Abstract
The motor pattern that drives coordinated movements of swimmerets in different segments during forward swimming characteristically begins with a power-stroke by the most posterior limbs, followed progressively by power-strokes of each of the more anterior limbs. To explain this caudal-to-rostral progression, the hypothesis was proposed that the neurons that drive the most posterior swimmerets are more excitable than their more anterior counterparts, and so reach threshold first.
To test this excitability-gradient hypothesis, I used carbachol to excite expression of the swimmeret motor pattern and used tetrodotoxin (TTX), sucrose solutions, and cutting to block the flow of information between anterior and posterior segments. I showed that the swimmeret activity elicited by carbachol is like that produced when the swimmeret system is spontaneously active and that blocking an intersegmental connective uncoupled swimmeret activity on opposite sides of the block.
When anterior and posterior segments were isolated from each other, the frequencies of the motor patterns expressed by anterior segments were not slower than those expressed by posterior segments exposed to the same concentrations of carbachol. This result was independent of the concentration of carbachol applied and of the number of segmental ganglia that remained connected. When TTX was used to block information flow, the motor patterns produced in segments anterior to the block were significantly faster than those from segments posterior to the block.
These observations contradict the predictions of the excitability-gradient hypothesis and lead to the conclusion that the hypothesis is incorrect.
Keywords: pattern-generation, locomotion, coordination, motor control, excitability-gradient
The cycles of movements made by its swimmerets when a crayfish moves forward usually begin with a power-stroke by the most posterior pair of swimmerets, followed progressively by power-strokes by each of the more anterior pairs of swimmerets (see Fig. 1). How does this orderly sequence of movements in different segments come about?
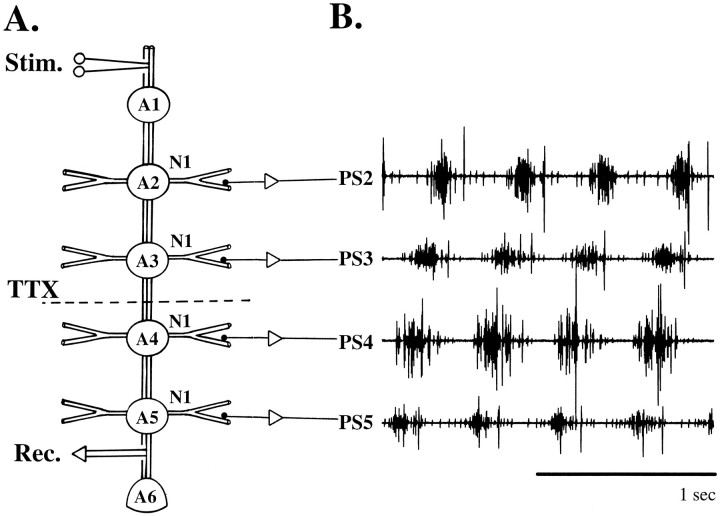
Fig. 1.
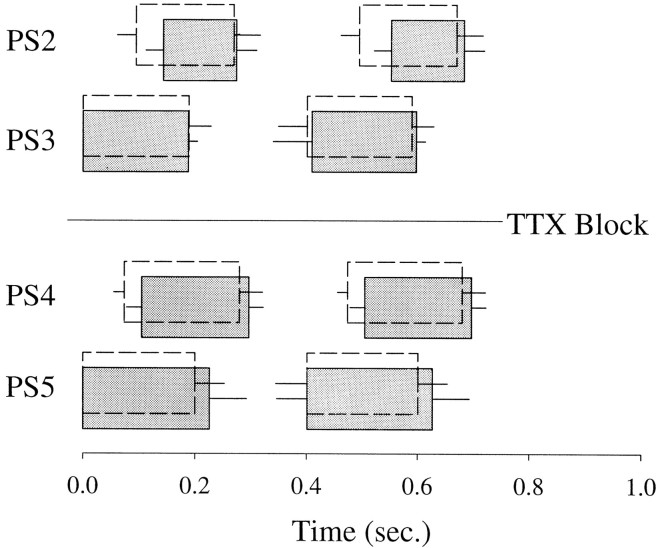
A, A diagram of the ventral nerve cord of the crayfish that shows the spatial relations of the six abdominal ganglia (A1..A6), the interganglionic connectives in which coordinating information flows, and the segmentally reiterated nerves (N1) that innervate the pair of swimmerets in each segment. The broken line (TTX) marks the position of the well that contained TTX to interrupt impulse conduction in the A3–A4 connectives. In some experiments, impulse conduction in the connectives was tested by stimulating the connective anterior to A1 (Stim.) while recording from the A5–A6 connective (Rec.). B, Impulses in axons of PS motor neurons were recorded with pin electrodes on the posterior branches of the N1 nerve that projected from ganglia A2, A3, A4, and A5 (PS2..PS5). When the swimmeret system was active, coordinated bursts of impulses were produced by PS axons from each ganglion (PS2..PS5). Each burst in axons from A5 preceded those in more anterior ganglia by a substantial phase difference. This phase difference causes the characteristic metachronal beating of the swimmerets in the intact animal.
Each swimmeret is innervated by its own set of motor neurons (Davis, 1968, 1969; Mulloney et al., 1990; Sherff and Mulloney, 1996) that are part of its pattern-generating module (Murchison et al., 1993). These modules are located in individual segmental ganglia (see Fig. 1) that are separated spatially but linked together by interganglionic connectives in which thousands of axons run (Wiersma, 1958). The motor neurons themselves do not send axon collaterals to neighboring ganglia (Mulloney et al., 1990; Sherff and Mulloney, 1996), but coordinating interneurons do conduct information to neighboring modules about the activities of modules in each segment (Hughes and Wiersma, 1960; Stein, 1971, 1974; Mulloney et al., 1993). The connections between neighboring ganglia are enough to permit a pair of ganglia to generate well coordinated swimmeret activity, with the same phase relations that they would exhibit in an intact animal (Paul and Mulloney, 1986). Thus, the nervous system that controls swimmeret movements seems to be a concatenated series of equivalent modules. If this is so, why does the most posterior pair of swimmerets normally begin each cycle of movements?
Ikeda and Wiersma (1964) tested the possibility that the most posterior ganglion of the ventral nerve cord was a unique trigger for these movements, and showed that this was not so. They then proposed that “a pacemaker (occurs) in each half ganglion, connected … with the next anterior pacemaker. Within this series, the pacemaker cells show a progressively diminishing excitability.” This hypothesis predicts that if an identical stimulus is provided separately to each “pacemaker,” the more anterior pacemakers would respond less to this stimulus and would oscillate at slower rates.
The discoveries that cholinergic agonists could elicit the normal swimmeret motor pattern from isolated crayfish ventral nerve cords (Chrachri and Neil, 1993) and that the period of this pattern was sensitive to the concentration of nicotinic agonists (Braun and Mulloney, 1993) made it possible to test this hypothesis experimentally. In this paper, I show that carbachol does excite the swimmeret system in a dose-dependent manner and that the motor patterns that carbachol elicits have the same structure as does spontaneously generated activity that drives coordinated swimmeret movements. To test the responses of the “pacemakers” in anterior and posterior ganglia, I isolated them by blocking impulse traffic in a middle connective and compared the frequencies of bursts in power-stroke (PS) axons in anterior and posterior ganglia. Contrary to the prediction of the hypothesis, burst frequencies in anterior ganglia were not slower than those in posterior ganglia. I conclude that there is no demonstrable gradient of excitability and that this hypothetical mechanism cannot explain the normal phase constancy of swimmeret movements.
MATERIALS AND METHODS
Animals. Crayfish, Pacifastacus leniusculus, were obtained from local suppliers and kept in aerated freshwater aquaria at 15°C. The normal saline solution contained 195 mm NaCl, 5.36 mm KCl, 2.6 mm MgCl2, 13.5 mmCaCl2, and 10 mm Tris-maleate buffer, at pH 7.4. Carbachol (Research Biochemicals International, Natick, MA) was dissolved in this saline. Carbachol solutions were bath-applied to isolated abdominal nerve cords by replacing the saline in the dish. Once bath-application of carbachol began, the preparation was allowed to reach a steady-state response before data were recorded. The order of presentation of different concentrations of carbachol was varied in each experiment.
Electrophysiological recordings. Before dissection, animals were chilled on ice. The abdominal nerve cord, which included ganglia A1 through A6 (see Fig. 1), was dissected free and pinned out in a dish under aerated saline. In this species, only A2 through A5 innervate swimmerets used for locomotion. The sheath around each of these four ganglia was opened surgically to facilitate diffusion of carbachol into the tissue. Action potentials in axons of PS motor neurons from ganglia A2 through A5 were recorded extracellularly from the posterior branch of the first segmental nerve, N1, the nerve that innervates each swimmeret (Stein, 1971; Sherff and Mulloney, 1997). In some experiments, I also recorded return-stroke (RS) activity from the anterior branches of N1. Signals were amplified, stored on videotape, and later displayed on a wide-bandwidth chart recorder or on a computer, using Axoscope (Axon Instruments, Foster City, CA).
Blocks of interganglionic connectives. To interrupt impulse traffic in selected interganglionic connectives, I either cut the connective between two ganglia or placed a Vaseline well containing 0.5 μm tetrodotoxin (TTX) (Calbiochem, La Jolla, CA) or isotonic sucrose on the connective. The sucrose- and TTX-blocks could be reversed by replacing the blocking solutions with normal saline.
To test the effectiveness of each block in some experiments, I placed a stimulating electrode on the T5–A1 connective anterior to A1 and a recording electrode on the A5–A6 connective and recorded impulses in axons that ran the length of the abdominal nerve cord. When the block was effective, these impulses failed to reach the recording electrode.
Data analysis. To describe the activity of the swimmeret system quantitatively, the times at which each burst of impulses started and stopped were measured from the chart record with a digitizing tablet. The periods, durations, and phases of these bursts were calculated from these times (Mulloney and Hall, 1987). The instantaneous frequency of each burst was calculated as the inverse of its period. Descriptive statistics of each of these parameters were calculated for each experiment.
Because a burst of impulses in PS motor neurons in A5 (PS5) normally signals the start of each cycle of swimmeret output, and PS bursts in each ganglion have the same period (Ikeda and Wiersma, 1964; Mulloney et al., 1990), when the ventral cord was intact the period of the motor pattern was calculated as the time from the start of one PS5 burst to the start of the next PS5 burst. The latency of each PS burst in more anterior ganglia was measured as the difference between the time at which that burst began and the time at which the preceding PS5 burst began. The phase of each burst in other ganglia was then defined as the ratio of the latency of that burst to the period of that cycle (Mulloney and Hall, 1987). When ganglia had been isolated from A5, the most posterior ganglion of the chain nonetheless initiated each cycle of swimmeret activity (Ikeda and Wiersma, 1964). Therefore, the PS bursts from this most posterior remaining ganglion were used to define the period of the cycle and as the reference for calculating phases of activity in more anterior ganglia.
To assess the independence of activity in ganglia anterior and posterior to a block on the connective, I also calculated the phases of PS bursts anterior to the block relative to A5 bursts. If impulse activity was blocked effectively, I predicted that there would be no preferred phase and that the distribution of phases would not be significantly different from a random distribution.
Statistical procedures. The deviation from randomness of phases of PS bursts measured across a blocked connective were estimated with Kolmogorov–Smirnov tests (Zar, 1984). Pairedt tests or ANOVAs were used to assess differences of frequencies, durations, and phases of PS bursts recorded from ganglia on opposite sides of a blocked connective or recorded under different conditions.
RESULTS
To test the hypothesis that posterior components of the swimmeret system were inherently more excitable than were anterior components, I first established that the response of the system to bath-applied carbachol had the same properties as other forms of excitation and described the dose–response relationship of carbachol and excitation. Then I bathed anterior and posterior segments of the ventral nerve cord in the same concentrations of carbachol and compared the properties of the activity produced in these different segments when they were separated from one another.
Carbachol excited the swimmeret system
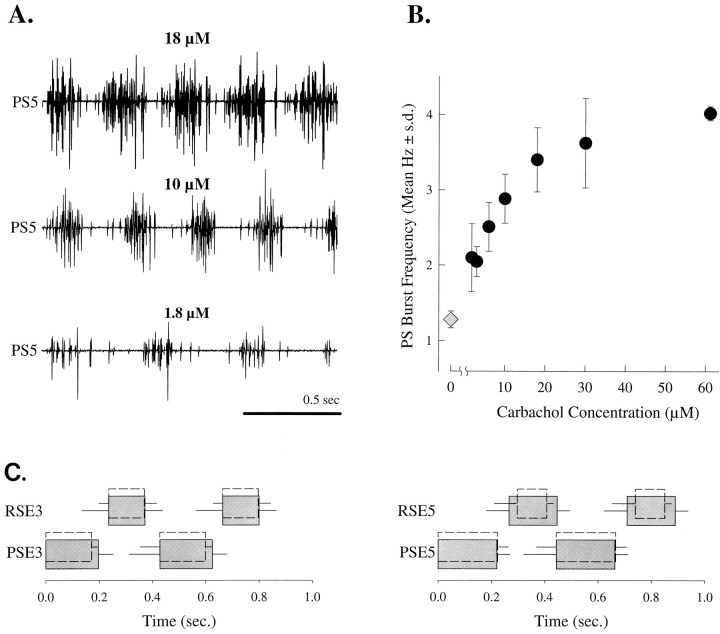
Our earlier work had shown that carbachol could elicit expression of coordinated swimmeret activity (Fig. 1) from a previously silent ventral nerve cord (Braun and Mulloney, 1993). In active preparations, both the intensities and frequencies of PS bursts increased as the concentration of carbachol in the bath rose (Fig.2A). The threshold concentration for this increase in burst frequency was ∼1.0 μm carbachol, and the response saturated at ∼50 μm (Fig.2B). ED50 of this response was 7.8 μm.
Fig. 2.
Carbachol excited the swimmeret system in a dose-dependent manner. A, Recordings of PS bursts from one preparation exposed to three concentrations of carbachol.B, The frequency of PS bursts increased as the concentration of carbachol increased. The solid circlesand error bars mark frequencies (mean ± SD) of PS bursts in preparations bathed in the given concentrations of carbachol (n = 3 preparations); the diamondmarks frequencies (mean ± SD) of PS bursts expressed spontaneously in saline (n = 3 preparations).C, The structures of the motor patterns produced spontaneously by each ganglion were similar to those produced when the system was excited by carbachol. The periods, durations, and latencies of the spontaneous patterns were normalized to the mean period of the carbachol-induced patterns. Shaded solid boxes show bursts recorded in carbachol; open dotted boxes show normalized spontaneous bursts. Each box shows the mean duration (+SD) of bursts in the named motor axons. The secondPSE5 and PSE3 box begins at the mean period (−SD) of the pattern. Each RSE5 and RSE3 box begins at the mean latency (−SD) of those bursts after the PSE burst, and so illustrates the mean phase difference between PSE and RSE activity to the swimmeret.
The periods of swimmeret motor patterns elicited by carbachol were shorter than those produced spontaneously by isolated nerve cords (p < 0.01), but the structures of the patterns were similar. To compare the structures of the motor patterns produced under these two conditions, I recorded both PS excitor (PSE) and RS excitor (RSE) bursts from A3 and A5 and normalized the durations of the bursts produced spontaneously to the shorter period of the carbachol-induced activity. PSE bursts alternated precisely with RSE bursts under both conditions (Fig. 2C). The phases of RSE bursts relative to PSE bursts in the same ganglion were similar under both conditions (t test; p > 0.58). The relative durations of PSE3, PSE5, and RSE3 bursts were similar under both conditions, but RSE5 bursts were relatively longer in carbachol (t test; p = 0.023).
The increase in burst intensity had two components (Fig.2A): individual units fired more frequently during a burst and new, larger units were recruited. In an intact crayfish, each of these features—the higher burst frequency, the increased spike frequency, and the recruitment of additional units—would contribute to more powerful swimmeret beating and faster forward movement. Therefore, the action of carbachol was like the action of the transmitters with which the crayfish CNS excites the swimmeret system and regulates the force of swimmeret movements.
Excitation by carbachol was persistent and reversible
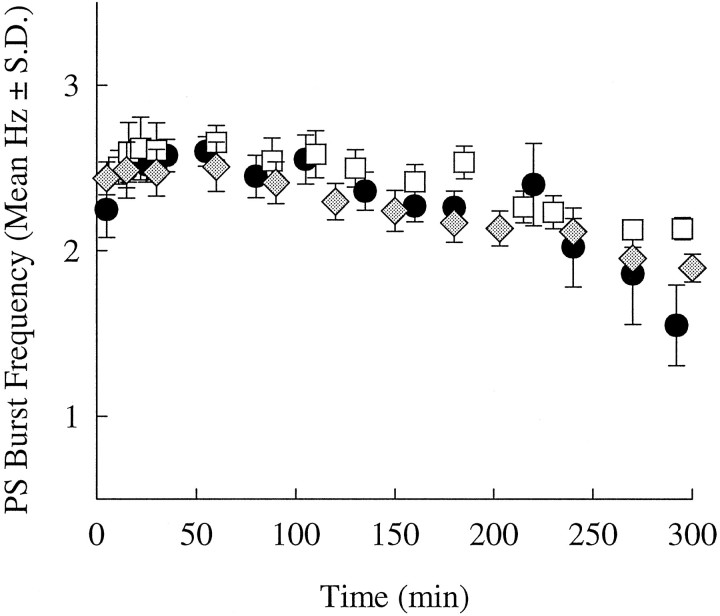
Many receptors for neurotransmitters desensitize soon after they bind their authentic transmitter or its analogs. Desensitization would complicate the interpretation of experiments that assumed that the response to an applied dose of carbachol was independent of time. To measure the time-dependence of the swimmeret system’s response to bath-applied carbachol, I applied 6 μm carbachol to three otherwise inactive preparations and recorded their activity periodically for 5 hr (Fig. 3). Each preparation reached a peak burst frequency within 30 min and continuously expressed the normal swimmeret motor pattern for the rest of the experiment. As time passed, the burst frequency of each preparation declined slowly but was still ∼80% of the maximum at 250 min.
Fig. 3.
Time course of responses to 6 μmcarbachol. The three different symbols mark data from three experiments. Each point is the burst frequency (mean ± SD) of a sample of PSE activity recorded at that time.
When carbachol solutions in the bath were replaced with normal saline, the system returned to a resting state similar to that expressed at the start of the experiment (data not shown). The time course of this recovery depended on the concentration of carbachol that had been used; higher concentrations took longer to wash out.
Blocking interganglionic axons uncoupled swimmeret circuits on opposite sides of the block
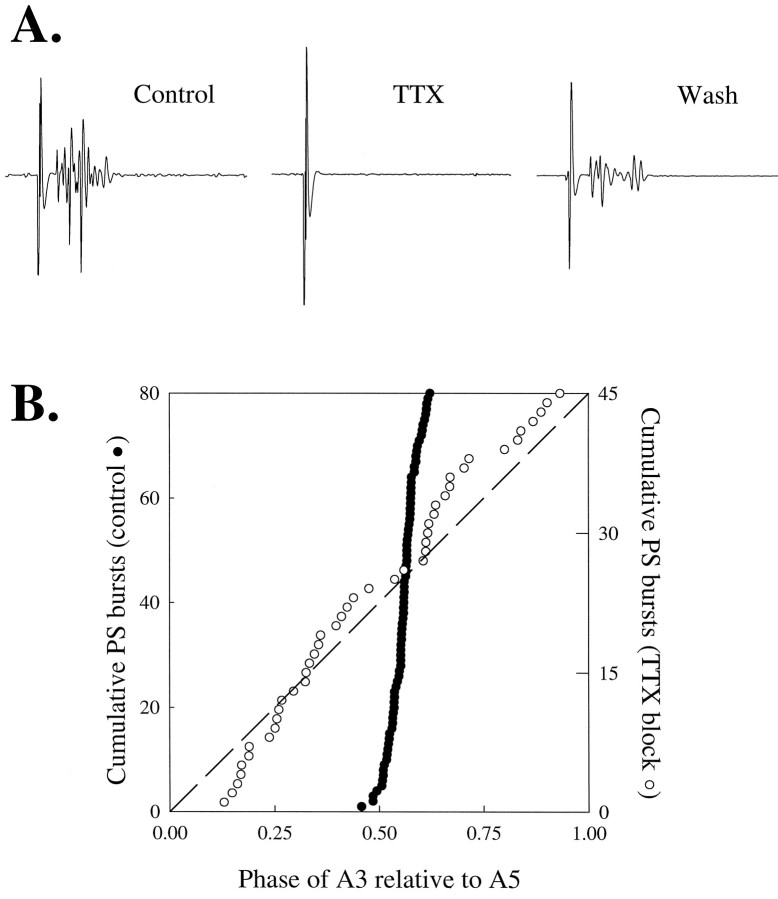
To test the hypothesis that swimmeret pacemakers in different segments had “progressively diminishing excitabilities,” I first had to halt conduction of information between segments. I used three methods to do this: sucrose blocks, TTX blocks, and cutting of selected interganglionic connectives. In each sucrose- and TTX-block experiment, I tested the effectiveness of the block by using a Kolmolgorov–Smirnov procedure (Zar, 1984) to see whether subsequent PS bursts on opposite sides of the block still had a preferred phase relationship (Fig.4). If ganglia were actually uncoupled, I predicted that PS bursts on opposite sides of the block would occur independently, and if the periods of these bursts differed, then the distribution of the phases of the anterior bursts relative to the posterior bursts would not be significantly different from a random distribution. In a cumulative–frequency plot of phases (Fig. 4B), randomly distributed phases would fall along a straight diagonal line. In each experiment included in this analysis, PS bursts across the block showed no preferred phase relationship (Fig.4B); the probability that the observed phases were not randomly distributed was <0.05. When the connectives were cut, all impulse traffic between the ganglia halted, and the thoroughness of the cut was apparent because the cut ends pulled apart.
Fig. 4.
Tetrodotoxin (TTX) applied locally to selected interganglionic connectives blocked impulse conduction and uncoupled swimmeret activity on opposite sides of the block. A, Impulses triggered by stimulation of the connectives anterior to A1 were recorded by electrodes on the connective between A5 and A6 under control conditions (compare Fig. 1), but disappeared when a TTX well was placed on the connectives between A3 and A4. This block was washed away by replacing the TTX solution with saline. B, When the TTX block was in place, the predictable phase-lag of PS bursts in ganglia anterior to the block disappeared. Solid circles show phases of A3 bursts from one preparation in each interval of A5 activity under control conditions; open circles are phases of the same A3 recorded when the TTX block was in place. In these cumulative–frequency plots, phases of the PS bursts were sorted and then plotted in ascending order. The data of A andB are from the same preparation.
Uncoupled anterior pacemakers ran slightly faster than did posterior pacemakers
To compare frequencies of swimmeret motor patterns produced by three-ganglion chains from anterior and posterior segments, I first recorded the response of the intact nerve cord (A1..A6) to 6 μm carbachol by recording simultaneously the activity in PS nerves of A2, A3, A4, and A5. I then cut the A3–A4 connective (n = 4 experiments) or blocked the A3–A4 connectives with isotonic sucrose (n = 3 experiments) or TTX (n = 4 experiments).
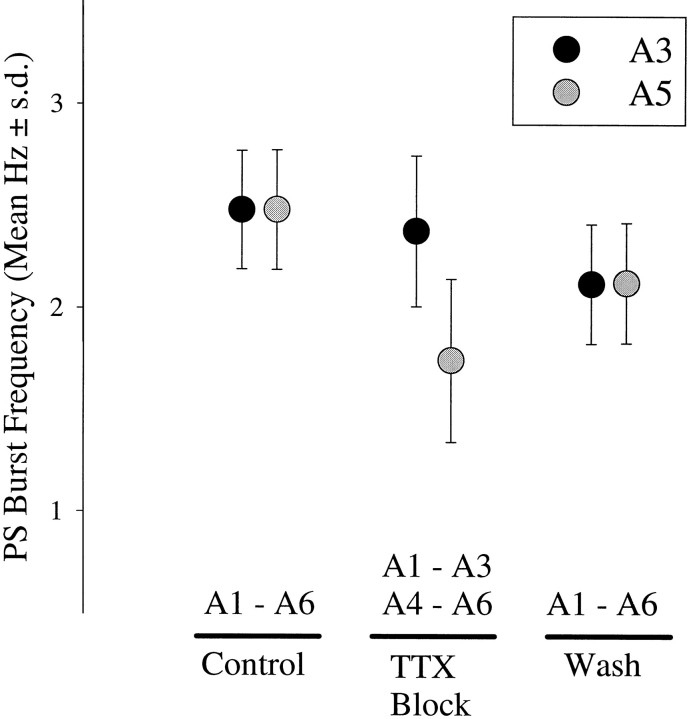
When anterior and posterior ganglia on opposite sides of a block continued to generate regular PS bursts, the phases of these bursts were independent (Fig. 4B), and mean frequency of bursts in posterior ganglia was lower than the mean in anterior ganglia (Fig. 5). Comparison of the mean frequency of A1..A3 (2.19 ± 0.37 Hz) with that of A4..A6 (2.00 ± 0.38 Hz) for all 11 experiments indicated that they were not different (pairedt test; p = 0.133; n = 11). The same comparison of the four TTX-block experiments (Fig. 5) indicated that the A1..A3 frequencies were faster than the A4..A6 frequencies (paired t test; p = 0.004).
Fig. 5.
When impulse traffic in the A3–A4 connective was blocked, the frequencies of PS bursts in posterior ganglia were not higher than those of PS bursts in anterior ganglia when both were excited by 6 μm carbachol. Circles mark mean PS frequency, ± SD, recorded before the block was imposed (A1 - A6), while it was in place (A1 - A3, A4 - A6), and after the block was washed out (A1 - A6). n = 4 experiments.
The downward trend of the mean frequencies observed in the course of these experiments (Fig. 5) is partially accounted for by the time needed to establish and later reverse the TTX blocks. The time between the “Control” and the “Wash” data averaged 253 min for the experiments included in Figure 5, and the drop in frequency observed here is similar to that seen in Figure 3 at 250 min.
To determine whether these results depended on the strength of excitation or on the numbers of connected ganglia, I tested the responses of two-ganglion chains to different concentrations of carbachol (Fig. 6). I first recorded the response of the intact nerve cord (A1..A6) to a particular concentration of carbachol by recording simultaneously from PS nerves of A2, A3, A4, and A5. I then blocked the A1–A2, A3–A4, and A5–A6 connectives with TTX (n = 4 experiments). In each of these experiments, PS bursts across the A3–A4 block showed no preferred phase relationship, and impulse traffic was blocked effectively (Fig.4A).
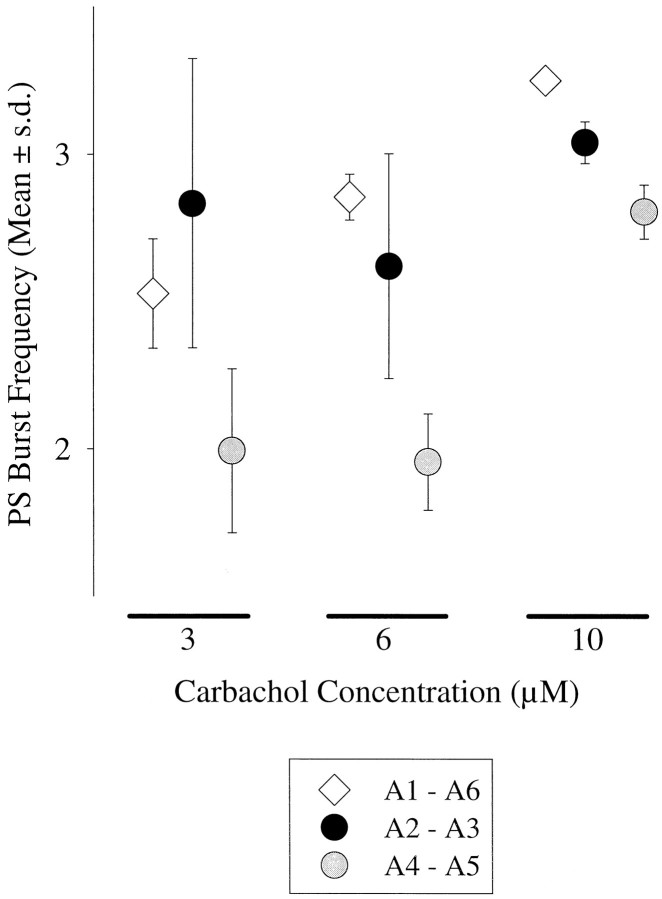
Fig. 6.
Independent of the strength of excitation applied, frequencies of PS bursts from isolated anterior pairs of ganglia (A2 - A3) were as high as or higher than those from isolated posterior pairs (A4 - A5). Pairs of ganglia were isolated by placing TTX blocks on the A1–A2, A3–A4, and A5–A6 connectives (compare Fig. 1). Circles mark the frequencies (mean ± SD) expressed when the two pairs were isolated. Diamonds mark the mean frequencies expressed by these same preparations in the specified concentrations of carbachol before the TTX blocks were imposed.
The responses of each preparation to 3 μm, 6 μm, or 10 μm carbachol were recorded. At each concentration, the responses of anterior and posterior pairs were similar (Fig. 6); anterior pairs had a higher mean frequency of PS bursts than did posterior pairs (paired t test: for 3 μm, p = 0.197; for 6 μm,p = 0.146; for 10 μm, p = 0.041)
Swimmeret motor patterns generated in isolated pairs of ganglia had the same structure as patterns generated by complete abdominal nerve cords
Each ganglion that innervates a pair of swimmerets can respond directly to excitation by command interneurons (Wiersma and Ikeda, 1964; Stein, 1971, 1973) and to pharmacological excitation (Paul and Mulloney, 1986; Acevedo et al., 1994; Braun and Mulloney, 1995). Any two pairs of neighboring ganglia can produce coordinated PS bursts with the same phase relations observed in the intact nerve cord (Paul and Mulloney, 1986), but previous work did not consider differences in the periods of activity in different ganglia.
I compared PS activity in A2 and A3 or in A4 and A5 produced under control conditions, with their activity produced when the A3–A4 connective was blocked (Fig. 7). The mean period of PS3 bursts in the isolated anterior ganglia A2..A3 was not significantly different from the mean period recorded from the intact cord. The durations of PS3 bursts were nearly identical in both conditions (Fig.7). PS2 bursts were slightly shorter in the isolated A1..A3 chains than they were in the controls (p = 0.212).
Fig. 7.
The structures of swimmeret motor patterns produced by isolated pairs of ganglia (A2..A3, A4..A5) compared with patterns produced by intact abdominal nerve cords exposed to 6 μm carbachol. Periods of isolated A2..A3 patterns were not significantly different from those of the intact system and are not normalized in this figure. Periods of isolated A4..A5 patterns were longer than those of the intact system, and the periods, durations, and latencies of bursts from isolated PS5 and PS4 recordings have been normalized to the mean period of PS5 bursts in intact nerve cords.Open dotted boxes show bursts from intact nerve cords;solid gray boxes show bursts from isolated pairs of ganglia. Each pattern begins with a burst in PS5 or PS3; the second PS5 and PS3 burst begins at the mean period (−SD) of the recorded motor pattern. Each box shows the mean duration (+SD) of PS bursts from the ganglion indicated. Each PS4 andPS2 box begins at the mean latency (−SD) of those bursts after the start of the contemporary PS5 orPS3 burst.
The mean period of PS5 bursts was significantly longer than controls when the A3–A4 connective was blocked (ANOVA; p = 0.031). To compare PS4 and PS5 activity under these two conditions, I normalized their burst durations measured with the block in place relative to the period recorded under control conditions with the cord intact (Fig. 7). These relative burst durations from blocked cords were not significantly different from normal controls (PS4,p = 0.102; PS5, p = 0.058). Both the phases of PS2 relative to PS3 and the phases of PS4 relative to PS5 were somewhat longer than those measured from the intact cord (Fig. 7). These differences were not statistically significant (p > 0.08), but they might be a sign that coordinating information spans more than one neighboring ganglion (Stein, 1971).
DISCUSSION
The excitability-gradient hypothesis as originally proposed (Ikeda and Wiersma, 1964) has the virtues of being intuitively plausible and potentially testable. One of its features, the existence of segmental pacemakers, has been demonstrated to be correct: each ganglion has two pattern-generating modules that can function independently as pacemakers (Murchison et al., 1993). Are posterior pacemakers more excitable, as the hypothesis states? The necessary conditions for a direct test of the hypothesis are that the excitation given to each be the same and that the different pacemakers be able to function in isolation, so that differences in their intrinsic frequencies or responses to excitation could be expressed. The experiments reported here meet those conditions. The structures of the motor patterns produced by anterior and posterior ganglia when the connective between them was blocked were similar to the structures produced under normal conditions (Fig. 7), but there was no longer any preferred phase (Fig.4B).
When pacemakers in different ganglia were isolated, I observed no evidence that the posterior pacemakers were more excitable than anterior ones (Figs. 5, 6). None of the preparations in which I could demonstrate that anterior pacemakers were uncoupled from posterior ones (e.g., Fig. 4B) had posterior motor patterns significantly faster than their anterior counterparts. Both sets responded to all concentrations of carbachol that I tested (Fig. 6). The mean frequencies of PS bursts in anterior ganglia tended to be faster than those from posterior ganglia (Fig. 5), and I did not observe systematic differences in the time of onset of their responses. These results contradict the hypothesis in a critical way: the posterior pacemakers are not more excitable than their anterior counterparts.
Before pharmacological methods for exciting the swimmeret system were discovered (Mulloney et al., 1987; Braun and Mulloney, 1993; Chrachri and Neil, 1993), the only method for testing the hypothesis was stimulation of command neurons (Wiersma and Ikeda, 1964; Stein, 1971,1973), a technically demanding procedure that is difficult to control satisfactorily. Stein (1973) did use cut command interneurons stimulated at different frequencies to excite differentially ganglia anterior and posterior to the cut. In those experiments, coordinating tracts between the anterior and posterior ganglia were preserved. He demonstrated that phases of activity across the excitation boundary would shift as the relative intensities of excitation changed, but the characteristic posterior-to-anterior metachronal progression was always expressed. A conceptually similar experiment constructed gradients of excitation by bathing different ganglia in different concentrations of carbachol (Braun and Mulloney, 1995) or proctolin (Acevedo et al., 1994), and it also showed that the normal metachronal progression was always expressed. These results imply that the intersegmental coordinating circuits of the swimmeret system are strong enough to override differences in the excitation given to different segments, but these earlier experiments did not test the excitability-gradient hypothesis explicitly because the modules in different ganglia were still linked together.
“Pacemaker cells” in the swimmeret system
The original statement of the excitability-gradient hypothesis speaks of pacemaker cells in each segment (Ikeda and Wiersma, 1964). Since then, we have learned that each swimmeret has its own pattern-generating module of motor neurons and nonspiking local interneurons, and that these modules are coordinated by a separate circuit of coordinating interneurons (for review, see Mulloney et al., 1993). There is no evidence for “pacemaker cells” in these modules like those envisioned in the hypothesis; the cycle of PS–RS activity is best accounted for by the properties of a circuit of nonspiking local interneurons that drive the motor neurons (Perkel and Mulloney, 1974; Paul and Mulloney, 1986; Skinner et al., 1994; Sharp et al., 1996; Sherff and Mulloney, 1996). A contemporary restatement of the hypothesis would refer instead to a gradient of excitability of these modules in different segments. The results of the experiments reported here also contradict this restatement: if the modules in different segments are quantitatively different, the difference is of a sign opposite to that predicted. The only evidence of systematic differences that I observed indicates that anterior modules might be more excitable than posterior modules (Figs. 5, 6).
Gradients of excitability or excitation in other motor systems
The idea that metachronal coordination was a consequence of segmental gradients of excitability or excitation has been proposed separately to explain normal coordination of forward swimming in leeches (Friesen and Pearce, 1993), crayfish (Ikeda and Wiersma, 1964), fish (Matsushima and Grillner, 1972), and tadpoles (Tunstall and Roberts, 1994). In leeches, the swimming muscles of each segment are innervated by a segmental ganglion, and the normal progression of contractions is anterior to posterior; however, chains of posterior ganglia produce motor output at higher frequencies than do anterior chains excited in the same way (Pearce and Friesen, 1985), and so this excitability gradient cannot explain the normal phase progression. The concatenated intersegmental coordinating circuits of the leech override the apparent segmental differences in excitability. I note that like the differences in leeches, the slightly higher frequencies of PS bursts in anterior ganglia (Fig. 6) also oppose the normal progression of swimmeret movements.
Swimming in fish and tadpoles is also accomplished by an anterior-to-posterior sequence of contractions by segmental muscles, and gradients of excitability (Matsushima and Grillner, 1992) and excitation (Tunstall and Roberts, 1994) have been proposed as mechanisms to account for this progression. From experiments like those described here, Cohen (1987a,b) has reported differences in the responses of anterior and posterior segments of the lamprey cord to uniform exposure to glutamate analogs, and differences in their responses to bath-applied serotonin. Although individual preparations were reported to maintain stable differences in the frequencies of anterior and posterior segments, these data showed a wide scatter and have been interpreted to show both the presence and absence of segmental gradients of excitability (Williams et al., 1990; Grillner et al., 1993; Sigvardt, 1993). In newly hatched tadpoles (stage 37/38),Tunstall and Roberts (1994) demonstrated segmental differences in the strength of tonic excitation during swimming and similar differences in the strengths of postsynaptic potentials from local interneurons. The performance of the spinal circuits of the tadpole continues to mature beyond the stage at which this gradient occurs, however. At stage 37/38, the tadpole cannot maintain a constant intersegmental phase as frequency changes (Tunstall and Sillar, 1993), and we do not know whether the excitation gradient is still present once more mature performance has appeared. I conclude that although excitability gradients are perennially attractive, it is still uncertain whether they contribute directly to production of phase-constant metachronal movements in any system that has been investigated.
Where is carbachol’s site of action in the swimmeret system?
This analysis has interpreted the changes in swimmeret activity caused by carbachol as the result of direct action on swimmeret modules in each ganglion. One alternative site of action would be on some of the command interneurons that excite the swimmeret system (Wiersma and Ikeda, 1964; Stein, 1971; Acevedo et al., 1994). The axons of each of these interneurons run the length of the abdominal nerve cord, and each ganglion can respond directly to stimulation of these axons (Wiersma and Ikeda, 1964; Stein, 1973). If this alternative site of action is correct, Figure 5 implies that in each ganglion the command axon responds in the same way to carbachol and that it excites the swimmeret module in the same way. If a segmental gradient of excitation is normally provided by the command axons, this gradient somehow must be neatly counteracted by a reverse gradient of their sensitivity to carbachol.
Because axons of many primary sensory afferents that enter each abdominal ganglion are cholinergic (Barker et al., 1972), a second alternative site of action would be on neurons postsynaptic to these afferents. There are hundreds of afferents from each swimmeret (Killian and Page, 1992), and perturbation of individual swimmerets during normal movements will cause changes in the coordinated activity of the system (West et al., 1979); therefore, this alternative is possible. The same constraints that apply to an action through command axons, however, apply to an action through segmental sensory pathways: the local effects of activating these sensory pathways in each ganglion must be quantitatively similar. I cannot distinguish these three alternatives from the evidence available, but I think that a direct action on the swimmeret modules is most probable because of the constraints that would be necessary for actions through sensory pathways or command axons to yield these results.
Swimmeret modules do not differ in excitability: the coordinating circuit is polarized
The observation that swimmeret modules in more posterior ganglia do not express motor patterns with higher frequencies than those expressed in more anterior ganglia is a fundamental contradiction of the excitability-gradient hypothesis. This, together with the observation that differential excitation of modules at the anterior end of the abdominal nerve cord did not reverse the usual posterior-to-anterior progression of PS bursts (Braun and Mulloney, 1995), leads to the conclusion that the hypothesis is incorrect. Instead, I think that the normal phase progression is caused by an inherent polarity or asymmetry in the intersegmental coordinating circuit (Skinner et al., 1997). This coordinating circuit thus imposes on a set of otherwise similar modules a particular sequence of PS firing, a sequence characteristic of the metachronal movements of swimmerets during forward swimming.
Footnotes
This work was supported by National Science Foundation Grants IBN 92-22470 and IBN 95-14889. I thank Wendy Hall for assistance at every level and thank Hisaaki Namba and Frances Skinner for critically reading this manuscript. Len White and Joe Hudson contributed data to Figure 2.
Correspondence should be addressed to Brian Mulloney, Section of Neurobiology, Physiology, and Behavior, Storer Hall, University of California, Davis, Davis, CA 95616-8755.
REFERENCES
- 1.Acevedo LD, Hall WM, Mulloney B. Proctolin and excitation of the crayfish swimmeret system. J Comp Neurol. 1994;345:612–627. doi: 10.1002/cne.903450411. [DOI] [PubMed] [Google Scholar]
- 2.Barker DL, Herbert E, Hildebrand JG, Kravitz EA. Acetylcholine and lobster sensory neurones. J Physiol (Lond) 1972;226:205–229. doi: 10.1113/jphysiol.1972.sp009981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun G, Mulloney B. Cholinergic modulation of the swimmeret system in crayfish. J Neurophysiol. 1993;70:2391–2398. doi: 10.1152/jn.1993.70.6.2391. [DOI] [PubMed] [Google Scholar]
- 4.Braun G, Mulloney B. Coordination in the crayfish swimmeret system: differential excitation causes changes in intersegmental phase. J Neurophysiol. 1995;73:880–885. doi: 10.1152/jn.1995.73.2.880. [DOI] [PubMed] [Google Scholar]
- 5.Chrachri A, Neil DM. Interaction and synchronization between two abdominal motor systems in crayfish. J Neurophysiol. 1993;69:1373–1383. doi: 10.1152/jn.1993.69.5.1373. [DOI] [PubMed] [Google Scholar]
- 6.Cohen AH. Intersegmental coordinating system of the lamprey central pattern generator for locomotion. J Comp Physiol [A] 1987a;160:181–193. [Google Scholar]
- 7.Cohen AH. Effects of oscillator frequency on phase-locking in the lamprey central pattern generator. J Neurosci Methods. 1987b;21:113–125. doi: 10.1016/0165-0270(87)90109-9. [DOI] [PubMed] [Google Scholar]
- 8.Davis WJ. The neuromuscular basis of lobster swimmeret beating. J Exp Zool. 1968;168:363–378. [Google Scholar]
- 9.Davis WJ. Neural control of swimmeret beating in the lobster. J Exp Biol. 1969;50:99–117. doi: 10.1242/jeb.50.1.99. [DOI] [PubMed] [Google Scholar]
- 10.Friesen WO, Pearce RA. Mechanisms of intersegmental coordination in leech locomotion. Semin Neurosci. 1993;5:41–47. [Google Scholar]
- 11.Grillner S, Matsushima T, Wadden T, Tegnér J, El Manira A, Wallén P. The neurophysiological bases of undulatory locomotion in vertebrates. Semin Neurosci. 1993;5:17–28. [Google Scholar]
- 12.Hughes GM, Wiersma CAG. The coordination of swimmeret movements in the crayfish, Procambarus clarkii. J Exp Biol. 1960;37:657–670. [Google Scholar]
- 13.Ikeda K, Wiersma CAG. Autogenic rhythmicity in the abdominal ganglion of the crayfish: the control of swimmeret movements. Comp Biochem Physiol. 1964;12:107–115. doi: 10.1016/0010-406x(64)90053-2. [DOI] [PubMed] [Google Scholar]
- 14.Killian KA, Page CH. Mechanosensory afferents innervating the swimmerets of the lobster. II. Afferents activated by hair deflection. J Comp Physiol [A] 1992;170:501–508. doi: 10.1007/BF00191465. [DOI] [PubMed] [Google Scholar]
- 15.Matsushima T, Grillner S. Neural mechanisms of intersegmental coordination in lamprey: local excitability changes modify the phase coupling along the spinal cord. J Neurophysiol. 1992;67:373–388. doi: 10.1152/jn.1992.67.2.373. [DOI] [PubMed] [Google Scholar]
- 16.Mulloney B, Hall WM. The PD programs: a method for the quantitative description of motor patterns. J Neurosci Methods. 1987;19:47–59. doi: 10.1016/0165-0270(87)90020-3. [DOI] [PubMed] [Google Scholar]
- 17.Mulloney B, Acevedo LD, Bradbury AG. Modulation of the crayfish swimmeret rhythm by octopamine and the neuropeptide proctolin. J Neurophysiol. 1987;58:584–597. doi: 10.1152/jn.1987.58.3.584. [DOI] [PubMed] [Google Scholar]
- 18. Mulloney B, Acevedo LD, Chrachri A, Hall WM, Sherff CM. A confederation of neural circuits: control of swimmeret movements by a modular system of pattern generators. Frontiers in crustacean neurobiology Wiese K, Krenz WD, Tautz J, Reichert H, Mulloney B. 1990. 439 447 Birkhauser; Basel: Verlag. [Google Scholar]
- 19.Mulloney B, Murchison D, Chrachri A. Modular organization of pattern-generating circuits in a segmental motor system: the swimmerets of crayfish. Semin Neurosci. 1993;5:49–57. [Google Scholar]
- 20.Murchison D, Chrachri A, Mulloney B. A separate local pattern-generating circuit controls the movements of each swimmeret in crayfish. J Neurophysiol. 1993;70:2620–2631. doi: 10.1152/jn.1993.70.6.2620. [DOI] [PubMed] [Google Scholar]
- 21.Paul DH, Mulloney B. Intersegmental coordination of swimmeret rhythms in isolated nerve cords of crayfish. J Comp Physiol [A] 1986;158:215–224. [Google Scholar]
- 22.Pearce RA, Friesen WO. Intersegmental coordination of the leech swimming rhythm. I. Roles of cycle period gradient and coupling strength. J Neurophysiol. 1985;54:1444–1459. doi: 10.1152/jn.1985.54.6.1444. [DOI] [PubMed] [Google Scholar]
- 23.Perkel DH, Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science. 1974;185:181–183. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- 24.Sharp AA, Skinner FK, Marder E. Mechanisms of oscillation in dynamic clamp constructed two-cell half-center circuits. J Neurophysiol. 1996;76:867–883. doi: 10.1152/jn.1996.76.2.867. [DOI] [PubMed] [Google Scholar]
- 25.Sherff CM, Mulloney B. Tests of the motor neuron model of the local pattern-generating circuits in the swimmeret system. J Neurosci. 1996;16:2839–2859. doi: 10.1523/JNEUROSCI.16-08-02839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherff CM, Mulloney B (1997) Passive properties of swimmeret motor neurons. J Neurophysiol, in press. [DOI] [PubMed]
- 27.Sigvardt KA. Intersegmental coordination in the lamprey central pattern generator for locomotion. Semin Neurosci. 1993;5:3–16. doi: 10.1016/0166-2236(92)90006-t. [DOI] [PubMed] [Google Scholar]
- 28.Skinner FK, Kopell N, Marder E. Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. J Comput Neurosci. 1994;1:69–88. doi: 10.1007/BF00962719. [DOI] [PubMed] [Google Scholar]
- 29.Skinner FK, Kopell N, Mulloney B (1997) How does the crayfish swimmeret system work? Insights from nearest neighbor coupled oscillator models. J Comput Neurosci, in press. [DOI] [PubMed]
- 30.Stein PSG. Intersegmental coordination of swimmeret motor neuron activity in crayfish. J Neurophysiol. 1971;34:310–318. doi: 10.1152/jn.1971.34.2.310. [DOI] [PubMed] [Google Scholar]
- 31.Stein PSG. The relationship of interlimb phase to oscillator activity gradients in crayfish. In: Stein RB, Pearson KG, Smith RS, Redford JB, editors. Control of posture and locomotion. Plenum; New York: 1973. pp. 621–623. [Google Scholar]
- 32.Stein PSG. Neural control of interappendage phase during locomotion. Am Zool. 1974;14:1003–1016. [Google Scholar]
- 33.Tunstall MJ, Sillar KT. Physiological and developmental aspects of intersegmental coordination in Xenopus embryos and tadpoles. Semin Neurosci. 1993;5:29–40. [Google Scholar]
- 34.Tunstall MJ, Roberts A. A longitudinal gradient of synaptic drive in the spinal cord of Xenopus embryos and its role in coordination of swimming. J Physiol (Lond) 1994;474:393–405. doi: 10.1113/jphysiol.1994.sp020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West L, Jacobs G, Mulloney B. Intrasegmental proprioceptive influences on the period of the swimmeret rhythm in crayfish. J Exp Biol. 1979;82:289–301. doi: 10.1242/jeb.82.1.281. [DOI] [PubMed] [Google Scholar]
- 36.Wiersma CAG. On the functional connections of single units in the central nervous system of the crayfish, Procambarus clarkii. J Comp Neurol. 1958;110:421–471. doi: 10.1002/cne.901100306. [DOI] [PubMed] [Google Scholar]
- 37.Wiersma CAG, Ikeda K. Interneurons commanding swimmeret movements in the crayfish, Procambarus clarkii. Comp Biochem Physiol Physiol. 1964;12:509–525. doi: 10.1016/0010-406x(64)90153-7. [DOI] [PubMed] [Google Scholar]
- 38.Williams TL, Sigvardt KA, Kopell N, Ermentrout GB, Remler MP. Forcing of coupled nonlinear oscillators: studies of intersegmental coordination in the lamprey locomotor central pattern generator. J Neurophysiol. 1990;64:862–871. doi: 10.1152/jn.1990.64.3.862. [DOI] [PubMed] [Google Scholar]
- 39.Zar JH. Biostatistical analysis. Prentice-Hall; Englewood Cliffs, NJ: 1984. [Google Scholar]