Abstract
To obtain a better understanding of the cortical representation of bimanual coordination, we measured regional cerebral blood flow (rCBF) with 15O-labeled water and positron emission tomography (PET). To detect areas with changes of rCBF during bimanual finger movements of different characteristics, we studied 12 right-handed normal volunteers. A complete session consisted of three rest scans and six scans with acoustically paced (1 Hz) bimanual, mirror, or parallel sequential finger movements. Activation of the right dorsal premotor area (PMd) extending to the posterior supplementary motor area (SMA) was significantly stronger during the parallel movements than during the mirror sequential movements (p < 0.05, at cluster level with correction for multiple comparisons). To determine whether these cortical areas truly represented bimanual coordination, a different group of nine normal volunteers was studied with a different task. Subjects performed acoustically paced (2 Hz) abduction–adduction movements of the index finger, making right only, left only, and bimanual mirror and parallel movements. Activation of the posterior SMA and right PMd was significantly greater during the parallel movements than during the bimanual mirror movements or the unimanual movements of either hand (p < 0.01, with anatomical constraint). Thus, the posterior SMA and right PMd appear to be related to the bimanual coordination of finger movements.
Keywords: regional cerebral blood flow, sequential finger movements, positron emission tomography, supplementary motor area, premotor cortex, bimanual coordination
The neuroanatomical basis of coupling of bimanual coordination is poorly understood. Because distal hand movement is controlled mainly by the contralateral hemisphere (Brinkman and Kuypers, 1972, 1973), there needs to be interhemispheric coupling. Inter- and intrahemispheric interconnections among primary motor cortex, premotor cortex, and supplementary motor area (SMA) suggest that interhemispheric connections may be mediated primarily by the SMA (Rouiller et al., 1994). Lesions of the SMA disrupt bimanually coordinated movement (Brinkman, 1981, 1984; Freund, 1990), but whether this is discoordination of the muscle activation pattern or failure to program or execute movement strategies, which would be more complex with bimanual interactions, remains unclear (Donoghue and Sanes, 1994). To depict the neuroanatomical substrate for bimanual coordination, we measured regional cerebral blood flow (rCBF) with15O-labeled water and positron emission tomography (PET) during bimanual, sequential finger movements. The comparison of bimanual and unimanual movements by the functional neuroimaging technique is made difficult by the overlap of the cortical regions representing the right and left unimanual movements. Because it is well known that sequential finger movements activate the primary and nonprimary motor cortex bilaterally (Rao et al., 1993; Shibasaki et al., 1993; Sadato et al., 1996), a simple subtraction method cannot be applied. Instead, we used detection of changes in rCBF in accordance with differences in characteristics of the bimanual movements. Because previous neurological and animal studies suggest that the “default” state for bimanual control produces mirror movements (Brinkman, 1981,1984; Chan and Ross, 1988), the areas that showed the greatest activation during the nonmirror bimanual movements are likely to have some role in bimanual coordination. We also used simpler bimanual and unimanual movements to determine whether these particular areas were more activated during bimanual movements than during unimanual movements, and whether the activation was sequence-dependent.
MATERIALS AND METHODS
The experiment consisted of two tasks in a total of 21 subjects, all of whom were right-handed according to the Edinburgh inventory (Oldfield, 1971). The protocol was approved by the Institutional Review Board, and all subjects gave their written informed consent for the study. A small plastic catheter was placed in the cubital vein of each subject’s left arm for injection of the radioisotope. The subjects lay in a supine position with their eyes closed and patched and their heads immobilized with an elastic band and sponge cushions.
Experiment 1: bimanual movements with different characteristics. We studied 12 normal volunteers, all men, aged 19–25 years (mean, 22.3 years). Each subject had nine consecutive PET scans, with a 10 min interval between scans. A complete session consisted of three rest scans and six scans with acoustically paced, bimanual, sequential finger movements in mirror and parallel conditions (Table 1). Subjects were trained before the PET scans to perform the sequences without difficulty. For the rest scans, subjects lay quietly, listening to a metronome sounding at a frequency of 1 Hz. No attempt was made to control the subjects’ thought content or attention during rest. Both hands were placed in a prone position on a flat board. For the movement scans, subjects briskly and precisely tapped the board with the fingers of both hands at a frequency of 1 Hz. The finger movements were paced to the beat of the metronome, which began sounding 8 sec before the isotope injection and continued for 3 min. The whole sequence was started at the same time and performed repeatedly for 45 times in each condition. The finger movements were monitored and recorded by a video camera. Performance of the sequence was assessed by calculating the percentage of correct sequences. No omission of taps was observed. The three conditions (rest, mirror, and parallel) consisted of one trial block. Within each trial block, the order of the three conditions was randomized across all subjects. Trial blocks (Trials 1, 2, 3) were repeated three times in each subject.
Table 1.
Sequences of bimanual sequential finger movements
| Group A (n = 6) | Group B (n = 6) | |
|---|---|---|
| Mirror | ||
| Right | 1,2,3,4 | 1,2,3,4 |
| Left | 1,2,3,4 | 1,2,3,4 |
| Parallel | ||
| Right | 4,3,2,1 | 1,2,3,4 |
| Left | 1,2,3,4 | 4,3,2,1 |
Sequence: 1, index finger; 2, middle finger; 3, ring finger; 4, little finger.
Experiment 2: simple abduction–adduction movements of the index finger. We studied nine different normal volunteers, men, aged 22–27 years (mean, 24.3 years). Each subject had 10 consecutive scans at 10 min intervals. Each subject performed acoustically paced (2 Hz) abduction–adduction movements of the index finger: right only, left only, mirror, and parallel, each of which was performed twice, on the same board as in Experiment 1. The direction of the movement was alternated at each beat of the metronome. In the mirror movements, simultaneous abduction was alternated with adduction of both index fingers. In the parallel movements, the task was simultaneous adduction of the other index finger, with repeated alternation of the direction. The first and last scans were performed under the rest conditions. The eight scans in between were for the task conditions. The order of the four conditions (right only, left only, mirror, and parallel) was counterbalanced and randomized across the subjects. Other settings were identical to Task 1.
PET scans. The PET scans were performed with General Electric Advance tomograph (GE/Yokogawa Medical System, Tokyo, Japan), with the interslice septa retracted. The physical characteristics of this scanner have been described in detail by De Grado et al. (1994)and Lewellen et al. (1996). This scanner acquires 35 slices with an interslice spacing of 4.25 mm. In the three-dimensional mode, the scanner acquires oblique sinograms with a maximum cross-coincidence of ±11 rings. A 10 min transmission scan using two rotating Ge-68 sources was performed for attenuation correction. Images of CBF were obtained by summing the activity during the 60 sec period after the first detection of an increase in cerebral radioactivity after the intravenous bolus injection of 10 mCi of 15O-labeled water (Sadato et al., 1995). The images were reconstructed with the Kinahan–Rogers reconstruction algorithm (Kinahan and Rogers, 1989). Hanning filters were used, giving transaxial and axial resolutions of 6 and 10 mm [full width at half maximum (FWHM)], respectively. The field of view and pixel size of the reconstructed images were 256 and 2 mm, respectively. No arterial blood sampling was performed, and thus the images collected were those of tissue activity. Tissue activity recorded by this method is nearly linearly related to rCBF (Fox et al., 1984; Fox and Mintun, 1989).
Anatomical MRI. For anatomical reference, a high-resolution whole-brain magnetic resonance image (MRI) for each subject was obtained separately. A regular head coil and a conventional T1-weighted, spoiled-Grass volume sequence with a flip angle of 30°, echo time of 5 msec, repetition time of 33 msec, and field of view of 24 cm were used. Matrix size was 256 × 256, slice thickness was 1.5 mm, and pixel size was 0.937 × 0.937 mm. These volume data of 124 sagittal slices were interpolated and resliced to transaxial images with voxel size of 0.937 × 0.937 × 0.937 mm. Each high-resolution image was normalized to the template T1-weighted images with linear transformation. Because mesial surface is variable in its gyral anatomy, the location of the activated areas in relation to the cingulate sulcus was examined on the normalized MRIs. The cingulate sulcus was identified on the contiguous sagittal images of each anatomically normalized high-resolution MRI with the method proposed bySteinmetz et al. (1989). The distance along z axis between the peak activation and the cingulate sulcus of each subject was measured.
Data analysis. The data were analyzed with statistical parametric mapping (SPM95; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks Inc., Sherborn, MA) (Friston et al., 1989, 1990, 1994, 1995a,b). The scans from each subject were realigned using the first image as a reference. After realignment, all images were transformed into a standard stereotaxic space (Talairach and Tournoux, 1988) and filtered with a Gaussian kernel of 10 mm FWHM in the x, y, andz axes. After the appropriate design matrix was specified, the condition, subject, and covariate effects were estimated according to the general linear model at each and every voxel. The design matrix included global activity as a confounding covariate, and this analysis can therefore be regarded as an ANCOVA (Friston et al., 1990). To test hypotheses about regionally specific condition effects, the estimates were compared using linear contrasts. The resulting set of voxel values for each contrast constitute a statistical parametric map of thet statistic SPM(t). The SPM(t) was transformed to the unit normal distribution [SPM(Z)]. The threshold of SPM(Z) was set at Z > 2.3. The resulting foci were characterized in terms of spatial extent (k) and peak height (u). The significance of each region was estimated using distributional approximation from the theory of Gaussian fields. This characterization is in terms of the probability that a region of the observed number of voxels could have occurred by chance [p(nmax > k)], giving the corrected p values at cluster levels for multiple comparisons over the entire volume analyzed, or that the peak height observed could have occurred by chance [p(Zmax > u)], giving the corrected p values at voxel levels. p < 0.05 of a corrected p value was used as a statistical threshold (Friston et al., 1994, 1995b). Because of generous threshold of Z > 2.3, activated regions during bimanual movement conditions compared with rest conditions constituted a large activated field. Hence significant activated foci were reported on the basis of the peak height: p < 0.05 with a correction for multiple comparisons (Friston et al., 1995b).
To identify the cortical areas related to the control of the bimanual finger movements, parallel and mirror sequential finger movement conditions in Experiment 1 were compared. From Experiment 1, we obtained a priori information for Experiment 2 as to which regions would show increase in rCBF during parallel movements compared with mirror movements. Because voxel-level significance has strong control over the regional specificity of the activation (Friston et al., 1996), activated foci that reached voxel-level significance (p < 0.05 with correction for multiple comparisons over the entire brain) were used for anatomically constraining hypothesis for Experiment 2. As the basis of statistical inference of any activations that fall within the FWHM of the prespecified location in SPM(Z), we used the pvalue of voxel-level with a Bonferroni correction for number of the prespecified locations (Friston et al., 1996). FWHM of SPM(Z), which indicates the extent of autocorrelation of the data or dependency of the Z value of one voxel on its neighbors, was estimated by the variance of the first derivatives of SPM(Z) in three directions (Friston et al., 1991,1995b).
RESULTS
Experiment 1
The performance of the mirror movements was slightly but significantly better than that of the parallel movements, but there was no significant difference between the mirror and the parallel movements for trial effect or interaction between sequence and trial effects (Table 2).
Table 2.
Performance of bimanual sequential finger movements
| Trial | Mirror | Parallel |
|---|---|---|
| 1 | 100.0 ± 0.0 | 96.9 ± 4.1 |
| 2 | 99.4 ± 1.9 | 98.5 ± 2.2 |
| 3 | 99.4 ± 1.4 | 99.3 ± 2.0 |
| Total | 99.6 ± 1.4 | 98.2 ± 3.0* |
Values are mean ± SD for percentage of correct sequences [% = correct sequences/ total sequences (n = 45) × 100; n = 12 for each trial].
Sequence effect was significant (F(1,66) = 7.05; p = 0.009). The difference in the percentage of correct sequences among the three trials was not significant in either the mirror or parallel movements (p > 0.05; two-way ANOVA).
Both mirror and parallel movements activated the primary sensorimotor cortex (SM1) extending to the premotor cortex (PM), inferior and superior parietal lobule (LPi and LPs), cerebellum, putamen, and thalamus bilaterally, and the posterior SMA. In addition, the midbrain and right prefrontal cortex were significantly activated by the parallel movements, but not the mirror movements, compared with the rest condition. (Tables 3,4).
Table 3.
Activation by sequential finger movement (n = 12): mirror versus rest
| Location | Coordinates | Z | %ΔCBF | Voxel-level corrected p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| SM1 | ||||||
| Left | −34 | −20 | 56 | 8.3 | 9.7 | <0.01 |
| Right | 34 | −20 | 56 | 10.1 | 10.5 | <0.01 |
| LPi | ||||||
| Left | −32 | −34 | 44 | 8.8 | 8.5 | <0.01 |
| Right | 32 | −34 | 44 | 8.0 | 8.4 | <0.01 |
| Cerebellum | ||||||
| Left | −20 | −56 | −28 | 8.6 | 8.6 | <0.01 |
| Right | 26 | −54 | −28 | 8.2 | 9.5 | <0.01 |
| Putamen | ||||||
| Left | −22 | −8 | 4 | 6.3 | 5.6 | <0.01 |
| Right | 22 | −8 | 4 | 5.5 | 5.0 | <0.01 |
| Thalamus | ||||||
| Left | −8 | −30 | 8 | 5.8 | 5.0 | <0.01 |
| Right | 14 | −24 | 8 | 6.2 | 5.2 | <0.01 |
| LPs | ||||||
| Left | −32 | −40 | 56 | 4.5 | 4.3 | 0.03 |
| Right | 32 | −60 | 52 | 4.9 | 4.0 | <0.01 |
| SMA | ||||||
| Right | 6 | −4 | 52 | 7.5 | 4.8 | <0.01 |
LPi, Inferior parietal lobule; LPs, superior parietal lobule; SM1, primary sensorimotor area; SMA, supplementary motor area.
Table 4.
Activation by sequential finger movement (n = 12): parallel versus rest
| Location | Coordinates | Z | %ΔCBF | Voxel-level corrected p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| SM1 | ||||||
| Left | −36 | −30 | 56 | 9.3 | 10.2 | <0.01 |
| Right | 32 | −18 | 56 | 11.7 | 12.2 | <0.01 |
| LPi | ||||||
| Left | −32 | −34 | 44 | 9.2 | 9.0 | <0.01 |
| Right | 32 | −34 | 44 | 8.2 | 8.7 | <0.01 |
| Cerebellum | ||||||
| Left | −20 | −56 | −28 | 10.5 | 12.6 | <0.01 |
| Right | 12 | −56 | −20 | 8.9 | 9.4 | <0.01 |
| Putamen | ||||||
| Left | −22 | −8 | 4 | 6.1 | 5.4 | <0.01 |
| Right | 22 | −8 | 4 | 5.8 | 5.4 | <0.01 |
| Thalamus | ||||||
| Left | −18 | −10 | 4 | 6.5 | 5.9 | <0.01 |
| Right | 14 | −22 | 8 | 6.5 | 5.8 | <0.01 |
| LPs | ||||||
| Left | −30 | −54 | 56 | 5.1 | 5.4 | <0.01 |
| Right | 14 | −68 | 56 | 4.6 | 4.2 | <0.01 |
| SMA | ||||||
| Right | 4 | −6 | 56 | 8.3 | 8.4 | <0.01 |
| Prefrontal cortex | ||||||
| Right | 54 | 2 | 24 | 6.5 | 4.7 | <0.01 |
| Midbrain | ||||||
| Left | −2 | −24 | −12 | 4.3 | 4.1 | 0.04 |
LPi, Inferior parietal lobule; LPs, superior parietal lobule; SM1, primary sensorimotor area; SMA, supplementary motor area.
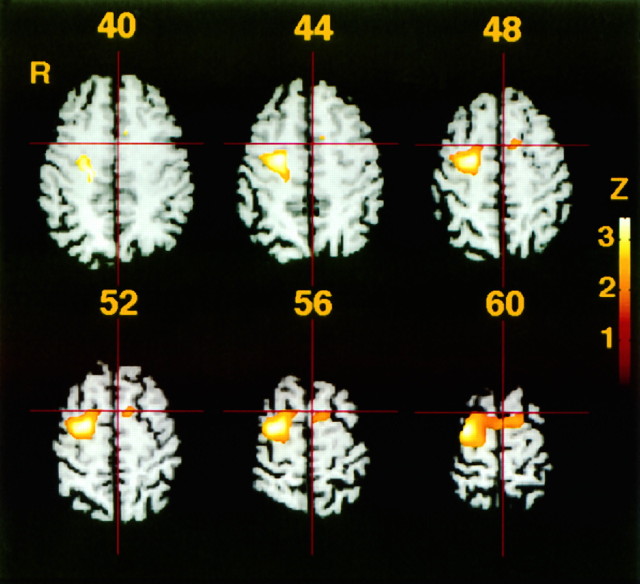
The right dorsal premotor area (PMd) extending to the posterior SMA showed significantly greater activation during the parallel movements compared with the mirror movements (correctedp < 0.05) (Fig. 1, Table5). These areas showed significant but less prominent activation during the mirror movements than during the parallel movements compared with the rest condition (Table 5).
Fig. 1.
Comparisons of adjusted mean rCBF between bimanual, sequential parallel movement and mirror finger movements, superimposed on typical magnetic resonance images unrelated to the study’s subjects. Six transaxial images of 40, 44, 48, 52, 56, and 60 mm above the anterior–posterior commissural line are shown. The pixels show levels of statistical significance above p < 0.05 for its spatial extension of activation after thresholding atZ > 2.3. Vertical red lineindicates midsagittal plane, crossing horizontal red line at the vertical anterior commissural line (VAC).
Table 5.
Activated foci by sequential finger movements: parallel versus mirror
| Region size (k) | Cluster-level correctedp | Z | Voxel-level correctedp5-150 | Coordinates | %ΔCBF | Location | Adjusted mean rCBF (mean ± SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Rest | Mirror5-160 | Parallel5-160 | ||||||
| 929 | 0.033 | 4.46 | 0.019 (<0.0001) | 22 | −10 | 52 | 3.9 | PMd (right) | 70.2 ± 2.3 | 72.5 ± 2.3 (3.9) | 75.3 ± 2.4 (7.4) |
| 4.28 | 0.040 (<0.0001) | 20 | −10 | 52 | 3.8 | PMd (right) | 67.0 ± 2.3 | 69.2 ± 2.2 (3.7) | 71.8 ± 2.4 (7.2) | ||
| 3.41 | 0.497 (<0.0001) | 16 | −20 | 64 | 2.6 | SMA (right) | 53.7 ± 1.8 | 55.1 ± 1.7 (3.3) | 56.6 ± 1.1 (6.2) | ||
| 3.35 | 0.558 (<0.0001) | −12 | −10 | 64 | 2.7 | SMA (left) | 55.2 ± 1.6 | 57.1 ± 1.7 (4.1) | 58.6 ± 2.0 (6.8) | ||
F5-150: Uncorrected p value.
F5-160: Z values for the comparison with rest condition. PMd, Dorsal premotor cortex; SMA, supplementary motor area.
Experiment 2
There were no erroneous movements in any of the tasks (right only, left only, mirror, or parallel movements of the index fingers) in all subjects in all trials.
Abduction–adduction movements of the right index finger activated the left SM1 extending to the posterior SMA, and the right cerebellum. Left index finger movements activated the right SM1 and a region extending from the anterior cingulate gyrus (ACG) to the posterior SMA, left ventral premotor cortex, and the left cerebellum. Bimanual movements activated the SM1 and a region extending from ACG to the posterior SMA, LPi, basal ganglia, and thalamus bilaterally, and the right prefrontal cortex (Tables 6,7).
Table 6.
Activation by abduction–adduction movements of the index finger: unimanual versus rest
| Location | Coordinates | Z | %ΔCBF | Voxel-level corrected p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right index finger | ||||||
| SM1 | ||||||
| Left | −32 | −26 | 56 | 7.3 | 12.4 | <0.01 |
| SMA | ||||||
| Left | −4 | −8 | 44 | 5.0 | 5.6 | <0.01 |
| Cerebellum | ||||||
| Right | 18 | −62 | −20 | 5.1 | 7.2 | <0.01 |
| Left index finger | ||||||
| SM1 | ||||||
| Right | 40 | −20 | 52 | 7.5 | 10.5 | <0.01 |
| ACG | ||||||
| Right | 2 | −8 | 40 | 5.1 | 4.6 | <0.01 |
| Cerebellum | ||||||
| Left | −6 | −64 | −16 | 5.7 | 9.0 | <0.01 |
| Right | 8 | −90 | −28 | 4.5 | 6.8 | 0.02 |
| LPi | ||||||
| Right | 30 | −26 | 40 | 6.7 | 9.5 | <0.01 |
| PMv | ||||||
| Left | −54 | −10 | 32 | 5.3 | 4.7 | <0.01 |
ACG, Anterior cingulate gyrus; LPi, inferior parietal lobule; PMv, ventral premotor cortex; SM1, primary sensorimotor area; SMA, supplementary motor area.
Table 7.
Activation by bimanual abduction–adduction movements of the index finger
| Location | Coordinates | Z | %ΔCBF | Voxel-level corrected p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Mirror versus rest | ||||||
| SM1 | ||||||
| Left | −34 | −22 | 52 | 7.6 | 13.1 | <0.01 |
| Right | 40 | −20 | 52 | 7.4 | 10.3 | <0.01 |
| ACG | ||||||
| Right | 10 | −2 | 36 | 5.1 | 6.9 | <0.01 |
| Cerebellum | ||||||
| Left | −4 | −64 | −16 | 6.3 | 10.1 | <0.01 |
| Right | 8 | −60 | −24 | 5.2 | 8.0 | <0.01 |
| LPi | ||||||
| Left | −28 | −38 | 40 | 5.7 | 7.1 | <0.01 |
| Right | 36 | −38 | 44 | 4.3 | 6.6 | 0.04 |
| PMd | ||||||
| Left | −16 | −20 | 48 | 5.1 | 6.9 | <0.01 |
| Right | 24 | −16 | 48 | 4.5 | 6.7 | 0.02 |
| Globus pallidus | ||||||
| Left | −22 | −14 | 4 | 5.0 | 5.7 | <0.01 |
| Prefrontal cortex | ||||||
| Right | 58 | 6 | 16 | 4.6 | 6.4 | <0.01 |
| Thalamus | 0 | −24 | 12 | 4.4 | 5.5 | 0.04 |
| Parallel versus rest | ||||||
| SM1 | ||||||
| Left | −36 | −22 | 52 | 7.5 | 13.3 | <0.01 |
| Right | 40 | −20 | 52 | 7.9 | 11.5 | <0.01 |
| LPi | ||||||
| Left | −30 | −26 | 40 | 5.5 | 8.2 | <0.01 |
| Right | 30 | −26 | 40 | 7.5 | 11.2 | <0.01 |
| PMd | ||||||
| Left | −16 | −20 | 48 | 5.2 | 7.2 | <0.01 |
| Right | 24 | −16 | 48 | 5.5 | 8.7 | <0.01 |
| Cerebellum | ||||||
| Left | −16 | −54 | −20 | 5.9 | 9.5 | <0.01 |
| Right | 26 | −56 | −28 | 5.9 | 10.2 | <0.01 |
| Thalamus | ||||||
| Left | −16 | −18 | 8 | 5.7 | 6.3 | <0.01 |
| Right | 2 | −24 | 12 | 5.0 | 6.7 | <0.01 |
| Globus pallidus | ||||||
| Left | −20 | −6 | 4 | 5.0 | 6.2 | <0.01 |
| Putamen | ||||||
| Right | 24 | −16 | 8 | 4.9 | 6.4 | <0.01 |
| Prefrontal cortex | ||||||
| Right | 58 | 4 | 16 | 5.3 | 7.3 | <0.01 |
ACG, Anterior cingulate gyrus; LPi, inferior parietal lobule; LPs, superior parietal lobule; PMd, dorsal premotor cortex; SM1, primary sensorimotor area; SMA, supplementary motor area.
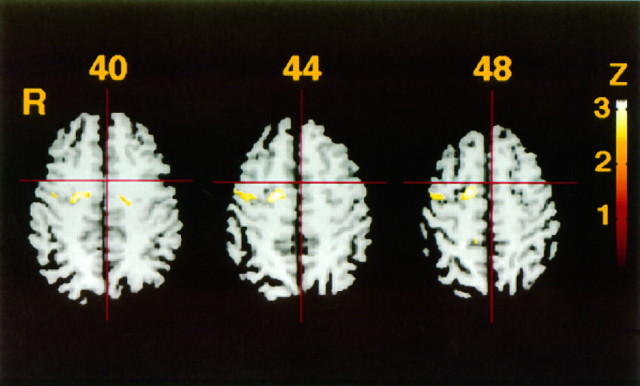
The right PMd with Talairach’s coordinates of x= 20 mm, y = −10 mm, and z = 52 mm, and x = 22 mm, y = −10 mm, and z = 52 mm showed activation during parallel sequential movements compared with mirror movements with voxel-level significance corrected for multiple comparisons (Table 5). Hence assessment of the activation during parallel abduction–adduction movements compared with during mirror movements was restricted to the regions that fall within the FWHM of this location. Estimated FWHM of SPM(Z) was 14.9 mm in x, 16.0 mm in y, and 19.4 mm inz axis. With this anatomical constraint, the areas of right PMd and right posterior SMA were significantly activated with a Bonferroni correction for two foci (p < 0.01) (Table 8, Fig.2). Measured with the normalized MRI, the location with coordinates (14, −6, 48) was 8.0 ± 3.3 mm (n = 9; mean ± SD) above the cingulate sulcus, confirming that it is in the SMA region in this particular group. Measured in the template MRI (shown in Figs. 1, 2), the location (14, −6, 48) is 9 mm above the cingulate sulcus.
Table 8.
Activated foci by the abduction–adduction movement in the SMA-premotor regions: parallel–mirror (n = 9)
| Location | Z | p8-150 | Coordinates | %ΔCBF | Adjusted rCBF (mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Rest | Right index | Left index | Mirror | Parallel | ||||
| SMA (right) | 3.16 | 0.0016 | 14 | −6 | 48 | 4.3 | 62.5 ± 3.0 | 63.3 ± 1.6 | 64.7 ± 2.3 | 64.7 ± 2.2 | 67.5 ± 2.4 |
| PMd (right) | 2.73 | 0.0064 | 22 | −10 | 44 | 3.8 | 60.3 ± 3.1 | 61.5 ± 1.9 | 61.9 ± 2.5 | 61.9 ± 1.9 | 64.3 ± 2.1 |
F8-150: With a priori an anatomical constraint that the right PMd with Talairach’s coordinates of x = 20, y = −10, z = 52, and x = 22,y = −10, z = 52, specified by the (parallel–mirror) comparison of bimanual sequential finger movements in Experiment 1, and its surrounding regions, which fall within the FWHM of the SPM(Z), are the regions of interest. A Bonferroni correction for the two locations was applied.
Fig. 2.
Comparisons of adjusted mean rCBF between bimanual, abduction–adduction, parallel, and mirror movements of the index fingers. Three transaxial images of 40, 44, and 48 mm above the anterior–posterior commissural line are shown. The pixels showZ > 2.3. The activated foci are corresponding to that of sequential finger movements, with lesser significance.
The parallel abduction–adduction movements activated these areas more prominently than unimanual movements of the index fingers, whereas no significant difference was observed between mirror bimanual movements and unimanual movements of either hand (Table 8).
DISCUSSION
Bimanual movements of different characteristics
Because both parallel and mirror are the characteristics of bimanual coordination, the difference between parallel and mirror is attributed to the difference of bimanual coordination. Hence areas showing increased rCBF during parallel rather than mirror should have a role in bimanual coordination. The right-sided larger activation also suggests that more work is necessary in the parallel movements than mirror movements in the right hemisphere, whereas left-sided neural networks might be used equally in both conditions. The slight difference in performance of sequential movements and no difference in nonsequential movements may suggest that the performance difference is related to sequence generation and not to bimanual coordination.
Supplementary motor area
Activation of the SMA is associated with the initiation of movement, motor programming (Roland et al., 1980), motor planning (Orgogozo and Larsen, 1979; Grafton et al., 1992; Rao et al., 1993), readiness to move (Fox et al., 1985), motor learning (Roland et al., 1989; Seitz et al., 1990; Grafton et al., 1992), complexity of the movement (Shibasaki et al., 1993), and responsiveness to internal cueing of movement (Halsband et al., 1993) or to the selection of movement (Deiber et al., 1991). The SMA is now argued to have two distinct areas with different functions, that is, the anterior SMA or pre-SMA, and the posterior SMA, or SMA proper (Luppino et al., 1993). They are roughly divided by the vertical anterior commissural line (Deiber et al., 1991).
Previous studies suggest that the SMA has a role in bimanual coordination. Brinkman (1981, 1984) found that unilateral ablation of the SMA in monkeys produced a long-lasting deficit in bimanual coordination, especially prominent when the SMA lesion was contralateral to the nonpreferred hand. Laplane et al. (1977) found that three patients with unilateral SMA excision for control of epilepsy showed an inability to perform alternating movements of the hand that required reciprocal coordination. Chan and Ross (1988)reported a patient with an infarct of the right SMA who showed pathological left-handed mirror writing and mirror movements during bimanual coordination. They hypothesized that the SMA may be responsible for nonmirror transformation of motor programs originating in the left hemisphere before execution by the primary motor area in the right hemisphere.
This hypothesis is supported by other studies. First, a leading role of the left hemisphere for bimanual movement is suggested by the hypothesis that the left hemisphere is dominant for motor programs. Studies involving brain lesions showed that the left cerebral hemisphere can exert some ipsilateral motor control (Wyke, 1966, 1967,1968, 1971; Kimura and Archibald, 1974; Kimura, 1977; De Renzi et al., 1980; Jason, 1985). Concerning the effects of task complexity on movement ipsilateral to lesions, Haaland et al. (1987) found that patients with left-sided cerebral strokes showed greater impairment with a less complex movement than with a more complex movement, whereas patients with right-sided cerebral strokes did not. They speculated that the less complex task was performed as a preprogrammed, open loop movement, whereas the more complex task was performed by visual guidance (closed loop movement), concluding that the left hemisphere is dominant for preprogrammed movements. These notions are consistent with the present study, because sequential finger movements were open loop, preprogrammed movements.
Second, cerebral dominance and asynchrony between bimanual movements have been reported. During a bimanual circular tracking task, the right hand leads the left hand by ∼25 msec (Stucchi and Viviani, 1993; Viviani et al., 1995). Because this delay is compatible with intercallosal transmission, the mechanism responsible for setting and maintaining the rhythm may be located in the left hemisphere, with the other hemisphere receiving time-keeping information through an interhemispheric connection.
Third, a study in nonhuman primates showed that the SMA hand representations are strongly interconnected via the corpus callosum, whereas the transcallosal interconnections of the M1 or between the SMA and the M1 are sparse (Rouiller et al., 1994).
Finally, using transcranial magnetic stimulation, which can demonstrate the contribution of a cortical area to a task by transiently disrupting its function, Pascual-Leone et al. (1994) showed that stimulation to the SMA disrupted the performance of parallel sequential finger movements and converted them to mirror movements. They concluded that the SMA was required for the synchrony of bimanual movements and for the bimanual coordination of parallel movements.
These findings, together with the present study, suggest that the posterior SMA is related to the nonmirror transformation of sequential finger movements.
Dorsal premotor area
The “premotor cortex” is a term originally applied to the lateral portion of the frontal agranular cortex rostral to the primary motor cortex (Dum and Strick, 1991). Premotor lesions can be characterized by the disintegration of the dynamics of the motor act and skilled movements (Kleist, 1907, 1911; Luria, 1966). Although some types of apraxia have been related mainly to left premotor lesions, a role for the right premotor area has occasionally been described (Halsband et al., 1993). The premotor cortex in the primate is heterogeneous and composed of multiple areas, including the PMd and the PMv (Dum and Strick, 1991; He et al., 1993). PMd is located around the precentral dimple of the monkey, and PMv is in the postarcuate region. The right premotor cortex, which showed more activation during parallel movements than during mirror movements in the present study, may be equivalent to the PMd because of its dorsal location.
Mushiake et al. (1991) showed that the motor set-related activity to perform a remembered sequential movement was more frequent in the PMd than in the PMv. In addition, sequence-specific neurons were more numerous in the PMd. During hand movement with instruction of the direction and amplitude, Kurata (1993) showed that set-related activity of the PMd was most active after instruction stimuli (ISs) were given for both amplitude and direction, not after IS for either one alone was presented. Thus PMd set-related activity may contribute to motor preparation by providing specified amplitude and direction. A majority of set-related neurons after obtaining two ISs showed activity reflecting both amplitude and direction, suggesting that these two parameters were integrated in PMd set-related activity. These findings suggest that the PMd may have a role in preprogrammed processes linked to sequential motor actions (Kurata, 1993). The PMd has dense corticocortical input from the SMA (Kurata, 1991). The functional significance of the input may be to provide the information necessary for motor set, so that the PMd could generate a program more closely reflecting motor aspects (Kurata, 1991). In nonhuman primates, the SMA and premotor cortex showed premovement activity. A portion of cells with movement-specific activity were observed exclusively in relation to the bilateral key press (Tanji et al., 1988). Hence the right PMd may integrate information such as the sequence of finger movements from the SMA to fit the left finger movements into that of the counterpart.
Abduction–adduction movement of index fingers
The present study showed that the activation of the posterior SMA and right PMd was significantly more prominent during parallel abduction–adduction finger movements than during mirror movements. The difference may originate from coordination of the muscular activity rather than programming sequences or execution. In these areas, there was no significant difference in activation between mirror abduction–adduction movements and unimanual movements, whereas the parallel movements were associated with greater activation than the unimanual movements. This implies that mirror movements do not need additional involvement of the SMA and right premotor cortex. Involuntary mirror movements on one side of the body that occur as mirror reversal of an intended movement on the other side of the body (Cohen et al., 1991) are common as normal phenomena in children and usually disappear after the first decade of life (Connolly and Stratton, 1968). The timing of this disappearance coincides with the completion of myelination of the corpus callosum (Yakovlev and Lecours, 1967), implying the importance of interhemispheric connections to generate nonmirror movement. These findings suggest that the SMA and right PMd may suppress the “default” mirror movements for nonmirror, bimanual coordination.
Other areas of activation during bimanual movements
Bimanual movements activated well established parts of the motor system. Additional activation in the midbrain was noted during sequential parallel movements, and in the right prefrontal cortex during sequential parallel and abduction–adduction movements. Because no significant difference between parallel and mirror was noted, the role of these areas may not be specific to bimanual coordination.
Jenkins et al. (1994) reported activation of the midbrain during the learning of a new sequence of finger tapping, whereas no significant activation occurred during performance of a prelearned sequence. They speculated that this activation represented activation of cerebellorubral pathways, although the effect of increased attention in learning a new sequence was another possibility. Because the tasks had a minimal learning component in the present study and because the subjects had to attend to their fingers and the auditory cues to perform the bimanual movements, the activation of the midbrain may be related to increased attention (Kinomura et al., 1996).
The right prefrontal cortex is also related to sustained attention (Pardo et al., 1991). To perform bimanual movement, on-line monitoring of the position of the fingers is necessary. Because the right prefrontal cortex is involved in spatial working memory (Jonides et al., 1993), activation of the right prefrontal cortex during bimanual movements may be related to increased spatial attention and spatial working memory.
Footnotes
We thank Dr. Mark Hallett, National Institute of Neurological Disorders and Stroke, for helpful discussion.
Correspondence should be addressed to Dr. Norihiro Sadato, Biomedical Imaging Research Center, Fukui Medical School, 23 Shimoaizuki, Matsuoka, Yoshida, Fukui, 910–11 Japan.
REFERENCES
- 1.Brinkman C. Lesions in supplementary motor area interfere with a monkey’s performance of a bimanual coordination task. Neurosci Lett. 1981;27:267–270. doi: 10.1016/0304-3940(81)90441-9. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman C. Supplementary motor area of the monkey’s cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkman J, Kuypers HGJM. Splitbrain monkeys: cerebral control of ipsilateral and contralateral arm, hand, and finger movements. Science. 1972;176:535–539. doi: 10.1126/science.176.4034.536. [DOI] [PubMed] [Google Scholar]
- 4.Brinkman J, Kuypers HGJM. Cerebral control of contralateral and ipsilateral arm, hand, and finger movements in the split-brain rhesus monkey. Brain. 1973;96:653–674. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- 5.Chan J-L, Ross ED. Left-handed mirror writing following right anterior cerebral artery infarction: evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology. 1988;38:59–63. doi: 10.1212/wnl.38.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Cohen LG, Meer J, Tarkka I, Bierner S, Leiderman DB, Dubinsky RM, Sanes JN, Jabbari B, Branscum B, Hallett M. Congenital mirror movements. Brain. 1991;114:381–403. doi: 10.1093/brain/114.1.381. [DOI] [PubMed] [Google Scholar]
- 7.Connolly K, Stratton P. Developmental changes in associated movements. Dev Med Child Neurol. 1968;10:49–56. doi: 10.1111/j.1469-8749.1968.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 8.De Grado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM. Performance characteristics of a whole-body PET scanner. J Nucl Med. 1994;35:1398–1406. [PubMed] [Google Scholar]
- 9.Deiber M-P, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RSJ. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- 10.De Renzi E, Motti F, Nichelli P. Imitating gestures: a quantitative approach to ideomotor apraxia. Arch Neurol. 1980;37:6–10. doi: 10.1001/archneur.1980.00500500036003. [DOI] [PubMed] [Google Scholar]
- 11.Donoghue JP, Sanes JN. Motor areas of the cerebral cortex. J Clin Neurophysiol. 1994;11:382–396. [PubMed] [Google Scholar]
- 12.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox PT, Mintun MA. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med. 1989;30:141–149. [PubMed] [Google Scholar]
- 14.Fox PT, Mintun MA, Raichle ME, Herscovitch P. A noninvasive approach to quantitative functional brain mapping with H215O and positron emission tomography. J Cereb Blood Flow Metab. 1984;4:329–333. doi: 10.1038/jcbfm.1984.49. [DOI] [PubMed] [Google Scholar]
- 15.Fox PT, Fox JM, Raichle ME, Burde RM. The role of cerebral cortex in the generation of voluntary saccades: a positron emission tomography study. J Neurophysiol. 1985;54:348–369. doi: 10.1152/jn.1985.54.2.348. [DOI] [PubMed] [Google Scholar]
- 16.Freund H-J. Premotor area and preparation of movement. Rev Neurol. 1990;146:543–547. [PubMed] [Google Scholar]
- 17.Friston KJ, Passingham RE, Nutt JG, Heather JD, Sawle GV, Frackowiak RSJ. Localisation in PET images: direct fitting of the intercommissural (AC-PC) line. J Cereb Blood Flow Metab. 1989;9:690–695. doi: 10.1038/jcbfm.1989.97. [DOI] [PubMed] [Google Scholar]
- 18.Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RSJ. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- 19.Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 20.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 21.Friston KJ, Ashburner J, Frith CD, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–188. [Google Scholar]
- 22.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- 23.Friston KJ, Holmes A, Poline J-B, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 24.Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RSJ, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci. 1992;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haaland KY, Harrington DL, Yeo R. The effects of task complexity on motor performance in left and right CVA patients. Neuropsychologia. 1987;25:783–794. doi: 10.1016/0028-3932(87)90116-3. [DOI] [PubMed] [Google Scholar]
- 26.Halsband U, Ito N, Tanji J, Freund H-J. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- 27.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jason GW. Manual sequence learning after focal cortical lesions. Neuropsychologia. 1985;23:483–496. doi: 10.1016/0028-3932(85)90003-x. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 31.Kimura D. Acquisition of a motor skill after left-hemisphere damage. Brain. 1977;100:527–542. doi: 10.1093/brain/100.3.527. [DOI] [PubMed] [Google Scholar]
- 32.Kimura D, Archibald Y. Motor functions of the left hemisphere. Brain. 1974;97:337–350. doi: 10.1093/brain/97.1.337. [DOI] [PubMed] [Google Scholar]
- 33.Kinahan PE, Rogers JG. Analytic three dimensional image reconstruction using all detected events. IEEE Trans Nucl Sci. 1989;36:964–968. [Google Scholar]
- 34.Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 35.Kleist K. Corticale (innervatorische) apraxie. Jahrbuch Psychiatrie Neurologie. 1907;28:46–112. [Google Scholar]
- 36.Kleist K. Der gang und der gegenwartige stand der apraxie-forschung. Ergebnisse Neurol Psychiatrie. 1911;1:342–452. [Google Scholar]
- 37.Kurata K. Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci Res. 1991;12:263–280. doi: 10.1016/0168-0102(91)90116-g. [DOI] [PubMed] [Google Scholar]
- 38.Kurata K. Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J Neurophysiol. 1993;69:187–200. doi: 10.1152/jn.1993.69.1.187. [DOI] [PubMed] [Google Scholar]
- 39.Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977;34:301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- 40.Lewellen TK, Kohlmeyer SG, Miyaoka RS, Kaplan MS. Investigation of the performance of the General Electric advance positron emission tomograph in 3D mode. IEEE Trans Nucl Sci. 1996;43:2199–2206. [Google Scholar]
- 41.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- 42.Luria AR. Higher cortical functions in man. Basic Books; New York: 1966. [Google Scholar]
- 43.Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- 44.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Orgogozo JM, Larsen B. Activation of the supplementary motor area during voluntary movement in man suggests it works as a supramotor area. Science. 1979;206:847–850. doi: 10.1126/science.493986. [DOI] [PubMed] [Google Scholar]
- 46.Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 47.Pascual-Leone A, Cohen L, Wassermann E, Hallett M (1994) The role of the supplementary motor area (SMA) in the coordination of bimanual movements. Neurology 44[Suppl 2]:A329.
- 48.Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, Wong EC, Haughton VM, Hyde JS. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- 49.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 50.Roland PE, Eriksson L, Widen L, Stone-Elander S. Changes in regional cerebral oxidative metabolism induced by tactile learning and recognition in man. Eur J Neurosci. 1989;1:3–18. doi: 10.1111/j.1460-9568.1989.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 51.Rouiller EM, Balalian A, Kazennikov O, Moret V, Yu X-H, Wiesendanger M. Transcallosal connections of the distal forelimb representation of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- 52.Sadato N, Carson RE, Daube-Witherspoon ME, Campbell G, Hallett M, Herscovitch P. Optimization of non-invasive activation study with 15O water and 3D PET. J Nucl Med. 1995;36:82P. doi: 10.1097/00004647-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Sadato N, Campbell G, Ibanez V, Deiber M-P, Hallett M. Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci. 1996;16:2693–2700. doi: 10.1523/JNEUROSCI.16-08-02691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seitz RJ, Roland PE, Bohm C, Greitz T, Stone-Elander S. Motor learning in man: a positron emission tomographic study. NeuroReport. 1990;1:57–60. doi: 10.1097/00001756-199009000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M, Fukuyama H, Asato R, Konishi J. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116:1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- 56.Steinmetz H, Furst G, Freund H-J. Cerebral cortical localization: application and validation of the proportional grid system in MR imaging. J Comput Assist Tomogr. 1989;13:10–19. [PubMed] [Google Scholar]
- 57.Stucchi N, Viviani P. Cerebral dominance and asynchrony between bimanual two-dimensional movements. J Exp Psychol Hum Percept Perform. 1993;19:1200–1220. doi: 10.1037//0096-1523.19.6.1200. [DOI] [PubMed] [Google Scholar]
- 58.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 59.Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- 60.Viviani P, Perani D, Grassi F, Stucchi N, Todde S, Fazio F. Hemispheric asymmetry in cerebral activity during rhythmic bimanual coordination. J Cereb Blood Flow Metab. 1995;15:S865. [Google Scholar]
- 61.Wyke M. Postural arm drift associated with brain lesions in man. Arch Neurol. 1966;15:329–334. doi: 10.1001/archneur.1966.00470150107016. [DOI] [PubMed] [Google Scholar]
- 62.Wyke M. Effects of brain lesions on the rapidity of arm movements. Neurology. 1967;17:1113–1120. doi: 10.1212/wnl.17.11.1113. [DOI] [PubMed] [Google Scholar]
- 63.Wyke M. The effects of brain lesions in the performance of an arm-hand precision task. Neuropsychologia. 1968;6:125–134. [Google Scholar]
- 64.Wyke M. The effects of brain lesions on the performance of bilateral arm movements. Neuropsychologia. 1971;9:33–42. doi: 10.1016/0028-3932(71)90059-5. [DOI] [PubMed] [Google Scholar]
- 65.Yakovlev PI, Lecours A-R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Blackwell; Oxford: 1967. pp. 3–70. [Google Scholar]