Abstract
Voltage-dependent inhibition of high voltage-activated (HVA) calcium currents by G-proteins can be transiently relieved (facilitated) by strong depolarizing prepulses. However, with respect to the physiological significance of facilitation, it remains to be established if it can be induced by action potentials (AP) in central neurons. With the use of whole-cell recordings of dissociated cholinergic basal forebrain neurons of the guinea pig, it is shown that the GTPγS-inhibited HVA currents that occur throughN-ethylmaleimide (NEM)-sensitive Gi–Go subtypes of G-proteins can be facilitated. Furthermore, although different types of HVA channels are present in these neurons, facilitation occurred mostly through disinhibition of the N-type current. On the basis of data indicating that the recovery from facilitation was relatively slow, we tested if more physiological stimuli that crudely mimicked APs (2 msec long depolarizations to 40 mV from a holding of −50 mV) potentially could induce facilitation of HVA currents inhibited by GTPγS and cholinergic agonists. Indeed, evidence is provided that the extent of facilitation is dependent on both the number and frequency of AP-like depolarizations. These results suggest that firing rates and patterns of discharge of neurons could influence their responsiveness to transmitters acting on N-type HVA calcium channels.
Keywords: facilitation, N-type channels, G-protein, action potentials, modulation, mode of discharge
It is now well established that calcium currents can be modulated by a variety of neurotransmitters (Anwyl, 1991; Hille, 1994). Most of these effects are reversible reductions of calcium currents, which may be mediated partly by fast and direct effects of G-proteins on the calcium channel itself (membrane-delimited effects) or indirectly via second messengers (Hille, 1994). Inhibition of calcium currents directly by G-proteins can occur by voltage-dependent and -independent mechanisms (Bean, 1989; Elmslie et al., 1990; Lubke and Dunlap, 1994; Diversé-Pierluissi et al., 1995; Dolphin, 1996). One particularly interesting facet of voltage-dependent reduction of calcium currents is the capacity to display transient disinhibition (also called facilitation), which is produced by strong depolarizing prepulses (Marchetti et al., 1986; Bean, 1989; Ikeda, 1991; Kasai, 1991; Lopez and Brown, 1991).
However, it remains unclear if facilitation evoked after G-protein activation can occur with action potentials. In a study using peripheral neurons (dorsal root ganglion neurons; Womack and McCleskey, 1995), only one-third of the tested cells displayed a weak (up to 20%) facilitation after depolarizations similar to action potentials (AP-like depolarizations). Moreover, in central neurons (raphe neurons;Penington et al., 1991), facilitation could not be elicited using AP-like depolarizations, whereas large depolarizations could restore calcium current inhibited by serotonin. Because it could bear on the physiological significance of facilitation in central neurons, the aim of the following study was thus to reinvestigate that question using cholinergic basal forebrain (BF) neurons as a model (see Materials and Methods).
These cells, which provide the major cholinergic input to the neocortex (Rye et al., 1984), can discharge either tonically at low frequency (up to a maximum of 15 Hz) or in a high frequency bursting mode, with up to 250 Hz within a burst (Khateb et al., 1992, 1995; Alonso et al., 1994;Griffith et al., 1994). These properties can be ascribed in part to the different types of calcium currents with which they are endowed (Allen et al., 1993; Griffith et al., 1994; S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data). In particular, cholinergic neurons display an important low voltage-activated (LVA) current that underlies the low-threshold spike (LTS), which permits them to fire on hyperpolarization with bursts of action potentials (Khateb et al., 1992; Allen et al., 1993; Griffith et al., 1994; S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data). In addition, these neurons possess at least five subtypes of high voltage-activated (HVA) calcium currents based on the particular pharmacological sensitivity to the L-type blocker nifedipine, the N-type blocker cono-GVIA (Allen et al., 1993), and the P/Q-type blocker AGA-IVA (S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data). It has not yet been determined if facilitation occurs in these cells.
The following study thus was designed to test the hypothesis that action potential-like depolarizations could trigger facilitation in BF cholinergic neurons when applied in a frequency domain corresponding to that which is known to occur for these cells. For that purpose it was necessary to determine first, the presence of G-protein-induced facilitation of calcium currents inhibited by GTPγS; second, the nature of the currents implicated in the facilitation process; third, the kinetics of the facilitation; and, finally, the ability of “physiological stimuli” such as AP-like depolarizations to promote facilitation.
MATERIALS AND METHODS
Dissociation. Slices from young guinea pigs (80–200 gm) were obtained using standard methods (Khateb et al., 1992, 1993) and dissociated with a slightly modified version of the method developed by Kay and Wong (1986). Briefly, guinea pigs were deeply anesthetized with Nembutal and decapitated. The brain was removed and rapidly transferred in cold (4°C) oxygenated (95% O2/5% CO2) physiological saline containing (in mm): 130 NaCl, 20 NaHCO3, 1.25 KH2PO4, 1.3 MgSO4, 5 KCl, 10 glucose, and 2.4 CaCl2, pH 7.35. Using a vibrotome (Campden Instruments, WPI, Berlin, Germany), two to three slices (400 μm thick) containing the basal forebrain were cut and left at room temperature for a period of 1 hr in physiological saline. The region of interest, which included the substantia innominata, the horizontal limb of the diagonal band, and the magnocellular preoptic nucleus [Paxinos and Watson (1986); seeGritti et al. (1993) for a discussion on basal forebrain cholinergic nuclei], then was dissected out (1 piece from each hemisphere) with a small razor blade. The regions of the medial septum and the vertical limb of the diagonal band that make up most of the septo-hippocampal projecting neurons were excluded. In this study, the term basal forebrain cholinergic neurons will be used to characterize those cells located within the above mentioned nuclei. For dissociations, slices first were immersed in a gassed 100% oxygenated PIPES solution containing (in mm): 120 NaCl, 5 KCl, 0.5 CaCl2, 1 MgCl2, 25 glucose, and 20 PIPES (pH-adjusted to 7.0 with NaOH). The tissue was placed in small test tubes containing an oxygenated PIPES solution with the enzyme trypsin (Sigma type XII, 0.8 mg/ml; St. Louis, MO) 2 hr at 30°C. The enzymatic reaction was stopped by rinsing the tissue with 10% goat or horse serum (Life Technologies, Basel, Switzerland) and left to rest in enzyme-free PIPES for at least another hour. Neurons were dispersed by triturating in HEPES (see below) with the help of two different sized fire-polished Pasteur pipettes. The suspension was transferred to a Cell-Tek-coated Petri dish, which was mounted on an inverted microscope (Zeiss, Oberkochen, Germany). Healthy neurons adhered to the bottom of the dish within 15 min.
Whole-cell recordings and solutions. Voltage-clamp recordings from neurons were obtained using the whole-cell version of the patch-clamp technique (Hamill et al., 1981). Patch pipettes were pulled (Sutter Instruments, Novato, CA) from borosilicate glass (1.5 o.d., 0.86 i.d.; Clark Instruments, Pangbourne, UK) and had resistances typically of 2–4 MΩ in the bath. Signals were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) and monitored on a 486 PC clone equipped with pClamp software (v. 6.0) and a 125 kHz interface (Digidata 1200, Axon Instruments). Records were low-pass-filtered at 2 or 5 kHz. Typical access resistances ranged from 4–8 MΩ, and compensation of 70–80% was used. Interferences from linear leak current, and capacitive transients were subtracted by using a P/4 protocol or by subtracting raw traces from those in the presence of cadmium (100–200 μm). The internal recording solution contained the following (in mm): 130 Cs acetate, 20 CsCl, 5 MgCl2, 5 HEPES, 3 Na2ATP, 0.2 GTPγS, 0.1 BAPTA, and 14 phosphocreatine (pH-adjusted to 7.3 with CsOH). In some experiments, GTPγS was replaced by 0.2 mm GTP-Na or 1 mm GDPβS. Neurons were perfused continuously (3 ml/min) with a HEPES medium containing (in mm): 150 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 8 glucose, and 10 HEPES (pH-adjusted to 7.3 with NaOH). To record barium currents, calcium was replaced with an equimolar concentration of barium in the HEPES solution. Calcium or barium currents were isolated by adding TTX (1 μm, Latoxan, Rosans, France), TEA (20 mm), and 4-AP (4 mm) to the HEPES medium. The effects of GTPγS could be observed when calcium or barium was used as the charge carrier. The calcium channel blocker conotoxin-GVIA (Alomone Labs, Jerusalem, Israel), muscarine, and carbachol (Sigma) were prepared in single-use aliquots and thawed on the day of the experiments. These agents were applied with a more rapid application system, which consisted of a multibarrel constructed with polyethylene tubings according to the method of Bertrand et al. (1997). All experiments were performed at room temperature (20–22°C). Measurements of the current amplitude were obtained 7–8 msec after the onset of the voltage step. Currents were activated once every 6 or 7 sec, and access resistance was checked periodically. For analysis of current kinetic slow-down, exponential fits were performed on the first 5 msec (excluding the artefactual first few 100 μsec) for the faster phase of activation and until the current reached a more steady-state level of activation for the slower phase. Statistical analysis was performed using a Student’s t test with p ≤ 0.05 chosen as statistically significant. All means are expressed as mean ± SE.
RESULTS
Facilitation of N-type calcium current
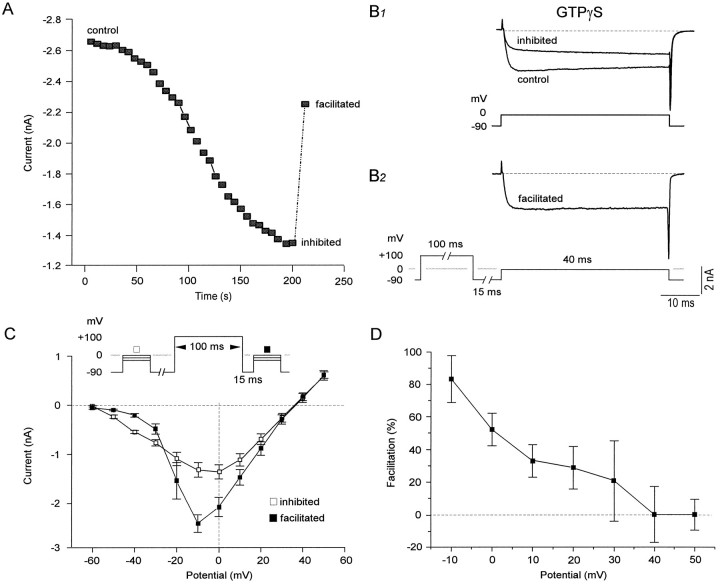
There is now strong evidence that G-proteins can block calcium currents by a direct membrane-delimited pathway (Bean, 1989; Elmslie et al., 1990; Bernheim et al., 1991, 1992; Beech et al., 1992; Hille, 1994; Herlitze et al., 1996; Ikeda, 1996). In BF cholinergic neurons, cell dialysis with the poorly hydrolyzable GTP analog GTPγS, in contrast to dialysis with GTP, produced a gradual and irreversible decrease in the HVA, but not the LVA (data not shown), calcium current. Figure 1A,B shows the reduction of the HVA barium current (evoked by a 40 msec test pulse to 0 mV from aVh of −90 mV) from −2.6 to −1.3 nA within 3 min after break-in in whole-cell mode. At the end of the reduction, the HVA current was facilitated by 84% when a 100 msec long prepulse to 100 mV was given 15 msec before the test pulse (to 0 mV fromVh −90 mV). For all neurons sampled (n = 18), 53 ± 5% of the barium current remaining in presence of GTPγS was facilitated after applying a prepulse (such as the one described above) 139 ± 19 sec after break-in. In a similar manner, 28 ± 2% (n = 29) of the calcium current was facilitated 146 ± 18 sec after break-in. Dialysis of the neurons with GTPγS also produced a slowing of the activation phase (also called “kinetic slowing”) of the barium current, which seemed relieved (data not shown) with the depolarizing prepulse (to 100 mV for 100 msec). In 10 neurons recorded in the presence of GTPγS, the activation onset of the barium current could be fit accurately with two time constants. The faster constant τ1 was 0.84 ± 0.05 msec, whereas the slower phase had a τ2 of 55.7 ± 14.2 msec. After the prepulse, τ1 was significantly decreased to 0.67 ± 0.05 msec, whereas the slower phase disappeared. Facilitation of the current flowing through calcium channels was never observed when GTPγS (200 μm) was replaced with GTP (200 μm,n = 15) or GDPβS (1 mm, n= 5). These results suggest that facilitation in BF cholinergic neurons is dependent on GTPγS and that a “tonic” G-protein-mediated inhibition of HVA currents such as that seen in other neuronal types (Ikeda, 1991; Kasai, 1991) is not present here.
Fig. 1.
Reduction of inward HVA barium current by GTPγS is voltage-dependent. A, Plot of barium current measured at the peak after break-in (control), after a maximal reduction (inhibited), and during facilitation of the current when it is preceded by a depolarizing prepulse (100 msec long to 100 mV, 15 msec before the test pulse from aVh of −90 mV). Traces are displayed inB1 and B2 to show the extent of inhibition and facilitation. C, Averaged I–V values for seven neurons obtained before (□) and 15 msec after (▪) a 100 msec prepulse to 100 mV. Notice that currents obtained after prepulses of −50 to −30 mV are reduced in size but are increased after those of more depolarized potentials. D, Plot of averaged percentage of facilitation expressed as a function of amplitude of the test pulse. Large facilitation was observed at the peak (−10 mV) current but gradually decreased to near null levels at very depolarized potentials of 40 and 50 mV.
Another method to visualize the voltage dependence of facilitation is to perform I–V values before and after a prepulse (100 msec long to 100 mV). The averaged I–V values for seven neurons obtained before and after a prepulse are shown in Figure 1C. Currents elicited at negative test pulses (−50 to −30 mV) were generally smaller when preceded by prepulses (which likely is attributable to LVA inactivation during the prepulse), but the facilitation was most important at the peak current (−10 mV) and decreased gradually thereafter for test potentials up to 50 mV. It is also noticeable for these averaged I–V values that the peak current in the presence of GTPγS seems to occur at more positive potentials (∼10 mV more) than the peak current elicited after the prepulse. As is shown in Figure 1D, voltage-dependent facilitation peaked at test potentials of −10 mV and 0 mV (83 ± 14% and 52 ± 10% of facilitation, respectively) and was null for very depolarized test pulses of 40 and 50 mV. These data suggest that the inhibition mediated by G-proteins is strongly voltage-dependent, being maximal when the current is elicited at the peak but minimal at depolarized potentials.
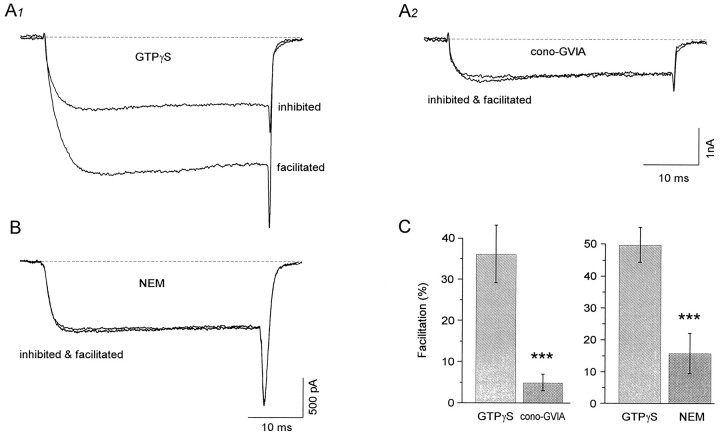
Previously it was determined pharmacologically that BF cholinergic neurons are endowed with an LVA as well as several HVA calcium currents, including L (17%), N (35%), P/Q (30%), and R (18%) types (S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data). There are indications in the literature that facilitation potentially could occur through disinhibition of most of these calcium current subtypes. For example, it was demonstrated that facilitation can occur through disinhibition of L-type current (Artalejo et al., 1992; Sculptoreanu et al., 1993a,b; Bourinet et al., 1994), N-type current (Elmslie et al., 1990; Boland and Bean, 1993;Swartz, 1993), and also P/Q-type currents (Mintz et al., 1992; Mintz and Bean, 1993; Toselli and Taglietti, 1995; Bourinet et al., 1996). However, as is shown for a representative neuron (Fig.2A), the facilitated current principally flowed through a single type of calcium channel in BF neurons. Indeed, this cell, the current flowing through calcium channels, was facilitated by 88% in control medium, but application of 400 nm cono-GVIA reduced it by 39% and completely abolished the facilitation. In 11 neurons tested, the application of 200–400 nm cono-GVIA reduced the facilitation from 36 ± 7% to 5 ± 2% (Fig. 2C). These data suggest that, in BF cholinergic neurons, facilitation occurred mostly through disinhibition of the N-type calcium current.
Fig. 2.
Facilitation of the N-type calcium current through Gi–Go G-proteins. A1, Overlay of current flowing through calcium channels maximally diminished by GTPγS (inhibited) and after a prepulse (facilitated; 100 msec to 100 mV, from aVh of −90 mV). A2, The inhibited current is reduced further by the application of the N-type inhibitor cono-GVIA (400 nm), and facilitation is abolished.B, In another cell, pre-exposure with NEM (50 μm) for 2 min before obtaining whole-cell configuration abolished facilitation (evoked by a 100 msec long prepulse to 80 mV, from a Vh of −50 mV). The mean facilitation obtained after exposure to either cono-GVIA (n = 11) or NEM (n = 8) is shown in histogram form in C.Asterisks correspond to a level of significance ofp < 0.01.
Many types of G-proteins are known to be implicated in inhibition of calcium currents; however, not all types have been shown to play a role in facilitation. In fact, pertussis and N-ethylmaleimide (NEM)-sensitive G-proteins (Gi and Go) seem to be of primary importance in mediating the voltage-dependent pathway in both peripheral (Hille, 1994) and central neurons (Foehring, 1996; Yan and Surmeier, 1996). To test if these subtypes of G-proteins also are involved in the facilitation observed in cholinergic basal forebrain neurons, we perfused NEM (50 μm; Shapiro et al., 1994a,b;Foehring, 1996; Yan and Surmeier, 1996) for 2 min before the whole-cell configuration was achieved and GTPγS dialyzed. The amount of facilitation (evoked with a 100 msec long prepulse to 100 mV) after perfusion of NEM was compared with that of control neurons recorded with the same recording solution and in the same Petri dish before NEM perfusion. Facilitation was present in all control neurons recorded (49.9 ± 5.5%, n = 15) but, in contrast, was only 15.9 ± 6.3% (n = 8) when neurons were exposed to NEM (Fig. 2B,C).
Kinetics of facilitation
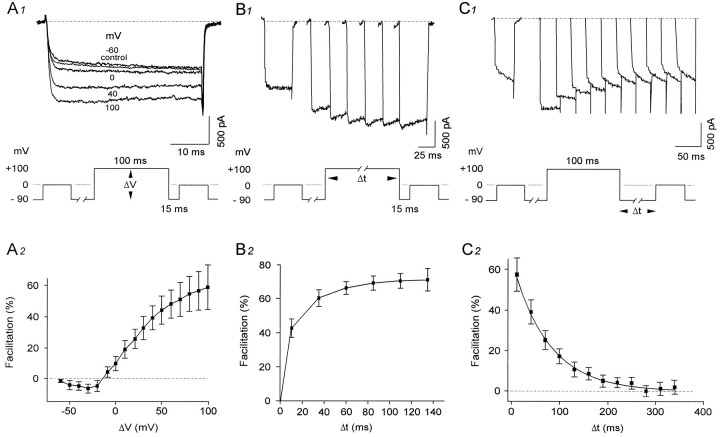
The effects of varying the amplitude or duration of the prepulse, as well as the interval between the pre- and test pulse, were verified on the test current. An example of varying the amplitude of the prepulse from −60 to 100 mV while keeping the values fixed for the duration of the prepulse (100 msec) and duration of interpulse (15 msec) is shown for a neuron in Figure 3A1. In the neuron illustrated, the current flowing through the calcium channels was decreased slightly by 6% when compared with control if it was preceded by a prepulse to −60 mV. With further depolarization to 0, 40, and 100 mV, the current was facilitated by 15, 56, and 90%, respectively. Mean results for all neurons sampled (n = 6) are shown in Figure 3A2. Although some decrease in current amplitude was observed for prepulses between −50 and −15 mV (which may be attributable partly to inactivation of the LVA current), an increase was observed thereafter for prepulses of increasing amplitude potentials up to 100 mV. The most important facilitation (57.0 ± 14%, n = 6) was obtained with prepulses to 100 mV. Increasing the duration of the prepulse also produced an increase in the extent of facilitation (Fig. 3B). The onset of facilitation was fast (<10 msec) and increased gradually to a quasi-plateau with prepulse duration of up to 140 msec.
Fig. 3.
Characteristics of facilitation. A, Facilitation is augmented by depolarizing steps of increasing amplitude. A1, Example of a barium current elicited 15 msec after a prepulse (100 msec long, from Vh −90 mV) to −60, 0, 40, and 100 mV. Currents were reduced by more negative prepulses such as that at −60 mV but increased steadily in amplitude with respect to more depolarized prepulse steps. A2, Plot of averaged facilitation (n = 6) as a function of membrane potential showing that the facilitation is augmented with depolarizing prepulses more positive than −10 mV. B, Onset of facilitation is fast. B1, Current traces in control and after prepulses of increasing duration from 10 to 135 msec (at 100 mV, from a Vh of −90 mV). Currents were facilitated with prepulses as short as 10 msec in duration and increased in size up to a duration of 135 msec (Δ t = 10, 35, 60, 85, 110, and 135 msec). B2, Plot displaying averaged facilitation (n = 6) as a function of prepulse duration. Onset of facilitation was fast and augmented until a plateau was reached near 135 msec. C, Recovery of facilitation is relatively slow.C1, Series of barium current traces in control and after interpulse intervals ranging from 10 to 280 msec (Δ t= 10, 40, 70, 100, 130, 160, 190, 220, 250, and 280 msec). Currents decreased in size as a function of augmenting time interval durations.C2, Graph displays a plot of averaged facilitation in six neurons as a function of duration of the interpulse interval. Facilitation decreased exponentially from 58 to 3% with a τ of 76 msec.
The time for recovery from facilitation was verified by varying the duration of the interval between pre- (100 msec to 100 mV) and test pulse (40 msec to 0 mV). As illustrated in Figure 3C1 for a representative neuron, facilitation steadily decreased in an exponential manner with larger duration interpulse intervals from 10 to 340 msec (from 84 to 0%). For all neurons recorded (Fig.3C2), facilitation decreased with a τ of 76 msec from 57.6 ± 8.2% to 3.3 ± 1.0% for intervals ranging from 10 to 340 msec.
AP-like depolarizations induce facilitation
Because of (1) the characteristics of the facilitation described above and (2) the extensive range of firing frequencies displayed by these neurons in situ (up to 250 Hz), we tested whether successive depolarizations more like those occurring during action potentials could elicit facilitation. Hence, series of action potential (AP)-like depolarizations (2 msec long to a test potential of 40 mV from a Vh of −50 mV) were given 15 msec before the test pulse (40 msec to 0 mV from Vh of −50 mV). A test potential of 40 mV was chosen because action potentials in these cells had an amplitude of 113 ± 18 mV (from a resting potential of approximately −60 mV, n = 57). Experiments were performed with two distinct facilitation paradigms: first, by increasing the number of AP-like depolarizations (from 2 to 20 events) applied at a frequency of 200 Hz or second, by giving 20 AP-like events at increasing frequencies (from 5 to 200 Hz). Results for a representative neuron are shown in Figure 4. Increasing the number of AP-like events from 2 to 20 increased the facilitation from 12 to 91%, respectively (Fig. 4A). In a similar manner (Fig. 4C), increasing the frequency also augmented the facilitation from 8 to 84%. For all the neurons sampled (Fig. 4B,D), increasing the number of AP-like events from 2 to 20 augmented facilitation from 19 ± 3% to 66 ± 8% (n = 13), whereas augmenting the frequency from 5 to 200 Hz produced an increase in facilitation from 10 ± 2% to 61 ± 4% (n = 11). Therefore, when more physiological stimulations crudely mimicking action potentials were used, the inhibition of the current could be partially reversed even by as few as two AP-like events.
Fig. 4.
Action potential-like depolarizations can induce facilitation. A, Example of barium current traces evoked by a test pulse that was preceded by a series of 2 msec long depolarizing pulses to 40 mV from a Vh of −50 mV. Increasing the number of AP-like depolarizations from 2 to 20 at a frequency of 200 Hz increased facilitation. The inset shows that, for the cell in A and C, applying a prepulse (100 msec duration to 40 mV from a Vh of −50 mV) before the test pulse facilitated the current by 112%. B, Graph displays a mean averaged increase in facilitation (n = 11) as a function of augmented number of AP-like events. C, For the same neuron shown in A, facilitation also can be augmented when the frequency of AP-like depolarizations is increased.D, The plot displays a mean average increase in facilitation (n = 11) when frequency of AP-like events is augmented.
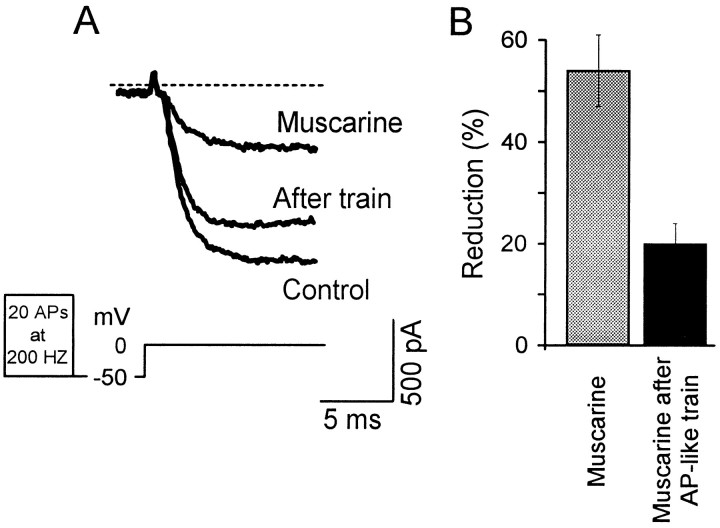
To verify further the physiological significance of facilitation, we tested whether the inhibition of the current by cholinergic agonists (20 μm carbachol or 10 μm muscarine; Allen and Brown, 1993) could be relieved by using AP-like depolarizations (20 AP-like events at 200 Hz). In the example shown in Figure5A, the application of 10 μmmuscarine caused a 70% reduction of the current. After the AP-like train, this inhibition was only 22%. In four neurons tested, the application of carbachol or muscarine reduced the current flowing through calcium channels by 54 ± 7%; the reduction was only 20 ± 4% after AP-like trains (Fig. 5B). When the facilitation elicited in the presence of cholinergic agonists was quantified in a similar manner to that obtained with the GTPγS (facilitated current/inhibited current), the facilitation was 83 ± 27%.
Fig. 5.
Action potential-like depolarizations induce facilitation of current inhibited by cholinergic agonists.A, Example of barium current traces evoked by a test pulse in control conditions, its inhibition during the application of 10 μm muscarine, and the facilitation of the current when 20 AP-like events were given at a frequency of 200 Hz. Recordings were performed using GTP instead of GTPγS in the recording pipette.B, Summary of results (n = 4) obtained using same protocol as in A.
DISCUSSION
Three important findings of this study are that, in basal forebrain cholinergic neurons, (1) more than one-half of the G-protein-inhibited HVA calcium current (in presence of GTPγS or a cholinergic agonist) can be disinhibited (facilitated) by action potential-like prepulses, (2) the facilitation is induced mainly through disinhibition of the N-type current (whereas L- and P/Q-type currents were not significantly involved), and (3) Gi–Go subtypes of G-proteins are implicated in this coupling.
Cell identification
The cholinergic nature of the vast majority of cells used in this study was estimated on the basis of a parallel study (S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data) in which it was shown that 80% of BF-dissociated neurons with a soma diameter greater than 25 μm were ChAT-positive and thus cholinergic, whereas the remaining 20% were noncholinergic and thus likely GABAergic (Freund and Meskenaite, 1992; Gritti et al., 1993). Furthermore, when these large dissociated neurons were selected for recordings in current-clamp mode, they were shown to have the capacity to fire in low-threshold bursts that were similar in every respect to those of identified ChAT-positive BF neurons in basal forebrain slices (Khateb et al., 1992). Therefore, in this area, selection of neurons based on size offers a reasonably accurate method of choosing their phenotypic identity (cholinergic vs noncholinergic). It must be stressed that the characteristics of facilitation were relatively similar for all neurons recorded. Hence, facilitation that could have been recorded in a small number of large-sized noncholinergic neurons must be similar to that observed in cholinergic neurons.
Mechanisms of facilitation
It is now well established that membrane-delimited G-proteins mediate inhibition of calcium currents in a variety of preparations (Hille, 1994). For BF cholinergic neurons, one study has shown a muscarinic m2 receptor-mediated reduction of HVA calcium currents via G-protein activation (Allen and Brown, 1993). However, the voltage dependency of this inhibition was not assessed. Here we present evidence that G-protein activation reduces HVA, but not LVA, currents of BF cholinergic neurons. Furthermore, it is shown that only a portion of this inhibition can be reversed or facilitated with depolarizing prepulses, suggesting that the inhibition can be both dependent and independent of voltage. Short pre-exposures of NEM, a sulfhydryl-alkylating agent known to block pertussis-sensitive Gi–Go (Shapiro et al., 1994a,b; Foehring, 1996; Yan and Surmeier, 1996), significantly reduced facilitation. This suggests that, as in peripheral neurons (Bean, 1989; Elmslie et al., 1990; Beech et al., 1992; Hille, 1994), Gi–Gosubtypes of G-proteins also may play an important role in voltage-dependent inhibition of central neurons. The inhibition of the HVA current as well as the “kinetic slowing” produced by G-proteins may be the result of a shift in the activation threshold to more depolarized potentials (Bean, 1989; Boland and Bean, 1993; Golard and Siegelbaum, 1993).
Disinhibition of N-type current
The current involved in facilitation in the present study seems to be carried principally through N-type cono-GVIA-sensitive channels and not through L, P, and Q types. There are indications that facilitation potentially can occur through different types of HVA calcium channels. For example, the α1A (corresponding to P/Q-type currents), and α1B (N-type) calcium channel subunits (Bourinet et al., 1994, 1996;Herlitze et al., 1996) expressed in cell lines or in oocytes, as well as N- and P-type currents in situ (Mintz et al., 1992; Mintz and Bean, 1993; Toselli and Taglietti, 1995), display agonist- or GTPγS-induced inhibition and facilitation. However, in a similar manner to what is observed in BF cholinergic neurons, facilitation in cortical neurons also occurs principally through disinhibition of the N current (Swartz, 1993), although other types of calcium currents (T, L, P, and R) are known to be present (Lorenzon and Foehring, 1995; Markram et al., 1995).
Facilitation by action potential-like depolarizations
Kinetics of facilitation onset was relatively rapid (<10 msec) in BF cholinergic neurons and comparable with other neuronal cell types either with cell dialysis with GTPγS or application of a cholinergic agonist. In contrast, recovery from inhibition was slower (τ = 76 msec) than that obtained for G-protein-dependent facilitation found in peripheral neurons (30–60 msec; Elmslie et al., 1990; Golard and Siegelbaum, 1993). This slower recovery from inhibition is important, because this criterion partly will determine the “summation” of facilitation that is shown to occur during AP-like stimulations. In fact, calcium current facilitation was shown to be substantial when AP-like depolarizations were used as prepulses, thus supporting our original hypothesis that facilitation in central neurons can be induced by “physiological stimuli.” Two aspects are of a particular interest in these results. First, the relationship between the frequency of the AP-like prepulses (2 msec in duration and 90 mV in amplitude) with the amount of facilitation indicates that at low frequencies (10–20 Hz) the amount of facilitation is quite small (∼15%), whereas at higher frequencies (150–200 Hz) a high degree of facilitation (60%) is attained. This possibly could explain the weak level of facilitation (20%) observed with AP-like depolarizations for a minority of DRG cells (Womack and McCleskey, 1995) and the near absence of facilitation (5%) found in raphe neurons (Penington et al., 1991). In both of these studies, however, only low frequency trains were used (up to 20 Hz), which could explain the weak level of facilitation obtained with respect to the present study (see Fig.4D for comparison). A lack of facilitation also was demonstrated in another study performed on sympathetic neurons using AP waveforms (Toth and Miller, 1995). However, whereas APs were applied at moderate frequencies (40–75 Hz), only a small number of APs (7) were given at this rate, indicating that this parameter is also a contributing factor in facilitation. The second interesting aspect concerns the number of AP-like depolarizations given at a high frequency that permit facilitation. Indeed, when tested at 200 Hz, it is noteworthy that two APs were enough to yield a 20% facilitation, whereas five APs could produce more than one-half (∼35%) of the maximal reachable level of facilitation (60%). This latter result could be particularly relevant for cells such as the BF cholinergic neurons, which have the ability to fire in high frequency bursts (see below).
Physiological significance
Identified cholinergic neurons in vitro and presumed cholinergic neurons in vivo have been shown to fire either tonically at a rather low frequency (up to 15 Hz in vitroand 25 Hz in vivo) or in high frequency bursts (up to 13 AP/burst at 250 Hz) (Khateb et al., 1993, 1995; Alonso et al., 1994,1996; Szymusiak, 1995; Nuñez, 1996; A. Khateb, unpublished data). Because of the characteristics of facilitation described here and the implication of the N current in the calcium-dependent potassium conductance that produces the slow after-hyperpolarization (AHP) and accommodation (S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data), it is most probable that voltage-dependent inhibition of the N current (by a transmitter) would greatly reduce the slow AHP when the neuron fires near rest (at a low frequency). One can speculate, however, that when the neuron is hyperpolarized and thus brought at the level where the LTS can trigger high-frequency bursting (Khateb et al., 1992; Alonso et al., 1996), the blocking effect on the consecutive AHP (which also depends importantly on the N current; (S. Williams, M. Serafin, M. Mühlethaler, L. Bernheim, unpublished data) would fade away, thereby allowing the burst to terminate. Such a mechanism could allow neurotransmitters that block AHPs (through the reduction of N-type current) to promote and shape the bursting pattern in BF cholinergic neurons. Thus, because of the mechanism of facilitation, the blocking intensity of the AHP by a transmitter would be dependent on the state of excitability of the neuron.
Footnotes
This work was supported by grants from the Swiss Fonds National to L.B. and M. M. S.W. was supported by a postdoctoral fellowship from the Medical Research Council of Canada. We thank Danièle Machard for her technical assistance and Gilbert von Kaenel for his help with graphics.
Correspondence should be addressed to Dr. M. Mühlethaler, Département de Physiologie, Centre Médical Universitaire, 1 rue Michel-Servet, 1211 Genève 4, Switzerland.
REFERENCES
- 1.Allen TG, Brown DA. M2 muscarinic receptor-mediated inhibition of the Ca2+ current in rat magnocellular cholinergic basal forebrain neurones. J Physiol (Lond) 1993;466:173–189. [PMC free article] [PubMed] [Google Scholar]
- 2.Allen TG, Sim JA, Brown DA. The whole-cell calcium current in acutely dissociated magnocellular cholinergic basal forebrain neurones of the rat. J Physiol (Lond) 1993;460:91–116. doi: 10.1113/jphysiol.1993.sp019461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso A, Faure MP, Beaudet A. Neurotensin promotes oscillatory bursting behavior and is internalized in basal forebrain cholinergic neurons. J Neurosci. 1994;14:5778–5792. doi: 10.1523/JNEUROSCI.14-10-05778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso A, Khateb A, Fort P, Jones BE, Mühlethaler M. Differential oscillatory properties of cholinergic and non-cholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8:169–182. doi: 10.1111/j.1460-9568.1996.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 5.Anwyl R. Modulation of vertebrate neuronal calcium channels by transmitters. Brain Res Rev. 1991;16:265–281. doi: 10.1016/0165-0173(91)90010-6. [DOI] [PubMed] [Google Scholar]
- 6.Artalejo CR, Rossie S, Perlman RL, Fox AP. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992;358:63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- 7.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 8.Beech DJ, Bernheim L, Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992;8:97–106. doi: 10.1016/0896-6273(92)90111-p. [DOI] [PubMed] [Google Scholar]
- 9.Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 10.Bernheim L, Mathie A, Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1992;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertrand D, Buisson B, Krause RM, Bertrand S (1997) Electrophysiology: a method to investigate the functional properties of ligand-gated channels. J Recept Signal Transduct Res, in press. [DOI] [PubMed]
- 12.Boland LM, Bean BP. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993;13:516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourinet E, Charnet P, Tomlinson WJ, Stea A, Snutch TP, Nargeot J. Voltage-dependent facilitation of a neuronal α1c L-type calcium channel. EMBO J. 1994;13:5032–5039. doi: 10.1002/j.1460-2075.1994.tb06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourinet E, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diversé-Pierluissi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 16.Dolphin AC. Facilitation of Ca2+ current in excitable cells. Trends Neurosci. 1996;19:35–43. doi: 10.1016/0166-2236(96)81865-0. [DOI] [PubMed] [Google Scholar]
- 17.Elmslie KS, Zhou W, Jones SW. LHRH and GTPγS modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- 18.Foehring RC. Serotonin modulates N- and P-type calcium currents in neocortical pyramidal neurons via a membrane-delimited pathway. J Neurophysiol. 1996;75:648–659. doi: 10.1152/jn.1996.75.2.648. [DOI] [PubMed] [Google Scholar]
- 19.Freund TF, Meskenaite V. Gamma-aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci USA. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golard A, Siegelbaum SA. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J Neurosci. 1993;13:3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith WH, Taylor L, Davis MJ. Whole-cell and single-channel currents in guinea-pig basal forebrain neurons. J Neurophysiol. 1994;71:2359–2376. doi: 10.1152/jn.1994.71.6.2359. [DOI] [PubMed] [Google Scholar]
- 22.Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- 23.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 24.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 25.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol (Lond) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 28.Kasai H. Tonic inhibition and rebound facilitation of a neuronal calcium channel by a GTP-binding protein. Proc Natl Acad Sci USA. 1991;88:8855–8859. doi: 10.1073/pnas.88.19.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kay AR, Wong RKS. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986;16:227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- 30.Khateb A, Mühlethaler M, Alonso A, Serafin M, Jones BE. Cholinergic nucleus basalis neurons display the capacity for rhythmic bursting activity mediated by low-threshold calcium spikes. Neuroscience. 1992;51:489–494. doi: 10.1016/0306-4522(92)90289-e. [DOI] [PubMed] [Google Scholar]
- 31.Khateb A, Fort P, Alonso A, Jones BE, Mühlethaler M. Pharmacological and immunohistochemical evidence for serotonergic modulation of cholinergic nucleus basalis neurons. Eur J Neurosci. 1993;5:541–547. doi: 10.1111/j.1460-9568.1993.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 32.Khateb A, Fort P, Serafin M, Jones BE, Mühlethaler M. Rhythmical bursts induced by NMDA in guinea-pig cholinergic nucleus basalis neurones in vitro. J Physiol (Lond) 1995;487:623–638. doi: 10.1113/jphysiol.1995.sp020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez HS, Brown AM. Correlation between G-protein activation and reblocking kinetics of Ca2+ channel currents in rat sensory neurons. Neuron. 1991;7:1061–1068. doi: 10.1016/0896-6273(91)90350-9. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzon NM, Foehring RC. Characterization of pharmacologically identified voltage-gated calcium channel currents in acutely isolated rat neocortical neurons. I. Adult neurons. J Neurophysiol. 1995;73:1430–1442. doi: 10.1152/jn.1995.73.4.1430. [DOI] [PubMed] [Google Scholar]
- 35.Luebke JI, Dunlap K. Sensory neurons N-type calcium currents are inhibited by both voltage-dependent and -independent mechanisms. Pflügers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti C, Carbone E, Lux HD. Effects of dopamine and noradrenaline on Ca2+ channels of cultured sensory and sympathetic neurons of chick. Pflügers Arch. 1986;406:104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- 37.Markram H, Helm JP, Sakmann B. Dendritic calcium transient evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol (Lond) 1995;485:1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mintz M, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 39.Mintz M, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 40.Nuñez A. Unit activity of rat basal forebrain neurons: relationship to cortical activity. Neuroscience. 1996;72:757–766. doi: 10.1016/0306-4522(95)00582-x. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 42.Penington NJ, Kelly JS, Fox AP. A study of the mechanism of Ca2+ current inhibition produced by serotonin in rat dorsal raphe neurons. J Neurosci. 1991;11:3594–3609. doi: 10.1523/JNEUROSCI.11-11-03594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and non-cholinergic components employing combined retrograde-tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 44.Sculptoreanu A, Rotman E, Takahashi M, Scheuer T, Catterall WA. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel α1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1993a;90:10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993b;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J Neurosci. 1994a;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro MS, Wollmuth LP, Hille B. Angiotensin II inhibits calcium and M current channels in rat sympathetic neurons via G-proteins. Neuron. 1994b;12:1319–1329. doi: 10.1016/0896-6273(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 48.Swartz KJ. Modulation of Ca2+ channels by protein kinase C in rat central and peripheral neurons: disruption of G-protein-mediated inhibition. Neuron. 1993;11:305–320. doi: 10.1016/0896-6273(93)90186-u. [DOI] [PubMed] [Google Scholar]
- 49.Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- 50.Toselli M, Taglietti V. Muscarine inhibits high-threshold calcium currents with two distinct modes in rat embryonic hippocampal neurons. J Neurophysiol. 1995;483:347–365. doi: 10.1113/jphysiol.1995.sp020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toth TP, Miller RJ. Calcium and sodium currents evoked by action potential waveform in rat sympathetic neurones. J Physiol (Lond) 1995;485:43–57. doi: 10.1113/jphysiol.1995.sp020711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Womack MD, McCleskey EW. Interaction of opioids and membrane potential to modulate Ca2+ channels in rat dorsal root ganglion neurons. J Neurophysiol. 1995;473:1793–1798. doi: 10.1152/jn.1995.73.5.1793. [DOI] [PubMed] [Google Scholar]
- 53.Yan Z, Surmeier JD. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]