Abstract
The assembly of multimeric protein complexes that include vesicle-associated membrane protein 2 (VAMP-2) and the plasma membrane proteins syntaxin 1A and synaptosome-associated protein of 25 kDa (SNAP-25) are thought to reflect the biochemical correlates of synaptic vesicle targeting, priming, or fusion. Using a variety of protein–protein interaction assays and a series of deletion and point mutations, we have investigated the domains of VAMP-2 required for the formation of binary complexes with either syntaxin 1A or SNAP-25 and ternary complexes with both syntaxin 1A and SNAP-25. Deletions within the central conserved domain of VAMP-2 eliminated binding to either syntaxin 1A or both syntaxin 1A and SNAP-25. Although all of the deletion mutants were able to form ternary complexes, only some of these complexes were resistant to denaturation in sodium dodecyl sulfate. These results demonstrate that cooperative interactions result in the formation of at least two biochemically distinct classes of ternary complex. Two point mutations previously shown to have effects on the intracellular trafficking of VAMP-2 (M46A, reduced endocytosis and sorting to synaptic vesicles; N49A, enhanced sorting to synaptic vesicles) lie within a domain required for both syntaxin 1A and SNAP-25 binding. Syntaxin 1A and SNAP-25 binding was reduced by the M46A mutation and enhanced by the N49A mutation, suggesting that a correlation exists between the membrane-trafficking phenotype of the two VAMP-2 point mutants and their competence to form complexes with either syntaxin 1A or SNAP-25.
Keywords: VAMP, syntaxin, SNAP-25, SNARE complex, synaptic vesicle, exocytosis
Synaptic transmission, the primary means by which neurons communicate with their target cells, is initiated by the regulated secretion of neurotransmitter from the presynaptic nerve terminal. The precise interaction of neurotransmitter-filled synaptic vesicles with the presynaptic plasma membrane and the rapid fusion of these membranes after an action potential-induced influx of calcium constitute the primary steps in neurotransmitter secretion. Recent biochemical and genetic studies have begun to elucidate the molecular mechanisms responsible for neurotransmitter secretion and reveal the fundamental similarity of these mechanisms to those underlying the targeting and fusion of other intracellular transport vesicle intermediates (Bennett and Scheller, 1993, 1994; Ferro-Novick and Jahn, 1994; Schwarz, 1994; Südhof, 1995).
Vesicle-associated membrane protein 2 (VAMP-2, also known as synaptobrevin), together with the presynaptic plasma membrane proteins syntaxin 1 and synaptosome-associated protein of 25 kDa (SNAP-25), has been proposed to participate in synaptic vesicle targeting or fusion. Several lines of evidence support such a role, including the observation that a class of potent presynaptic neurotoxins (including tetanus toxin and six types of botulinum toxin) selectively target syntaxin 1, SNAP-25, or VAMP-2 for proteolysis (Niemann et al., 1994;Schiavo et al., 1994). In addition, Drosophila mutants defective in either syntaxin 1 or VAMP exhibit profound deficits in synaptic transmission (Broadie et al., 1995), whereas soluble fragments of syntaxin 1A and VAMP can perturb neurotransmitter release from PC12 cells (Bennett et al., 1993) and squid nerve terminal (Hunt et al., 1994), respectively. Furthermore, a complex composed of syntaxin 1, SNAP-25, and VAMP-2 serves as a receptor for a family of general membrane trafficking factors known as solubleN-ethylmaleimide sensitive factor (NSF) attachment proteins (SNAPs) (Söllner et al., 1993). Because of this, the complex of syntaxin 1, VAMP-2, and SNAP-25 is commonly referred to as the synaptic SNAP receptor (SNARE) complex. A general role in membrane trafficking for proteins related to syntaxin 1A, SNAP-25, and VAMP-2 is supported further by the observation that each is related to gene products required for the proper functioning of the yeast secretory pathway (Bennett and Scheller, 1993; Ferro-Novick and Jahn, 1994). Together, these observations highlight the central role of syntaxin 1, SNAP-25, and VAMP-2 in the membrane trafficking events that underlie neurotransmitter secretion.
Recent biochemical studies have demonstrated that direct interactions among VAMP-2, syntaxin 1A, and SNAP-25 contribute to the formation of the synaptic SNARE complex (Calakos et al., 1994; Chapman et al., 1994;Hayashi et al., 1994; Pevsner et al., 1994; Kee et al., 1995). Each of the components of the synaptic SNARE complex contains heptad repeat domains (Lupas et al., 1991), suggesting that coiled-coil structures may contribute to complex assembly. The central conserved domain of VAMP-2, which is important for both syntaxin 1 and SNAP-25 binding (Calakos et al., 1994; Hayashi et al., 1994), consists of two heptad repeats. Interestingly, recent studies have demonstrated that mutations within this region of VAMP-2 influence both its sorting to synaptic vesicles (Grote et al., 1995) and its endocytosis (Grote and Kelly, 1996). These results suggest that the same domain of VAMP-2 required for SNARE complex formation also may contribute to its endocytosis and targeting to synaptic vesicles. To characterize the structure and function of VAMP-2 further and to define the overlap in domain usage better, we have investigated the effects that systematic deletion and point mutations in the cytoplasmic domain of VAMP-2 have on the assembly of synaptic SNARE complexes.
MATERIALS AND METHODS
Materials. Rabbit polyclonal antibodies against rat VAMP-2 either were provided by William Trimble [University of Toronto (Gaisano et al., 1994)] or prepared by Berkeley Antibody (Richmond, CA). A rabbit polyclonal antiserum against mouse SNAP-25 was generated by Berkeley Antibody. Molecular biology reagents were obtained from New England Biolabs (Beverly, MA), and all analytical grade chemicals were from Sigma (St. Louis, MO).
Preparation of fusion proteins. Constructs for the bacterial expression of glutathione S-transferase (GST) fusion proteins incorporating the full cytoplasmic domain of rat syntaxin 1A (amino acids 4–267) and mouse SNAP-25 (amino acids 1–206) were prepared in the pGEX-KG vector (Guan and Dixon, 1991), as previously described (Calakos et al., 1994; Pevsner et al., 1994). A construct encoding a GST fusion protein containing amino acids 1–164 of SNAP-25 [SNAP-25 (ΔC)] was prepared by digesting a vector consisting of full-length SNAP-25 in pGEX-KG with XbaI (which cuts both in the SNAP-25 insert and at the 3′ end of the pGEX-KG polylinker), followed by religation. Constructs for the expression GST–VAMP-2 fusion proteins were prepared by PCR amplification of the DNA fragment encoding the cytoplasmic domain of rat VAMP-2 (amino acids 1–94) and various deletion and point mutant forms of VAMP-2 (Grote et al., 1995). Previously described mammalian expression vector constructs were used as templates, and primers were designed to introduce EcoRI (5′) and SacI (3′) restriction sites at the ends of the amplified fragments. The amplified fragments were directionally cloned into EcoRI- and SacI-digested pGEX-KG, and their orientation and structure were confirmed by DNA sequencing.
Immobilized GST fusion proteins (for use in affinity chromatography experiments) were prepared from bacterial lysates, as previously described (Pevsner et al., 1994). Soluble recombinant proteins were purified from the GST fusion protein by cleavage with thrombin in 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2.5 mm CaCl2, and 0.1% β-mercaptoethanol as described (Calakos et al., 1994; Pevsner et al., 1994). Protein concentrations were estimated by Coomassie blue staining of protein bands after SDS-PAGE with bovine serum albumin as a standard.
Affinity chromatography assay. For affinity chromatography binding studies, GST fusion proteins (either syntaxin 1A or SNAP-25) bound to glutathione-agarose beads were incubated with wild-type or mutant VAMP-2 either in the absence or presence of SNAP-25 (as indicated in the figure legends) in 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2.5 mm CaCl2, 0.1% Triton X-100, 0.1% gelatin, and 0.1% bovine serum albumin. After a 2 hr incubation at 4°C, the beads were washed three times with 500 μl of 10 mm HEPES-KOH, pH 7.5, 140 mm potassium acetate, 1 mm MgCl2, 0.1 mm EGTA, and 0.1% Triton X-100 (buffer A) containing 0.1% gelatin and once with buffer A without gelatin. Proteins on the beads were recovered by boiling in SDS-PAGE sample buffer, resolved by SDS-PAGE, and analyzed by Western blotting. The blots were probed with affinity-purified rabbit anti-VAMP-2 antibodies. After incubation with125I-labeled goat anti-rabbit secondary antibody (ICN Biochemicals, Costa Mesa, CA), VAMP-2 immunoreactivity was visualized by autoradiography and quantitated by phosphorimaging (Molecular Dynamics, Sunnyvale, CA). Known amounts of bacterially expressed VAMP-2 proteins were analyzed simultaneously to normalize for potential differences in immunoreactivity. For titration experiments (see Figs.2, 4), the 50% effective concentration (EC50), defined as the concentration of VAMP-2 at which half-maximal binding occurs, was estimated from plots of phosphorimaging pixel intensity versus input VAMP-2 protein concentration.
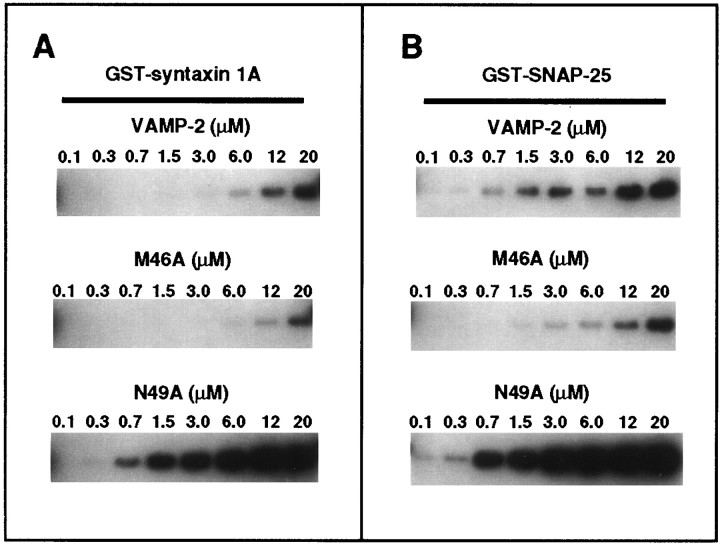
Fig. 2.
Titration of the in vitro binding of wild-type, M46A, and N49A mutant VAMP-2 to GST–syntaxin 1A and GST–SNAP-25. Shown are in vitro binding of wild-type and mutant VAMP-2 at the indicated concentrations to (A) immobilized GST–syntaxin 1A (0.3 μm) and (B) immobilized GST–SNAP-25 (0.3 μm). Bound VAMP-2 was recovered and visualized by Western blotting, as described in Materials and Methods.
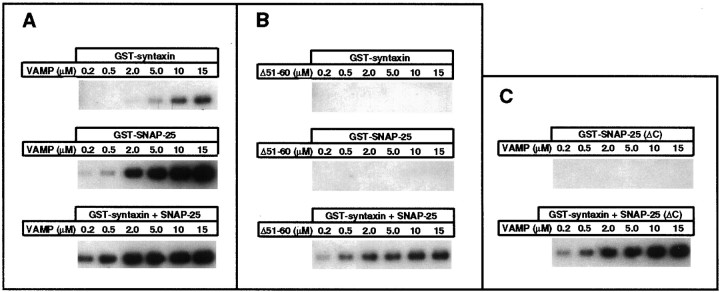
Fig. 4.
Potentiation of wild-type and Δ51–60 mutant VAMP-2 binding to GST–syntaxin 1A in the presence of soluble SNAP-25.In vitro binding of wild-type (A) and Δ51–60 mutant VAMP-2 (B) at the indicated concentrations to immobilized GST–syntaxin 1A (0.3 μm), immobilized GST–SNAP-25 (0.3 μm), and immobilized GST–syntaxin 1A in the presence of soluble SNAP-25 (1 μm). C, In vitro binding of wild-type VAMP-2 at the indicated concentrations to immobilized GST–SNAP-25 (ΔC) (0.3 μm) and immobilized GST–syntaxin 1A (0.3 μm) in the presence of soluble SNAP-25 (ΔC) (1 μm). Bound VAMP-2 was recovered and visualized by Western blotting, as described in Materials and Methods.
Yeast two-hybrid analysis. To allow in-frame insertion into the two-hybrid expression plasmids, we introduced convenient restriction endonuclease sites at each end of the desired cDNA fragment. The coding sequence of the cytoplasmic domain of rat VAMP-2 (amino acids 1–94) and a series of VAMP-2 deletion and point mutants (Grote et al., 1995) were amplified by PCR and inserted viaEcoRI and SalI restriction sites into pGBT9 in-frame with the DNA binding domain of GAL4. The coding sequence of the cytoplasmic domain of rat syntaxin 1A (amino acids 1–266) and mouse SNAP-25 (amino acids 1–206) were amplified by PCR and inserted via EcoRI and BglII sites into pGAD424 in-frame with the activation domain of GAL4. The yeast expression vectors were obtained from Clontech (Palo Alto, CA).
Yeast strain SFY526 (MATa, ura3-52, his 3-200, ade 2-101, lys 2-801, trp 1-901, leu 2-3, 112, canr, gal4-542, gal80-538, URA::GAL1-lacZ) (Clontech) was grown in YPD medium (yeast extract, peptone, and dextrose) or on synthetic defined dropout yeast medium lacking the appropriate amino acids (BIO 101, La Jolla, CA). SFY526 was transformed simultaneously with two of the hybrid plasmids by the improved lithium acetate method of Gietz et al. (1992). Cotransformants were plated on medium lacking tryptophan and leucine to select for the pGBT9 and pGAD424 derivatives, respectively. Typically, after 3 d at 30°C, the Trp+, Leu+transformants were tested for β-galactosidase activity by a color filter assay with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) as the substrate. For quantitative studies, β-galactosidase activity was assayed in 100 μl of cell lysate witho-nitrophenyl-β-d-galactopyranoside as the substrate.
Ligand overlay blotting. Wild-type and mutant VAMPs (100 ng each) were separated by SDS-PAGE and transferred to nitrocellulose. Four identical nitrocellulose blots containing the immobilized VAMP-2 proteins were blocked first for 30 min at room temperature in 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.05% Tween-20, and 5% nonfat dry milk (blocking buffer). Then the blots were incubated in blocking buffer containing 2 μg/ml of either syntaxin 1A or SNAP-25 alone or a mixture of both proteins. After a 1 hr incubation, the blots were washed in blocking buffer for 5 min and then probed with a monoclonal antibody against syntaxin 1A (HPC-1) or an affinity-purified anti-SNAP-25 antibody. After incubation with125I-labeled goat anti-mouse or goat anti-rabbit antibodies, the bound proteins were visualized by autoradiography and quantitated by phosphorimaging.
Analysis of SDS-resistant SNARE complexes. Combinations of soluble wild-type or mutant VAMP-2, syntaxin 1A, and SNAP-25 (2 μm each) were incubated overnight (16 hr) at 4°C in 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 2.5 mm CaCl2. After addition of 6× SDS-PAGE sample buffer, samples were divided into two aliquots and either boiled for 3 min or incubated at 37°C for 3 min. Samples then were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with an affinity-purified monoclonal antibody against syntaxin 1A [HPC-1 (Barnstable et al., 1985)]. After incubation with125I-labeled goat anti-mouse secondary antibody (ICN Biochemicals), immunoreactive species were visualized by autoradiography. For analysis of the heat lability of the SDS-resistant complexes, samples were divided into 14 aliquots and incubated at temperatures between 25–97°C (in 6°C increments) for 3 min using a programmable thermal cycler (MJ Research, Watertown, MA). One aliquot was boiled for 3 min. Samples were resolved by SDS-PAGE (at 4°C), transferred to nitrocellulose, and analyzed by immunoblotting with an affinity-purified antibody against VAMP-2 via a chemiluminescence detection system (DuPont-NEN, Boston, MA).
RESULTS
Deletions within the conserved domain of VAMP-2 eliminate binary interactions with syntaxin 1A and SNAP-25
To identify the domains of VAMP-2 involved in interactions with syntaxin 1A and SNAP-25, we analyzed the binding properties of a series of VAMP-2 mutants (Fig. 1A) bothin vitro with an affinity chromatography assay and in vivo with the yeast two-hybrid system. For the affinity chromatography assay, the interaction of soluble recombinant VAMP-2 (either wild-type or mutant) with immobilized GST–syntaxin 1A or GST–SNAP-25 was examined. As shown in Figure 1B,C, the VAMP-2 deletion mutants fell into three categories: (1) those able to interact with either syntaxin 1A or SNAP-25 (Δ2–31 and Δ71–80), (2) those able to interact with SNAP-25, but not syntaxin 1A (Δ31–38, Δ61–70), and (3) those unable to interact with either syntaxin 1A or SNAP-25 (Δ41–50, Δ51–60). Within each category, the relative amount of VAMP-2 binding was variable.
Fig. 1.
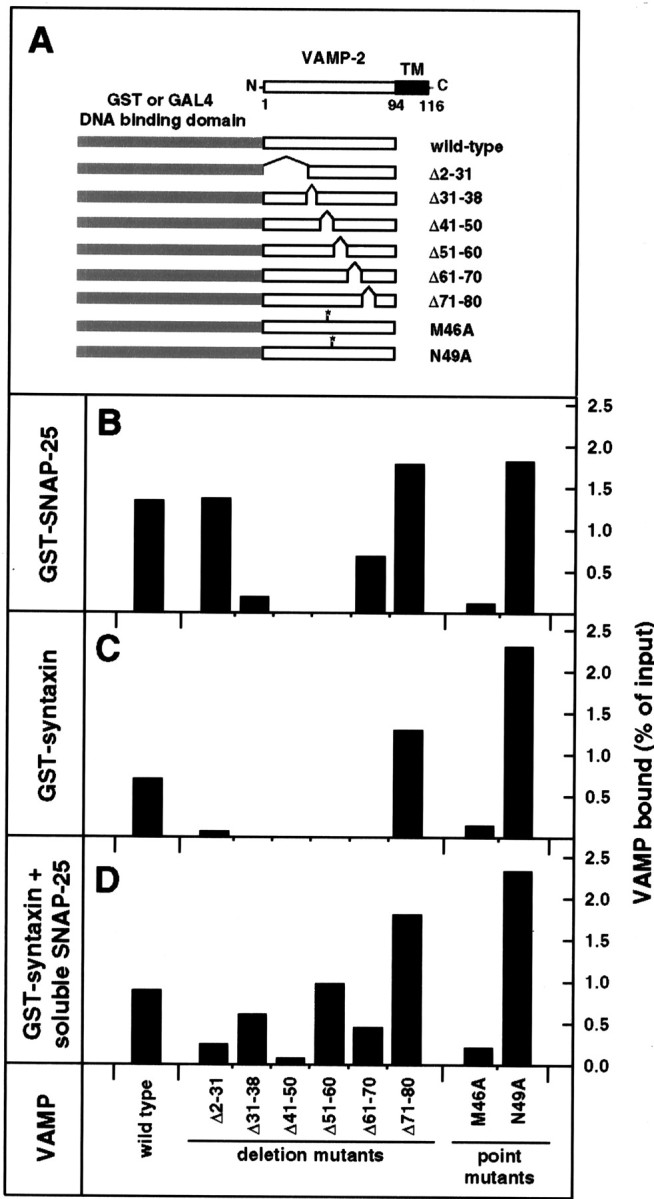
Affinity chromatography survey of binary and ternary interactions among wild-type and mutant VAMP-2, GST–syntaxin 1A, and GST–SNAP-25. A, Schematic diagram of wild-type VAMP-2 and the VAMP-2 mutants used in the present study. The constructs encode the cytoplasmic domain of wild-type or mutant VAMP-2 fused to the C terminus of either GST or the GAL4 DNA binding domain.TM, Transmembrane domain. B–D, In vitro binding of wild-type and mutant VAMP-2 (10 μm) to (B) immobilized GST–SNAP-25 (0.5 μm), (C) immobilized GST–syntaxin 1A (0.5 μm), and (D) immobilized GST–syntaxin 1A (0.5 μm) in the presence of soluble SNAP-25 (1 μm). Bound VAMP-2 was recovered and quantitated by Western blotting, as described in Materials and Methods. The relative strengths of the interactions are expressed as the percentage of VAMP-2 input recovered on the immobilized GST fusion proteins.
To investigate the in vivo interactions between wild-type or deletion mutants of VAMP-2 and either syntaxin or SNAP-25, the yeast two-hybrid system was used (Fields and Song, 1989). The two-hybrid system exploits the fact that the yeast transcription factor GAL4 consists of two separate domains, the DNA binding domain and the transcription activating domain, that must be in close proximity to function (Ma and Ptashne, 1987). For these studies, yeast were cotransformed with a “bait” vector [engineered to express a fusion protein consisting of the cytoplasmic domain of VAMP-2 or VAMP-2 mutants (Fig. 1A) linked to the DNA-binding domain of GAL4] and a “prey” vector (engineered to express a fusion protein consisting of the cytoplasmic domain of syntaxin 1A or full-length SNAP-25 linked to the activation domain of GAL4). After selection of cotransformants, in vivo interactions between VAMP-2 and syntaxin 1A or SNAP-25 were monitored by the activation of β-galactosidase, a GAL4-regulated reporter gene.
Qualitative results from the two-hybrid analysis were obtained by blue/white screening with X-gal indicator. Control experiments in which the yeast were transformed with one or both vectors lacking the insert or with vectors containing a cDNA not related to the SNARE complexes (SV40 large T-antigen) failed to produce detectable β-galactosidase activity (data not shown). In contrast, expression of wild-type VAMP-2 in combination with either syntaxin 1A or SNAP-25 yielded positive (blue) transformants (Table 1). Furthermore, all of the VAMP-2 deletion mutants that failed to interact with either syntaxin 1A or SNAP-25 by affinity chromatography also failed to interact with the yeast two-hybrid approach. However, two of the deletion mutants that were capable of interacting with both syntaxin 1A and SNAP-25 in vitro (Δ2–31 and Δ71–80) failed to interact detectably with either syntaxin 1A or SNAP-25 with the yeast two-hybrid system. This may reflect a greater sensitivity of the affinity chromatography assay (Hata and Südhof, 1995) or an inaccessibility of binding sites or conformational limitations for the VAMP-2 mutants when present in the context of the GAL4 fusion protein.
Table 1.
Analysis of in vivo interactions between wild-type or mutant VAMP-2 and syntaxin 1A or SNAP-25 with the yeast two-hybrid system
| VAMP-2 “bait” | Syntaxin 1A “prey” | SNAP-25 “prey” |
|---|---|---|
| Wild-type | + | ++ |
| Δ2–30 | − | − |
| Δ31–40 | − | + |
| Δ41–50 | − | − |
| Δ51–60 | − | − |
| Δ61–70 | − | + |
| Δ71–80 | − | − |
| M46A | + | + |
| N49A | ++ | +++ |
Two-hybrid constructs were prepared and cotransfected into the reporter strain SFY526, as described in Materials and Methods. β-Galactosidase activity was monitored by using X-gal as a substrate and is expressed as either a + (blue colonies) or − (white colonies) signal. The number of +’s reflects the intensity of the blue color.
Point mutations in VAMP-2 (M46A and N49A) have opposing effects on binary interactions with syntaxin 1A and SNAP-25
The region of VAMP-2 removed by the Δ41–50 deletion mutant, one of the mutants that is defective in binding to both syntaxin 1A and SNAP-25, corresponds to the region of VAMP-2 recently identified as a synaptic vesicle targeting signal (Grote et al., 1995). To further characterize the role of this region in interactions with syntaxin 1A and SNAP-25, we investigated the binding properties of two point mutants, M46A and N49A. These two point mutants were selected because they display very distinct trafficking phenotypes in vivo in PC12 cells: M46A is defective in endocytosis and in sorting to synaptic vesicles, whereas N49A displays enhanced sorting to synaptic vesicles (Grote et al., 1995; Grote and Kelly, 1996). In both the affinity chromatography assay (Fig. 1B,C) and the yeast two-hybrid assay (Table 1), the M46A mutant exhibited reduced binding to both syntaxin 1A and SNAP-25 (relative to wild-type), whereas the N49A mutant displayed enhanced binding to both syntaxin 1A and SNAP-25.
To better characterize the effect of the VAMP-2 point mutations on the in vitro interactions with syntaxin 1A and SNAP-25, we performed affinity chromatography assays with increasing concentrations of soluble VAMP-2. As shown in Figure2A, the N49A mutant bound to GST–syntaxin 1A to a greater extent and with a higher apparent affinity than wild-type VAMP-2. In comparison with the binding of wild-type VAMP-2 (which was not saturable at concentrations up to 20 μm), the binding of the N49A mutant was strongly potentiated and saturable, with an EC50 of ∼4 μm. Similarly, as shown in Figure 2B, the N49A mutant bound to GST–SNAP-25 to a greater extent and with higher apparent affinity (EC50 of 2.5 μm) than wild-type VAMP-2 (EC50 of 6 μm). In contrast to the N49A mutant, the binding of the M46A mutant to both GST–syntaxin 1A (Fig. 2A) and GST–SNAP-25 (Fig.2B) was significantly weaker than wild-type VAMP-2, failing to reach saturation at even the highest concentration tested (20 μm).
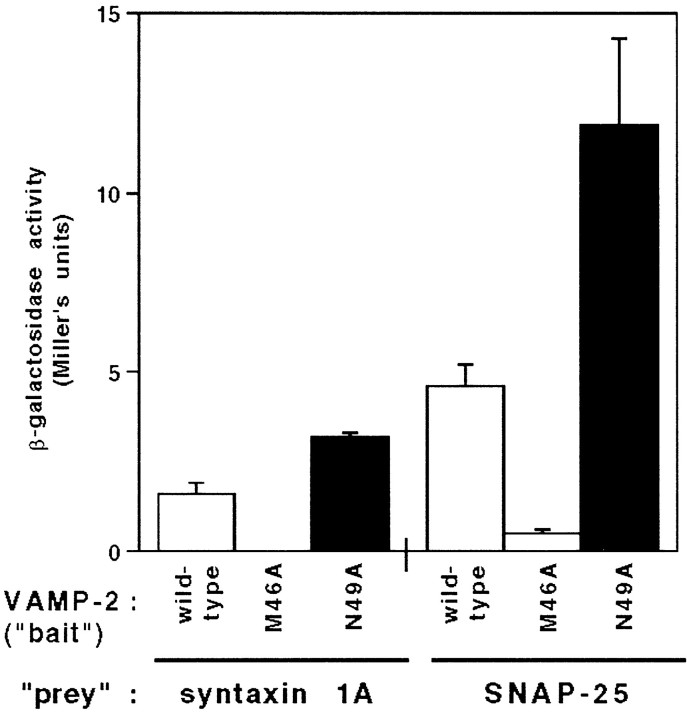
To determine whether the point mutations in VAMP-2 influence the formation of protein complexes in vivo, we used the yeast two-hybrid assay. A quantitative enzyme assay was used to assess the level of β-galactosidase activity associated with each of the transformants and to infer the relative strength of the corresponding protein–protein interactions (Fig. 3). Consistent with the affinity chromatography assay [Figure 2 (Pevsner et al., 1994)], the interaction between VAMP-2 and SNAP-25 was stronger than that between VAMP-2 and syntaxin 1A (threefold higher β-galactosidase activity). Compared with wild-type VAMP-2, the M46A mutant exhibited a reduced interaction with both syntaxin 1A and SNAP-25 (10-fold less β-galactosidase activity), whereas the N49A mutant exhibited an enhanced interaction with both syntaxin 1A and SNAP-25 (2- to 2.5-fold more β-galactosidase activity). Together with the affinity chromatography results, these observations demonstrate that point mutations within the synaptic vesicle targeting domain of VAMP-2 can generate pronounced and opposing effects on VAMP-2 binding properties.
Fig. 3.
Quantitative analysis of the in vivo binding of wild-type, M46A, and N49A mutant VAMP-2 to syntaxin 1A and SNAP-25 with the yeast two-hybrid system. Two-hybrid “bait” (wild-type, M46A, and N49A mutant VAMP-2) and “prey” (syntaxin 1A and SNAP-25) plasmids were prepared as described in Materials and Methods. The reporter strain SFY526 was cotransformed with the indicated plasmid pairs, and the level of β-galactosidase activity in the transformants was determined by usingo-nitrophenyl-β-d-galactopyranoside as the substrate. The activities are expressed in Miller’s units and represent the average (±SE) taken from four independent transformants. β-Galactosidase activity was not detectable after cotransfection of the M46A mutant VAMP-2 with syntaxin 1A.
Mutations within the conserved domain of VAMP-2 do not eliminate the formation of ternary SNARE complexes
Another characteristic of VAMP-2 is its ability to form ternary complexes with the combination of syntaxin 1A and SNAP-25. The formation of the ternary complexes can be monitored by the potentiation of VAMP-2 binding to GST–syntaxin 1A in the presence of soluble SNAP-25 (Pevsner et al., 1994). As illustrated in Figure1D, all of the VAMP-2 deletion mutants, with varying efficiency, were able to form ternary complexes with GST–syntaxin 1A and soluble SNAP-25. Surprisingly, even those mutants that failed to interact with either syntaxin 1A or SNAP-25 in binary reactions (Δ41–50 and Δ51–60) were able to form ternary complexes. The potentiating effect of soluble SNAP-25 was characterized further via titration binding experiments (Fig. 4). Wild-type VAMP-2 binding to GST–syntaxin 1A was strongly enhanced in the presence of soluble SNAP-25. The apparent affinity of VAMP-2 for ternary complex formation (EC50 of 1.5 μm) was greater than that for its interactions with either syntaxin 1A (unsaturated up to 15 μm) or SNAP-25 (EC50 of 6 μm). The effect of soluble SNAP-25 on the binding of the Δ51–60 mutant was even more striking. Although this mutant did not interact with either GST–fusion protein alone (even at the highest concentration tested), the addition of all three proteins resulted in the assembly of a ternary complex with an estimated EC50 for Δ51–60 of 2.5 μm.
Previous studies have demonstrated that the N-terminal 80 amino acids of SNAP-25 are sufficient for syntaxin 1A binding, whereas the C-terminal 25 amino acids are necessary for VAMP-2 binding (Chapman et al., 1994; Hayashi et al., 1994). To better define the requirements for ternary complex formation, we tested the ability of SNAP-25 deletion mutants to potentiate the binding of wild-type VAMP-2 to GST–syntaxin 1A. SNAP-25 with an N-terminal deletion (lacking amino acids 1–124) was unable to bind syntaxin 1A and was also without effect on VAMP-2 binding (data not shown). In contrast, SNAP-25 with a C-terminal deletion [SNAP-25 (ΔC), lacking amino acids 165–206], although unable to interact directly with VAMP-2, was nearly as effective as full-length SNAP-25 at potentiating the interaction of VAMP-2 with GST–syntaxin 1A (Fig. 4C). Taken together, these results demonstrate that stable binary interactions between VAMP-2 and syntaxin 1A or SNAP-25 are not essential for the cooperative assembly of ternary SNARE complexes.
Ternary, but not binary, SNARE complex formation can be detected by ligand overlay blotting
To further characterize the interactions of VAMP-2 with syntaxin 1A and SNAP-25, we performed ligand overlay blotting. The VAMP-2 proteins were immobilized by electrophoretic transfer to nitrocellulose membranes after SDS-PAGE. Then the membranes were incubated with either syntaxin 1A, SNAP-25, or a mixture of syntaxin 1A and SNAP-25. Sites of syntaxin 1A or SNAP-25 binding were visualized (Fig.5A,B) and quantitated (Fig. 5C) by Western blotting with syntaxin 1A and SNAP-25 specific antibodies. Neither syntaxin 1A alone or SNAP-25 alone was capable of interacting with immobilized wild-type or VAMP-2 mutants. However, in the presence of both syntaxin 1A and SNAP-25, ternary complexes were able to form with each of the immobilized VAMP-2 proteins. The level of syntaxin 1A and SNAP-25 binding varied among the different mutants, with the lowest level of binding observed with deletion mutants Δ2–31, Δ51–60, Δ61–70, and Δ41–50 (Fig. 5C). Although the ratio of syntaxin 1A immunoreactivity to SNAP-25 immunoreactivity was constant for all of the VAMP-2 proteins analyzed, the absolute amount of syntaxin 1A recovered in the complexes was always two- to threefold greater than the amount of SNAP-25, as determined by comparison with syntaxin 1A and SNAP-25 standards run on parallel Western blots (data not shown). Whether this reflects an inaccessibility of SNAP-25 epitopes in the ternary complexes, the assembly of nonstoichiometric complexes, or the partial dissociation of SNAP-25 during the assay remains to be determined.
Fig. 5.
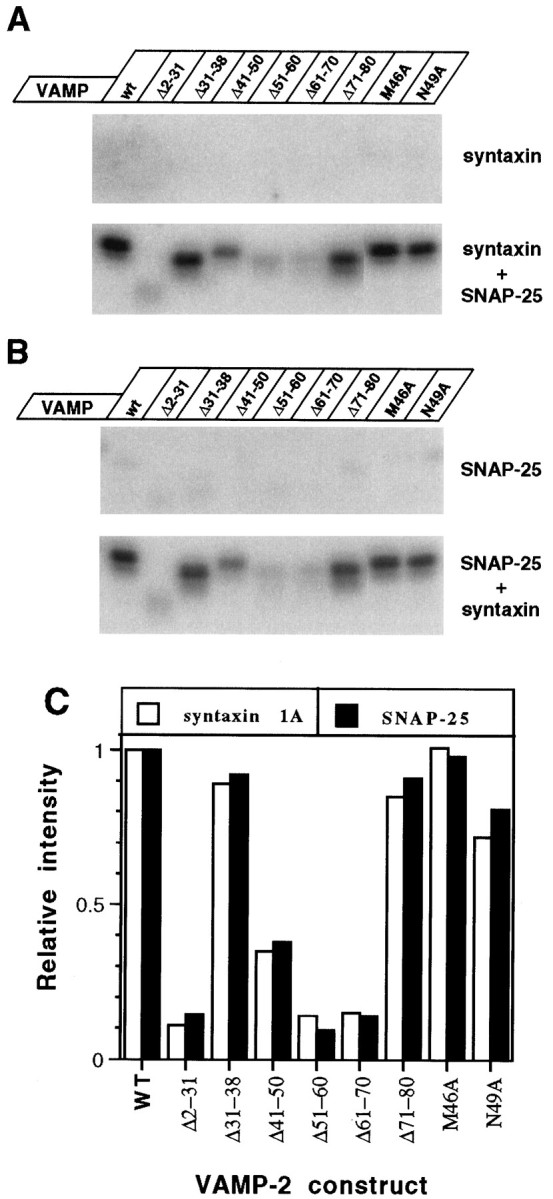
Analysis of binary and ternary SNARE protein interactions via ligand overlay blotting. A, Wild-type and mutant VAMP-2 proteins (100 ng each) were resolved by SDS-PAGE and transferred to nitrocellulose. After an incubation with 2 μg/ml of either syntaxin 1A (upper) or both syntaxin 1A and SNAP-25 (lower), the blots were probed for syntaxin 1A immunoreactivity, as described in Materials and Methods.B, Blots identical to those described inA were incubated with 2 μg/ml of either SNAP-25 (upper) or both SNAP-25 and syntaxin 1A (lower) and probed for SNAP-25 immunoreactivity.C, Relative binding intensity of syntaxin 1A (open bars) and SNAP-25 (solid bars) to immobilized VAMP-2 proteins, as determined by quantitation of the blots shown in the lower panels of A andB. All values were normalized to those for wild-type VAMP-2.
A subset of VAMP-2 deletion mutants eliminates the formation of SDS-resistant ternary SNARE complexes
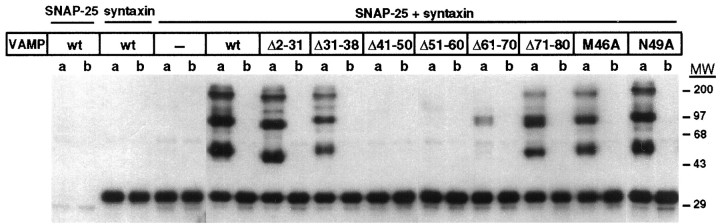
The formation of heat-labile, SDS-resistant multimeric complexes composed of VAMP-2, syntaxin 1A, and SNAP-25 initially was described byHayashi et al. (1994). To define the domain of VAMP-2 required for the acquisition of this unusual property, we examined the ability of ternary SNARE complexes (assembled from recombinant VAMP-2, syntaxin 1A, and SNAP-25) to resist SDS denaturation at low temperatures by Western blotting with an anti-syntaxin 1A antibody (Fig.6). Control samples containing only syntaxin 1A and VAMP-2 or syntaxin 1A and SNAP-25 did not generate any heat-labile, SDS-resistant complexes. However, samples containing syntaxin 1A, SNAP-25, and wild-type VAMP-2 generated a set of three prominent heat-labile complexes (migrating at apparent molecular weights of 50, 100, and 200 kDa), which may represent monomers, dimers, and tetramers of the stable SNARE complex. Each of the SDS-resistant complexes was also immunoreactive for VAMP-2 (Fig. 7) and SNAP-25 (data not shown) and contained equimolar amounts of the three proteins (Hayashi et al., 1994; J. Hao, unpublished observation). Interestingly, all of the VAMP-2 mutants, with the exception of Δ41–50, Δ51–60, and Δ61–70, were able to generate prominent SDS-resistant complexes when combined with SNAP-25 and syntaxin 1A. These results demonstrate that ternary complexes can exist in either SDS-resistant or SDS-sensitive states and that a central region within VAMP-2 (between amino acids 41 and 70) is required to generate the SDS-resistant state. Consistent with these observations, the proteolytic cleavage of VAMP-2 within this central region by botulinum toxins D and F prevents the assembly of SDS-resistant SNARE complexes (Hayashi et al., 1994).
Fig. 6.
Formation of heat-labile, SDS-resistant ternary complexes with wild-type and mutant forms of VAMP-2. Combinations of soluble wild-type or mutant VAMP-2, syntaxin 1A, and SNAP-25 (2 μm each) were incubated for 16 hr at 4°C. After SDS-PAGE sample buffer was added, samples were divided into two aliquots and either incubated at 37°C (a) or boiled (b). After SDS-PAGE and transfer to nitrocellulose, multimeric complexes were detected by using an antibody against syntaxin 1A and visualized by autoradiography. Binary controls are shown in the three pairs of lanes at the far left. The molecular weight (MW) markers are in kDa.
Fig. 7.
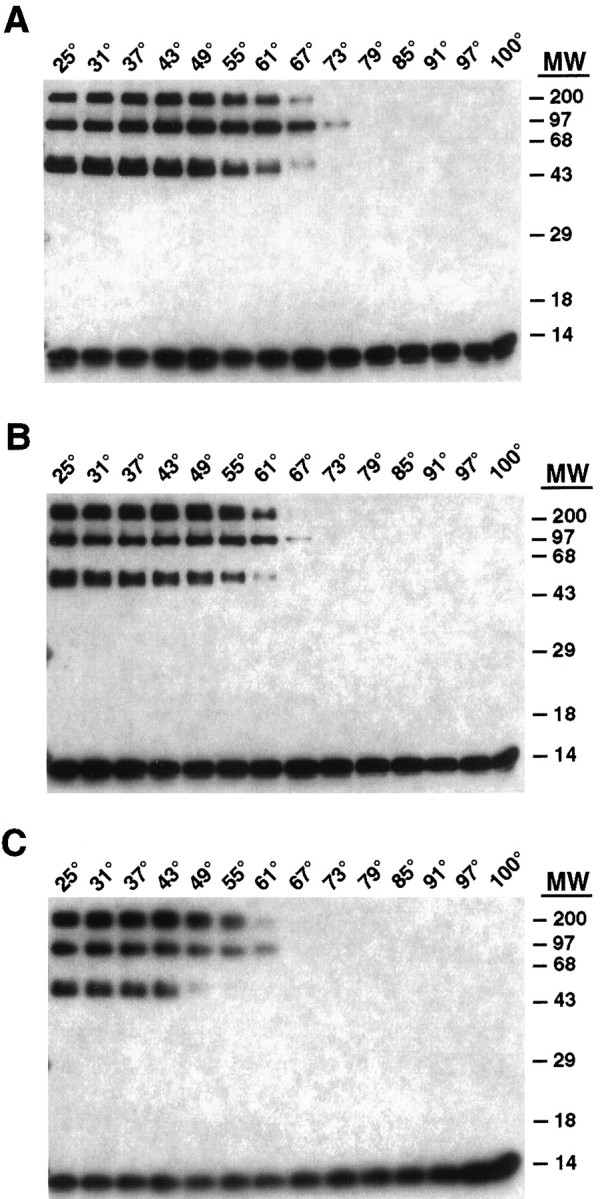
Heat lability of SDS-resistant ternary complexes generated by using wild-type, M46A, and N49A mutant VAMP-2. Ternary SNARE complexes containing wild-type VAMP-2 (A), M46A (B), or N49A (C) were assembled as described in Figure 6 and divided into 14 aliquots. After incubation at the indicated temperatures, the samples were resolved by SDS-PAGE and analyzed by immunoblotting with an affinity-purified anti-VAMP-2 antibody and visualized with a chemiluminescence detection system. The molecular weight (MW) markers are expressed in kDa.
To investigate the possible relationship among the different SDS-resistant complexes and to determine whether the complexes formed with the VAMP-2 point mutants display differential stability, we performed melting curve experiments. Mixtures of syntaxin 1A, SNAP-25, and either wild-type VAMP-2, M46A, or N49A were incubated at increasing temperatures before SDS-PAGE and Western blot analysis with an antibody against VAMP-2. With wild-type VAMP-2 (Fig. 7A), the 50 and 200 kDa complexes began to dissociate at a slightly lower temperature (55–61°C) than the 100 kDa complex (61–67°C), with all three complexes being fully dissociated by 79°C. Because none of the complexes increases in abundance during the dissociation process, it is unlikely that temperature-induced interconversion of the complexes is occurring. The SDS-resistant complexes formed with the M46A point mutant seemed to be less stable than wild-type VAMP-2 complexes (Fig.7B), whereas the SDS-resistant complexes formed with the N49A point mutant were less stable than both wild-type VAMP-2 and M46A mutant complexes (Fig. 7C). This observation demonstrates that the enhanced ability of the N49A mutant to form binary complexes with both syntaxin 1A and SNAP-25 (Figs. 2, 3) does not result in the formation of SDS-resistant ternary complexes with enhanced thermal stability. Rather, the reduced stability of the M46A and N49A mutant complexes may be a consequence of disruption of the integrity of the central region of VAMP-2, the importance of which in SDS resistance was clearly established by the behavior of the Δ41–50 deletion mutant (Fig. 6).
DISCUSSION
Formation of binary complexes
We have examined the binary interactions between VAMP-2 and either syntaxin 1A or SNAP-25, both in vitro with an affinity chromatography assay and in vivo with the yeast two-hybrid system. The similar results obtained with the two assays provide confidence that the interactions being monitored accurately reflect those responsible for complex assembly at the synapse. Deletion mutagenesis was used to delineate the regions of VAMP-2 required for its binding to syntaxin 1A and SNAP-25. Our results suggest that VAMP-2 contains a SNAP-25 binding site that encompasses at least amino acids 41–60 and a syntaxin 1A binding site that encompasses at least amino acids 31–70. The fact that deletion mutations in either of the two domains in VAMP-2 predicted to form coiled coils (amino acids 30–56 or 57–88) can eliminate syntaxin 1A or SNAP-25 binding is consistent with the prospect that coiled coils play an important role in these interactions. Two amino acid substitutions within the conserved domain of VAMP-2, M46A and N49A, have strong but opposite effects on sorting to synaptic vesicles (Grote et al., 1995). When the binding properties of these mutant VAMP-2 proteins were analyzed both in vitroand in vivo, the mutation that enhanced sorting to synaptic vesicles, N49A, displayed higher affinity for both syntaxin 1A and SNAP-25. The point mutation that was defective in sorting to synaptic vesicles, M46A, showed reduced interaction in both cases.
These results clearly demonstrate that point mutations that influence the sorting of VAMP-2 also affect its association with members of the SNARE complex. However, the correlation between binary complex formation and synaptic vesicle targeting does not extend to the deletion mutants of VAMP-2. For example, the Δ51–60 mutant of VAMP-2 fails to interact detectably with either syntaxin 1A or SNAP-25 but is targeted to synaptic vesicles at a level comparable with wild-type VAMP-2 (Grote et al., 1995). Likewise, the Δ31–38 mutant of VAMP-2 is capable of interacting with only SNAP-25 but is targeted inefficiently to synaptic vesicles (Grote et al., 1995). Thus, it is very unlikely that the influence of the point mutants on VAMP-2 targeting is a direct consequence of enhanced or reduced interactions with syntaxin 1A or SNAP-25. Another explanation for the behavior of the point mutants is that the amino acids mutated directly contribute to a binding site shared among syntaxin 1A, SNAP-25, and the sorting machinery. If this were the case, one would anticipate that syntaxin 1A and SNAP-25 recognize VAMP-2 in similar ways. Again, the behavior of the VAMP-2 deletion mutants does not support this notion, because the domain requirements for binary complex formation with syntaxin 1A and SNAP-25, although overlapping, are clearly distinct. Furthermore, because the combination of syntaxin 1A, SNAP-25, and VAMP-2 assembles into a ternary complex, it is unlikely (although not impossible) that both syntaxin 1A and SNAP-25 are binding to the same amino acids of VAMP-2 in binary complexes.
An alternative explanation for the properties of the VAMP-2 point mutants is that they differentially influence the conformational state of VAMP-2. Although a synthetic peptide corresponding to the cytoplasmic domain of VAMP-2 is not highly structured in solution (Cornille et al., 1994), this region of VAMP-2 must be able to adopt specific conformations in the presence of the appropriate binding partner(s). We suggest that VAMP-2 may exist in two conformational states: a “closed” state, favored by the M46A mutation, and an “open” state, favored by the N49A mutation. It is likely that reactive proteins able to facilitate membrane fusion are kept in an inactive or closed conformation until needed. The regulatory actions of n-sec 1 and α-SNAP/NSF may include the generation of a closed conformation of syntaxin 1A that is unable to interact with either VAMP-2 or SNAP-25 (Pevsner et al., 1994; Hanson et al., 1995; Kee et al., 1995). The potentially negative regulatory interaction of VAMP-2 with synaptophysin (Calakos and Scheller, 1994; Edelmann et al., 1995; Washbourne et al., 1995) similarly might stabilize a closed VAMP-2 conformation. The closed state of VAMP-2 might consist of a folded conformation or homodimer (Calakos and Scheller, 1994) that masks the binding sites for both syntaxin 1A and SNAP-25. The conversion of VAMP-2 to the open conformation would allow association with the plasma membrane components of the synaptic vesicle targeting and fusion machinery (syntaxin 1A and SNAP-25) as well as the components of the sorting machinery during recycling.
Assembly of ternary complexes
Several methods have been used to investigate the requirements for assembly of ternary SNARE complexes. Two of these methods, the affinity chromatography assay and the ligand overlay assay, clearly demonstrate that assembly is a cooperative process. Although some (affinity chromatography assay) or all (ligand overlay assay) of the VAMP-2 proteins failed to form binary complexes with either syntaxin 1A or SNAP-25, they all were able to form ternary complexes. In addition, a truncated form of SNAP-25 [SNAP-25 (ΔC)] lacking the C-terminal domain required for binary interactions with VAMP-2 is fully capable of potentiating VAMP-2 binding in a ternary complex. The proteolytic fragments of VAMP-2 and SNAP-25 generated by certain clostridial neurotoxins display similar cooperative binding properties (Hayashi et al., 1994). A number of mechanisms might contribute to the cooperative nature of ternary complex formation. One possibility is that the binary complex between syntaxin 1A and SNAP-25 generates a distinct binding site for VAMP-2 that is only modestly influenced by the mutations tested. This distinct binding site could be generated by a conformational change in either component of the syntaxin 1A/SNAP-25 heterodimer. The observation that ternary complexes can form with SNAP-25 mutants that do not form detectable binary complexes with VAMP-2 suggests that SNAP-25 may promote ternary complex formation by enhancing the interaction between VAMP-2 and syntaxin 1A. If this is the case, some forms of ternary complex may not include a direct interaction between SNAP-25 and VAMP-2. Alternatively, a high-affinity VAMP binding site may be generated by a unique surface at the interface between syntaxin 1A and SNAP-25. Another possibility is that the formation of a trimeric coiled coil among syntaxin 1A, SNAP-25, and VAMP-2 results in ternary complex assembly. In this case, the differential effects of VAMP-2 mutations on the assembly of binary and ternary complexes could be a reflection of the relative stability of the corresponding dimeric or trimeric coiled-coil structures. Finally, the present observations do not exclude the possibility that weak binary interactions involving VAMP-2 or VAMP-2 mutants fall below the level of detectability of the affinity chromatography, yeast two-hybrid, or ligand overlay assays. Such weak interactions still might be of sufficient strength to result in the cooperative assembly of ternary complexes.
One of the striking properties of the ternary complexes that form among syntaxin 1A, SNAP-25, and VAMP-2 is their resistance to SDS denaturation at low temperature. Evidence that these SDS-resistant complexes may be physiologically important is provided by the following observations: (1) they are detectable in SDS extracts of brain tissue (Hayashi et al., 1994); (2) they form less efficiently when the component proteins have been subjected to proteolysis with clostridial neurotoxins (Hayashi et al., 1994); (3) they are the preferential substrate for the binding of α-SNAP and NSF and subsequent ATP-hydrolysis-induced complex dissociation (Pellegrini et al., 1995). In the present study, we have found that several of the VAMP-2 deletion mutants fail to form SDS-resistant complexes. The two mutants that were least effective (Δ41–50, Δ51–60) were those that eliminate the binary interaction between SNAP-25 and VAMP-2. Thus, it can be postulated that an interaction between VAMP-2 and SNAP-25, although not absolutely required for its assembly, contributes to the stability of the ternary complex. Consistent with this possibility, the ternary complexes formed with SNAP-25 proteins lacking the C-terminal VAMP-2 binding domain (generated by mutagenesis or by cleavage with botulinum toxin E) are not stable in SDS (Hayashi et al., 1994; J. Hao, unpublished observation).
Although the in vitro assembly of SNARE complexes is well established, much less is known about their structure or in vivo functional importance. Our results demonstrate that the domains of VAMP-2 required for the assembly of binary, ternary, and SDS-resistant ternary complexes are distinct. These observations establish a foundation for the more detailed structural studies that will be required to define precisely the protein–protein interactions that lead to the assembly of distinct binary and ternary SNARE complexes. Furthermore, mutant forms of VAMP with well characterized effects on the assembly of SNARE complexes should prove to be useful tools for localization of SNARE complex functions within the ordered set of reactions that lead to neurotransmitter secretion.
These authors contributed equally to this work.
This work was supported by National Institutes of Health Grants NS 09878 and DA 10154 (R.B.K.) and GM 51313 (M.K.B.), the Alfred P. Sloan Foundation (M.K.B.), the McKnight Fund for Neuroscience (M.K.B.), and a Fonds National de la Recherche Suisse fellowship (N.S.). We thank William Trimble and Colin Barnstable for antibodies to VAMP-2 and syntaxin 1, respectively, and Eric Grote for VAMP-2 deletion and point mutant constructs and valuable discussions throughout the course of this work.
Correspondence should be addressed to Dr. Mark K. Bennett, Department of Molecular and Cell Biology, Life Sciences Addition, University of California, Berkeley, CA 94720.
REFERENCES
- 1.Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett MK, Scheller RH. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 5.Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 6.Calakos N, Scheller RH. Vesicle-associated membrane protein and synaptophysin are associated on the synaptic vesicle. J Biol Chem. 1994;269:24534–24537. [PubMed] [Google Scholar]
- 7.Calakos N, Bennett MK, Peterson KE, Scheller RH. Protein–protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- 8.Chapman ER, An S, Barton N, Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- 9.Cornille F, Goudreau N, Ficheux D, Niemann H, Roques BP. Solid-phase synthesis, conformational analysis, and in vitro cleavage of synthetic human synaptobrevin II 1-93 by tetanus toxin L chain. Eur J Biochem. 1994;222:173–181. doi: 10.1111/j.1432-1033.1994.tb18855.x. [DOI] [PubMed] [Google Scholar]
- 10.Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- 12.Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 13.Gaisano HY, Sheu L, Foskett JK, Trimble WS. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. J Biol Chem. 1994;269:17062–17066. [PubMed] [Google Scholar]
- 14.Gietz D, St. Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grote E, Kelly RB. Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grote E, Hao JC, Bennett MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 17.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 18.Hanson PI, Otto H, Barton N, Jahn R. The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- 19.Hata Y, Südhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J Biol Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt JM, Bommert K, Charlton MP, Kistner A, Habermann E, Augustine GJ, Betz H. A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 22.Kee Y, Lin RC, Hsu SC, Scheller RH. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 23.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Ptashne M. A new class of yeast transcriptional activators. Cell. 1987;51:113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- 25.Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini LL, O’Connor V, Lottspeich F, Betz H. Clostridial neurotoxins compromise the stability of a low energy SNARE complex mediating NSF activation of synaptic vesicle fusion. EMBO J. 1995;14:4705–4713. doi: 10.1002/j.1460-2075.1995.tb00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 28.Schiavo G, Rossetto O, Montecucco C. Clostridial neurotoxins as tools to investigate the molecular events of neurotransmitter release. Semin Cell Biol. 1994;5:221–229. doi: 10.1006/scel.1994.1028. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz TL. Genetic analysis of neurotransmitter release at the synapse. Curr Opin Neurobiol. 1994;4:633–639. doi: 10.1016/0959-4388(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 30.Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion [see comments]. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 31.Südhof TC. The synaptic vesicle cycle: a cascade of protein–protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 32.Washbourne P, Schiavo G, Montecucco C. Vesicle-associated membrane protein-2 (synaptobrevin-2) forms a complex with synaptophysin. Biochem J. 1995;305:721–724. doi: 10.1042/bj3050721. [DOI] [PMC free article] [PubMed] [Google Scholar]