneous sense organs of the North American siluroid fishes. J Comp Neurol. 1901;11:177–249. [Google Scholar]
- 27.Herrick CJ. The organ and sense of taste in fishes. Bull US Fish Comm. 1904;22:237–272. [Google Scholar]
- 28.Herrick CJ. The central gustatory paths in the brains of bony fishes. J Comp Neurol. 1905;15:375–456. [Google Scholar]
- 29.Johnsen PB, Teeter JH. Spatial gradient detection of chemical cues by catfish. J Comp Physiol. 1980;140:95–99. [Google Scholar]
- 30.Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studies by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- 31.Kanwal JS, Caprio J. Central projections of the glossopharyngeal and vagal nerves in the channel catfish, Ictalurus punctatus: clues to differential processing of visceral inputs. J Comp Neurol. 1987;264:216–230. doi: 10.1002/cne.902640207. [DOI] [PubMed] [Google Scholar]
- 32.Kanwal JS, Finger TE, Caprio J. Forebrain connections of the gustatory system in Ictalurid catfishes. J Comp Neurol. 1988;278:353–376. doi: 10.1002/cne.902780306. [DOI] [PubMed] [Google Scholar]
- 33.Kimmel CB, Powell SL, Metcalfe WK. Brain neurons which project to the spinal cord in young larvae of the zebrafish. J Comp Neurol. 1982;205:112–127. doi: 10.1002/cne.902050203. [DOI] [PubMed] [Google Scholar]
- 34.Knudsen EI. Auditory and visual maps of space in the optic tectum of the owl. J Neurosci. 1982;2:1177–1194. doi: 10.1523/JNEUROSCI.02-09-01177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamb CF, Caprio J. Convergence of oral and extraoral information in the superior secondary gustatory nucleus of the channel catfish. Brain Res. 1992;588:201–211. doi: 10.1016/0006-8993(92)91576-z. [DOI] [PubMed] [Google Scholar]
- 36.Lamb CF, Caprio J. Taste and tactile responsiveness of neurons in the posterior diencephalon of the channel catfish. J Comp Neurol. 1993;337:419–430. doi: 10.1002/cne.903370306. [DOI] [PubMed] [Google Scholar]
- 37.Lamb CF, Finger TE. Axonal projection patterns of neurons in the secondary gustatory nucleus of channel catfish. J Comp Neurol. 1996;365:585–593. doi: 10.1002/(SICI)1096-9861(19960219)365:4<585::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee RK, Eaton RC. Identifiable reticulospinal neurons of the adult zebrafish, Brachydanio rerio. J Comp Neurol. 1991;304:34–52. doi: 10.1002/cne.903040104. [DOI] [PubMed] [Google Scholar]
- 39.Lee RKK, Eaton RC, Zottoli SJ. Segmental arrangement of reticulospinal neurons in the goldfish hindbrain. J Comp Neurol. 1993;329:539–556. doi: 10.1002/cne.903290409. [DOI] [PubMed] [Google Scholar]
- 40.Marui T, Caprio J. Electrophysiological evidence for the topographical arrangement of taste and tactile neurons in the facial lobe of the channel catfish. Brain Res. 1982;231:185–190. doi: 10.1016/0006-8993(82)90017-8. [DOI] [PubMed] [Google Scholar]
- 41.Masino T. Brainstem control of orienting movements: intrinsic coordinate systems and underlying circuitry. Brain Behav Evol. 1992;40:98–111. doi: 10.1159/000113906. [DOI] [PubMed] [Google Scholar]
- 42.Mendelson B. Development of reticulospinal neurons of the zebrafish. II. Early axonal outgrowth and cell body position. J Comp Neurol. 1986;251:172–184. doi: 10.1002/cne.902510204. [DOI] [PubMed] [Google Scholar]
- 43.Mendelson B, Kimmel CB. Identified vertebrate neurons that differ in axonal projection develop together. Dev Biol. 1986;118:309–313. doi: 10.1016/0012-1606(86)90098-9. [DOI] [PubMed] [Google Scholar]
- 44.Mesulam M-M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing general afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- 45.Metcalfe WK, Mendelson B, Kimmel CB. Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larva. J Comp Neurol. 1986;251:147–159. doi: 10.1002/cne.902510202. [DOI] [PubMed] [Google Scholar]
- 46.Molenaar GJ. An additional trigeminal system in certain snakes possessing infrared receptors. Brain Res. 1974;78:340–344. doi: 10.1016/0006-8993(74)90560-5. [DOI] [PubMed] [Google Scholar]
- 47.Morita Y, Finger TE. Reflex connections of the facial and vagal gustatory systems in the brainstem of the bullhead catfish, Ictalurus nebulosus. J Comp Neurol. 1985;231:547–558. doi: 10.1002/cne.902310411. [DOI] [PubMed] [Google Scholar]
- 48. Orlovsky GN. Activity of reticulospinal neurons during locomotion. Biofzika 15 1970. 278 764 (English translation, 761–771). [Google Scholar]
- 49.Prasada Rao PD, Jadhao AG, Sharma SC. Descending projection neurons to the spinal cord of the goldfish, Carassius auratus. J Comp Neurol. 1987;265:96–108. doi: 10.1002/cne.902650107. [DOI] [PubMed] [Google Scholar]
- 50.Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Müller and Mauthner cells. J Neurophysiol. 1967;30:1000–1023. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- 51.Rovainen CM. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974;154:207–224. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- 52.Steiner J. Human facial expression in response to taste and smell stimulation. Adv Child Dev. 1979;13:257–295. doi: 10.1016/s0065-2407(08)60349-3. [DOI] [PubMed] [Google Scholar]
- 53.Ter Horst GJ, Copray JCVM, Liem RSB, VanWilligen JD. Projections from the rostral parvocellular reticular formation to pontine and medullary nuclei in the rat: involvement in autonomic regulation and orofacial motor control. Neuroscience. 1991;40:735–758. doi: 10.1016/0306-4522(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 54.Timerick S, Roberts BL, Paul DH. Brainstem neurons projecting to different levels of the spinal cord of the dogfish, Scyliorhinus canicula. Brain Behav Evol. 1992;39:93–100. doi: 10.1159/000114107. [DOI] [PubMed] [Google Scholar]
- 55.Valentincic T, Caprio J. In: Chemical signals in vertebrates VI (Doty RL, ed), pp 365–369. Plenum; New York: 1993. [Google Scholar]