Abstract
Microphotolysis and confocal microscopy were used to investigate the timing of calcium release and of the electrical response inLimulus polyphemus ventral photoreceptors. The fluorescent dyes Fluo-3 and Calcium Green-5N were used to monitor local Ca2+ elevations. Photolysis of caged inositol trisphosphate (InsP3) close to the plasma membrane of the light-sensitive rhabdomeral (R-) lobe resulted in Ca2+elevation within 10–20 msec, 20–45 msec before the physiological response to light normally would be detected. Inward ionic current flow and depolarization followed InsP3-induced calcium release within 2.5 ± 3.3 msec. Voltage-clamping the cells and removal of extracellular Ca2+ did not affect the timing of the Ca2+ elevation that followed the photolysis of caged InsP3 or its relationship to the electrical response. In contrast to the physiological response to light, which only released calcium within the R-lobe, photolysis of InsP3 elevated Cai in both lobes, although with much greater effect in the R-lobe, as compared with the bulk of the A-lobe, suggesting the presence of InsP3-sensitive calcium stores in both lobes. Photolysis of caged calcium [o-nitrophenyl EGTA (NPE)] at the edge of the R-lobe activated an inward ionic current within 1.8 ± 0.7 msec. This NPE-induced current reversed at a membrane potential of 10 ± 6 mV in the range typical of that of the light-activated current under physiological conditions. Calcium release, therefore, activates an inward current rapidly enough to contribute to the electrical response to light.
Keywords: phototransduction, Limulus polyphemus, photoreceptor, nitrophenyl EGTA (NPE), caged InsP3, confocal microscopy
The light response in invertebrate photoreceptors is thought to be mediated by the ubiquitous phosphoinositide-signaling (PI) pathway (Bloomquist et al., 1988; Hardie and Minke, 1995;Ranganathan et al., 1995). The major known products of the PI pathway are inositol 1,4,5 trisphosphate (InsP3), which releases stored calcium ions, and diacylglycerol (Berridge, 1993). However, there is no clear understanding of how the products of the PI pathway can be linked to the opening of the ion channels that depolarize the membrane potential of the photoreceptor. For the ventral photoreceptors of the horseshoe crab (Limulus polyphemus), intracellular pressure injections of InsP3 or Ca2+ ions activate, in darkness, an inward current having a reversal potential (Erev) similar to that of the light-activated current (Brown et al., 1984; Fein et al., 1984). The injections also subsequently desensitize the light response. Therefore, elevation of the cytosolic Ca2+ ion concentration (Cai) is thought to play a role in both excitation and adaptation ofLimulus photoreceptors (Frank and Fein, 1991; Nagy, 1991;Shin et al., 1993; Contzen et al., 1995). However, it has not been possible so far to verify that released calcium can activate ion channels sufficiently rapidly to contribute to the generation of inward current during the light response. In excised patches of light-sensitive membrane, cyclic guanosine monophosphate (cGMP), but not calcium ions, activates ion channels (Bacigalupo et al., 1991). Calcium-activated production of cGMP has, therefore, been proposed to couple the elevation of Cai to the activation of ion channels (Shin et al., 1993). The proposal of this additional step raises further doubts that calcium can act rapidly enough. Therefore, the time taken for released calcium to activate an inward current is clearly critical for determining the role of light-induced calcium release in mediating the electrical response to light. To investigate the timing of the response to released calcium ions, we have used fluorescent calcium-sensitive dyes and photolysis of caged InsP3 and nitrophenyl EGTA (NPE) to create and measure spatially localized calcium transients with a millisecond time resolution (Walker et al., 1989; Ellis-Davies and Kaplan, 1994).
MATERIALS AND METHODS
Ventral optic nerves were dissected as described by Millecchia and Mauro (1969) and placed in artificial seawater (ASW) containing (in mm): 435 NaCl, 10 KCl, 20 MgCl2, 25 MgSO4, 10 CaCl2, and 10 HEPES, pH 7.0. For some experiments the preparation was kept on ice in ASW containing 40 mm hydroxylamine for 15 min under bright white light (Faddis and Brown, 1992). After treatment with 1% Pronase, ventral photoreceptor cells were impaled with a glass micropipette. A solution containing 10–25 mm GDP-βS [guanosine-5′-O-(2-thiodiphosphate); Calbiochem, La Jolla, CA], 1 mm Fluo-3 or Calcium Green-5N (Molecular Probes, Eugene, OR), 10 mm caged InsP3 (Calbiochem), 100 mm K-aspartate, and 10 mm HEPES, pH 7.0, was pressure-injected from the micropipette into the cells. Approximately 50–100 injections of 1–10 pl were delivered before the beginning of the experiment. Caged ATP (10 mm; Calbiochem) replaced caged InsP3 in the injection solution for control experiments.
For experiments using caged calcium, 40 mm NPE (o-nitrophenyl EGTA hexapotassium salt; Molecular Probes) mixed with 32 mm CaCl2 replaced caged InsP3 in the micropipette solution. A 0.8 Ca–NPE mixture should yield a Ca2+ concentration of ∼1 μm(Ellis-Davies and Kaplan, 1994). After multiple UV flashes, Cai was increased irreversibly in some cells, presumably because of the cumulative shift in the Ca:NPE ratio. This sustained elevation of Cai was associated in some cells with a sustained inward current (Shin et al., 1993).
Ventral nerves were viewed with a Zeiss LSM 410 laser-scanning confocal microscope equipped with a 488 nm argon laser (Uniphase) focused through a Zeiss Neofluor 40×/0.75 objective lens (for details, seeUkhanov and Payne, 1995). A 351/364 nm ion argon laser (Innova Technologies) also was focused through the same objective lens and was used to photolyze caged compounds. The UV laser intensity was attenuated by neutral density filters and is expressed here by an arbitrary relative scale. High-speed shutters (Uniblitz model 26L, Vincent Associates, Rochester, NY) were placed in the path of the laser beams to control the timing and duration of flashes. A procedure similar to that described by Wang and Augustine (1996) was used to check that the focus and position of the UV spot were coaxial with the 488 nm laser beam. Briefly, photobleaching of a thin polysterene film stained with Nile Red was used to determine the position of the focused spots created by the 488 nm and UV lasers.
For line scans, the laser beam swept every 4 msec across the cell, each scan containing 512 pixels. In all, 512 successive scans were stacked to create a raw image of fluorescence that represented 2.048 sec of recording time. Changes of Cai on illumination of the photoreceptors were displayed as a ratio of fluorescence relative to the fluorescence, Fo, recorded during the latent period of the response. National Institutes of Health IMAGE software (written by Wayne Rasband at National Institutes of Health and available via anonymous ftp from zippy.nimh.nih.gov) was used for off-line image processing, including smoothing and ratio calculations. Smoothing reduced the spatial resolution of line scans to 4 μm. By photobleaching Nile Red-stained polystyrene film, we also determined the uniformity of illumination during laser beam scans. We found that the intensity of the laser beams varied by <30% over the first 412 pixels of the scan. However, the beam intensity increased fourfold over the final 100 pixels because of a slowing of the movement of the beam toward the end of each scan. To avoid artifacts because of increased photolysis in this region of the scan, we deleted from our analysis the 100 pixels at the right-hand side of every line in the stack.
Membrane voltage or ionic current were recorded with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) and digitally sampled at a rate of at least 1 kHz. For current recording and measuring of the Erev under two-electrode voltage clamp, the cells were impaled with a second micropipette filled with 3 m KCl (resistance < 10 MΩ).
RESULTS
Use of GDP-βS and hydroxylamine to prolong response latency
We previously have used steps of light delivered by the 488 nm laser of our confocal microscope to excite ventral photoreceptors and simultaneously to monitor Cai (Ukhanov and Payne, 1995). To release caged compounds, we delivered a flash from a 364 nm laser at the onset of the 488 nm step, both lasers being focused onto the same point (see Materials and Methods). The application of caged compounds to functioning photoreceptor cells presents a challenge, because rhodopsin absorbs the UV light required for photolysis. This would be expected to accelerate calcium release and the electrical response through the normal physiological pathway. It is, therefore, important to reduce the sensitivity of the physiological pathway as much as possible. In the case of Limulus ventral photoreceptors, the physiological response to the intense laser light delivered by our confocal microscope is delayed by a latent period of ∼20 msec (Ukhanov and Payne, 1995). To reduce the gain of phototransduction and to increase the latent period even further, we followed the protocol ofFaddis and Brown (1992), treating the cells with hydroxylamine and injecting them with GDP-βS. By bleaching rhodopsin (Hubbard and Wald, 1960) and inhibiting GTP-binding proteins (Fein, 1986), respectively, these agents slow excitation of the cells by light without affecting the response to injected InsP3 (Faddis and Brown, 1992). As a result, the latency of the normal physiological response to both the 488 nm laser and the flash from the UV laser is prolonged to 30–50 msec (Payne and Ukhanov, 1996). This latency enabled us to observe the effects of the release of caged compounds, which photolyze within 1–3 msec, before any physiological response caused by the activation of rhodopsin is detectable.
Photolysis of caged InsP3 rapidly elevates Cai in ventral photoreceptors
Ventral photoreceptors were injected with GDP-βS, caged InsP3, and the calcium indicator dye Fluo-3 and were viewed with a laser- scanning confocal microscope. After orientation of the laser beam, a line scan was performed in a plane that passed through the interior of the light-sensitive rhabdomeral (R-) lobe of the photoreceptor, the insensitive arhabdomeral (A-) lobe (Calman and Chamberlain, 1982), and the axon (Fig.1A). The 488 nm laser used for this line scan excited the cell through the normal physiological mechanism without photolyzing the cage and simultaneously elicited fluorescence from Fluo-3 so as to monitor accompanying changes in Cai. After a latent period of 40 msec, the elevation of Caibegan at the extreme edge of the R-lobe beneath the photoreceptive microvillar membrane (Ukhanov and Payne, 1995) and then spread over the next 400 msec toward the boundary between the R-lobe and the light-insensitive A-lobe (Fig. 1B, upper frame). The elevation of Cai barely penetrated the A-lobe. In five cells, Fluo-3 fluorescence recorded during illumination by the 488 nm laser within 10 μm of the edge of the R-lobe rose to a peak of 3.7 ± 0.8 times its initial level (mean ± SD). At 70 μm from the edge of the R-lobe, the fluorescence remained at 1.1 ± 0.11 times its initial level over the same time period. A 10 msec flash from a UV laser then was superimposed at the beginning of a second 488 nm scan. This flash initiated fast cleavage of caged InsP3, with consequent elevation of Cai within 30 msec along the entire length of the scanned line within the R-lobe (Fig. 1B, lower frame). The elevation of Cai along the scanned line in the A-lobe was much less than that in the R-lobe, with the exception of a region close to the axon. For the same five cells, the peak Fluo-3 fluorescence after the UV flash recorded within 10 μm of the edge of the R-lobe was 4.0 ± 0.7 times its initial level (mean ± SD). At 70 μm from the edge of the R-lobe, the peak fluorescence was 1.6 ± 0.6 times its initial level.
Fig. 1.
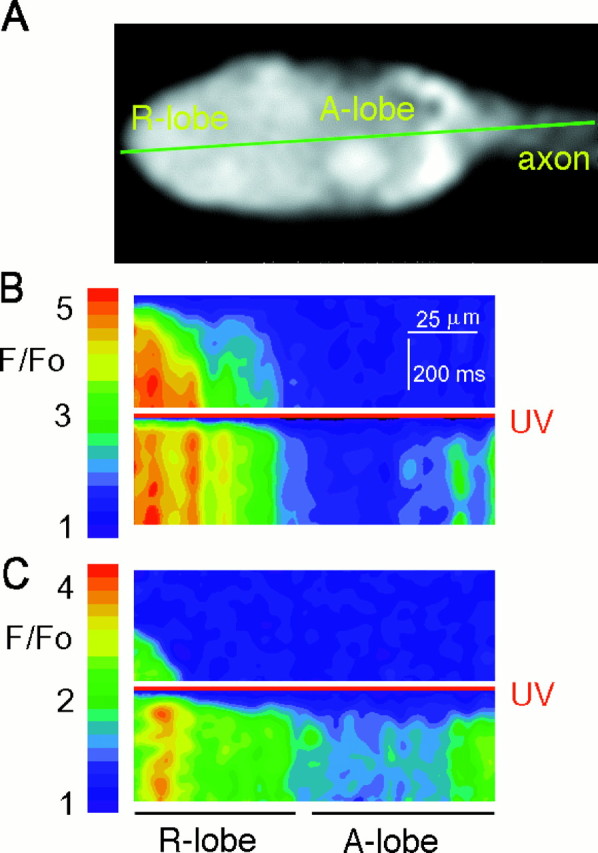
Photolysis of caged InsP3 initiates calcium release throughout Limulus ventral photoreceptors. A, Ventral photoreceptor loaded with 10 mm GDP-βS, 1 mm Fluo-3, and 10 mmcaged InsP3. The laser beam scanned along the green line every 4 msec, and the resulting lines of fluorescence data were stacked to create the images below.B, Upper frame, Fluorescence recorded during a line scan with a 488 nm laser beam, which excited the photoreceptor through the physiological mechanism. The scan shows the temporal progression of the physiological elevation of Caifrom the edge of the R-lobe membrane (left-hand edge of scan) into the A-lobe (right-hand side of scan). The laser beam started scanning across the cell at a time indicated by the first line of the image. The left-hand side of each scanned line was cropped to eliminate pixels beyond the edge of the R-lobe (determined as the point at which total fluorescence drops by 50%). The right-hand edge of each scanned line also was cropped to eliminate the final 100 pixels in which laser illumination was not uniform (see Materials and Methods). B,Lower frame, A 10 msec flash from a UV (351/364 nm) laser, relative intensity 0.5 (red line), was superimposed at the beginning of the scan. The resulting photolysis of caged InsP3 induced fast calcium release throughout the cell. C, To exclude any contribution of Ca2+influx, we kept a different cell in darkness for 30 min in ASW containing 1 mm EGTA instead of 10 mmCa2+. This increased the latency of response to the 488 nm laser via the physiological mechanism (upper frame) but did not alter the latency or pattern of elevation of Cai after photolysis of InsP3 (lower frame).
To verify that most of the Cai rise comes from the release of internal calcium stores, we removed Ca2+ from the ASW bathing another ventral nerve. The nerve was kept in darkness for 30 min in ASW containing 1 mm EGTA instead of 10 mm Ca2+. As expected from previous work (Lisman, 1976; Payne and Flores, 1992), removal of extracellular Ca2+ increased the latent period of the physiological response to the 488 nm scan (Fig. 1C, upper frame) to 230 msec. Nevertheless, a brief UV flash elicited as rapid a response to photolysis of InsP3 along the entire scanned line as for cells bathed in 10 mm Ca2+(Fig. 1C, lower frame). These results might be explained if prolonged exposure of the cell to zero extracellular Ca2+ and the accompanying reduction of resting levels of Cai (Levy and Fein, 1985; Ukhanov et al., 1995) resulted in a slowing of light-induced phospholipase-C activity (Rack et al., 1994;Mitchell et al., 1995).
Rapid coupling of InsP3-induced elevation of Cai to the electrical response
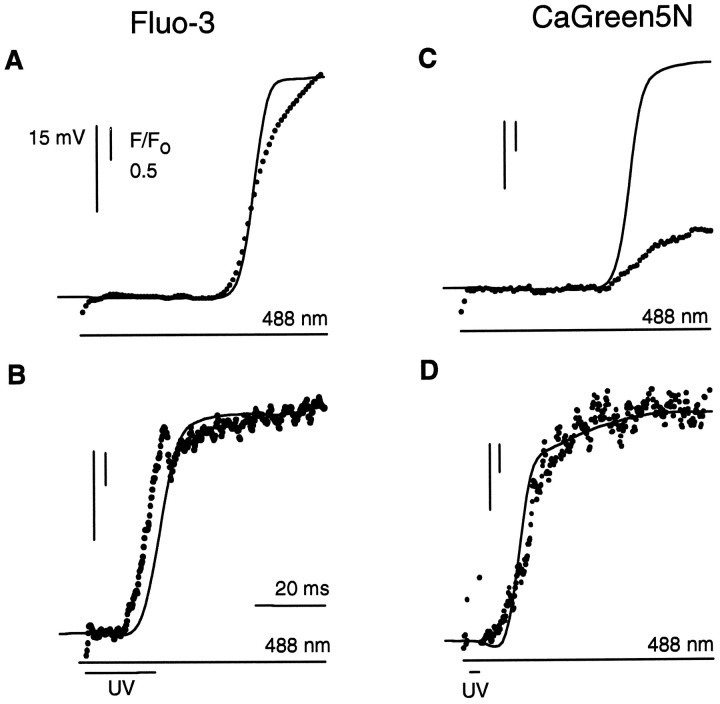
Positioning the laser beam at a stationary spot allowed detection of fluorescence with the highest temporal resolution as well as microphotolysis at the fastest rate. The spot chosen was located at the edge of the R-lobe where the fastest elevations of calciumvia the physiological mechanism were observed. Because this placement was based on the spatially smoothed line scan images of calcium release, resolution of the edge of R-lobe was limited to ∼4 μm (see Materials and Methods). During a 488 nm flash delivered to this spot, calcium release via the physiological mechanism began after a latent period of 42 msec and rapidly saturated the Fluo-3 (Fig.2A). Membrane depolarization was detected simultaneously with the calcium release. A brief superimposed UV flash, which photolyzed caged InsP3, initiated calcium release with a greatly reduced latency of 13 msec (Fig.2B). Membrane depolarization followed 2 msec later. We attribute this earlier calcium elevation and electrical response to photolytic release of InsP3 by the UV flash.
Fig. 2.
Timing of calcium release by using the stationary spot mode of the laser. Photoreceptors were loaded with either Fluo-3 or Calcium Green-5N, caged InsP3, and GDP-βS. The 488 nm and UV (351/364 nm) laser beams were focused onto the edge of the R-lobe, equivalent to the extreme left-hand edge of the photoreceptor in Figure 1A. A, Membrane potential (solid line) and Fluo-3 fluorescence (dots) recorded during illumination by the 488 nm laser. Laser stimulation started at the beginning of the fluorescence trace.B, Effect of superimposing a 20 msec duration UV flash.C, Membrane potential and Calcium Green-5N fluorescence recorded from a photoreceptor illuminated by the 488 nm laser.D, Effect of superimposing a 3-msec-duration UV flash. The rapid reduction in fluorescence in B andD on termination of the UV flash is attributable to cessation of autofluorescence and additional Fluo-3 fluorescence created by the UV illumination; relative intensity 0.05 inB and 0.5 in D. Fluorescence was sampled every 0.9 msec in A and C and every 0.2 msec in B and D.
The low-affinity calcium indicator dye Calcium Green-5N (Haugland, 1992) was used to better compare peak elevations of Cai. Calcium Green-5N saturates at much higher levels of Caithan Fluo-3 and is therefore more suitable for following the time course and magnitude of large elevations of Cai. The 488 nm laser again was focused onto a spot at the edge of the R-lobe. Calcium Green-5N fluorescence during a 488 nm step rose similarly to the Fluo-3 signals (Fig. 2C), reaching a peak within ∼100 msec. Photolysis of caged InsP3 at the same spot induced a peak fluorescence increase that was nearly threefold larger than that activated by 488 nm light alone (Fig. 2D) and reached its peak within 20 msec, implying an extremely fast and large Cai rise.
We investigated the effect of removing extracellular Ca2+and of voltage-clamping photoreceptors. Figure 3 shows inward current and fluorescence recorded from a voltage-clamped photoreceptor that had been filled with Fluo-3, GDP-βS, and caged InsP3. Before stimulating the cell, the photoreceptor was bathed in darkness for 30 min in ASW containing 1 mm EGTA instead of 10 mm CaCl2. As expected from the line scans (Fig. 1C), the latent period of the response to the 488 nm flash alone (Fig. 3A), but not to the superimposed UV flash (Fig. 3B), was greatly prolonged, as compared with control conditions (compare Figs. 2 and 3, noting the different time scales). The relationship between the timing of the electrical response and the fluorescence traces that followed photolysis of caged InsP3 by the UV flash was also similar to that seen under control conditions (Fig. 3, inset). Removal of extracellular calcium for many minutes therefore did not greatly affect the coupling of InsP3-induced calcium release to the generation of inward current.
Fig. 3.
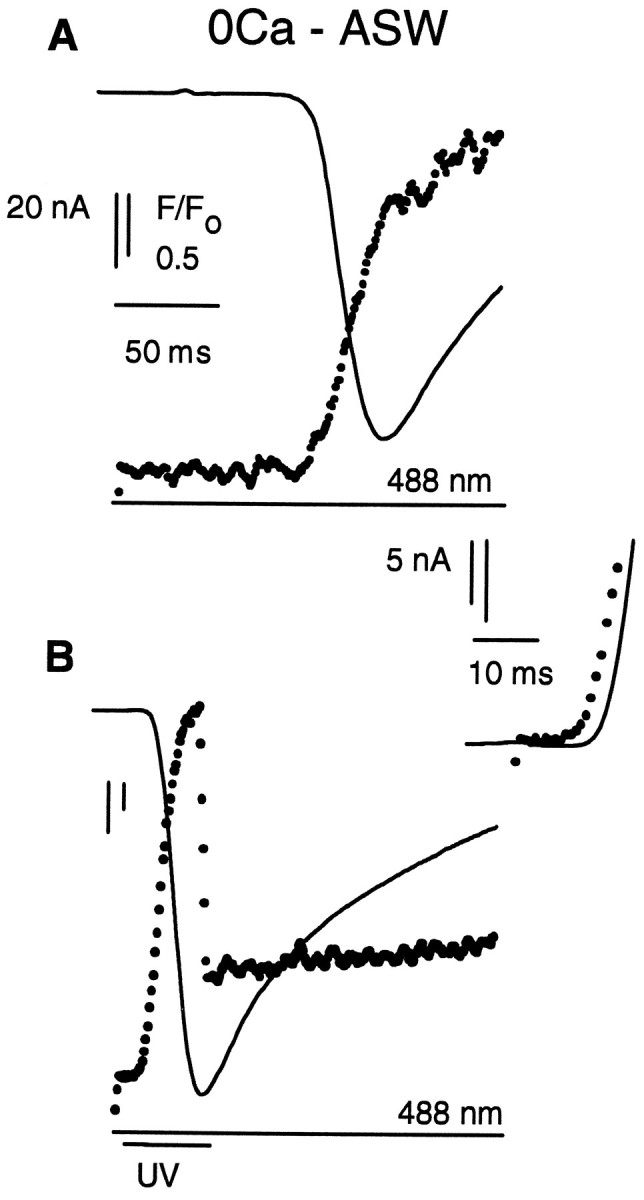
Removal of extracellular Ca2+ does not affect InsP3-induced calcium release inLimulus ventral photoreceptors. A, Membrane current (solid line) and Fluo-3 fluorescence (dots) recorded from a photoreceptor voltage-clamped to its resting membrane potential and illuminated by a 488 nm laser. The photoreceptor was bathed in artificial seawater containing 1 mm EGTA instead of 10 mm Ca2+. Note the change in time scale, as compared with Figure 2. B, Effect of superimposing a 40 msec duration UV flash. The rapid reduction in fluorescence in B on termination of the UV flash is attributable to cessation of autofluorescence and additional Fluo-3 fluorescence created by the UV illumination; relative intensity 0.5. Fluorescence was sampled every 0.9 msec.
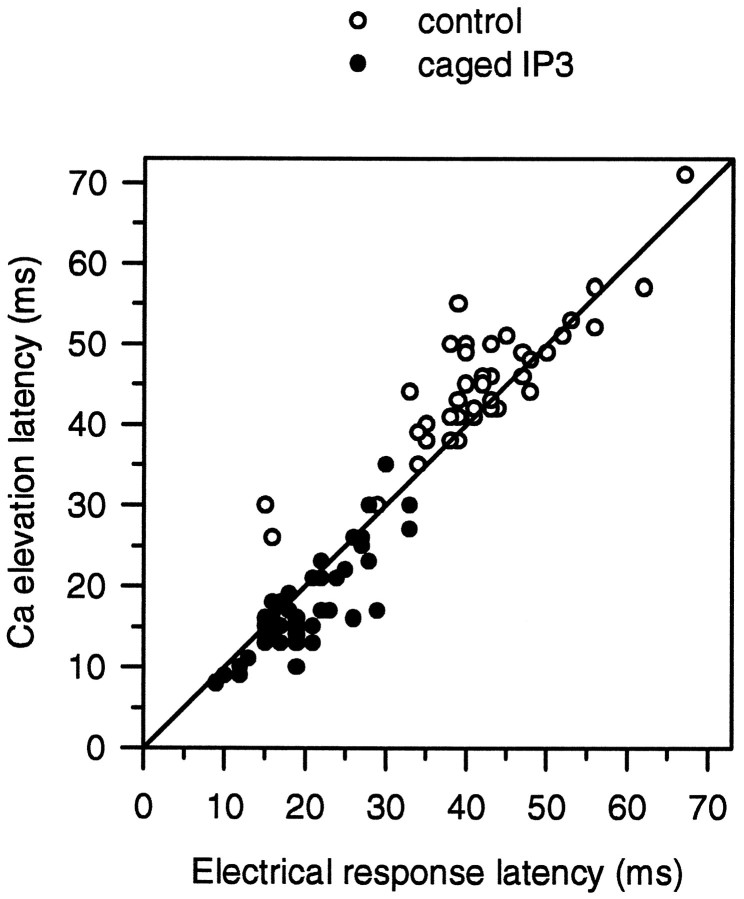
For further analysis of the data obtained in normal ASW, we compared the latency of the electrical response and of the Caielevation induced by the photolysis of caged InsP3 at the edge of the R-lobe. The complete data are plotted as a scatter diagram in Figure 4 (solid circles). The mean latency of the calcium signals (the first detectable increase in Fluo-3 fluorescence) that followed photolysis of caged InsP3 by the UV flash was 17 ± 6 msec (mean ± SD; n= 42; 14 cells), whereas the mean latency of the electrical response was 20 ± 6 msec. The variable latency of the InsP3-induced calcium signal may be a general property of InsP3-induced calcium release, shared with other cells (Parker and Ivorra, 1993). However, the relative timing of the calcium signal and the electrical response exhibited less variability. On average, the InsP3-induced calcium signal led the electrical response by 2.5 ± 3.3 msec.
Fig. 4.
A scatter plot of the latencies of the calcium signal (both Fluo-3 and Calcium Green-5N fluorescence) and of the electrical responses (either photocurrent or changes in membrane potential) after photolysis of caged InsP3(filled circles) or during the physiological response to 488 nm light (open circles). Astraight diagonal line indicating equality of the two latencies is drawn to show the slightly different distributions of the two sets of data points.
Latencies for the responses to physiological stimulation of the cell by 488 nm illumination alone were longer than those for the responses to the release of caged InsP3 (Fig. 4, open circles). The mean latency of the physiological electrical response was 45 ± 12 msec (n = 52; 14 cells), whereas the latency of the accompanying calcium signals was 48 ± 12 msec. The comparatively large SD of these data reflect, in part, the known variability inherent in the process that determines the latency of the physiological response to individual quanta (Yeandle and Spiegler, 1973) including, presumably, the time taken to generate InsP3 and for InsP3 to release calcium (see above). The distribution of the latencies also differed from those elicited after photolysis of caged InsP3. The majority (32 of 52) of the latency data for the physiological responses falls above the diagonal line in Figure 4, indicating that the detection of calcium release often slightly lagged the initiation of the physiological electrical response. One explanation for the difference in the distributions of latencies might be the time taken for calcium to diffuse small distances. Even at the highest diffusion rate of 227 μm2/sec (Allbritton et al., 1992), Ca2+ ions will take ∼3 msec to travel 1 μm. The resolution of the edge of the R-lobe, determined from line scan images of calcium release, was limited by spatial smoothing to ∼4 μm. Thus, the confocal spot could have been placed a few micrometers from the microvillar membrane of the R-lobe. This error in placement might account for the systematic difference in the timing of the physiological and InsP3-induced calcium signals. Calcium released by the physiological mechanism would be expected to be initiated from calcium stores directly beneath the microvillar membrane, where light-induced production of InsP3 occurs. This physiological release of calcium immediately would open channels in the plasma membrane. However, if the confocal measuring spot were placed more than a micrometer from the microvillar membrane, the calcium ions would have to diffuse to the spot to be detected. The detection of the calcium signal would, therefore, be expected to lag the physiological electrical response by a few milliseconds. On the other hand, calcium ions released by photolysis of caged InsP3 from stores at the same distant confocal spot would be detected immediately, but the calcium ions would have to diffuse to the microvillar membrane to initiate an electrical response. If the confocal measuring spot were placed more than a micrometer from the microvillar membrane, the calcium signal might, therefore, be expected to lead the electrical response to photolysis of caged InsP3 by a few milliseconds but to lag the physiological electrical response to 488 nm light.
Photolysis of caged ATP had little effect on calcium release or the electrical response
We were concerned that the absorption of UV light by rhodopsin, in addition to the release of caged InsP3, might accelerate the response of the cell when the UV flash was superimposed on the 488 nm step. We therefore substituted caged ATP for caged InsP3in the solution injected into the cells and repeated some of the above experiments. Responses from cells coinjected with 10 mmcaged ATP, Fluo-3, and GDP-βS did not exhibit a large reduction in the latency of their response when the UV flash was superimposed on the 488 nm flash (Fig. 5). The mean latencies of the calcium signals after the 488 nm laser flash were 42 ± 2 msec without the UV flash and 39 ± 5 msec (n = 8) with the superimposed UV flash (relative intensity 0.5, duration 30 msec), whereas those of the electrical responses were 37 ± 5 and 33 ± 7 msec, respectively. We presume that the UV flash is relatively ineffective because the 488 nm illumination alone locally saturates the physiological response to light. The UV flash, however, does elicit increased autofluorescence and Fluo-3 fluorescence (Fig.5B). Because the UV flash, by itself, does not significantly alter the initial response of the cell to the 488 nm light, we attribute the effects of UV stimulation of cells loaded with caged compounds to the release of active InsP3 or free Ca2+.
Fig. 5.
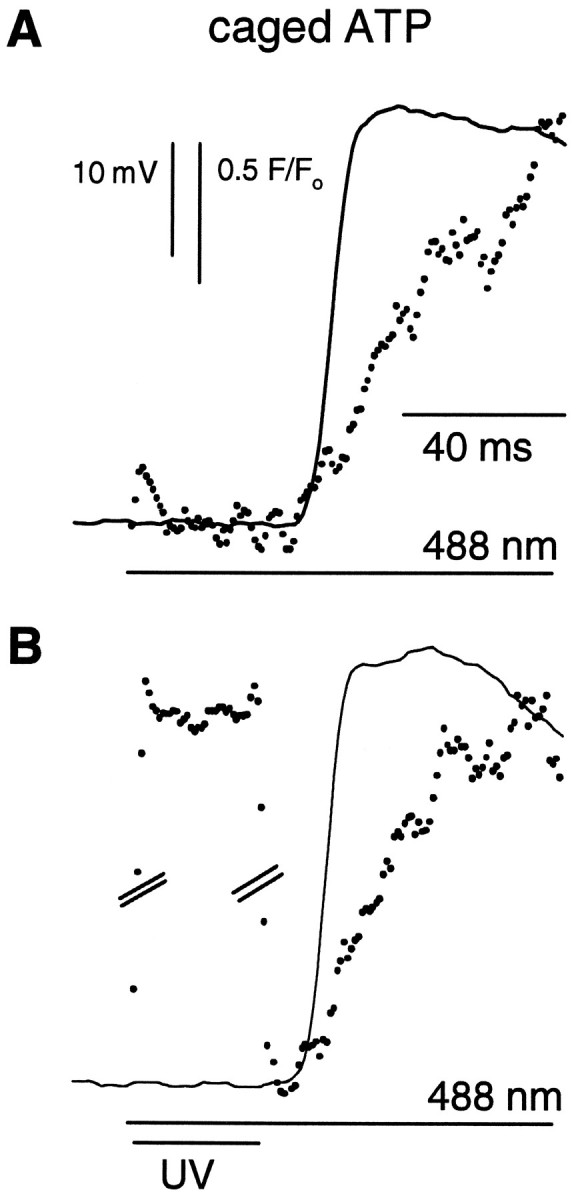
Photolysis of caged ATP does not alter either electrical excitation or light-induced calcium release inLimulus ventral photoreceptors. Photoreceptors were loaded with Fluo-3, caged ATP, and GDP-βS. The 488 nm and UV (351/364 nm) laser beams were focused onto the edge of the R-lobe, equivalent to the extreme left-hand edge of the photoreceptor in Figure 1A. A, Membrane potential (solid line) and Fluo-3 fluorescence (dots) recorded during illumination by a 488 nm laser. Laser stimulation began at the beginning of the fluorescence trace.B, Effect of superimposing a 30 msec UV flash; relative intensity 0.5. The break in the vertical scale represents 7.5 F/Fo units and is needed to show the time course of autofluorescence excited by the UV light.
Use of caged calcium
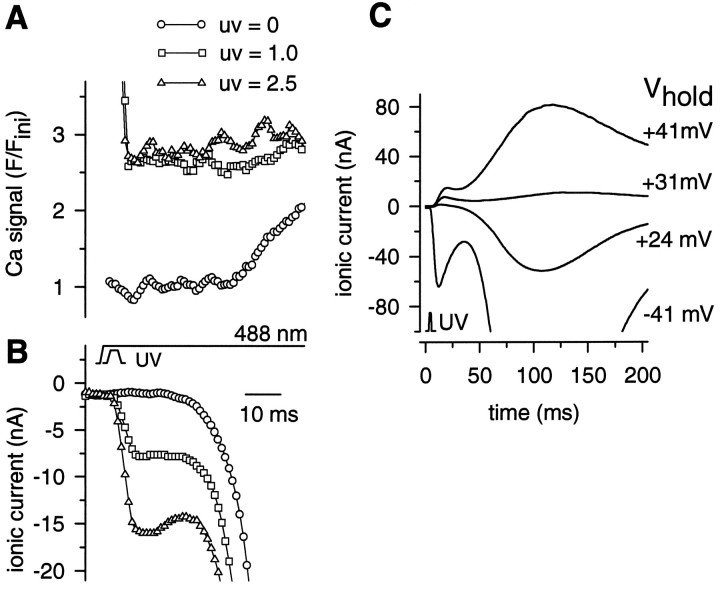
To determine the speed of action of released Ca independently, we directly released calcium from NPE. Injection of photoreceptors with 40 mm 0.8 Ca–NPE decreased the latent period of the physiological response to 488 nm light to <30 msec (compare Fig.6B). This effect can be ascribed to the higher Cai, 1 μm, in the injection solution, as compared with the normal resting Cai of 0.4 μm, because calcium elevation is known to reduce response latency (Fein and Charlton, 1977). NPE injection also slowed the rise time of the fluorescence increase caused by calcium releasedvia the physiological mechanism [compare the fluorescence trace in Fig. 2A with the control (UV = 0) trace in Fig. 6A]. We ascribe this to the buffering of Cai by NPE. Photolytic release of Ca2+ ions in the vicinity of the photoreceptor membrane by a 3 msec UV flash induced an inward current that clearly consisted of more than one component (traces labeled UV = 1.0, UV = 2.5 in Fig. 6B). An early transient inward current, rising to peak within 10 msec, was observed only after the UV flash and was attributed to the photolysis of NPE. The physiological response to the 488 nm light would not be expected to exhibit such an early transient current because of the 30–60 msec latent period associated with the physiological release of calcium (traces labeledUV = 0 in Fig. 6). A later larger current component induced by the UV flash rose at approximately the same time as the response to the 488 nm step and was, therefore, attributed to the physiological response. To verify that most of this early ionic current flows via ion channels rather than via the activation of an electrogenic Na/Ca exchanger, we determined its reversal potential under voltage clamp. Reversal of the early NPE-induced current is shown in Figure 6C. The reversal potential (Erev) lay between 3 and 22 mV, averaging 10 ± 6 mV (n = 4). The bulk of the current that followed the early NPE-induced transient reversed at a slightly more positive potential in all cells, averaging 19 ± 6 mV. In separate experiments, reversal of the inward current activated by the photolysis of caged InsP3 also was observed (data not shown). At high intensities of the UV laser, the latency of the electrical response to photolysis of caged NPE followed the onset of the UV flash by 1.8 ± 0.7 msec (mean ± SD, 5 cells). There is, therefore, a good agreement with the 2.5 ± 3.3 msec delay of the electrical response with respect to the calcium signal after photolysis of caged InsP3.
Fig. 6.
UV flashes activated an early inward ionic current in Limulus ventral photoreceptors loaded with 40 mm 0.8 Ca–NPE, Fluo-3, and GDP-βS. A 3 msec flash from a UV (351/364 nm) laser was superimposed on a 488 nm laser step. The relative intensity of the UV laser is as indicated in A. Both lasers were focused on a spot at the edge of the R-lobe of a ventral photoreceptor. A, Fluo-3 fluorescence, plotted as the ratio of the Fluo-3 fluorescence, F, observed during the response to that, Fini, sampled before the onset of the first UV flash in the series delivered to the cell. The detection of the elevation of Cai inducedvia the normal physiological mechanism by the 488 nm laser alone (UV = 0) was slowed significantly as a result of the loading of the photoreceptor with NPE. The control trace (UV = 0) was recorded after the presentation of the UV flashes. B, Inward current activated within the 3 msec duration of the UV flashes. C, Reversal of inward ionic current generated in another cell loaded with NPE and GDP-βS and illuminated by a 3 msec UV laser flash; relative intensity 50. For this cell, Erev of the early current transient was estimated as +22 mV.
DISCUSSION
Faddis and Brown (1992) investigated the electrical response of ventral photoreceptors after flash photolysis of InsP3produced by diffuse illumination. They observed that release of caged InsP3 increased the peak current produced through the physiological mechanism via the activation of rhodopsin. However, they could not separate the InsP3-induced current from the physiological light-induced current. Our confocal method reduces overall illumination of the photoreceptor while concentrating the UV light. This technique reduces the overall stimulation of the cell via the physiological mechanism while maximizing the concentration of InsP3 released at its site of action. As a result, we were able to observe an electrical response to the release of caged InsP3 during the latent period when no physiological response to light is detectable. Our results confirm their main conclusion—that the 10–20 msec delay between photolysis of InsP3 and the electrical response indicates that the latter is not caused by the direct rapid interaction of InsP3 with a channel in the microvillar membrane. Rather, our simultaneous measurements of Cai indicate that the electrical response immediately follows and is caused by the release of Ca2+ions (Payne et al., 1986).
Location of InsP3-sensitive calcium stores
Photolysis of caged InsP3 released calcium from stores within both the lobes of Limulus ventral photoreceptors. We note that not all of the calcium release necessarily passes through InsP3-gated channels in the endoplasmic reticulum. Some might be the consequence of a secondary wave of calcium-induced calcium release. The consequent elevation of Cai is larger in the R-lobe than in most regions of the A-lobe, with the exception of an area close to the axon. Earlier experiments using aequorin as a calcium indicator failed to reveal any significant calcium release on microinjection of InsP3 into the A-lobe (Payne and Fein, 1986). More sensitive calcium-selective microelectrode recordings, however, detected a relatively slow and small (∼2 μm) Ca2+ transient after injection of 100 μmInsP3 into the A-lobe (Levy and Payne, 1993). Our results support the latter observations and suggest that the aequorin imaging system was not sufficiently sensitive to detect release in the A-lobe. Our results are also consistent with a recent anatomical study showing that smooth endoplasmic reticulum (SER) is not confined to the submicrovillar region but that it forms a dense continuous network that extends from beneath the microvillar membrane into the center of the R-lobe. A less dense tubular reticulum, continuous with SER in the R-lobe, extends into the A-lobe (Feng et al., 1994). The 488 nm light would result in InsP3 production at the microvillar plasma membrane of the R-lobe through the PI pathway, creating a wave of calcium release from the SER that spreads into the center of the R-lobe and diminishes in magnitude as the InsP3 diffuses into the A-lobe. Release of caged InsP3, however, would elevate Cai throughout the cell, with the magnitude of the elevation of Cai in different cell regions being graded according to the density of the SER. This might explain the larger elevation of Cai in the R-lobe, as compared with the bulk of the A-lobe.
The prominent InsP3-induced calcium release in the region of the A-lobe where the axon originates suggests another concentration of SER there. We know of no published study on the distribution of SER in this particular region of Limulus photoreceptors. However, the presence of prominent ER and of the InsP3receptor protein in the axons of mammalian neurons has been well documented (Broadwell and Cataldo, 1984; Mignery et al., 1989). Because we do not detect any elevation of calcium concentration in the axon during the first second of the physiological response to light (Fig.1B), we assume that the calcium stores in the axon are not involved in the immediate transmission of the light response along the axon. However, it may be that, during more prolonged illumination, InsP3 produced in the cell body may diffuse down the axon and regulate axonal function or structure. Alternatively, the calcium stores in the axon may be vesicles that are being transported to fulfill a role in the function of the axon terminal.
Speed of coupling of calcium release to depolarization
If, as is indicated by experiments on excised patches (Bacigalupo et al., 1991), calcium does not bind directly to and open ion channels in the microvillar membrane, then the intermediate steps that couple calcium to the activation of ion channels impose a delay of only 1–3 msec. The significance of this result for visual excitation depends on when calcium is released during the light response. Analysis of the control responses to 488 nm illumination (Fig. 4, open circles) indicates that the mean latency of the physiological electrical response was 45 ± 12 msec, whereas the elevation of Cai was first detected after 48 ± 12 msec. Because it then takes ∼50 msec for the photocurrent to reach its peak, there seems to be sufficient time for released calcium ions to contribute to the activation of the photocurrent during the rising edge of the response to light. This assertion is consistent with the large attenuation of the rate of rise of the photocurrent produced by the intracellular injection of calcium chelators such as EGTA (Payne and Fein, 1986) and di-Bromo-BAPTA (Shin et al., 1993). However, because the detection of the physiological calcium signals often lagged that of the electrical response by a few milliseconds, we cannot assert that calcium elevation is the sole initiator of the electrical response. As noted in Results, a simple explanation for this lag is the time taken for the diffusion of calcium into the confocal spot after its release from SER immediately below the microvillar membrane. However, it is also possible that a parallel pathway of visual excitation exists, one which can initiate an electrical response but which does not require calcium release (Frank and Fein, 1991; Contzen et al., 1995). The possibility of such a pathway is discussed further below.
Reversal potential of the calcium-activated current
Previous experiments in which calcium was pressure-injected into the R-lobe concluded that both the calcium-induced current and the light-induced current reversed between +10 and +20 mV (Payne et al., 1986). The present experiments confirm this approximate range for the reversal potentials but indicate a consistent difference between the reversal potentials of current components activated by photolysis of caged calcium and by the physiological mechanism in the presence of NPE. The early transient, ascribed to calcium release, reversed at 10 ± 6 mV, whereas the much larger later component, ascribed to the physiological mechanism, reversed at 19 ± 6 mV. This difference in Erev may be significant. The response of dark-adapted ventral photoreceptors to bright light flashes does not reverse at a unique potential and has been interpreted as consisting of three components, each having aErev differing by a few millivolts (Deckert et al., 1992). The second of these components, C2, reverses at a potential a few millivolts below that of the other components. Pharmacological experiments (Nagy, 1991; Contzen and Nagy, 1995) have suggested that the C2 is mediated viaInsP3-induced calcium release, whereas the other components possibly are mediated via cyclic nucleotide metabolism. If multiple pathways of excitation exist, then under the conditions of our flash photolysis experiment we would expect the buffering of calcium by NPE to reduce the contribution of calcium release to the physiological response relative to other mechanisms. According to the proposal of Nagy and collaborators, the early calcium-activated component of the response to the photolysis flash may, therefore, be expected to display a more negative reversal potential than the later physiological response.
Nagy and collaborators ascribe the different components of the light response to the activation of different channels by the various messenger pathways (Deckert et al., 1992). We think it is unlikely that further analysis of small differences in reversal potential will yield a definitive answer as to whether more than one channel mediates the light response. A very large increase in Cai accompanies the light response (Ukhanov and Payne, 1995), and the light-activated channels may be calcium-permeable, or the voltage-gated channels that mediate the current induced by depolarizing voltage steps may be calcium-sensitive. These factors may distort the waveform of the photocurrent close to the reversal potential (O’Day et al., 1993). A future combination of local flash photolysis of NPE with patch-clamp recording, however, may allow the direct comparison of single channels activated by released calcium and by the physiological mechanism under different conditions.
Comparison with Drosophila photoreceptors
It is noteworthy that experiments recently performed onDrosophila photoreceptors with similar methods have failed to observe any ionic current directly activated by the photolysis of caged calcium. Flash photolysis of DM-Nitrophen loaded by dialysis into dissociated Drosophila photoreceptors activates only the Na/Ca exchanger (Hardie, 1996). Instead, a physical link has been proposed between the activation of the InsP3 receptor and the opening of the putative channel protein TRP (Hardie and Minke, 1995). This difference may indicate that microvillar photoreceptors of invertebrates have evolved a variety of linkages of the PI pathway to ion channel activation.
Footnotes
This work was supported by National Institutes of Health Grant EY-07743. We thank Dr. Ian Mather of the Department of Animal Sciences, University of Maryland, College Park, for the use of the confocal microscope and Drs. Roger Hardie and Mark Gray-Keller for their comments.
Correspondence should be addressed to Dr. Richard Payne, Department of Zoology, University of Maryland, College Park, MD 20742.
Dr. Ukhanov’s present address: Institut fuer Zellphysiologie, Universitaet Potsdam, Lennestrasse 7a, D-14471 Potsdam, Germany.
REFERENCES
- 1.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5 trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo J, Johnson EC, Vergara C, Lisman JE. Light-dependent channels in excised patches of Limulus ventral photoreceptors are opened by cyclic GMP. Proc Natl Acad Sci USA. 1991;88:7938–7942. doi: 10.1073/pnas.88.18.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 4.Bloomquist BT, Shortridge RD, Schneuwly S, Pedrew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila norpA and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 5.Broadwell RD, Cataldo AM. The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. II. Axons and terminals. J Comp Neurol. 1984;230:231–248. doi: 10.1002/cne.902300208. [DOI] [PubMed] [Google Scholar]
- 6.Brown JE, Rubin LJ, Ghalayini AJ, Tarver AL, Irvine RF, Berridge MJ, Anderson RE. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984;311:160–162. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- 7.Calman BS, Chamberlain S. Distinct lobes of Limulus photoreceptors. II. Structure and ultrastructure. J Gen Physiol. 1982;80:839–862. doi: 10.1085/jgp.80.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contzen K, Nagy K. Current components stimulated by different G-proteins in Limulus ventral photoreceptor. NeuroReport. 1995;6:1905–1908. doi: 10.1097/00001756-199510020-00020. [DOI] [PubMed] [Google Scholar]
- 9.Contzen K, Richter K-H, Nagy K. Selective inhibition of the phospholipase C pathway blocks one light-activated current component in Limulus photoreceptor. J Comp Physiol. 1995;177:601–610. [Google Scholar]
- 10.Deckert A, Nagy K, Helrich CS, Stieve H. Three components in the light-induced current of the Limulus ventral photoreceptors. J Physiol (Lond) 1992;453:69–96. doi: 10.1113/jphysiol.1992.sp019219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis-Davies GCR, Kaplan JH. Nitrophenyl EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci USA. 1994;91:187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faddis MN, Brown JE. Flash photolysis of caged compounds in Limulus ventral photoreceptors. J Gen Physiol. 1992;100:547–570. doi: 10.1085/jgp.100.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fein A. Blockade of visual excitation in Limulus photoreceptor by GDP-βS. Science. 1986;232:1543–1545. doi: 10.1126/science.3487116. [DOI] [PubMed] [Google Scholar]
- 14.Fein A, Charlton JS. Enhancement and phototransduction in the ventral eye of Limulus. J Gen Physiol. 1977;69:553–569. doi: 10.1085/jgp.69.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fein A, Payne R, Corson DW, Berridge MJ, Irvine RF. Photoreceptor excitation and adaptation by inositol 1,4,5 trisphosphate. Nature. 1984;311:157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Carson JH, Morgan F, Walz B, Fein A. Three-dimensional organization of endoplasmic reticulum in the ventral photoreceptors of Limulus. J Comp Neurol. 1994;341:172–183. doi: 10.1002/cne.903410204. [DOI] [PubMed] [Google Scholar]
- 17.Frank TM, Fein A. The role of inositol phosphate cascade in visual excitation of invertebrate microvillar photoreceptors. J Gen Physiol. 1991;97:697–723. doi: 10.1085/jgp.97.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie RC. INDO-1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors. J Neurosci. 1996;16:2924–2933. doi: 10.1523/JNEUROSCI.16-09-02924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardie RC, Minke B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium. 1995;18:256–274. doi: 10.1016/0143-4160(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 20.Haugland RP. Handbook of fluorescent probes and research chemicals, 5th Ed. Molecular Probes; Eugene, OR: 1992. [Google Scholar]
- 21.Hubbard R, Wald G. Visual pigment of the horseshoe crab, Limulus polyphemus. Nature. 1960;186:212–215. doi: 10.1038/186212b0. [DOI] [PubMed] [Google Scholar]
- 22.Levy S, Fein A. Relationship between light-sensitivity and intracellular free calcium in Limulus ventral photoreceptors. J Gen Physiol. 1985;85:805–841. doi: 10.1085/jgp.85.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy S, Payne R. A lingering elevation of Cai accompanies inhibition of inositol 1,4,5 trisphosphate-induced Ca release in Limulus ventral photoreceptors. J Gen Physiol. 1993;101:67–84. doi: 10.1085/jgp.101.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisman JE. Effects of removing extracellular Ca2+ on excitation and adaptation in Limulus ventral photoreceptors. Biophys J. 1976;16:1331–1335. doi: 10.1016/S0006-3495(76)85777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mignery GA, Suedhoff TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5 trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 26.Millecchia R, Mauro A. The ventral photoreceptor cells of Limulus. III. A voltage-clamp study. J Gen Physiol. 1969;54:331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell J, Gutierrez J, Northup JK. Purification, characterization, and partial amino acid sequence of a G-protein-activated phospholipase C from squid photoreceptors. J Biol Chem. 1995;270:854–859. doi: 10.1074/jbc.270.2.854. [DOI] [PubMed] [Google Scholar]
- 28.Nagy K. Biophysical processes in invertebrate photoreceptors: recent progress and a critical overview based on Limulus photoreceptors. Q Rev Biophys. 1991;24:165–226. doi: 10.1017/s0033583500003401. [DOI] [PubMed] [Google Scholar]
- 29.O’Day PM, Johnson EC, Freeman S. A single class of light-activated channel can yield biphasic light-induced currents at Erev. Biophys J. 1993;64:A134. [Google Scholar]
- 30.Parker I, Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol (Lond) 1993;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne R, Fein A. The initial response of Limulus ventral photoreceptors to bright flashes: released calcium as a synergist to excitation. J Gen Physiol. 1986;87:243–269. doi: 10.1085/jgp.87.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne R, Flores TM. The latency of the response of Limulus photoreceptors to inositol trisphosphate lacks the calcium-sensitivity of that to light. J Comp Physiol [A] 1992;170:311–316. doi: 10.1007/BF00191419. [DOI] [PubMed] [Google Scholar]
- 33.Payne R, Ukhanov K. Latencies of calcium elevation and depolarization in Limulus ventral photoreceptors injected with GDP-βS. J Photochem Photobiol [B] 1996;35:91–95. doi: 10.1016/1011-1344(96)07302-2. [DOI] [PubMed] [Google Scholar]
- 34.Payne R, Corson DW, Fein A. Pressure injection of calcium both excites and adapts Limulus ventral photoreceptors. J Gen Physiol. 1986;88:107–126. doi: 10.1085/jgp.88.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rack M, Xhonneux-Cremers B, Schraermeyer U, Stieve H. On the Ca2+ dependence of inositol-phospholipid-specific phospholipase C of microvillar photoreceptors from Sepia officinalis. Exp Eye Res. 1994;58:659–664. doi: 10.1006/exer.1994.1063. [DOI] [PubMed] [Google Scholar]
- 36.Ranganathan R, Malicki DM, Zuker C. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- 37.Shin J, Richard EA, Lisman JE. Ca2+ is an obligatory intermediate in the excitation cascade of Limulus photoreceptors. Neuron. 1993;11:845–855. doi: 10.1016/0896-6273(93)90114-7. [DOI] [PubMed] [Google Scholar]
- 38.Ukhanov KY, Payne R. Light-induced calcium release in Limulus ventral photoreceptors as revealed by laser confocal microscopy. Cell Calcium. 1995;18:301–313. doi: 10.1016/0143-4160(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 39.Ukhanov KY, Flores TM, Hsiao H-S, Mohapatra P, Pitts CH, Payne R. Measurement of cytosolic Ca2+ concentration in Limulus ventral photoreceptors using fluorescent dyes. J Gen Physiol. 1995;105:95–116. doi: 10.1085/jgp.105.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker JW, Feeney J, Trentham DR. Photolabile precursors of inositol phosphates: preparation and properties of 1-(2-nitrophenyl)ethyl esters of myo-inositol 1,4,5 trisphosphate. Biochemistry. 1989;28:3272–3280. doi: 10.1021/bi00434a023. [DOI] [PubMed] [Google Scholar]
- 41.Wang SS-H, Augustine GJ. Confocal imaging and local photolysis of caged compounds: dual probes of synaptic function. Neuron. 1996;15:755–760. doi: 10.1016/0896-6273(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 42.Yeandle S, Spiegler JB. Light-evoked and spontaneous discrete waves in the ventral nerve photoreceptor of Limulus. J Gen Physiol. 1973;61:552–571. doi: 10.1085/jgp.61.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]