Abstract
We investigated the effects of sleep on wake-induced c-fos expression in the cerebral cortex of rats and c-fos-lacZ transgenic mice. In the cortex of rats, the levels of c-Fos, detected both by immunocytochemistry and Western blot, remained high during 6 or 12 hr of enforced wakefulness but declined rapidly (within 1 hr) with increasing time of recovery sleep. Similarly, in the transgenic mice in which lacZexpression is driven from the c-fos promoter, β-galactosidase activity was high after enforced wakefulness and declined with increasing amounts of sleep. These results suggest that the decrease in c-Fos protein in cortical neurons during sleep may be attributable to cessation of c-fos expression, activation of a process that degrades the wake-induced c-Fos, or both.
Keywords: sleep, c-fos expression, c-fos-lacZtransgenic mice, cingulate cortex, immunohistochemistry, Western blot, β-gal activity
Sleep and wakefulness are marked by dramatic shifts in neuronal firing. During wakefulness most brain neurons, including cortical neurons, are active, whereas non-rapid eye movement (REM) sleep is marked by a relatively quiescent and distinctively different pattern of neuronal activity. Such shifts in neuronal firing and the accompanying release of neurotransmitters most likely produce alterations at the molecular level.
To find evidence that gene expression occurs during sleep–wakefulness, investigators have examined the cellular immediate early gene c-fos. This gene is rapidly and transiently induced in response to various stimuli (for review, see Morgan and Curran, 1991). The expression of c-fos has also been shown to change in different parts of the brain during spontaneous sleep–wake episodes (Cirelli et al., 1993a; Pompeiano et al., 1994; Grassi-Zucconi et al., 1994; Sherin et al., 1996). Fos protein levels in the cortex and other parts of the rat brain are high during the dark period when rats are awake and low during the light period when rats are asleep (Grassi-Zucconi et al., 1993). Moreover, enforced wakefulness increases c-Fos levels in cortex, and with ensuing sleep there is a decline of c-Fos in rats (Pompeiano et al., 1992; Cirelli et al., 1993b, 1996) and in cats (Shiromani et al., 1994). Cirelli et al. (1996) recently demonstrated that the wake-induced c-fos expression in cortical neurons is dependent on noradrenergic input from the locus coeruleus (LC).
We investigated the effects of recovery sleep on enforced wake-induced c-Fos immunoreactivity (c-Fos-ir) using immunocytochemistry and Western blot techniques. Transgenic c-fos-lacZ mice with the bacterial gene lacZ, encoding β-galactosidase (β-gal), fused into the fourth exon of c-fos (Smeyne et al., 1992), were also studied. An increase in β-gal activity and c-Fos protein occurred during enforced wakefulness, indicating activation of c-fos expression. In addition, there was a decrease in β-gal activity and c-Fos-ir with sleep. This suggests that during sleep there is a cessation of c-fos expression, an activation of a proteolytic pathway that degrades c-Fos in wake-active neurons, or both. The time course of the sleep-induced decline of c-Fos in rats and mice resembles the time course described in cats (Shiromani et al., 1994), suggesting a similar process across different species.
MATERIALS AND METHODS
Animals and sleep deprivation paradigm. Rats and mice used in this study were housed in a room with controlled temperature (21°C) and a 12 hr light/dark cycle (lights on 7:00 A.M.), with food and water provided ad libitum.
The first set of experiments was performed using male Sprague Dawley rats (300–350 gm). Surgical implantation of sleep-recording electrodes was done under anesthesia [mixture of (in mg/kg): 0.75 acepromazine, 2.5 xylazine, and 22 ketamine]. One week after recovery from surgery, the animals were connected to flexible cables that permitted undisturbed recording of sleep–wake episodes. After 3 d of adaptation to the recording cables the experimental paradigm was started. The rats were kept awake for 12 hr (7:00 A.M.–7:00 P.M.) by gently touching them or by tapping the cage whenever the animals produced EEG signs of sleep. Six animals were killed after the 12 hr period of wakefulness (0% sleep), and the rest were allowed 1 hr of recovery sleep (n = 7), during which the rats spontaneously slept 0–95% of the time. The EEG records were scored in 12 sec epochs (paper speed, 5 mm/sec) for wakefulness and non-REM and REM sleep. All animals were perfused transcardially with 50 ml of ice-cold normal saline followed by 350 ml of ice-cold 2% formaldehyde in 0.1 m PBS. The brains were removed, placed overnight in the same fixative, and then transferred and stored in buffer containing 20% sucrose and 0.1 m PBS, pH 7.4, at 4°C for immunohistochemical analysis.
A second set of experiments was performed using c-fos-lacZtransgenic mice (Smeyne et al., 1992). In these mice a bacterial β-galactosidase (lacZ) gene was fused in frame into the C-terminal region of the c-fos gene. The c-Fos-lacZ fusion protein expressed from the c-fos promoter contains 315 N-terminal amino acids from c-Fos and 1015 C-terminal amino acids from β-galactosidase. As a result, these transgenic mice can be used for the simultaneous detection of both c-Fos protein and β-galactosidase activity in adjacent sections.
A total of 16 mice were used. Wakefulness was prolonged (10:00 A.M.–1:00 P.M.) by gentle handling of the animals. Six mice were killed after 3 hr of prolonged wakefulness, and the remaining 10 mice were allowed 1 hr of recovery sleep. The amount of sleep and wakefulness was determined by behavioral observations as described byFranken et al. (1992). If the animals were immobile, i.e., without gross body, head, or whisker movements, then they were considered to be asleep and were awakened by tapping the cage or by gentle touch. During recovery sleep, such periods of immobility were followed by the animal rapidly assuming a curled sleep posture.
c-Fos immunohistochemistry. Coronal sections (40 μm thick) were cut with a freezing microtome. For detection of Fos-ir, a rabbit polyclonal antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:1000 dilution. Control sections were processed without primary or secondary antibodies. Antibodies for c-Fos from other sources (Oncogene Science) yielded similar results. The sections (one in four series) were washed with 0.1 m PBS containing 0.25% Triton X-100 and then incubated overnight at room temperature in the presence of antibody. The following day the sections were washed in 0.1 m PBS and incubated with anti-rabbit secondary antibody (donkey anti-rabbit serum). After washing, the sections were incubated for 1 hr in avidin–biotin complex (ABC kit; Vector Laboratories, Burlingame, CA) followed by washes and 5 min of treatment with the chromogen DAB-nickel chloride-hydrogen peroxide solution (Vector). The sections were then mounted onto gelatin-coated slides and coverslipped after dehydration in alcohol–xylene solutions.
β-Galactosidase histochemistry. Sections (40 μm thick) were cut with a freezing microtome, and visualization of β-gal activity was performed as described by Oberdick et al., (1990). Briefly, the sections were mounted on gelatin-coated slides and air dried. Sections were stained for 16–20 hr at 37°C in the dark with 5-bromo-4-chloro-3-indolyl-β-d-galactosidase (X-gal; Boehringer Mannheim, Indianapolis, IN) to determine β-gal activity. Adjacent sections were stained for Fos-ir as described above.
Quantitation of labeled nuclei. c-Fos- or β-gal-labeled cells from the cingulate cortex were counted. Darkly labeled cells were counted in a 100 × 300 μm area from one cortical hemisphere. Counts were done on 8–10 tissue sections (one in four series), and an average count per section was determined for each animal.
Western blot analysis. In another set of experiments, for Western blot analysis, rats were killed by rapid decapitation at 7:00 P.M. (lights off) after 6 hr of prolonged wakefulness (1:00–7:00 P.M.) (n = 4) or after 1 hr of recovery sleep (n = 4). The cortical tissue samples were dissected and frozen in liquid nitrogen. Nuclear extracts from cortical tissue were prepared as described by Sonnenberg et al., (1989). Tissue was homogenized in a Dounce homogenizer in 10 vol 0.25 mm sucrose, 15 mm EGTA, 0.15 mm spermine, 0.5 mmspermidine, 5 mm DTT, 2 μg/ml leupeptin, 5 μg/ml aprotinin, and 0.2 mm PMSF. The homogenates were centrifuged at 2000 × g for 6 min at 4°C, and the pellets were resuspended in the original volume of (in mm): 10 HEPES, pH 7.9, 1.5 MgCl2, 10 NaCl, and 5 DTT, containing protease inhibitors. After centrifugation at 4000 ×g for 10 min at 4°C, the nuclear pellets were resuspended in 50 mm HEPES, pH 7.9, 0.75 mmMgCl2, 0.5 mm EDTA, 0.5 mNaCl, 12.5% glycerol, 5 mm DTT, and protease inhibitors and incubated on a rotating shaker for 1 hr at 4°C and centrifuged at 15,000 × g for 30 min. The supernatant containing the final nuclear extract was dialyzed overnight at 4°C in 5 mm Tris-HCl, pH 7.9, and 0.1 mm PMSF and lyophilized. The pellets were resuspended in water, and protein content was estimated using the Bradford method. Protein samples (50 μg) were electrophoresed in 10% SDS-PAGE at 150 V for 3 hr. The samples were electroblotted overnight at 20 V onto nitrocellulose membranes. The membranes were blocked using 3% nonfat dry milk in PBST buffer (10 mm sodium phosphate, pH 7.4, 150 m sodium chloride, and 0.1% Tween-20) and incubated overnight on a shaker with anti-c-Fos rabbit antibody (SC-52G) at 1:1000 dilution (Santa Cruz Biotechnology). A 62 kDa band was identified as c-Fos protein. In control experiments this band disappeared after preincubation with the blocking peptide (SC-52P) recommended by the manufacturer (Santa Cruz Biotechnology). The next day the blots were washed using PBST and incubated for 1 hr with anti-rabbit, peroxidase-conjugated, secondary antibody. The protein bands were detected using an ECL detection kit (Amersham, Arlington Heights, IL) as described by the manufacturer.
RESULTS
Fos-ir decreases with sleep
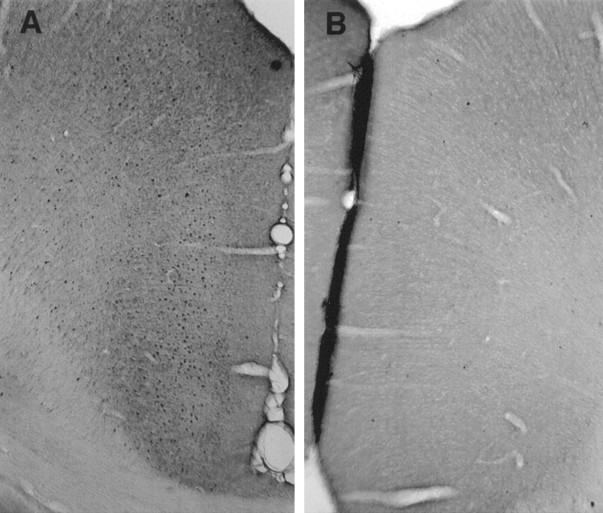
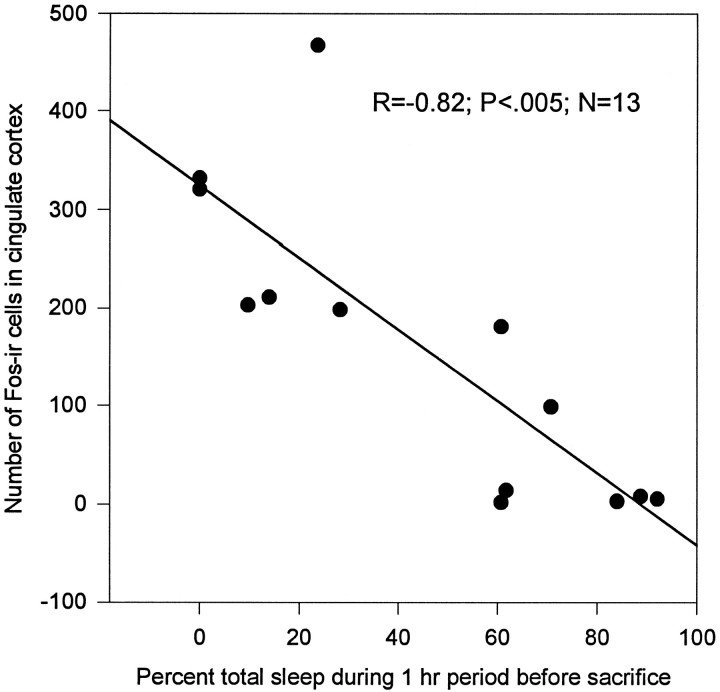
Fos-ir was elevated in the cingulate cortex of rats kept awake for 12 hr compared with animals that were permitted to sleep after enforced wakefulness (Fig.1A,B). The number of Fos-positive neurons was inversely related to the percentage of total sleep (slow wave plus REM sleep, r = −0.82;p < 0.005) during the hour preceding death (Fig.2). Similar results were observed in another set of experiments in which c-Fos-ir concentration was high in the cortex after 6 hr of enforced wakefulness. The number of c-Fos-ir cells decreased after 1 hr of recovery sleep (data not shown).
Fig. 1.
Immunohistochemical detection of c-Fos in cingulate cortex. Rats were kept awake by gentle handling for 12 hr (7:00 A.M.–7:00 P.M.) and then killed (n = 6) or allowed 1 hr of sleep (n = 7). c-Fos-labeled cells in cingulate cortex were detected using immunohisochemistry after prolonged wakefulness (A) and after 1 hr of sleep (B).
Fig. 2.
Enforced wakefulness and recovery from sleep-induced changes in the number of c-Fos-positive neurons in cortex. The number of c-Fos-positive cells was counted in cingulate cortex of rats that were kept awake for 12 hr or were allowed sleep after enforced wakefulness. Percent of sleep was calculated as total sleep time/60 min × 100. c-Fos levels declined in those animals who slept when they were permitted (n = 7) compared with those animals not permitted to sleep (n = 6). The number of c-Fos-positive cells declined with increasing percentage of sleep (n = 13; r = −0.82;p < 0.005).
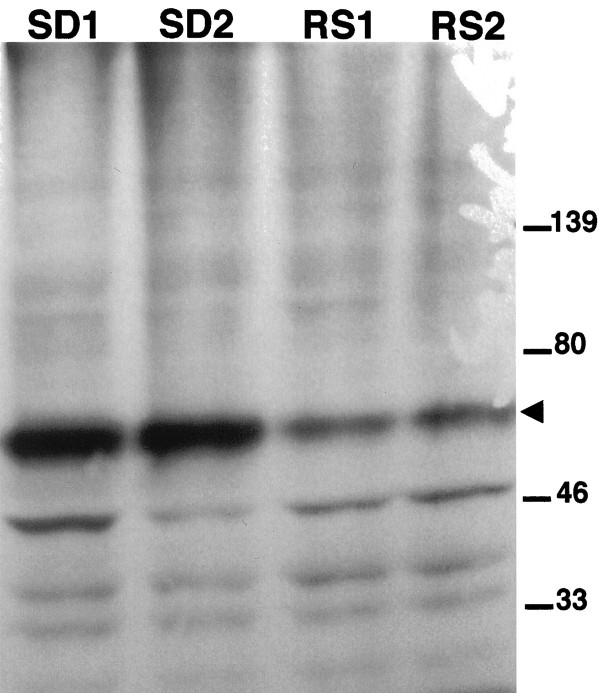
To determine whether the antibody recognizes the 62 kDa c-Fos protein, Western blot analysis was used to compare the level of c-Fos in cingulate cortex from rats that were kept awake or allowed 1 hr of recovery sleep. Figure 3 shows that the antibody detected a 62 kDa c-Fos protein that selectively decreased after sleep. This size is consistent with other studies of c-Fos (Curran and Morgan, 1986). Other bands of Fos-related antigens (FRAs) at 35 and 47 kDa did not decrease in cortical extracts of rats killed after 1 hr of recovery sleep.
Fig. 3.
Western blot analysis of nuclear proteins. To determine the size of c-Fos protein, Western blot analysis was performed using tissue extracts of cingulate cortex from rats after 6 hr of enforced wakefulness (SD1, SD2), and followed by 1 hr of sleep (RS1, RS2) before death. c-Fos protein (molecular weight, 62 kDa; arrowhead) decreased in rats that were allowed sleep before death.
Decrease in β-Galactosidase activity after 1 hr of recovery sleep
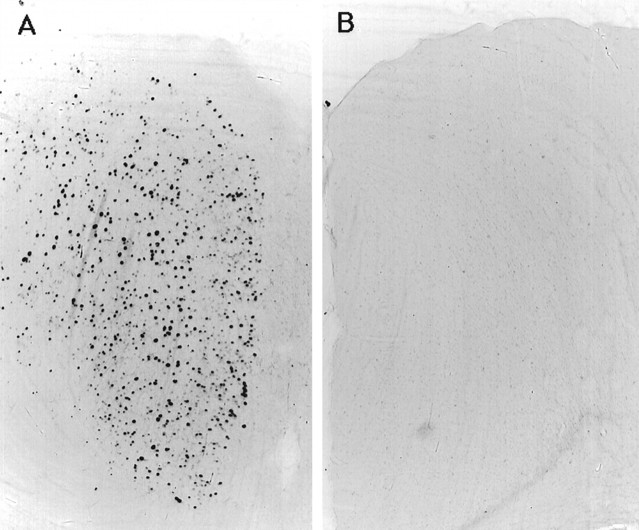
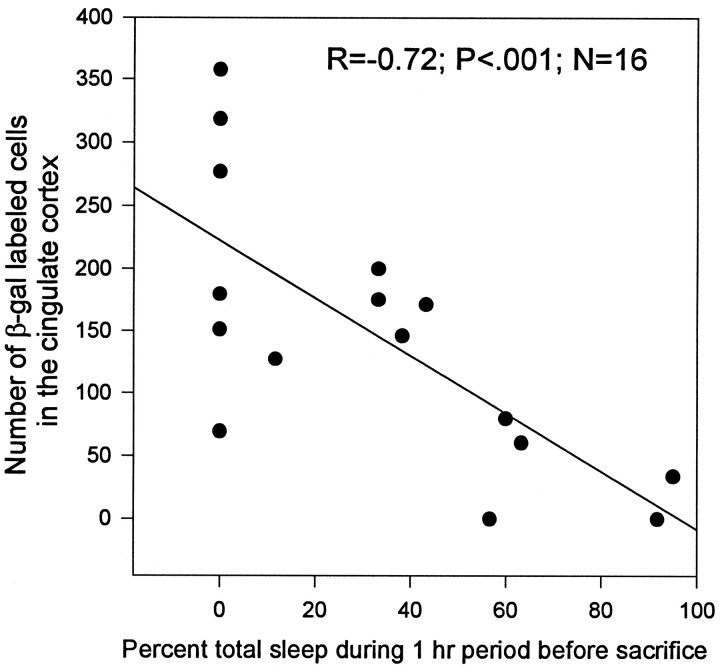
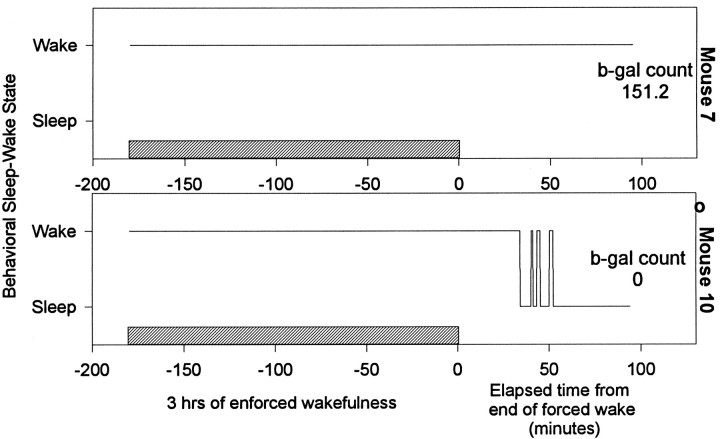
Similar results were obtained with c-fos-lacZ mice. The β-gal activity in cingulate cortex was high after 3 hr of enforced wakefulness (Fig. 4A) and declined significantly after 1 hr of recovery sleep (Fig.4B). The β-gal activity decreased as a function of the time spent sleeping (Fig. 5) (r = −0.72; p < 0.001). Figure6 shows the time spent sleeping before death for two representative mice. One exhibited high levels of β-gal-positive neurons; this animal did not sleep when permitted (mouse 7), whereas another showed a loss of activity after 45 min of sustained sleep (mouse 10). Because β-gal is driven from the c-fos promoter, this result supports the conclusion that c-Fos (rather than or in addition to a Fos-related antigen) is elevated during wakefulness.
Fig. 4.
Detection of β-galactosidase activity in cingulate cortex. The presence of β-gal activity in the cingulate cortex of c-fos-lacZ transgenic mice was detected using x-gal after 3 hr of enforced wakefulness (A) and then followed by 1 hr of sleep (B).
Fig. 5.
Sleep-induced decrease in β-galactosidase activity. The number of cells showing β-gal activity was correlated with the percent of sleep during the 60 min period before death of c-fos-lacZ transgenic mice. β-Galactosidase activity decreased with increasing amount of sleep (n = 16;r = −0.72; p < 0.001).
Fig. 6.
The wake–sleep profile of two representative mice. Both mice were kept awake for 3 hr and then left undisturbed for 1 hr. Mouse 7 did not sleep even when permitted to do so, and high levels of β-galactosidase staining were found in the cingulate cortex. Mouse 10 was asleep for 45 min before death, and it showed no β-galactosidase stained cells. The hatched barrepresents 3 hr period of enforced wakefulness.
DISCUSSION
Several studies have found that the expression of c-fos(mRNA and protein) is increased in many brain regions during spontaneous (Cirelli et al. 1993a; Grassi-Zucconi et al. 1993, 1994;Pompeiano et al. 1994; Sherin et al. 1996) or enforced wakefulness in rats (Pompeiano et al. 1992; O’Hara et al. 1993, Cirelli et al. 1995,1996). Similarly in cats, Fos-ir increases with 24 hr of enforced wakefulness and declines after 45 min of recovery sleep (Shiromani et al. 1994). The levels of c-fos expression have been determined in different species, using various techniques, and for different durations of enforced wakefulness. However, in these reports, c-Fos was detected only by immunohistochemical analysis using polyclonal antisera against c-Fos. Such antisera can cross-react with other FRAs. Using Western blot analysis, we demonstrated that the increased Fos-ir during enforced wakefulness and its reduction during sleep are specific for 62 kDa c-Fos. This was further confirmed in c-fos-lacZ mice showing similar changes in β-gal expression, which is transcribed from the c-fos promoter. In addition, in previous studies the animals were examined immediately at the end of the enforced-wake period. Therefore, the effects of short periods of ensuing sleep on wake-induced c-Fos levels were not examined. The present study was conducted to clarify further the effects of sleep on wake-induced c-Fos levels.
Specifically, the present study examined c-Fos during the 1 hr sleep period immediately before death. This provides important insight into the dynamics of c-fos transcription and degradation in response to rapidly changing sleep–wake conditions. A previous study (Pompeiano et al., 1994) examined c-fos (mRNA and protein) in rats that were killed after a 5 hr period of spontaneous sleep–wakefulness (either in the day or night). In that study, c-Fos was found to be lower in rats that were asleep 80% of the 5 hr period compared with rats that were asleep 25% of the 5 hr period.Grassi-Zucconi et al. (1994) measured sleep–wakefulness during a 4 hr period and also found that c-Fos levels decreased with sleep. Our results are similar in that there was a decrease in c-Fos in response to sleep. However, by using a 1 hr sleep period, the temporal decline in c-Fos can be better understood.
It was observed that in c-fos-lacZ transgenic mice, in whichlacZ expression is driven from the c-fospromoter, β-gal activity was high in awake mice but declined with increasing amounts of sleep. Although mRNA levels were not examined in this study, other investigators have observed a decrease in c-fos mRNA with sleep using Northern blot and in situ hybridization (Grassi-Zucconi et al. 1993; Cirelli et al. 1995), which is indicative of the levels of mRNA at the time of death. Our results with c-fos-lacZ mice also showed a decrease in sleep-related β-galactosidase activity. Observations of both endogenous Fos and the fusion transgene thus support the conclusion that sleep is associated with reduced transcription from the c-fos promoter, or increased degradation of c-fosmRNA, which has a short half-life because of the Shaw-Kamen degradation sequence (AUUUA) in its 3′-untranslated region (Gillis and Malter, 1991). It is difficult to determine the relative contributions of these two processes.
The molecular events leading to the initiation of c-fos gene transcription include phosphorylation of the c-AMP response element binding (CREB) protein, bound to CRE sequence upstream from the c-fos promoter (Morgan and Curran, 1991). An increase in phosphorylated CREB throughout the brain after wakefulness was observed by us (unpublished data) and Cirelli et al. (1996). Another report (Cirelli and Tononi, 1996) has shown an increase in serine/threonine kinase-specific phosphorylation in wakefulness. Activation of such events might, in part, be attributable to noradrenergic stimulation (Cirelli et al.,1996). Because during sleep neuronal activity in the LC is considerably reduced (McCarley and Hobson, 1975; Aston-Jones and Bloom, 1981), it is possible that a decline in norepinephrine tone from the LC may result in a reduction of CREB phosphorylation and a subsequent decline in c-fos expression. Our results using c-fos-lacZ mice also showed a sleep-induced decrease in β-gal activity, suggesting a decrease in transcription from the c-fos promoter during sleep.
Our observation, that after 1 hr of sleep, not only Fos-ir but also β-gal activity is decreased, is instructive. Pulse–chase studies performed on PC12 cells showed that Fos degrades with a half-life of ∼45 min but is still detectable after 2 hr (Curran and Morgan, 1986). In B104 cells, Fos-lacZ has a longer half-life than that of endogenous c-Fos (Schilling et al., 1991). Such studies are difficult to performin vivo. However, in c-fos-lacZ rats the β-gal activity in hippocampus and cortex after pentylenetetrazole (PTZ) treatment was highest at 2 hr and declined after 3 hr but was still detectable at 4 hr (Kasof et al., 1995). Moreover, in those studies the disappearance of Fos-ir was faster than β-gal activity after PTZ treatment. Our observations indicate that after 1 hr of sleep, both Fos-ir and β-gal activity are reduced. This strongly suggests the possibility of activation of a proteolytic pathway during sleep that targets both Fos and the Fos-LacZ fusion protein. Rechsteiner and Rogers (1996) have proposed that the presence of polypeptide sequences enriched in proline (P), glutamic acid (E), serine (S), and threonine (T) (PEST) in c-Fos renders it more susceptible to proteolytic degradation. There is also emerging evidence that Fos is eliminated by ubiquitinylation (Hermida-Matsumoto et al., 1996). The decline in Fos and β-gal staining with sleep shows that the fused protein that is detected in the nucleus is degraded in its entirety. In addition, β-galactosidase does not contain PEST sequence. It is therefore more likely that the fusion protein is ubiquitinylated, resulting in its total degradation by trafficking to the proteosome. The exact nature of this pathway and its possible activation at the onset of sleep is yet to be studied.
Our results thus confirm previous observations that levels of Fos increase during enforced wakefulness. These levels decrease within 1 hr of sleep. The regulation of transcription of c-fos and activation of putative proteolytic pathways may contribute to these changes. These observations demonstrate that the regulation of gene expression and modulation of their protein products can occur with short periods of sleep. Moreover, this process appears to be similar across different species.
Footnotes
This research was supported by the Veterans Administration Medical Research Service and National Institutes of Health Grant NS30140. J.I.M. was supported in part by National Institutes of Health Cancer Center Support CORE Grant P30 CA21765 and by the American Lebanese Syrian Associated Charities. Parts of this study were presented at the 1995 Annual Meeting of The Society for Neuroscience. We thank Dr Mahesh Thakkar for helpful discussions.
Correspondence should be addressed to Priyattam J. Shiromani, Veterans Administration Medical Center and Harvard Medical School, Research 151, 940 Belmont Street, Brockton, MA 02401.
REFERENCES
- 1.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirelli C, Tononi G. Changes in protein phosphorylation patterns in the brain during the sleep-waking cycle. Soc Neurosci Abstr. 1996;22:688. [Google Scholar]
- 3.Cirelli C, Pompeiano M, Tononi G. Fos-like immunoreactivity in the rat brain in spontaneous wakefulness and sleep. Arch Ital Biol. 1993a;131:327–330. [PubMed] [Google Scholar]
- 4.Cirelli C, Pompeiano M, Tononi G. Fos expression in the rat brain after variable periods of sleep deprivation. Sleep Res. 1993b;22:595. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 5.Cirelli C, Pompeiano M, Tononi G. Sleep deprivation and c-fos expression in the rat brain. J Sleep Res. 1995;4:92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 6.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: A role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 7.Curran T, Morgan JI. Barium modulates c-fos expression and post-translational modification. Proc Natl Acad Sci USA. 1986;83:8521–8524. doi: 10.1073/pnas.83.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franken P, Tobler I, Borbely AA. Cortical temperature and EEG slow-wave activity in the rat: analysis of vigilance state related changes. Pflügers Arch. 1992;420:500–507. doi: 10.1007/BF00374625. [DOI] [PubMed] [Google Scholar]
- 9.Gillis P, Malter PS. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine and oncogene mRNAs. J Biol Chem. 1991;266:3172–3177. [PubMed] [Google Scholar]
- 10.Grassi-Zucconi G, Menegazzi M, Carcereri De Prati A, Bassetti A, Montagnese P, Mandile P, Cosi C, Bentivoglio M. c-fos mRNA is spontaneously induced in the rat brain during the activity period of the circadian cycle. Eur J Neurosci. 1993;5:1071–1078. doi: 10.1111/j.1460-9568.1993.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 11.Grassi-Zucconi G, Giuditta A, Mandile P, Chen S, Vescial S, Bentivoglio M. c-fos spontaneous expression during wakefulness is reversed during sleep in neuronal subsets of the rat cortex. J Physiol (Paris) 1994;88:91–93. doi: 10.1016/0928-4257(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 12.Hermida-Matsumoto ML, Chock PB, Curran T, Yang DCH. Ubiquitinylation of transcription factors c-Jun and c-Fos using reconstituted ubiquitinylating enzymes. J Biol Chem. 1996;271:4930–4936. doi: 10.1074/jbc.271.9.4930. [DOI] [PubMed] [Google Scholar]
- 13.Kasof GM, Mandelzys A, Maika SD, Hammer RE, Curran T, Morgan JI. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J Neurosci. 1995;15:4238–4249. doi: 10.1523/JNEUROSCI.15-06-04238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- 15.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 16.Oberdick J, Smeyne RJ, Mann JR, Zackson S, Morgan JI. A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science. 1990;248:223–226. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- 17.O’Hara BF, Young KA, Watson FL, Heller HC, Kilduff TS. Immediate early gene expression in brain during sleep deprivation: Preliminary observations. Sleep. 1993;16:1–7. doi: 10.1093/sleep/16.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Pompeiano M, Cirelli C, Tononi G. Effects of sleep-deprivation on Fos-like immunoreactivity in the rat brain. Arch Ital Biol. 1992;130:325–335. [PubMed] [Google Scholar]
- 19.Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 20.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Pharmacol. 1996;21:267–271. [PubMed] [Google Scholar]
- 21.Schilling K, Luk D, Morgan JI, Curran T. Regulation of a fos-lacZ fusion gene: A paradigm for quantitative analysis of stimulus-transcription coupling. Proc Natl Acad Sci USA. 1991;88:5665–5669. doi: 10.1073/pnas.88.13.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 23.Shiromani PJ, Winston S, Malik M, McCarley RW. Rapid decline in Fos-LI in association with recovery sleep that follows total sleep deprivation. Sleep Res. 1994;23:36. [Google Scholar]
- 24.Smeyne RJ, Schilling K, Robertson L, Luk D, Oberdick J, Curran T, Morgan JI. fos-lacZ transgenic mice: mapping sites of gene induction in the central nervous system. Neuron. 1992;8:13–23. doi: 10.1016/0896-6273(92)90105-m. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg JL, Macgregor-Leon PF, Curran T, Morgan JI. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes and seizure. Neuron. 1989;3:359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]